Abstract
Background
Alcoholic hepatitis (AH) is a major cause of liver-related hospitalization. The profile, treatment patterns, and outcomes of subjects admitted for AH in routine clinical practice are unknown. Also, it is not known whether these are changing over time. This study is thus aimed to identify temporal trends in hospitalization rates, clinical characteristics, treatment patterns, and outcomes of subjects admitted for AH in a routine clinical setting.
Methods
A retrospective analysis of adults admitted for AH from 2000 to 2011 was performed using an anonymized EMR database of patient-level data from 169 U.S. medical centers.
Results
(i) Epidemiology: The proportion of baby boomers admitted for AH increased from 2000 to 2011 (26 to 31%, p < 0.0001). (ii) Clinical: The median Model for End-Stage Liver Disease (MELD) score increased over time from 12 to 14 (p = 0.0014) driven mainly by increased international normalized ratio (1.2 to 1.4, p < 0.0001). The median Charlson Comorbidity Index increased from 0 to 1 (p < 0.0001) with increased diabetes, chronic obstructive pulmonary disease, and heart disease. (iii) Complications: The following increased from 2001 to 2011: Gastrointestinal bleed—7 to 10% (p = 0.03); hepatic encephalopathy—7 to 13% (p < 0.0001); hepatorenal syndrome—1.8 to 2.8% (p = 0.0003); sepsis—0 to 6% (p < 0.0001); and pancreatitis—11 to 16% (p = 0.0061). (iv) Treatment patterns and mortality: Eight to 9% of subjects received steroids while pentoxifylline use increased to 2.2%. In those with MELD ≥ 22, mortality remained between 19 and 20% and only steroids modestly improved survival in this subset.
Conclusions
Severe AH continues to have a high mortality. The severity and comorbidities and complications associated with AH have worsened. Drug therapy remains suboptimal.
Keywords: Alcoholic Hepatitis, Model for End-Stage Liver Disease, Lille Score, Steroids, Prednisolone, Pentoxifylline, Cirrhosis, Alcohol-Induced Cirrhosis, Sepsis, Liver Failure
Alcohol-related liver disease remains a leading cause of liver-related morbidity and mortality in many parts of the world (Liang et al., 2011; Liangpunsakul, 2011; Sandahl et al., 2011). Alcoholic hepatitis (AH) is an important contributor to alcohol-related morbidity and mortality and is a major cause of liver-related admissions to hospitals (Jinjuvadia et al., 2015). It is one of the principal manifestations of alcohol-related liver disease and is classically characterized by jaundice, hepatomegaly, and neutrophilia along with an aspartate transaminase:alanine transaminase ratio of 2 or more (Basra and Anand, 2011). The histological findings of AH include macro- and microvesicular steatosis, cellular ballooning, lobular inflammation along with variable degrees of cholestasis, and pericellular fibrosis which can progress to cirrhosis (Altamirano et al., 2014; Sherlock, 1990). It is now appreciated that the clinical phenotype associated with histological evidence of alcohol-related steatohepatitis can be variable. While many subjects with histological features of alcoholic steatohepatitis are relatively asymptomatic and remain largely in an outpatient setting (Cortez-Pinto et al., 2003), others develop jaundice and can develop severe liver failure and often require hospitalization (Casanova and Bataller, 2014).
The classic descriptions of AH date back to several decades ago. At the time, the Maddrey discriminant index was used to predict clinical outcomes; this index is based on the serum bilirubin and the prothrombin time (Maddrey et al., 1978). The interlaboratory variability in the prothrombin time has led to increasing adoption of the Model for End-Stage Liver Disease (MELD) which relies on the international normalized ratio (INR) rather than the prothrombin time (Dunn et al., 2005; Singal and Shah, 2011; Vaa et al., 2011). Both the Maddrey index and the MELD score have also been used to determine the need for pharmacological treatment with steroids or pentoxifylline.
Since the early descriptions of AH and the response to various treatments, the general population has aged, become more ethnically diverse, obese, and the prevalence of type 2 diabetes and related comorbidities has increased substantially (Forouhi and Wareham, 2014; Greiver et al., 2014). Conceivably, these could modify the clinical phenotype of affected subjects and even impact their response to therapy. It is also not well known whether the rates of hospitalization, the spectrum of severity particularly among hospitalized subjects, duration of hospitalization, and clinical outcomes are changing over time. Similarly, there are no data from “real-world” cohorts of subjects hospitalized with AH regarding treatment patterns and the response to treatment. Such data are needed to guide policy makers regarding resource allocation to tackle the disease and also to provide evidence of how well current therapies perform in everyday practice.
This study is a retrospective analysis of subjects hospitalized with AH from medical centers that use the Cerner® electronic medical record system (Cerner Corporation, Kansas City, MO). The aims of this analysis were to define temporal patterns of rates of hospitalization, race distribution among those admitted with AH, the related comorbidities, clinical features, treatments provided, and the responses to such treatments.
MATERIALS AND METHODS
This was a retrospective cohort study using Cerner Health Facts® data between the years 2000 and 2011. Health Facts® is a HIPAA compliant, de-identified, longitudinal collection of individual level information generated from the Cerner® electronic medical record system which is utilized by both community and academic facilities across the United States. Due to the de-identified nature of the data sets obtained, this study was not considered human subjects research and was considered exempt from review by our University Institutional Review Board.
Population Studied
The entry criteria for this study included those who were admitted with AH to an inpatient facility at any center contributing data to Health Facts®. All admissions to any inpatient unit were included for this analysis. This was done to avoid missing individuals admitted to specific areas given the diversity of triaging admissions across the large number of centers involved. Adult subjects (>21 years of age) with AH were identified by an International Classification of Diseases-9 (ICD-9) code of 571.1 as an admission diagnosis code. It also required demographic, clinical, and laboratory data to be available.
Data Collected
Baseline demographic data along with laboratory data were extracted on each individual. All comorbid conditions present such as type 2 diabetes mellitus and cardiovascular disease were also recorded. Similarly, the presence of concomitant viral hepatitis and cirrhosis was also noted. Cirrhosis was assessed by the ICD-9 codes 571.2 and 571.5. Clinical criteria for cirrhosis were not used for the diagnosis due to the difficulties in separating AH from decompensated cirrhosis by clinical criteria alone. Blood counts, hepatic panel, and INR were used to evaluate the severity of the AH. The medications provided at admission and through the course of hospitalization were also obtained from the Health Facts® database. The use of steroids and their dosage as well as the use of pentoxifylline were noted. Data on clinical outcomes such as mortality, transfer to hospice, need for ICU care, development of ascites and renal dysfunction, hepatic encephalopathy, sepsis, and gastrointestinal bleeding were also obtained from laboratory data and clinical diagnostic codes during the hospitalization or as secondary diagnostic codes at discharge. The ICD-9 codes used to collect these data are provided in Table S1.
Statistical Analysis
All statistical analyses were performed using the Statistical Analysis Software version 9.4 (SAS Inc., Cary, NC). First, data on the admission rates for AH were computed as the proportion of all admissions over time and a Cochran–Armitage test for trend used to determine significance. Patient characteristics, age, sex, race (non-Hispanic Caucasian, non-Hispanic Black, and Other), were obtained and compared between patients with AH and the general population (all the admissions in the year).
Next, the presence of cirrhosis, associated liver disease, for example, viral hepatitis and other comorbidities were quantified, and temporal trends over the study duration were identified. Specific comorbid diseases such as diabetes, asthma/chronic obstructive pulmonary disease, and heart disease, HIV were extracted, and yearly prevalence trends were calculated among those with AH. The presence of concomitant liver diseases of other etiology such as hepatitis C and the presence of underlying cirrhosis were also analyzed. To account for a wider range of comorbid categories (a total of 17), the Charlson Comorbidity Index (CCI) was obtained as described previously (Deyo et al., 1992). Each comorbidity category has an associated weight based on the adjusted risk of 1-year mortality. A total score of 0 indicates no comorbidities were found.
The severity of the AH was estimated using the MELD score which was calculated as follows (Malinchoc et al., 2000):
Those with severe AH were defined as having a MELD score of ≥ 22. This measure predicts 30-day mortality with a sensitivity of 75% and specificity of 75% with diagnostic accuracy (c-statistic 0.83) comparable to the Maddrey’s Discriminant Function (Dunn et al., 2005). The individual values and MELD scores were log transformed and then assessed for linear trend by year using Spearman’s correlation coefficient.
The main outcome measure was in-hospital mortality. A logistic regression model was constructed to determine the predictors of in-hospital mortality among AH patients, after controlling for calendar year, patient demographics, complications of chronic liver disease and AH (cirrhosis, hepatic encephalopathy, sepsis, gastrointestinal bleeding, and acute pancreatitis), and pharmaceutical interventions with either pentoxifylline or corticosteroids. The results of logistic regression were reported as odds ratios (ORs) with a 95% confidence interval (CI). The prevalence rates of other complications and their temporal trends were quantified as described above for the prevalence of AH admissions.
RESULTS
Over the course of the study analysis (2000 to 2011), data from a total of 169 medical centers including both academic- and community-based tertiary care facilities and smaller community hospitals were obtained. The hospitals were distributed throughout the United States but were mostly in the northeastern part of the country. During this time, there were 8.1 million admissions that involved 6.1 million individuals. Of note, there were only 49 hospitals that provided data to Health Facts® in 2000 of which only 12 had admissions related to AH. By 2011, the total number of participating centers had increased to 169 of which 54 reported admissions due to AH.
Trends for Hospitalizations for Alcoholic Hepatitis
In 2000, a total of 119 admissions involving 111 unique subjects were identified in 12 of the 49 participating centers (Table 1). This accounted for 0.06% of all hospitalizations and 0.08% of all individuals admitted to these hospitals. The rates of hospitalizations as a proportion of total hospitalizations increased initially to 0.12% and then remained around 0.09% until 2008 after which it trended down to about 0.06%. There were no significant differences in rates of hospitalizations in centers from various regions of the United States. Similarly, there were no significant differences between academic and community medical centers after adjusting for the total volumes of admissions at these centers.
Table 1.
Hospitalization Rates for Alcoholic Hepatitis (AH)
| Year | General population | AH population | % of all admits |
% of all patients |
MELDa | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of hospitals |
Number of admits |
Number of unique patients |
Number of hospitals |
Number of admits |
Number of unique patients |
Median | % ≥22 |
|||
| 2000 | 49 | 190,160 | 142,717 | 12 | 119 | 111 | 0.06 | 0.08 | 12 | 22.1 |
| 2001 | 69 | 309,695 | 230,999 | 18 | 384 | 363 | 0.12 | 0.16 | 13 | 16.1 |
| 2002 | 74 | 323,235 | 242,715 | 22 | 374 | 335 | 0.12 | 0.14 | 12 | 13.5 |
| 2003 | 95 | 308,864 | 243,182 | 27 | 272 | 252 | 0.09 | 0.10 | 14 | 15.5 |
| 2004 | 92 | 324,409 | 265,929 | 25 | 307 | 292 | 0.09 | 0.11 | 13 | 17.5 |
| 2005 | 92 | 356,894 | 292,797 | 34 | 407 | 382 | 0.11 | 0.13 | 13 | 20.3 |
| 2006 | 97 | 476,557 | 387,629 | 39 | 430 | 401 | 0.09 | 0.10 | 13 | 16.9 |
| 2007 | 99 | 619,741 | 491,685 | 39 | 435 | 403 | 0.07 | 0.08 | 13 | 23.9 |
| 2008 | 130 | 799,312 | 621,596 | 52 | 691 | 631 | 0.09 | 0.10 | 16 | 29.2 |
| 2009 | 142 | 1,511,595 | 1,228,355 | 56 | 916 | 889 | 0.06 | 0.07 | 14 | 23.3 |
| 2010 | 151 | 1,511,058 | 1,275,312 | 61 | 994 | 952 | 0.07 | 0.07 | 14 | 24.9 |
| 2011 | 169 | 1,398,678 | 1,204,605 | 54 | 784 | 734 | 0.06 | 0.06 | 14 | 18.1 |
| Total | 211 | 8,130,198 | 6,184,108 | 111 | 6,113 | 5,603 | 0.08 | 0.09 | 14 | 21.4 |
Information to calculate Model for End-Stage Liver Disease (MELD) was available for 3,425 patients.
Demographic Trends Among Those Admitted with Alcoholic Hepatitis
Among those with AH, the mean age (48 ± 10 years) remained unchanged; however, the proportion of those ages 40 to 49 years decreased (46 to 36%) while those aged 50 to 59 years increased (26 to 31%) (p < 0.0001 for both) (Table 2). Baby boomers born between 1945 and 1965 accounted for 28 to 35% of all admissions but almost 58 to 77% of AH admissions (chi-square p < 0.0001). Compared to other generations admitted in the year 2011, those born between 1945 and 1965, that is, baby boomers (OR: 5.5, CI: 4.5, 6.7) and those born between 1966 and 1976 (OR: 7.1, CI: 5.7, 8.9) were more likely to be admitted with a diagnosis of AH. The proportions of males being admitted remained stable over the study period (39.5 to 40.6%) but accounted for 66 to 75% of AH-related admissions. There were no significant trends with respect to gender among admissions for AH over time. Non-Hispanic Blacks accounted for 10 to 18% of all admissions but only 6 to 17% of AH-related admissions (chi-square p < 0.0001).
Table 2.
Trends in Demographic Characteristics of All Hospitalized Subjects and Those Admitted with Alcoholic Hepatitis (AH)
| Year | Age (years) | Generation | Gender % Male |
Ethnicity | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | % Aged 40 to 49 years |
% Aged 50 to 59 years |
% Born 1945 to 1965 |
% Born 1966 to 1976 |
% Non-Hispanic White |
% Non-Hispanic Blacks |
||
| General population | |||||||||
| 2000 | 57.3 | 19.6 | 13.3 | 14.5 | 28.5 | 13.4 | 40.0 | 80.68 | 16.15 |
| 2001 | 57.8 | 19.6 | 13.3 | 14.7 | 28.4 | 13.1 | 40.3 | 83.03 | 13.64 |
| 2002 | 58.5 | 19.7 | 13.2 | 14.3 | 28.2 | 12.9 | 39.8 | 84.71 | 10.82 |
| 2003 | 59.2 | 19.7 | 12.6 | 14.3 | 27.7 | 12.2 | 39.5 | 84.19 | 10.44 |
| 2004 | 58.7 | 19.7 | 13.3 | 14.8 | 29.2 | 12.1 | 40.0 | 83.64 | 10.85 |
| 2005 | 59.3 | 19.6 | 13.0 | 15.1 | 29.5 | 11.6 | 40.3 | 84.34 | 11.43 |
| 2006 | 58.8 | 19.8 | 13.3 | 15.2 | 30.2 | 12.1 | 40.5 | 77.92 | 15.62 |
| 2007 | 58.2 | 19.9 | 13.2 | 15.4 | 30.8 | 12.6 | 40.5 | 75.96 | 16.26 |
| 2008 | 58.0 | 19.8 | 12.8 | 15.7 | 31.7 | 12.3 | 40.2 | 74.02 | 18.12 |
| 2009 | 57.3 | 19.5 | 13.2 | 16.7 | 34.0 | 12.3 | 40.2 | 75.64 | 17.67 |
| 2010 | 57.7 | 19.5 | 12.8 | 16.9 | 34.4 | 12.1 | 40.5 | 75.22 | 18.38 |
| 2011 | 57.7 | 19.5 | 12.4 | 17.0 | 35.0 | 12.0 | 40.6 | 74.98 | 18.70 |
| AH population | |||||||||
| 2000 | 48.8 | 9.7 | 46.2 | 26.1 | 77.3 | 3.4 | 74.8 | 86.6 | 8.4 |
| 2001 | 47.3 | 10.1 | 42.7 | 22.7 | 71.4 | 9.9 | 66.9 | 83.6 | 13.3 |
| 2002 | 46.8 | 9.9 | 42.0 | 22.7 | 71.1 | 12.3 | 70.3 | 84.0 | 9.4 |
| 2003 | 48.8 | 9.8 | 41.2 | 24.3 | 71.3 | 10.3 | 69.5 | 82.4 | 8.1 |
| 2004 | 48.3 | 11.2 | 36.5 | 26.1 | 65.8 | 16.3 | 66.5 | 84.7 | 9.1 |
| 2005 | 48.7 | 10.9 | 36.4 | 29.5 | 67.6 | 16.7 | 68.8 | 81.8 | 10.6 |
| 2006 | 48.6 | 10.3 | 34.7 | 34.0 | 69.3 | 17.0 | 70.0 | 82.1 | 14.7 |
| 2007 | 49.6 | 10.8 | 36.1 | 31.7 | 66.9 | 17.2 | 68.1 | 78.2 | 16.3 |
| 2008 | 48.6 | 10.6 | 36.3 | 32.6 | 64.7 | 23.4 | 66.9 | 82.5 | 14.9 |
| 2009 | 48.5 | 10.7 | 35.3 | 32.4 | 64.3 | 20.7 | 66.5 | 78.5 | 16.6 |
| 2010 | 49.4 | 10.6 | 32.4 | 35.9 | 64.6 | 22.2 | 73.7 | 83.6 | 11.7 |
| 2011 | 48.6 | 11.0 | 36.4 | 30.5 | 58.3 | 25.6 | 67.0 | 86.0 | 6.6 |
Worsening Comorbidity Profile Among Those Admitted for Alcoholic Hepatitis
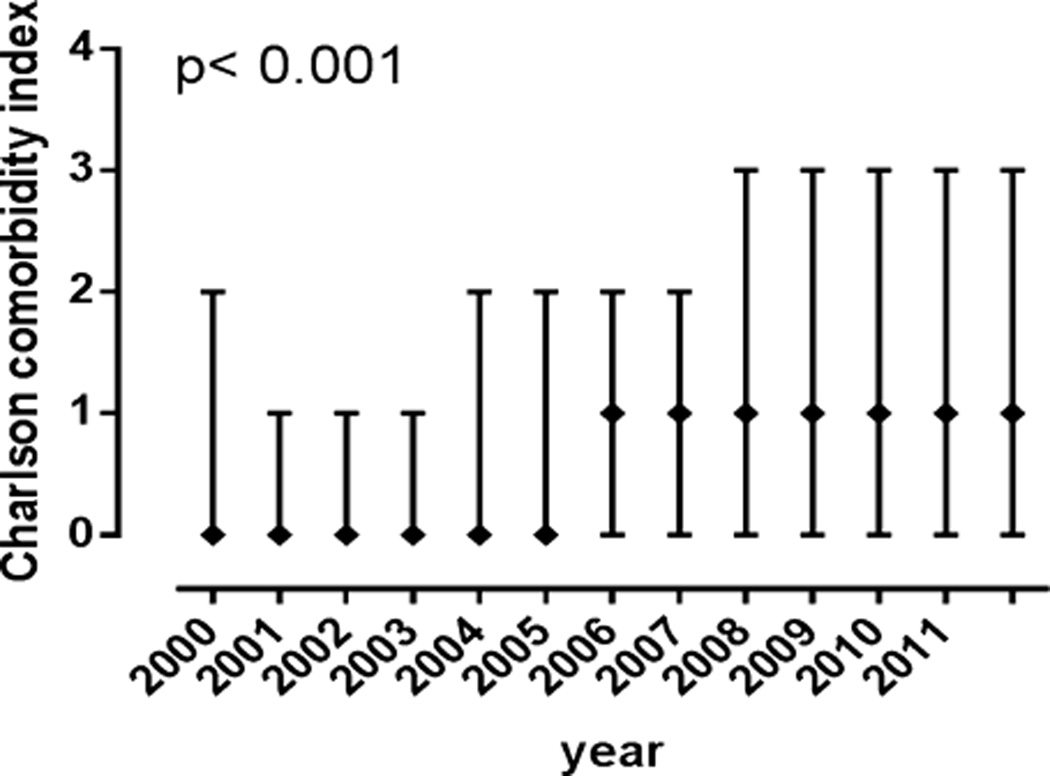
The proportion of subjects with AH who also had type 2 diabetes increased substantially from 4.2% in 2000 to 11.7% in 2011 (p < 0.0001) (Table 3). This was accompanied by a similar and highly significant increase in heart (mainly coronary artery disease) and lung diseases (mainly chronic obstructive pulmonary disease) (p < 0.0001 for both). On the other hand, the proportion of subjects with concomitant HIV infection declined from 1.68% to 0.89% over the same duration. This did not, however, reach statistical significance. Viral hepatitis was overrepresented among those with AH (HCV: 6 to 12%, HBV: 1 to 4%) and was relatively stable over time (Cochran, HCV p = 0.31, HBV p = 0.06). The diagnosis of cirrhosis increased significantly between 2000 (28%) to 2011 (32%) (p < 0.0001). These changes reflected general patterns across the spectrum of all hospitalizations. There were no significant regional differences or differences among University versus community centers. The overall CCI increased over time (Fig. 1).
Table 3.
Patient Comorbidity and Laboratory Data in Those with Alcoholic Hepatitis
| Year | Diabetes (%) | Lung disease (%) | Heart disease (%) | HIV (%) | CCI Med |
AST Med |
AST Med |
Bili Med |
Cr Med |
INR Med |
HCV (%) | HBV (%) | Cirrhosis (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2000 | 4.20 | 0.84 | 2.52 | 1.68 | 0 | 144 | 63 | 2.0 | 0.8 | 1.2 | 8.4 | 2.5 | 27.73 |
| 2001 | 8.59 | 5.73 | 5.21 | 1.04 | 0 | 142 | 66 | 1.7 | 0.8 | 1.2 | 6.5 | 0.8 | 20.05 |
| 2002 | 7.75 | 6.95 | 6.68 | 0.00 | 0 | 130 | 74 | 1.5 | 0.8 | 1.2 | 12.0 | 0.5 | 18.72 |
| 2003 | 11.03 | 5.51 | 8.09 | 1.47 | 0 | 152 | 72 | 2.2 | 0.9 | 1.3 | 6.3 | 2.6 | 22.06 |
| 2004 | 7.17 | 5.54 | 5.21 | 0.33 | 0 | 166 | 74 | 1.7 | 0.8 | 1.3 | 10.1 | 3.6 | 25.08 |
| 2005 | 8.60 | 8.11 | 8.35 | 0.49 | 1 | 147 | 73 | 2.1 | 0.8 | 1.2 | 8.9 | 2.7 | 25.55 |
| 2006 | 7.67 | 8.14 | 7.44 | 1.16 | 1 | 144 | 68 | 2.0 | 0.8 | 1.2 | 7.7 | 2.8 | 27.91 |
| 2007 | 8.51 | 9.66 | 9.66 | 1.15 | 1 | 139 | 68 | 2.0 | 0.8 | 1.3 | 10.8 | 0.9 | 31.95 |
| 2008 | 11.29 | 10.13 | 10.42 | 0.58 | 1 | 132 | 67 | 2.2 | 0.8 | 1.3 | 9.4 | 2.6 | 33.86 |
| 2009 | 9.72 | 9.61 | 10.92 | 0.98 | 1 | 137 | 67 | 2.1 | 0.8 | 1.3 | 9.8 | 1.4 | 29.59 |
| 2010 | 11.37 | 9.36 | 13.08 | 0.60 | 1 | 140 | 69 | 2.0 | 0.9 | 1.3 | 9.0 | 0.9 | 29.88 |
| 2011 | 11.73 | 9.06 | 9.44 | 0.89 | 1 | 144 | 73 | 2.0 | 0.8 | 1.3 | 9.8 | 0.6 | 31.51 |
ALT, alanine transaminase; AST, aspartate transaminase; Bili, bilirubin; CCI, Charleson Comorbidity Index; Cr, creatinine; HBV, hepatitis B virus; HCV, hepatitis C virus; INR, international normalized ratio; Med, median.
Fig. 1.
The median Charlson Comorbidity Index with upper and lower quartiles from 2000 to 2011. The median increased significantly (p < 0.001) from 2000 to 2011.
Increasing Severity of Alcoholic Hepatitis Over Time Among Hospitalized Subjects
The median MELD score increased from 12 to 14 over time (Spearman’s p = 0.0014). This was driven by a modest increase in INR (1.2 to 1.4, Spearman’s p < 0.0001) while median bilirubin and creatinine remained stable around 2 and 0.8 mg/dl, respectively. After an initial drop in the proportion of subjects with severe AH from 22 to 13% from 2000 to 2002, there was an increase in such cases to 18 to 23%over time (Cochrane p < 0.0006).
Treatment Patterns for Alcoholic Hepatitis Over Time
Steroid utilization remained stable at about 10% among all cases of AH (Cochrane p = 0.41) while the use of pentoxifylline increased from 0% in 2000 to 3% in 2011 (Cochrane p = 0.0035). Among those with severe AH (MELD ≥ 22), the proportion of patients who received corticosteroids remained stable averaging 28% (Cochran p = 0.6786). In this population, the use of pentoxifylline increased significantly from 0% in 2000 to 17% in 2011 (Cochran p = 0.0001).
Outcomes and the Impact of Treatment
Mortality
AH was associated with a significantly higher mortality compared to all admission diagnoses. The rate of in-hospital mortality stayed between 1 and 2% for all admissions from 2000 to 2011. On the other hand, of the 6,113 AH-related admissions, there were 267 (4.37%) deaths. In 2000, 4.2% of AH admissions resulted in death. This rate decreased to 1.6% in 2002, and then increased back up to 4 to 5% between 2007 and 2011 (Cochran p = 0.0361).
Among those that died, the mean age was 52.6 (SD 10) years and there was no significant change in average age of death with each increasing year of the study period (Spearmans’s p = 0.25). Of those that died, 70% were males, and 87%were non-Hispanic Whites.
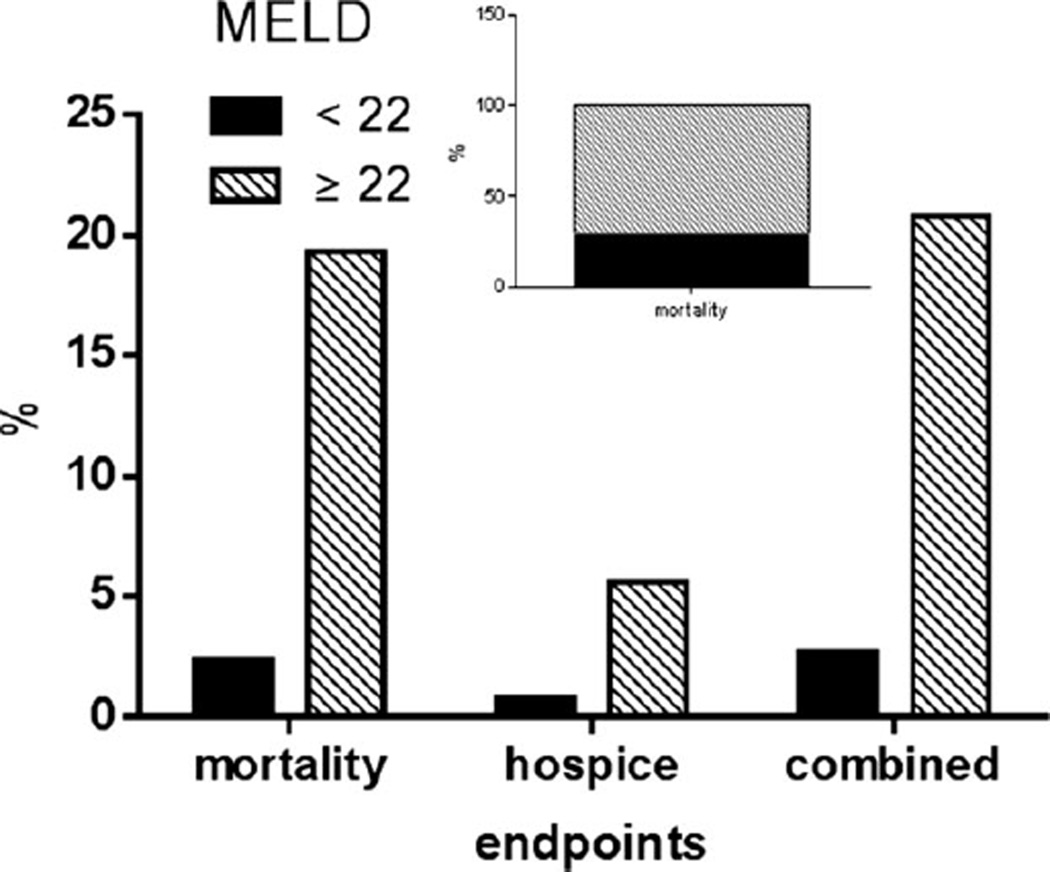
The MELD score was closely linked to 30-day mortality and inpatient mortality. Whereas 2.3% of those with a MELD score < 22 died, 19.3% of those with a MELD ≥ 22 died during the first hospitalization (p < 0.0001) (Fig. 2). Similarly, 0.7 and 5.6% of those below and above these MELD thresholds, respectively, were sent to hospice for terminal care, and the overall composite endpoints of death or transfer to hospice for terminal care in these groups were 2.6 and 20.8%, respectively.
Fig. 2.
Mortality in hospitalized subjects with alcoholic hepatitis: 2.3% of those with a Model for End-Stage Liver Disease (MELD) score < 22 died, while 19.3% of those with a MELD ≥ 22 died during the first hospitalization (p < 0.0001). Also, 0.7 and 5.6% of those below and above these MELD thresholds, respectively, were sent to hospice for terminal care, and the overall composite endpoints of death or transfer to hospice for terminal care in these groups were 2.6 and 20.8%, respectively. Of all deaths (inset), 71.2% occurred in those with MELD ≥ 22 whereas 29.8% occurred in those with lower MELD scores.
Surprisingly, among all those that died with available admission MELD data (n = 191), only 71.2% had severe AH with a MELD ≥ 22 (Fig. 2). Most subjects (81%) had 3 or more organ system dysfunction at the time of death. Infections (bacteremia and pneumonias) and sepsis (positive blood cultures) were the most common associated conditions related to mortality even in those who initially had MELD scores < 22.
Three separate models were developed to evaluate the factors associated with mortality (Table 4). In the first, only demographic parameters and associated comorbidities were included. Using stepwise multiple logistic regression analysis, the CCI and increasing age were related to mortality. Non-Hispanic Blacks had 62%lower risk for in-hospital mortality (OR: 0.38, CI: 0.22 to 0.66) compared to non-Hispanic Whites. Patients with concomitant viral hepatitis C (4.8 vs. 4.3%) or cirrhosis (9.4 vs. 2.4%) had higher mortality rates, but this did not reach statistical significance.
Table 4.
Multilevel Multivariate Logistic Regression Analyses to Determine Predictors of In-Hospital Mortality from 2000 to 2011
| Variables | Total N |
Died N |
Died % |
Model 1 | Model 2 | Model 2 | |||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | ||||
| Calendar year | 6,113 | 267 | 4.4 | 0.9 | 0.9 to 1.03 | 0.94 | 0.90 to 0.99 | 0.94 | 0.90 to 0.99 |
| CCI | |||||||||
| 0 | 2,797 | 27 | 1.0 | – | – | – | – | – | – |
| 1 | 1,372 | 36 | 2.6 | 2.49 | 1.50 to 4.14 | 2.07 | 1.22 to 3.50 | 2.05 | 1.21 to 3.48 |
| 2 | 360 | 14 | 3.9 | 3.40 | 1.75 to 6.60 | 2.70 | 1.34 to 5.43 | 2.67 | 1.32 to 5.39 |
| ≥3 | 1,584 | 190 | 12.0 | 13.15 | 8.70 to 19.89 | 4.98 | 2.97 to 8.33 | 4.92 | 2.93 to 8.26 |
| Age (years) | |||||||||
| <40 | 1,140 | 28 | 2.5 | – | – | – | – | – | – |
| 40 to 49 | 2,235 | 74 | 3.3 | 1.24 | 0.79 to 1.94 | 1.28 | 0.79 to 2.07 | 1.28 | 0.79 to 2.09 |
| 50 to 59 | 1,871 | 98 | 5.2 | 1.75 | 1.12 to 2.72 | 1.83 | 1.14 to 2.95 | 1.84 | 1.14 to 2.97 |
| ≥60 | 867 | 67 | 7.7 | 2.86 | 1.79 to 4.58 | 3.16 | 1.91 to 5.24 | 3.18 | 1.92 to 5.30 |
| Sex | |||||||||
| Male | 4,208 | 188 | 4.5 | – | – | – | – | – | – |
| Female | 1,905 | 79 | 4.1 | 0.91 | 0.69 to 1.20 | 0.92 | 0.68 to 1.24 | 0.91 | 0.68 to 1.23 |
| Ethnicity | |||||||||
| Non-Hispanic White | 5,042 | 232 | 4.6 | – | – | – | – | – | – |
| Non-Hispanic Blacks | 746 | 14 | 1.9 | 0.38 | 0.22 to 0.66 | 0.35 | 0.19 to 0.63 | 0.35 | 0.20 to 0.64 |
| Other | 325 | 21 | 6.5 | 1.58 | 0.97 to 2.56 | 1.49 | 0.88 to 2.52 | 1.49 | 0.88 to 2.51 |
| GI bleeding | |||||||||
| Negative | 5,549 | 201 | 3.6 | – | – | – | – | – | – |
| Present | 564 | 66 | 11.7 | 1.85 | 1.31 to 2.62 | 1.85 | 1.30 to 2.62 | ||
| Encephalopathy | |||||||||
| Negative | 5,460 | 168 | 3.1 | – | – | – | – | – | – |
| Present | 653 | 99 | 15.2 | 1.54 | 1.09 to 2.18 | 1.54 | 1.09 to 2.18 | ||
| HRS | |||||||||
| Negative | 5,960 | 212 | 3.6 | – | – | – | – | – | – |
| Present | 153 | 55 | 35.9 | 5.11 | 3.36 to 7.75 | 5.05 | 3.32 to 7.70 | ||
| Pancreatitis | |||||||||
| Negative | 5,206 | 245 | 4.7 | – | – | – | – | – | – |
| Positive | 907 | 22 | 2.4 | 0.96 | 0.59 to 1.56 | 0.96 | 0.59 to 1.56 | ||
| Sepsis | |||||||||
| Negative | 5,925 | 200 | 3.4 | – | – | – | – | – | – |
| Positive | 188 | 67 | 35.6 | 9.07 | 6.14 to 13.38 | 9.02 | 6.11 to 13.34 | ||
| HCV | |||||||||
| Negative | 5,548 | 240 | 4.3 | – | – | – | – | – | – |
| Positive | 565 | 27 | 4.8 | 0.76 | 0.48 to 1.21 | 0.76 | 0.48 to 1.21 | ||
| Hepatocellular carcinoma | |||||||||
| Negative | 6,077 | 257 | 4.2 | – | – | – | – | – | – |
| Positive | 36 | 10 | 27.8 | 3.92 | 1.63 to 9.42 | 3.95 | 1.64 to 9.48 | ||
| Cirrhosis | |||||||||
| Negative | 4,384 | 105 | 2.4 | – | – | – | – | – | – |
| Positive | 1,729 | 162 | 9.4 | 1.35 | 0.99 to 1.85 | 1.35 | 0.99 to 1.85 | ||
| Medication use | |||||||||
| Negative | 5,411 | 206 | 3.8 | – | – | – | – | – | – |
| Pentoxifylline | 539 | 41 | 7.6 | 1.16 | 0.56 to 2.40 | ||||
| Corticosteroid | 103 | 12 | 11.7 | 1.06 | 0.71 to 1.56 | ||||
| Both | 60 | 8 | 13.3 | 1.03 | 0.39 to 2.68 | ||||
Charleson Comorbidity Index; GI, gastrointestinal; HCV, hepatitis C virus; HRS, hepatorenal syndrome.
When the concomitant presence or development of additional cirrhosis-related complications such as ascites and encephalopathy were included in the model (model 2), these were not found to significantly affect mortality which remained tightly linked to the MELD score.
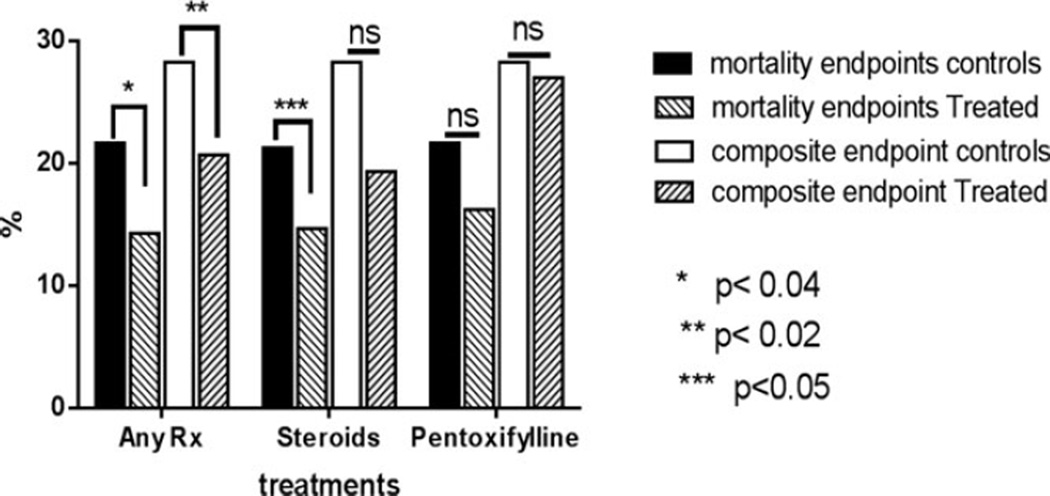
Finally, when treatments provided were included in the model (Model 3), neither steroids nor pentoxifylline significantly impacted mortality in the entire cohort of 5,603 subjects involved in 6,113 admissions after accounting for age and other comorbidities. To further refine the model, only subjects who had a MELD ≥ 22 and who also had an established indication for pharmacological intervention were analyzed (Fig. 3). In this group of subjects, when any pharmacological treatment was compared to no treatment, drug therapy improved 30-day mortality (14.3 vs. 21.6%, p < 0.02) and a composite end point including both mortality and end-of-life hospice care (20.7 vs. 28.3%, p < 0.04). This was largely driven by steroid therapy which decreased mortality (14.7 vs. 21.6%, p < 0.05) but only produced a trend for improvement of the 30 day composite end point (19.3 vs. 28.3%, p = n.s.). In contrast, pentoxifylline had minimal impact on either mortality (16.2 vs. 21.6%, p = n.s.) or the 30-day composite end point (27 vs. 28.3%).
Fig. 3.
Effectiveness of drug therapy in routine clinical practice: Mortality and a composite end point including in-hospital mortality and transfer to hospice are shown for subjects with alcoholic hepatitis and Model for End-Stage Liver Disease (MELD) ≥ 22. Those receiving any drug therapy had a significantly improved in-hospital mortality and the composite end point compared to MELD-matched controls who did not receive these drugs. Steroids improved mortality but only produced a trend toward improved composite end point. On the other hand, pentoxifylline was not associated with either significantly improved in-hospital mortality or the composite end point.
Of those who were treated with steroids, 59.6%of subjects had a Lille score ≥ 0.45 at day 7 (Louvet et al., 2007). These individuals had a 27.8%30-day mortality compared to 2.7% in those with a Lille score < 0.45. Those with a Lille score > 0.45 at day 7 also had a higher rate of death or discharge to hospice (29.6 vs. 10.8%, p < 0.01) and hepatorenal syndrome (25.9 vs. 8.1%, p < 0.03). The Lille score did not predict outcomes in those receiving pentoxifylline therapy.
Other Clinical Outcomes
The incidence of complications in those admitted with AH also increased from 2001 to 2011 (Table 5). Specifically, the rates of gastrointestinal bleeding increased from 7 to 10% (p < 0.03). Hepatic encephalopathy rates increased from 7 to 13% (p < 0.0001) while sepsis increased from 0.0 to 6% (p < 0.0001) and hepatorenal syndrome increased from 1.8 to 2.8% (p < 0.0003). Of note, acute flares of pancreatitis increased from 11 to 16% (p < 0.006).
Table 5.
Percentage of Subjects with Alcoholic Hepatitis-Related Complications
| Year | GI bleed | Hepatic encephalopathy |
Hepatorenal syndrome |
Sepsis | Pancreatitis |
|---|---|---|---|---|---|
| 2000 | 10.9 | 13.5 | 1.7 | 0.0 | 16.0 |
| 2001 | 6.8 | 7.0 | 1.8 | 0.0 | 10.7 |
| 2002 | 8.6 | 4.8 | 0.8 | 0.0 | 11.0 |
| 2003 | 7.7 | 6.6 | 0.7 | 0.0 | 15.8 |
| 2004 | 9.5 | 8.5 | 2.9 | 0.7 | 15.0 |
| 2005 | 9.6 | 9.1 | 2.7 | 1.7 | 11.3 |
| 2006 | 6.5 | 10.7 | 0.7 | 1.9 | 15.4 |
| 2007 | 8.5 | 10.3 | 1.4 | 3.0 | 15.9 |
| 2008 | 10.4 | 12.6 | 2.8 | 3.3 | 16.5 |
| 2009 | 8.2 | 10.6 | 3.5 | 4.6 | 16.1 |
| 2010 | 11.5 | 13.3 | 3.7 | 4.7 | 15.4 |
| 2011 | 10.0 | 13.3 | 2.8 | 5.9 | 15.6 |
GI, gastrointestinal.
Compared to those with an admission MELD score < 22, those with an admission MELD score ≥ 22 had a higher incidence of gastrointestinal bleeding (14.9 vs. 10.6%, p < 0.002), encephalopathy (32.2 vs. 8.8%, p < 0.0001), sepsis (10.9 vs. 2.1%, p < 0.0001), and hepatorenal syndrome (14.4 vs. 0.16%, p < 0.0001). On the other hand, concomitant acute flare of alcohol-related pancreatitis was more common in those with low MELD scores (14.6 vs. 9.1%, MELD < 22 vs. ≥ 22, p < 0.0003).
DISCUSSION
Data-mining efforts using electronic medical records represent an important method to evaluate diseases in a “realworld” clinical care setting. It can provide information about disease presentation, spectrum of comorbidities, and information about “effectiveness” of therapeutic interventions which provide a more relevant picture of the health burden due to specific diseases compared to classic descriptions of limited cohorts in 1 or a few academic centers or even in a tightly controlled randomized controlled trial setting (Raghupathi and Raghupathi, 2014). The current study provides novel information about such trends in AH-related admissions in the United States from 169 medical centers in geographically diverse regions and representing the full spectrum of medical centers from small community hospitals to large university tertiary care hospitals.
Probably, the most important finding of this analysis is that severe AH continues to be associated with an unacceptably high mortality. While considerable advances in management have resulted in a marked improvement in-hospital mortality for major “killers” such as acute myocardial infarction to around 5% or less (Mehta et al., 2005; Rosamond et al., 2012; Simms et al., 2015), there has been little progress in the management of severe AH which continues to have an approximately 20% in-hospital mortality. These data underscore the need for a substantial investment of research resources to improve AH-related outcomes.
Another important finding is that almost 30% of those who died had a MELD score less than 22. Historically, the use of a Maddrey Discriminant Index of 32 to enter subjects into clinical trials of steroids for AH has led to a widespread use of this threshold or a MELD value to 22 to recommend pharmacological therapy for this condition. The ongoing mortality in those with lower MELD score indicates that these subjects should not be ignored and further research testing approaches to improve the outcomes of such patients is warranted. The current National Institute on Alcohol Abuse and Alcoholism funded trial of obeticholic acid in this population (NCT # 02039219) is thus a step in the right direction.
Infections and sepsis also emerge as major contributors to mortality in this “real-life” setting and are in line with data related to cirrhosis and cirrhosis-related mortality in recent years (Bajaj et al., 2012; Jalan et al., 2014). Further studies are now needed to identify the bacteriology and spectrum of such infections in more focused prospective studies. These will inform future approaches to reduce the risks of infection and infection-related mortality in this population. Potential approaches to improve intestinal barrier function and reduce bacterial translocation as well as the risks of hospital acquired pneumonia in this population are attractive targets for clinical trials.
This study further demonstrates that while the overall prevalence of AH-related admissions remains around 0.06 to 0.08% of all admissions, the subjects being admitted are older and have more comorbidities that are both linked to outcomes. These admission rates are at variance with a recent report from the Agency for Healthcare Research and Quality (Liangpunsakul, 2011). A potential explanation is that not all hospitals reported admissions for AH which probably reflects the nature of services provided there. However, as all admissions were counted, they increased the denominator without contributing to the numerator, that is, admissions due to AH. The increasing proportion of subjects with type 2 diabetes is particularly of concern as their glycemic control is likely to be adversely impacted by steroid therapy. Furthermore, a combination of diabetes and steroids is expected to enhance the risks of sepsis a major contributor to mortality in this population.
An increasing proportion of individuals over time with established cirrhosis were also identified. Cirrhosis was identified from ICD-9 codes which present the potential for error due to miscoding. It is furthermore difficult to clinically distinguish AH from decompensated alcoholic cirrhosis with sepsis without a liver biopsy in many instances. The current study indicates that liver biopsies are generally not performed in U.S. centers for this indication, and thus, histology could not be used to determine the true proportions of those with cirrhosis. Therefore, the data regarding the prevalence of cirrhosis should be considered “soft” and the lack of histology data remains a limitation of this analysis.
It is also disturbing to note the relatively modest impact of drug therapy for severe AH. Although one may argue that the data on treatment outcomes were not obtained in a randomized clinical trial setting and thus irrelevant, they represent outcomes in a “real-world” setting and a measure of the effectiveness of the therapy as opposed to their efficacy. When the entire cohort was evaluated, neither steroids nor pentoxifylline was associated with improved outcomes. Steroids did, however, demonstrate modest efficacy in this “real-life” nonrandomized setting for those with severe AH (MELD ≥ 22) while pentoxifylline did not demonstrate any signal for improvement. Taken together with a large randomized clinical trial demonstrating a lack of efficacy of pentoxifylline as a stand-alone treatment as well in combination with steroids (Mathurin et al., 2013; Thursz et al., 2015), these data provide a strong rationale for discarding pentoxifylline from the therapeutic armamentarium for AH and investment of resources to develop additional therapies for AH.
Electronic medical record-based data-mining approaches are not without pitfalls. The data are not controlled or randomized. Also, given the median levels of bilirubin and MELD scores, at least half the subjects had only mild AH. Thus, the potential for hidden biases that could account for the lack of efficacy of drug therapies exists. The data also reflect clinical practice and laboratory tests are not obtained systematically at predetermined time points to allow rigorous comparisons of various subgroups at specific points in the course of the disease. Recently, using criteria available in routine EMRs hospital-based outcomes models have been developed for acute myocardial infarction (McNamara et al., 2015). Similar models need to be developed for AH to allow better analysis of outcomes in a “real-life” setting and also to compare the impact of interventions where it actually matters, that is, in routine clinical settings.
In summary, the current study indicates that severe AH continues to have mortality rates that are substantially higher than general inpatient mortality rates and those reported for major illnesses such as acute myocardial infarction. It further indicates that the population of subjects with AH is getting older and has more comorbidities which adversely affect their outcomes. The incidence of complications of end-stage liver disease in those admitted with AH is also rising, and almost a third of all deaths in this population occurs in those with moderate AH. Steroids demonstrate modest effectiveness in reducing 30-day mortality with borderline significance while pentoxifylline is ineffective. It is hoped that these data will stimulate further studies to reduce the burden of AH.
Supplementary Material
Acknowledgments
GRANT SUPPORT
T32 DK 007150-38, UL1000058, UO1 AA 029891, K23 AA021179.
Footnotes
CONFLICT OF INTERESTS
The authors declare that they have no conflict of interests.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article:
Table S1. ICD-9 utilized for patient classification.
REFERENCES
- Altamirano J, Miquel R, Katoonizadeh A, Abraldes JG, Duarte-Rojo A, Louvet A, Augustin S, Mookerjee RP, Michelena J, Smyrk TC, Buob D, Leteurtre E, Rincon D, Ruiz P, Garcia-Pagan JC, Guerrero-Marquez C, Jones PD, Barritt AST, Arroyo V, Bruguera M, Banares R, Gines P, Caballeria J, Roskams T, Nevens F, Jalan R, Mathurin P, Shah VH, Bataller R. A histologic scoring system for prognosis of patients with alcoholic hepatitis. Gastroenterology. 2014;146:1231.e6–1239.e1. doi: 10.1053/j.gastro.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj JS, O’Leary JG, Reddy KR, Wong F, Olson JC, Subramanian RM, Brown G, Noble NA, Thacker LR, Kamath PS, Nacseld Second infections independently increase mortality in hospitalized patients with cirrhosis: the North American consortium for the study of end-stage liver disease (NACSELD) experience. Hepatology. 2012;56:2328–2335. doi: 10.1002/hep.25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basra S, Anand BS. Definition, epidemiology and magnitude of alcoholic hepatitis. World J Hepatol. 2011;3:108–113. doi: 10.4254/wjh.v3.i5.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova J, Bataller R. Alcoholic hepatitis: prognosis and treatment. Gastroenterol Hepatol. 2014;37:262–268. doi: 10.1016/j.gastrohep.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Cortez-Pinto H, Baptista A, Camilo ME, De Moura MC. Nonalcoholic steatohepatitis—a long-term follow-up study: comparison with alcoholic hepatitis in ambulatory and hospitalized patients. Dig Dis Sci. 2003;48:1909–1913. doi: 10.1023/a:1026152415917. [DOI] [PubMed] [Google Scholar]
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- Dunn W, Jamil LH, Brown LS, Wiesner RH, Kim WR, Menon KV, Malinchoc M, Kamath PS, Shah V. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology. 2005;41:353–358. doi: 10.1002/hep.20503. [DOI] [PubMed] [Google Scholar]
- Forouhi NG, Wareham NJ. Epidemiology of diabetes. Medicine (Abingdon) 2014;42:698–702. doi: 10.1016/j.mpmed.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiver M, Williamson T, Barber D, Birtwhistle R, Aliarzadeh B, Khan S, Morkem R, Halas G, Harris S, Katz A. Prevalence and epidemiology of diabetes in Canadian primary care practices: a report from the Canadian Primary Care Sentinel Surveillance Network. Can J Diabetes. 2014;38:179–185. doi: 10.1016/j.jcjd.2014.02.030. [DOI] [PubMed] [Google Scholar]
- Jalan R, Fernandez J, Wiest R, Schnabl B, Moreau R, Angeli P, Stadlbauer V, Gustot T, Bernardi M, Canton R, Albillos A, Lammert F, Wilmer A, Mookerjee R, Vila J, Garcia-Martinez R, Wendon J, Such J, Cordoba J, Sanyal A, Garcia-Tsao G, Arroyo V, Burroughs A, Gines P. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60:1310–1324. doi: 10.1016/j.jhep.2014.01.024. [DOI] [PubMed] [Google Scholar]
- Jinjuvadia R, Liangpunsakul S Translational Research and Evolving Alcoholic Hepatitis Treatment Consortium. Trends in alcoholic hepatitis-related hospitalizations, financial burden, and mortality in the United States. J Clin Gastroenterol. 2015;49:506–511. doi: 10.1097/MCG.0000000000000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W, Chikritzhs T, Pascal R, Binns CW. Mortality rate of alcoholic liver disease and risk of hospitalization for alcoholic liver cirrhosis, alcoholic hepatitis and alcoholic liver failure in Australia between 1993 and 2005. Intern Med J. 2011;41:34–41. doi: 10.1111/j.1445-5994.2010.02279.x. [DOI] [PubMed] [Google Scholar]
- Liangpunsakul S. Clinical characteristics and mortality of hospitalized alcoholic hepatitis patients in the United States. J Clin Gastroenterol. 2011;45:714–719. doi: 10.1097/MCG.0b013e3181fdef1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvet A, Naveau S, Abdelnour M, Ramond MJ, Diaz E, Fartoux L, Dharancy S, Texier F, Hollebecque A, Serfaty L, Boleslawski E, Deltenre P, Canva V, Pruvot FR, Mathurin P. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology. 2007;45:1348–1354. doi: 10.1002/hep.21607. [DOI] [PubMed] [Google Scholar]
- Maddrey WC, Boitnott JK, Bedine MS, Weber FL, Jr, Mezey E, White RI., Jr Corticosteroid therapy of alcoholic hepatitis. Gastroenterology. 1978;75:193–199. [PubMed] [Google Scholar]
- Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, Ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864–871. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- Mathurin P, Louvet A, Duhamel A, Nahon P, Carbonell N, Boursier J, Anty R, Diaz E, Thabut D, Moirand R, Lebrec D, Moreno C, Talbodec N, Paupard T, Naveau S, Silvain C, Pageaux GP, Sobesky R, Canva-Delcambre V, Dharancy S, Salleron J, Dao T. Prednisolone with vs without pentoxifylline and survival of patients with severe alcoholic hepatitis: a randomized clinical trial. JAMA. 2013;310:1033–1041. doi: 10.1001/jama.2013.276300. [DOI] [PubMed] [Google Scholar]
- McNamara RL, Wang Y, Partovian C, Montague J, Mody P, Eddy E, Krumholz HM, Bernheim SM. Development of a hospital outcome measure intended for use with electronic health records: 30-day risk-standardized mortality after acute myocardial infarction. Med Care. 2015;53:818–826. doi: 10.1097/MLR.0000000000000402. [DOI] [PubMed] [Google Scholar]
- Mehta SR, Yusuf S, Diaz R, Zhu J, Pais P, Xavier D, Paolasso E, Ahmed R, Xie C, Kazmi K, Tai J, Orlandini A, Pogue J, Liu L Investigators C-ETG. Effect of glucose-insulin-potassium infusion on mortality in patients with acute ST-segment elevation myocardial infarction: the CREATE-ECLA randomized controlled trial. JAMA. 2005;293:437–446. doi: 10.1001/jama.293.4.437. [DOI] [PubMed] [Google Scholar]
- Raghupathi W, Raghupathi V. Big data analytics in healthcare: promise and potential. Health Inf Sci Syst. 2014;2:3. doi: 10.1186/2047-2501-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosamond WD, Chambless LE, Heiss G, Mosley TH, Coresh J, Whitsel E, Wagenknecht L, Ni H, Folsom AR. Twenty-two-year trends in incidence of myocardial infarction, coronary heart disease mortality, and case fatality in 4 US communities, 1987–2008. Circulation. 2012;125:1848–1857. doi: 10.1161/CIRCULATIONAHA.111.047480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandahl TD, Jepsen P, Thomsen KL, Vilstrup H. Incidence and mortality of alcoholic hepatitis in Denmark 1999–2008: a nationwide population based cohort study. J Hepatol. 2011;54:760–764. doi: 10.1016/j.jhep.2010.07.016. [DOI] [PubMed] [Google Scholar]
- Sherlock S. Alcoholic hepatitis. Alcohol Alcohol. 1990;25:189–196. doi: 10.1093/oxfordjournals.alcalc.a044992. [DOI] [PubMed] [Google Scholar]
- Simms AD, Weston CF, West RM, Hall AS, Batin PD, Timmis A, Hemingway H, Fox K, Gale CP. Mortality and missed opportunities along the pathway of care for ST-elevation myocardial infarction: a national cohort study. Eur Heart J Acute Cardiovasc Care. 2015;4:241–253. doi: 10.1177/2048872614548602. [DOI] [PubMed] [Google Scholar]
- Singal AK, Shah VH. Alcoholic hepatitis: prognostic models and treatment. Gastroenterol Clin North Am. 2011;40:611–639. doi: 10.1016/j.gtc.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Thursz MR, Forrest EH, Ryder S, Investigators S. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med. 2015;373:282–283. doi: 10.1056/NEJMc1506342. [DOI] [PubMed] [Google Scholar]
- Vaa BE, Asrani SK, Dunn W, Kamath PS, Shah VH. Influence of serum sodium on MELD-based survival prediction in alcoholic hepatitis. Mayo Clin Proc. 2011;86:37–42. doi: 10.4065/mcp.2010.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.