Abstract
Antidepressants are widely used during pregnancy. Several studies have shown that the use of antidepressants during pregnancy is linked to adverse outcomes, including congenital malformations, prematurity, and low birth weight. However, there is a knowledge gap regarding the potential association between gestational exposure to antidepressants and the risk of autism spectrum disorders (ASD). The etiology of ASD remains unclear, although studies have implicated genetic predispositions and environmental risk factors in the development of ASD in children. In this review, we describe the association between gestational use of antidepressants, specifically selective serotonin reuptake inhibitors, and the risk of ASD.
Keywords: SSRI, pregnancy, autism spectrum disorders, review
Introduction
Antidepressants are widely used during gestation for the treatment of depression. In the United States, the prevalence of antidepressant medication use during pregnancy increased from 5.7% in 1999 to 13.3% in 20031; in Canada, 4.5% of pregnant women reported using them between 2001 and 2006.2 It remains, however, that there is continued confusion regarding their appropriate use during this critical time period. Gestational exposure to antidepressants has been associated with an increased risk of spontaneous abortion,3 major congenital malformations,4 5 prematurity,6 7 low birth weight,6 7 neonatal withdrawal,8 and pregnancy-induced hypertension.9 Discontinuation of antidepressants during pregnancy in severely depressed women was associated with relapse of maternal depression in some studies.10 11 Nevertheless, up to 20% of women who continue antidepressant use during pregnancy remain depressed,12 suggesting a lack of efficacy in some pregnant women. Currently, few studies have investigated the effects of antidepressant use during pregnancy on the neurodevelopment of children, including autism spectrum disorders (ASD). Given recent projections by the World Health Organization that depression will be the second leading cause of death by 2020,13 antidepressants are likely to remain widely used, including during pregnancy. Therefore, a better understanding of their long-term neurodevelopmental effects on children, when used during gestation, is a public health priority.
Autism Spectrum Disorders
Autism spectrum disorder (ASD) is defined as a neurodevelopmental disorder which is characterized by pervasive impairment of communication, language, and social interaction, and by repetitive/restricted and stereotyped patterns of behavior.14 According to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), this complex disorder includes five subgroups: autistic disorder, Rett syndrome, childhood disintegrative disorder, pervasive developmental disorder—–not otherwise specified (PDD–NOS), and Asperger syndrome.15 The diagnosis of ASD is made at 3 years of age on average. The estimated prevalence of ASD has increased over time from 0.04% in 1966 to approximately 1% today16 17 in the United States. This observed rise in the prevalence of ASD can be partly attributed to extrinsic factors, including changes in the diagnostic criteria and their overlap with other diagnoses, better screening of the overall population (higher detection), and greater awareness of the general population.18 19 Nevertheless, both genetic and environmental factors could also be important in the increase in ASD prevalence over time.20 These factors may include de novo mutations,21 specific genes conferring susceptibility,15 advanced maternal age,22 maternal diseases such as diabetes and hypertension,23 24 and a maternal history of psychiatric disorder.25 26
Genetic Predispositions to Autism Spectrum Disorders
Accumulating evidence suggests that genetic factors contribute to the development of ASD. The genetic etiology of ASD was first reported in a twin study in 1977,27 and numerous twin studies have since been conducted. In particular, in Denmark, a study including 21 pairs of twins (11 monozygotic [MZ]; 10 dizygotic [DZ]) showed that 91% of MZ twins and 0% of DZ twins28 were concordant with regard to the presence of ASD. A British twin study suggested that 60% of MZ pairs were concordant for autism versus 0% for DZ pairs; in the same study, 92% of MZ pairs were concordant for a broader spectrum of related cognitive or social abnormalities versus 10% of DZ pairs.29 This later study also suggested that genetic susceptibility contributed strongly to autism and that multiple genes were involved. It remains, however, that the influence of genetic factors on the development of ASD is complex and the cause of ASD is unknown in the majority of cases.
On the other hand, family history studies have shown that the risk of ASD recurrence in another child was higher than the general population prevalence (1%),30 and ranged from 3 to 14%.31 32 A recent study indicated an even higher probability of ASD recurrence among siblings (18.7%; 95% confidence interval [CI]: 13.34–25.5).33
Gestational Use of Antidepressants, Especially SSRIs, and the Risk of ASD—Biological Plausibility
Several biological mechanisms might underlie the association between prenatal selective serotonin reuptake inhibitor (SSRI) exposure and the occurrence of neurodevelopmental disorders, including ASD. Serotonin plays a critical role in the development of the brain.34 35 Studies have shown that serotonin modulates numerous pre- and postnatal processes, as well as developmental processes, including cell division, neuronal migration, cell differentiation, and synaptogenesis.36 Furthermore, some studies have suggested that serotonin acts as a morphogen during embryonic development, influencing the maturation of the brain.37 Experimental studies using rodent models have also indicated that transient inhibition of the serotonin transporter with fluoxetine hydrochloride, an SSRI, during brain development has consequences for behavior in later life, indicating a critical role for serotonin in the maturation of the brain systems.38 39 Brain imaging research has shown atypical development of the capacity for serotonin synthesis in the brains of children with ASD,40 41 and abnormalities in serotonin receptor 2A binding in the cerebral cortex.42 An intrinsic feedback mechanism affects circulating serotonin levels and the morphological modification of serotonergic neurons. There is some evidence that the serotonergic system plays a role in ASD. SSRIs block the 5-hydroxytryptamine transporter (5-HTT), leading to increased levels of serotonin in the extracellular space, and it was reported that autistic individuals have elevated 5-HT levels in their blood platelets.43 44 Studies of the phenomenon of elevated platelet serotonin levels, termed hyperserotonemia, in rodents have shown an association between blood 5-HT levels and autistic-like behaviors.45 Furthermore, hyperserotonemia was observed in 30% of individuals with autism.46 The mechanism of hyperserotonemia is still unknown. Some studies suggested an increase uptake of serotonin into platelet,47 diminishing release from platelets,48 and decreased catabolism of serotonin.49 Hence, dysfunction of the serotonin system during pregnancy following SSRI exposure could directly induce changes in fetal brain development.50
Gestational Use of Antidepressants, Especially SSRIs, and the Risk of ASD—Review of Published Studies
To date, seven published studies have explored the association between antidepressant use during pregnancy and the risk of ASD. The results of these studies are presented in Table 1 and are illustrated in Figs. 1 and 2.
Table 1. Characteristics of the studies examining the association between antidepressant use during pregnancy and the risk of ASD.
| Authors | Source Population, country | Design | Exposure definition | Results (n, %) No. exposure cases/total no. of cases |
Overall results OR/HR/RR 95%CI |
|---|---|---|---|---|---|
| Croen et al (2011)51 | N = 1,805 | Population-based case–control study | SSRIs: 12 mo prior delivery |
Any AD 20/298 (6.7%) | 12 mo before delivery SSRI OR: 2.1 (1.0–4.4) |
| Kaiser Permanente Medical Care Program (KPNC) Northern California |
1st trimester | SSRIs 15/298 (5.0) | First trimester SSRI OR: 3.5 (1.5–7.9) |
||
| 2nd trimester | DAAs 2/298 (0.7) | Second trimester SSRI OR: 1.5 (0.5–5.0) |
|||
| 3rd trimester SSRI |
TCAs 5/298 (1.7) | Third trimester SSRI OR: 2.2 (0.7–6.9) |
|||
| Sørensen et al (2013)53 | N = 668,468 | Cohort study | During pregnancy | Any AD: 104/5,437 (1.9%) | Any AD during pregnancy HR: 1.2 (0.7–2.1) |
| Danish Civil Registration System Denmark |
1st trimester | Any SSRI: 91/5,437 (1.6%) | SSRI during pregnancy HR: 1.4 (0.8–2.4) |
||
| 2nd trimester + 3rd trimester | Sibling design: Any AD during pregnancy HR: 1.1 (0.5–2.3) SSRI during pregnancy HR: 0.9 (0.4–2.0) |
||||
| SSRIs SNRIs TCAs |
|||||
| Rai et al (2013)52 | N = 54,472 | Nested case–control study | During pregnancy: Any AD |
21/1679 (1.25%) | Any AD during pregnancy OR: 1.90 (1.15–3.14) |
| Stockholm Youth cohort Sweden |
During pregnancy: SSRIs |
SSRIs during pregnancy OR: 1.65 (0.90–3.03) |
|||
| Nonselective monoamine reuptake inhibitors | |||||
| Hviid et al (2013)54 | N = 626,875 | Cohort study | SSRI: during pregnancy | 122.6 per 100,000 person-year | |
| Hviid et al (2013)54 | Nationwide Medical Birth Registry Denmark |
SSRI: From 2 y before pregnancy through delivery | During pregnancy 52/3,892 (1.33%) | Rate ratio: 1.20 (0.90–1.61) | |
| SSRI: Only during pregnancy | From 2 y before pregnancy through delivery 29/3,892(0.7%) | Rate ratio: 1.08 (0.74–1.58) | |||
| SSRI: During 1st trimester | Only during pregnancy 23/3,892(0.6%) | Rate ratio: 1.40 (0.92–2.13) | |||
| During first trimester 40/3,892 (1.02%) |
Rate ratio: 1.35 (0.97–1.87) | ||||
| Harrington et al (2014)55 | N = 966 | Population-based Case–control study | SSRI during pregnancy | During pregnancy Boys and girls 29/492 (5.9%) |
ASD vs. TD Boys and girls; OR: 1.55 (0.59–4.08) |
| Genetics and the Environment (CHARGE) Study California |
SSRI: 1st trimester | During pregnancy Boys only 25/421 (5.9%) |
ASD vs. TD Boys only; OR: 2.92 (1.07–7.93) Among maternal mental health subset: Boys and girls; OR: 1.86 (0.76–4.58) Boys only; OR: 3.17 (0.91–11.00) |
||
| SSRI: 2nd trimester | |||||
| SSRI: 3rd trimester | |||||
| Clements et al (2014)56 | N = 5,399 | Case–control study | During pregnancy | During pregnancy: 2.9% | During pregnancy OR: 1.10 (0.70–1.70) |
| New England USA |
AD: 1st trimester | First trimester: 2.3% | First trimester OR: 1.43 (0.85–2.38) |
||
| AD: 2nd trimester | Second trimester: 2.0% | Second trimester OR: 1.34 (0.77–2.27) |
|||
| AD: 3rd trimester | Third trimester: 1.8% | Third trimester OR: 1.08 (0.61–1.88) |
|||
| Boukhris et al (2014)57 | N = 145,456 | Cohort study | AD during 1st trimester |
First trimester: 40/1,054 (3.80%) |
|
| Quebec Pregnancy Cohort Québec Canada |
AD during 2nd/3rd trimester | 2nd/3rd trimester: 31/1,054 (2.94%) |
Any AD during 2nd/3rd trimesters HR: 1.87 (1.15–3.04) SSRIs in 2nd/3rd trimesters HR: 2.17 (1.20–3.93) Stratification on family history of ASD With family history of ASD HR: 8.36 (0.31–221.71) |
||
| SSRIs | Without family history of ASD HR: 1.87 (1.14–3.06) |
||||
| SNRIs | Any AD use during 2nd/3rd trimesters: Stratification on maternal depression With maternal depression HR:1.75(1.03–2.97) Without maternal depression HR:1.36 (0.34–5.50) |
||||
| TCAs | |||||
| Others AD |
Abbreviations: AD, antidepressants; ASD, autism spectrum disorder; CI, confidence interval; DAA, dual-acting antidepressant; HR, hazard ratio; OR, odds ratio; RR, rate ratio; SNRI, serotonin–norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant; TD, typical development.
Fig. 1.
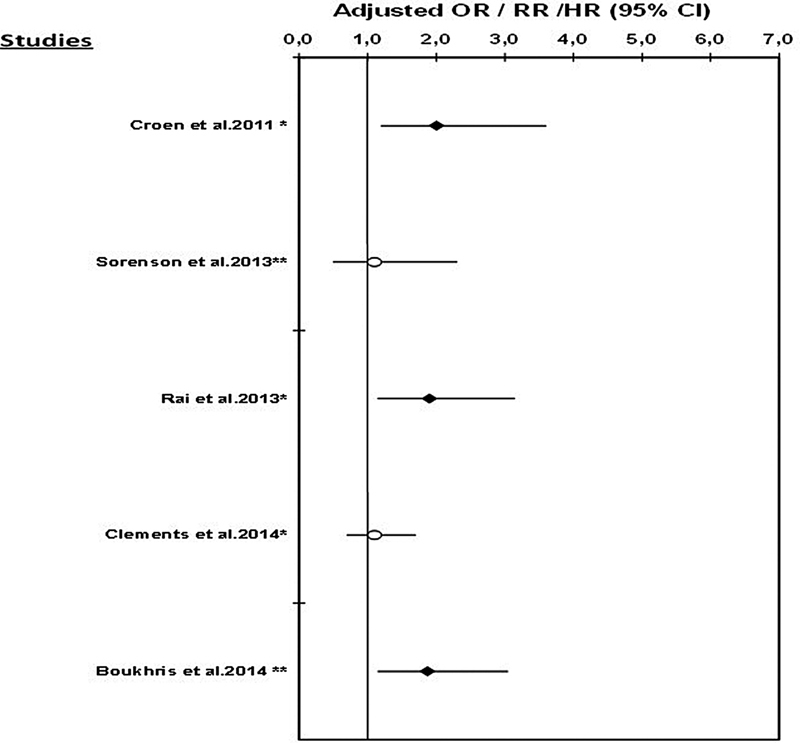
Risk of autism spectrum disorder associated with the use of any antidepressant during pregnancy. Note: no data were available on the risk of ASD associated with AD use during pregnancy in the studies by Harrington et al (2014)55 and Hviid et al (2013).54 *Odds ratio (OR); **hazards ratio (HR)/rate ratio (RR).
Fig. 2.
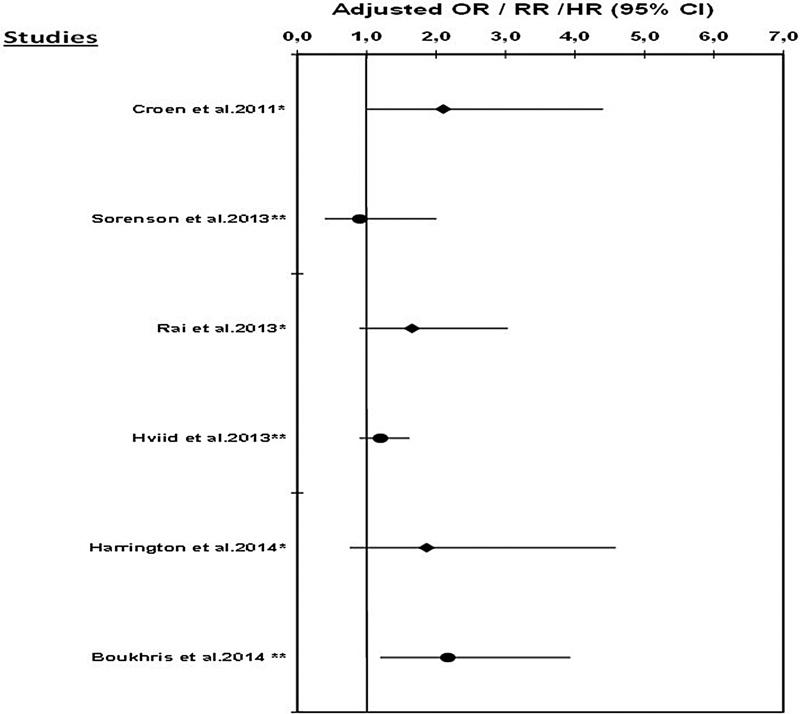
Risk of autism spectrum disorder associated with the use of SSRIs during pregnancy. Note: no data are available on the risk of ASD associated with SSRI use during pregnancy in the study by Clements et al (2014).56 *Odds ratio (OR); **hazards ratio (HR)/rate ratio (RR).
Croen et al51 performed a population-based case–control study using data from the Kaiser Permanent Medical Care Program in Northern California (KPNC). Cases were defined as infants born at KPNC facilities between 1995 and 1999 who had at least one International Classification of Diseases, 9th Revision (ICD-9) diagnosis of ASD between 1995 and 2002. Children without ASD were randomly selected from the remaining cohort of live births and were matched to cases on sex, birth year, and hospital of birth (ratio of 5:1). Authors restricted the analysis to one child per mother. There were 298 cases of ASD, 20 (6.7%) of whom were exposed to an antidepressant, and 15 (5.0%) were exposed to SSRIs (13 were exposed to SSRIs only and 2 were exposed to SSRIs in combination with other antidepressants). After adjusting for maternal age, race/ethnicity, education, birth year, sex, hospital of birth, and birth weight, investigators found that antidepressant use during pregnancy was associated with an increased risk of ASD in the offspring (adjusted odds ratio [OR] = 2.0; 95% CI: 1.2–3.6). Authors also showed that cases of ASD were more than twice as likely to have been exposed to an SSRI in utero than the controls (adjusted OR = 2.1; 95% CI: 1.0–4.4), after adjustment for maternal history of any mental disorders was made. The use of SSRIs during any trimester was also associated with an increased risk of ASD, but the association was only statistically significant during the first trimester (adjusted OR = 3.5; 95% CI: 1.5–7.9). In an analysis restricted to a subgroup of women with mental disorders in the year before delivery, the risk of ASD was associated with any SSRI use during pregnancy (OR = 1.6; 95% CI: 0.6–4.0), but this association was not statistically significant. However, authors highlighted the possible misclassification of history of maternal mental disorders, which could have introduced bias in the stratified analyses. A second concern involved the small number of ASD-exposed cases in the second (n = 5) and third trimesters of pregnancy (n = 6), which limited statistical power for these analyses. Although they have used ICD9 codes for the identification of ASD, a subset of 50 children underwent clinical evaluation with the Autism Diagnostic Interview—Revised (ADI-R) and the Autism Diagnostic Observation Schedule–Generic (ADOS), and 94% (47/50) of them met the criteria for ASD. Although rates of ASD diagnoses have increased markedly over the past 20 years, analyses were adjusted for birth year.
In 2013, Rai et al52 (Table 1, Figs. 1 and 2) conducted a nested case–control study within the Stockholm Youth Cohort, including all young people (aged 0–17 years) registered between 2001 and 2007. Cases of ASD were defined using ICD-9 and ICD-10 codes, or DSM-IV criteria, and a multisource case ascertainment method, with registers covering all pathways of autism diagnosis and care within Stockholm County. Each case of ASD was matched to 10 controls without ASD on date of birth (month and year) and sex. Antidepressant use during pregnancy was reported by mothers at their first antenatal interview. A parental history of depression was obtained using the Stockholm County Adult Psychiatric Outpatient Register, which records the dates and diagnoses of individuals with visits to any outpatient psychiatric services in Stockholm County, and the Swedish National Patient Register, which contains the dates and discharge diagnoses of all inpatients and outpatients visits in Sweden. In this cohort, 1,679 cases of ASD were identified; 21 were exposed to antidepressants in utero. After adjustment for potential confounders, including any maternal psychiatric disorders, Rai et al52 reported a statistically significantly increased risk of ASD associated with antidepressant use during pregnancy (adjusted OR = 1.90; 95% CI: 1.15–3.14). They also found that the risk of ASD was 1.65 (95% CI: 0.90–3.03) and 2.69 (95% CI: 1.04–6.96) for the use of SSRIs and nonselective monoamine reuptake inhibitors during pregnancy, respectively. A history of depression and antidepressant use in pregnant mothers was strongly associated with ASD in their offspring (adjusted OR = 3.34; 95% CI: 1.50–7.47). In contrast, an association between antidepressant use during pregnancy and ASD was not seen among women reporting the use of these medications for reasons other than for depression. Again analyses were underpowered, and given that the prevalence of ASD has increased over time, detection bias could not be ruled out. However, authors attempted to control for this bias by matching cases and controls by date of birth.
Sørensen et al53 (Table 1, Figs. 1 and 2) conducted a cohort study of all children born in Denmark between 1996 and 2006, identified using the Danish Civil Registration System. Authors obtained information on maternal use of antidepressants during pregnancy from the Danish National Prescription Registry. Information on ASD diagnoses and parental psychiatric disorders were obtained from the Danish Psychiatric Central Register. Analyses were adjusted for parental age at conception, parental psychiatric history, gestational age at delivery, birth weight, sex of the newborn, and parity. Overall, 655,615 children were included in the cohort, 5,437 of who had a diagnosis of ASD; 8,833 (1.3%) were exposed to antidepressants in utero. When analyses were restricted to children of mothers with affective disorders, no statistically significant association was found between gestational use of antidepressants and the risk of ASD (adjusted hazard ratio [HR] = 1.2; 95% CI: 0.7–2.1). Similarly, no statistically significant association was found between SSRI exposure during pregnancy and the risk of ASD (adjusted HR = 1.4; 95% CI: 0.8–2.4). Findings did not vary by trimester of exposure. Using a sibling design, authors further restricted the cohort to include only families with at least two siblings and in which at least one had been diagnosed with ASD. In this analysis, no statistically significant association was found between prenatal exposure to any antidepressant (adjusted HR = 1.1; 95% CI: 0.5–2.3) or to SSRIs (adjusted HR = 0.9; 95% CI: 0.4–2.0) and ASD in children, suggesting that genetic predisposition is a stronger risk factor for ASD than gestational antidepressant use. However, sibling pairs with no ASD diagnosis were excluded from this analysis, resulting in partial adjustment for genetic predispositions. Indeed, exclusion of sibling pairs without ASD potentially led to overestimation of the association between family history of ASD and the risk of ASD in another child, and potentially underestimated the association between antidepressant use during pregnancy and ASD.
Hviid et al54 (Table 1, Fig. 2) conducted a large population-based prospective cohort study in Denmark, using data from national registries (Medical Birth Registry, National Patient Register, National Prescription Registry, Danish Civil Registration System, and Danish Psychiatric Central Register) from 1996 to 2005, with follow-up through 2009. Only singleton births were included, and newborns with conditions associated with an increased risk of ASD, such as congenital rubella syndrome, were excluded. Only exposure to SSRI during pregnancy was considered. The authors took into account the effects of potential confounders, such as maternal age at pregnancy onset, smoking status during pregnancy, maternal psychiatric conditions, year of birth, use of medications other than SSRIs, and maternal level of education. Children were prospectively followed up from birth until January 1, 2010, or until the child's 10th birthday. A total of 626,875 children were included, 6,068 (0.97%) of who were exposed to SSRIs in utero. Exposure to SSRIs any time from 4 weeks before the beginning of pregnancy until delivery was associated with a 20% increased risk of ASD (adjusted rate ratio [RR] = 1.20; 95% CI: 0.90–1.61), but this estimate was not statistically significant. Results were similar when the analysis was restricted to SSRI use during pregnancy (adjusted RR = 1.40; 95% CI: 0.92–2.13). However, when SSRI exposure was limited to 6 to 24 months before pregnancy, the adjusted RR was 1.46 (95% CI: 1.17–1.81), suggesting that the observed association may be partly due to confounding by indication. To control for detection bias, Hviid et al54 adjusted for calendar year during which the follow-up assessment was made.
Harrington et al55 (Table 1, Fig. 2) performed a population-based case–control study to investigate the association between prenatal SSRI exposure and the risk of ASD. The participants were families enrolled in the Childhood Autism Risks from Genetics and the Environment (CHARGE) study between April 2003 and August 2010. The population controls were identified using state birth files, and were matched to autism cases by age, sex, and regional center. ASD diagnoses were confirmed with ADI-R, and ADOS. The Vineland Adaptive Behavior Scales and the Mullen Scales of Early Learning were used to define developmental delay (DD). Maternal interviews were conducted to ascertain prenatal SSRI use, maternal mental health, and sociodemographic status. A total of 966 mother–child pairs were included: 492 with ASD, 154 with DD, and 320 with typical development; 48 (5%) mothers were prenatally exposed to SSRIs. After adjustment for potential confounders (regional center, child's year of birth, and birthplace of mother), prenatal exposure to SSRIs was associated with a nonsignificant increased risk of ASD (adjusted OR = 1.55; 95% CI: 0.59–4.08); the association was, however, statistically significant among male children (adjusted OR = 2.92; 95% CI: 1.07–7.93). No significant associations were noted when restricting analyses to mothers with anxiety or mood disorder at any time before delivery. No adjustment was made for genetic predisposition to ASD; detection bias was taken into account by adjusting for the child's year of birth.
Recently, Clements and colleagues (Table 1, Fig. 1) published a case–control study on prenatal antidepressant exposure and the risk of ASD in a large health system database.56 Data on maternal health, ASD diagnosis, and antidepressant prescription were collected from electronic health records of a large health care system in Massachusetts, and were linked to birth records in the Massachusetts Registry of Vital Records and Statistics. Children with an ASD diagnosis were matched in a ratio of 1:3 with non-ASD children (controls) according to year of birth, delivery hospital, sex, insurance type, race/ethnicity, and prematurity status. Exposure to antidepressants was defined as (1) any time during pregnancy and (2) according to trimester of use. After adjustment for history of maternal depression, the risk of ASD associated with antidepressant exposure during pregnancy was elevated but not statistically significant (adjusted OR in first trimester = 1.43; 95% CI: 0.85–2.38; adjusted OR in second trimester = 1.34; 95% CI: 0.77–2.27). Considering the possibility of confounding by indication, the authors evaluated the association between the use of antidepressants before pregnancy (any time before the last menstrual period) and the risk of ASD. They reported that the use of antidepressants before pregnancy was significantly associated with ASD (adjusted OR = 1.62; 95% CI: 1.17–2.23) after adjustment for maternal history of depression. The authors stressed that a potential source of bias was the systematic misclassification of case and control status, which could have led to the nonsignificant estimates. Again, detection bias was adjusted for by taking into account year of birth.
Finally, Boukhris et al57 (Table 1, Figs. 1 and 2) assessed the risk of ASD in children exposed to gestational antidepressants, taking into account potential confounding factors and any family history of ASD. A cohort study was conducted using data from the Quebec Pregnancy Cohort, and included data on all pregnancies and children in Quebec in 1998 to 2009. Only singleton births were considered in this study. Antidepressant use during pregnancy was defined as having at least one prescription filled during gestation, and according to the trimester of use; antidepressant classes were also studied (Table 2). ASD outcomes were defined as infants with at least one diagnosis of ASD before December 31, 2009 (Table 2). The cohort included 145,456 live-born children in 1998 to 2009; 1,054 (0.72%) children had a diagnosis of ASD. The mean age of children at the end of follow-up was 6.24 years (standard deviation [SD] = 3.19). Adjusting for potential confounders, overall antidepressant use during the second or third trimester of pregnancy was statistically significantly associated with ASD (adjusted HR = 1.87; 95% CI: 1.15–3.04); SSRI use during the second or third trimester of pregnancy (adjusted HR = 2.17; 95% CI: 1.20–3.93) and the combined use of more than one antidepressant class during the same period (adjusted HR = 4.39; 95% CI: 1.44–13.32) was associated with the risk of ASD. To separate the effects of antidepressants from the underlying indication, a stratified analysis on maternal depression status was performed, resulting in a significantly increased risk of ASD associated with antidepressant use during the second or third trimester among children of depressed mothers (adjusted HR = 1.75; 95% CI: 1.03–2.97); no statistically significant association was found among nondepressed mothers (adjusted HR = 1.36; 95% CI: 0.34–5.50; 2 exposed cases). Genetic predispositions, defined as having a sibling with ASD, were further taken into account. In the subgroup of children with no genetic predisposition to ASD, second/third trimester use of antidepressants was increasing the risk of ASD (adjusted HR = 1.87; 95% CI: 1.14–3.06); having a child with ASD increased the risk of having another child with the disorder but nonstatistically significant (adjusted HR = 8.36; 95% CI: 0.31–221.71), which could be explained by the lack of power for analyses on this stratum. All analyses were adjusted for calendar year of birth to control for detection bias.
Table 2. Characteristics of the study by Boukhris et al57examining the association between antidepressant use during pregnancy and the risk of ASD.
| Authors | Definition of outcome | Years of Dx | Definition of exposure | Exposure period | Indication control |
|---|---|---|---|---|---|
| Boukhris et al (2014)57 | Children with ASD diagnosis between birth and the end of the study period (2009): Childhood autism (ICD-9 299.0 or ICD-10 F84.0) Atypical autism (ICD-9 299.0 or ICD-10 F84.1) Asperger syndrome (ICD-9 299.8 or ICD-10 F84.5) Other pervasive developmental disorders (ICD-9 299.8 or ICD-10 F84.8) Pervasive developmental disorder unspecified (ICD-9 299.9 or ICD-10 F84.9) |
1998–2009 | AD exposure was defined as having at least one prescription filled AD classes considered: SSRIs, SNRIs, TCAs, MAOIs, and others AD. A single class exposure was defined as the filling of prescriptions for only one AD classes. Combined classes of ADs were defined as the filling of a prescription for at least two or more different AD classes |
1. The year before the first day of gestation (1DG) defined as the first day of the last menstrual period 2. The 1st trimester of pregnancy defined as the time from the 1DG until the 14th completed week of gestation 3. The 2nd/3rd trimesters (between the 15th week until the end of pregnancy) |
Maternal psychiatric disorders other than depression in the year prior (adjusted) Maternal depression/anxiety/bipolar disorder (stratified) |
Abbreviations: AD, antidepressants; ASD, autism spectrum disorder; MAOI, monoamine oxydase inhibitor; SNRI, serotonin–norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant.
Conclusion
Taken together, studies are suggestive of an increased risk of ASD associated with antidepressant use during pregnancy. The hyperserotonemia in autistic patients provides a possible biological explanation for this association. Although, some findings are not statistically significant, this could partly be explained by lack of statistical power. Lack of statistical power should not be mistaken for lack of effect.
Presented studies51 52 53 54 55 56 57 used different approaches to control for the possible confounding effect of the indication (i.e., adjustment for maternal depression and other psychiatric disorders, sibling analysis, restricting the analysis to the children of mothers with affective disorders, and stratified analyses according to maternal depression status). Nevertheless, although confounding by indication was adjusted for in the majority of studies presented, we cannot completely rule out residual confounding by indication given the observational nature of the data. None of the seven studies controlled for the severity of maternal depression per se; however, Boukhris et al57 adjusted for proxies for severity, such as use of antidepressants before pregnancy, use of other antipsychotics, maternal diagnoses of other psychiatric disorders such as schizophrenia, and personality disorders. It remains that given the strength of the reported associations, serotonin inhibition during gestation seems to play an important role in the occurrence of ASD. Further research is needed to specifically assess the risk of ASD associated with antidepressant types and dosages during pregnancy. More research is also required to determine the most relevant temporal window of exposure during pregnancy.
Acknowledgments
Dr. Anick Bérard is a recipient of a career award from the Fonds de la Recherche en Santé du Québec (FRQ-S), and is on the endowment research Chair of the Famille Louis-Boivin for “Medications, Pregnancy and Lactation” at the Faculty of Pharmacy of the University of Montreal. This study was supported by the CIHR, “Quebec Training Network in Perinatal Research.”
References
- 1.Cooper W O, Willy M E, Pont S J, Ray W A. Increasing use of antidepressants in pregnancy. Am J Obstet Gynecol. 2007;196(6):5440–5.44E7. doi: 10.1016/j.ajog.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 2.Daw J R, Mintzes B, Law M R, Hanley G E, Morgan S G. Prescription drug use in pregnancy: a retrospective, population-based study in British Columbia, Canada (2001-2006) Clin Ther. 2012;34(1):239–24900. doi: 10.1016/j.clinthera.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 3.Kjaersgaard M I, Parner E T, Vestergaard M. et al. Prenatal antidepressant exposure and risk of spontaneous abortion - a population-based study. PLoS ONE. 2013;8(8):e72095. doi: 10.1371/journal.pone.0072095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gentile S. Selective serotonin reuptake inhibitor exposure during early pregnancy and the risk of birth defects. Acta Psychiatr Scand. 2011;123(4):266–275. doi: 10.1111/j.1600-0447.2011.01673.x. [DOI] [PubMed] [Google Scholar]
- 5.Wurst K E, Poole C, Ephross S A, Olshan A F. First trimester paroxetine use and the prevalence of congenital, specifically cardiac, defects: a meta-analysis of epidemiological studies. Birth Defects Res A Clin Mol Teratol. 2010;88(3):159–170. doi: 10.1002/bdra.20627. [DOI] [PubMed] [Google Scholar]
- 6.Ross L E, Grigoriadis S, Mamisashvili L. et al. Selected pregnancy and delivery outcomes after exposure to antidepressant medication: a systematic review and meta-analysis. JAMA Psychiatry. 2013;70(4):436–443. doi: 10.1001/jamapsychiatry.2013.684. [DOI] [PubMed] [Google Scholar]
- 7.Källén B. Neonate characteristics after maternal use of antidepressants in late pregnancy. Arch Pediatr Adolesc Med. 2004;158(4):312–316. doi: 10.1001/archpedi.158.4.312. [DOI] [PubMed] [Google Scholar]
- 8.Nordeng H, Lindemann R, Perminov K V, Reikvam A. Neonatal withdrawal syndrome after in utero exposure to selective serotonin reuptake inhibitors. Acta Paediatr. 2001;90(3):288–291. [PubMed] [Google Scholar]
- 9.De Vera M A, Bérard A. Antidepressant use during pregnancy and the risk of pregnancy-induced hypertension. Br J Clin Pharmacol. 2012;74(2):362–369. doi: 10.1111/j.1365-2125.2012.04196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yonkers K A, Gotman N, Smith M V. et al. Does antidepressant use attenuate the risk of a major depressive episode in pregnancy? Epidemiology. 2011;22(6):848–854. doi: 10.1097/EDE.0b013e3182306847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen L S, Nonacs R M, Bailey J W. et al. Relapse of depression during pregnancy following antidepressant discontinuation: a preliminary prospective study. Arch Women Ment Health. 2004;7(4):217–221. doi: 10.1007/s00737-004-0059-3. [DOI] [PubMed] [Google Scholar]
- 12.Berard A, Karam F, Sheehy O. et al. Antidepressant use during pregnancy and the risk of delay in overall cognitive development at one year old: results from the OTIS Antidepressants Study. Birth Defects Res A Clin Mol Teratol. 2012;94:253–290. [Google Scholar]
- 13.Christopher M Alan L, eds. The Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability from Diseases, Injuries, and Risk Factors in 1990 and Projected to 2020 Geneva: Harvard School of Public Health; 1996 [Google Scholar]
- 14.American Psychiatric Association . Washington, DC: American Psychiatric Association; 2000. Diagnostic and Statistical Manual of Mental Disorders.4th ed. [Google Scholar]
- 15.Muhle R, Trentacoste S V, Rapin I. The genetics of autism. Pediatrics. 2004;113(5):e472–e486. doi: 10.1542/peds.113.5.e472. [DOI] [PubMed] [Google Scholar]
- 16.Kogan M D, Blumberg S J, Schieve L A. et al. Prevalence of parent-reported diagnosis of autism spectrum disorder among children in the US, 2007. Pediatrics. 2009;124(5):1395–1403. doi: 10.1542/peds.2009-1522. [DOI] [PubMed] [Google Scholar]
- 17.Centers for disease Control and Prevention (CDC) MMWR Surveill Summ Prevalence of Autism Spectrum Disorders—Autism and Developmental Disabilities Monitoring Network, 14 Sites, United States, 2008. 2012;61(3):2–18 Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/ss6103a1.htm. Accessed March 30, 2012 [PubMed]
- 18.Hertz-Picciotto I, Delwiche L. The rise in autism and the role of age at diagnosis. Epidemiology. 2009;20(1):84–90. doi: 10.1097/EDE.0b013e3181902d15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hertz-Picciotto I Commentary: Diagnostic change and the increased prevalence of autism Int J Epidemiol 20093851239–1241., author reply 1243–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hertz-Picciotto I, Croen L A, Hansen R, Jones C R, van de Water J, Pessah I N. The CHARGE study: an epidemiologic investigation of genetic and environmental factors contributing to autism. Environ Health Perspect. 2006;114(7):1119–1125. doi: 10.1289/ehp.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ronemus M, Iossifov I, Levy D, Wigler M. The role of de novo mutations in the genetics of autism spectrum disorders. Nat Rev Genet. 2014;15(2):133–141. doi: 10.1038/nrg3585. [DOI] [PubMed] [Google Scholar]
- 22.Sandin S, Hultman C M, Kolevzon A, Gross R, MacCabe J H, Reichenberg A. Advancing maternal age is associated with increasing risk for autism: a review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 2012;51(5):477–486. doi: 10.1016/j.jaac.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 23.Lyall K, Pauls D L, Spiegelman D, Ascherio A, Santangelo S L. Pregnancy complications and obstetric suboptimality in association with autism spectrum disorders in children of the Nurses' Health Study II. Autism Res. 2012;5(1):21–30. doi: 10.1002/aur.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore G S, Kneitel A W, Walker C K, Gilbert W M, Xing G. Autism risk in small- and large-for-gestational-age infants. Am J Obstet Gynecol. 2012;206(4):3140–3.14E11. doi: 10.1016/j.ajog.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daniels J L, Forssen U, Hultman C M. et al. Parental psychiatric disorders associated with autism spectrum disorders in the offspring. Pediatrics. 2008;121(5):e1357–e1362. doi: 10.1542/peds.2007-2296. [DOI] [PubMed] [Google Scholar]
- 26.Jokiranta E, Brown A S, Heinimaa M, Cheslack-Postava K, Suominen A, Sourander A. Parental psychiatric disorders and autism spectrum disorders. Psychiatry Res. 2013;207(3):203–211. doi: 10.1016/j.psychres.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Folstein S, Rutter M. Infantile autism: a genetic study of 21 twin pairs. J Child Psychol Psychiatry. 1977;18(4):297–321. doi: 10.1111/j.1469-7610.1977.tb00443.x. [DOI] [PubMed] [Google Scholar]
- 28.Steffenburg S, Gillberg C, Hellgren L. et al. A twin study of autism in Denmark, Finland, Iceland, Norway and Sweden. J Child Psychol Psychiatry. 1989;30(3):405–416. doi: 10.1111/j.1469-7610.1989.tb00254.x. [DOI] [PubMed] [Google Scholar]
- 29.Bailey A, Le Couteur A, Gottesman I. et al. Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med. 1995;25(1):63–77. doi: 10.1017/s0033291700028099. [DOI] [PubMed] [Google Scholar]
- 30.Piven J, Vieland V J, Parlier M. et al. A molecular genetic study of autism and related phenotypes in extended pedigrees. J Neurodev Disord. 2013;5(1):30. doi: 10.1186/1866-1955-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sumi S, Taniai H, Miyachi T, Tanemura M. Sibling risk of pervasive developmental disorder estimated by means of an epidemiologic survey in Nagoya, Japan. J Hum Genet. 2006;51(6):518–522. doi: 10.1007/s10038-006-0392-7. [DOI] [PubMed] [Google Scholar]
- 32.Ritvo E R, Jorde L B, Mason-Brothers A. et al. The UCLA-University of Utah epidemiologic survey of autism: recurrence risk estimates and genetic counseling. Am J Psychiatry. 1989;146(8):1032–1036. doi: 10.1176/ajp.146.8.1032. [DOI] [PubMed] [Google Scholar]
- 33.Ozonoff S, Young G S, Carter A. et al. Recurrence risk for autism spectrum disorders: a Baby Siblings Research Consortium study. Pediatrics. 2011;128(3):e488–e495. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitaker-Azmitia P M. Serotonin and brain development: role in human developmental diseases. Brain Res Bull. 2001;56(5):479–485. doi: 10.1016/s0361-9230(01)00615-3. [DOI] [PubMed] [Google Scholar]
- 35.Sodhi M S, Sanders-Bush E. Serotonin and brain development. Int Rev Neurobiol. 2004;59:111–174. doi: 10.1016/S0074-7742(04)59006-2. [DOI] [PubMed] [Google Scholar]
- 36.Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4(12):1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- 37.Il'ková G, Rehák P, Veselá J. et al. Serotonin localization and its functional significance during mouse preimplantation embryo development. Zygote. 2004;12(3):205–213. doi: 10.1017/s0967199404002862. [DOI] [PubMed] [Google Scholar]
- 38.Ansorge M S, Zhou M, Lira A, Hen R, Gingrich J A. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306(5697):879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- 39.Oberlander T F, Gingrich J A, Ansorge M S. Sustained neurobehavioral effects of exposure to SSRI antidepressants during development: molecular to clinical evidence. Clin Pharmacol Ther. 2009;86(6):672–677. doi: 10.1038/clpt.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chugani D C, Muzik O, Behen M. et al. Developmental changes in brain serotonin synthesis capacity in autistic and nonautistic children. Ann Neurol. 1999;45(3):287–295. doi: 10.1002/1531-8249(199903)45:3<287::aid-ana3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 41.Chandana S R, Behen M E, Juhász C. et al. Significance of abnormalities in developmental trajectory and asymmetry of cortical serotonin synthesis in autism. Int J Dev Neurosci. 2005;23(2-3):171–182. doi: 10.1016/j.ijdevneu.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 42.Murphy D G, Daly E, Schmitz N. et al. Cortical serotonin 5-HT2A receptor binding and social communication in adults with Asperger's syndrome: an in vivo SPECT study. Am J Psychiatry. 2006;163(5):934–936. doi: 10.1176/ajp.2006.163.5.934. [DOI] [PubMed] [Google Scholar]
- 43.Hanley H G, Stahl S M, Freedman D X. Hyperserotonemia and amine metabolites in autistic and retarded children. Arch Gen Psychiatry. 1977;34(5):521–531. doi: 10.1001/archpsyc.1977.01770170031002. [DOI] [PubMed] [Google Scholar]
- 44.Tordjman S, Anderson G M, Cohen D. et al. Presence of autism, hyperserotonemia, and severe expressive language impairment in Williams-Beuren syndrome. Mol Autism. 2013;4(1):29. doi: 10.1186/2040-2392-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kahne D, Tudorica A, Borella A. et al. Behavioral and magnetic resonance spectroscopic studies in the rat hyperserotonemic model of autism. Physiol Behav. 2002;75(3):403–410. doi: 10.1016/s0031-9384(01)00673-4. [DOI] [PubMed] [Google Scholar]
- 46.Schain R J, Freedman D X. Studies on 5-hydroxyindole metabolism in autistic and other mentally retarded children. J Pediatr. 1961;58:315–320. doi: 10.1016/s0022-3476(61)80261-8. [DOI] [PubMed] [Google Scholar]
- 47.Marazziti D, Muratori F, Cesari A. et al. Increased density of the platelet serotonin transporter in autism. Pharmacopsychiatry. 2000;33(5):165–168. doi: 10.1055/s-2000-7588. [DOI] [PubMed] [Google Scholar]
- 48.Cook E H, Leventhal B L. The serotonin system in autism. Curr Opin Pediatr. 1996;8(4):348–354. doi: 10.1097/00008480-199608000-00008. [DOI] [PubMed] [Google Scholar]
- 49.Anderson G M. Monoamines in autism: an update of neurochemical research on a pervasive developmental disorder. Med Biol. 1987;65(2-3):67–74. [PubMed] [Google Scholar]
- 50.Ansorge M S, Hen R, Gingrich J A. Neurodevelopmental origins of depressive disorders. Curr Opin Pharmacol. 2007;7(1):8–17. doi: 10.1016/j.coph.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 51.Croen L A, Grether J K, Yoshida C K, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry. 2011;68(11):1104–1112. doi: 10.1001/archgenpsychiatry.2011.73. [DOI] [PubMed] [Google Scholar]
- 52.Rai D, Lee B K, Dalman C, Golding J, Lewis G, Magnusson C. Parental depression, maternal antidepressant use during pregnancy, and risk of autism spectrum disorders: population based case-control study. BMJ. 2013;346:f2059. doi: 10.1136/bmj.f2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sørensen M J, Grønborg T K, Christensen J. et al. Antidepressant exposure in pregnancy and risk of autism spectrum disorders. Clin Epidemiol. 2013;5:449–459. doi: 10.2147/CLEP.S53009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hviid A, Melbye M, Pasternak B. Use of selective serotonin reuptake inhibitors during pregnancy and risk of autism. N Engl J Med. 2013;369(25):2406–2415. doi: 10.1056/NEJMoa1301449. [DOI] [PubMed] [Google Scholar]
- 55.Harrington R A, Lee L C, Crum R M, Zimmerman A W, Hertz-Picciotto I. Prenatal SSRI use and offspring with autism spectrum disorder or developmental delay. Pediatrics. 2014;133(5):e1241–e1248. doi: 10.1542/peds.2013-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clements C C, Castro V M, Blumenthal S R. et al. Prenatal antidepressant exposure is associated with risk for attention-deficit hyperactivity disorder but not autism spectrum disorder in a large health system. Mol Psychiatry. 2014 doi: 10.1038/mp.2014.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boukhris T, Sheehy O, Bérard A. Antidepressant use during pregnancy is increasing the risk of autism spectrum disorder even after taking genetic predisposition into account. J Popul Ther Clin Pharmacol. 2014;21(2):e308–e337. [Google Scholar]


