Abstract
Purpose
To characterize the pharmacokinetics of temsirolimus and its major metabolite, sirolimus, in patients receiving enzyme-inducing antiepileptic drugs (EIAED) compared with patients receiving non-EIAEDs. An additional objective was to determine whether concentrations of temsirolimus or sirolimus were achieved in brain tumor tissue.
Experimental Design
Patients with recurrent malignant gliomas not receiving EIAEDs initially received temsirolimus weekly at a dose of 250 mg i.v. The dose was subsequently reduced to 170 mg due to intolerable side effects. For patients taking EIAEDs, the starting dose of temsirolimus was 250 mg with standard dose escalation until the maximal tolerated dose was established. Ten whole blood samples were obtained over a period of 24 h after administration of temsirolimus for pharmacokinetic assessments. Patients eligible for cytoreductive surgery received temsirolimus before tumor resection. Whole blood and tumor tissue were obtained for analysis.
Results
Significant differences in the pharmacokinetic variables for temsirolimus and sirolimus were observed between the two patient groups at a comparable dose level of 250 mg. For patients receiving EIAEDs, the systemic exposure to temsirolimus was lower by 1.5-fold. Likewise, peak concentrations and exposure to sirolimus were lower by 2-fold. Measurable concentrations of temsirolimus and sirolimus were observed in brain tumor specimens. The average tissue to whole blood ratio for temsirolimus was 1.43 and 0.84 for sirolimus.
Conclusions
Drugs that induce cytochrome P450 3A4, such as EIAEDs, significantly affect the pharmacokinetics of temsirolimus and its active metabolite, sirolimus. Total exposure to temsirolimus and sirolimus was lower in the EIAED group at the maximum tolerated dose of 250 mg compared with the non-EIAED group at the maximum tolerated dose of 170 mg. However, brain tumor tissue concentrations of temsirolimus and sirolimus were relatively comparable in both groups of patients at their respective dose levels. Correlative analyses of the tissue for the inhibition of the key regulators (p70S6 kinase and 4E-binding protein 1) of mammalian target of rapamycin are necessary to define the therapeutic significance of the altered exposure to temsirolimus.
Temsirolimus (CCI-779, Torisel) is an ester of sirolimus (rapamycin). Both temsirolimus and its major metabolite, sirolimus, bind with equipotency to the cytosolic protein FKBP-12 (1). This protein-drug complex inhibits mammalian target of rapamycin – mediated phosphorylation of p70S6 kinase and the eukaryotic initiation factor 4E-binding protein 1, which are involved in the regulation of key molecules involved in cell cycle control and apoptosis (2, 3). Phosphatidylinositol 3-kinase and AKT are the upstream key regulators of the phosphorylation and activation of mammalian target of rapamycin. A significant number of patients with recurrent glioblastoma multiforme have altered PTEN gene suppression function, which results in increased activity of the phosphatidylinositol 3-kinase/AKT/mammalian target of rapamycin signaling pathway (4).
Preclinical studies have documented temsirolimus activity in glioma cell lines as well as in orthotopic glioblastoma nude mouse models (5). Additionally, incubation of the human-derived glioma U87 cell line (PTEN deficient) with 100 nmol/L (91.4 ng/mL) sirolimus for 1 h completely inhibited the mammalian target of rapamycin–dependent phosphorylation of p70S6 kinase (6). Recently, high baseline brain tumor levels of phosphorylated p70S6 have been associated with radiographic response in patients receiving temsirolimus for recurrent glioblastoma multiforme (7).
Hydrolysis and oxidation reactions are both involved in the biotransformation of temsirolimus. Sirolimus is formed by deesterification (noncytochrome process) of temsirolimus. Both temsirolimus and sirolimus are extensively metabolized by cytochrome P450 3A enzymes to various demethylated and hydroxylated metabolites (8). We previously reported on the results of a phase I study of temsirolimus in patients who were receiving enzyme-inducing antiepileptic drugs (EIAED). The starting dose was 250 mg and this was escalated to 330 mg in a standard phase I design; however, because of dose-limiting toxicities sustained at that dose, the phase II dose was established at 250 mg for patients on EIAEDs. Results of the pharmacokinetic findings in a limited number of patients were also reported (9). We also presented efficacy results of the phase II component of this trial in patients with recurrent glioblastoma multiforme. Patients not on EIAEDs initially received 250 mg temsirolimus; however, because of poor tolerance, the dose was subsequently reduced to 170 mg.
In this report, we characterize the pharmacokinetics of temsirolimus in two patient groups: those receiving EIAEDs (group B) and patients receiving non-EIAEDs (group A). In the phase II study, there was also a subset of patients who received temsirolimus before a planned surgical removal of recurrent tumor. These patients were subsequently treated with temsirolimus following surgery and were assessed for efficacy. This subgroup provided an additional opportunity to determine for the first time whether measurable concentrations of temsirolimus or sirolimus were achieved in brain tumor tissue.
Materials and Methods
Patient eligibility
Patients were accrued to the pharmacokinetic study if they met the protocol eligibility criteria for the phase I and II studies and signed an institutionally approved Committee on Human Research Consent form (9, 10). Briefly, eligible patients with a recurrent malignant glioma were ≥18 years of age, with Karnofsky performance status ≥60%. Their hematologic, renal, and hepatic variables were within normal limits. Cholesterol and triglyceride levels were less than 350 and 400 mg/dL, respectively.
Clinical study design
As previously published (9, 10), patients were stratified into two groups according to their pretreatment anticonvulsant medications (Table 1). For patient not receiving EIAEDs (group A), the initial dose of temsirolimus was 250 mg as a 30-min i.v. infusion weekly with no rest period required (phase II). However, the dose was subsequently reduced to 170 mg weekly because of intolerable side effects (stomatitis). Patients receiving EIAEDs were treated at an initial dose level of 250 mg i.v. weekly. Standard phase I dose escalations were planned using the modified Fibonacci dose escalation schema and standard three to six patient cohorts per dose level. Toxicities were graded according to the National Cancer Institute Common Toxicity Criteria (version 2.0). For the purpose of evaluation, a cycle was defined as every 4 weeks. The maximum tolerated dose was based on the tolerability observed during the first 4 weeks of treatment. The 250 mg dose level was defined as the maximum tolerated dose and subsequently patients receiving EIAEDs were treated at the 250 mg dose levels (phase II). Because of temsirolimus-induced hyperlipidemia, lipid-lowering agents were allowed during the study. None of the patients were receiving lipid-lowering agents at the time of their pharmacokinetic evaluation.
Table 1.
EIAEDs and non-EIAEDs
| EIAEDs | |
|---|---|
| Carbamazepine | (Tegretol, Tegretol XR, Carbatrol) |
| Oxcarbazepine | (Trileptal) |
| Phenytoin | (Dilantin, Phenytek) |
| Fosphenytoin | (Cerebyx) |
| Phenobarbital | |
| Primidone | (Mysoline) |
| Non-EIAEDs | |
| Valporic acid | (Depakote, Depakene) |
| Gabapentin | (Neurontin) |
| Lamotrigine | (Lamictil) |
| Topriamate | (Topamax) |
| Tiagabine | (Gabatril) |
| Zonisamide | (Zonegran) |
| Levetiracetam | (Keppra) |
| Clonazepam | (Klonopin) |
| Clonozam | (Frisium) |
During the phase II component of the study, eligible patients for whom a surgical resection was recommended received temsirolimus at the protocol-defined dose 2 h before surgery. Following surgery, these patients continued on temsirolimus and were included in the assessment of efficacy.
Pharmacokinetic Evaluation
Sample collection
Whole blood (7 mL) was collected in EDTA-containing tubes on day 1, cycle 1 at each of the following times: baseline (before infusion); end of infusion; and 0.5, 1, 1.5, 2, 3, 5, 8, and 24 h after administration. Baseline samples were also obtained on cycle 1, day 7 and cycle 2, day 1. The whole blood was transferred into two polypropylene tubes (one for temsirolimus determination and one for sirolimus) and stored at −70°C until analyzed. Whole blood was selected as the biological matrix for analysis due to the preferential distribution of temsirolimus and sirolimus into RBCs and limited storage stability in plasma.
Whole blood and tumor tissue were obtained at the time of resection from patients who received temsirolimus before surgery. Before analysis, the tumor tissue was weighed and homogenized in 1 mL of analytic grade methanol.
Analytic methods
Analysis of temsirolimus and sirolimus was done by two validated high-performance liquid chromatography assays using electrospray ionization mass spectrometry as described previously (9). Temsirolimus and the deuterated temsirolimus internal standard as well as sirolimus and its internal standard (desmethoxyrapamycin) were obtained from Wyeth-Ayerst Research. The calibration curves for both temsirolimus and sirolimus were linear (R2 > 0.99) over the range from 6 to 500 ng/mL. End-of-infusion samples of temsirolimus were diluted 1:100 into the linear range of the calibration curve. The lower limit of detection was 3 ng/mL for both temsirolimus and sirolimus. The interday assay precision for temsirolimus (sirolimus in parentheses) expressed as the percentage coefficient of variation of the estimated concentration of quality control samples is 1.7% (12.1%) and 10.5% (6.7%), respectively, for the low and high quality control samples. The interday percentage accuracy for temsirolimus (sirolimus) was 95% (101%) for the low quality controls and 97% (106%) for the high quality controls.
Pharmacokinetic analyses
Temsirolimus and sirolimus whole blood concentrations were analyzed by noncompartmental methods (11). The time intervals relative to the start of the temsirolimus infusion and the actual sample times were used for the determination of time to peak (Tmax) and the area under the whole blood concentration-time curves (AUC). Peak concentration (Cmax) was determined by inspection of each individual’s whole blood concentration-versus-time curve. The terminal disposition rate constants were estimated by linear regression analysis of the log concentration versus time. Terminal half-lives (t1/2) were calculated by dividing 0.693 by the elimination rate constants. The AUC was calculated using the linear trapezoidal rule up to the last measurable data point (for AUC0-24) and then extrapolated to infinity (AUC). The systemic clearance for temsirolimus was determined by dividing the dose by the AUC. The apparent volume of distribution at steady state (Vdss) was determined by the following relationship: Vdss = (dose × AUMC / AUC2) - (dose × duration of infusion) / (2 × AUC), where AUMC is the area under the moment curve extrapolated to infinity. A metabolic ratio estimated as the ratio of the AUCsir to the AUCtem was used as a measure of the relative extent of conversion of temsirolimus to sirolimus. AUCsum represents the aggregate of the temsirolimus AUC (unadjusted for molecular weight differences).
Statistical considerations
Pharmacokinetic variables are reported as mean values ± SD. Difference between the two groups with respect to the kinetic variables was evaluated using the unpaired two-tailed (t) test. Two-tailed probability values of <0.05 were regarded as statistically significant.
Results
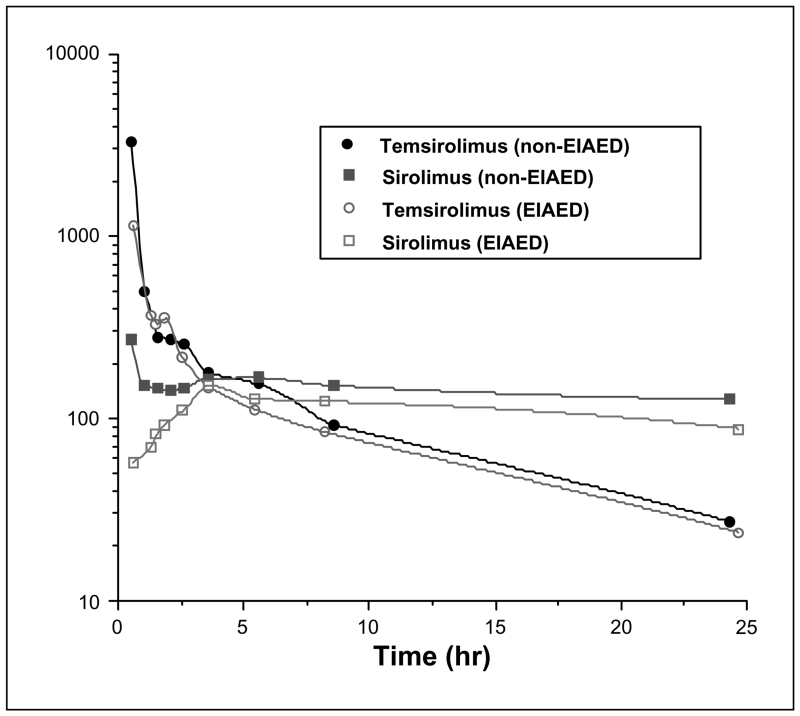
Thirty-six patients with recurrent malignant glioma were accrued for pharmacokinetic analyses to this North American Brain Tumor Consortium phase I/II trial. The pharmacokinetic characteristics for temsirolimus and its metabolite sirolimus are summarized in Table 2. A representative blood concentration-time curve generated from three randomly selected patients from each of the two groups at the 250 mg dose level is depicted in Fig. 1. There seemed to be an interesting difference in the behavior of the metabolite (sirolimus) early in the plasma concentration-time profile related to the time to reach maximum concentration (Tmax) between the two groups. However, there was no significant difference (P = 0.12) between the mean Tmax in either group. Pharmacokinetic comparisons between the two groups (non-EIAED versus EIAED) and the total patient population are displayed in Table 3. The majority of the patients (47%) in the EIAED group were receiving phenytoin with or without dexamethasone.
Table 2.
Mean (±SD) pharmacokinetic variables of temsirolimus and sirolimus
| Temsirolimus |
||||||
|---|---|---|---|---|---|---|
| Dose (mg) | Cmax (μg/mL) | t1/2 (h)* | AUC (μg × h/mL) | CL (L/h) | Vdss (L) | |
| Group A (n = 13) † | 170 | 1.66 (±0.85) | 9.81 (±3.19) | 3.53 (±1.53) | 53.97 (±17.29) | 533 (±265) |
| Group A (n = 6) | 250 | 2.36 (±1.64) | 8.83 (±2.71) | 5.03 (±2.92) | 66.15 (±41.69) | 470 (±362) |
| Group B (n = 13) ‡ | 250 | 1.45 (±0.89) | 9.38 (±2.16) | 3.32 (±0.84) | 79.60 (±18.61) | 699 (±241) |
| Group B (n = 4) | 330 | 2.95 (±1.70) | 10.37 (±3.01) | 3.58 (±0.67) | 94.46 (±16.90) | 654 (±414) |
| Sirolimus |
||||||||
|---|---|---|---|---|---|---|---|---|
|
Cmax (ng/mL) |
Tmax (h) |
t1/2* (h) |
AUC (μg × h/mL) |
Troughs (ng/mL) |
AUC ratio (SIR/TEM) |
AUCsum (μg × h/mL) |
||
| 168 h | C2D1 | |||||||
| Group A † (n = 13) | 185.47 (±87.11) |
3.47 (±3.41) |
38.34 (±56.05) |
10.97 (±5.93) |
15.48 (±8.19), n = 10 |
12.67 (±7.73), n = 3 |
3.56 (±2.29) |
14.49 (±5.74) |
| Group A (n = 6) | 260.60 (±125) |
1.66 (±1.48) |
51.35 (±24.43) |
14.49 (±4.94) |
14.97 (±6.30), n = 4 |
9.55 (±2.29), n = 3 |
3.37 (±1.27) |
19.52 (±6.65) |
| Group B ‡ (n = 13) | 131.83 (±46.37) |
5.01 (±1.94) |
28.86 (±13.55) |
6.83 (±3.28) |
6.82 (±0.38), n = 4 |
– | 2.12 (±0.93) |
9.74 (±3.67) |
| Group B (n = 4) | 246.11 (±1.08) |
1.81 (±1.00) |
23.69 (±4.17) |
7.93 (±2.79) |
13.15 (n = 1) |
9.90 (n = 1) |
2.35 (±1.04) |
11.50 (±2.30) |
Abbreviations: CL, clearance; C2D1 cycle 2, day 1; SIR, sirolimus; TEM, temsirolimus.
Harmonic mean.
Group A (non-EIAEDs).
Group B (EIAEDs).
Fig. 1.
Comparative temsirolimus and sirolimus whole blood curves at the 250 mg dose level for patients receiving non-EIAEDs (n = 3) or EIAEDs (n = 3).
Table 3.
Pharmacokinetic relationships
| Pharmacokinetic variables (250 mg dose levels) | Non-EIAD (n = 6) | EIAED (n = 13) | P |
|---|---|---|---|
| Temsirolimus | |||
| t1/2 (h) | 8.83 (±2.71) | 9.38 (±2.16) | 0.64 |
| Cmax (μg/mL) | 2.36 (±1.64) | 1.45 (±0.89) | 0.13 |
| AUC (μg × h/mL) | 5.03 (±2.92) | 3.32 (±0.84) | 0.06 |
| CL (L/h) | 66.15 (±41.69) | 79.60 (±18.61) | 0.34 |
| Vdss (L) | 470 (±362) | 699 (±241) | 0.12 |
| Sirolimus | |||
| t1/2 (h) | 51.35 (±24.43) | 28.86 (±13.55) | 0.02 |
| Cmax (ng/mL) | 260.60 (±125) | 131.83 (±46.37) | 0.004 |
| AUC (μg × h/mL) | 14.49 (±4.94) | 6.82 (±3.28) | 0.008 |
| AUCsum (μg × h/mL) | 19.52 (±6.65) | 9.74 (±3.67) | 0.006 |
| AUCratio | 3.37 (±1.27) | 2.12 (±0.93) | 0.002 |
| Population pharmacokinetic variables | Non-EIAD (n = 19) | EIAED (n = 17) | P |
|---|---|---|---|
| Temsirolimus | |||
| t1/2 (h) | 9.48 (±2.93) | 9.57 (±2.25) | 0.92 |
| Cmax (μg/mL/mg)* | 0.012 (±0.013) | 0.010 (±0.01) | 0.41 |
| AUC (μg × h/mL/mg)* | 0.02 (±0.009) | 0.01 (±0.004) | 0.002 |
| CL (L/h) | 57.82 (±26.76) | 83.10 (±18.86) | 0.003 |
| Vdss (L) | 513 (±290) | 689 (±276) | 0.07 |
| Sirolimus | |||
| t1/2 (h) | 42.04 (±49.42) | 27.45 (±10.98) | 0.24 |
| Cmax (ng/mL/mg)* | 1.08 (±0.50) | 0.58 (±0.24) | 0.0007 |
| AUC (μg × h/mL/mg)* | 0.062 (±0.03) | 0.027 (±0.01) | 0.00006 |
| AUCratio | 3.50 (±1.99) | 2.18 (±0.93) | 0.018 |
Dose normalized.
Significant differences were observed between the two patient populations. For patients receiving EIAED, the systemic exposure to temsirolimus was lower due to an increase in clearance and apparent volume of distribution. Likewise, peak concentrations and exposure to sirolimus were lower by 46% and 57%, respectively. Trough levels for temsirolimus 168 h after dose (day 7) and cycle 2, day 1 were nondetectable. In contrast, trough levels for sirolimus were detectable in most patients in the non-EIAED group and in a lesser number of patients in the EIAED group. Total exposure to temsirolimus plus sirolimus was lower at the maximum tolerated dose (250 mg) for the EIAED group [AUCsum, 9.74 (±3.67) μg × h/mL] compared with the maximum tolerated dose (170 mg) for the non-EIAED [AUCsum, 14.49 (±5.74) μg × h/mL]. No association of drug exposure (AUCtem, AUCsir, or AUCsum) and toxicity (e.g., elevated cholesterol or triglyceride) was found.
Following a 30-min infusion of temsirolimus before surgery, tumor tissue and a blood sample were obtained from six patients at the time of tumor resection. The time from temsirolimus administration and tumor tissue acquisition ranged from 2.7 to 5.6 h. The relative tumor concentrations of temsirolimus and sirolimus are summarized in Table 4. The tissue to whole blood ratio of temsirolimus and sirolimus ranged from 0.69 to 3.37 and 0.52 to 1.81, respectively.
Table 4.
Brain tumor concentrations of temsirolimus and sirolimus
| Dose (group) |
Whole blood concentration, ng/mL (TEM/SIR) |
Time (h) from BOI to tumor acquisition |
Tumor concentration, ng/g dry weight (TEM/SIR) |
Tissue/blood ratio (TEM/SIR) |
|---|---|---|---|---|
| 170 mg (A) | 108/133 | 5.1 | 129/BLD | 1.19/BLD |
| 170 mg (A) | 150/164 | 5.0 | 213/86 | 1.42/0.52 |
| 250 mg (B) | 290/70 | 2.7 | 200/127 | 0.69/1.81 |
| 250 mg (B) | 207/35 | 5.6* | 192/22 | 0.93/0.63 |
| 250 mg (B) | 127/96 | 3.8 | 428/61 | 3.37/0.63 |
| 250 mg (B) | 162/86 | 5.4 | 155/50 | 0.97/0.58 |
NOTE: A, non-EIAED group; B, EIAED group.
Abbreviations: BOI, beginning of infusion; BLD, below limit of detection.
Three and a half hours between whole blood and tumor acquisition.
Discussion
Drugs that induce cytochrome P450 3A4, such as EIAEDs, significantly alter the pharmacokinetics of temsirolimus and its active metabolite, sirolimus. The pharmacokinetics of both temsirolimus and sirolimus are similar in both patient groups to those previously published (Table 5). Our pharmacokinetic results for patients receiving EIAEDs are in close agreement to those recently reported by the North Central Cancer Treatment Study Group (7). Total exposure (AUCsum) of temsirolimus plus sirolimus was lower in the EIAED group at both the 250 and 330 mg dose levels compared with either of the non-EIAED groups at the dose levels of 170 or 250 mg (Table 2). The EIAEDs seemed to have a greater effect on sirolimus rather than temsirolimus because, at the respective dose levels of 250 mg, the temsirolimus AUCs were not significantly different, whereas there was significant 2-fold difference in the sirolimus AUCs. Coadministration of the strong cytochrome P450 3A4/5 inducer rifampin with temsirolimus had no significant effect on temsirolimus AUC but decreased sirolimus AUC by 56%.13 The significant differences in the clearance and the volume of distribution for temsirolimus between the two groups for the total population (Table 3) may be confounded. The differences in the clearance values of temsirolimus at the 170 mg dose level (54 L/h) and the 250 mg dose level (66 L/h) in the non-EIAED group may account for this observation. Dose-dependent volume of distribution and clearance of temsirolimus has been reported by other investigators (12 – 14). The reported clearance values for temsirolimus in patients with renal cell cancer at dose levels of 25, 75, and 250 mg are 16, 42, and 98 L/h, respectively (15).
Table 5.
Relative pharmacokinetic comparisons of i.v. temsirolimus
| Study | Temsirolimus |
Sirolimus |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Dose (mg) |
Cmax (μg/mL) |
AUC (μg × h/mL) |
CL (L/h) |
Vdss (L) |
Cmax (ng/mL) |
AUC (μg × h/mL) |
AUCsum (μg × h/mL) |
168 h trough (ng/mL) |
|
| Non-EIAEDs | |||||||||
| Atkins et al. (15) | 250 (n = 6) |
2.83 (±0.87) |
2.70 (±0.72) |
98 (±26) |
897 (±316) |
266 (±93) |
13.30 (±3.70) |
16.00 (±3.59) |
– |
| Current study | 250 (n = 6) |
2.36 (±0.16) |
5.03 (±2.92) |
66 (±42) |
470 (±362) |
261 (±125) |
14.49 (±4.94) |
19.52 (±6.65) |
14.97 (±6.30), n =4 |
| EIAEDs | |||||||||
| Galanis et al. (7) | 250 (n = 6) |
1.07 (±0.68) |
3.39 (±1.34) |
83 (±31) |
978 (±292) |
134 (±26) |
5.89 (±1.80) |
9.28 (±0.40) |
2.80 (±1.66) |
| Current study | 250 (n = 13) |
1.45 (±0.89) |
3.32 (±0.84) |
80 (±18) |
699 (±241) |
132 (±46) |
6.83 (±3.28) |
9.74 (±3.67) |
6.82 (±0.38), n = 4 |
Following a single 30-min infusion of temsirolimus, tumor tissue concentrations of both temsirolimus and sirolimus were observed. The average tissue to whole blood ratio for temsirolimus was 1.43 and 0.84 for sirolimus. To our knowledge, this is the first report of the ability of temsirolimus and its metabolite, sirolimus, to penetrate the brain tumor barrier. One potential confounding factor associated with the tissue/blood concentration ratio is the possibility of residual blood trapped in the tumor. However, because temsirolimus tissue concentration is greater than its ng/g blood concentration (mean tissue concentration of 220 ng/g versus mean whole blood concentration of 174 ng/mL) and its volume of distribution (533-699 L) is much greater than blood volume (6 L), the correction of tissue concentration for blood contamination is theoretically unnecessary (16). Correlative analyses of the tumor tissue (pending) for the inhibition of the key regulators (p70S6 kinase, eIF4E, PTEN, etc.) of mammalian target of rapamycin will be necessary to define the therapeutic significance of the finding along with the ongoing temsirolimus combination trials in gliomas.
Acknowledgments
Grant support: University ofTexas Health Science Center (San Antonio, TX) grants UO1CA62426 and P30CA54174, University of California at San Francisco grants U01CA62422 and GCRC# M01-RR00079, Dana-Farber Cancer Center grant U01CA62407, University of California at Los Angeles grants U01CA62339 and GCRC# M01-RR0865, University of Michigan grants U01CA62399 and M01-RR00042, University of Texas M. D. Anderson Cancer Center grants CA62412 and CA16672, University of Texas Southwestern Medical Center grants CA62455 and M01-RR00633, University of Wisconsin Hospital grants U01CA62421and GCRC# M01-RR03186, and Memorial Sloan-Kettering Cancer Center grant U01CA62399.
Footnotes
U.S. Food and Drug Administration, U.S. Department of Health and Human Services, 5600 Fishers Lane, Rockville, MD 20857-0001 or online at http://www.Fda.gov/cda/foi/label/2007/022088lbl.pdf.
References
- 1.Vogt PK. PI3-kinase, mTOR, protein synthesis and cancer. Trends Mol Med. 2001;7:482–4. doi: 10.1016/s1471-4914(01)02161-x. [DOI] [PubMed] [Google Scholar]
- 2.Smolewski P. Recent developments in targeting the mammalian target of rapamycin (mTOR) kinase pathway. Anti-Cancer. 2006;17:487–94. doi: 10.1097/00001813-200606000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov. 2006;5:671–88. doi: 10.1038/nrd2062. [DOI] [PubMed] [Google Scholar]
- 4.Knobbe CB, Merlo A, Reifenberger G. Pten signaling in gliomas. Neuro-Oncol. 2002;4:196–211. [PMC free article] [PubMed] [Google Scholar]
- 5.Gibbons JJ, Discafani C, Peterson R. The effect of CCI-779, a novel macrolide anti-tumor agent, on the growth of human tumor cells in vitro and in nude mouse xenograft in vivo [abstract] Proc Am Assoc Cancer Res. 1999;40:301. [Google Scholar]
- 6.Eshleman JS, Carlson BL, Mladek AC, Kastner BD, Shide KL, Sarkaria JN. Inhibition of the mammalian target of rapamycin sensitizes U87 xenografts to fractionated radiation therapy. Cancer Res. 2002;62:7291–7. [PubMed] [Google Scholar]
- 7.Galanis E, Buckner J, Maurer MJ, et al. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group Study. J Clin Oncol. 2005;23:5294–304. doi: 10.1200/JCO.2005.23.622. [DOI] [PubMed] [Google Scholar]
- 8.Cai P, Tsao R, Ruppen ME. In vitro metabolic study of temsirolimus: preparation isolation and identification of the metabolites. Drug Metab Dispos. 2007;35:1554–63. doi: 10.1124/dmd.107.014746. [DOI] [PubMed] [Google Scholar]
- 9.Chang SM, Kuhn J, Wen P, et al. Phase I/pharmacokinetic study of CCI-779 in patients with recurrent malignant glioma on enzyme-inducing antiepileptic drugs. Invest New Drugs. 2004;22:427–35. doi: 10.1023/B:DRUG.0000036685.72140.03. [DOI] [PubMed] [Google Scholar]
- 10.Chang SM, Wen P, Cloughesy T, et al. Phase II study of CCI-779 in patients with recurrent glioblastoma multiforme. Invest New Drugs. 2005;23:357–61. doi: 10.1007/s10637-005-1444-0. [DOI] [PubMed] [Google Scholar]
- 11.Gibalidi M. Biopharmaceutics and clinical pharmacokinetics. 3rd Lea & Febiger; Philadelphia (PA): 1984. [Google Scholar]
- 12.Raymond E, Alexandre I, Faivre S, et al. Safety and pharmacokinetics of escalated doses of weekly intravenous infusion of CCI-779, a novel mTOR inhibitor, in patients with cancer. J Clin Oncol. 2004;22:2336–47. doi: 10.1200/JCO.2004.08.116. [DOI] [PubMed] [Google Scholar]
- 13.Hidalgo M, Buckner JC, Erlichman C, et al. A phase I and pharmacokinetic study of temsirolimus (CCI-779) administered intravenously daily for 5 days every 2 weeks to patients with advanced cancer. Clin Cancer Res. 2006;12:5755–63. doi: 10.1158/1078-0432.CCR-06-0118. [DOI] [PubMed] [Google Scholar]
- 14.Boni JP, Leister C, Bender G, et al. Population pharmacokinetics of CCI-779: correlations ot safety and pharmacogenomic responses in patients with advanced renalcancer. Clin Pharmacol Ther. 2005;77:76–89. doi: 10.1016/j.clpt.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 15.Atkins MB, Hidalgo M, Stadler, et al. Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol. 2004;22:909–18. doi: 10.1200/JCO.2004.08.185. [DOI] [PubMed] [Google Scholar]
- 16.Guidicelli C, Dricot E, Moati F, Strolin-Benedetti M, Giudicelli J-C. Is it important to correct apparent drug tissue concentrations for blood contamination in the dog? Fund Clin Pharmacol. 2004;18:281–6. doi: 10.1111/j.1472-8206.2004.00246.x. [DOI] [PubMed] [Google Scholar]