Abstract
Domoic acid (DA) is a toxin produced by marine algae and known primarily for its role in isolated outbreaks of Amnestic Shellfish Poisoning and for the damage it inflicts on marine mammals, particularly California sea lions. Lethal effects of DA are often preceded by seizures and coma. Exposure to DA during development can result in subtle and highly persistent effects on brain development and include behavioral changes that resemble diagnostic features of schizophrenia and anomalies in social behavior we believe are relevant to autism spectrum disorder (ASD). To more fully examine this hypothesis, we chose to examine adolescent mice exposed in utero to DA for endpoints relevant to ASD, specifically changes in social behavior and network structure, the latter measured by resting state functional connectivity (rsfcMRI). We found that male offspring exposed in utero to DA expressed reproducible declines in social interaction and atypical patterns of functional connectivity in the anterior cingulate, a region of the default mode network that is critical for social functioning. We also found disruptions in global topology in regions involved in the processing of reward, social, and sensory experiences. Finally, we found that DA exposed males expressed a pattern of local over-connectivity. These anomalies in brain connectivity bear resemblance to connectivity patterns in ASD and help validate DA-exposed mice as a model of this mental disability.
Keywords: Resting state functional connectivity, mouse, social behavior, brain imaging, autism
1. Introduction
Domoic acid (DA) is an algal neurotoxin that accumulates in marine fish and shellfish [1]. Human consumption of highly DA-contaminated seafood can result in Amnestic Shellfish Poisoning, with symptoms that include memory loss, seizures, and death [1-3]. Human exposure to DA typically results from consumption of oysters, mussels, and clams in their entirety, including the contaminated viscera. In other cases, commercial processing promotes leaching of DA from the viscera into the consumable muscle meat [4, 5]. The regulatory limit for DA harvest, 20 mg of DA/kg tissue, is designed to safeguard from acute Amnestic Shellfish Poisoning [6]. In the Pacific Northwest, Chesapeake Bay and along the coasts of Western Europe, peak annual levels of DA in shellfish can exceed this limit, resulting in intermittent restrictions on shellfish harvests. Lower levels of DA exposure exert more subtle effects that are often observed in pinnepeds stranded along the West Coast of North America. California sea lions exposed to levels of DA that are not acutely lethal can display diminished spatial memory [7], blunted habituation to auditory stimuli [8], heightened aggression [9], highly repetitive behaviors [10], and abnormal locomotion in both water [11] and land [9]. Experimental studies that involve the administation of low doses of DA to adult rodents show that exposed subjects express hypomobility [12], impaired spatial learning [13] (Petrie et al., 1992), aggression [14], and significantly increased stereotypic behaviors [15].
In addition to adult exposures, developmental exposures to low levels of DA can have pernicious and highly persistent effects [16, 17]. At ED 13 in rat development, a timepoint corresponding to the onset of neurogenesis and equivalent to the mid-first trimester of human development [18], DA interacts with kainate receptors, depolarizes neurons, and mediates hippocampal damage, with long term effects including a reduction in seizure thresholds [19]. After a single exposure to DA at either ED 14.5 or 17.5, adults express profound decreases in conditioned fear memory and adaptation to novel circumstances [20]. Neonatal rats exposed to DA express, as adults, hypoactivity [21] and impairments in spatial learning [22] and sensory motor gating [23] and, if exposed to DA between 8 and 14 days of age, express as adults lower seizure thresholds [24-26], altered novelty-related behavior [27], altered conditioned responses to drug reward [28], persistent changes in learning and memory [29] and diminished social interaction [30].
This broad timespan of susceptibility, representing the equivalent to a 3-month period extending from the end of the first trimester to the end of the second trimester in humans [18], is not surprising given that DA interacts with kainate receptors 1 and 2 (KA1&2) and glutamate receptors (GluR) 5 and 6 are expressed in the rat hippocampus by E13 and throughout the cortex by E17 [31]. These receptors are also expressed during human fetal brain development [32] in regions including cortical and limbic areas that regulate social behavior. Mechanisms of action for DA likely change over the course of development. DA binds its receptors, promoting gene expression [33, 34] and engendering neuroexcitation [16, 35], but may also alter the trajectory of development via hippocampal in selective loss of interneurons and oligodendrocytes, or by moderating glutamate tone [19], axon and dendrite morphology [36, 37] or myelination [20]. At each stage of fetal and postnatal rodent development, neurons respond to DA exposure in the manner that is relevant for receptor engagement and neuronal development [38]. Effects of DA on early development may be exacerbated by elevated exposures to DA in utero [39] and by elevated serum retention during early postnatal development [40].
Early exposure to DA may render the developing fetus at risk for schizophrenia [23, 30]. Autism is also a possibility, as California sea lions exposed to DA present with seizures, diminished social regulation, and repetitive behaviors [9] and these behaviors bear striking resemblance to the clinical features of this disorder.
Rodent models gain greater construct validity when behavioral, anatomical, and physiological dimensions of their biology bear resemblance to the human mental disorder they represent. Until recently, imaging techniques commonly used to evaluate the human brain were unavailable for studies of rodent models. Resting state functional connectivity (rs-fcMRI), first described in humans [41] has been used extensively to examine brain network abnormalities in psychiatric disorders [42-45]. Rs-fcMRI offers insight to the default mode network, which has been repeatedly implicated in psychiatric disorders [46]. This network plays a normative role in complex cognitive tasks such as social cognition [47, 48] and may be involved in evaluations of a self-versus other model building of the social environment and self-referential evaluation [48, 49]. Indeed, areas involved in a variety of social tasks largely overlap with areas in the default system [48, 50].
The default network has recently been shown to be conserved in mice [51, 52] and Rs-fcMRI has been adapted to identify robust and biologically plausible functional networks in mice [51-53], a development that helps bridge the gap between human and animal models of disease [52, 54]. The default network spans a distributed network of regions including the anterior cingulate cortex (ACC), retrosplenial cortex, and orbital area [52]. As the ACC plays a critical role in social functioning [55-57] and because DA exposure can target glutamatergic cells which are highly expressed in the cingulate [31], we focused on connectivity of the ACC of the default mode.
Larger scale examinations of global topology offer useful insights into psychiatric disorders. For example, graph theoretical techniques help reveal overall network topology in ASD [45] and schizophrenia [58]. One such metric is “degree”, an index of the overall number of connections of a given region, a useful account of a regions contribution to a network. In addition to node degree, other measures have aimed to distinguish network topology based on the spatial proximity of functional connections. A popular hypothesis in the ASD literature is that children with ASD show an excess of short-range connectivity and a decrease in connectivity between longer-range connections. Multiple studies have demonstrated local over-connectivity in ASD [59-64], but the extent these phenomena are related to early brain insults remains unclear.
We asked whether in utero exposure to DA generates anomalous adolescent social behaviors and corresponding changes in functional connectivity. We investigated functional connectivity in both the ACC region of the default mode and in the larger scale network structure. Global network topology was assessed in order to determine whether DA exposure preferentially affects regions associated with social behavior. Local connectivity and longer-range patterns of connectivity were also assessed for similarities to ASD.
2. Materials and Methods
2.1. Behavior Methods
2.1.1 Mouse husbandry
C57BL/6J (B6) mice, purchased from Jackson Laboratories (Bar Harbor, ME, USA), were bred and housed at Oregon Health and Science University (OHSU) under controlled temperature (21 ± 1°C) and humidity (40–60%). Lighting was maintained on a 12:12 h light/dark (dark period 0900–2100 hours) cycle. Mice were housed in standard polypropylene cages (290 × 180 × 130 mm) lined with pelleted paper bedding (ECOfresh, Absorption Corp.) and provided ad libitum access to chow (Lab Rodent Diet 5001, Purina Mills) and water. Animal care and experimental protocols were conducted in accordance with the regulations of the institutional care and use committee at the Oregon Health and Science University and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Our own laboratory personnel carried out all aspects of the mouse husbandry under strict guidelines to insure gentle and consistent handling of the mice.
2.1.2 Domoic Acid Administration
Pregnant mice were weighed daily. When at a weight of 30 grams (M = 30.157 grams, SD = 1.513), embryonic day (ED) 16 (M = 16.2, SD = 1.66), gravid females were injected subcutaneously with either 1.5 mg/kg of DA or saline. We chose this dose of DA based upon results of our own experimental evidence that exposure to this dose causes deficits in adolescent social approach behavior in FVB mice [65] and based upon evidence of effectiveness in comparable doses of DA administered via intraperitoneal injection [20] and below the threshold expected to induce convulsive effects or inducing fetal loss [66]. Weaning occurred on postnatal day (PD) 20-22; pups were separated by sex and placed in separate clean colony cages. Housing groups of siblings were made up of two males and two females. During sexing, mice were randomly assigned as the test or stimulus mouse role for social behavior testing. Test mice were marked on the tail with a black Sharpie and stimulus mice were ear-punched on the top left ear. To avoid euthanizing litters with uneven sex distributions, on rare occasions, two male and female housing groups were combined across litters if both litters were the same age and treatment condition.
2.1.3 Social Investigation (SI)
Juveniles were tested for SI at PD 21, PD 25, and PD 35. All testing was conducted during the dark period of the light dark cycle between 1400 and 1800 under dim red illumination. Prior to testing on PD21 ± 1, and immediately following weaning, mice were individually isolated into clean new home cages. Cages were returned to a housing rack where they remained for 30 min, then briefly stationed in a laminar flow hood for five minutes. They were then transferred to the adjacent experimental room where they remained for an additional five minutes. The stimulus mouse was then added to the cage of the test mouse, placed in an opposing corner of the cage. Ultrasonic vocalizations (USVs) and social behaviors of the test mouse were recorded for five minutes (particular behaviors are described below). At the end of 5 minutes, mice were removed from the experimental room and returned to their social housing group for subsequent testing.
Juvenile mice were isolated 24 hours prior to SI testing (within one day of PD 25 and 35), into a clean cage containing fresh bedding without nesting material and ad libitum access to food and water. After the 24 hours, the stimulus mouse was introduced to the cage of the test mouse where USVs and social interactions were recorded for 15 minutes. The behaviors were video recorded (Sony, DCR-VX2100, Japan) and stored on a on a desktop computer (Precision T3400, Dell, Round Rock, TX, USA) for additional analysis. All behaviors were scored with the aid of computer assisted analysis software (ButtonBox v.5.0, Behavioral Research Solutions, Madison, WI, USA) by two different observers blind to the age and treatment group of the interacting mice. At the conclusion of behavioral testing on PD35, animals were euthanized by CO2 in accordance with institutional animal care and use committee (IACUC) protocols.
Individual SI scores were coded for test mouse behavior directed towards the stimulus mouse, including: [i] sniffing or snout contact with the head/ neck/mouth area, [ii] sniffing or snout contact with the flank area, [iii] direct contact with the anogenital area, [iv] social pursuit within one body-length as the stimulus mouse moved continuously throughout the cage and [v] social grooming. These variables were combined into a composite measure of SI (Panksepp et al., 2007). SI testing was conducted during the dark phase (4.5 to 9.5 hours after lights-off.), under dim red illumination, in a sound-dampened room. SI was recoded for each experiment and two independent raters blind to the experimental conditions scored each video until the interrater reliability was greater than r = .90 for each observation (up to three times per rater). The presentation of all SI data and statistical outcomes in this study are based on an average of these two independent measurements.
In total, 52 same sex B6 pairs were tested for SI. DA exposed mice included 22 testing pairs (11 male pairs, 11 female pairs), and 30 exposed mice included 30 testing pairs (16 male, 14 female). Two mice exposed to saline and one exposed to DA died before the conclusion of the experiment and four mice were excluded due to climbing on the equipment during SI testing (two DA mice at PD25 and two saline mice at PD35).
2.1.3 Ultrasonic vocalizations
Ultrasonic vocalizations (USVs) were recorded during SI tests with an ultrasound microphone (10–180 kHz flat-frequency range) lowered through a 1/8“thick transparent Plexiglas cage top with a 30-mm diameter hole in its center. USVs were collected with an UltraSoundGate 116 acquisition system (UltraSoundGate model CM16, Avisoft Bioacoustics, Berlin, Germany) and Avisoft-Recorder v.2.97 (Avisoft Bioacoustics), and stored as wav files for subsequent analysis. A 40-kHz band-pass filter was used to minimize background noise during recordings. Using SASLab Pro v.4.39 (Avisoft Bioacoustics), each sonogram was automatically tallied for the total number of USVs produced with during the SI period. However, some wav files contained some ‘non-USV’ signal that compromised the precision of the automated parameter-measurements. Thus, extraneous noise was manually identified and removed. When a rater encountered a signal that was difficult to interpret, the USV was evaluated by a minimum of one additional trained observer. For the final count, two blind observers manually removed noise from the sonograms, and the total USVs were averaged across the two raters (inter-rater reliability, r > .9).
2.1.4 Behavioral Statistical analyses
The effect of DA exposure on social behavior and USVs were analyzed using a full factorial repeated measures analysis of variance (ANOVA). Treatment group and sex of each mouse pair were between-group factors and ages at social interaction test (post-natal day (PD) 25 and 35) were repeated measures. Trend level interactions and pre-planned post hoc analyses comparing the effect of DA for males and females, were followed up with t-tests. All statistical analyses were conducted using JMP version 6.0 (SAS Institute Inc.) and p < 0.05 was considered statistically significant.
2.2 MRI Methods
2.2.1 Subjects
A total of 16 C57Bl/6J adult male mice (Jackson Laboratory, Bar Harbor, ME), nine control males (prenatal exposure to a saline injection) and seven DA males (prenatal exposure to 1.5 mg/kg DA), ranging in age from PD32-40 were subjected to the MRI portion of the experiment. Mice were maintained on a 12-h light/dark cycle (lights on at 0600 h), room temperature 21 ± 1°C, and allowed food and water ad libitum. All experiments were performed during the animal’s light cycle. Protocols were approved by the OHSU Institutional Animal Use and Care Committee and were conducted in accordance with National Institutes of Health (NIH) “Principles of Laboratory Animal Care.”
2.2.2 Animal Preparation
All mice were anesthetized throughout the MR imaging procedure. Anesthesia was induced by 3-4% isoflurane and maintained with 1-1.5% isoflurane. The head was kept stationary using a custom-built head holder designed to fit in the RF coil. Respiration (80-100 bpm) and animal temperature (maintained at 37°C) were monitored and controlled by a small animal monitoring system (Model 1030 Monitoring and Gating System, SA Instruments, Stony Brook, NY).
2.2.3 Imaging Acquisition
Imaging was performed during a single session for each animal on an 11.75T Bruker BioSpec scanner equipped with a Resonance Research, Inc high-bandwidth shim power supply. A 20mm ID RF quadrature volume coil (M2M, Cleveland, OH) was used for all studies. All scans were performed with Paravision 5. Using MAPSHIM, a 3D Fieldmap phase image was acquired; TR=20ms, TE1=2ms, inter echo time = 4.003ms, FA=20°, FOV=40 mm × 18 mm × 25 mm, matrix = 80 × 90 × 125 (voxel size of 0.5 × 0.2 × 0.2 mm3, matching the EPI voxel size). This was followed by a T2-weighted structural image (RARE, TR = 4590ms, effective TE = 32ms, RARE factor = 8, 30 contiguous slices (0.5 mm thick) with interleaved acquisition, FOV = 18 × 18 mm, matrix = 150 × 150, voxel size 0.12 × 0.12 × 0.5 mm3, 2 repetitions). Global (volume) and local (brain voxel) shimming with MAPSHIM were performed to calculate first and second order shims prior to the functional MRI scan. The resting-state fMRI consisted of a single shot gradient echo-planar imaging (EPI) sequence with the following parameters: 450 repetitions (total scan time = 15min), TR = 2000ms, TE = 10ms, FA = 60°, 30 contiguous slices (0.5 mm thick) with interleaved acquisition, FOV = 25.6 × 16 mm, matrix = 128 × 80, voxel size 0.2 × 0.2 × 0.5 mm3.
2.2.4 General fMRI BOLD preprocessing
Functional images were first processed to reduce artifacts as previously described (Stafford et al., 2014). These steps include: 1) removal of a central spike caused by MR signal offset; 2) correction of odd vs. even slice intensity differences attributable to interleaved acquisition without gaps; 3) correction of field in homogeneities by applying the fieldmap phase information using FEAT[67]; 4) movement correction; 5) within run intensity normalization to a whole brain mode value of 1000. Processed functional data was transformed to an anatomical atlas for each individual via the T2 scan. Functional data was registered to the rodent atlas supplied by the caret software (map_015 atlas) [68-70]. Each run then was resampled in atlas space on an isotropic 0.2 mm grid combining movement correction and atlas transformation in one interpolation [71].
2.2.5 rs-fcMRI pre-processing
As previously described [52], several additional preprocessing steps were used to reduce spurious variance unlikely to reflect neuronal activity (e.g. heart rate and respiration). These steps included: 1) a temporal band-pass filter (0.009 Hz < f < 0.1 Hz); 2) spatial smoothing (0.4 mm full width at half maximum); 3) regression of six parameters obtained by rigid body head motion correction; 4) regression of the whole brain signal; and 5) regression of the first order derivative of the whole brain and motion parameters.
2.2.6 Regions of Interest (ROI)
168 cortical pre-defined areas, based on the connectional and architectonic subdivisions in the mouse were used. These areas were obtained by combining a brain atlas as defined by the Allen Institute for Brain Science [72] with that of Paxinos [73], upon which it is based and many regions are identical, including the main olfactory bulb (MOB) and accessory olfactory bulb (AOB). However, as the Allen MOB and AOB regions were directly from histological preparation tissue distortion made it difficult to directly register these ROIs to the mouse MR data (tissue warping from histology at the rostral fringes of the mouse brain are most susceptible to tissue warping). Thus, we chose to use regions directly mapped to the caret atlas as they were 1) MRI based and 2) free of the distortion from histological preparation (Caret, map_015, available at: http://sumsdb.wustl.edu/sums/mousemore.do). The remaining 164 ROIs were derived from the ALLEN institute defined cortical areas, for a total of 168 ROIs.
2.2.7 Extraction and computation of regionwise resting state correlations
For each animal, we collected 15 minutes of resting state BOLD data. For each ROI, a resting time series was extracted separately, and correlated region-by-region for each animal to create correlation matrices [68-70]. For statistical analysis, r-values were Fisher Z transformed to improve normality. Finally, for each volume ROI Fisher Z transformed data was averaged across all subjects and used for analysis.
2.3 Analysis methods
2.3.1 Graph Metrics Methods
In order to assess similarities and differences in functional connectomes between DA and saline exposed mice we assessed node degree, which is a measure that identifies the most connected nodes (or ROIs) by counting the number of direct connections to all other nodes. A node with high degree will have strong, direct connections to many other nodes in the network [74, 75]. Degree is a binary classifier, for our analysis if a connection was in the top 15% strongest positive functional connections it was considered functionally connected. The Matlab code was used for degree and can be obtained in the brain connectivity toolbox (see https://sites.google.com/site/bctnet/measures/list; [76]). Graph metrics are plotted on the same scale for both groups, with the strongest values in red. To assess the significance of differences between DA and saline exposed mice, a null distribution was built by randomizing the group labels across 10,000 permutations. For each randomized group an average matrix was generated (i.e. Schmidt–Hunter method for meta-analyses) and differences between the two groups were calculated. Finally, the percentage of null values that exceeded the true observed value was calculated. A resulting two-tailed p-value for each ROI was then assessed for significance.
2.3.2 Group Comparisons of seed based functional connectivity
Group differences in functional connectivity of the left and right dorsal anterior cingulate were assessed between DA and saline exposed mice. First, each regions resting state time series was computed and a correlation coefficient was computed between the seed region (left or right cingulate) and all other brain regions for each subject. The strength of correlation coefficients from the seed region to all other regions were compared by a t-test and plotted on the brain. Connections with group differences greater than p < .05 are plotted on the brain (see figure 3).
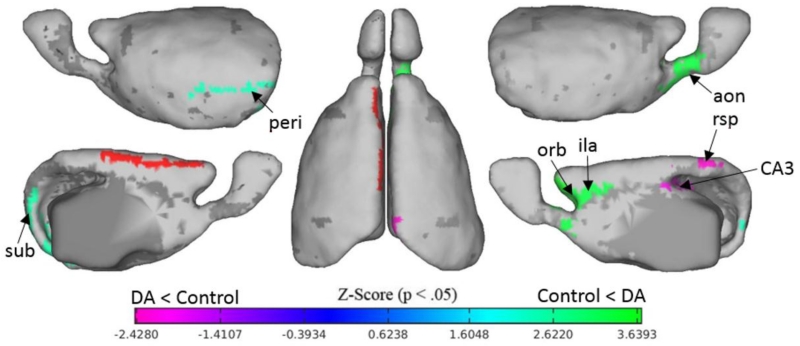
Figure 3.
Group differences in left anterior cingulate functional connectivity. The red region is the left dorsal anterior cingulate (seed region), green colors correspond to connections which are stronger in DA exposed animals and pink colors represent connections with weaker connectivity in DA exposed animals. All regions shown represent connections with group differences of p<.05, and a z-score of above or below 1.98, uncorrected. Noteworthy differences include decreased connectivity to posterior nodes of the default system (rsp and CA3) and overconnectivity to anterior regions (orb, ila). Regions shown are the perirhinal area (peri), orbital (orb), infralimbic area (ila), hippocampal CA3 (CA3), the rsp (retrosplenial cortex), and the subiculum (sub).
2.3.3 Short Range connectivity
For this study, short-range connections were defined as ROI pairs that were directly adjacent and long-range connectivity was defined as interactions between non-adjacent regions. This resulted in a comparison of 1000 short-range connections and 13,028 long-range connections.
To assess differences in short- and long-range connectivity, group connectivity matrices were averaged, connection strengths for adjacent (short-range) and non-adjacent (long-range) connections were computed, and paired two-sample t-tests to test were used to evaluate differences in long- and short- range connectivity strengths.
3. Results
3.1 Social interaction tests
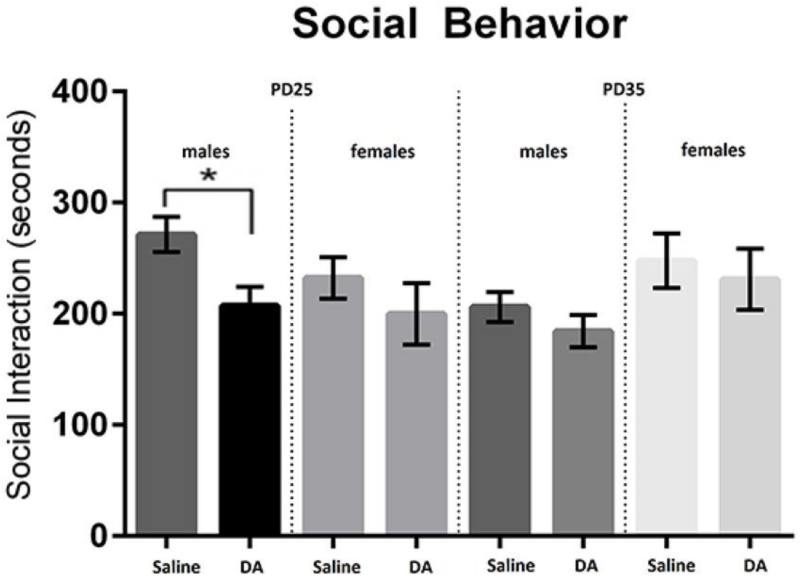
A major aim of this study was to examine whether prenatal exposure to DA reduces social behavior and whether these deficits were exaggerated in male mice. We found a significant time by sex interaction (F(1,44) = 13.1796, p = .0007) where females tended to increase in SI while males decreased in SI across from PD25 to 35. There was also a trend for lower SI in DA animals at PD25 (F(1,44) = 3.018, p = .0893). As expected, post hoc analyses showed that at test date PD25, males exposed to DA expressed lower SI responses than saline exposed animals (p = .0072). However, this effect was not seen at the PD35 test date. Female SI responses were not sensitive to prenatal DA exposure and there was no main effect of DA across gender and test date (p = .11). Results from the repeated measures ANOVA and post hoc t-tests are shown in figure 1. No group or sex differences in SI were found during the shorter, 5-minute SI test at PD21.
Figure 1.
DA exposed males show reduced time spent in social interaction which is mainly driven by observations at PD25 (*p = .0072). We also found that males tended to decrease their SI across time, where females increased their SI behavior (p = .0007). Means and standard errors are shown.
3.2 Ultrasonic Vocalizations
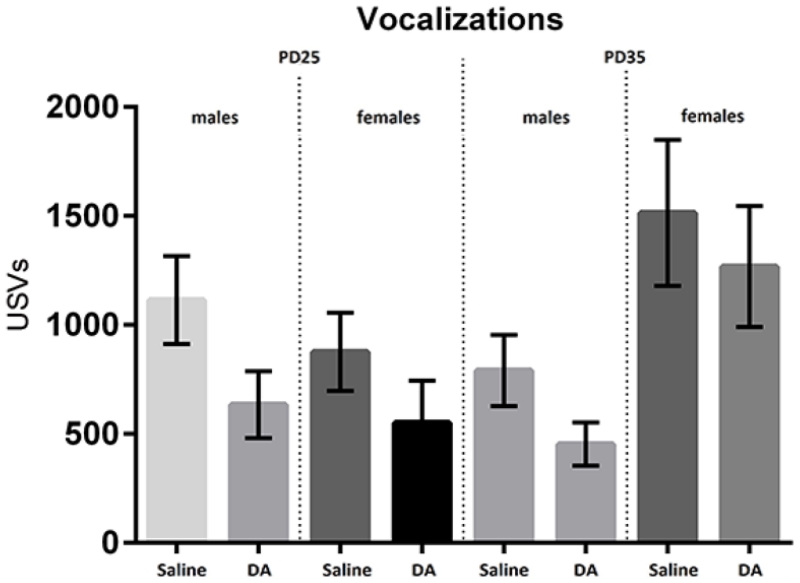
Ultrasonic vocalizations were quantified across two 15-minute SI tests. Although correlated with SI (r = .41, p < .0001) across each of the tests DA exposure did not significantly affect the number of USVs (F(1,48) = 1.78, p = .18). Males showed a trend towards fewer calls than females (F(1,44)=2.925, p = .094), driven by increase in call number for female mice at test date PD35. We also found a significant time by sex interaction (F(1,44) = 26.45, p < .0001) and effect of time (F(1,44) = 5.95, p < .018), driven by an increase in USVs in female mice at PD35. As seen in figure 2, there is a trend towards lower USVs in DA mice most clearly in male mice at PD25 (p=.070). Means and standard errors are shown for each subgroup.
Figure 2.
Number of USVs in saline and DA treated rats at PD25 and PD35. Overall, the effect of DA did not reach significance (p = .17), however, there was a trend towards fewer USVs in male mice on PD25 (p = .07). Means and standard errors are shown.
3.3 Rs-fcMRI Network Results
In order to characterize connectivity change resulting from prenatal DA exposure employed three general analyses. We expected altered connectivity within the default mode, global connectivity alterations would target areas involved in social behavior, and we expected differences in local and long range connectivity between DA and control animals.
3.4 Anterior cingulate functional connectivity
We examined group differences in ACC connectivity because it has been implicated in DA exposure and because this region is a major hub of the default mode network. Unexpectedly, we found a pattern of overconnectivity from the dorsal anterior cingulate to the anterior nodes of the default system, including infralimbic and orbital regions. In contrast, we found that posterior nodes of the default system including the dorsal retrosplenial and CA3 of the hippocampus showed reduced connectivity. Group difference maps for whole brain connectivity are shown in figure 3 for the left dorsal ACC and in figure S1 for the right dorsal ACC.
3.5 Group Differences in Node Degree
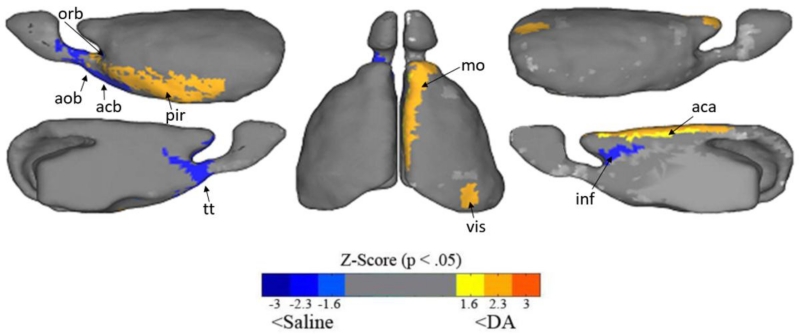
Node degree is a central measurement for analyzing network dynamics which quantifies the number of connections each region has to the rest of the brain. Regions that are connected to many other areas (high degree regions) are referred to as “hubs.” We found group differences in hub architecture in a number of regions (p < .05, 10,000 permutations). Specifically, we found that mice with prenatal exposure to DA have lower degree in the left ventrolateral orbital area, left nucleus accumbens, left accessory and anterior olfactory area, and the right infralimbic area. Conversely, the DA had higher node degree in the right visual cortex, secondary motor area, the dorsal anterior cingulate, and the left piriform cortex. See figure 4.
Figure 4.
Group differences in node degree. The total number of functional connections from a region to all other regions, are plotted on the mouse brain. Warm colors are regions with higher node degree in DA exposed males and cool colors are areas with higher node degree in saline exposed males. DA exposed animals have global connectivity, that is, more connections (higher degree) to the piriform cortex (pir), secondary motor area (mo), visual (vis), and dorsal anterior cingulate (aca). DA exposed animals have lower degree to the infralimbic cortex (inf), taenia tecta (tt), nucleus accumbens (acb), anterior olfactory (aob), and ventral lateral orbital area (orb). All z-scores visualized are p < .05 based on 10,000 random permutations.
3.6 Local vs distributed connectivity
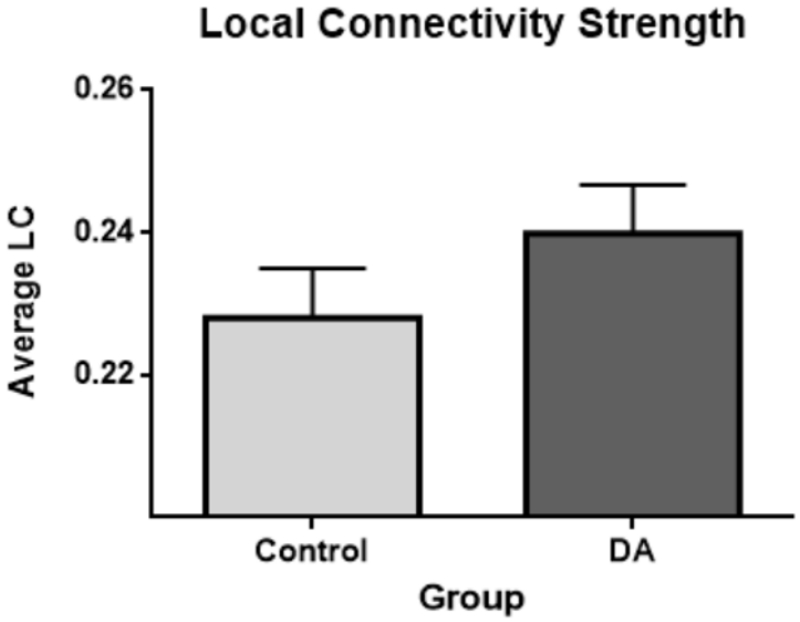
Next, we examined differences in local versus long-range connectivity. For this analysis, adjacent ROIs were considered short range connections and long range connections were non-adjacent ROI pairs. Average connectivity for each pair was assessed and paired sample t-tests were run to assess group differences. No difference in non-adjacent, longer-range, connections were found between groups (DA M = −.0053, control = −.0065, p = .34). However, DA exposed animals had greater connectivity between adjacent, short range, connections compared to controls (DA M = .22399, control M = .2280, t(999) = 2.614, p = .0091). See figure 5.
Figure 5.
DA exposed animals have greater local connectivity compared to controls (p = .0091). Local connectivity (LC) is the group averaged connectivity strength (r) between all adjacent ROI pairs. Error bars represent the standard error of measuremnet for each group.
4. Discussion
Diagnostic assessments of California sea lions and experimental studies with laboratory rodents have shown that DA exposure can result in heightened seizure activity, diminished motor function, aberrant and even maladaptive social behavior, and repetitive behaviors; observations that suggest exposure to DA engenders behaviors resembling features of autism spectrum disorder. To determine whether exposure to DA also results in patterns of brain connectivity that share similarity with ASD, we evaluated resting state functional connectivity in our mouse model.
We exposed mice to DA at ED 16, a timepoint equivalent to ED 18 in rats [18]. This timepoint clearly follows the most frequently targeted period of development for DA studies, ED 13 in rats [77], when the hippocampus undergoes peak neurogenesis [78] and is most sensitive to DA exposure [19]. Critically, DA exposure is neurotoxic throughout development, including demonstrated effects throughout late gestation in mice [20] and extending to PD 14 in rats [23, 24, 28, 29].
Kainate receptors are expressed during late-gestation in the rodent [31] and human [32] brain, which corresponds with E20 in the rat [79] and the first trimester of human fetal development [79]. To engage these receptors in regions outside the time and place of hippocampal neurogenesis, we injected a single dose to DA (1.5 mg/kg) to pregnant B6 dams on ED16. Exposure at this dose and developmental timepoint diminshes social investigation in adolescent FVB mice and results in an increase in parvalbumin immnoreactivity, an anatomical finding that suggests DA exposure dysregulates excitatory/inhibitory tone [65]. We do not know how DA disrupts mouse social behavior because prenatal exposure to straddling timepoints, ED14.5 and ED17.5, which also causes profound changes in conditioned fear memory and adaptation to novel circumstances, effects myelination and growth of neuronal processes [20]. One likely possibility is that domoic acid exposure during development has pleiotropic effects on brain functioning because glutamate receptors are fundamental to many aspects of neuronal development.
Adolescent B6 mice prenatally exposed to DA expressed diminished social interaction, as indicated by our measure of social investigation. We also found a trend toward lower USV production, suggesting a relationship between social investigation and USV production that we have noted previously [80, 81]. Effects of DA on adolescent social investigation by B6 mice are modest in the current report and are similar to our previous studies with FVB mice [65]. Our finding of DA effects on adolescent social behavior is consistent with the juvenile onset of social deficits diagnosed in autism [82]. Our finding that prenatal exposure of mice to DA more robustly diminished adolescent male social interactions is consistent with a previous report that postnatal exposure of rats to DA more effectively diminishes social interactions among adult males [30]. Our results are also consistent with evidence that human males are more sensitive to DA exposure than females [83] and consistent with the sex ratio associated with autism [84]. We urge caution in placing stock in the greater sensitivity of males to both DA exposure and autism risk because developing males are generally more sensitive to developmental insults than are females.
Like other animal models of human disease and disability, our model of ASD may be challenged for lack of construct validity. Common to all laboratory models, housing of rodents in standard caging denies them the spatial and temporal variation they require for robust anatomical [85], or social-emotional development [86]. In this view, our model benefits from parallel assessments of California sea lions, what we might consider a free-range sentinel species. In general, experimental models also gain construct validity is when they express multiple features of the disorder they are designed to simulate. We might have improved upon our construct validity by studying more behavioral phenotypes, such as deficits in empathy [87] or social reward [88], other behavioral phenotypes relevant to ASD, rather than just SI [89-91]. In this study, we chose instead to assess construct validity by examining functional connectivity.
The human default mode network is comprised of brain regions that support social cognition, including processing related to self versus other [47, 48]. With recent success identifying an analagous default network in mice [52], and anomalies in the human default mode associated with autism and schizophrenia [46], we chose to examine this network in DA-exposed mice. We focused on the ACC as it plays a critical role in the functionality of the default network and in social interaction [55-57], including perception of distress in others [92], an ability is diminished in autism [93, 94]. Indeed, the cingulate cortex can have impaired function in autism [95-97]. The ACC is a likely target of DA exposure, as it binds with high affinity to the glutamate receptor GluR6 [98], which is expressed in the developing cingulate cortex [31]. The ACC of our DA-exposed mice expressed a complex and atypical patterns of connectivity, with over-connectivity between the ACC an frontal regions, such as the orbital and infralimbic cortex, and under-connectivity in posterior regions, including the retrospenial cortex, and the CA3 region of the hippocampus.
Though the exact nature of connectivity within the default mode remains incompletely characterized in ASD [99] and schizophrenia [100, 101], adults with ASD can express weak connectivity between the anterior and posterior cingulate [102] and over-connectivity between default mode components [45]. As ASD represents a spectrum of social disability, we expect connectivity to vary across th disorder. For instance, if one child has difficulties with empathy and another with emotional expression, we might assume these discrete social deficits are associated with distinct patterns of functional connectivity. To futher explore these relationships, future studies might incorporate caging that offers mice temporal and spatial complexity for more healthy social development, and with these mice identify variations a range of social behaviors (social approach, social reward, empathy, etc) and in default mode connectivity, and then consider within-subject relationships between discrete social behaviors (social approach, social reward, empathy, etc) and default mode connectivity.
Node degree is a metric of global network structure that characterizes connectivity between each region and the rest of the brain. We hypothesized that regions most impacted would be involved in social behavior. Accordingly, a number of regions relevant to the social brain distinguished DA from saline exposed mice. DA-exposed mice expressed a lower node degree (i.e. fewer connections to the rest of the brain) in the infralimbic and ventral lateral orbital areas, both default network regions critical for higher cognitive processing and sensory integration. Additionally, we found underconnectivity of the nucleus accumbens in DA-exposed mice, which could potentially relate to reduced reward seeking associated with social interaction [88, 103]. DA-exposed mice also expressed increased node degree in sensory regions including the visual cortex, secondary motor cortex, and piriform cortex. Incidentally, children with ASD show similar increases in node degree in sensory systems such as the visual cortex and decreases in degree in regions of the frontal cortex [104], where node degree is predominately decreased in schizophrenia [105]. Although we primarily find a pattern of under-connectivity of higher order regions and over-connectivity in sensory regions of DA-exposed animals, this pattern was not globally observed. DA-exposed mice also express under-connectivity in the sensory related anterior olfactory nucleus, and over-connectivity in the right dorsal anterior cingulate of the default mode. Overall, these results suggest that DA exposure leads to a complex reorganization of network topology affecting both higher order as well as sensory networks and share similarities with network abnormalities associated with ASD.
Mice exposed to in utero to DA also expressed an increase in local connectivity measured by higher connectivity between adjacent brain regions. Local over-connectivity and long-range under-connectivity has been associated with ASD [59, 61-64]. Multiple studies have demonstrated decreased long-range connectivity (between more distant regions) in children with ASD. These variations in connectivity may promote atypical reliance on local, rather than distributed processing, and an inefficient integration between brain systems. Although, we found increased local connectivity, we did not observe a difference in long-range connectivity in DA exposed mice (measured by connectivity between non-adjacent regions). However, a comprehensive analysis of how connectivity changes as a function of each regions relative distance may yet identify group differences as a function of longer-range connections. Nevertheless, our findings suggest that prenatal DA exposure results in a brain organization characterized by a higher local connectivity.
In response to an outbreak of Amnestic Shellfish Poisoning in 1988, Canada promulgated a regulatory action limit for DA in mussels of 20 mg of DA/kg bivalve tissue designed to protect against the acute effects of DA exposure [83, 106, 107]. The US and the European Union have adopted this regulatory limit, though studies conducted since promulgation of this regulatory action limit suggest that developmental exposure to low levels of DA can have pernicious and long-term behavioral effects [40, 108]. Findings that the mammalian fetal brain expresses receptors with high affinity to DA over a prolonged window of development, that DA concentrates in amniotic fluid, and that juveniles are more sensitive to DA than adults, also suggest that low concentrations of DA in the environment could have adverse effects on a developing fetus [1, 39, 109, 110]. These developmental sensitivities may have real world implications. Prenatal exposures to DA may render juvenile California sea lions with subtle neurological disabilities and less capable of survival [111]. Some reports suggest humans may confront analogous risks. A high prevalence of ASD has been associated with coastal communities adjacent to marine environments with high levels of DA [84, 112-114]. Taken together, developmental exposures to domoic acid and other natural and synthetic ligands of glutamate receptors deserve attention for their potential contributions to ASD susceptibility.
Supplementary Material
Figure S1. Right anterior cingulate connectivity. Group differences in right anterior cingulate functional connectivity. The red region is the right dorsal anterior cingulate (seed region), hot and cool colors correspond regions with group differences in functional connectivity to the anterior cingulate (p<.05).
Research Highlights.
-
-
Prenatal domoic acid (DA) exposure results in behavioral and neural phenotypes associated with developmental disorders
-
-
Prenatal DA exposure leads to reduced social behavior
-
-
Functional connectivity MRI abnormalities include atypical default mode connectivity, disrupted network structure in social, reward, and sensory areas, and local over-connectivity.
Acknowledgements
This study was approved by the Institutional Animal Care and Use Committee (IACUC) of Oregon Health & Science University. This report was supported by the developmental account of Dr. Lahvis and inspired by sea lion studies supported by Oregon Sea Grant, NA06OAR4170059, project number R/BT-50-PD from the National Oceanic and Atmospheric Administration’s National Sea Grant College Program, U.S. Department of Commerce, and by appropriations made by the Oregon State Legislature. The statements, findings, conclusions, and recommendations are those of the authors and do not necessarily reflect the views of these funders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Lefebvre KA, Robertson A. Domoic acid and human exposure risks: A review. Toxicon. 2010;56(2):218–230. doi: 10.1016/j.toxicon.2009.05.034. [DOI] [PubMed] [Google Scholar]
- [2].Grant KS, Burbacher TM, Faustman EM, Gratttan L. Domoic acid: neurobehavioral consequences of exposure to a prevalent marine biotoxin. Neurotoxicology & Teratology. 2010;32(2):132–41. doi: 10.1016/j.ntt.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Apeldoorn M.E.v., Egmond H.P.v., Speijers GJA. Dutch National Institute for Public Health and the Environment (RIVM) Rijksinstituut voor Volksgezondheid en Milieu RIVM; 1999. Amnesic shellfish poisoning: A review; pp. 1–53. [Google Scholar]
- [4].Hatfield CL, Gauglitz EJJ, Barnett HJ, Lund JAK, Wekell JC, Eklund M. The fate of domoic acid in Dungeness crab (Cancer magister) as a function of processing. Journal of Shellfish Research. 1995;14(2):359–363. [Google Scholar]
- [5].McCarron P, Hess P. Tissue distribution and effects of heat treatments on the content of domoic acid in blue mussels, Mytilus edulis. Toxicon. 2006;47(4):473–479. doi: 10.1016/j.toxicon.2006.01.004. [DOI] [PubMed] [Google Scholar]
- [6].Todd E. Amnesic shellfish poisoning-- a new seafood toxin syndrome. 1990 [Google Scholar]
- [7].Cook PF, Reichmuth C, Rouse AA, Libby LA, Dennison SE, Carmichael OT, Kruse-Elliott KT, Bloom J, Singh B, Fravel VA, Barbosa L, Stuppino JJ, Van Bonn WG, Gulland FMD, Ranganath C. Algal toxin impairs sea lion memory and hippocampal connectivity,with implications for strandings. Science. 2015;350(6267):1545–1547. doi: 10.1126/science.aac5675. [DOI] [PubMed] [Google Scholar]
- [8].Cook P, Reichmuth C, Gulland F. Rapid behavioural diagnosis of domoic acid toxicosis in California sea lions. Biology Letters. 2011;7(4):536–538. doi: 10.1098/rsbl.2011.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gulland F, Haulena M, Fauquier D, Langlois G, Lander M, Zabka T, Duerr R. Domoic acid toxicity in Californian sea lions (Zalophus californianus): clinical signs, treatment and survival. Veterinary Record. 2002;150(15):475–480. doi: 10.1136/vr.150.15.475. [DOI] [PubMed] [Google Scholar]
- [10].Wittmaack C, Lahvis GP, Keith EO, Self-Sullivan C. Diagnosing domoic acid toxicosis in the California sea lion (Zalophus californianus) using behavioral criteria: A novel approach. Zoo biology. 2015 doi: 10.1002/zoo.21217. [DOI] [PubMed] [Google Scholar]
- [11].Thomas K, Harvey JT, Goldstein T, Barakos J, Gulland F. Movement, dive behavior, and survival of California sea lions ( Zalophus californianus) posttreatment for domoic acid toxicosis. Marine Mammal Science. 2010;26(1):36–52. [Google Scholar]
- [12].Sobotka TJ, Brown R, Quander DY, Jackson R, Smith M, Long SA, Barton CN, Rountree RL, Hall S. Domoic acid: Neurobehavioral and neurohistological effects of low-dose exposure in adult rats. Neurotoxicology and Teratology. 1996;18(6) doi: 10.1016/s0892-0362(96)00120-1. domoic acid-domoic acid. [DOI] [PubMed] [Google Scholar]
- [13].Petrie B, Pinsky C, Standish N, Bose R, Glavin G. Parenteral domoic acid impairs spatial learning in mice. Pharmacology Biochemistry and Behavior. 1992;41(1):211–214. doi: 10.1016/0091-3057(92)90084-s. [DOI] [PubMed] [Google Scholar]
- [14].Fuquay JM, Muha N, Pennington PL, Ramsdell JS. Domoic acid induced status epilepticus promotes aggressive behavior in rats. Physiology & Behavior. 2012;105(2):315–320. doi: 10.1016/j.physbeh.2011.08.013. [DOI] [PubMed] [Google Scholar]
- [15].Baron AW, Rushton SP, Rens N, Morris CM, Blain PG, Judge SJ. Sex differences in effects of low level domoic acid exposure. Neurotoxicology. 2013;34:1–8. doi: 10.1016/j.neuro.2012.10.010. [DOI] [PubMed] [Google Scholar]
- [16].Ramsdell JS. 13 The Molecular and Integrative Basis to Domoic Acid Toxicity. Phycotoxins: Chemistry and biochemistry. 2008:223. [Google Scholar]
- [17].Costa LG, Giordano G, Faustman EM. Domoic acid as a developmental neurotoxin. Neurotoxicology. 2010;31(5):409–423. doi: 10.1016/j.neuro.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105(1):7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- [19].Dakshinamurti K, Sharma SK, Sundaram M, Watanabe T. Hippocampal changes in developing postnatal mice following intrauterine exposure to domoic acid. Journal of Neuroscience. 1993;13(10):4486–95. doi: 10.1523/JNEUROSCI.13-10-04486.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tanemura K, Igarashi K, Matsugami T-R, Aisaki K.-i., Kitajima S, Kanno J. Intrauterine environment-genome interaction and children’s development (2): Brain structure impairment and behavioral disturbance induced in male mice offspring by a single intraperitoneal administration of domoic acid (DA) to their dams. The Journal of toxicological sciences. 2009;34(Special Issue 2):SP279–SP286. doi: 10.2131/jts.34.sp279. [DOI] [PubMed] [Google Scholar]
- [21].Levin EDED, Pang WGWG, Harrison JJ, Williams PP, Petro AA, Ramsdell JSJS. Persistent neurobehavioral effects of early postnatal domoic acid exposure in rats. Neurotoxicology and Teratology. 2006;28(6):673–680. doi: 10.1016/j.ntt.2006.08.005. [DOI] [PubMed] [Google Scholar]
- [22].Doucette TAT, Ryan CLC, Tasker RAR. Gender-based changes in cognition and emotionality in a new rat model of epilepsy. Amino Acids. 2007;32(3):317–322. doi: 10.1007/s00726-006-0418-7. [DOI] [PubMed] [Google Scholar]
- [23].Adams AL, Doucette TA, Ryan CL. Altered pre-pulse inhibition in adult rats treated neonatally with domoic acid. Amino Acids. 2008;35(1):157–160. doi: 10.1007/s00726-007-0603-3. [DOI] [PubMed] [Google Scholar]
- [24].Perry MA, Ryan CL, Tasker R. Effects of low dose neonatal domoic acid administration on behavioural and physiological response to mild stress in adult rats. Physiology & Behavior. 2009;98(1-2):53–59. doi: 10.1016/j.physbeh.2009.04.009. [DOI] [PubMed] [Google Scholar]
- [25].Doucette TA, Bernard PB, Husum H, Perry MA, Ryan CL, Tasker RA. Low doses of domoic acid during postnatal development produce permanent changes in rat behaviour and hippocampal morphology. Neurotoxicity Research. 2004;6(7-8):555–63. doi: 10.1007/BF03033451. [DOI] [PubMed] [Google Scholar]
- [26].Gill DA, Bastlund JF, Watson WP, Ryan CL, Reynolds DS, Tasker RA. Neonatal exposure to low-dose domoic acid lowers seizure threshold in adult rats. Neuroscience. 2010;169(4):1789–1799. doi: 10.1016/j.neuroscience.2010.06.045. [DOI] [PubMed] [Google Scholar]
- [27].Burt MA, Ryan CL, Doucette TA. Altered responses to novelty and drug reinforcement in adult rats treated neonatally with domoic acid. Physiology & Behavior. 2008;93(1-2):327–336. doi: 10.1016/j.physbeh.2007.09.003. [DOI] [PubMed] [Google Scholar]
- [28].Burt MA, Ryan CL, Doucette TA. Low dose domoic acid in neonatal rats abolishes nicotine induced conditioned place preference during late adolescence. Amino Acids. 2008;35(1):247–9. doi: 10.1007/s00726-007-0584-2. [DOI] [PubMed] [Google Scholar]
- [29].Adams AL, Doucette TA, James R, Ryan CL. Persistent changes in learning and memory in rats following neonatal treatment with domoic acid. Physiology & Behavior. 2009;96(4/5):505–512. doi: 10.1016/j.physbeh.2008.11.019. [DOI] [PubMed] [Google Scholar]
- [30].Ryan CL, Robbins MA, Smith MT, Gallant IC, Adams-Marriott AL, Doucette TA. Altered social interaction in adult rats following neonatal treatment with domoic acid. Physiology & Behavior. 2011;102(3-4):291–295. doi: 10.1016/j.physbeh.2010.11.020. [DOI] [PubMed] [Google Scholar]
- [31].Bahn S, Volk B, Wisden W. Kainate receptor gene expression in the developing rat brain. The Journal of Neuroscience. 1994;14(9):5525–5547. doi: 10.1523/JNEUROSCI.14-09-05525.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ritter LM, Unis AS, Meadorw-Woodruff JH. Ontogeny of ionotropic glutamate receptor expression in human fetal brain. Developmental Brain Research. 2001;127(2):123–133. doi: 10.1016/s0165-3806(01)00126-2. [DOI] [PubMed] [Google Scholar]
- [33].Robertson HH, Renton KK, Kohn JJ, White TT. Patterns of Fos expression suggest similar mechanisms of action for the excitotoxins domoic and kainic acid. Annals of the New York Academy of Sciences. 1992;648(0077-8923, 0077-8923):330–334. doi: 10.1111/j.1749-6632.1992.tb24572.x. [DOI] [PubMed] [Google Scholar]
- [34].Peng YG, Ramsdell JS. Brain Fos induction is a sensitive biomarker for the lowest observed neuroexcitatory effects of domoic acid. Fundamental & Applied Toxicology. 1996;31(2):162–8. doi: 10.1006/faat.1996.0087. [DOI] [PubMed] [Google Scholar]
- [35].Teitelbaum JS, Zatorre RJ, Carpenter S, Gendron D, Evans AC, Gjedde A, Cashman NR. Neurologic sequelae of domoic acid intoxication due to the ingestion of contaminated mussels. New England Journal of Medicine. 1990;322(25):1781–1787. doi: 10.1056/NEJM199006213222505. [DOI] [PubMed] [Google Scholar]
- [36].Colman JR, Nowocin KJ, Switzer RC, Trusk TC, Ramsdell JS. Mapping and reconstruction of domoic acid-induced neurodegeneration in the mouse brain. Neurotoxicology & Teratology. 2005;27(5):753–67. doi: 10.1016/j.ntt.2005.06.009. [DOI] [PubMed] [Google Scholar]
- [37].Scallet AC, Binienda Z, Caputo FA, Hall S, Paule MG, Rountree RL, Schmued L, Sobotka T, Slikker W., Jr. Domoic acid-treated cynomolgus monkeys (M. fascicularis): effects of dose on hippocampal neuronal and terminal degeneration. Brain Research. 1993;627(2):307–13. doi: 10.1016/0006-8993(93)90335-k. [DOI] [PubMed] [Google Scholar]
- [38].Doucette TA, Bernard PB, Yuill P, Tasker R, Ryan CL. Low doses of non-NMDA glutamate receptor agonists alter neurobehavioural development in the rat. Neurotoxicology and Teratology. 2003;25(4):473–479. doi: 10.1016/s0892-0362(03)00034-5. [DOI] [PubMed] [Google Scholar]
- [39].Maucher JM, Ramsdell JS. Maternal-fetal transfer of domoic acid in rats at two gestational time points. Environmental Health Perspectives. 2007;115(12):1743–6. doi: 10.1289/ehp.10446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Xi D, Peng YG, Ramsdell JS. Domoic acid is a potent neurotoxin to neonatal rats. Natural Toxins. 1997;5(2):74–9. doi: 10.1002/(SICI)(1997)5:2<74::AID-NT4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- [41].Biswal B, Zerrin Yetkin F, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo- planar mri. Magnetic resonance in medicine. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- [42].Dias TGC, Wilson VB, Bathula DR, Iyer SP, Mills KL, Thurlow BL, Stevens CA, Musser ED, Carpenter SD, Grayson DS. Reward circuit connectivity relates to delay discounting in children with attention-deficit/hyperactivity disorder. European Neuropsychopharmacology. 2013;23(1):33–45. doi: 10.1016/j.euroneuro.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Fair DA, Bathula D, Nikolas MA, Nigg JT. Distinct neuropsychological subgroups in typically developing youth inform heterogeneity in children with ADHD. Proceedings of the National Academy of Sciences. 2012;109(17):6769–6774. doi: 10.1073/pnas.1115365109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Current opinion in neurology. 2008;21(4):424–430. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- [45].Ray S, Miller M, Karalunas S, Robertson C, Grayson DS, Cary RP, Hawkey E, Painter JG, Kriz D, Fombonne E. Structural and functional connectivity of the human brain in autism spectrum disorders and attention- deficit/hyperactivity disorder: A rich club- organization study. Human brain mapping. 2014;35(12):6032–6048. doi: 10.1002/hbm.22603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJ. Default-mode brain dysfunction in mental disorders: a systematic review. Neuroscience & biobehavioral reviews. 2009;33(3):279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- [47].Mars RB, Neubert F-X, Noonan M, Sallet J, Toni I, Rushworth MF. On the relationship between the″ default mode network″ and the″ social brain″. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Schilbach L, Eickhoff SB, Rotarska-Jagiela A, Fink GR, Vogeley K. Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Consciousness and cognition. 2008;17(2):457–467. doi: 10.1016/j.concog.2008.03.013. [DOI] [PubMed] [Google Scholar]
- [49].Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proceedings of the National Academy of Sciences. 2001;98(7):4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58(3):306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Sforazzini F, Schwarz AJ, Galbusera A, Bifone A, Gozzi A. Distributed BOLD and CBV-weighted resting-state networks in the mouse brain. Neuroimage. 2014;87:403–415. doi: 10.1016/j.neuroimage.2013.09.050. [DOI] [PubMed] [Google Scholar]
- [52].Stafford JM, Jarrett BR, Miranda-Dominguez O, Mills BD, Cain N, Mihalas S, Lahvis GP, Lattal KM, Mitchell SH, David SV. Large-scale topology and the default mode network in the mouse connectome. Proceedings of the National Academy of Sciences. 2014;111(52):18745–18750. doi: 10.1073/pnas.1404346111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Guilfoyle DN, Gerum SV, Sanchez JL, Balla A, Sershen H, Javitt DC, Hoptman MJ. Functional connectivity fMRI in mouse brain at 7T using isoflurane. Journal of neuroscience methods. 2013;214(2):144–148. doi: 10.1016/j.jneumeth.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Miranda-Dominguez O, Mills BD, Grayson D, Woodall A, Grant KA, Kroenke CD, Fair DA. Bridging the gap between the human and macaque connectome: a quantitative comparison of global interspecies structure-function relationships and network topology. The Journal of Neuroscience. 2014;34(16):5552–5563. doi: 10.1523/JNEUROSCI.4229-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- [56].Lavin C, Melis C, Mikulan E, Gelormini C, Huepe D, Ibañez A. The anterior cingulate cortex: an integrative hub for human socially-driven interactions. Neural basis of social learning, social deciding, and other-regarding preferences. 2015:8. doi: 10.3389/fnins.2013.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Rudebeck P, Buckley M, Walton M, Rushworth M. A role for the macaque anterior cingulate gyrus in social valuation. Science. 2006;313(5791):1310–1312. doi: 10.1126/science.1128197. [DOI] [PubMed] [Google Scholar]
- [58].Liu Y, Liang M, Zhou Y, He Y, Hao Y, Song M, Yu C, Liu H, Liu Z, Jiang T. Disrupted small-world networks in schizophrenia. Brain. 2008;131(4):945–961. doi: 10.1093/brain/awn018. [DOI] [PubMed] [Google Scholar]
- [59].Barttfeld P, Wicker B, Cukier S, Navarta S, Lew S, Sigman M. A big-world network in ASD: dynamical connectivity analysis reflects a deficit in long-range connections and an excess of short-range connections. Neuropsychologia. 2011;49(2):254–263. doi: 10.1016/j.neuropsychologia.2010.11.024. [DOI] [PubMed] [Google Scholar]
- [60].Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: local over-connectivity but long-distance disconnection. Current Opinion in Neurobiology. 2005;15(2):225–230. doi: 10.1016/j.conb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- [61].Dajani DR, Uddin LQ. Local brain connectivity across development in autism spectrum disorder: A cross- sectional investigation. Autism Research. 2015 doi: 10.1002/aur.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Hernandez L, Rudie JD, Kilroy EM, Colich NL, Bookheimer SY, Iacoboni M. Increased Local Connectivity and Decreased Long Range Connectivity In Autism Is Consistent with Immaturity of Cortical Networks; International Meeting for Autism Research; San Diego, CA. 2011. [Google Scholar]
- [63].Keown CL, Shih P, Nair A, Peterson N, Mulvey ME, Müller R-A. Local functional overconnectivity in posterior brain regions is associated with symptom severity in autism spectrum disorders. Cell reports. 2013;5(3):567–572. doi: 10.1016/j.celrep.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Maximo JO, Keown CL, Nair A, Muller R. Approaches to local connectivity in autism using resting state functional connectivity MRI. Front Hum Neurosci. 2013;7:605. doi: 10.3389/fnhum.2013.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Zuloaga D, Lahvis G, Mills B, Pearce H, Turner J, Raber J. Fetal domoic acid exposure affects lateral amygdala neurons, diminishes social investigation and alters sensory-motor gating. NeuroToxicology. 2016 doi: 10.1016/j.neuro.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Tryphonas L, Truelove J, Iverson F. Acute parenteral neurotoxicity of domoic acid in cynomolgus monkeys (M. fascicularis) Toxicologic Pathology. 1990;18(2):297–303. doi: 10.1177/019262339001800208. Erratum appears in Toxicol Pathol 1990;18(3):431. [DOI] [PubMed] [Google Scholar]
- [67].Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- [68].Van Essen DC. Cortical cartography and Caret software. Neuroimage. 2012;62(2):757–764. doi: 10.1016/j.neuroimage.2011.10.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Van Essen DC, Drury HA, Dickson J, Harwell J, Hanlon D, Anderson CH. An integrated software suite for surface-based analyses of cerebral cortex. Journal of the American Medical Informatics Association. 2001;8(5):443–459. doi: 10.1136/jamia.2001.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Van Essen DC, Dierker DL. Surface-based and probabilistic atlases of primate cerebral cortex. Neuron. 2007;56(2):209–225. doi: 10.1016/j.neuron.2007.10.015. [DOI] [PubMed] [Google Scholar]
- [71].Lancaster JL, Glass TG, Lankipalli BR, Downs H, Mayberg H, Fox PT. A modality- independent approach to spatial normalization of tomographic images of the human brain. Human brain mapping. 1995;3(3):209–223. [Google Scholar]
- [72].Oh SW, Harris JA, Ng L, Winslow B, Cain N, Mihalas S, Wang Q, Lau C, Kuan L, Henry AM. A mesoscale connectome of the mouse brain. Nature. 2014;508(7495):207–214. doi: 10.1038/nature13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Paxinos G, Franklin KBJ. The Mouse Brain in Sterotactic Coordinates. Compact second edition Elsevier; Amsterdam: 2004. [Google Scholar]
- [74].Wasserman S, Faust K. Social network analysis: Methods and applications. Cambridge university press; 1994. [Google Scholar]
- [75].Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nature Reviews Neuroscience. 2009;10(3):186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- [76].Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52(3):1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- [77].Levin ED, Pizarro K, Pang WG, Harrison J, Ramsdell JS. Persisting behavioral consequences of prenatal domoic acid exposure in rats. Neurotoxicology & Teratology. 2005;27(5):719–25. doi: 10.1016/j.ntt.2005.06.017. [DOI] [PubMed] [Google Scholar]
- [78].Angevine JB., Jr Time of neuron origin in the hippocampal region: An autoradiographic study in the mouse. Experimental Neurology. 1965 [PubMed] [Google Scholar]
- [79].Clancy B, Darlington R, Finlay B. Translating developmental time across mammalian species. Neuroscience. 2001;105(1):7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- [80].Kabir ZD, Kennedy B, Katzman A, Lahvis GP, Kosofsky BE. Effects of prenatal cocaine exposure on social development in mice. Developmental neuroscience. 2014;36(3-4):338–346. doi: 10.1159/000360524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Panksepp JB, Jochman K, Kim JU, Koy JJ, Wilson ED, Chen Q, Wilson CR, Lahvis GP. Affiliative behavior, ultrasonic communication and social reward are influenced by genetic variation in adolescent mice. PLoS ONE [Electronic Resource] 2007;2(e351) doi: 10.1371/journal.pone.0000351. doi:10.1371/journal.pone.0000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, Liaw J. Early Social Attention Impairments in Autism: Social Orienting, Joint Attention, and Attention to Distress. Developmental Psychology. 2004;40(2):271–283. doi: 10.1037/0012-1649.40.2.271. [DOI] [PubMed] [Google Scholar]
- [83].Perl TM, Bedard L, Kosatsky T, Hockin JC, Todd ECD, Remis RS. An Outbreak of Toxic Encephalopathy Caused by Eating Mussels Contaminated with Domoic Acid. New England Journal of Medicine. 1990;322(25):1775–1780. doi: 10.1056/NEJM199006213222504. [DOI] [PubMed] [Google Scholar]
- [84].Kim YS, Leventhal BL, Koh YJ, Fombonne E, Laska E, Lim EC, Cheon KA, Kim SJ, Kim YK, Lee H, Song DH, Grinker RR. Prevalence of autism spectrum disorders in a total population sample. American Journal of Psychiatry. 2011;168(9):904–12. doi: 10.1176/appi.ajp.2011.10101532. [DOI] [PubMed] [Google Scholar]
- [85].Benefiel AC, Greenough WT. Effects of experience and environment on the developing and mature brain: Implications for laboratory animal housing. Ilar Journal. 1998;39(1):5–11. doi: 10.1093/ilar.39.1.5. [DOI] [PubMed] [Google Scholar]
- [86].Lahvis GP. Animal Models of Autism, Epigenetics, and the Inescapable Problem of Animal Constraint. In: Kim Y-K, Gewirtz J, editors. Animal Models of Behavior Genetics Research. Springer Publishing Company; New York, NY: in press. [Google Scholar]
- [87].Chen Q, Panksepp JB, Lahvis GP. Empathy is moderated by genetic background in mice. PLoS ONE [Electronic Resource] 2009;4(2) doi: 10.1371/journal.pone.0004387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Panksepp JB, Lahvis GP. Social reward among juvenile mice. Genes, Brain, & Behavior. 2007;6(7):661–71. doi: 10.1111/j.1601-183X.2006.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Casey B, Oliveri ME, Insel T. A neurodevelopmental perspective on the research domain criteria (RDoC) framework. Biological psychiatry. 2014;76(5):350–353. doi: 10.1016/j.biopsych.2014.01.006. [DOI] [PubMed] [Google Scholar]
- [90].Bishop SL, Lahvis GP. The autism diagnosis in translation: shared affect in children and mouse models of ASD. Autism Research. 2011;4(5):317–335. doi: 10.1002/aur.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Lahvis GP, Black LM. Social Interactions in the Clinic and the Cage: Toward a More Valid Mouse Model of Autism. Animal Models of Behavioral Analysis. 2011:153–192. [Google Scholar]
- [92].Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303(5661):1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- [93].Hansman-Wijnands M, Hummelen J. Differential diagnosis of psychopathy and autism spectrum disorders in adults. Empathie deficit as a core symptom. Tijdschrift voor Psychiatrie. 2006;48(8):627–636. [PubMed] [Google Scholar]
- [94].Smith A. The empathy imbalance hypothesis of autism: A theoretical approach to cognitive and emotional empathy in autistic development. The Psychological Record. 2009;59(2):273–294. [Google Scholar]
- [95].Haznedar MM, Buchsbaum MS, Wei TC, Hof PR, Cartwright C, Bienstock CA, Hollander E. Limbic circuitry in patients with autism spectrum disorders studied with positron emission tomography and magnetic resonance imaging. American Journal of Psychiatry. 2000;157(12):1994–2001. doi: 10.1176/appi.ajp.157.12.1994. [DOI] [PubMed] [Google Scholar]
- [96].Ohnishi T, Matsuda H, Hashimoto T, Kunihiro T, Nishikawa M, Uema T, Sasaki M. Abnormal regional cerebral blood flow in childhood autism. Brain. 2000;123(Pt 9):1838–44. doi: 10.1093/brain/123.9.1838. [DOI] [PubMed] [Google Scholar]
- [97].Haznedar M, Buchsbaum MS, Wei T-C, Hof PR, Cartwright C, Bienstock CA, Hollander E. Limbic circuitry in patients with autism spectrum disorders studied with positron emission tomography and magnetic resonance imaging. The American Journal of Psychiatry. 2000;157(12):1994–2001. doi: 10.1176/appi.ajp.157.12.1994. [DOI] [PubMed] [Google Scholar]
- [98].Tasker RA, Tracy D. Domoic Acid, Seafood and Freshwater Toxins. CRC Press. 2008:397–429. [Google Scholar]
- [99].Müller R-A, Shih P, Keehn B, Deyoe JR, Leyden KM, Shukla DK. Underconnected, but how? A survey of functional connectivity MRI studies in autism spectrum disorders. Cerebral Cortex. 2011;21(10):2233–2243. doi: 10.1093/cercor/bhq296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].van den Heuvel MP, Fornito A. Brain networks in schizophrenia. Neuropsychology review. 2014;24(1):32–48. doi: 10.1007/s11065-014-9248-7. [DOI] [PubMed] [Google Scholar]
- [101].Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annual review of clinical psychology. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- [102].Monk CS, Peltier SJ, Wiggins JL, Weng S-J, Carrasco M, Risi S, Lord C. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. Neuroimage. 2009;47(2):764–772. doi: 10.1016/j.neuroimage.2009.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Dolen G, Darvishzadeh A, Huang KW, Malenka RC. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501(7466):179–184. doi: 10.1038/nature12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Di Martino A, Yan C-G, Li Q, Denio E, Castellanos FX, Alaerts K, Anderson JS, Assaf M, Bookheimer SY, Dapretto M. The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Molecular psychiatry. 2014;19(6):659–667. doi: 10.1038/mp.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Bassett DS, Nelson BG, Mueller BA, Camchong J, Lim KO. Altered resting state complexity in schizophrenia. Neuroimage. 2012;59(3):2196–2207. doi: 10.1016/j.neuroimage.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Tryphonas LL, Truelove JJ, Todd EE, Nera EE, Iverson FF. Experimental oral toxicity of domoic acid in cynomolgus monkeys (Macaca fascicularis) and rats. Preliminary investigations, Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 1990;28(10):707–715. doi: 10.1016/0278-6915(90)90147-f. [DOI] [PubMed] [Google Scholar]
- [107].Todd ECD. Domoic acid and amnesic shellfish poisoning—a review. Journal of Food Protection. 1993;56:69–83. doi: 10.4315/0362-028X-56.1.69. [DOI] [PubMed] [Google Scholar]
- [108].Doucette TA, Strain SM, Allen GV, Ryan CL, Tasker RAR. Comparative behavioural toxicity of domoic acid and kainic acid in neonatal rats. Neurotoxicology and Teratology. 22(6):863–869. doi: 10.1016/s0892-0362(00)00110-0. [DOI] [PubMed] [Google Scholar]
- [109].Doucette TA, Tasker RA. Perinatal Domoic Acid as a Neuroteratogen. 2015. [DOI] [PubMed] [Google Scholar]
- [110].Maucher JM, Ramsdell JS. Domoic Acid Transfer to Milk: Evaluation of a Potential Route of Neonatal Exposure. Environmental Health Perspectives. 2005;113(4):461–464. doi: 10.1289/ehp.7649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Ramsdell JS, Zabka TS. In Utero Domoic Acid Toxicity: A Fetal Basis to Adult Disease in the California Sea Lion (Zalophus californianus) Marine Drugs. 2008;6(2):262–290. doi: 10.3390/md20080013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Ouellette-Kuntz H, Coo H, Yu CT, Chudley AE, Noonan A, Breitenbach M, Ramji N, Prosick T, Bedard A, Holden JJA. Prevalence of pervasive developmental disorders in two Canadian provinces. Journal of Policy and Practice in Intellectual Disabilities. 2006;3(3):164–172. [Google Scholar]
- [113].Choi KD, Lee JS, Lee JO, Oh KS, Shin IS. Investigation of domoic acid in shellfish collected from Korean fish retail outlets. Food Science and Biotechnology. 2009;18(4):842–848. [Google Scholar]
- [114].Waldman M, Nicholson S, Adilov N, Williams J. Autism Prevalence and Precipitation Rates in California, Oregon, and Washington Counties. Archives of Pediatrics Adolescent Medicine. 2008;162(11):1026–1034. doi: 10.1001/archpedi.162.11.1026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Right anterior cingulate connectivity. Group differences in right anterior cingulate functional connectivity. The red region is the right dorsal anterior cingulate (seed region), hot and cool colors correspond regions with group differences in functional connectivity to the anterior cingulate (p<.05).