Abstract
Purpose
The purpose of the study is to determine short-term reproducibility of apparent diffusion coefficient (ADC) estimated from diffusion-weighted magnetic resonance (DW-MR) imaging of the prostate.
Methods
Fourteen patients with biopsy-proven prostate cancer were studied under an Institutional Review Board-approved protocol. Each patient underwent two, consecutive and identical DW-MR scans on a 3T system. ADC values were calculated from each scan and a deformable registration was performed to align corresponding images. The prostate and cancerous regions of interest (ROIs) were independently analyzed by two radiologists. The prostate volume was analyzed by sextant. Per-voxel absolute and relative percentage variations in ADC were compared between sextants. Per-voxel and per-ROI variations in ADC were calculated for cancerous ROIs.
Results
Per-voxel absolute difference in ADC in the prostate ranged from 0 to 1.60 × 10−3 mm2/s (per-voxel relative difference 0% to 200%, mean 10.5%). Variation in ADC was largest in the posterior apex (0% to 200%, mean 11.6%). Difference in ADC variation between sextants was not statistically significant. Cancer ROIs’ per-voxel variation in ADC ranged from 0.001 × 10−3 to 0.841 × 10−3 mm2/s (0% to 67.4%, mean 11.2%) and per-ROI variation ranged from 0 to 0.463 × 10−3 mm2/s (mean 0.122 × 10−3 mm2/s).
Conclusions
Variation in ADC within the human prostate is reasonably small, and is on the order of 10%.
Keywords: DWI, ADC, Prostate, Reproducibility, MRI
Diffusion-weighted MR (DW-MR) imaging has emerged as an important component of the multi-parametric prostate MR examination paradigm over the last decade [1–5]. DW-MR imaging has been shown to complement T2-weighted imaging in the detection of cancer in both peripheral and transition zones [1, 4]. In a recent meta-analysis, Tan et al. reported that the sensitivity and specificity of T2-weighted imaging improved from 0.57–0.62 to 0.74–0.78, respectively, to 0.69–0.72 and 0.80–0.85, respectively, when combined with DW-MR imaging [2]. Apparent diffusion coefficient (ADC) values obtained from DW-MR imaging have been shown to correlate with the Gleason scores of prostate cancer and D'Amico clinical risk scores [4, 6–9]. Recent studies have also shown that decreased baseline ADC values measured from prostate cancers of men who are active surveillance candidates are associated with tumor upgrade on repeat biopsy [10, 11]. These studies suggest that there may be a role for ADC to serve as a predictive biomarker in patients undergoing active surveillance. Developing ADC as a quantitative imaging biomarker would allow better detection of prostate cancer by reducing inter-radiologist subjectivity and better differentiation of aggressive prostate cancer from indolent disease, thus reducing overtreatment while minimizing under diagnosis [12, 13].
A fundamental limitation of either qualitative or quantitative approaches to diagnostic characterization, however, is the lack of characterization of the reproducibility of the measurement. ADC values can be influenced by several imaging-dependent factors, such as gradient systems, coil systems, pulse sequence design, imaging parameters, and artifacts related to susceptibility effects or eddy currents [14]. However, the variability of ADC values in normal prostate and prostate cancer has not been well established. In this study we evaluate the short-term reproducibility of DW-MR imaging of the prostate in terms of changes in ADC maps between two consecutive scans without repositioning the same patient using the same scanner and identical imaging parameters. Such an evaluation allows us to quantify the minimum expected variation in ADC maps, providing an effective lower bound on the uncertainty in DW-MRI acquisition. Clinically, this uncertainty represents the most fundamental limitation of DW-MRI usage; this is the component of variability that cannot be reduced by standardizing the imaging protocol or equipment. A second-phase study of long-term reproducibility will be needed to account for variability from these sources as well as longer-term biological variability.
Materials and methods
Study subjects
Fourteen patients with biopsy-proven prostate cancer treated at our institution between April 2011 and September 2011 (age range 38–80 years, median 58.7 years; prostate-specific antigen (PSA) level range 4.5–12.7 ng/mL, median 9.97 ng/mL; and Gleason score range 6–8, median 6.5) were evaluated under an Institutional Review Board-approved protocol. Informed consent was obtained from each patient and the study was compliant with the Health Insurance Portability and Accountability Act. All patients underwent a 12-core transrectal ultrasound (TRUS)-guided biopsy of the prostate (one medial and one lateral core in each sextant of the prostate: right base, left base, right middle, left middle, right apex, and left apex) and had at least one core that was positive for prostate cancer. Each patient underwent two identical DW-MR scans using spin-echo Echo-Planar Imaging (EPI) sequences (TR/TE 5000 ms/64 ms, b values of 0, 50, 150, 990, and 1500 s/mm2, 3-mm slice thickness, 26 slices, 1.5 mm × 1.5 mm in plane resolution, 128 × 128 matrix size, 180 × 180 mm2 field of view, EPI factor 65, number of averages 4, scan time per sequence 7 min 25 s). The patient remained on the table between the scans to ensure that no significant prostate motion or deformation was introduced. All imaging was performed on a 3T Philips Achieva MRI scanner (Philips Healthcare, Best, Netherlands) using an endorectal coil in combination with an 8-channel cardiac coil positioned around the pelvis. 1 mg glucagon (Glucagon; Lilly, Indianapolis, IN) was injected intramuscularly before scanning to limit peristalsis of the rectum.
Image processing and prostate delineation
Following image acquisition, ADC maps were generated using custom-built software written in MATLAB (Mathworks Inc., Natick, MA), that estimated ADC values via least squares fit of the negative log of the ratio of signal intensities, −ln(Sb/S0), vs. b values.
A radiologist (XX with 3 years of experience in prostate MRI) manually delineated the prostate on both series of DW-MR images for all patients using a custom-written graphical user interface in MATLAB. Segmentation was performed on the b = 0 s/mm2 image because these images had the highest signal intensity. A second radiologist (YY with 11 years of experience in prostate MRI) drew regions of interest (ROIs) of the cancerous lesions for nine patients (Gleason Score 6–8, mean 7) based on the multi-parametric MRI results and 12 section TRUS-guided biopsy results. Dark spots on the ADC map were outlined if there was a corresponding, hypointense, well-defined focus on T2 images and they were in a sextant with a positive biopsy core. The three-dimensional ROI volumes were delineated using a Stentor PACS clinical software (Philips Healthcare, Best, Netherlands) on the b = 0 s/mm2 images on the first DW-MRI sequence only.
Image registration
To estimate the true difference in ADCs between the two consecutive scans, any variability due to motion must be removed. Factors such as bowel gas and patient discomfort may cause the prostate to shift between the first and second DW-MR scans, thus causing bias in a voxel-based analysis. We performed deformable registration between the two DW-MR image sequences using the open-source software package Plastimatch (v. 1.5.12-beta). For each patient, the prostate outlines on the ADC maps derived from the first and second DW-MR scans were overlaid on the corresponding, two, b = 0 s/mm2 images. The surrounding tissue was masked out by setting image intensity to zero and the second image was registered to the first using a Demons registration [15]. The estimated deformation was then applied to warp the second-scan ADC maps onto the first-scan ADC maps. For each patient, an “ADC difference map” was then obtained by subtracting the two sets of ADC maps.
Data analysis
The reproducibility of ADC values in the prostate was evaluated in terms of the ADC difference maps. The prostate volume was divided into sextants corresponding to the left base, right base, left medial, right medial, and left and right apex regions. The absolute (|ADCscan1 - ADCscan2|) and relative percent differences () per voxel were then calculated across each of these regions as well as for the entire prostate. Additionally, the cancer ROIs were overlaid on the ADC difference maps and the per-voxel and per-ROI relative and absolute differences were calculated for the cancer ROIs.
Statistical analysis
The differences between sextants were determined to be statistically significant or not, using one way ANOVA (p < 0.05). The mean difference in each sextant per patient was calculated and listed as an independent measurement and ANOVA was performed on all six measurements.
Results
Overall absolute and relative percentage variations
The per-voxel absolute difference in ADC within the prostate ranged from 0 to 1.60 × 10−3 mm2/s (relative difference 0.00% to 200%, mean 10.52%). Table 1 shows the per-voxel ADC relative and absolute differences averaged across all patients within each sextant and over the entire prostate.
Table 1.
Voxel-to-voxel relative and absolute ADC variations within each sextant and the entire prostate across all patients
| Anterior Apex | Posterior Apex | Anterior Medial | Posterior Medial | Anterior Base | Posterior Base | Whole Prostate | |
|---|---|---|---|---|---|---|---|
| Relative percentage variation | |||||||
| Mean difference (%) | 10.6 | 11.6 | 10.0 | 10.5 | 10.8 | 10.3 | 10.5 |
| Stan. Dev. of difference (%) | 12.0 | 12.5 | 12.9 | 11.4 | 13.3 | 10.6 | 12.0 |
| Median difference (%) | 7.07 | 8.00 | 6.42 | 7.23 | 6.76 | 7.24 | 7.05 |
| Absolute variation (10–3 mm2/s) | |||||||
| Mean difference | 0.103 | 0.138 | 0.100 | 0.126 | 0.116 | 0.117 | 0.117 |
| Stan. Dev. of difference | 0.0980 | 0.135 | 0.104 | 0.129 | 0.154 | 0.113 | 0.125 |
| Median difference | 0.0751 | 0.0974 | 0.0699 | 0.0878 | 0.0728 | 0.0836 | 0.0802 |
Variations within sextant
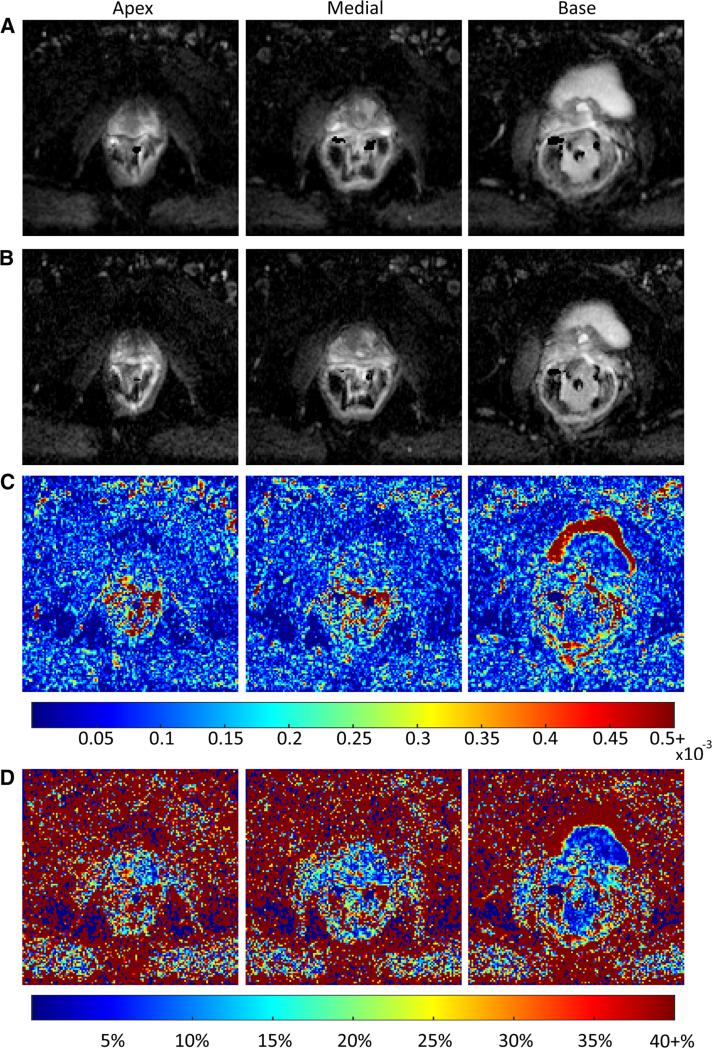
The amount of variation in ADC did not change substantially with location in the prostate. The amount of variation was relatively uniform across the entire prostate with slightly higher variability seen in the apex. The largest difference was seen in the posterior apex (mean 11.55%). These results did not demonstrate a statistically significant difference in the level of variation between the different sextants. Variation in ADC within the apex, medial region, and base of the prostate is illustrated in Fig. 1 for a single patient.
Fig. 1.
Axial ADC maps generated from back-to-back scans of a single patient (57 years, prostatic adenocarcinoma, Gleason score 8, PSA 10.37) through the center of the apex, medial section, and base of the prostate. Slices shown do not contain cancerous tissue. Absolute difference in ADC per voxel for corresponding slices as well as the relative percentage difference in ADC are shown. High percentage variation shown outside of the prostate, particularly in the distal, left bladder may be attributable to the motion effects and is not of much concern, as the ADC maps were registered using a warping template derived based on the masked prostate images, and do not take other tissues into account.
Variations within cancer ROIs
Per-voxel analysis of the voxels located in cancer ROIs showed a mean and standard deviation in relative ADC difference of 17.15% ± 16.51%, respectively, higher than that of the whole prostate. The mean ± standard deviation of absolute voxel-by-voxel difference in cancer is 0.188 ± 0.213 × 10−3 mm2/s, and in the whole prostate is 0.117 ± 0.125 × 10−3 mm2/s (Table 2).
Table 2.
Relative percentage and absolute difference in ADC for cancer ROIs and whole prostate derived using voxel-based approach, and relative percentage and absolute difference in mean ADC of cancer ROIs between the two scans
| Absolute (10–3 mm2/s) |
Relative percentage |
|||
|---|---|---|---|---|
| Range | Median | Range | Median | |
| Per-voxel variation | ||||
| Cancer ROI | [0.0, 0.84] | 0.097 | [0.0%, 67%] | 11% |
| Whole prostate | [0.0, 1.6] | 0.080 | [0.0%, 200%] | 7.1% |
| Per-ROI variation | ||||
| Cancer ROI | [0.0, 0.46] | 0.036 | [1.0%, 38%] | 4.2% |
| Mean + SD ADC of whole prostatea: 1.2 ± 0.27 × 10–3 mm2/s | ||||
| Mean + SD ADC of cancersa: 0.95 ± 0.32 × 10–3 mm2/s | ||||
Voxelwise ADC across all patients
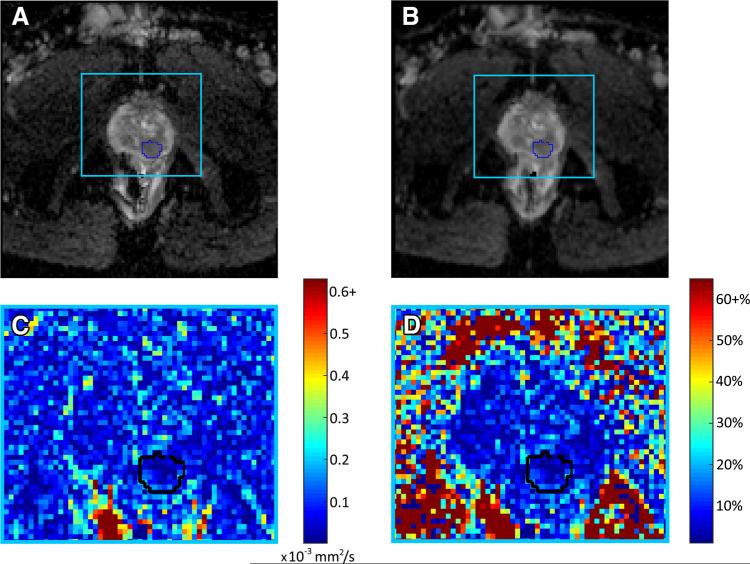
Per-ROI analysis of the cancer ROIs showed the absolute ADC difference ranged from 0 to 0.463 × 10−3 mm2/s, with a mean of 0.122 × 10−3 mm2/ (1.02% to 37.7%, mean 10.6%). Figure 2 shows an example of variation within a cancer ROI and surrounding prostate tissue for a single patient.
Fig. 2.
A–B Pair of axial ADC maps generated from back-to-back DW-MR images for a single patient (55 years, prostatic adenocarcinoma, PSA 12.7) with cancerous ROI outlined in blue, C absolute difference in ADC between scan a and scan b for area within light blue box, and D relative percentage difference in ADC between scan a and scan b for area within light blue box. High areas of variability are common outside of the prostate, particularly in the rectum, and relative variation is high outside of the prostate where the signal intensity is very low. Although the variability in ADC is low within the prostate there are foci of 25% to 30% visible, demonstrating how variability within DW-MRI may affect not only quantitative imaging results, but also results of a qualitative, clinical assessment.
Discussion
Our results suggest that the reproducibility of ADC measurements in prostate is reasonable. The posterior prostate consistently showed higher variability than the anterior portion which may be attributable to the influence of the rectum. However, we were not able to demonstrate statistical significance in the variations between the sextants.
Chenevert and Malyarenko have both performed studies evaluating the repeatability of ADC measurements made using ice water phantoms and found variability to be less than 5% [16, 17]. Chenevert evaluated 20 MRI scanners from three vendors over 25 days with a phantom of distilled water in an ice water bath and found that reproducibility in ADC for a single system was within ±5%. Malyarenko performed a study in which a DW-MRI exam was performed on an ice water phantom at 35 institutions on 3 different vendor systems, each exam consisting of 4 back-to-back DW-MRI acquisitions. Intra-exam repeatability of ADC values, defined as 2 standard deviations in ADC expressed as a percentage of mean ADC, was found to be within 1% (i.e., 95% of measurements are expected to fall within 1% of the mean) for 70 ROIs drawn within the phantom. Comparing with the results of these phantom studies, we consider the reproducibility demonstrated in our study to be reasonable.
Our results show lower variability in ADC than recent reports of reproducibility in the abdomen and liver [18, 19], which show variability of 25% to 30%. Our study is unique in evaluating back-to-back prostate scans while the patient remains on the table, thus eliminating effects of scanner variability over time and inter-scanner and patient variability from repositioning the patient in the scanner. These sources of variation that were not considered in this study have been evaluated recently in other studies. Litjens et al. performed a study on 51 patients with prostate cancer in the peripheral zone and another 10 patients with high PSA levels but negative TRUS-guided biopsy results to determine inter-patient variability of ADC in the peripheral zone and its effect on the prediction of prostate cancer aggressiveness, by comparing median ADCs within an ROI between repeat imaging scans [20]. Results of this study indicate that ADC measurement variability in normal peripheral zone tissue attributable to coil and imaging sequence parameter choices was significantly lower (p = 0.0058) (mean 0.068 × 10−3 mm2/s ±27 × 10−3) than inter-patient variability that they hypothesized being attributable to tissue physiological variations (1.2–2.0 × 10−3 mm2/s). Gibbs et al. evaluated the reproducibility of DW-MRI of the prostate in a study of 8 healthy volunteers undergoing two DW-MRI sessions timed approximately 1 month apart, with each volunteer scanned twice in the second session. ROIs were outlined in the prostate for comparison of ADCs. Results indicate that both short-and medium-term repeatability (defined as 2.77 times the within-subject standard deviation) were under 35% [21].
Motion and deformation of the prostate inevitably have influence on a reproducibility study. We minimized such effects by performing deformable registration, however, it is impossible to completely remove motion-related variability. Glucagon was also injected immediately before the MR exam to limit peristalsis of the rectum but due to the nature of the study in which the patient remained on the table between scans and time between scans was minimized, it was not practical to re-administer glucagon between scans. Thus, it is possible that the true short-term variability may be slightly lower than that reported here. Note also that the time between our back-to-back image acquisitions was so small that motion between the consecutive scans was minimal.
Limitations of our study include a limited number of study patients. Also, our reference standard was TRUS-guided biopsy results rather than histopathology. This may be important for our analysis of the cancer ROIs, but is likely not critical for our analysis of the reproducibility over the entire prostate. Lastly, in this study we evaluated only short-term reproducibility and did not evaluate contributions from scanner model, coil choice, and imaging parameters, etc. Future studies that take into account the contributions from all of these sources will be necessary, particularly for using ADC as an imaging biomarker because reproducibility between institutions and scanners will be important.
In conclusion, ADC has strong potential to become a powerful quantitative imaging biomarker for prostate cancer but it is important to understand the reproducibility of DW-MR imaging for this potential to be realized. Our results show that ADC variation within the prostate is reasonable, on the order of 10%, and lower than those reported for other abdominal organs. This study of an evaluation of short-term reproducibility is a first step toward a more comprehensive assessment of the reproducibility of DW-MR imaging of prostate cancer.
Acknowledgement
This work was supported in part by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under grant number T32 EB002103.
References
- 1.Haider MA, van der Kwast TH, Tanguay J, et al. Combined T2-weighted and diffusion-weighted MRI for localization of prostate cancer. Am J Roentgenol. 2007;189(2):323–328. doi: 10.2214/AJR.07.2211. [DOI] [PubMed] [Google Scholar]
- 2.Tan CH, Wei W, Johnson V, Kundra V. Diffusion-weighted MRI in the detection of prostate cancer: meta-analysis. Am J Roentgenol. 2012;199(4):822–829. doi: 10.2214/AJR.11.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kajihara H, Hayashida Y, Murakami R, et al. Usefulness of diffusion-weighted imaging in the localization of prostate cancer. Int J Radiat Oncol Biol Phys. 2009;74(2):399–403. doi: 10.1016/j.ijrobp.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 4.Oto A, Yang C, Kayhan A, et al. Diffusion-weighted and dynamic contrast-enhanced MRI of prostate cancer: correlation of quantitative MR parameters with gleason score and tumor angio-genesis. Am J Roentgenol. 2011;197(6):1382–1390. doi: 10.2214/AJR.11.6861. [DOI] [PubMed] [Google Scholar]
- 5.Rosenkrantz AB, Mannelli L, Kong XT, et al. Prostate cancer: utility of fusion of T2-weighted and high b-value diffusion-weighted images for peripheral zone tumor detection and localization. J Magn Reson Imaging. 2011;34(1):95–100. doi: 10.1002/jmri.22598. [DOI] [PubMed] [Google Scholar]
- 6.Lim HK, Kim JK, Kim KA, Cho K-S. Prostate cancer: apparent diffusion coefficient map with T2-weighted images for detection-A Multireader study. Radiology. 2009;250(1):145–151. doi: 10.1148/radiol.2501080207. [DOI] [PubMed] [Google Scholar]
- 7.Woodfield CA, Tung GA, Grand DJ, et al. Diffusion-weighted MRI of peripheral zone prostate cancer: comparison of tumor apparent diffusion coefficient with Gleason score and percentage of tumor on core biopsy. Am J Roentgenol. 2010;194(4):W316–W322. doi: 10.2214/AJR.09.2651. [DOI] [PubMed] [Google Scholar]
- 8.Kitajima K, Kaji Y, Fukabori Y, et al. Prostate cancer detection with 3 T MRI: comparison of diffusion-weighted imaging and dynamic contrast-enhanced mri in combination with T2-weighted imaging. J Magn Reson Imaging. 2010;31(3):625–631. doi: 10.1002/jmri.22075. [DOI] [PubMed] [Google Scholar]
- 9.Mehta R, Kyshtoobayeva A, Kurosaki T, et al. Independent association of angiogenesis index with outcome in prostate cancer. Clin Cancer Res. 2001;7(1):81–88. [PubMed] [Google Scholar]
- 10.Hosseinzadeh K, Schwarz SD. Endorectal diffusion-weighted imaging in prostate cancer to differentiate malignant and benign peripheral zone tissue. J Magn Reson Imaging. 2004;20(4):654–661. doi: 10.1002/jmri.20159. [DOI] [PubMed] [Google Scholar]
- 11.Issa B. In vivo measurement of the apparent diffusion coefficient in normal and malignant prostatic tissues using echo-planar imaging. J Magn Reson Imaging. 2002;16(2):196–200. doi: 10.1002/jmri.10139. [DOI] [PubMed] [Google Scholar]
- 12.Padhani AR, Liu G, Chenevert TL, et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia. 2009;11(2):102–125. doi: 10.1593/neo.81328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clarke LP, Croft BS, Nordstrom R, et al. Quantitative imaging for evaluation of response to cancer therapy. Transl Oncol. 2009;2(4):195–197. doi: 10.1593/tlo.09217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sasaki M, Yamada K, Watanabe Y, et al. Variability in absolute apparent diffusion coefficient values across different platforms may be substantial: a multivendor, multi-institutional comparison study. Radiology. 2008;249(2):624–630. doi: 10.1148/radiol.2492071681. [DOI] [PubMed] [Google Scholar]
- 15.Sharp GC, Kandasamy N, Singh H, Folkert M. GPU-based streaming architectures for fast cone-beam CT image reconstruction and demons deformable registration. Phys Med Biol. 2007;52(19):5771–5783. doi: 10.1088/0031-9155/52/19/003. [DOI] [PubMed] [Google Scholar]
- 16.Chenevert TL, Galban CJ, Ivancevic MK, et al. Diffusion coefficient measurement using a temperature-controlled fluid for quality control in multicenter studies. J Magn Reson Imaging. 2011;34(4):983–987. doi: 10.1002/jmri.22363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malyarenko D, Galban CJ, Londy FJ, et al. Multi-system repeatability and reproducibility of apparent diffusion coefficient measurement using an ice-water phantom. J Magn Reson Imaging. 2012;37(5):1238–1246. doi: 10.1002/jmri.23825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braithwaite AC, Dale BM, Boll DT, Merkle EM. Short- and midterm reproducibility of apparent diffusion coefficient measurements at 3.0-T diffusion-weighted imaging of the abdomen. Radiology. 2009;250(2):459–465. doi: 10.1148/radiol.2502080849. [DOI] [PubMed] [Google Scholar]
- 19.Kim SY, Lee SS, Byun JH, et al. Malignant hepatic tumors: short-term reproducibility of apparent diffusion coefficients with breath-hold and respiratory-triggered diffusion-weighted MR imaging. Radiology. 2010;255(3):815–823. doi: 10.1148/radiol.10091706. [DOI] [PubMed] [Google Scholar]
- 20.Litjens GJS, Hambrock T, Hulsbergen-van de Kaa C, Barentsz JO, Huisman HJ. Interpatient variation in normal peripheral zone apparent diffusion coefficient: effect on the prediction of prostate cancer aggressiveness. Radiology. 2012;265(1):260–266. doi: 10.1148/radiol.12112374. [DOI] [PubMed] [Google Scholar]
- 21.Gibbs P, Pickles MD, Turnbull LW. Repeatability of echo-planar-based diffusion measurements of the human prostate at 3 T. Magn Reson Imaging. 2007;25(10):1423–1429. doi: 10.1016/j.mri.2007.03.030. [DOI] [PubMed] [Google Scholar]