Abstract
Brain reward circuits are implicated in stress-related psychiatric disorders. Exercise reduces the incidence of stress-related disorders, but the contribution of exercise reward to stress resistance is unknown. Exercise-induced stress resistance is independent of exercise controllability; both voluntary and forced wheel running protect rats against anxiety- and depression-like behavioral consequences of stress. Voluntary exercise is a natural reward, but whether rats find forced wheel running rewarding is unknown. Moreover, the contribution of dopamine (DA) and striatal reward circuits to exercise reward is not well characterized. Adult, male rats were assigned to locked wheels, voluntary running (VR), or forced running (FR) groups. FR rats were forced to run in a pattern resembling rats' natural wheel running behavior. Both VR and FR increased the reward-related plasticity marker ΔFosB in the dorsal striatum (DS) and nucleus accumbens (NAc), and increased activity of DA neurons in the lateral ventral tegmental area (VTA), as revealed by immunohistochemistry for tyrosine hydroxylase (TH) and pCREB. Both VR and FR rats developed conditioned place preference (CPP) to the side of a CPP chamber paired with exercise. Re-exposure to the exercise-paired side of the CPP chamber elicited conditioned increases in cfos mRNA in direct pathway (dynorphin-positive) neurons in the DS and NAc in both VR and FR rats, and in TH-positive neurons in the lateral VTA of VR rats only. Results suggest that the rewarding effects of exercise are independent of exercise controllability and provide insight into the DA and striatal circuitries involved in exercise reward and exercise-induced stress resistance.
Keywords: dopamine, ventral tegmental area, rat, immediate early genes, striatum
Introduction
Exercise can increase resistance to the development of stress-related psychiatric disorders, including depression, anxiety, and post-traumatic stress disorder (Sothmann et al., 1996; Greenwood & Fleshner, 2011; Sciolino et al., 2012; Asmundson et al., 2013; Greenwood & Fleshner, 2013). Rats allowed voluntary access to running wheels, for example, are protected against anxiety- and depression-like behavioral effects of stress (Dishman et al., 1997; Solberg, 1999; Greenwood et al., 2003a; Zheng et al., 2006). Central reward circuitry, including dopamine (DA) neurons originating in the midbrain substantia nigra (SN) or ventral tegmental area (VTA) and projecting to the dorsal striatum (DS; nigrostriatal pathway) or nucleus accumbens (NAc; mesolimbic pathway), are implicated in the pathophysiology and treatment of stress-related disorders (Nestler & Carlezon, 2006). Rodents find exercise a naturally reinforcing and rewarding activity (Iversen, 1993; Lett et al., 2000; Greenwood et al., 2011; Trost & Hauber, 2014). Indeed, voluntary wheel running produces adaptations within DA reward pathways consistent with repeated activation of these circuits (Foley & Fleshner, 2008; Greenwood et al., 2011). Reward-related phasic, “burst” firing of DA neurons activates low-affinity DA 1 receptors (D1) located on direct-pathway striatal neurons (Bertran-Gonzalez et al., 2008; Bertran-Gonzalez et al., 2010b; Dreyer et al., 2010). ΔFosB, a marker of repeated neuronal activity (Perrotti et al., 2004; Ulery-Reynolds et al., 2009) and reward-related plasticity (Nestler, 2008; Wallace et al., 2008), increases specifically in direct-pathway neurons of the NAc following voluntary exercise (Werme et al., 2002). Moreover, over-expression of ΔFosB in the DS or NAc can elicit stress resistance in a manner similar to exercise (Vialou et al., 2010; Nestler, 2015). These data suggest that rewarding effects of exercise could include activation of nigrostriatal and mesolimbic DA pathways, and that repeated activation of these circuits could contribute to exercise-induced stress resistance. Despite this possibility, the contribution of DA pathways to exercise reward is not well characterized.
One goal of the current studies was to identify the neural circuits sensitive to exercise that might contribute to reward and stress resistance from exercise. A second goal was to determine whether behavioral and neurochemical indices of exercise reward depend on exercise controllability. Using a novel forced wheel running paradigm in which rats are forced to run on motorized wheels in a pattern that closely resembles their natural running behavior, we have observed that stress resistance is independent of exercise controllability (Greenwood et al., 2013). Therefore, comparing the effects of voluntary and forced wheel running on reward-related circuitry could provide important insights into the contribution of exercise reward to exercise-induced stress resistance. The results of these studies suggest that both voluntary and forced wheel running are rewarding and activate similar circuits implicated in reward, including DA neurons of the lateral VTA and direct-pathway neurons of the DS and NAc. These data are consistent with exercise reward being an important factor for the stress resistance produced by both voluntary and forced exercise. The mechanism through which exercise recruits reward-related striatal circuits, however, may differ depending on the controllability of exercise.
Materials and Methods
Subjects
A total of 47, young adult (between P42-P49 upon arrival), male Fischer 344 rats (Harlan SPF, Indianapolis, IN., USA) were used in all experimental procedures. Rats were housed in a temperature (22°C) and humidity-controlled environment and were maintained on a 12:12 hour light:dark cycle (lights on 07:00 - 19:00 h). All rats had ad libitum access to food (lab chow) and water and were weighed weekly. All rats were individually housed in Nalgene Plexiglas home cages (45 cm × 25.2 cm × 14.7 cm). Rats acclimated to these housing conditions for 7 days prior to any experimental manipulation. Care was taken to minimize animal discomfort during all procedures. The University of Colorado Boulder Animal Care and Use Committee approved all experimental protocols.
Exercise Protocols
Rats were randomly assigned to Locked, voluntary wheel running (VR), or forced wheel running (FR) conditions. Because experience with wheel running is required to minimize non-running behaviors (e.g. tumbling, hanging onto wheel rungs) during forced wheel running (Greenwood et al., 2013), motorized wheels belonging to rats in the FR group were removed from the motor and were allowed to spin freely, and all rats in the VR and FR conditions were allowed voluntary access to their wheels for 5 consecutive active (dark) cycles prior to the start of differential group treatment. Wheels belonging to rats in the FR group were re-connected to motors following these 5 nights of voluntary running, and all subsequent running by the FR group was controlled by the motorized wheels. Nights during which rats ran in wheels, rats were transported immediately prior to the start of the active cycle from their home cages to their assigned locked (Locked), freely mobile (VR), or motorized (FR) running wheels (1.1 m circumference; Lafayette Instruments, Lafayette, IN., USA). The motorized running wheels cannot be turned voluntarily by the rats. Instead, forced wheels are driven by a motor controlled by the Activity Wheel Monitor software (Lafayette Instruments, Lafayette, IN., USA) according to a protocol pre-programed by the experimenters and designed to closely approximate a rat's natural voluntary running behavior based on analyses of prior experiments. In order to reduce tumbling in the wheel, speed, bout length and distance run by FR rats was slowly increased during the first few days of FR. Moreover, the average and maximum running speed of the FR condition was less than that of the VR condition (Greenwood et al., 2013). Because of this reduction in speed, rats in the FR group run less distance per day than rats in the VR group. However, the pattern of running is similar. Running bout length (average 2.04±1.95) and duration of rest periods (range 0.33-30 min) were carefully matched between VR and FR conditions. Rats in the Locked, VR and FR groups were confined to their wheels for their assigned running bouts which were either the entire 12h duration of the active cycle (Experiment 1) or for the first 2 h of the active cycle (Experiment 2). A food tray and water bottle mounted on the side of the wheel allowed access to food and water while rats were confined to the wheel. We have previously observed that rats eat and drink equal amounts regardless of exercise condition (Greenwood et al., 2013). Wheel revolutions were automatically recorded by the Lafayette Activity Wheel Monitor software. A visual depiction of the voluntary and forced running wheels, as well as detailed analyses of voluntary and forced running patterns, can be found in our prior report (Greenwood et al., 2013).
Experimental Procedures
Experiment 1
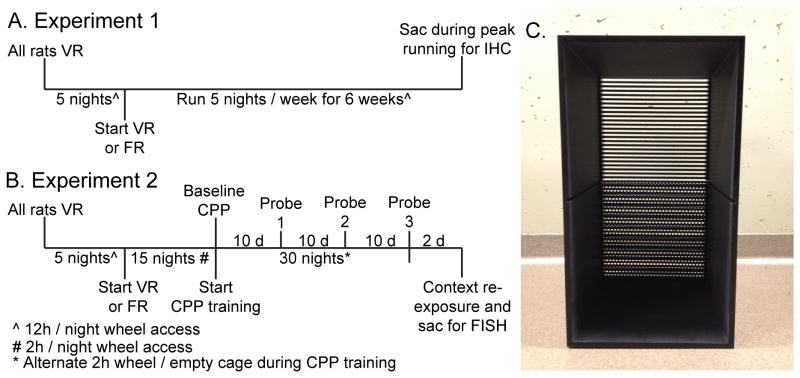
The experimental timeline used for Experiment 1 appears in Figure 1A. In order to characterize the sensitivity of reward-related circuits to voluntary and forced exercise, rats were randomly assigned to Locked (n = 9), VR (n = 8), and FR (n = 7) groups. In this study rats were placed into their assigned locked, mobile, or motorized wheel 5 nights / week for 7 weeks. For the first 5 nights of the study, wheels belonging to rats in the FR condition were rendered freely mobile and all rats in the VR and FR conditions were allowed to run freely in their wheels. Following this wheel running-acclimatization procedure, rats in the VR group continued nightly exposure to their voluntary wheels, whereas rats in the FR group began running according to the forced wheel protocol. On the last evening of the study, following 6 weeks of Locked, VR, or FR conditions, all rats were placed into their assigned wheels at the onset of the active (dark) cycle as usual. Then, between 2-3 h later, all rats were sacrificed and brains were removed for IHC detection of double TH/pCREB and ΔFosB immunoreactivity in midbrain and striatum, respectively.
Figure 1.
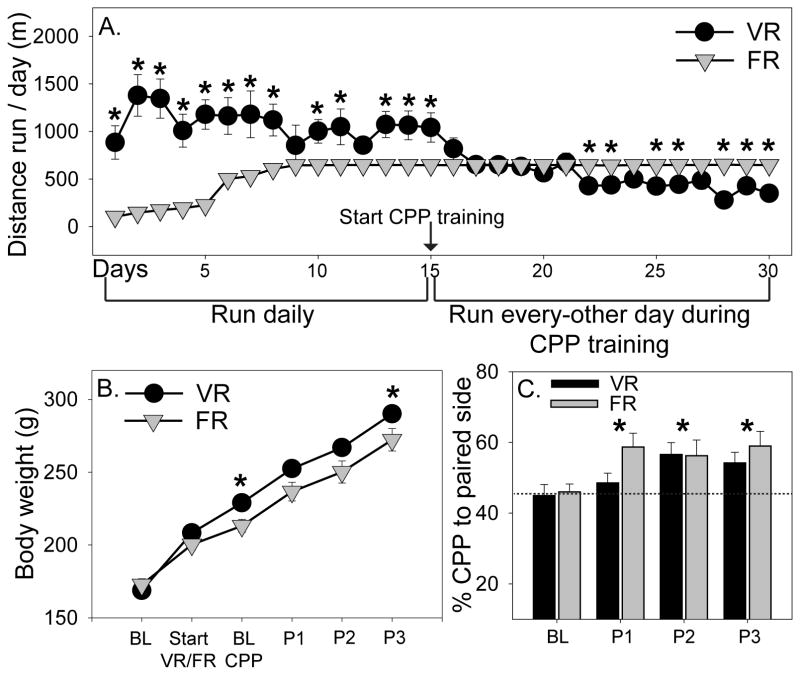
Experimental timelines. A) Following 5 nights of voluntary wheel running, rats were assigned to Locked, voluntary running (VR) or forced running (FR) groups. Rats were placed into their assigned Locked, VR, or FR wheels 5 nights a week for 6 weeks prior to sacrifice for immunohistochemistry (IHC) for tyrosine hydroxylase / pCREB or ΔFosB in the midbrain or striatum, respectively. B) Following 5 nights of voluntary wheel running, rats were assigned to voluntary running (VR) or forced running (FR) groups and nightly running was subsequently restricted to 2 h. Rats were placed into their assigned voluntary or motorized wheels for the first 2 h of the active cycle for 15 consecutive nights prior to assessment of baseline conditioned place preference (CPP). Following baseline CPP, CPP training was initiated. During CPP training, rats were exposed to the paired side of the CPP chamber following 2 h of either VR or FR during the first 2 h of the active cycle, or, on alternating nights, the unpaired side of the CPP chamber following 2 h of exposure to an empty rat cage. Probe trials, during which the barrier separating the 2 sides of the CPP chamber was removed, were conducted every 10 days. One additional day of paired CPP training took place the day after the 3rd probe trial. Then, the next day, rats were re-exposed to either the unpaired or paired side of the CPP chamber for 30 min prior to sacrifice via rapid decapitation for assessment of neural activation patterns with double fluorescent in situ hybridization (FISH). C) CPP apparatus used in experiment 2. The barrier between the two halves of the CPP chamber is removed to show the floor textures.
Experiment 2
The experimental timeline for experiment 2 is shown in Figure 1B. After the 5 nights of wheel acclimation during which all rats ran voluntarily in their wheels for the entirety of the active cycle, rats were randomly assigned to VR or FR groups (n = 12/group). Rats were then placed nightly into their assigned voluntary or motorized wheel for 2 h, starting at the beginning of the active cycle, for 15 days in order to establish voluntary and forced running behavior. Baseline CPP testing occurred after 15 days of VR or FR. CPP training started after baseline CPP testing. During CPP training, rats were placed into their assigned VR or FR wheels or empty cage for 2 h on alternating nights for 30 days. Because most voluntary running behavior occurs within the first 3 h of the active cycle (Eikelboom & Mills, 1988; Greenwood et al., 2011), rats in the current study were exposed to CPP training after only 2 h of running, during peak running, in order to provide a discrete exercise stimulus to pair with the paired side of the CPP chamber and facilitate CPP. In order to determine if CPP to exercise depends upon exercise controllability, a probe trial occurred after every 10 days of CPP training, for a total of 3 probe trials.
In order to identify the reward-related neural circuits that might contribute to the development of exercise CPP, rats were exposed to 1 additional evening of a wheel running/paired CPP training bout following the 3rd and final probe trial. Rats were then randomly assigned to be re-exposed to either the paired or unpaired side of the CPP chamber 24 h after the last running bout, yielding the following groups: Voluntary / Unpaired (n = 6), Voluntary / Paired (n = 6), Forced / Unpaired (n = 6), and Forced / Paired (n = 6). During re-exposure the wall separating the 2 sides of the CPP chamber remained in place, and rats were placed into the assigned unpaired or paired side for 30 min, during which time locomotor activity was recorded and scored in a subset of rats (n = 4 /group) using TopScan. Rats were sacrificed after the 30 min re-exposure and activity of midbrain DA neurons was quantified using double fluorescent in situ hybridization (FISH) for cfos and TH. Reward-related DA release in the striatum increases signaling at low-affinity D1 receptors, located on medium spiny neurons associated with the direct pathway of the DS and NAc (Bertran-Gonzalez et al., 2008; Bertran-Gonzalez et al., 2010b; Dreyer et al., 2010). Since emerging data suggest that D1 signaling on direct pathway neurons in both the DS and NAc can contribute to reward and reinforcement (Kravitz & Kreitzer, 2012a; Kravitz et al., 2012a; Ilango et al., 2014), conditioned activation of DS and NAc direct pathway neurons was also assessed with double FISH for cfos and dynorphin mRNAs. Dynorphin is nearly 100% co-localized with D1 on direct pathway neurons in the DS and NAc core (NAcC)(Anderson & Reiner, 1990; Gerfen et al., 1990; Bertran-Gonzalez et al., 2010a), thus serves as a convenient genotypic marker of D1-expressing, direct pathway neurons. FISH was used in this experiment rather than immunohistochemistry because the presence of dynorphin labeling in striatal neuropil makes co-label protein quantification challenging, whereas dynorphin mRNA is restricted to cell bodies. Quantification of immediate early genes in both of these experiments was performed in the nigrostriatal and mesolimbic pathways because of the overlapping roles of these pathways in reward and reinforcement (Wise, 2009; Kravitz & Kreitzer, 2012b).
Double Immunohistochemistry
Rats were deeply anesthetized with sodium pentobarbital between 2-3 h after the start of the active (dark) cycle, during peak running. Rats were perfused transcardially with cold saline, followed by 300-400 ml of 4% paraformaldehyde (PF) in 0.1 M phosphate buffer (PB). Brains were extracted, post-fixed in PF overnight, and then transferred to 30% sucrose solution for 3 days. Brains were then rapidly frozen in isopentane with dry ice and subsequently stored at -70°C until coronal sectioning at 35 μm on a cryostat. Brain slices were stored in cryoprotectant at -20°C until staining. All tissue from each region was processed simultaneously in 25-well staining dishes so that direct comparisons between could be made.
Immunohistochemistry was performed as previously described (Greenwood et al., 2003a; Greenwood et al., 2003b; Greenwood et al., 2011). Briefly, 35 μm brain sections representing the rostral to caudal extent of the striatum (1.6mm to 0.2mm rostral from Bregma) or SN and VTA (5.30mm to -6.04mm caudal from Bregma) were rinsed in 0.01 M phosphate buffered saline (PBS) followed by a 15 min incubation in 0.6% hydrogen peroxide. Sections were incubated at room temperature (RT) for 48 h at 4°C in blocking solution containing 0.5% Triton X-100, 5% normal goat serum, and rabbit anti-ΔFosB (1:450; catalog number 9890, Cell Signaling, Danvers, MA) or rabbit anti-pCREB antibody (1:5000; catalog number 06-519, Millipore, Billerica, MA). The ΔFosB antibody was chosen because of its high selectivity for the stable, larger (33-37-kDa) isoforms of ΔFosB induced by chronic manipulations, and its lack of cross-reactivity to smaller isoforms of FosB induced transiently by acute manipulations (Chen et al., 1997; McClung et al., 2004). CREB is phosphorylated rapidly after cellular activity, including in the VTA following rewarding drugs such as morphine (Walters et al., 2003). Therefore, pCREB is a useful immediate early gene to use as a marker of recent neural activity in midbrain DA neurons following reward-eliciting stimuli.
Incubation in primary antisera was followed by another series of washes in PBS after which the sections were incubated at RT for 90 min in blocking solution containing a 1:300 dilution of biotinylated goat anti-rabbit IgG (Jackson Immunoresearch, West Grove, PA). Sections were then incubated with avidin-biotin-horseradish peroxidase complexes (ABC; Vecastain Elite ABC kit, Vector Laboratories, Burlingame, CA) in PBS containing 0.5% Triton X-100 for 2 hr. After washes with PB, sections were placed in a solution containing 3,3′-diaminobenzidene tetrahydrochloride (DAB), ammonium chloride, cobalt chloride, nickel ammonium sulfate, and glucose oxidase in PB for 10 min. The peroxidase reaction was started by addition of glucose solution and reacted for ∼15 min, yielding a dark brown / black reaction product. The reaction was stopped by rinses in PBS. For double labeled sections through the midbrain, staining for pCREB preceded TH. For double-labeling, sections through the midbrain labeled with pCREB were incubated in blocking solution for 30 min, followed by 48 h incubation at 4°C in blocking buffer containing a 1:100,000 dilution of rabbit anti-TH (Pel Freeze Biologicals, Rogers, AR). Sections were then placed in goat anti-rabbit IgG (1:300; Jackson ImmunoResearch) for 90 min, followed by rinses and a 2 h incubation in ABC. Tissue was rinsed in PB and reacted with DAB and glucose oxidase. The peroxidase reaction was initiated by addition of glucose and allowed to proceed for approximately 15 minutes, yielding a light brown reaction product. The reaction was stopped by PBS washes.
Controls for the cross-reactivity of ΔFosB, pCREB, and TH antisera consisted of processing additional sections except without addition of the primary antibody. Sections processed this way demonstrated a lack of labeling. Stained sections were mounted onto gelatin coated, glass slides and air-dried overnight. Slide-mounted sections were dehydrated in a series of alcohols, rinsed in Histoclear, and cover-slipped with DPX.
Quantification of immunohistochemistry
Images of the medial and lateral portions of the DS, NAcC, NAc shell (NAcS), VTA and SN pars compacta regions (see Figures 3A and 4A) were captured digitally on a Nikon Eclipse 55i microscope at 20X. At least 6 hemispheres per rat were imaged for each region counted, resulting in a total of at least 36 images per rat (6 images per brain region). Quantification was done with Image J by multiple experimenters blind to treatment condition of the rats. A 450 μm by 450 μm square was drawn around the region of interest and quantification of single ΔFosB (dark stained nucleus in striatal slices), single pCREB (dark stained nucleus in midbrain slices), TH (light brown cytoplasmic stain), and double-labeled neurons (light brown cytoplasm containing a darkly-stained nucleus) took place within that area.
Figure 3.
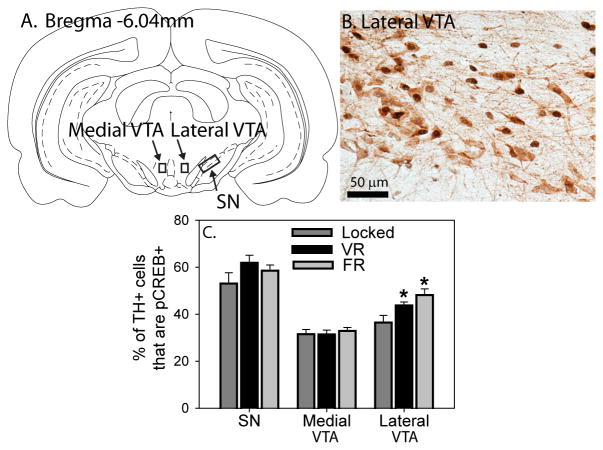
Exercise, regardless of controllability, activates dopamine neurons in the lateral ventral tegmental area (VTA). A) Figure from the atlas of Paxinos and Watson showing the regions analyzed in the VTA and the substantia nigra (SN). B) Photomicrograph showing tyrosine hydroxylase (TH; light brown) and pCREB (dark brown/black) immunostaining in the lateral VTA (400x magnification). C) Six weeks of voluntary (VR) and forced (FR) running increased the percentage of TH neurons in the lateral VTA that contained pCREB, relative to Locked controls (* p < 0.05 relative to Locked group). Bars represent group means ± SEM.
Figure 4.
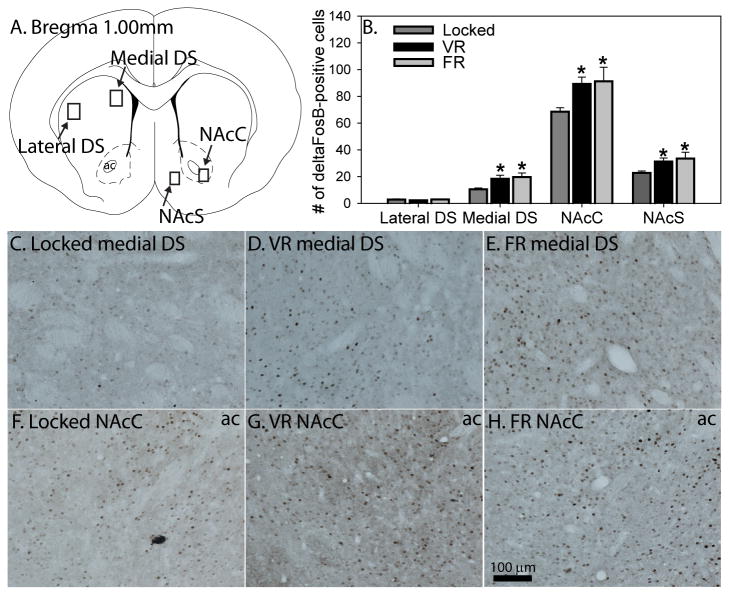
Exercise, regardless of controllability, increases ΔFosB in the medial dorsal striatum (DS) and nucleus accumbens (NAc). A) Figure from the atlas of Paxinos and Watson showing the regions analyzed in the DS, NAc core (NAcC) and NAc shell (NAcS). B) Six weeks of voluntary (VR) of forced (FR) running increased ΔFosB in the medial DS, NAcC, and NAcS, but not the lateral DS (* p < 0.05 relative to Locked controls). C-E) Representative photomicrographs depicting immunostaining of ΔFosB in the medial dorsal striatum. F-H) Representative photomicrographs depicting immunostaining of ΔFosB in the NAcC. Bars represent group means ± SEM.
Conditioned Placed Preference
CPP Apparatus
The CPP apparatus (38 cm tall × 57 cm long × 38 cm high) was composed of opaque black Plexiglas (5mm thick) and contained 2 joined compartments (Figure 1C). The 2 compartments of the conditioning apparatus were separated by a removable black Plexiglas wall (10 cm tall × 12 cm wide × 5 mm thick), and had distinctive flooring. One side (the “bar” side) consisted of black, anodized stainless steel bars 29 cm long and 5 mm thick, separated by 4 mm between each bar. The flooring of the opposite side (the “hole” side) consisted of a perforated, black anodized steel sheet covered in small holes 6.5 mm in diameter, and spaced 3.5 mm apart edge-to-edge.
Baseline preference
Rats were placed into the CPP apparatus for 20 minutes to assess baseline CPP. The wall separating the 2 halves of the CPP chamber was replaced with a black Plexiglass divider (10 cm tall × 12 cm wide × 5 mm thick) with a doorway cutout (10 cm tall × 12 cm wide × 5 mm thick) to allow rats free access to both sides of the apparatus. Behavior was videotaped for time spent on each side of the CPP apparatus and scored using TopScan (CleverSys Inc., Reston, VA., USA) software. If rats demonstrated a baseline preference to a particular side of the CPP chamber, the opposite side of the chamber was assigned as the side paired with exercise, so that rats would have to overcome their baseline preference in order to develop CPP to the exercise-paired side.
CPP training
Running occurred every-other night during CPP training. On running nights, rats were placed into their assigned running wheel (voluntary or forced) for 2 h. Following 2 h of running, rats were removed from their wheels and immediately placed into one side of the CPP apparatus (the “paired” side) for 20 min. During CPP training the wall separating the 2 sides of the CPP chambers was inserted, so rats were confined to 1 side. On alternating nights, rats were placed individually into an empty cage (Nalgene Plexiglas cage; 45 cm × 25.2 cm × 14.7 cm) for 2 h, rather than into their assigned running wheel. We have observed previously that CPP to the side of the CPP chamber paired with VR occurs even when compared to the side paired with a locked wheel (Greenwood et al., 2011), indicating that CPP to exercise can overcome potential rewarding effects a locked wheel may provide. In the current study exposure to an empty cage, rather than a locked wheel, was paired with the “unpaired” side of the CPP chamber in an attempt to speed development of exercise CPP. After 2 h in this empty cage, rats were placed for 20 min into the opposite side of the CPP chamber, deemed the “unpaired” side. This protocol insured that each rat was exposed to both sides of the CPP apparatus equally, but only one side was associated with wheel running. Rats were returned to their home cages following the CPP training. CPP chambers were thoroughly cleaned with water between each rat exposure. CPP training was conducted for a total of 30 days.
Probe trials
To determine which side of the CPP apparatus (unpaired or paired side) the rats preferred, the wall separating the 2 halves of the CPP chamber was again replaced with the Plexiglass insert containing a doorway cutout as for baseline testing. Rats were placed (1 at a time) onto one side of the apparatus in a counterbalanced manner. Rats were allowed to explore both sides of the apparatus for 10 min. Time spent in each side of the CPP chamber was recorded and scored automatically using TopScan. Probe trials occurred 24 h following a wheel running/paired training bout, and occurred immediately following the start of the active cycle.
Fluorescent In Situ Hybridization
Rats were sacrificed by rapid decapitation after 30 min of re-exposure to the paired or unpaired side of the CPP chamber, brains were extracted, frozen in chilled isopentane (-40° C), and stored at -80 °C until sectioning. Coronal sections (10μm) containing the striatum (between 1.6mm to 0.2mm rostral from Bregma) or midbrain (between -5.30mm to -6.04mm caudal from Bregma) were cut on a cryostat, thaw-mounted directly on to FisherBrand Colorfrost Plus (Fisherbrand, Pittsburg, PA., USA), and stored at -80° C until processing for double label fluorescent in situ hybridization (FISH). FISH was used to detect the proportion of TH or dynorphin neurons co-expressing cfos mNRA in the midbrain or striatum, respectively. The protocol for FISH followed our previously published procedures (Clark et al., 2014; Mika et al., 2015). cRNA ribroprobes complementary to TH (300 mer), dynorphin (744 mer), or cfos (680 mer) prepared from cDNA subclones in transcription vectors were labeled with fluorescein-12-UTP (for cfos; Roche) and digoxigenin-11-UTP (for TH or dynorphin; Roche) using standard transcription methods. Riboprobes were diluted in 50% hybridization buffer containing 50% formamide, 10% dextran sulfate, 2X saline sodium citrate (SSC), 50 mM PBS (pH = 7.4), 1X Denhardt's solution, and 0.1 mg/ml yeast tRNA. Brain sections were hybridized with the probe overnight (55°C). The next day, sections were washed in 2X SSC, treated with RNase A (200 mg/ml) for 1 h at 37°C, and washed to a final stringency of 0.1X SSC at 65°C for 1 h, then placed into 0.05 M PBS overnight at 4°C. Endogenous peroxidases were quenched in 2% H2O2 in PBS with agitation (30 min, RT). Subsequently, slides were washed with 1X TBS containing 0.05% Tween-20 (pH 7.5; TBS-T), incubated in blocking buffer (30 min, RT; FP1012; Perkin Elmer), then incubated with anti-fluorescein-HRP (1:100 in blocking buffer, 80 μl/slide; NEF710, Perkin Elmer) for 2 h in humidified chambers. Next, slides were washed in TBS-T, and the fluorescein–UTP–probe complex was detected with a tyramide signal amplification kit with fluorescein as the fluorophore (1h at RT in humidified chambers; 1:100, 80 μl/slide; TSA-Plus Kit, Perkin Elmer). Slides were washed in TBS-T, rinsed in 1X TBS and transferred to PBS. Subsequently, slides were washed with TBS-T and incubated with anti-digoxigenin–peroxidase (1:750 in blocking buffer, 80 μl/slide; Roche) for 30 min at RT in humidified chambers. The digoxigenin–UTP–cfos complex was detected as stated above with cyanine-3 as the fluorophore. Slides were then cover-slipped with Vectashield hard-set mounting medium (containing DAPI as a counterstain; H-1500, Vector Labs). Control slides of the same tissue without the addition of probe or without amplification were included.
Image analysis for FISH
Images were captured at 20X magnification on a Zeiss AX10 with axioscan Z1 fluorescent microscope, interfaced to a computer operating AxioVision software (Zeiss, Oberkochen, Germany). Digoxigenin (dynorphin or TH), Cy3 (cfos), and DAPI (nuclei) emission channels were merged to construct a single image. For each rat, 2 images were acquired in both the left and right, medial and lateral DS from each of 2 striatal sections (total of 4 images per slice; 8 images per animal; coordinates between 1.6mm to 0.2mm from bregma). Additionally, images of both the left and right medial and lateral VTA (2 slices per rat) were taken for each rat (total 4 images per slice; 8 images per animal; coordinates between -5.30mm to -6.04mm from bregma). Regions sampled are shown in Figure 3A (midbrain) and 4A (striatum). The number of cfos, TH, dynorphin, and co-labeled (TH/cfos or dynorphin/cfos) neurons were counted by multiple experimenters blind to treatment conditions of the animals with Zeiss Zen Vision software using the ‘measure events’ tool to prevent multiple counts of the same cell. If less than 2 images of a brain region could not be obtained for a particular rat due to tissue damage, that rat was excluded from analysis of that particular brain region.
Statistical Analyses
Group differences in body weight and running distance were analyzed utilizing repeated measures analysis of variance (ANOVA). ANOVA was used for comparison of protein expression data between Locked, VR, and FR groups in Experiment 1. CPP was expressed as a percent preference for the side of the CPP chamber paired with wheel running, using the following formula: (time spent on the paired side/total time spent on both sides) × 100. Preference scores were compared with repeated measured ANOVA. Activation of midbrain and striatal circuits following re-exposure to the unpaired or paired side of the CPP chamber (Experiment 2) were compared using 2 × 2 ANOVA with exercise type (VR or FR) and side of the chamber (unpaired or paired) as the factors. Percentage of TH (midbrain) or dynorphin (striatum) neurons expressing IEGs was calculated using the following formula: # of double-labeled cells / total # of single TH or dynorphin-labeled cells. Regression analyses was used to determine relationships between distance run by rats in the VR group and behavioral and neurochemical outcomes. Analyses were followed by Fisher's protected least significant difference (PLSD) post hoc tests when appropriate. Group differences were considered different when p ≤ 0.05.
Results
Voluntary and forced wheel running recruit similar midbrain and striatal neural circuits implicated in reward
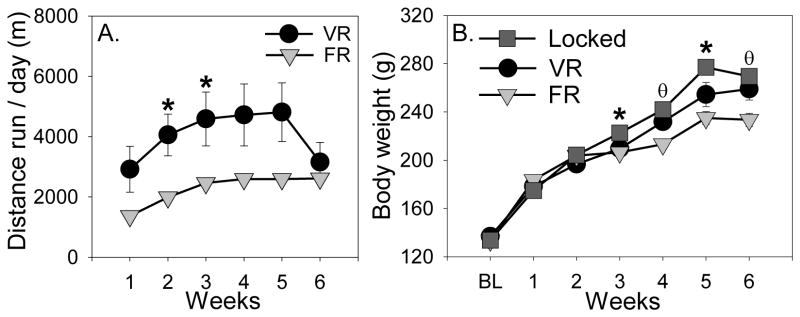
All rats were allowed unrestricted nightly access to voluntary wheels for 5 active cycles prior to being randomly assigned to VR or FR groups. Running data during this time were lost due to a hardware failure, but Figure 2A shows running distance over the course of the experiment starting from the time rats were split into VR and FR groups. All rats increased running distance over time (F (5, 65) = 11.7; p < 0.0001), and VR rats ran a greater distance than FR rats during several weeks (exercise × time interaction: F (5, 65) = 3.4; p = 0.0085; see Figure 2A for results of post-hoc tests). Rats in the VR (406.6 ± 121.2 m) and FR (431.3 ± 137.1 m) groups ran equivalent distances during the hours prior to sacrifice on the last night of the study (data not shown). Body weights are shown in Figure 2B. Body weights of rats in all groups increased over time (F (6, 126) = 524.4; p < 0.0001), but the rate of weight gain depended on the exercise condition (exercise × time interaction: F (12, 126) = 9.9; p < 0.0001). Similar to our prior observations (Greenwood et al., 2013), Locked rats gained the most weight and rats in the FR group gained the least (Figure 2B).
Figure 2.
Running distance and body weights of rats used in Experiment 1. A) Voluntarily running (VR) rats ran more than forced running (FR) rats during weeks 2 and 3 (* p < 0.05). B) Locked rats weighed more than exercising rats during weeks 3 and 5 (* p < 0.05 relative to both VR and FR groups). Additionally, FR rats weighed less than Locked and VR rats during weeks 4 and 6 (Ɵp < 0.05 relative to Locked and VR groups). Points represent group means ± SEM.
Following 6 weeks of either Locked, VR, or FR conditions, rats were sacrificed during peak running and IHC for double TH / pCREB in the midbrain or single ΔFosB in the striatum was performed in order to determine whether increases in pCREB and ΔFosB in these regions depend on exercise controllability. Figure 3A shows a figure from the atlas of Paxinos and Watson (Paxinos, 1998) with the regions sampled in the SN and VTA outlined. Figure 3B shows a representative photomicrograph (40X magnification) of TH / pCREB double-labeling in the lateral VTA, and Figure 3C shows the mean % of double TH / pCREB-labeled neurons in the midbrain. No group differences were observed in the % of TH+ neurons containing pCREB in the SN or the medial VTA. However, group differences were observed in the lateral VTA (F (2, 21) = 5.44; p = 0.01), where the % of double TH / pCREB-labeled cells were higher in both the VR and FR groups relative to the Locked group. No group differences in the number of total TH or single pCREB-immunoreactive cells were observed (Table 1), with the exception of the lateral VTA, where wheel running increased the number of pCREB-labeled cells regardless of controllability.
Table 1.
| Dependent Variable | Brain Region | Sedentary | Voluntary Run | Forced Run |
|---|---|---|---|---|
| # of TH+ cells | SN | 22.8 ± 2.2 | 19.6 ± 2.2 | 15.8 ± 2.6 |
| Lateral VTA | 73.2 ± 5.7 | 73.7 ± 5.2 | 74.6 ± 4.8 | |
| Medial VTA | 72.6 ± 3.5 | 66.6 ± 2.9 | 68.5 ± 4.2 | |
| # of pCREB+ cells | SN | 9.6 ± 0.8 | 12.7 ± 0.9 | 13.3 ± 1.7 |
| Lateral VTA* | 36.5 ± 3.1A | 43.8 ±1.4 | 48.1 ± 2.7 | |
| Medial VTA | 31.5 ± 2.0 | 31.4 ± 1.8 | 32.9 ± 1.5 |
main effect of exercise (p<0.05)
different from all other groups (p<0.05)
Figure 4A shows a figure from the atlas of Paxinos and Watson (Paxinos, 1998) depicting the striatal regions sampled. Figure 4 also shows representative photomicrographs of ΔFosB-labeling in the medial DS (Figures 4C-E) and NAcC (Figures 4F-H). Figure 4B shows the mean number of ΔFosB-positive cells in the striatum. No group differences were observed in the lateral DS. However, group differences were observed in the medial DS (F (2, 21) = 5.1; p = 0.01), the NAcC (F (2, 21) = 4.1; p = 0.03), and the NAcS (F (2, 21) = 3.6; p = 0.04). ΔFosB in each of these regions was elevated in both VR and FR rats relative to Locked controls (see Figure 4H for results of post hoc tests). No significant correlations were found between distance run by rats in the VR group and % double pCREB/TH neurons or ΔFosB labeling.
Both voluntary and forced wheel running produce conditioned place preference
Distance run by rats subsequently assigned to VR (2,140 ± 432.3 m/night) and FR (1,575.74 ± 291.1 m/night) conditions during the first 5 nights of unrestricted access to voluntary running wheels did not differ. Following assignment to VR and FR groups, rats were placed into their assigned VR or FR wheel for the first 2 h of the active cycle each night for 15 days, in order to acquire wheel running behavior. Figure 5A shows the average nightly running distance once rats were split into VR and FR groups. Interestingly, rats in the VR group initially ran more than rats in the FR group, but distance run by the VR group began to decline around day 15, and by day 22 of running rats in the VR run group were running less than the FR group (time × exercise interaction: F (29, 609) = 18.7; p < 0.0001; see Figure 5A for results of post hoc analyses). Body weights are shown in Figure 5B. Again, rats in the FR group gained less weight over time compared to VR rats (time × exercise interaction: F (5, 105) = 3.4; p = 0.007; see Figure 5B for post hoc analyses).
Figure 5.
Both voluntary (VR) and forced (FR) wheel running produce conditioned place preference (CPP). A) Rats in the VR condition ran more than FR rats during the first phase of Experiment 2, when rats were placed in their wheels for the entire active cycle for 15 consecutive nights. After the start of CPP training, when running was restricted to 2 h per night for every-other night, VR rats began to run less than FR rats (* p < 0.05). B) FR rats weighed less than VR rats at the time of baseline (BL) CPP testing and during the time of the 3rd probe trial (P3; * p < 0.05). C) Both VR and FR produced an increase in preference for the paired side of the CPP chamber (*p < 0.05 relative to baseline CPP). P1, probe 1; P2, probe 2; P3, probe 3. Data represent group means ± SEM.
To determine if CPP to the after-effects of wheel running depends on exercise controllability, VR or FR rats were exposed to CPP training for 30 days and probe trials were conducted at baseline (after 15 consecutive days of 2 h/day VR or FR), and every 10 days thereafter in order to determine preference. There was no difference between the VR and FR groups in preference to the side of the CPP chamber paired with exercise (Figure 5C). However, preference to the paired side of the CPP chamber increased over time in both exercise groups (F (3, 63) = 6.1; p = 0.001; Figure 5C), indicating that rats develop CPP to the after effects of wheel running regardless of the controllability of the exercise. Distance run by rats in the VR group did not correlate with their preference to the paired side of the CPP chamber.
Neural pathways responsive to exercise reward differ depending on exercise controllability
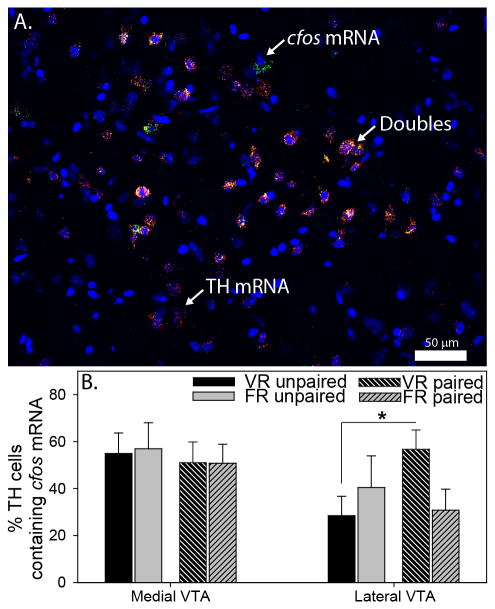
In order to investigate the contribution of midbrain DA and striatal direct-pathway neurons to exercise reward, rats used in the CPP study were confined to either the unpaired or paired side of the CPP chamber for 30 min and cfos mRNA in TH mRNA-positive neurons (midbrain) or dynorphin mRNA-positive neurons (striatum) was quantified with double FISH. Top-scan analyses revealed that locomotor activity during re-exposure to the CPP chamber did not differ between groups (data not shown). Regions sampled in the SN and VTA were identical to regions shown in Figure 3A. Numbers of cfos mRNA-positive cells in the SN were close to zero, thus SN data are not shown. Figure 6A depicts a representative photomicrograph of TH / cfos mRNA double-labeling in the lateral VTA, and Figure 6B shows the mean % of double TH / cfos mRNA-labeled neurons in the VTA. There were no group differences in the number of double-labeled neurons in the medial VTA. Interestingly, however, re-exposure to the side of the CPP chamber previously paired with exercise, relative to the unpaired side, elicited an increase in the number of double-labeled neurons in the lateral VTA of VR, but not FR, rats (exercise × side interaction: F (1, 19) = 4.4; p = 0.04). There were no group differences in the total number of TH mRNA-positive cells or single cfos mRNA-positive cells in the VTA (Table 2).
Figure 6.
Activation of ventral tegmental area (VTA) dopamine neurons in response to voluntary and forced exercise reward. A) Photomicrograph (200x magnification) showing DAPI (blue), tyrosine hydroxylase (TH) mRNA (red), cfos mRNA (green), and triple-labeled (yellow, designated by arrows) cells in the lateral VTA. B) Re-exposure to the side of the conditioned place preference chamber paired with voluntary running (VR), but not forced running (FR), elicited an increase in the percentage of double TH/cfos mRNA-labeled cells in the lateral, but not medial, VTA of VR rats (*p < 0.05). Bars represent group means ± SEM.
Table 2.
| Dependent Variable | Brain Region | Vol Run Unpaired | Vol Run Paired | Forced Run Unpaired | Forced Run Paired |
|---|---|---|---|---|---|
| Midbrain | |||||
| Total # of TH+ cells | Lateral VTA | 8.8 ± 3.7 | 8.4 ± 1.2 | 7.0 ± 2.0 | 6.3 ± 1.3 |
| Medial VTA | 48.0 ± 4.8 | 47.0 ± 4.0 | 45.5 ± 2.5 | 48.8 ± 2.9 | |
| # of single cfos+ cells | Lateral VTA | 8.8 ± 3.9 | 8.4 ± 1.2 | 7.0 ± 2.0 | 6.3 ± 1.3 |
| Medial VTA | 12.8 ± 2.3 | 7.1 ± 2.0 | 6.3 ± 2.2 | 6.8 ± 1.3 | |
| Striatum | |||||
| Total # of dynorphin+ cells | Medial DS*# | 81.9 ± 5.5 | 62.0 ± 5.1 | 61.2 ± 7.9 | 46.0 ± 6.3 |
| Lateral DS | 44.9 ± 5.1 | 37.7 ± 5.0 | 40.9 ± 5.7 | 29.0 ± 2.0 | |
| NAcC | 73.9 ± 6.4 | 61.8 ± 7.1 | 68.0 ± 4.0 | 63.8 ± 5.8 | |
| NAcS | 65.6 ± 6.3 | 61.0 ± 8.4 | 65.2 ± 7.1 | 68.0 ± 11.7 | |
| # of single cfos+ cells | Medial DS# | 10.4 ± 1.7 | 15.6 ± 2.7 | 9.6 ± 2.4 | 21.6 ± 3.6 |
| Lateral DS# | 12.6 ± 2.2 | 19.5 ± 1.8 | 14.9 ± 3.2 | 26.2 ± 4.2 | |
| NAcC#Ɵ | 3.8 ± 0.7 | 4.5 ± 1.2 | 3.5 ± 0.4 | 8.4 ± 1.1A | |
| NAcS | 5.0 ± 0.6 | 7.7 ± 1.5 | 7.8 ± 2.5 | 9.4 ± 1.5 | |
main effect of exercise (p<0.05)
main effect of side (p<0.05)
exercise × side interaction (p<0.05)
different from all other groups (p<0.05)
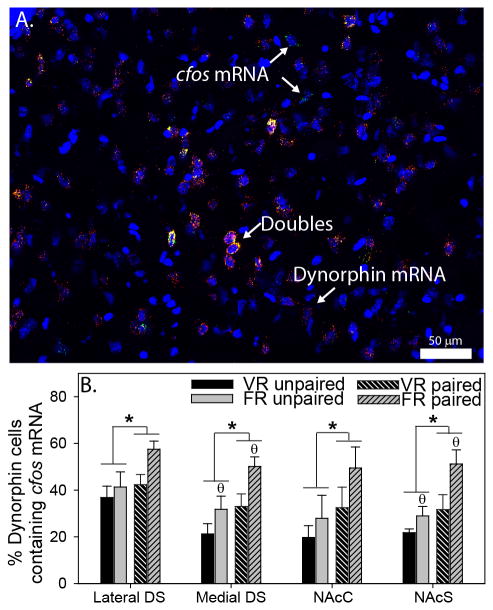
Figure 7A shows a representative photomicrograph of dynorphin / cfos mRNA double-labeling in the medial DS. Regions sampled in the striatum were identical to regions shown in Figure 4A. Figure 7B shows the mean percentage of dynorphin mRNA-positive cells that also contained cfos mRNA. Exposure to the paired side of the CPP chamber increased the percent of double-labeled cells in the lateral DS (F (1, 15) = 4.7; p = 0.04), medial DS (F (1, 15) = 9; p = 0.009), NAcC (F (1, 19) = 4.3; p = 0.05), and NAcS (F (1, 19) = 10.2; p = 0.005) regardless of exercise condition. Interestingly, relative to the VR group, rats in the FR group had a greater percentage of double-labeled neurons in the medial DS (F (1, 15) = 7.6; p = 0.01) and NAcS (F (1, 19) = 7; p = 0.01), but not the lateral DS or NAcC, regardless of which side of the CPP chamber rats were re-exposed to. The total numbers of dynorphin mRNA-positive and single cfos mRNA-positive cells in the striatum are shown in Table 2. No significant correlations were found between distance run by rats in the VR group and % double cfos/dynorphin neurons.
Figure 7.
Activation of dorsal striatum (DS) and nucleus accumbens (NAc) regions in response to voluntary and forced exercise reward. A) Photomicrograph (200x magnification) showing DAPI (blue), dynorphin mRNA (red), cfos mRNA (green), and triple-labeled (yellow, designated by arrows in inset) cells in the DS. B) Re-exposure to the side of the conditioned place preference chamber paired with voluntary running (VR) or forced running (FR) elicited an increase in the percentage of double dynorphin/cfos mRNA-labeled cells in the lateral DS, medial DS, NAc core (NAcC), and NAc shell (NAcS; * p < 0.05 paired vs. unpaired; Ɵ p < 0.05 FR vs. VR). Bars represent group means ± SEM.
Discussion
We report the novel observations that neurochemical and behavioral indices of reward elicited by wheel running are independent of the controllability of the wheel running. Rats forced to run in motorized wheels lacked any control over the onset or offset of the wheel revolutions, yet demonstrated an increase in activation of midbrain DA neurons, an accumulation of the reward-related plasticity marker ΔFosB in the DS and NAc, and CPP to the aftereffects of wheel running; effects similar to those observed in rats allowed to run voluntarily in wheels. Re-exposure to the side of the CPP chamber paired with exercise, relative to the side paired with a sedentary cage, also elicited conditioned activation of dynorphin-positive neurons in the DS and NAc regardless of exercise controllability, suggesting that striatal direct-pathway neurons could contribute to the rewarding effects of exercise. Interestingly, however, the mechanisms by which exercise reward activates direct pathway neurons might differ depending on exercise controllability. VR rats re-exposed to the side of the CPP chamber paired with VR demonstrated a conditioned activation of DA neurons of the lateral VTA, suggesting that activation of subpopulations of midbrain DA neurons could contribute to direct pathway activation during voluntary exercise reward. In contrast, no such cfos mRNA increases were observed in midbrain DA neurons of FR rats re-exposed to the side of the CPP chamber paired with FR. Together, these data are consistent with the idea that rewarding effects of exercise contribute to exercise-induced stress resistance, as both VR and FR produce stress resistance (Greenwood et al., 2013) and our data suggest are also both rewarding. Moreover, these data offer insight into the neural circuitries and mechanisms underlying exercise reward and provide potential targets for future investigations of exercise reward and exercise-induced stress resistance.
The accumulation of ΔFosB in the NAc has been observed in response to a variety of drug and natural rewards, and ΔFosB is thought to mediate the rewarding effects of these manipulations (Hope et al., 1994; Nestler et al., 1999; Perrotti et al., 2008; Wallace et al., 2008; Pitchers et al., 2013). Consistent with ΔFosB being responsive to natural rewards, Werme et al. (2002) reported that 4 weeks of voluntary exercise increased ΔFosB in the NAcC of mice (Werme et al., 2002). Using an antibody non-selective for FosB/ΔFosB, we observed a similar increase in the NAcC and NAcS following 6 weeks of voluntary wheel running in rats (Greenwood et al., 2011). Interestingly, the increase in ΔFosB in the NAcC occurs selectively in dynorphin-positive, D1-expressing direct pathway neurons, and overexpression of ΔFosB in this neuronal population, but not enkephalin-positive, D2-expressing indirect pathway neurons, increases wheel running behavior (Werme et al., 2002). Direct-pathway neurons are well known to be responsive to increases in reward-related phasic activity of midbrain DA neurons, and direct pathway neurons can themselves contribute to reinforcement and reward, as indicated by an increase in lever pressing and CPP in response to optogenetic stimulation of DS and NAc direct pathway neurons (Kravitz et al., 2012b). It is therefore possible that the increase in ΔFosB following voluntary exercise could be a consequence of DA signaling in the striatum and be an important contributing factor to exercise reward and the motivation to exercise.
Using a ΔFosB antibody with little cross-reactivity to the transient FosB, we show that the accumulation of ΔFosB in response to exercise occurs not only in the ventral striatum, but also the medial DS (Figure 3), a region implicated in movement, goal-directed behavior, and reward (Kravitz & Kreitzer, 2012b). Moreover, increases in ΔFosB were not selective to voluntary exercise. FR also increased ΔFosB in these same regions, suggesting that exercise-related signaling responsible for the induction of ΔFosB occurs regardless of exercise controllability. The current results also are consistent with a role for DA in mediating the observed increase in striatal ΔFosB. Rats exposed to VR or FR conditions were sacrificed during the peak of their active cycle and activation of midbrain DA neurons was assessed with double-label TH / pCREB IHC. Although exercise-induced increases in double-labeling in the SN, the main origin of DA projections to the DS, did not reach significance (Figure 2), both VR and FR increased the percentage of activated DA neurons in the lateral VTA, relative to Locked wheel treatment. These data suggest that activation of a distinct population of VTA DA neurons could contribute to the increase in ΔFosB observed following VR or FR. It is interesting that lateral, but not medial, VTA neurons seem to be most sensitive to exercise. Emerging data suggest that distinct circuits within the VTA differ in their roles in reward (Lammel et al., 2014). Lammel et al. (2012), for example, reported that whereas optogenetic stimulation of the laterodorsal tegmentum elicited a strong c-Fos response in the lateral VTA and CPP to the location where the optical stimulation occurred, optogenetic stimulation of the lateral habenula produced a strong c-Fos response in the medial VTA and conditioned place aversion (Lammel et al., 2012). Based on these data, the IEG response observed in DA neurons of the lateral VTA could be contributing to the rewarding effects of exercise, in part, by increasing ΔFosB in the striatum. However, because contribution of the VTA to DS DA is minimal (Fallon, 1988), it is unlikely that lateral VTA activity significantly influenced the increase of ΔFosB observed in the DS. Instead, it is possible that activity of SN DA neurons that is inadequate to elicit a detectible increase in pCREB, or other mechanisms within the DS, could be responsible for the increase in DS ΔFosB following exercise.
An increase in ΔFosB in direct pathway neurons in response to exercise could directly contribute to the stress-protective effects of exercise. Indeed, overexpression of ΔFosB in direct pathway neurons of the DS or NAc (Vialou et al., 2010; Nestler, 2015) and higher levels of dynorphin mRNA in the DS (Berube et al., 2013), which is specific to direct pathway neurons in this region, are both associated with resistance to social defeat stress. Although increase in ΔFosB in response to voluntary exercise occur specifically in direct pathway neurons (Werme et al., 2002), it remains unknown whether the observed increase in ΔFosB after FR occurred in similar neuronal populations. Exposure to chronic stress can increase ΔFosB in both direct and indirect pathway neurons in the NAc (Perrotti et al., 2004; Lobo et al., 2013). Because FR rats gain less weight over time compared to VR (Figures 2 and 5, and (Greenwood et al., 2013)), and our prior study indicated that FR elicited other indices of chronic stress such as adrenal hypertrophy and thymic involution (Greenwood et al., 2013), it is possible that a portion of the ΔFosB induced by FR occurred within indirect pathway neurons as a consequence of stress. This seems unlikely when considered in light of the observation that FR produces robust protection against anxiety- and depression-like behavioral effects of subsequent acute uncontrollable stress (Greenwood et al., 2013). Interestingly, a proportion of mice exposed to chronic social defeat stress are resistant to the depression-like consequences of the defeat, and these stress-resistant mice display an increase in ΔFosB selectively within direct-pathway neurons of the NAc (Lobo et al., 2013). Thus, it is possible that manipulations that foster stress resistance, such as FR, increase ΔFosB in direct pathway neurons despite the potential presence of a stress response. Future studies will be required to characterize the contribution of stress and to identify the specific neuronal population in which ΔFosB increases following FR. We also cannot completely rule out the possibility that the ΔFosB antibody used bound to the smaller isoforms of ΔFosB which, rather than accumulating over weeks of running, could have increased rapidly during the acute exercise session prior to sacrifice.
In addition to increasing ΔFosB and activating DA neurons in the lateral VTA, both VR and FR increase CPP to the side of a chamber paired with the after-effects of the exercise (Figure 5C). CPP is a common rodent model of reward, and CPP can be observed following drugs of abuse or naturally rewarding stimuli (Mucha et al., 1982; Chesworth et al., 2015; Mustroph et al., 2015). It may be surprising that rats will develop CPP to forced exercise, especially in light of the classic signs of chronic stress adaptations present in FR rats. Indeed, because the wheels were not actually contained within the paired side of the CPP chambers, it is possible that the CPP observed in FR rats developed not to the rewarding after-effects of the exercise, but developed instead to the cessation of forced exercise. Although this possibility cannot be ruled out, the interpretation that the CPP developed to rewarding effects of exercise is supported by the observations that FR elicits stress resistance (Greenwood et al., 2013), increases striatal ΔFosB and activity of lateral VTA DA neurons, and that rats will learn to lever press for forced rotations of a rotating drum, despite the fact that the rats lacked control over the revolutions of the drum after it was switched on (Kavanau, 1967). These data suggest that rats find wheel running rewarding regardless of its controllability.
Whether the current results would extend to treadmill training, another type of forced exercise, remains unknown. The common use of aversive stimuli such as electric foot shock to motivate the animals to treadmill run would present a difficult confound to overcome. However, an important difference between typical treadmill training paradigms and the forced wheel running used in the current studies could be the pattern of running. Typical treadmill training studies, including our prior study in which treadmill training failed to produce stress resistance (Greenwood et al., 2013), force rats to run continuously at one speed for durations up to an hour. In contrast, FR rats were forced to run in brief running bouts (average of 2.04 ± 1.95 min), and at constantly varying speeds, characteristic of voluntary wheel running (Greenwood et al., 2013). Rather than using treadmill training, future experiments using motorized wheels can manipulate the pattern of running in order to determine if there are preferred running patterns that produce CPP and stress resistance.
CPP is a Pavlovian learning phenomenon in which a rewarding stimulus is associated with one side of the CPP chamber (the paired side), and a neutral stimulus is associated with the opposite (unpaired) side. Since rats preferred the side of the chamber paired with the after effects of exercise, an effect of exercise that lingers beyond the termination of acute exercise likely became associated with the paired side of the CPP chamber during CPP training. It is therefore possible that re-exposure to the paired side of the CPP chamber would illicit conditioned activation of these same after-effects through similar Pavlovian processes. To investigate whether midbrain DA neurons and striatal direct pathway neurons become activated during re-exposure to the paired side of the CPP chamber, versus the unpaired side, rats that went through 30 days of CPP training were re-exposed to either the paired or unpaired side and double TH / cfos mRNA (midbrain) or dynorphin / cfos mRNA (striatum) neurons were labeled with FISH. Re-exposure to the paired side, relative to the unpaired side, elicited an increase in the percentage of dynorphin-positive, direct pathway neurons in the DS, NAcC, and NAcS (Figure 7B). These data suggest that activation of striatal direct pathway neurons occurs concurrently with exercise CPP. Importantly, cfos mRNA within dynorphin-positive neurons does not appear to be a consequence of locomotor activity. Although there was a pattern of greater overall cfos mRNA in both dynorphin-positive (Figure 7B) and -negative (Table 2) neurons in the striatum of FR rats compared to VR rats, rats within the VR and FR groups had similar histories of exercise, and rats re-exposed to the paired side displayed locomotor activity during re-exposure equal to rats re-exposed to the unpaired side.
DA signaling through D1 receptors is one mechanism by which striatal direct pathway neurons can be activated. Since cfos may not have been the optimal IEG with which to assess activation of the SN given its low levels of expression within the SN, the potential contribution of SN DA neurons to DS direct pathway activation remains unknown. In the lateral VTA, however, re-exposure to the paired side of the CPP chamber elicited an increase in the percentage of TH-positive neurons that contained cfos mRNA in VR rats (Figure 6B). Thus, re-exposure to the side of the CPP chamber increases activity of a portion of VTA DA neurons that could contribute to NAc direct pathway activation and the rewarding effects of exercise. No such pattern of VTA activation was observed in FR rats (Figure 6B). Therefore, in FR rats, activation of direct pathway neurons during re-exposure to the paired side of the CPP chamber may be driven by a source other than activity of VTA neurons. Activity of striatal direct pathway neurons is modulated by a variety of factors including glutamate, endocannabinoids, adenosine, acetylcholine, endogenous opioids, and GABA. Of these, there is evidence that an opioidergic mechanism of DA and/or direct pathway modulation and reward occurs in exercising rats. Voluntary exercise produces plasticity in opioidergic systems in the striatum (Werme et al., 2000; Brene et al., 2007; Greenwood et al., 2011), and CPP to the after-effects of acute voluntary exercise can be blocked by the opioid receptor antagonist naloxone (Lett et al., 2001).
In conclusion, we present the novel findings that the rewarding effects of exercise are independent of exercise controllability. Both voluntary and forced wheel running increase ΔFosB in the striatum, activate midbrain DA circuits implicated in reward, and produce CPP to the after-effects of exercise. These data are especially interesting in light of the clear consequences of chronic stress present in the FR rats. Despite apparent chronic physiological stress, FR still elicits neurochemical and behavioral indices of reward, although a contribution of stress to these observation can't be ruled out. Our data also implicate direct pathway neurons of the DS and NAc in the rewarding effects of exercise. Interestingly, however, whereas data are consistent with activity of VTA DA neurons driving striatal direct pathway activation during voluntary exercise reward, similar evidence does not exist for FR. Together, these data suggest that striatal direct pathway neurons represent a promising target for modulation of rewarding / reinforcing effects of exercise, exercise motivation, and stress resistance. Should these data translate to humans, they imply that midbrain DA and striatal circuits implicated in reward and stress resistance may be recruited during exercise even in those individuals who perceive exercise as “forced,” such as military personnel, professional athletes, those to whom exercise is prescribed by health care providers, or those who perceive exercise as a stressor.
Acknowledgments
This work was funded by NIH R01-MH068283-06A1 and NIH R03-MH086665-01.
References
- Anderson KD, Reiner A. Extensive co-occurrence of substance P and dynorphin in striatal projection neurons: an evolutionarily conserved feature of basal ganglia organization. J Comp Neurol. 1990;295:339–369. doi: 10.1002/cne.902950302. [DOI] [PubMed] [Google Scholar]
- Asmundson GJ, Fetzner MG, Deboer LB, Powers MB, Otto MW, Smits JA. Let's get physical: a contemporary review of the anxiolytic effects of exercise for anxiety and its disorders. Depress Anxiety. 2013;30:362–373. doi: 10.1002/da.22043. [DOI] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Herve D, Valjent E, Girault JA. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:5671–5685. doi: 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Herve D, Girault JA, Valjent E. What is the Degree of Segregation between Striatonigral and Striatopallidal Projections? Front Neuroanat. 2010a;4 doi: 10.3389/fnana.2010.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Herve D, Girault JA, Valjent E. What is the Degree of Segregation between Striatonigral and Striatopallidal Projections? Frontiers in neuroanatomy. 2010b;4 doi: 10.3389/fnana.2010.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berube P, Laforest S, Bhatnagar S, Drolet G. Enkephalin and dynorphin mRNA expression are associated with resilience or vulnerability to chronic social defeat stress. Physiol Behav. 2013;122:237–245. doi: 10.1016/j.physbeh.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Brene S, Bjornebekk A, Aberg E, Mathe AA, Olson L, Werme M. Running is rewarding and antidepressive. Physiol Behav. 2007 doi: 10.1016/j.physbeh.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Kelz MB, Hope BT, Nakabeppu Y, Nestler EJ. Chronic Fos-related antigens: stable variants of deltaFosB induced in brain by chronic treatments. J Neurosci. 1997;17:4933–4941. doi: 10.1523/JNEUROSCI.17-13-04933.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesworth R, Brown RM, Kim JH, Ledent C, Lawrence AJ. Adenosine 2A receptors modulate reward behaviours for methamphetamine. Addict Biol. 2015 doi: 10.1111/adb.12225. [DOI] [PubMed] [Google Scholar]
- Clark PJ, Ghasem PR, Mika A, Day HE, Herrera JJ, Greenwood BN, Fleshner M. Wheel running alters patterns of uncontrollable stress-induced cfos mRNA expression in rat dorsal striatum direct and indirect pathways: A possible role for plasticity in adenosine receptors. Behav Brain Res. 2014;272:252–263. doi: 10.1016/j.bbr.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishman RK, Renner KJ, Youngstedt SD, Reigle TG, Bunnell BN, Burke KA, Yoo HS, Mougey EH, Meyerhoff JL. Activity wheel running reduces escape latency and alters brain monoamine levels after footshock. Brain research bulletin. 1997;42:399–406. doi: 10.1016/s0361-9230(96)00329-2. [DOI] [PubMed] [Google Scholar]
- Dreyer JK, Herrik KF, Berg RW, Hounsgaard JD. Influence of phasic and tonic dopamine release on receptor activation. J Neurosci. 2010;30:14273–14283. doi: 10.1523/JNEUROSCI.1894-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikelboom R, Mills R. A microanalysis of wheel running in male and female rats. Physiol Behav. 1988;43:625–630. doi: 10.1016/0031-9384(88)90217-x. [DOI] [PubMed] [Google Scholar]
- Fallon JH. Topographic organization of ascending dopaminergic projections. Ann N Y Acad Sci. 1988;537:1–9. doi: 10.1111/j.1749-6632.1988.tb42093.x. [DOI] [PubMed] [Google Scholar]
- Foley TE, Fleshner M. Neuroplasticity of dopamine circuits after exercise: implications for central fatigue. Neuromolecular Med. 2008;10:67–80. doi: 10.1007/s12017-008-8032-3. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Fleshner M. Exercise, stress resistance, and central serotonergic systems. Exercise and sport sciences reviews. 2011;39:140–149. doi: 10.1097/JES.0b013e31821f7e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Fleshner M. Mechanisms Underlying the Relationship Between Physical Activity and Anxiety: Animal data. Routledge; New York, NY: 2013. [Google Scholar]
- Greenwood BN, Foley TE, Day HE, Campisi J, Hammack SH, Campeau S, Maier SF, Fleshner M. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. J Neurosci. 2003a;23:2889–2898. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Le TV, Strong PV, Loughridge AB, Day HE, Fleshner M. Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behavioural brain research. 2011;217:354–362. doi: 10.1016/j.bbr.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Kennedy S, Smith TP, Campeau S, Day HE, Fleshner M. Voluntary freewheel running selectively modulates catecholamine content in peripheral tissue and c-Fos expression in the central sympathetic circuit following exposure to uncontrollable stress in rats. Neuroscience. 2003b;120:269–281. doi: 10.1016/s0306-4522(03)00047-2. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Spence KG, Crevling DM, Clark PJ, Craig WC, Fleshner M. Exercise-induced stress resistance is independent of exercise controllability and the medial prefrontal cortex. The European journal of neuroscience. 2013;37:469–478. doi: 10.1111/ejn.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope BT, Nye HE, Kelz MB, Self DW, Iadarola MJ, Nakabeppu Y, Duman RS, Nestler EJ. Induction of a long-lasting AP-1 complex composed of altered Fos-like proteins in brain by chronic cocaine and other chronic treatments. Neuron. 1994;13:1235–1244. doi: 10.1016/0896-6273(94)90061-2. [DOI] [PubMed] [Google Scholar]
- Ilango A, Kesner AJ, Keller KL, Stuber GD, Bonci A, Ikemoto S. Similar roles of substantia nigra and ventral tegmental dopamine neurons in reward and aversion. J Neurosci. 2014;34:817–822. doi: 10.1523/JNEUROSCI.1703-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen IH. Techniques for establishing schedules with wheel running as reinforcement in rats. J Exp Anal Behav. 1993;60:219–238. doi: 10.1901/jeab.1993.60-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanau JL. Behavior of captive white-footed mice. Science. 1967;155:1623–1639. doi: 10.1126/science.155.3770.1623. [DOI] [PubMed] [Google Scholar]
- Kravitz AV, Kreitzer AC. Striatal mechanisms underlying movement, reinforcement, and punishment. Physiology. 2012a;27:167–177. doi: 10.1152/physiol.00004.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Kreitzer AC. Striatal mechanisms underlying movement, reinforcement, and punishment. Physiology. 2012b;27:167–177. doi: 10.1152/physiol.00004.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nature neuroscience. 2012a;15:816–818. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nature neuroscience. 2012b;15:816–818. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Malenka RC. Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology. 2014;76 Pt B:351–359. doi: 10.1016/j.neuropharm.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lett BT, Grant VL, Byrne MJ, Koh MT. Pairings of a distinctive chamber with the aftereffect of wheel running produce conditioned place preference. Appetite. 2000;34:87–94. doi: 10.1006/appe.1999.0274. [DOI] [PubMed] [Google Scholar]
- Lett BT, Grant VL, Koh MT. Naloxone attenuates the conditioned place preference induced by wheel running in rats. Physiol Behav. 2001;72:355–358. doi: 10.1016/s0031-9384(00)00427-3. [DOI] [PubMed] [Google Scholar]
- Lobo MK, Zaman S, Damez-Werno DM, Koo JW, Bagot RC, DiNieri JA, Nugent A, Finkel E, Chaudhury D, Chandra R, Riberio E, Rabkin J, Mouzon E, Cachope R, Cheer JF, Han MH, Dietz DM, Self DW, Hurd YL, Vialou V, Nestler EJ. DeltaFosB induction in striatal medium spiny neuron subtypes in response to chronic pharmacological, emotional, and optogenetic stimuli. J Neurosci. 2013;33:18381–18395. doi: 10.1523/JNEUROSCI.1875-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA, Ulery PG, Perrotti LI, Zachariou V, Berton O, Nestler EJ. DeltaFosB: a molecular switch for long-term adaptation in the brain. Brain Res Mol Brain Res. 2004;132:146–154. doi: 10.1016/j.molbrainres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Mika A, Bouchet CA, Bunker P, Hellwinkel JE, Spence KG, Day HE, Campeau S, Fleshner M, Greenwood BN. Voluntary exercise during extinction of auditory fear conditioning reduces the relapse of fear associated with potentiated activity of striatal direct pathway neurons. Neurobiol Learn Mem. 2015;125:224–235. doi: 10.1016/j.nlm.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucha RF, van der Kooy D, O'Shaughnessy M, Bucenieks P. Drug reinforcement studied by the use of place conditioning in rat. Brain Res. 1982;243:91–105. doi: 10.1016/0006-8993(82)91123-4. [DOI] [PubMed] [Google Scholar]
- Mustroph ML, Merritt JR, Holloway AL, Pinardo H, Miller DS, Kilby CN, Bucko P, Wyer A, Rhodes JS. Increased adult hippocampal neurogenesis is not necessary for wheel running to abolish conditioned place preference for cocaine in mice. Eur J Neurosci. 2015;41:216–226. doi: 10.1111/ejn.12782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Review. Transcriptional mechanisms of addiction: role of DeltaFosB. Philos Trans R Soc Lond B Biol Sci. 2008;363:3245–3255. doi: 10.1098/rstb.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. FosB: a transcriptional regulator of stress and antidepressant responses. Eur J Pharmacol. 2015;753:66–72. doi: 10.1016/j.ejphar.2014.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Kelz MB, Chen J. DeltaFosB: a molecular mediator of long-term neural and behavioral plasticity. Brain Res. 1999;835:10–17. doi: 10.1016/s0006-8993(98)01191-3. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1998. [Google Scholar]
- Perrotti LI, Hadeishi Y, Ulery PG, Barrot M, Monteggia L, Duman RS, Nestler EJ. Induction of deltaFosB in reward-related brain structures after chronic stress. J Neurosci. 2004;24:10594–10602. doi: 10.1523/JNEUROSCI.2542-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotti LI, Weaver RR, Robison B, Renthal W, Maze I, Yazdani S, Elmore RG, Knapp DJ, Selley DE, Martin BR, Sim-Selley L, Bachtell RK, Self DW, Nestler EJ. Distinct patterns of DeltaFosB induction in brain by drugs of abuse. Synapse. 2008;62:358–369. doi: 10.1002/syn.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchers KK, Vialou V, Nestler EJ, Laviolette SR, Lehman MN, Coolen LM. Natural and drug rewards act on common neural plasticity mechanisms with DeltaFosB as a key mediator. J Neurosci. 2013;33:3434–3442. doi: 10.1523/JNEUROSCI.4881-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciolino NR, Dishman RK, Holmes PV. Voluntary exercise offers anxiolytic potential and amplifies galanin gene expression in the locus coeruleus of the rat. Behavioural brain research. 2012;233:191–200. doi: 10.1016/j.bbr.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg LC, Horton TH, Turek FW. Circadian rhythms and depression: effect of exercise in an animal model. American Journal of Physiology. 1999;276:R152–R161. doi: 10.1152/ajpregu.1999.276.1.R152. [DOI] [PubMed] [Google Scholar]
- Sothmann MS, Buckworth J, Claytor RP, Cox RH, White-Welkley JE, Dishman RK. Exercise training and the cross-stressor adaptation hypothesis. Exerc Sport Sci Rev. 1996;24:267–287. [PubMed] [Google Scholar]
- Trost A, Hauber W. Dopamine D1/D2 receptors do not mediate the expression of conditioned place preference induced by the aftereffect of wheel running. BMC Neurosci. 2014;15:124. doi: 10.1186/s12868-014-0124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulery-Reynolds PG, Castillo MA, Vialou V, Russo SJ, Nestler EJ. Phosphorylation of DeltaFosB mediates its stability in vivo. Neuroscience. 2009;158:369–372. doi: 10.1016/j.neuroscience.2008.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialou V, Robison AJ, Laplant QC, Covington HE, 3rd, Dietz DM, Ohnishi YN, Mouzon E, Rush AJ, 3rd, Watts EL, Wallace DL, Iniguez SD, Ohnishi YH, Steiner MA, Warren BL, Krishnan V, Bolanos CA, Neve RL, Ghose S, Berton O, Tamminga CA, Nestler EJ. DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci. 2010;13:745–752. doi: 10.1038/nn.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DL, Vialou V, Rios L, Carle-Florence TL, Chakravarty S, Kumar A, Graham DL, Green TA, Kirk A, Iniguez SD, Perrotti LI, Barrot M, DiLeone RJ, Nestler EJ, Bolanos-Guzman CA. The influence of DeltaFosB in the nucleus accumbens on natural reward-related behavior. J Neurosci. 2008;28:10272–10277. doi: 10.1523/JNEUROSCI.1531-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters CL, Kuo YC, Blendy JA. Differential distribution of CREB in the mesolimbic dopamine reward pathway. J Neurochem. 2003;87:1237–1244. doi: 10.1046/j.1471-4159.2003.02090.x. [DOI] [PubMed] [Google Scholar]
- Werme M, Messer C, Olson L, Gilden L, Thoren P, Nestler EJ, Brene S. Delta FosB Regulates Wheel Running. J Neurosci. 2002;22:8133–8138. doi: 10.1523/JNEUROSCI.22-18-08133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werme M, Thoren P, Olson L, Brene S. Running and cocaine both upregulate dynorphin mRNA in medial caudate putamen. Eur J Neurosci. 2000;12:2967–2974. doi: 10.1046/j.1460-9568.2000.00147.x. [DOI] [PubMed] [Google Scholar]
- Wise RA. Roles for nigrostriatal--not just mesocorticolimbic--dopamine in reward and addiction. Trends Neurosci. 2009;32:517–524. doi: 10.1016/j.tins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Liu Y, Li W, Yang B, Chen D, Wang X, Jiang Z, Wang H, Wang Z, Cornelisson G, Halberg F. Beneficial effects of exercise and its molecular mechanisms on depression in rats. Behav Brain Res. 2006;168:47–55. doi: 10.1016/j.bbr.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]