Abstract
Iron is an essential micronutrient and is important not only in carrying oxygen but also to the catalytic activity of a variety of enzymes. In the fetus, it is vital to the synthesis of hemoglobin and in brain development. Iron deficiency (ID) anemia in pregnancy is a common problem, even in high-income country settings. Around 50% of pregnant women worldwide are anemic, with at least half of this burden due to ID. Iron supplements are widely recommended and used during pregnancy globally. However, the evidence on the extent of benefit they contribute to the offspring's health is not well established, and their routine use has its side effects and drawbacks. Dietary iron intake is difficult to assess accurately and it is unlikely to be sufficient to meet the demands of pregnancy if women start with inadequate body iron stores at conception. Evidence from experimental animal models suggests that maternal ID during pregnancy is associated with fetal growth restriction, as well as offspring obesity and high blood pressure later in life. The possible biological mechanisms for this observed association may be due to ID-induced changes in placental structure and function, enzyme expression, nutrient absorption, and fetal organ development. However, such evidence is limited in human studies. Prenatal ID in experimental animal models also adversely affected the developing brain structures, neurotransmitter systems, and myelination resulting in acute brain dysfunction during the period of deficiency and persistence of various postnatal neurobehavioral abnormalities as well as persistent dysregulation of some genes into adult life after iron repletion pointing to the possibility of gene expression changes. The evidence from human population studies is limited and heterogeneous and more research is needed in the future, investigating the effects of ID in pregnancy on future offspring health outcomes.
Keywords: iron, pregnancy, nutrition, cognitive function, cardiovascular disease, developmental origins
Introduction
Why Is Iron Important?
The human body requires iron for essential physiological functions including oxygen transport, hemoglobin (Hb) and myoglobin synthesis, and cell growth and differentiation.1 It is vital for the function of body enzymes necessary for electron transfer and oxidation-reduction reactions.2 Its deficiency limits oxygen delivery to cells. In the fetus, iron is used to synthesize Hb,3 and is essential in brain development.4 The size of iron stores required at each stage of pregnancy to ensure an optimal outcome for the mother and the child is still not exactly known.5
Iron balance in the body is determined by several elements: iron intake and absorption, iron loss, and body iron stores. Inadequate iron intake leads to enhancing dietary iron absorption, mobilizing body iron stores, reducing iron transport to the bone marrow, and eventually lowering Hb levels leading finally to anemia.2 Genetics also influence body iron. Women who carry a C282Y mutation in the hemochromatosis (HFE) gene are more likely, in the homozygous state, to suffer from hemochromatosis, a condition which is characterized by iron overload in the liver, through increased intestinal absorption of iron. About 12 to 20% of northern Europeans are heterozygotes for this mutation.6 These HFE gene mutation carriers are usually asymptomatic. However, they tend to have higher total body iron stores.7 8
During pregnancy, all the iron delivered to the fetus comes from maternal stores, absorption of dietary iron, or turnover of maternal erythrocytes.9 As there is an increased demand for iron during this period to cover the mother and the baby's needs, this is likely to affect iron balance in the body leading to maternal iron deficiency, particularly if the pregnancy starts with inadequate iron stores.
Stages of Iron Deficiency
Iron deficiency refers to a spectrum ranging from iron depletion to iron deficiency anemia (IDA). Women can experience one or more of these stages at different time points prior to conception, during pregnancy, and postpartum. The first stage is iron depletion when the amount of stored iron, which is measured by serum ferritin (sF) concentrations, is reduced; however, the amount of transport and functional iron may not be affected. Those with iron depletion do not have iron stores to mobilize if the body requires additional iron, as in the case of pregnancy.10 The cutoff level for iron depletion according to World Health Organization guidelines is sF less than 15 μg/L.11 This is the most common clinical test used to diagnose iron depletion in pregnancy. This leads to the second stage, which is iron-deficient erythropoiesis (IDE). In this stage, stored iron is depleted and transport iron, measured by transferrin saturation (TS), is reduced further. The amount of iron absorbed is not sufficient to replace the amount lost or to provide the amount needed for growth and function. The shortage of iron limits red blood cell production and results in increased erythrocyte protoporphyrin concentration and increased serum transferrin receptor (sTfR) levels.2 10 This in turn leads to the development of IDA.
Anemia accounts for 9% of the total disability from all conditions in 2010, with children younger than 5 years and women having the highest burden.10 IDA is the most common etiology of anemia. It is defined as anemia accompanied by depleted iron stores and signs of a compromised iron supply to the tissues. It is the most severe form of ID. Shortage of iron stores, transport, and functional iron result in reduced Hb production leading to a fall in its blood levels, in addition to low sF, low TS, increased sTfR, and erythrocyte protoporphyrin concentrations.10
Iron Requirements during Pregnancy
During pregnancy, extra iron is required to cover the increasing red cell mass, plasma volume, and the growth of the fetoplacental unit. The body's capacity to increase absorption during pregnancy starts with around 8% of ingested iron in the first trimester and progressively increases to 37% by 36 weeks' gestation.12 One study, when all women had sF >12 μg/L at recruitment in the first trimester, found the proportion of ferrous iron absorbed to be 7, 36, and 66% in gestational weeks 12, 24, and 36, respectively, compared with around 11% in nonpregnant women.13 It appears that the increased absorption of iron during pregnancy is elicited by depleted iron stores. This is demonstrated an inverse relationship between sF and iron absorption during pregnancy.13 14 15
The average total amount of iron which a women needs to mobilize during her pregnancy is 1,200 mg.16 The fetus takes up approximately 400 mg over full gestation, with up to 175 mg accumulating in the placenta. Pregnant women require an extra 1 mg/day in the first trimester, 4 to 5 mg/day in the second trimester, and a minimum of an extra 6 mg/day in the third trimester if they were to meet their pregnancy iron demands.12 However, it is still unlikely that iron requirements during late pregnancy can be met through diet alone, even with optimal absorption, if the pregnancy starts with inadequate iron stores.17 Therefore, a woman must enter pregnancy with iron stores of ≥300 mg if she is to meet her requirements fully.18 In fact, in a study that assessed iron status in early pregnancy, women with initial iron depletion (sF < 12 μg/L) were more likely to have iron depletion and ID (sF < 12 μg/L and TS <16%) throughout pregnancy compared with women who start their pregnancy noniron depleted (sF > 12 μg/L), despite the iron-depleted women receiving iron supplements.19
Epidemiology of Iron Deficiency in Pregnancy
ID remains the leading single nutrient deficiency in the world.20 It is estimated to affect 1 to 2 billion people, with women of childbearing age, infants, and young children particularly at risk. Around 50% of pregnant women worldwide are anemic, with at least half of this burden due to ID.20 21 The prevalence of IDA varies from 31% of pregnant women in South America to 64% in South Asia, with approximately 88% in India alone.2 Up to 40% of women worldwide have very low iron stores.22 ID during pregnancy is not just a problem in low- and middle-income countries. It is also common in high-income countries.23 24 25 About 25 to 40% of pregnant women in Western societies are estimated to have ID.26 with this problem being more pronounced in lower socioeconomic groups.27 In Denmark, for example, 42% of women of childbearing age were found to have small iron reserves.24
In the next two sections, this review presents the evidence for the association of maternal iron status in pregnancy with cardiovascular disease (CVD) risk in the offspring and the long-term neurobehavioral consequences of fetal/neonatal ID.
Maternal Iron Status in Pregnancy and Cardiovascular Disease Risk in the Offspring
Fetal life is a period of rapid development. Inadequate or imbalanced maternal nutrition during this period can alter physiological structures and/or functions leading to an increased risk of chronic disease in later life.28 Fetal growth and development is likely to be most sensitive to maternal dietary deficiencies during early pregnancy.9 Birth weight has been strongly linked to CVD morbidity and mortality. The initial finding of Barker and colleagues 25 years ago of an association between weight at birth and mortality from ischemic heart disease has resulted in extensive research into the developmental origins of health and disease.29 30
Maternal ID during pregnancy in animal models results in the development of offspring obesity, hypertension, and other adverse cardiovascular outcomes in the long term.31 32 33 34 35 36 This effect is observed even when the pups maintain normal iron levels throughout postnatal life.31 32 33 34
Pups of rat models fed an iron-deficient diet prior to and throughout pregnancy have higher mortality rate, are born smaller, and have larger hearts and smaller kidneys and spleens. Raised blood pressure in males born to mothers in the intervention group was observed despite the offspring having a normal iron status.33 In another study, systolic blood pressure was raised in both males and female offspring of iron-restricted dams at 3 months of age.37 The postnatal rise in systolic blood pressure associated with maternal anemia during pregnancy was not related to the greater placental to birth weight ratio.31 Iron supplementation during early, but not late, pregnancy reverses the effect of ID on birth size and the expression of iron metabolism genes.38
Postulated Biological Mechanisms for the Association between Maternal ID and CVD Risk in the Offspring
Evidence from animal studies shows that birth weight is partly dependent on the mother's iron status and not that of the neonate.39 Thus, maternal ID may affect fetal development by indirect mechanisms. ID-induced changes in maternal metabolism may have downstream effects on placental structure and function, enzyme expression, nutrient interactions, and fetal organ development.39 40 These potential biological pathways are reviewed later and illustrated in Fig. 1.
Fig. 1.
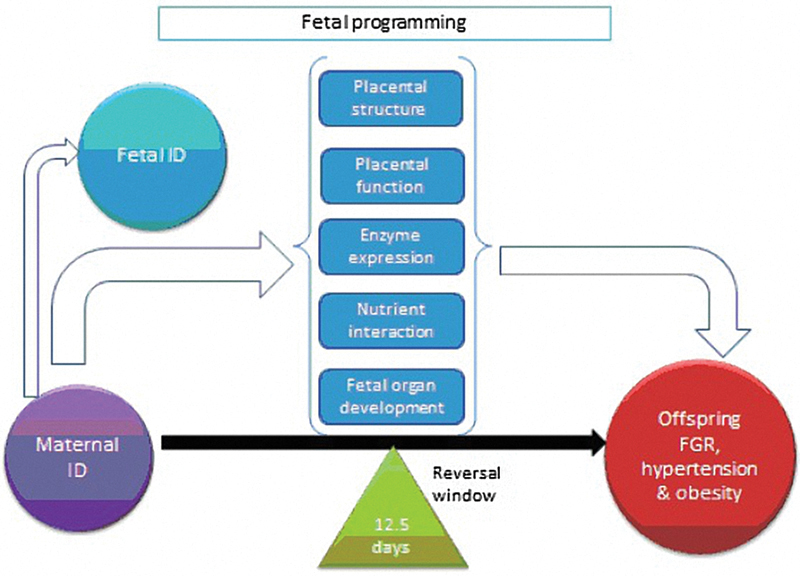
Potential biological pathways for the observed effect of maternal iron deficiency on offspring cardiovascular disease risk. Reversal window of adverse offspring outcomes if iron is administered by 12.5 days of rat gestation.49 ID, iron deficiency; FGR, fetal growth restriction.
Placental Structure and Function
The placenta is the principal organ that delivers nutrients to the developing fetus. Therefore, any stress that interferes with placental development or function is likely to have adverse consequences for the developing fetus. The placental structure is altered in maternal anemia. Changes due to maternal IDA include reduced capillary length and surface area and increased placental vascularization at term with reduced maternal sF and Hb levels.32 37 The surface area of capillaries involved in gas exchange is strongly and inversely related to maternal sF concentrations.41 Maternal anemia has also been shown to be associated with increased placental weight and reduced ratio of fetal weight to placental weight.27 42 The relationship between maternal ID with placental size and birth weight exists across the normal range for these measures and is not just restricted to severely anemic mothers. This increase in placental weight has been interpreted as compensatory placental hypertrophy.
In terms of placental function, increased proinflammatory cytokine, leptin, and tumor necrosis factor levels in the placenta have been associated with ID. Maternal ID has also been shown to cause fetal plasma amino acid and cholesterol and triacylglycerol levels to be decreased, suggesting decreased placental transport of amino acid and nonesterified fatty acids to the fetus.37 The exact mechanisms for these changes are yet unclear.
Enzyme Expression
ID in the mother may reduce the activity of the enzymes that use iron as a cofactor, for example, DNA polymerases as well as enzymes involved in neurotransmitter synthesis and neuronal energy.39 There is some evidence proposing that ID leads to failure in correct protein folding and transport through enzymatic and protein synthesis dysfunction, which in turns leads to cellular dysfunction and apoptosis.43 There is also evidence that maternal ID leads to a reduction in gamma-aminobutyric acid metabolism that is not reversible by postnatal iron supplementation.44 In addition, areas of the brain that are involved in higher cognitive functions have lower cytochrome c oxidase activity in neonatal rats born to ID mothers.2 This will be discussed further in this review under the effects of ID and offspring neurodevelopment.
Nutrient Interactions
The effect of altered iron status on the metabolism of other metals, such as copper, and mediators of cell function during pregnancy has been observed. Generally, ID results in increased copper levels in the liver and rise in serum ceruloplasmin concentrations.39 In pregnancy, maternal ID has a differential effect on copper metabolism in the mother and fetus.34 In the maternal liver, copper levels are inversely correlated with those of iron, while in the fetus both iron and copper levels are reduced. Animal studies have shown that neonatal copper deficiency is associated with developmental abnormalities and altered thyroid and immune function.38
A similar differential effect between mother and fetus is also seen in vitamin A metabolism. Maternal liver retinol levels are reduced in maternal ID, while in the fetus the opposite is seen. As the level of maternal iron decreases, the level of retinol in the fetal liver increases.45 This restriction in nutrient supply may have an impact on fetal development. There is evidence from animal and human studies of teratogenicity of natural and synthetic retinoids through affecting the development of cephalic neural-crest cells and their derivatives and perhaps interfering with the closure of the neural tube.46
Fetal Organ Development
ID may also interfere with normal fetal kidney development by reducing nephron number.39 This may result in the observed association between maternal ID and high blood pressure in the offspring as kidney nephron number is an important determinant of blood pressure. Nephron number is established during kidney development, beyond which point the number cannot be increased.47 Low nephron number reduces the surface area available for filtration, and therefore limits the ability of the kidney to excrete sodium and maintain normal extracellular fluid volume and blood pressure.48 49 Expanding on their earlier work with the Wistar ID model, Lisle et al35 have investigated the effect of maternal ID on the renal morphology of the adult offspring in rats. Their results show a reduction in the number of glomeruli in the kidney of offspring born to ID mothers. Offspring from both control and ID mothers also show an inverse relationship between glomerular number and blood pressure.
Epidemiological Studies
There is some indirect epidemiological evidence supporting the associations observed in animal studies described earlier. Godfrey et al27 found that maternal ID during pregnancy in humans is associated with a high ratio of placental weight to birth weight, which is considered a predictor of adult hypertension. The relationship between maternal iron status in pregnancy and children's blood pressure has been directly investigated in few studies (Table 1). All of them have used maternal Hb as a proxy for maternal iron status in pregnancy, with three of them including iron intake from diet and supplements as an additional marker for maternal iron status.50 51 Table 1 summarizes the characteristics of studies that have investigated the associations of indicators of maternal iron status in pregnancy with long-term health outcomes in the offspring.
Table 1. Characteristics of epidemiological studies investigating the association of maternal iron status and long-term offspring blood pressure levels.
| Study identification | Design | Study size (number) | Exposure assessment | Main outcome | Main finding |
|---|---|---|---|---|---|
| Belfort et al (2008)50 | Prospective cohort | 1,167 | Semiquantitative FFQ in the first second trimesters, Hb and MCV extracted from electronic laboratory database | Offspring BP at 3 y | Maternal iron intake positively associated with offspring BP |
| Brion et al (2008)51 | Prospective cohort | 1,255 (Hb) 7,484 (supplement) 7,130 (diet) |
FFQ at 32 wk, questionnaires at 18 and 32 wk for supplements, Hb extracted from medical records |
Offspring BP at 7 y | Maternal anemia associated with lower offspring BP in women not taking supplements |
| Law et al (1991)54 | Historical cohort | 405 | Lowest Hb during pregnancy extracted from medical records | Offspring BP at 4 y | Maternal anemia associated with higher offspring BP |
| Godfrey et al (1994)55 | Prospective cohort | 77 | Lowest Hb out of a maximum of 6 measurements throughout pregnancy | Offspring BP at 10–12 y | Negative association |
| Whincup et al (1994)56 | Historical cohort | 662 | Lowest Hb in pregnancy and change in MCV extracted from medical records | Offspring BP at 9–11 y | No association |
| Bergel et al (2000)53 | Prospective RCT follow-up | 518 | Lowest Hb during pregnancy recorded in the trial | Offspring BP at 5–9 y | Positive association |
| Alwan et al (2014)52 | Prospective cohort | 2,958 | Early pregnancy Hb | Offspring BP, arterial stiffness and endothelial function at age 10 | No association |
| Alwan et al (2012)57 | Prospective cohort, Mendelian randomization | 348 | Maternal genotype at single nucleotide polymorphisms in the HFE gene as instrumental variables | Offspring BP, waist circumference and body mass index in adulthood | No association |
Abbreviations: BP, blood pressure; FFQ, food frequency questionnaire; HFE, hemochromatosis; MCV, mean corpuscular volume; RCT, randomized controlled trial.
Brion et al51 analyzed data from the Avon Longitudinal Study for Parents and Children cohort with a sample size of 1,255 women in Bristol, United Kingdom, with Hb information. In this study, there was an association between maternal anemia and lower offspring systolic blood pressure at 7 years only in women who did not take iron supplements during pregnancy.51 This is a direction of association opposite to what is expected from animal study findings. A more recent analysis of the Avon Longitudinal Study for Parents and Children data examining the links between early pregnancy maternal Hb and offspring blood pressure, arterial stiffness, and endothelial function at age 12 years found no evidence of association.52 In this study, there was evidence of a modest association between taking iron supplements in pregnancy and lower offspring systolic blood pressure. In another study with a sample size of 1,167 pregnant American women, there was no association between first and second trimester maternal Hb and anemia with offspring blood pressure at 3 years. However, offspring blood pressure was positively associated with first trimester iron intake, again in contrast to animal studies findings.50
In a follow-up of a calcium supplementation trial in pregnancy in Argentina, Bergel et al53 found a positive association between maternal Hb during pregnancy and offspring systolic blood pressure at 5 to 9 years. In contrast, Law et al found an association between maternal anemia in pregnancy (<10 g/dL) and higher offspring systolic blood pressure at 4 years of 405 British children.54 Godfrey et al55 also found a negative association between systolic blood pressure of 77 Jamaican children with an average age of 11 years and lowest maternal Hb during pregnancy.55 Whincup et al56 found no evidence of association between lowest maternal Hb and change in mean corpuscular volume during pregnancy with blood pressure at 9 to 11 years of 662 children. In an instrumental variable analysis, using maternal C282Y mutation on the HFE gene as an instrument for mother's iron status, there was no evidence of association between maternal iron status and adult offspring's blood pressure and adiposity.57
To summarize, the direction of association between maternal Hb and offspring blood pressure during childhood in two of these studies was against that expected from the results of animal studies.51 53 Two studies supported the direction of association observed in animal studies, that is, maternal anemia associated with higher offspring blood pressure,54 55 while the remaining three found no association.50 52 56 57 However, it is important to note that all of these studies did not use a direct biomarker of iron status such as sF, and only three of them assessed maternal iron intake as an exposure.50 51 52 57
Although epidemiological studies support a strong association with immediate offspring birth outcomes, such as size at birth, such an association seems to attenuate the older offspring. Therefore, the effect of ID could be real but potentially modifiable by later diet and environment exposures throughout childhood and adolescence. This is hard to examine with the available data. A long-term follow-up of a birth cohort with detailed dietary and biomarker assessment of iron status during and, preferably, before pregnancy and measurement of a combination of offspring cardiovascular markers from birth to later in life could potentially answer this question. A long-term follow-up of a randomized controlled trial of iron supplements during pregnancy might also shed light on these relationships.
Another explanation for the inconclusive findings of epidemiological studies compared with animal studies is that the range of variation in iron status/intake is too narrow in humans to show an association. Extreme ID in animals cannot be replicated in observational studies in high-income countries where most participants are nutrient-replete. This can only be explored further in low-income countries where pregnant populations have more severe ID, spanning a long period of time before conception, or in natural human experiments such as the Dutch hunger winter58 and the siege of Leningrad.59
The offspring adverse changes observed in animal studies could be due to severe ID in the mother rather than moderate or mild. Therefore, generalizability of study results should be carefully considered when considering evidence from different parts of the world, particularly concerning the effect of iron supplements. Most supplement trials are conducted in low- or middle-income countries, while most observational studies that assess dietary intake in detail are conducted in high-income countries.
The findings can also be potentially masked by measurement bias in population studies. Animal studies usually involve accurate exposure assessment, as they are usually controlled intervention studies. This compares to the big potential measurement bias involved in observational population studies, particularly when it comes to dietary assessment methods from which iron intake is derived. Measurement error is profoundly associated with all of the commonly used dietary assessment methods in population health research such as food frequency questionnaires, food diaries, and interviewer-administered dietary recalls.
Fetal Iron Deficiency and Neurodevelopment
Significant brain growth occurs from 34 weeks postconception until 2 years of age with peak synapse development. During late fetal and early neonatal life periods, regions such as the hippocampus, the visual and auditory cortices, and the striatum undergo rapid development characterized by the morphogenesis and synaptogenesis that make them functional.60 During these periods, environmental influences may modify gene expression through epigenetic mechanisms, whereby gene function is altered through the processes of DNA methylation, histone modification, and the modulating effect of noncoding RNAs, without the alteration of the gene sequence per se. It has been shown in animal and in human studies that nutrition is one of the most salient environmental factors, and that nutrition can have a direct effect on gene expression. Evidence suggests that the timing of nutritional deficiencies can significantly affect both the morphological and the neurochemistry and neurophysiology brain development.61
It is well established that there is a correlation between IDA early in life and poor neurodevelopmental outcome.62 A large number of studies in humans and in animal models with reviews by Lozoff et al,63 64 Georgieff,65 and Beard66 67 demonstrated that early ID has a negative effect on the brain processes with concurrent neurobehavioral abnormalities. In rodent models, the effects of gestation ID were most prominent on dendritic structure, monoamine transmitter metabolism, and myelination,63 64 as well as in altering gene expression68 with a region-specific impact on neuronal development.69
Iron Deficiency and Human Neurodevelopment
Late gestational and neonatal ID could be related to maternal conditions during pregnancy, including severe IDA, placental vascular insufficiency, diabetes mellitus, and cigarette smoking.70 It has also been shown that infants exposed to alcohol prenatally are at an increased risk of IDA.71 Maternal anemia also increases the risk of low birth weight, either due to premature birth or fetal growth restriction, which is associated with IDA as well as with delayed neurocognitive development. Unfortunately, the neurocognitive complications of iron deficiency during the critical prenatal period of brain development may be difficult to correct, persisting into adulthood.72
The average concentration of iron in the brain is far higher than that of all other metals, except zinc. Iron is required by enzymes involved in specific brain functions, including myelination and synthesis of the neurotransmitters serotonin and dopamine, a precursor to epinephrine and norepinephrine. Unless maternal ID is severe, term infants are generally considered to be protected from IDA through the first few months of life.73
At birth, the iron status can be roughly assessed by determining the umbilical cord sF levels. Ferritin concentrations were lower for the preterm infants compared with term infants, although both groups had similar 5th centile values: <40 μg/L in term infants and <35 μg/L in preterm infants. Infants with sF in the lower quartile may benefit from close monitoring of their iron status.74 Maternal and neonatal iron status are related only if maternal iron status is compromised.75
An association between relatively low umbilical cord sF levels and lower scores on certain mental and psychomotor tests at 5 years of age has been reported, where a poor intrauterine iron status (low ferritin) was associated with less favorable mental and psychomotor development. Compared with children with cord ferritin in the 2 median quartiles, those in the lowest quartile scored lower on every test and had significantly worse language ability, fine-motor skills, and tractability. They were also 4.8-fold more likely to score poorly in fine-motor skills and 2.7-fold more likely to have poor tractability than children in the median quartiles.76
Infants who experience ID during the first 6 to 12 months of life are likely to experience persistent effects of the deficiency that alter functioning in adulthood. Depending on the stage of development at the time of ID, there may be an opportunity to reverse adverse effects, but the success of repletion efforts appear to be time dependent.67
A systematic review of trials on non-ID subjects that addressed the effects of iron supplementation during early life on the psychomotor and mental development of children concluded that iron supplementation of infants may positively affect the psychomotor development of children but may not influence their mental development and behavior.77
Epidemiologic Studies on Perinatal ID and Neurodevelopment in Humans
ID during pregnancy showed a relation to the neonate's general autonomous response, motor performance, and self-regulation capabilities as demonstrated in a study in Spain among 216 healthy and well-nourished pregnant women and their term, normal weight newborns. The neonatal behavior was assessed by the Neonatal Behavior Assessment Scale (NBAS). ID during pregnancy was related to some aspects of neonatal behavior and the associations are different depending on the time of gestation. During the initial stages of pregnancy, ID is related to the general autonomous response of the neonate, whereas during the later stages of pregnancy, ID is also related to neonatal motor maturity and self-regulation.78
IDA adversely affects the allocation of neurophysiologic resources to attention and recognition memory during the processing of information about familiar and unfamiliar stimuli. This delay in cognitive development may reflect alterations in efficiency of central nervous system functions that seem related to early ID. Several important developing central nervous system processes, such as myelination, dendritogenesis, synaptogenesis, and neurotransmission, are highly dependent on iron-containing enzymes and hemoproteins, especially in the striatum and the hippocampus.79 In humans, the hippocampus undergoes a period of rapid growth early in development from late gestation through the first year of life, coincident with the emergence of hippocampal-dependent recognition memory.80
Infant motor development in a cohort of 418 pregnant women in Ha Nam province in Vietnam was assessed by the Bayley of Infant and Toddler Development Motor Scales (BSID-M) at the age of 6 months. There were direct adverse effects on infant BSID-M scores at 6 months of age due to antenatal anemia in late pregnancy.81
In a sample of 148 low-income Peruvian women and their newborn infants, results indicated that lower levels of neonatal Hb and serum iron were related to higher levels of negative emotionality and to lower levels of alertness and soothability. For the most part, relations between neonatal iron measures and neonatal temperament were linear, operating across the full range of iron values.82
Data indicated that low iron status, both measured by anemia and ferritin levels, is related to poorer neurobehavioral status in premature infants who showed increased reflex scores, reflecting a greater percentage of abnormal reflexes.83
In utero latent ID as measured by cord ferritin levels was associated with abnormal auditory neural myelination in preterm infants.84 Iron-deficient infants of diabetic mothers (IDM) with low neonatal ferritin concentrations (<35 μg/L) have impaired auditory recognition memory processing at birth compared with iron-sufficient IDM (ferritin >35 μg/L)85 Also, infants with cord sF concentrations <35 μg/L had electrophysiologic evidence of abnormal auditory recognition memory, where they did not discriminate a familiar stimulus (e.g., maternal voice) from a novel stimulus (e.g., stranger's voice) with the same robustness as iron-sufficient infants.85 86
Diabetic pregnancies are characterized by chronic metabolic insults, including ID, that place the developing brain at risk for memory impairment later in life. Electrophysiological results in 3.5-year-old children suggested that both encoding and retrieval processes were compromised. These findings support the hypothesis that prenatal iron deficiency leads to alterations in neural development that have a lasting impact on memory ability.87
Early Iron Supplementation
There is a dearth of epidemiological evidence from well-designed intervention trials demonstrating the impact of maternal iron supplementation on the cognitive development of healthy children. Available studies have shown variable effects on offspring cognition in different populations.
In a randomized placebo-controlled iron supplementation trial among 430 Australian pregnant women, the authors reported that they could not find any difference between an iron supplemented versus placebo group in the IQ of children 4 years of age on the Stanford-Binet Intelligence Scale, despite maternal iron status having improved by supplementation, suggesting that supplementing pregnant women who are generally well nourished with iron may not confer any additional health benefits.88
In a small sample size of mothers from a higher socioeconomic background and with better feeding practice, a study in Canada found no evidence that better maternal iron status enhanced cognitive development in 6-month-old infants, measured on the Brunet-Lezine Scale of Psychomotor Development of Early Childhood and the Bayley Scales of Infant Development.89
On the other hand, a cohort follow-up of 676 children aged 7 to 9 years found evidence that maternal prenatal supplementation with iron and folic acid was positively associated with general intellectual ability, some aspects of executive function, and motor function, including fine motor control, in offspring at 7 to 9 years of age in a rural area of Nepal, where ID is highly prevalent.90 The study, however, does not clarify the effects of iron and folic acid separately.
A study that included 2-year follow-up of 850 children born to women who participated in a double-blind cluster randomized controlled trial of prenatal micronutrient supplementation in western rural China showed that the prenatal IDA group showed a significantly lower mental development index at 12, 18, and 24 months of age. Prenatal supplementation with sufficient iron protects child development even when the woman's IDA was not properly corrected in pregnancy.91
Animal Models of Prenatal Iron Deficiency
The neurobehavioral aspects of early ID have been explored in several animal models including mice, rats, and nonhuman primates. The structures of the brain can become abnormal because of ID either in utero or in early postnatal life because iron is essential for proper neurogenesis and differentiation of certain brain cells and brain regions. Oligodendrocytes, which are responsible for making myelin, are particularly sensitive to iron deprivation, which results in altered composition and amount of myelin in white matter.66 The brain is not metabolically homogenous with certain areas demonstrating greater iron-dependent metabolic activity early in life than other areas. Studies in rodents clearly identify the hippocampus and striatum among the main areas in which morphology is altered.67 Most studies focused on the major domains of the effects of early life ID on the brain, which included abnormalities in myelination, monoaminergic and glutamatergic neurotransmission, hippocampal morphology and metabolism, and gene expression.63 65 92 93 Other studies explored the effect of iron interventions, which may reverse the abnormalities in the affected brain regions depending on when in development the iron repletion occurs. Knock out mice models were developed to elucidate the specific role of iron in the development of particular brain cell types independent of the neuropathological processes such as anemia, tissue hypoxia, and stress resulting from total body ID.69 80 94
The effect of dietary iron on fetal growth in pregnant mice was evident when pregnant mice fed an iron-deficient diet had fewer viable pups, and had pups with shorter crown–rump length, decreased body weight, and decreased brain iron.95
Rodent studies showed that the effects of ID during gestation and lactation could persist into adulthood despite restoration of iron status at weaning.67 Long-term effects of prenatal ID on the brain may be due to altered regulation of genes caused by epigenetic phenomena altering chromatin structure and gene expression early in life.96
Nonhuman primate models, where more human-like behaviors can be assessed, show that prenatal IDA leads to more impulsive behavior, while postnatal IDA results in more passive, withdrawn behavior that is reminiscent of the findings in humans.97 A study in rhesus monkey explored the association of monoamine oxidase A (MAOA) gene polymorphisms and gestational ID on cognitive tasks in the offspring. ID combined with low-MAOA genotype showed distinctive effects on reward preference and problem solving, while ID in hi-MAOA juveniles modified response inhibition. Given the incidence of ID and MAOA polymorphisms in humans, this interaction could be a significant determinant of cognitive performance.98
Studies on Iron Deficiency and the Hippocampus
Animal studies showed structural impairments of the hippocampus in prenatal ID and altered composition and amount of myelin in white matter.99 ID affected neurogenesis and neurochemistry during brain development and altered the dendritic structure in the hippocampus.100 The hippocampus also exhibited altered neurometabolism and gene expression, decreased energy availability and growth factor expression, abnormal dendritogenesis, decreased long-term potentiation, and abnormal hippocampus-based learning and memory.65
Comparing the effects on offspring when pregnant rats were fed a diet that either contained iron or deficient in iron showed that there was less myelination of subcortical white matter and the fimbria of the hippocampus in the pups of iron-deficient mothers leading to altered behavioral outcomes such as novel object recognition task.101 Gestational ID effects on the hippocampus functions can persist to adulthood as seen in the study where formerly ID rats exhibited delayed acquisition of the hippocampus-dependent task with no differences from controls on the striatum- and amygdala-dependent tasks. These findings likely reflect long-term reduction in hippocampus-dependent learning and preserved function in other brain structures.102 Fetal/neonatal IDA altered dendrite branching and spine morphology in rat hippocampus area cornu ammonis 1 (CA1) during its period of rapid apical dendrite growth between P15 and P30.103 A heart rate trace fear conditioning procedure in rats showed that perinatal nutritional ID impaired hippocampus-dependent learning. The ID pups were impaired in trace fear conditioning which persisted to adulthood.104
Transgenic mice models that express tetracycline transactivator regulated, dominant negative transferrin receptor (DNTfR1) in hippocampal neurons, disrupting TfR1-mediated iron uptake specifically in CA1 pyramidal neurons, were developed to examine long-term effects of early ID on these neurons independent of total body ID. Findings demonstrated a critical requirement for iron during the period of rapid hippocampal structural and functional development where ID in CA1 neurons resulted in reduced brain-derived neurotrophic factor (BDNF) expression and appearance of critical period markers that likely contribute to deficits in spatial memory and apical dendrite structure. Early, but not late, iron repletion restored spatial memory, dendrite structure, and critical period markers in adult mice.80
Similarly, in the solute carrier family 11, member 2 (Slc11a2) knockout mouse model where the effect of ID without anemia can be explored in hippocampal neurons in vivo, there was lower hippocampal iron content; altered developmental expression of genes involved in iron homeostasis, energy metabolism, and dendrite morphogenesis; reductions in markers for energy metabolism and glutamatergic neurotransmission on magnetic resonance spectroscopy; and altered pyramidal neuron dendrite morphology in area 1 of Ammon horn in the hippocampus.94
A study on rat hippocampus prenatally showed that phosphocreatine, glutamate, N-acetylaspartate, aspartate, gamma-aminobutyric acid, phosphorylethanolamine and taurine concentrations, and the phosphocreatine/creatine ratio were elevated in the iron-deficient group of rats. These neurochemical alterations suggest persistent changes in resting energy status, neurotransmission, and myelination in perinatal ID.105 The iron supplementation dose for perinatal ID in rats differentially altered the neurochemical profile of the prefrontal cortex and hippocampus in adults. The neurochemical changes suggest altered glutamatergic neurotransmission, hypomyelination, and abnormal phospholipid metabolism in the formerly iron-deficient (FID) hippocampus.106
The study by Clardy et al92 reported 334 significantly changed genes in the 21-day-old rats that were born by ID dams. Several significant gene clusters were identified from the 334 significantly changed genes, including myelin-related, signal transduction; channel pore class transporter and α-type channel activity; ion channel activity; DNA binding; transitional metal binding; and solute carrier family members representing.92 After postnatal day 20, all animals were fed iron-sufficient diet and in the 6-month-old animals, five significant downregulated genes were identified. The low-level relevant proteins in the cytoplasm and nucleus led to decreased cytoskeletal stability, decreased nucleic acid translation, and decreased responsiveness to oxidative stress. The gene changes at this later time indicate that iron repletion was not capable of overcoming all the development perturbations.92
Hippocampal BDNF regulates multiple aspects of hippocampal development and function. BDNF was assessed in adult rats that had been iron deficient during the fetal and neonatal periods. Fetal–neonatal iron deficiency lowered BDNF function beyond the period of iron deficiency in the hippocampus. The lower adult hippocampal BDNF activity may underlie the persistence of learning deficits seen after early-life iron deficiency.70 BDNF is a nerve growth factor that affects neuronal maturation, synaptic plasticity, and processes important for hippocampal-dependent learning and memory. Reduced BDNF signaling seen in the FID adult rat could underlie the persistent cognitive impairments. The mechanisms underlying the downregulation of BDNF remain unclear, but one possibility of long-term dysregulation is that of stable epigenetic modifications. However, this dysregulation was not associated with epigenetic modifications across multiple generations because the adverse effects of early ID on hippocampal gene expression observed in the F1 generation were not present in the F2 generation.107 A recent study indicated that epigenetic modifications could be the potential mechanism to explain the long-term repression of Bdnf following fetal and early postnatal iron deficiency.68
IDA altered expression of nine cytoplasmic and transmembrane proteins that are critical for dendrite growth and regulate cytoskeletal structure in the rat hippocampus. Expression of these genes recovered with iron treatment during development, but these genes were ultimately suppressed in adulthood in FID animals, suggesting a programming effect consistent with the developmental origins of adult disease hypothesis.103 Early-life IDA altered the expression of critical genes for the expression of parvalbumins and perineuronal nets involved in neuronal dendritic structural plasticity of the hippocampus, thus contributing to delayed maturation of electrophysiology, and learning and memory behavior in rats.108
Enzymes, Proteins, and Neurotransmitters
It is believed that iron is involved with different enzyme systems in the brain, including the cytochrome c oxidase enzyme system in energy production, tyrosine hydroxylase for dopamine receptor synthesis, delta-9-desaturase for myelination, fatty acid synthesis, and ribonucleotide reductase for brain growth regulation.61 109 110 111 Regionally distributed losses of cytochrome c oxidase, a marker of neuronal energy status, were particularly prevalent in the hippocampus and frontal cortex.55 112
Whole brain genomic effects 6 months after early ID anemia in the rat include reductions in myelin basic protein expression and microtubule-associated protein-2, which codes for a scaffolding program important for cytoskeletal stability.92 A major neuropathology was defined by several investigators who noted altered fatty acid concentrations in the iron-deficient brain and postulated that iron-containing enzymes responsible for their synthesis into myelin were compromised.113 114
Perinatal ID was induced using a low-iron diet during gestation and the first postnatal week in male rats. Hippocampal size and neurochemical profile, consisting of 17 metabolites indexing neuronal and glial integrity, energy reserves, amino acids, and myelination, were quantified. The cross-sectional area of the hippocampus was decreased by 12% in the FID group and the hippocampal neurochemical profile was altered (creatine, lactate, N-acetylaspartylglutamate, and taurine and glutamine concentrations). The neurochemical changes suggest suppressed energy metabolism, neuronal activity, and plasticity in the FID hippocampus.115
In rats that had been on iron- deficient diets from midgestation onward, early iron treatment (at 4 days postpartum), but not later iron repletion, normalized brain iron concentrations, monoamine concentrations, and monoamine transporter and receptor densities in most brain regions. These findings suggest the existence of a critical window of opportunity for reversing the detrimental effects of ID in utero on brain development, at least in rats and probably also in humans.99 Another study sought to determine whether earlier iron treatment can normalize deficits of IDA in rats and what iron dose is optimal. Iron treatment at P8 in rats did not normalize all monoamine or behavioral measures after early IDA. Moderate iron treatment improved adult behavior, but higher iron treatment caused brain and behavioral patterns similar to total ID in the short and long term.116
Because insulin-like growth factor (IGF) modulates early postnatal cellular growth, differentiation, and survival, a dietary-induced rat model to assess the effects of gestational ID on activity of the IGF system suggested that IGF dysfunction is in part responsible for hippocampal abnormalities in untreated ID. Early postnatal iron treatment of gestational ID reactivates the IGF system and promotes neurogenesis and differentiation in the hippocampus during a critical developmental period.117
Combined Effects of Iron Deficiency and Other Dysfunctions
Both ID and infection are common during pregnancy and studies have described altered brain development in offspring as a result of these individual maternal exposures. A study in a rat model paired dietary ID during pregnancy and induced prenatal immune activation by bacterial endotoxin liposaccharide. Findings showed that long-term effects of maternal ID and prenatal liposaccharide were additive, such that offspring exposed to both insults displayed more adult behavioral abnormalities than offspring exposed to one alone.118 Another study on the association of ID with thyroid hormone levels during brain development suggested that some of the brain defects associated with Fe deficiency may be mediated through altered thyroidal status and the concomitant alterations in TH-responsive gene transcription.119
Conclusion
Prenatal ID adversely affects the developing brain structures, neurotransmitter systems, and myelination that result in acute brain dysfunction during the period of deficiency as well as persistence of neurobehavioral abnormalities even after complete brain iron repletion. The persistent dysregulation of genes into adult life after iron repletion points to possible changes in expression of these genes. In terms of cardiovascular outcomes in the offspring, there is substantial evidence from animal studies of a negative effect of maternal ID in pregnancy on offspring cardiovascular risk profile. However, evidence from human population studies is limited and heterogeneous. More evidence-based research in the long-term effects of ID during pregnancy is recommended. Evidence from birth cohorts with valid and reliable dietary and biomarker assessment at multiple points in pregnancy, measuring offspring cardiovascular indicators including arterial stiffness and adiposity measures among other conventionally measured birth outcomes, is urgently needed. Follow-up of iron supplementation through randomized controlled trials could shed a light on the long-term effects of increasing iron intake during pregnancy on offspring outcomes.
References
- 1.Cetin I, Berti C, Calabrese S. Role of micronutrients in the periconceptional period. Hum Reprod Update. 2010;16(1):80–95. doi: 10.1093/humupd/dmp025. [DOI] [PubMed] [Google Scholar]
- 2.Vigayaraghaven K. Oxford: Blackwell Publishing; 2004. Iron-deficiency anemias. [Google Scholar]
- 3.Milman N. Iron prophylaxis in pregnancy—general or individual and in which dose? Ann Hematol. 2006;85(12):821–828. doi: 10.1007/s00277-006-0145-x. [DOI] [PubMed] [Google Scholar]
- 4.Lozoff B. Perinatal iron deficiency and the developing brain. Pediatr Res. 2000;48(2):137–139. doi: 10.1203/00006450-200008000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Lynch S. Improving the assessment of iron status. Am J Clin Nutr. 2011;93(6):1188–1189. doi: 10.3945/ajcn.111.015214. [DOI] [PubMed] [Google Scholar]
- 6.Rhodes D A, Raha-Chowdhury R, Cox T M, Trowsdale J. Homozygosity for the predominant Cys282Tyr mutation and absence of disease expression in hereditary haemochromatosis. J Med Genet. 1997;34(9):761–764. doi: 10.1136/jmg.34.9.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cade J E, Moreton J A, O'Hara B. et al. Diet and genetic factors associated with iron status in middle-aged women. Am J Clin Nutr. 2005;82(4):813–820. doi: 10.1093/ajcn/82.4.813. [DOI] [PubMed] [Google Scholar]
- 8.Beutler E, Felitti V J, Koziol J A, Ho N J, Gelbart T. Penetrance of 845G—> A (C282Y) HFE hereditary haemochromatosis mutation in the USA. Lancet. 2002;359(9302):211–218. doi: 10.1016/S0140-6736(02)07447-0. [DOI] [PubMed] [Google Scholar]
- 9.Wu G, Bazer F W, Cudd T A, Meininger C J, Spencer T E. Maternal nutrition and fetal development. J Nutr. 2004;134(9):2169–2172. doi: 10.1093/jn/134.9.2169. [DOI] [PubMed] [Google Scholar]
- 10.Pavord S Myers B Robinson S Allard S Strong J Oppenheimer C; British Committee for Standards in Haematology. UK guidelines on the management of iron deficiency in pregnancy Br J Haematol 20121565588–600. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization . Geneva; 2011. Serum Ferritin Concentrations for the Assessment of Iron Status and Iron Deficiency in Populations. [Google Scholar]
- 12.Whittaker P G, Lind T, Williams J G. Iron absorption during normal human pregnancy: a study using stable isotopes. Br J Nutr. 1991;65(3):457–463. doi: 10.1079/bjn19910104. [DOI] [PubMed] [Google Scholar]
- 13.Barrett J F, Whittaker P G, Williams J G, Lind T. Absorption of non-haem iron from food during normal pregnancy. BMJ. 1994;309(6947):79–82. doi: 10.1136/bmj.309.6947.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Brien K O, Zavaleta N, Caulfield L E, Yang D X, Abrams S A. Influence of prenatal iron and zinc supplements on supplemental iron absorption, red blood cell iron incorporation, and iron status in pregnant Peruvian women. Am J Clin Nutr. 1999;69(3):509–515. doi: 10.1093/ajcn/69.3.509. [DOI] [PubMed] [Google Scholar]
- 15.O'Brien K O, Zavaleta N, Abrams S A, Caulfield L E. Maternal iron status influences iron transfer to the fetus during the third trimester of pregnancy. Am J Clin Nutr. 2003;77(4):924–930. doi: 10.1093/ajcn/77.4.924. [DOI] [PubMed] [Google Scholar]
- 16.Milman N. Iron and pregnancy—a delicate balance. Ann Hematol. 2006;85(9):559–565. doi: 10.1007/s00277-006-0108-2. [DOI] [PubMed] [Google Scholar]
- 17.Milman N. Prepartum anaemia: prevention and treatment. Ann Hematol. 2008;87(12):949–959. doi: 10.1007/s00277-008-0518-4. [DOI] [PubMed] [Google Scholar]
- 18.Bothwell T H Iron requirements in pregnancy and strategies to meet them Am J Clin Nutr 200072(1, Suppl):257S–264S. [DOI] [PubMed] [Google Scholar]
- 19.Ribot B, Aranda N, Viteri F, Hernández-Martínez C, Canals J, Arija V. Depleted iron stores without anaemia early in pregnancy carries increased risk of lower birthweight even when supplemented daily with moderate iron. Hum Reprod. 2012;27(5):1260–1266. doi: 10.1093/humrep/des026. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organisation Micronutrient Deficiencies Geneva 2011
- 21.World Health Organisation Iron Deficiency Anemia Assessment Prevention and Control: A Guide for Program Managers Geneva 2011
- 22.London: Department of Health; 2011. Scientific Advisory Committee on Nutrition. Iron and Health. [Google Scholar]
- 23.Beard J L Iron deficiency: assessment during pregnancy and its importance in pregnant adolescents Am J Clin Nutr 199459(2, Suppl):502S–508S., discussion 508S–510S [DOI] [PubMed] [Google Scholar]
- 24.Milman N, Clausen J, Byg K E. Iron status in 268 Danish women aged 18-30 years: influence of menstruation, contraceptive method, and iron supplementation. Ann Hematol. 1998;77(1-2):13–19. doi: 10.1007/s002770050405. [DOI] [PubMed] [Google Scholar]
- 25.Robinson S, Godfrey K, Denne J, Cox V. The determinants of iron status in early pregnancy. Br J Nutr. 1998;79(3):249–255. doi: 10.1079/bjn19980042. [DOI] [PubMed] [Google Scholar]
- 26.Bergmann R L, Gravens-Müller L, Hertwig K. et al. Iron deficiency is prevalent in a sample of pregnant women at delivery in Germany. Eur J Obstet Gynecol Reprod Biol. 2002;102(2):155–160. doi: 10.1016/s0301-2115(01)00609-1. [DOI] [PubMed] [Google Scholar]
- 27.Godfrey K M, Redman C W, Barker D J, Osmond C. The effect of maternal anaemia and iron deficiency on the ratio of fetal weight to placental weight. Br J Obstet Gynaecol. 1991;98(9):886–891. doi: 10.1111/j.1471-0528.1991.tb13510.x. [DOI] [PubMed] [Google Scholar]
- 28.Hanson M, Fall C, Robinson S, Baird J. London: British Medical Association; 2009. Early Life Nutrition and Lifelong Health. [Google Scholar]
- 29.Barker D J, Winter P D, Osmond C, Margetts B, Simmonds S J. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2(8663):577–580. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 30.Lawlor D A, Smith G D. Early life determinants of adult blood pressure. Curr Opin Nephrol Hypertens. 2005;14(3):259–264. doi: 10.1097/01.mnh.0000165893.13620.2b. [DOI] [PubMed] [Google Scholar]
- 31.Crowe C, Dandekar P, Fox M, Dhingra K, Bennet L, Hanson M A. The effects of anaemia on heart, placenta and body weight, and blood pressure in fetal and neonatal rats. J Physiol. 1995;488(Pt 2):515–519. doi: 10.1113/jphysiol.1995.sp020986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gambling L, Danzeisen R, Fosset C. et al. Iron and copper interactions in development and the effect on pregnancy outcome. J Nutr. 2003;133(5) 01:1554S–1556S. doi: 10.1093/jn/133.5.1554S. [DOI] [PubMed] [Google Scholar]
- 33.Gambling L Dunford S Wallace D I et al. Iron deficiency during pregnancy affects postnatal blood pressure in the rat J Physiol 2003552(Pt 2):603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gambling L, Dunford S, McArdle H J. Iron deficiency in the pregnant rat has differential effects on maternal and fetal copper levels. J Nutr Biochem. 2004;15(6):366–372. doi: 10.1016/j.jnutbio.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 35.Lisle S J, Lewis R M, Petry C J, Ozanne S E, Hales C N, Forhead A J. Effect of maternal iron restriction during pregnancy on renal morphology in the adult rat offspring. Br J Nutr. 2003;90(1):33–39. doi: 10.1079/bjn2003881. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J, Lewis R M, Wang C, Hales N, Byrne C D. Maternal dietary iron restriction modulates hepatic lipid metabolism in the fetuses. Am J Physiol Regul Integr Comp Physiol. 2005;288(1):R104–R111. doi: 10.1152/ajpregu.00343.2004. [DOI] [PubMed] [Google Scholar]
- 37.Lewis R M, Petry C J, Ozanne S E, Hales C N. Effects of maternal iron restriction in the rat on blood pressure, glucose tolerance, and serum lipids in the 3-month-old offspring. Metabolism. 2001;50(5):562–567. doi: 10.1053/meta.2001.22516. [DOI] [PubMed] [Google Scholar]
- 38.Gambling L, Andersen H S, Czopek A, Wojciak R, Krejpcio Z, McArdle H J. Effect of timing of iron supplementation on maternal and neonatal growth and iron status of iron-deficient pregnant rats. J Physiol. 2004;561(Pt 1):195–203. doi: 10.1113/jphysiol.2004.068825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gambling L, McArdle H J. Iron, copper and fetal development. Proc Nutr Soc. 2004;63(4):553–562. doi: 10.1079/pns2004385. [DOI] [PubMed] [Google Scholar]
- 40.McArdle H J Andersen H S Jones H Gambling L Fetal programming: causes and consequences as revealed by studies of dietary manipulation in rats — a review Placenta 200627(Suppl A):S56–S60. [DOI] [PubMed] [Google Scholar]
- 41.Steer P, Alam M A, Wadsworth J, Welch A. Relation between maternal haemoglobin concentration and birth weight in different ethnic groups. BMJ. 1995;310(6978):489–491. doi: 10.1136/bmj.310.6978.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ronnenberg A G, Wood R J, Wang X. et al. Preconception hemoglobin and ferritin concentrations are associated with pregnancy outcome in a prospective cohort of Chinese women. J Nutr. 2004;134(10):2586–2591. doi: 10.1093/jn/134.10.2586. [DOI] [PubMed] [Google Scholar]
- 43.Swali A, McMullen S, Hayes H, Gambling L, McArdle H J, Langley-Evans S C. Processes underlying the nutritional programming of embryonic development by iron deficiency in the rat. PLoS ONE. 2012;7(10):e48133. doi: 10.1371/journal.pone.0048133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cockell K A, Miller D C, Lowell H. Application of the Dietary Reference Intakes in developing a recommendation for pregnancy iron supplements in Canada. Am J Clin Nutr. 2009;90(4):1023–1028. doi: 10.3945/ajcn.2009.27561. [DOI] [PubMed] [Google Scholar]
- 45.Gambling L, Danzeisen R, Gair S. et al. Effect of iron deficiency on placental transfer of iron and expression of iron transport proteins in vivo and in vitro. Biochem J. 2001;356(Pt 3):883–889. doi: 10.1042/0264-6021:3560883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rothman K J, Moore L L, Singer M R, Nguyen U S, Mannino S, Milunsky A. Teratogenicity of high vitamin A intake. N Engl J Med. 1995;333(21):1369–1373. doi: 10.1056/NEJM199511233332101. [DOI] [PubMed] [Google Scholar]
- 47.Golub M S, Hogrefe C E, Tarantal A F. et al. Diet-induced iron deficiency anemia and pregnancy outcome in rhesus monkeys. Am J Clin Nutr. 2006;83(3):647–656. doi: 10.1093/ajcn.83.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brenner B M, Garcia D L, Anderson S. Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens. 1988;1(4, Pt 1):335–347. doi: 10.1093/ajh/1.4.335. [DOI] [PubMed] [Google Scholar]
- 49.Andersen H S, Gambling L, Holtrop G, McArdle H J. Maternal iron deficiency identifies critical windows for growth and cardiovascular development in the rat postimplantation embryo. J Nutr. 2006;136(5):1171–1177. doi: 10.1093/jn/136.5.1171. [DOI] [PubMed] [Google Scholar]
- 50.Belfort M B, Rifas-Shiman S L, Rich-Edwards J W, Kleinman K P, Oken E, Gillman M W. Maternal iron intake and iron status during pregnancy and child blood pressure at age 3 years. Int J Epidemiol. 2008;37(2):301–308. doi: 10.1093/ije/dyn002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brion M J, Leary S D, Smith G D, McArdle H J, Ness A R. Maternal anemia, iron intake in pregnancy, and offspring blood pressure in the Avon Longitudinal Study of Parents and Children. Am J Clin Nutr. 2008;88(4):1126–1133. doi: 10.1093/ajcn/88.4.1126. [DOI] [PubMed] [Google Scholar]
- 52.Alwan N A, Cade J E, Greenwood D C, Deanfield J, Lawlor D A. Associations of maternal iron intake and hemoglobin in pregnancy with offspring vascular phenotypes and adiposity at age 10: findings from the Avon Longitudinal Study of Parents and Children. PLoS ONE. 2014;9(1):e84684. doi: 10.1371/journal.pone.0084684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bergel E, Haelterman E, Belizán J, Villar J, Carroli G. Perinatal factors associated with blood pressure during childhood. Am J Epidemiol. 2000;151(6):594–601. doi: 10.1093/oxfordjournals.aje.a010247. [DOI] [PubMed] [Google Scholar]
- 54.Law C M, Barker D J, Bull A R, Osmond C. Maternal and fetal influences on blood pressure. Arch Dis Child. 1991;66(11):1291–1295. doi: 10.1136/adc.66.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Godfrey K M, Forrester T, Barker D J. et al. Maternal nutritional status in pregnancy and blood pressure in childhood. Br J Obstet Gynaecol. 1994;101(5):398–403. doi: 10.1111/j.1471-0528.1994.tb11911.x. [DOI] [PubMed] [Google Scholar]
- 56.Whincup P, Cook D, Papacosta O, Walker M, Perry I. Maternal factors and development of cardiovascular risk: evidence from a study of blood pressure in children. J Hum Hypertens. 1994;8(5):337–343. [PubMed] [Google Scholar]
- 57.Alwan N A, Lawlor D A, McArdle H J, Greenwood D C, Cade J E. Exploring the relationship between maternal iron status and offspring's blood pressure and adiposity: a Mendelian randomization study. Clin Epidemiol. 2012;4:193–200. doi: 10.2147/CLEP.S33833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lumey L H, Stein A D, Kahn H S. et al. Cohort profile: the Dutch Hunger Winter families study. Int J Epidemiol. 2007;36(6):1196–1204. doi: 10.1093/ije/dym126. [DOI] [PubMed] [Google Scholar]
- 59.Stanner S A, Yudkin J S. Fetal programming and the Leningrad Siege study. Twin Res. 2001;4(5):287–292. doi: 10.1375/1369052012498. [DOI] [PubMed] [Google Scholar]
- 60.Anjos T, Altmäe S, Emmett P. et al. Nutrition and neurodevelopment in children: focus on NUTRIMENTHE project. Eur J Nutr. 2013;52(8):1825–1842. doi: 10.1007/s00394-013-0560-4. [DOI] [PubMed] [Google Scholar]
- 61.Nyaradi A, Li J, Hickling S, Foster J, Oddy W H. The role of nutrition in children's neurocognitive development, from pregnancy through childhood. Front Hum Neurosci. 2013;7:97. doi: 10.3389/fnhum.2013.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fleming R E. Cord serum ferritin levels, fetal iron status, and neurodevelopmental outcomes: correlations and confounding variables. J Pediatr. 2002;140(2):145–148. doi: 10.1067/mpd.2002.121931. [DOI] [PubMed] [Google Scholar]
- 63.Lozoff B Beard J Connor J Barbara F Georgieff M Schallert T Long-lasting neural and behavioral effects of iron deficiency in infancy Nutr Rev 2006645, Pt 2S34–S43., discussion S72–S91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lozoff B, Georgieff M K. Iron deficiency and brain development. Semin Pediatr Neurol. 2006;13(3):158–165. doi: 10.1016/j.spen.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 65.Georgieff M K. Long-term brain and behavioral consequences of early iron deficiency. Nutr Rev. 2011;69 01:S43–S48. doi: 10.1111/j.1753-4887.2011.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beard J. Recent evidence from human and animal studies regarding iron status and infant development. J Nutr. 2007;137(2):524S–530S. doi: 10.1093/jn/137.2.524S. [DOI] [PubMed] [Google Scholar]
- 67.Beard J L. Why iron deficiency is important in infant development. J Nutr. 2008;138(12):2534–2536. doi: 10.1093/jn/138.12.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tran P V, Kennedy B C, Lien Y C, Simmons R A, Georgieff M K. Fetal iron deficiency induces chromatin remodeling at the Bdnf locus in adult rat hippocampus. Am J Physiol Regul Integr Comp Physiol. 2015;308(4):R276–R282. doi: 10.1152/ajpregu.00429.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Greminger A R, Lee D L, Shrager P, Mayer-Pröschel M. Gestational iron deficiency differentially alters the structure and function of white and gray matter brain regions of developing rats. J Nutr. 2014;144(7):1058–1066. doi: 10.3945/jn.113.187732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tran P V, Fretham S J, Carlson E S, Georgieff M K. Long-term reduction of hippocampal brain-derived neurotrophic factor activity after fetal-neonatal iron deficiency in adult rats. Pediatr Res. 2009;65(5):493–498. doi: 10.1203/PDR.0b013e31819d90a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carter R C, Jacobson S W, Molteno C D, Jacobson J L. Fetal alcohol exposure, iron-deficiency anemia, and infant growth. Pediatrics. 2007;120(3):559–567. doi: 10.1542/peds.2007-0151. [DOI] [PubMed] [Google Scholar]
- 72.Radlowski E C, Johnson R W. Perinatal iron deficiency and neurocognitive development. Front Hum Neurosci. 2013;7:585. doi: 10.3389/fnhum.2013.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McCann J C, Ames B N. An overview of evidence for a causal relation between iron deficiency during development and deficits in cognitive or behavioral function. Am J Clin Nutr. 2007;85(4):931–945. doi: 10.1093/ajcn/85.4.931. [DOI] [PubMed] [Google Scholar]
- 74.Siddappa A M, Rao R, Long J D, Widness J A, Georgieff M K. The assessment of newborn iron stores at birth: a review of the literature and standards for ferritin concentrations. Neonatology. 2007;92(2):73–82. doi: 10.1159/000100805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shao J, Lou J, Rao R. et al. Maternal serum ferritin concentration is positively associated with newborn iron stores in women with low ferritin status in late pregnancy. J Nutr. 2012;142(11):2004–2009. doi: 10.3945/jn.112.162362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tamura T, Goldenberg R L, Hou J. et al. Cord serum ferritin concentrations and mental and psychomotor development of children at five years of age. J Pediatr. 2002;140(2):165–170. doi: 10.1067/mpd.2002.120688. [DOI] [PubMed] [Google Scholar]
- 77.Szajewska H, Ruszczynski M, Chmielewska A. Effects of iron supplementation in nonanemic pregnant women, infants, and young children on the mental performance and psychomotor development of children: a systematic review of randomized controlled trials. Am J Clin Nutr. 2010;91(6):1684–1690. doi: 10.3945/ajcn.2010.29191. [DOI] [PubMed] [Google Scholar]
- 78.Hernández-Martínez C, Canals J, Aranda N, Ribot B, Escribano J, Arija V. Effects of iron deficiency on neonatal behavior at different stages of pregnancy. Early Hum Dev. 2011;87(3):165–169. doi: 10.1016/j.earlhumdev.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 79.Burden M J, Westerlund A J, Armony-Sivan R. et al. An event-related potential study of attention and recognition memory in infants with iron-deficiency anemia. Pediatrics. 2007;120(2):e336–e345. doi: 10.1542/peds.2006-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fretham S J, Carlson E S, Wobken J, Tran P V, Petryk A, Georgieff M K. Temporal manipulation of transferrin-receptor-1-dependent iron uptake identifies a sensitive period in mouse hippocampal neurodevelopment. Hippocampus. 2012;22(8):1691–1702. doi: 10.1002/hipo.22004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tran T D, Tran T, Simpson J A. et al. Infant motor development in rural Vietnam and intrauterine exposures to anaemia, iron deficiency and common mental disorders: a prospective community-based study. BMC Pregnancy Childbirth. 2014;14:8. doi: 10.1186/1471-2393-14-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wachs T D, Pollitt E, Cueto S, Jacoby E, Creed-Kanashiro H. Relation of neonatal iron status to individual variability in neonatal temperament. Dev Psychobiol. 2005;46(2):141–153. doi: 10.1002/dev.20049. [DOI] [PubMed] [Google Scholar]
- 83.Armony-Sivan R, Eidelman A I, Lanir A, Sredni D, Yehuda S. Iron status and neurobehavioral development of premature infants. J Perinatol. 2004;24(12):757–762. doi: 10.1038/sj.jp.7211178. [DOI] [PubMed] [Google Scholar]
- 84.Amin S B, Orlando M, Wang H. Latent iron deficiency in utero is associated with abnormal auditory neural myelination in ≥ 35 weeks gestational age infants. J Pediatr. 2013;163(5):1267–1271. doi: 10.1016/j.jpeds.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 85.Siddappa A M, Georgieff M K, Wewerka S, Worwa C, Nelson C A, Deregnier R A. Iron deficiency alters auditory recognition memory in newborn infants of diabetic mothers. Pediatr Res. 2004;55(6):1034–1041. doi: 10.1203/01.pdr.0000127021.38207.62. [DOI] [PubMed] [Google Scholar]
- 86.Deregnier R A, Nelson C A, Thomas K M, Wewerka S, Georgieff M K. Neurophysiologic evaluation of auditory recognition memory in healthy newborn infants and infants of diabetic mothers. J Pediatr. 2000;137(6):777–784. doi: 10.1067/mpd.2000.109149. [DOI] [PubMed] [Google Scholar]
- 87.Riggins T, Miller N C, Bauer P J, Georgieff M K, Nelson C A. Consequences of low neonatal iron status due to maternal diabetes mellitus on explicit memory performance in childhood. Dev Neuropsychol. 2009;34(6):762–779. doi: 10.1080/87565640903265145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhou S J, Gibson R A, Crowther C A, Baghurst P, Makrides M. Effect of iron supplementation during pregnancy on the intelligence quotient and behavior of children at 4 y of age: long-term follow-up of a randomized controlled trial. Am J Clin Nutr. 2006;83(5):1112–1117. doi: 10.1093/ajcn/83.5.1112. [DOI] [PubMed] [Google Scholar]
- 89.Rioux F M, Bélanger-Plourde J, Leblanc C P, Vigneau F. Relationship between maternal DHA and iron status and infants' cognitive performance. Can J Diet Pract Res. 2011;72(2):76. doi: 10.3148/72.2.2011.e140. [DOI] [PubMed] [Google Scholar]
- 90.Christian P, Murray-Kolb L E, Khatry S K. et al. Prenatal micronutrient supplementation and intellectual and motor function in early school-aged children in Nepal. JAMA. 2010;304(24):2716–2723. doi: 10.1001/jama.2010.1861. [DOI] [PubMed] [Google Scholar]
- 91.Chang S, Zeng L, Brouwer I D, Kok F J, Yan H. Effect of iron deficiency anemia in pregnancy on child mental development in rural China. Pediatrics. 2013;131(3):e755–e763. doi: 10.1542/peds.2011-3513. [DOI] [PubMed] [Google Scholar]
- 92.Clardy S L, Wang X, Zhao W. et al. Acute and chronic effects of developmental iron deficiency on mRNA expression patterns in the brain. J Neural Transm Suppl. 2006;(71):173–196. doi: 10.1007/978-3-211-33328-0_19. [DOI] [PubMed] [Google Scholar]
- 93.Carlson E S, Stead J D, Neal C R, Petryk A, Georgieff M K. Perinatal iron deficiency results in altered developmental expression of genes mediating energy metabolism and neuronal morphogenesis in hippocampus. Hippocampus. 2007;17(8):679–691. doi: 10.1002/hipo.20307. [DOI] [PubMed] [Google Scholar]
- 94.Carlson E S, Tkac I, Magid R. et al. Iron is essential for neuron development and memory function in mouse hippocampus. J Nutr. 2009;139(4):672–679. doi: 10.3945/jn.108.096354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hubbard A C, Bandyopadhyay S, Wojczyk B S, Spitalnik S L, Hod E A, Prestia K A. Effect of dietary iron on fetal growth in pregnant mice. Comp Med. 2013;63(2):127–135. [PMC free article] [PubMed] [Google Scholar]
- 96.Georgieff M K. The role of iron in neurodevelopment: fetal iron deficiency and the developing hippocampus. Biochem Soc Trans. 2008;36(Pt 6):1267–1271. doi: 10.1042/BST0361267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Golub M S. Recent studies of iron deficiency during brain development in nonhuman primates. Biofactors. 2010;36(2):111–116. doi: 10.1002/biof.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Golub M, Hogrefe C. Prenatal iron deficiency and monoamine oxidase A (MAOA) polymorphisms: combined risk for later cognitive performance in rhesus monkeys. Genes Nutr. 2014;9(2):381. doi: 10.1007/s12263-013-0381-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Osendarp S J, Murray-Kolb L E, Black M M. Case study on iron in mental development—in memory of John Beard (1947-2009) Nutr Rev. 2010;68 01:S48–S52. doi: 10.1111/j.1753-4887.2010.00331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jorgenson L A, Wobken J D, Georgieff M K. Perinatal iron deficiency alters apical dendritic growth in hippocampal CA1 pyramidal neurons. Dev Neurosci. 2003;25(6):412–420. doi: 10.1159/000075667. [DOI] [PubMed] [Google Scholar]
- 101.Wu L L, Zhang L, Shao J, Qin Y F, Yang R W, Zhao Z Y. Effect of perinatal iron deficiency on myelination and associated behaviors in rat pups. Behav Brain Res. 2008;188(2):263–270. doi: 10.1016/j.bbr.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 102.Schmidt A T, Waldow K J, Grove W M, Salinas J A, Georgieff M K. Dissociating the long-term effects of fetal/neonatal iron deficiency on three types of learning in the rat. Behav Neurosci. 2007;121(3):475–482. doi: 10.1037/0735-7044.121.3.475. [DOI] [PubMed] [Google Scholar]
- 103.Brunette K E, Tran P V, Wobken J D, Carlson E S, Georgieff M K. Gestational and neonatal iron deficiency alters apical dendrite structure of CA1 pyramidal neurons in adult rat hippocampus. Dev Neurosci. 2010;32(3):238–248. doi: 10.1159/000314341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McEchron M D, Paronish M D. Perinatal nutritional iron deficiency reduces hippocampal synaptic transmission but does not impair short- or long-term synaptic plasticity. Nutr Neurosci. 2005;8(5-6):277–285. doi: 10.1080/10284150500499644. [DOI] [PubMed] [Google Scholar]
- 105.Rao R, Tkac I, Townsend E L, Gruetter R, Georgieff M K. Perinatal iron deficiency alters the neurochemical profile of the developing rat hippocampus. J Nutr. 2003;133(10):3215–3221. doi: 10.1093/jn/133.10.3215. [DOI] [PubMed] [Google Scholar]
- 106.Rao R, Tkac I, Unger E L. et al. Iron supplementation dose for perinatal iron deficiency differentially alters the neurochemistry of the frontal cortex and hippocampus in adult rats. Pediatr Res. 2013;73(1):31–37. doi: 10.1038/pr.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Blegen M B, Kennedy B C, Thibert K A, Gewirtz J C, Tran P V, Georgieff M K. Multigenerational effects of fetal-neonatal iron deficiency on hippocampal BDNF signaling. Physiol Rep. 2013;1(5):e00096. doi: 10.1002/phy2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Callahan L S, Thibert K A, Wobken J D, Georgieff M K. Early-life iron deficiency anemia alters the development and long-term expression of parvalbumin and perineuronal nets in the rat hippocampus. Dev Neurosci. 2013;35(5):427–436. doi: 10.1159/000354178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dallman P R. Biochemical basis for the manifestations of iron deficiency. Annu Rev Nutr. 1986;6:13–40. doi: 10.1146/annurev.nu.06.070186.000305. [DOI] [PubMed] [Google Scholar]
- 110.Beard J L, Connor J R. Iron status and neural functioning. Annu Rev Nutr. 2003;23:41–58. doi: 10.1146/annurev.nutr.23.020102.075739. [DOI] [PubMed] [Google Scholar]
- 111.Youdim M B, Sills M A, Heydorn W E, Creed G J, Jacobowitz D M. Iron deficiency alters discrete proteins in rat caudate nucleus and nucleus accumbens. J Neurochem. 1986;47(3):794–799. doi: 10.1111/j.1471-4159.1986.tb00681.x. [DOI] [PubMed] [Google Scholar]
- 112.de Deungria M, Rao R, Wobken J D, Luciana M, Nelson C A, Georgieff M K. Perinatal iron deficiency decreases cytochrome c oxidase (CytOx) activity in selected regions of neonatal rat brain. Pediatr Res. 2000;48(2):169–176. doi: 10.1203/00006450-200008000-00009. [DOI] [PubMed] [Google Scholar]
- 113.Beard J L, Wiesinger J A, Connor J R. Pre- and postweaning iron deficiency alters myelination in Sprague-Dawley rats. Dev Neurosci. 2003;25(5):308–315. doi: 10.1159/000073507. [DOI] [PubMed] [Google Scholar]
- 114.Connor J R, Menzies S L. Relationship of iron to oligodendrocytes and myelination. Glia. 1996;17(2):83–93. doi: 10.1002/(SICI)1098-1136(199606)17:2<83::AID-GLIA1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 115.Rao R, Tkac I, Schmidt A T, Georgieff M K. Fetal and neonatal iron deficiency causes volume loss and alters the neurochemical profile of the adult rat hippocampus. Nutr Neurosci. 2011;14(2):59–65. doi: 10.1179/1476830511Y.0000000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Unger E L, Hurst A R, Georgieff M K. et al. Behavior and monoamine deficits in prenatal and perinatal iron deficiency are not corrected by early postnatal moderate-iron or high-iron diets in rats. J Nutr. 2012;142(11):2040–2049. doi: 10.3945/jn.112.162198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tran P V, Fretham S J, Wobken J, Miller B S, Georgieff M K. Gestational-neonatal iron deficiency suppresses and iron treatment reactivates IGF signaling in developing rat hippocampus. Am J Physiol Endocrinol Metab. 2012;302(3):E316–E324. doi: 10.1152/ajpendo.00369.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Harvey L, Boksa P. Additive effects of maternal iron deficiency and prenatal immune activation on adult behaviors in rat offspring. Brain Behav Immun. 2014;40:27–37. doi: 10.1016/j.bbi.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 119.Bastian T W, Anderson J A, Fretham S J, Prohaska J R, Georgieff M K, Anderson G W. Fetal and neonatal iron deficiency reduces thyroid hormone-responsive gene mRNA levels in the neonatal rat hippocampus and cerebral cortex. Endocrinology. 2012;153(11):5668–5680. doi: 10.1210/en.2012-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]


