Abstract
The endocannabinoid system comprises the two well characterized Gi/o‐protein coupled receptors (cannabinoid receptor 1 (CB1) and CB2), their endogenous lipid ligands, and the enzymes involved in their biosynthesis and biotransformation. Drug discovery efforts relating to the endocannabinoid system have been focused mainly on the two cannabinoid receptors and the two endocannabinoid deactivating enzymes fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MGL). This review provides an overview of cannabinergic agents used in drug research and those being explored clinically.
Cannabis has been used as a therapeutic for many millennia. However, it was not until the 1960s that its key ingredient, (‐)‐Δ9‐tetrahydrocannabinol (Δ9‐THC), was isolated and synthesized. In parallel, research in the pharmaceutical industry and academic laboratories produced a plethora of new and structurally related compounds with very potent biological properties.1 Notwithstanding these worthy medicinal chemistry efforts, nabilone (Eli Lilly) and Δ9‐THC remained, thus far, the only synthetic drugs in the market, whereas other programs never led to additional medications and the interest in developing cannabis‐based therapeutic medications tapered off. The reason for this decrease in interest can be attributed to the new compounds’ psychotropic side effects and to the absence of any well‐identified pharmacological mechanisms of action for newer analogs. A major breakthrough in the field occurred in the mid‐1980s with the discovery, cloning, expression, and imaging of the first cannabinoid receptor, named CB1, which was followed by the identification of a second cannabinoid target, CB2. Both CB1 and CB2 belong to the Class A G‐protein‐coupled receptor family and exhibit primarily Gi/o signaling mechanisms.
The discovery of CB1 and CB2 was followed by intensive research efforts aimed at exploring this intriguing biochemical system and identifying the key proteins involved in its modulation. Almost four decades later, we now have a better understanding of the key physiological roles played by the endocannabinoid receptors, the endocannabinoid ligands, and the different enzymes involved in their biosynthesis and biotransformation. A summary of these components is provided in Figure 1.2, 3
Figure 1.
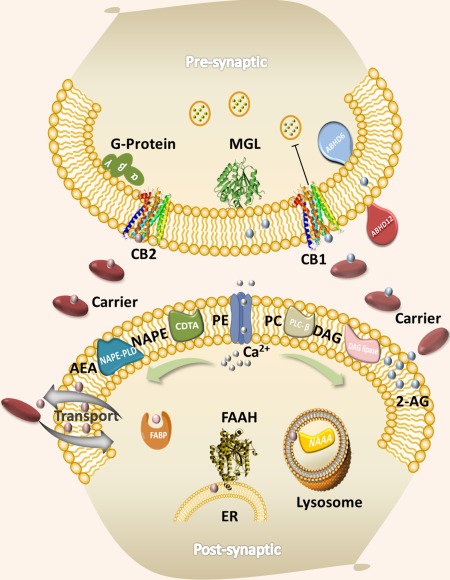
The endocannabinoid signaling system. CB1, cannabinoid receptor 1; CB2, cannabinoid receptor 2; FAAH, fatty acid amide hydrolase; MGL, monoacylglycerol lipase; ABHD6, α‐β hydrolase domain‐containing protein 6; ABHD12, α‐β hydrolase domain‐containing protein 12; NAPE, N‐arachidonoyl phosphatidylethanolamine; PE, phosphatidylethanolamine; PC, phospholipase C; PD, phospholipase D; DGL, diacylglycerol lipase; FABP, fatty‐acid‐binding protein; AEA, arachidonoylethanolamide; 2‐AG, 2‐arachidonoylglycerol; ER, endoplasmic reticulum. Figure adapted from M. Nasr and A. Makriyannis, unpublished results.
Cannabinoid receptors localize in the presynaptic junction and are thus engaged in retrograde signaling, a signature feature of this biochemical system. Both receptors are activated by two distinct families of lipid mediators represented by arachidonoylethanolamide (AEA) and 2‐arachidonoylgycerol (Figure 2 a). Unlike many other neurotransmitters, endocannabinoids are produced upon demand, have a relatively slow timeframe of action, and are biosynthesized from endogenous membrane components by a series of enzymes. The levels of endocannabinoids (endocannabinoid tone) are also controlled by endocannabinoid deactivating enzymes, the most prominent of which are FAAH for AEA and MGL for 2‐arachidonoylgycerol.2, 3 It is thus possible to affect this tone by chemically attenuating the functions of the above enzymes. Such approaches can lead to the development of suitable enzyme inhibitors capable of modulating the endocannabinoid tone, and thus serve as a basis for the design of therapeutic medications.
Figure 2.
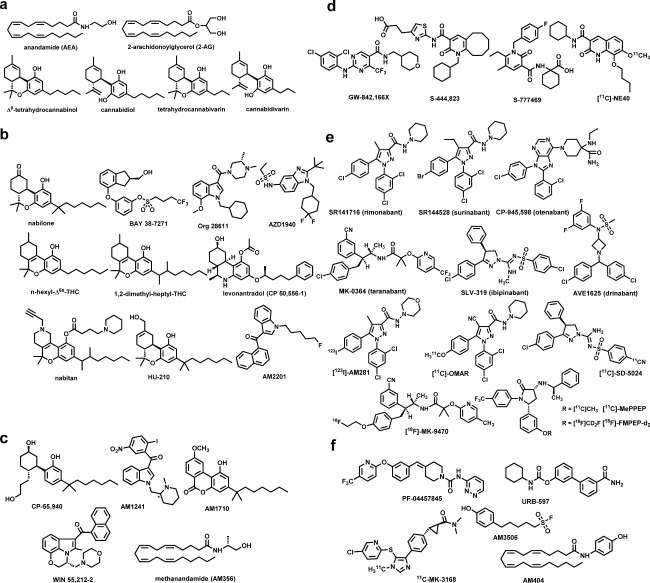
Distinct families of cannabinergic ligands. (a) Endo‐ and phytocannabinoids; (b) cannabinoid receptors 1 and 2 (CB1/CB2) agonists; (c) cannabinoid agonists as pharmacological tools; (d) CB2 agonists; (e) CB1 antagonists; and (f) fatty acid amide hydrolase (FAAH) and AEA transport inhibitors.
Endocannabinoid‐based drug discovery
Approaches for the development of cannabinoid receptor‐based medications include CB1 or CB2 agonists1, 2, 4, 5 and antagonists2 that are suitably designed to impart them with drug‐like properties and reduced undesirable side effects. Other approaches include the inhibition of FAAH and MGL, inhibition of endocannabinoid transport, as well as the design of ligands capable of modulating endocannabinoid function by binding to allosteric sites (positive, negative, and silent allosteric modulators).2 A summary of the current status of these compounds is discussed below while emphasizing cannabinergic compounds that have clinical significance (Table 1).
Table 1.
Clinical information on key phytocannabinoids and select synthetic cannabinoids a
| Compound | Established therapeutic indication(s) | Dose regimen/available strength | Clinical status, trial/PubMed identifier number | Sponsor institution/company |
|---|---|---|---|---|
| CB1 and CB1/CB2 agonists (Figures 2a,b) | ||||
| Tetrahydrocannabinol (synthetic) | Anorexia associated with weight loss in patients with AIDS, chemotherapy‐induced nausea and vomiting | 2.5 mg, 5 mg, 10 mg oral capsules as prescribed | Marketed | Actavis, Par Pharmaceuticals, Insys Therapeutics (Dronabinol), AbbVie (Marinol) |
| Nabilone | Chemotherapy‐induced nausea and vomiting | 1 mg oral capsules as prescribed | Marketed | Meda Pharmaceuticals, Valeant Pharmaceuticals (Cesamet) |
| Tetrahydrocannabinol‐cannabidiol mixture (as soft extracts from Cannabis sativa L., folium cum flore ‐ Cannabis leaf and flower) | Spasticity and neuropathic pain due to multiple sclerosis, pain in adult patients with advanced cancer | 100 µL oromucosal spray containing 2.7 mg of THC, 2.5 mg CBD and up to 0.04 g ethanol ‐ varying doses titrated as 1–12 sprays/day | Marketed; multiple trials up to phase 3 scheduled, FDA Fast Track drug designation, NCT01868048, NCT01337089, NCT01262651 | GW Pharmaceuticals (Sativex, Nabiximols) (alone, add‐on, or as adjunctive therapy) |
| Cannabidiol (both as plant extract and nonplant pharmaceutical grade drug substance) | Lennox‐Gastaut syndrome, Dravet syndrome, glioblastoma multiform, glioma, and pediatric schizophrenia | Purified plant extract ‐ varying doses p.o. and recommendations by the Data Safety Monitoring Committee nonplant based ‐ no higher than 40 mg/kg/day p.o. | Multiple trials up to phase 3 scheduled, FDA Orphan/Fast Track drug designation granted, NCT02224573, NCT02318563, NCT02318537 | GW Pharmaceuticals (Epidiolex) (plant extract) Insys Therapeutics (non‐plant‐based adjunctive therapy) |
| Cannabidivarin (plant extract) | Epilepsy | 400, 600, 800 mg, escalating, p.o. twice daily | Phase 2 scheduled, NCT02365610 | GW Pharmaceuticals (GWP42006) (add‐on therapy) |
| Cannabidiol‐tetrahydrocannabivarin mixture (plant extract) | Dyslipidemias, type 2 diabetes | 100 mg (CBD), 5 mg (THCV) each alone and as 20: 1 mixture; at 5 mg 1:1 mixture p.o. twice daily | Phase 2 complete, NCT01217112 | GW Pharmaceuticals (GWP42003‐GWP42004) |
| Levonantradol | Chemotherapy‐induced emesis | 0.5‐2 mg i.m. every 4 hours | Phase 2 terminated, PMID: 6392353 | Pfizer, Cooper Hospital |
| KN38‐7271 | Comatose after severe traumatic brain injury | 500, 1000 µg over 24 h i.v. | Phase 2 positive/unknown, EUCTR2006‐001589‐17‐DE, PMID: 22696266 | KeyNeurotek Pharmaceuticals AG, Otto‐von‐Guericke‐Universität Magdeburg |
| Org 28611 | Postoperative pain | 3 µg/kg i.v. | Phase 2 complete, NCT00782951, PMID: 18635703 | Merck Sharp & Dohme Corp, Centre for Human Drug Research, Leiden, NV Organon |
| AZD1940 | Pain (peripheral) | 400–800 µg p.o. | Phase 2 complete, NCT00659490, PMID: 23324098 | AstraZeneca |
| CB2 agonists (Figure 2d) | ||||
| GW842166X | Postoperative pain | 100–800 mg p.o. | Phase 2 complete, NCT00444769, PMID: 21540741 | GlaxoSmithKline |
| S‐777469 | Atopic dermatitis | 400–800 mg, p.o. twice daily | Phase 2 complete/unknown, NCT00703573 | Shionogi |
| CB1 antagonists (Figure 2e) | ||||
| Rimonabant | Obesity or excess weight in combination with type 2 diabetes or dyslipidemia | 20 mg/day oral tablets | Marketed and withdrawn; multiple trials up to phase 4 completed | Sanofi‐Aventis (Acomplia) |
| Surinabant | Nicotine addiction | 2.5, 5, 10 mg/day p.o. | Phase 2 complete, NCT00432575, PMID: 22219220 | Sanofi‐Aventis, Oslo University |
| BMS‐646,256 | Weight loss | 5, 10, 25, 50 mg/day p.o. | Phase 2 terminated, NCT00388609 | Bristol‐Myers Squibb |
| Taranabant | Nicotine addiction | 2–8 mg/day p.o. | Phase 2 complete, NCT00109135, PMID: 20191360 | Merck Sharp & Dohme Corp |
| CP‐945,598 | Weight loss during type 2 diabetes | 0.5, 1, 2 mg/day p.o. | Phase 3 terminated, NCT00430742, PMID: 20212496 | Merck Sharp & Dohme Corp, University of Melbourne |
| Weight loss | 10–20 mg/day p.o. | Phase 3 terminated, PMID: 21293451 | Pfizer, University College London | |
| AVE1625 | Alzheimer's disease | 10–40 mg/day p.o. | Phase 2 complete/unknown, NCT00380302 | Sanofi‐Aventis |
| FAAH inhibitors (Figure 2f) | ||||
| PF‐04457845 | Pain | 4 mg/day p.o. | Phase 2 complete, NCT00981357, PMID: 22727500 | Pfizer |
| Cannabis withdrawal PTSD with alcohol dependence | 4 mg/day p.o. 4 mg/day p.o. | Phase 2 recruiting, NCT01618656 SRCTN56834832, recruiting | Yale University, National Institute of Drug Abuse (NIH) Stockholm Centre for Dependency Disorders |
CB1, cannabinoid receptor‐1; CB2, cannabinoid receptor‐2; CBD, cannabidiol; FAAH, fatty acid amide hydrolase; FDA, US Food and Drug Administration; i.m. intramuscularly; NIH, National Institutes of Health; p.o., prescribed orally; PTSD, posttraumatic stress disorder; THC, tetrahydrocannabinol; THCV, tetrahydrocannabivarin.
The information provided in Table 1 is limited and a more comprehensive dataset relating to cannabinoid‐based medications, their therapeutic indication(s), dose regimen, clinical trials, first‐in‐man case studies, and relevant publications can be found using the following web resources available free of charge or through subscription:
http://www.fda.gov/Drugs/default.htm (US Food and Drug Administration)
http://www.ema.europa.eu/ema/ (European Medicines Agency)
http://www.medicines.org.uk/emc/ (U.K. Electronic Medicines Compendium)
https://www.medicinescomplete.com/mc/ (Royal Pharmaceutical Society)
http://apps.who.int/trialsearch/Default.aspx (WHO International Clinical Trials Registry Platform)
https://www.clinicaltrials.gov/ (Clinical trials registry service of U.S. National Institutes of Health)
https://www.clinicaltrialsregister.eu/ctr‐search/search (E.U. Clinical Trials Register)
http://www.ncbi.nlm.nih.gov/pubmed (Reference databases of U.S. National Library of Medicine)
http://www.cochranelibrary.com/ (Cochrane Central Register of Controlled Trials, Wiley Online Library)
http://thomsonreuters.com/cortellis‐clinical‐trials‐intelligence/ (Cortellis‐Thomson Reuters)
http://www.isrctn.com/ (ISRCTN registry ‐ Springer Science)
CB1 receptor agonists
The natural phytocannabinoids in cannabis include the psychotropic Δ9‐THC, Δ8‐THC, along with the nonpsychotropic Δ9‐tetrahydrocannabivarin, (‐)‐cannabidiol (CBD), cannabidivarin, cannabinol, all of which have been tested in humans (Figure 2 a, Table 1). Among these, Δ9‐THC (dronabinol, marinol) is useful as an analgesic, an antiemetic agent, and is approved for treating anorexia associated with weight loss in patients with AIDS. The combination of Δ9‐THC and CBD (sativex, nabiximols) is useful in treating multiple sclerosis‐related spasticity and pain in patients with advanced cancer. More recently, a purified formulation of CBD (Epidiolex) was granted orphan drug designation for treating epilepsy in humans. Pharmacologically, Δ9‐THC behaves as a CB1 partial agonist with moderate affinities for CB1 and CB2. On the other hand, tetrahydrocannabivarin and cannabinol behave as a weak antagonist and a weak agonist for CB1 respectively, whereas showing modest agonist activity for CB2. CBD and cannabidivarin display very low affinities for either CB1 or CB2, and the mechanism through which CBD acts remains elusive. Synthetic analogs of Δ9‐THC, such as n‐hexyl‐Δ6a‐THC, 1,2‐dimethylheptyl‐THC, its hydroxyl analog HU‐210, the nitrogen containing analogs levonantradol and nabitan, as well as the nonclassical analogs of the potent CB1/CB2 agonist CP‐55,940 were initially tested, but none of these ever reached market status (Figure 2 b,c). Nabilone (Cesamet), a potent CB1/CB2 agonist, and Δ9‐THC are both currently approved to treat chemotherapy‐induced nausea and vomiting in humans (Table 1).
The first new distinct chemotype to be introduced in cannabinoid medicinal chemistry by Sterling Winthrop was named aminoalkylindole and included pravadoline and its very potent CB1/CB2 agonist analog WIN 55212‐2, which is being used routinely as a successful pharmacological probe. Later generation aminoalkylindoles include analogs with potent antinociceptive properties, such as AM678 (JWH‐018) and AM2201 (Figure 2 c). Org 28611, an aminoalkylindole analog, was tested in humans both as an analgesic and a sedative (Table 1). However, the illicit use of these compounds and their analogs as “fake cannabis” (“Spice”) has marred their therapeutic value and many of these have been classified as Schedule 1 Controlled Substances.5 Among other classes, BAY 38–7271 (KN 38–7271), a potent CB1/CB2 agonist was found to be clinically efficacious in patients with severe traumatic brain injury (Figure 2 b, Table 1). The metabolically more stable methylated AEA analog (R)‐methanandamide (AM356) is widely used as a substitute for its endogenous counterpart in preclinical research.2
A more recent approach aimed at eliminating the undesirable CB1 central effects was the development of peripherally restricted agonists. Among these, the structurally distinct SAB378 and AZD1940 have undergone evaluation in humans (Figure 2 b, Table 1).
CB2 receptor agonists
An important breakthrough in the cannabinergic therapeutics came with the discovery that CB2 receptors, whose presence is almost entirely peripheral under normal homeostatic conditions, when activated, exhibit antinociceptive and anti‐inflammatory properties without the undesirable CB1 central effects. This opened the door for the development of CB2 selective agonists, the most effective of which early on were the aminoalkylindole AM1241 and the cannabilactone AM1710 (Figure 2 c), both of which produced excellent results when tested in rodent models for neuropathic and inflammatory pain.2 However, the early clinical development of CB2 agonists in industry, which include GW‐842,166x and cannabinor, have met with limited success as peripherally acting analgesic agents. More recently, CB2 agonists S‐777469 and S‐444823 were advanced into late stage trials in humans for treating atopic dermatitis (Table 1). Notably, since the early work using the first cannabinoid positron emission tomography agent [18F]‐Δ8‐THC in nonhuman primates, as agonists, the two radiotracers [11C]‐GW‐842,166X and [11C]‐NE40 have been used to study drug biodistribution and for imaging CB2 receptors in humans (Figure 2 d).
CB1 receptor antagonists
The first CB1 antagonist, SR141716 (rimonabant), a biarylpyrazole with inverse agonist functional properties and capable of blocking “cannabis” effects, was launched in Europe as an effective appetite suppressant for the treatment of obesity. Globally, a multitude of human trials were conducted using rimonabant, most studies involving conditions related to the metabolic syndrome. However, rimonabant was not approved by the US Food and Drug Administration and was later withdrawn from the European market because of certain gastrointestinal, nervous system, and psychiatric disorders that occurred in a small fraction of patients. In parallel, a number of companies developed structurally distinct inverse CB1 antagonists with similar pharmacological profiles.2 These included SR147778 (surinabant), CP‐945,598 (otenabant), BMS‐646256 (ibipinabant), MK‐0364 (taranabant), and AVE1625 (drinabant) (Table 1). However, all of these were shown to exhibit identical undesirable side effect profiles. Nonetheless, several novel CB1 antagonists have been developed as radiotracers for single photon emission computed tomography and positron emission tomography imaging for studying a variety of conditions involving overactivity of the endocannabinoid system. These include the first generation rimonabant analogs [123I]‐AM281, [124I]‐AM281, [125I]‐SD7015, and [11C]‐OMAR, the taranabant analog [18F]‐MK‐9470, the ibipinabant analog [11C]‐SD5024, and the pyrrolidinones [11C]‐MePPEP and [18F]‐FMPEP‐d2 (Figure 2 e).2
Recently, an effort to develop safer CB1 antagonists devoid of or with reduced undesirable side effects produced the first CB1 neutral antagonists having no or low inverse agonist activity as measured via cAMP levels (e.g., AM4113, AM6527, and peripherally restricted AM6545). The therapeutic implications for the new generation CB1 antagonists exhibiting improved safety and favorable therapeutic index profiles in advanced clinical settings are currently being explored.2
Indirectly acting cannabinoid agonists
A major effort was directed toward the development of FAAH inhibitors where the early inhibitors included the sulfonyl fluoride analogs AM374 and AM3506, both of which inhibit the enzyme irreversibly while exhibiting substantial selectivity. Later generation inhibitors, also acting irreversibly, included carbamates (e.g., URB597) and ureas (e.g., PF‐04457845), analogs that produced their effects by carbamylation of the active serine of the enzyme.2 AM3506, when tested in rodents, was shown to be potentially useful for the treatment of posttraumatic stress disorder, whereas PF‐04457845 was tested in humans for alleviating pain with trials currently underway for treating cannabis withdrawal and certain neuropsychiatric conditions, including Tourette's syndrome and posttraumatic stress disorder (Table 1). Positron emission tomography imaging of the enzyme is also being pursued with the irreversible inhibitors [11C]‐CURB and [11C]‐carbonyl‐URB694. [11C]‐MK‐3168 is notably the first reversible FAAH inhibitor to be used in humans for mapping brain FAAH activity (Figure 2 f).
Other approaches aimed at indirectly activating the endocannabinoid system include inhibition of MGL with a number of inhibitors being developed, as well as through inhibition of endocannabinoid transport, where AM404, VDM‐11, and the metabolically stable “reverse” amide AM1172 are among the earliest and best known examples.2
Future directions
In conclusion, CB1, CB2, and FAAH continue to be promising targets for drug development while keeping in mind that it is critical to identify the optimal therapeutic indication(s). CB1 peripheral agonists for analgesia, CB2 agonists, and FAAH inhibitors for conditions associated with inflammation, neurodegeneration, and neuropsychiatric conditions offer great promise. In addition, peripheral CB1 antagonists for metabolic disorders and CB1 neutral antagonists for addiction offer equally good opportunities as well. MGL/FAAH dual inhibitors and the more recent targets, which include the cannabinergic enzymes MGL and ABHD6, hold immense promise. A dark horse under very early development among many is the allosteric CB1 modulator approach that seems to offer unseen benefits over existing therapies.
CONFLICT OF INTEREST
The authors declared no conflict of interest.
References
- 1. Makriyannis, A . & Rapaka, R.S . The medicinal chemistry of cannabinoids: an overview. NIDA Res. Monogr. 79, 204–210 (1987). [PubMed] [Google Scholar]
- 2. Makriyannis, A . 2012 Division of medicinal chemistry award address. Trekking the cannabinoid road: a personal perspective. J. Med. Chem. 57, 3891–3911 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maccarrone, M ., Guzmán, M ., Mackie, K ., Doherty, P . & Harkany, T . Programming of neural cells by (endo)cannabinoids: from physiological rules to emerging therapies. Nat. Rev. Neurosci. 15, 786–801 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thakur, G.A ., Tichkule, R ., Bajaj, S . & Makriyannis, A . Latest advances in cannabinoid receptor agonists. Expert Opin. Ther. Pat. 19, 1647–1673 (2009). [DOI] [PubMed] [Google Scholar]
- 5. Castaneto, M.S ., Gorelick, D.A ., Desrosiers, N.A ., Hartman, R.L ., Pirard, S . & Huestis, M.A . Synthetic cannabinoids: epidemiology, pharmacodynamics, and clinical implications. Drug Alcohol Depend. 144, 12–41 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]


