Abstract
The first-in-class JAK1/JAK2 inhibitor ruxolitinib inhibits JAK/STAT signaling, inducing durable reductions in splenomegaly and constitutional symptoms in patients with myelofibrosis. However, the association of ruxolitinib therapy with myelosuppression indicates the continued need for optimal treatment choices in myelofibrosis. Pacritinib, a dual JAK2 and FLT3 inhibitor, improves disease-related symptoms and signs with manageable gastrointestinal toxicity in patients with myelofibrosis with splenomegaly and high-risk features, without causing overt myelosuppression, and therefore may provide an important treatment option for a range of patients with myelofibrosis. This article examines the role of JAK2 and FLT3 signaling in myelofibrosis and provides an overview of the clinical development of pacritinib as a new therapy for myelofibrosis.
Keywords: myelofibrosis, tyrosine kinase inhibitor, JAK2, FLT3, pacritinib, safety, efficacy
Introduction
Epidemiology and pathobiology of myelofibrosis
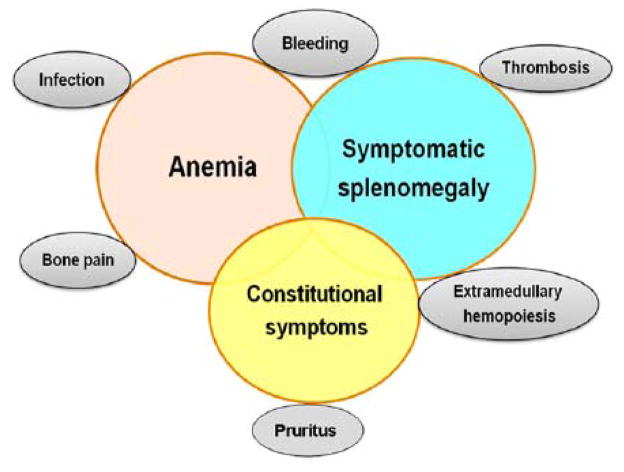
Myelofibrosis (MF), which primarily affects patients older than 60 years of age, is one of the classical BCR-ABL1-negative chronic myeloproliferative neoplasms (MPNs) that also includes essential thrombocythemia (ET) and polycythemia vera (PV); it is characterized by clonal proliferation of myeloid cells with variable morphologic maturity and hematopoietic efficiency 1–3. The clinical manifestations of MF are heterogeneous (Figure 1) 1. A hallmark of MF, the most aggressive of the MPNs, is bone marrow fibrosis that contributes to the severely impaired hematopoiesis that leads to anemia in most patients, as well as thrombocytopenia and leukopenia. Patients with MF may also suffer from marked splenomegaly, extramedullary hematopoiesis, and severe constitutional symptoms (e.g., fatigue, night sweats, fever, and weight loss) 1–3. Studies have shown that these symptoms can severely impair quality of life in patients with MF 4,5. In addition, MF can progress to acute myeloid leukemia (AML) and is associated with thrombotic and hemorrhagic complications 1,2. Survival for patients with MF can range from 1.3 to 15 years, depending on prognostic factors.6 Few patients are eligible for allogeneic stem cell transplantation, the only curative therapy for MF and traditional therapeutic options are often of limited benefit.7 MF, both primary and secondary, is estimated to have a yearly incidence of one to 1.5 per 100,000 persons8. However, the true incidence is not known and may be higher than estimated due to underdiagnosis, and differences in reporting methods used9–12.
Figure 1. Clinical manifestations of myelofibrosis.
Most patients with myelofibrosis typically present with symptoms of anemia or splenomegaly, or with constitutional symptoms. Evolution of the disease can result in marrow failure, abdominal symptoms from advancing splenomegaly, and constitutional symptoms such as weight loss, night sweats, and low-grade fever. Extramedullary hematopoiesis in sites other than the spleen and liver can occur in advanced stages of the disease. Reproduced with permission from the American Society of Hematology 1.
JAK2 & FLT3 signaling in myelofibrosis
In 2005, discovery of the gain-of-function mutation (a valine to phenylalanine substitution at codon 617 in the JAK2 gene) JAK2V617F 13–17 in approximately 50% of patients with primary MF (PMF) suggested the clinical utility of JAK2-targeted therapies for MF. The JAK family comprises four intracellular receptor tyrosine kinases (JAK1-3 and TYK2) that bind proximally to the cell membrane of multiple transmembrane cytokine and growth factor receptors18–21. The roles of each of these kinases in regulation of signaling downstream of cytokine and growth factor receptors have been examined in multiple kinase deficiency models22–26.
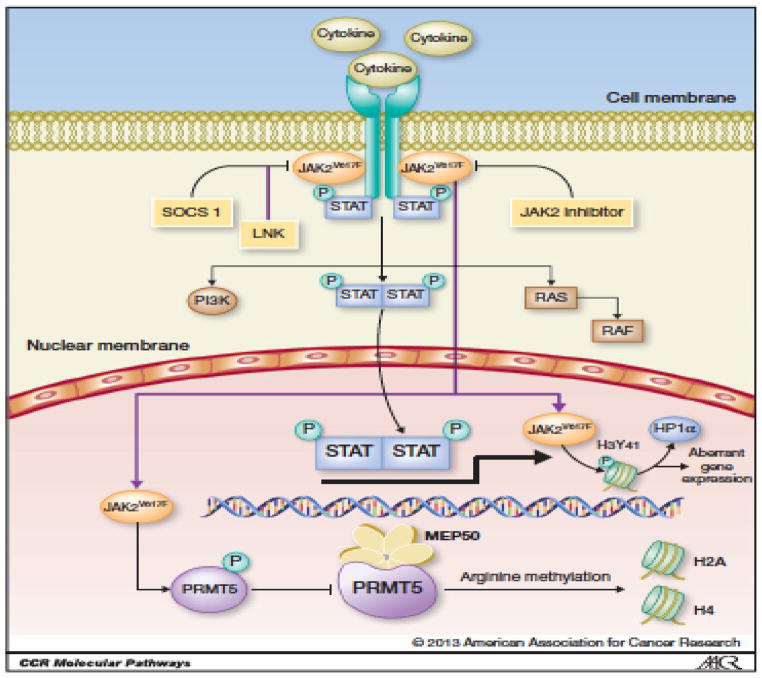
Dysregulation of the JAK/STAT pathway has been linked to the pathogenesis of multiple hematologic malignancies. JAK2 plays a critical role in hematopoiesis, in signaling downstream of common β-chain receptor cytokines (IL-3, IL-5, GM-CSF), single-chain cytokine receptors (EPOR, MPL, CSF3R), and IL-10 family cytokines, as well as in thymic stromal lymphopoietin (TSLP) signaling18, 23, 27. Activating JAK2 fusion proteins have been described in chronic myelogenous leukemia (CML), AML, and acute lymphoblastic leukemia (ALL)28–30. In 2005, the activating point mutation JAK2V617F was found to be associated with a majority of BCR-ABL–negative MPNs. This point mutation results in the constitutive activation of downstream signaling through the JAK/STAT (STAT3/STAT5), MAP kinase, and PI3K/AKT pathways, which are essential for the aberrant proliferation and cellular transformations associated with MPNs (Figure 2)31, 32. The JAK2V617F mutation has been found in up to 90% of patients with PV and 50% of patients with ET and PMF13–17. The majority of the remainder of patients with ET and MF have mutations in either CALR or MPL33, 34. Delineation of the central role of JAK/STAT signaling in these diseases has resulted in the clinical development of multiple JAK2 inhibitor therapies for MPNs, including MF (Table 1)35–37.
Figure 2. Aberrant JAK/STAT signaling in myeloproliferative neoplasms.
Upon cytokine binding, JAK2 molecules are recruited to cytokine receptors and phosphorylated, with subsequent recruitment and phosphorylation of STAT proteins. Phosphorylated STAT proteins dimerize and translocate to the nucleus, where they are involved in the transcription and regulation of multiple genes involved in cell proliferation, differentiation, and apoptosis. The JAK2V617F mutation causes constitutive activation of this signaling pathway and activation of STAT5 molecules in MF, as well as activation of PI3K/AKT and RAS/MAPK signaling. JAK2 inhibitors abrogate the JAK/STAT pathway through inhibition of the kinase activity of JAK2V617F. Reproduced with permission from the American Association of Cancer Research32.
Table 1.
JAK2 inhibitors in clinical development for MF
| Target and IC50 Activity (nM) | |||||||
|---|---|---|---|---|---|---|---|
| Drug | JAK1 | JAK2 | JAK3 | TYK2 | FLT3 | Phase | Patient population |
| Ruxolitinib35 | 3.3 | 2.8 | 428 | 19 | — | Approved | Intermediate- and high-risk MF patients, including PMF, post-PV and post-ET MF |
|
Pacritinib36
(SB1518) NCT02055781 NCT01773187 |
>100 | 6 | 18.3 | 27 | 14.8 | Phase III | Intermediate- and high-risk anemic/thrombocytopenic patients with MF, including post-PV and post-ET MF |
|
Momelotinib37
(CYT387) NCT02101268 |
11 | 18 | 155 | 17 | — | Phase III | Patients with MF with anemia/thrombocytopenia including PMF, post-PV, and post-ET MF |
|
NS-01837 NCT01423851 |
33 | <1 | 39 | 22 | — | Phase I/II | Patients with PMF, post-PV, or post-ET MF |
ET: Essential thrombocythemia; IC50: Half-maximal inhibitory concentration; MF: Myelofibrosis; PMF: Primary myelofibrosis; PV: Polycythemia vera.
In addition to the JAK2V617F mutation and JAK2 exon 12 mutation (present in PV), JAK/STAT signaling in MF can be dysregulated through mutations in MPN-related genes such as CBL, LNK, MPL and CALR3,33–34,38–39. Dysregulation of the JAK-STAT pathway is though to be the unifying pathobiologic abnormality in MPNs, as even patients without a JAK2 gene mutation can respond to a JAK2 inhibitor40,41. Recently other gene mutations have been described in MF. In particular, the ASXL-1 gene mutation has been associated with poor outcomes in MF42. In contrast, mutation in CALR that is mutually exclusive of the JAK2 mutation is associated with favorable outcomes33,42. Indeed, the response to JAK2 inhibitors among patients harboring the CALR mutation is similar to that of patient with the JAK2V617F mutation. Patients who are labeled as ‘triple negative’, in other words, without mutation in JAK2, CALR OR MPL tend to have worse outcomes43.
FMS-like receptor tyrosine kinase 3 (FLT3) is a member of the family of type 3 receptor tyrosine kinases that include KIT, FMS, and PDGF receptor44,45. FLT3 is expressed on hematopoietic stem cells and myeloid progenitors, playing an important role in the survival and proliferation of these cells44,45. Activation of FLT3 occurs after binding of FLT3 ligand to the receptor, dimerization of FLT3 and initiation of intracellular kinase activity, including phosphorylation and activation of PI3K/AKT, MAP kinase, and STAT5 signaling, which regulates multiple apoptotic, proliferation, and differentiation pathways44,45. Preclinical studies in a murine model have shown that FLT3 inhibition can block the development of myeloproliferative disease by targeting multipotent progenitors expressing FLT346. In addition, patients with primary MF who have a higher proportion of circulating FLT3-expressing CD34+ CD41+ megakaryocytic cells exhibit increased effector MAP kinase phosphorylation independent of JAK2V617F 47. Moreover, signaling through the FLT3 ligand (the levels of which are also increased in patients with primary MF), and FLT3-mediated activation of p38 MAPK play a role in the inflammatory dysmegakaryopoiesis characteristic of primary MF47. Megakaryocytes in MF are thought to be the source of cytokines such as PDGF, FGF, and TGF-β, which stimulate fibroblast proliferation in the bone marrow of patients with MF48. These data suggest that targeting the FLT3 kinase pathway, in addition to JAK2, in patients with MF may help mediate the inflammatory effects associated with MF.
JAK-2 inhibitor therapies in myelofibrosis
Ruxolitinib
Ruxolitinib, a first-in-class, orally available inhibitor of JAK1 and JAK2, is the only JAK inhibitor currently approved for the treatment of intermediate- and high-risk MF in the United States and European Union49. Ruxolitinib was also recently approved for treatment of PV, and has been shown to be superior to standard therapy in controlling hematocrit, reducing spleen volume and improving symptoms associated with PV50. Approval of ruxolitinib in MF was based on the results of the randomized Phase III studies COMFORT-I (ruxolitinib vs. placebo) and COMFORT-II (ruxolitinib vs. best available therapy [BAT]) in patients with PMF, post-PV or post-ET MF. Patients receiving ruxolitinib in COMFORT I and COMFORT II experienced significantly greater reduction in spleen volume, as well as improvements in symptoms compared with patients in the control arms. Responses were seen across MF subtypes and in patients with or without the JAK2V617F mutation. The most common grade 3 and 4 toxicities were anemia and thrombocytopenia, potentially due to the effects of ruxolitinib on red cell platelet precursors40,41; patient with low platelet counts (≤100,000) were not included in these Phase III studies. These data showed that ruxolitinib therapy resulted in clinically significant and sustained reduction in spleen size and prolonged symptom improvement in these patients, with modest decrease in JAK2 allele burden and reversal of fibrosis, and no improvement in transfusion requirements40,41. With good control of signs and symptoms of MF, ruxolitinib may prolong survival in patients with advanced MF. However, the benefits of ruxolitinib may come at the cost of toxicities such as anemia, that is often transfusion-dependent, and thrombocytopenia. In addition, ruxolitinib is not indicated for patients with platelet counts <50,000/μl, highlighting the continuing need for therapies that would improve and control disease characteristics with a favorable toxicity profile37,51.
Pacritinib
Multiple JAK2 tyrosine kinase inhibitors are currently in development as single-agent therapy for MF. Out of these, pacritinib, a dual JAK2 and FLT3 tyrosine kinase inhibitor, is being compared with BAT in Phase III trials in patients with MF. Currently, there are no FLT3 inhibitors approved for treatment of hematologic malignancies, although multiple trials are ongoing, particularly in FLT3-mutant AML. In this article, we discuss the role of JAK2 and FLT3 tyrosine kinase signaling in MF and present data from preclinical and clinical studies (Table 2)52–58 evaluating the mechanism of action and efficacy and safety of pacritinib in MF.
Table 2.
Results of clinical studies of pacritinib in myelofibrosis
| Title/NCT identifier | Disease/endpoints | Efficacy/safety outcomes |
|---|---|---|
| A Phase 1/2 study of SB1518 for the Treatment of Advanced Myeloid Malignancies (NCT00719836)52–54 | AML, CML, MF Primary: maximum tolerated dose of oral SB1518, spleen response rate by MRI Secondary: safety and tolerability |
RP2D: 400 mg daily 57% of patients with ≥ 25% spleen reduction by MRI at 24 weeks Common grade 1/2AEs: diarrhea, nausea, vomiting, fatigue, abdominal pain, extremity pain, insomnia, rash Common grade 3/4 AEs: diarrhea, thrombocytopenia, rash |
| A Phase I/II study of oral SB1518 in patients (NCT00745550)55,56 | PV, ET, MF Primary: maximum tolerated dose of oral SB1518, ≥ 35% reduction in spleen volume at 24 weeks by MRI Secondary: safety and tolerability |
RP2D: 400 mg daily 31% of patients with ≥ 35% spleen reduction by MRI at 24 weeks Common grade 1/2 AEs: diarrhea, nausea, fatigue, anemia, vomiting, abdominal pain, pruritus, thrombocytopenia Common grade 3/4 AEs: anemia, thrombocytopenia, diarrhea, fatigue, pruritus |
| PERSIST 1: randomized, Phase III study of oral pacritinib vs BAT in patients with MF (NCT01773187)57 | Primary MF, post-PV MF, post-ET MF Primary: ≥ 35% reduction in spleen volume at 24 weeks by MRI or CT Secondary: ≥ 50% reduction in total symptom score |
≥ 35% reduction in spleen volume in 25% (pacritinib) vs. 5.9% (BAT) of evaluable patients at 24 weeks Common grade 3 AEs: diarrhea, nausea, vomiting |
| PERSIST 2: randomized, controlled, Phase III study of oral pacritinib vs BAT in patients with thrombocytopenia with MF (NCT02055781) | Primary MF, post-PV MF, post-ET MF Primary: ≥ 35% reduction in spleen volume at 24 weeks by MRI or CT and ≥ 50% reduction in total symptom score Secondary: efficacy of once daily vs twice daily dosing schedules |
Currently recruiting |
AE: Adverse event; AML: Acute myeloid leukemia; BAT: Best available therapy; CML, Chronic myelogenous leukemia; CT: Computed topography; ET: Essential thrombocythemia; MF: Myelofibrosis; PV: Polycythemia vera; RP2D: Recommended Phase II dose.
Preclinical studies
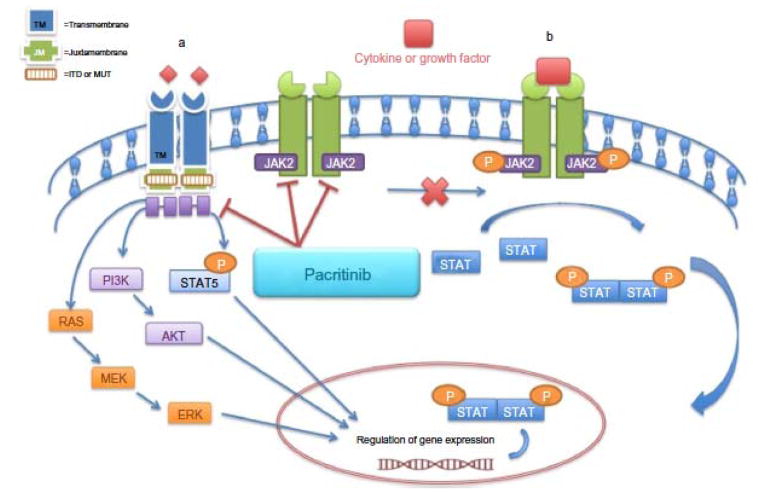
Pacritinib is a low molecular weight, macrocyclic compound with a unique kinome profile that inhibits multiple members of the JAK/FLT signaling pathways (Figure 3)20,59–60. The specificity of pacritinib for the JAK/STAT signaling pathway was demonstrated in wild-type JAK2- or JAK2V617F-expressing cell lines, where pacritinib inhibited phosphorylation-induced apoptosis and cell cycle arrest, and diminished cell proliferation of wild-type JAK2- and mutant JAK2V617F-dependent cancer cell lines61. Pacritinib also induced apoptosis and cell-cycle arrest and blocked proliferation of wild-type FLT3– and mutant FLT3–dependent cancer cell lines62.
Figure 3. Proposed mechanism of action of pacritinib inhibition in JAK2 and FLT3 signaling pathways.
Pacritinib has inhibitory effects in both the (A) FLT3 and (B) JAK/STAT signaling pathways. ITD in FLT3 and mutations in the tyrosine kinase domain results in constitutive activation of FLT3 kinase and downstream signaling pathways, including RAS/MAPK and PI3K/AKT. In addition, FLT3-ITD induces activation of STAT5 signaling. Pacritinib inhibits FLT3, FLT3-ITD, and the FLT3 tyrosine kinase domain mutant FLT3D835Y. ITD: Internal tandem duplication. Reproduced with permission from Dove Medical Press Ltd20.
Initial analysis of the spectrum of kinase inhibitory activity of pacritinib conducted against a panel of more than 50 tyrosine kinases showed that pacritinib is a potent inhibitor of wild-type JAK2 (half-maximal inhibitory concentration [IC50] = 23 nM) and mutant JAK2V617F (IC50 = 19 nM), with less potent activity against other JAK family members, such as TYK2 (IC50 = 50 nm), JAK3 (IC50 = 520 nm), and JAK1 (IC50 = 1280 nM)61. Pacritinib was also shown to exhibit potent activity against FLT3 (IC50 = 22 nM) and mutant FLT3D835Y (IC50 = 6 nM)61. The results of this initial kinase analysis are consistent with data from a 429-member kinome-wide analysis recently presented at the annual American Society of Hematology meeting36. This analysis showed that in addition to being potently inhibitory for JAK2 (IC50 = 6nM), JAK2V617F (IC50 = 9.4nM) and wild-type FLT3 (IC50 = 14.8 nM), pacritinib has specificity for kinases in several unrelated families, with inhibitory activity exhibited against non-JAK/FLT3 tyrosine kinases such as TNK1 (IC50 = 15 nM), TRKC and ROS1 (IC50 = 18.4 nM), and nontyrosine kinases such as IRAK1 (IC50 = 13.6 nM) and HIPK4 (IC50 = 14.5 nM)36. Pacritinib does not have specificity for JAK1 (IC50 >100 nM) at physiologically relevant levels (Table 1), in contrast with ruxolitinib, which is inhibitory for both JAK1 (IC50 = 3.3 nM) and JAK2 (IC50 = 2.8 nM). This lack of inhibition for JAK1 and specificity for FLT3 may assist with the side effects associated with pacritinib therapy and suggests a putative role for FLT3 in pacritinib function.
Phase I/II clinical studies
To date, two Phase I/II clinical studies of pacribinib in patients with MF have been conducted (Table 2), with subsequent presentation of multiple analyses examining the effect of pacritinib therapy in these patients. In the Phase I dose-finding part of the first Phase I/II study evaluating the pharmacokinetics, safety, and efficacy of pacritinib in patients with advanced myeloid malignancies and MF, pacritinib was well tolerated at doses ≤ 500 mg once daily (q.d.)53,54. Of patients with MF who were evaluable for response (n=21), 41% had ≥ 35% reduction in spleen size. The most commonly reported toxicities included diarrhea and nausea, the majority of which were grade 1/2 (Table 2). The most common, albeit rare, grade 3/4 toxicity was thrombocytopenia 4% 54. A dose of 400 mg oral pacritinib daily was selected as the recommended Phase II dose based on reduction in splenomegaly and safety of long-term administration52,53. Preliminary results from the Phase II part of the same study, that evaluated continuous oral dosing with 400 mg q.d. pacritinib in previously treated patients with MF (n = 33), including in those with splenomegaly, showed reductions in splenomegaly as measured by MRI and physical examination. The most commonly observed treatment-related toxicities were gastrointestinal, including diarrhea, nausea, vomiting, and abdominal pain. There were no grade 3 or 4 neutropenia or thrombocytopenia events52,53. Similar conclusions were reached in the second Phase I/II study55,56.
Final data from the Phase II study that further characterized the efficacy and safety of pacritinib in patients with intermediate- and high-risk MF (n = 35), including those with any anemia, thrombocytopenia, or neutropenia, were recently published55. Following treatment with pacritinib for up to 24 weeks, eight of 26 evaluable patients (31%) experienced a ≥ 35% reduction in spleen volume as measured by MRI, with 14 of 33 patients (42%) achieving ≥ 50% reduction in spleen size as assessed by physical examination. Median MF symptom scores, except for fatigue, improved by ≥ 50%. The most common treatment-emergent toxicities were grade 1 or 2 diarrhea (69%) and nausea (46%). Significant myelosuppression was not observed even in those with thrombocytopenia. Discontinuation due to toxicities occurred in 9 patients (26%); toxicities in four of these patients were serious adverse events55.
In a report of preliminary data from a long-term efficacy and safety analysis of 63 patients with palpable splenomegaly (56 with MF, seven with AML) enrolled in the Phase I of the two previously mentioned Phase I/II studies, patients had a median time on study of 13.3 months (range, 1–29+). The most common treatment-related toxicities were low-grade, manageable gastrointestinal events, with grade 3 and 4 toxicities comprising of grade 3 diarrhea (7.9%), and grade 3 nausea, abdominal pain, and grade 4 vomiting (1.6% each). Grade 4 treatment-related hematologic toxicities were rare, with two patients with anemia and one patient with thrombocytopenia63. Out of patients evaluable for a spleen response (n = 41), 44% experienced clinical improvement (as defined by International Working Group criteria), with three experiencing clinical improvement in platelet counts and one patient with clinical improvement in hemoglobin count. Median duration of progression-free survival (PFS) in enrolled patients was 563 days (range: 1–875). The 12-month PFS rate was 67%63.
Data from recent exposure-safety and exposure-efficacy analyses support the safety and limited myelosuppression of 400 mg q.d. pacritinib. The incidence and severity of anemia, thrombocytopenia, and gastrointestinal toxicities did not show a clear exposure-safety relationship with pacritinib64–66. In an analysis of all patients treated with pacritinib in clinical studies (n = 191)66, most toxicities were grade 1 and 2, with grade 3 and 4 toxicities occurring in 123 of 191 patients (64.4%). Serious adverse events were reported in 37.2% of patients, and 22 patients (11.5%) died (21 deaths were due to nondrug-related events, and one was due to drug-related subdural hematoma). Gastrointestinal toxicities resulted in drug discontinuation in 2.6% of patients. Importantly, no dose reduction for worsening thrombocytopenia was required in 11 patients with MF with baseline platelet counts <50,000/μL66.
An integrated analysis of 129 pacritinib-treated patients with advanced myeloid malignancies and MF enrolled in the two Phase I/II studies, examined outcomes in 28 of 65 patients with MF receiving 400 mg oral pacritinib q.d., who had platelet counts ≤ 100,000/μl. In total, 37% and 43% of all evaluable patients and patients with baseline platelet counts ≤ 100,000/μl, respectively, had a ≥ 35% reduction in spleen volume from baseline67. Together, data from these studies support the viability of pacritinib as a potential treatment option for patients with MF, including those with anemia and thrombocytopenia, and provide rationale for further investigation of pacritinib in larger, Phase III, randomized studies.
Phase III clinical studies
Currently, pacritinib is being evaluated in two ongoing randomized, controlled, Phase III clinical studies. PERSIST-1 (NCT01773187) is comparing the efficacy and safety of oral pacritinib 400 mg q.d. with BAT in patients with intermediate-1– or intermediate-2–risk MF, high-risk PMF, post-PV MF, or post-ET MF57. Primary and secondary efficacy outcomes include ≥ 35% reduction in spleen volume as assessed by MRI or CT scan and ≥ 50% improvement in MF symptoms from baseline to week 24. Importantly, no minimum baseline platelet count was required for trial eligibility. Recently reported results show that pacritinib met its primary endpoint in the PERSIST-1 study57. Patients were randomized 2:1 to pacritinib (n = 220) versus BAT (n = 107). At baseline, 32 and 15% of patients had baseline platelet counts of <100,000/μL or <50,000/μL, respectively, and 44% were thrombocytopenic. The median duration of treatment was 16.2 months for pacritinib versus 5.9 months for BAT. In the intent-to-treat population, 19.1% of patients receiving pacritinib versus 4.7% of those receiving BAT achieved ≥ 35% reduction in spleen volume (P = 0.003). In patients evaluable for response, the rates of reduction in spleen volume were 25% for pacritinib versus 5.9% for BAT (P = 0.0001). Pacritinib consistently improved rates of ≥ 35% reduction in spleen volume regardless of baseline platelet counts. In addition, pacritinib compared with BAT resulted in improvement in severe thrombocytopenia and anemia, and achievement of red blood cell transfusion independence (25.7% vs. 0%; p = 0.043). In addition, patients treated with pacritinib experienced sustained improvement in MF-associated symptoms. The most common toxicities occurring in ≥ 10% of patients with pacritinib versus BAT were mild-to-moderate diarrhea (53 vs 12%), nausea (27 vs 6%), anemia (22 vs 20%), thrombocytopenia (17 vs 13%) and vomiting (16 vs 6%). Three patients receiving pacritinib discontinued therapy and 13 had dose interruption for diarrhea. Gastrointestinal symptoms were manageable, and no grade 4 gastrointestinal events were reported in pacritinib-treated patients. Hematologic toxicities occurred at a similar rate between the two arms57. A full report of these data is eagerly anticipated.
PERSIST-2 (NCT02055781) will compare the efficacy and safety of two dosing schedules of oral pacritinib (200 mg twice daily or 400 mg qd) with BAT (including ruxolitinib) in patients with thrombocytopenia (only patients with platelet counts ≤ 100,000/μL are eligible) and PMF, post-PV MF, or post-ET MF. Primary efficacy outcomes include the proportion of patients achieving ≥ 35% reduction in spleen volume from baseline and proportion of patients with >50% reduction in total symptom score from baseline to week 2458.
Additional JAK2 inhibitors in development for myelofibrosis
Several inhibitors of JAK/STAT signaling are currently in development for treatment of MF. These include momelotinib, currently under evaluation in Phase III clinical studies, as well as NS-018 (NCT01423851) (Table 1). Clinical development of fedratinib, XL019 and AZD1480 was terminated following the emergence of debilitating neurologic toxicities68,69.
Momelotinib is a JAK inhibitor with specificity for JAK1 (IC50 = 11 nM), JAK2 (IC50 = 18 nM), and JAK3 (IC50 = 17 nM).37 Treatment with momelotinib has been shown to induce a favorable reduction in anemia and spleen size in patients with MF. However, longer duration of momelotinib therapy appears to be associated with treatment-emergent peripheral neuropathy, and investigations are ongoing into the associated risk factors70,71. Two Phase III trials of momelotinib are currently underway. SIMPLIFY-1 (NCT01969838) is a randomized, double-blind, active-controlled study evaluating momelotinib versus ruxolitinib in patients with primary or secondary MF. Primary and secondary outcomes include splenic and symptom response rates after 24 weeks of therapy. SIMPLIFY-2 (NCT02101268), a randomized, open-label study, will evaluate the efficacy of momelotinib versus BAT in anemic or thrombocytopenic patients with primary or secondary MF who were previously treated with ruxolitinib.
NS-018 is a potent and selective inhibitor of JAK2 (IC50 = <1 nM) (Table 1) and JAK2V617F 72. NS-018 has less activity against other members of the JAK family, including JAK1 (IC50 = 33 nM), JAK3 (IC50 = 39 nM), and TYK2 (IC50 = 22 nM). Oral NS-018 is currently in early-phase development in an ongoing phase 1/2 open-label, dose-escalation study (NCT01423851) evaluating its safety and tolerability in patients with primary and secondary MF.
Conclusion
Myelofibrosis is a clinically heterogeneous disease associated with debilitating constitutional symptoms, splenomegaly, and cytopenias. Until the development of JAK2 inhibitors, treatment options for patients with advanced MF in particular were limited. Development of small-molecule JAK2 inhibitor therapy for MPNs and MF has progressed rapidly since the approval of ruxolitinib in 2011. Several JAK2 inhibitors are currently in late- and early-stage development for treatment of MF. Ruxolitinib is the first approved JAK2 inhibitor therapy for patients with intermediate- or high-risk MF in the USA, and it can effectively improve quality of life and reduce splenomegaly. However, it may also lead to undesirable cytopenias, specifically anemia and thrombocytopenia. In addition, ruxolitinib is not indicated for patients with very low platelet levels. The development of myelosuppression with ruxolitinib is a reminder that responses to these therapies must be continually monitored.
Pacritinib is a novel JAK inhibitor with dual activity against JAK2 and FLT3. Pacritinib has activity in patients with MF, including those with anemia and thrombocytopenia, and is currently being tested in Phase III clinical trials. Therapy with pacritinib is not associated with myelosuppression; the most common toxicity is manageable gastrointestinal toxicity. Such therapies with inhibitory activity again JAK2 and FLT3 and limited myelosuppression may be the future for development of optimal therapies for MF and other malignancies. Specifically, the specificity of pacritinib for FLT3, which is commonly found mutated in AML, makes it a promising anticancer agent. The efficacy of pacritinib is also being evaluated in advanced lymphoid malignancies73–75.
EXECUTIVE SUMMARY.
Background
Myelofibrosis (MF), the most debilitating of the classical BCR-ABL1–negative chronic myeloproliferative neoplasms, also includes essential thrombocythemia and polycythemia vera.
There continues to be a need for therapies with a favorable toxicity profile that would improve and control disease characteristics in patients with MF.
JAK2 and FLT3 signaling in MF
JAK2, JAK/STAT signaling and the activating JAK2 mutation, JAK2V617F, play a central role in pathogenesis of MF. JAK2V617F was found to be associated with disease in 30–50% of patients with MF.
Discovery of the JAK2V617F mutation in MF led to the development of multiple small-molecule inhibitors of JAK2 for the treatment of MF.
JAK2 inhibitors in MF
Currently, ruxolitinib, a JAK1/JAK2 inhibitor, is the only JAK inhibitor approved for treatment of patients with MF.
Ruxolitinib has shown efficacy in reducing splenomegaly and symptoms associated with MF. However, important toxicities include anemia and thrombocytopenia.
Pacritinib, a dual JAK2/FLT3 inhibitor, has shown efficacy in reducing splenomegaly and symptoms associated with MF, including in patients with anemia and thrombocytopenia. The most common toxicity is manageable gastrointestinal toxicity.
The efficacy and safety of pacritinib in patients with MF is currently being evaluated in two Phase III clinical studies, PERSIST-1 and PERSIST-2.
References
Papers of special note have been highlighted as:
* of interest;
** of considerable interest
- 1.Cervantes F. How i treat myelofibrosis. Blood. 2014;124:2635–2642. doi: 10.1182/blood-2014-07-575373. [DOI] [PubMed] [Google Scholar]
- 2.Geyer HL, Mesa RA. Therapy for myeloproliferative neoplasms: When, which agent, and how? Hematology Am Soc Hematol Educ Program. 2014;2014(1):277–286. doi: 10.1182/asheducation-2014.1.277. [DOI] [PubMed] [Google Scholar]
- 3.Tefferi A. Primary myelofibrosis: 2013 update on diagnosis, risk-stratification, and management. American journal of hematology. 2013;88:141–150. doi: 10.1002/ajh.23384. [DOI] [PubMed] [Google Scholar]
- 4.Emanuel RM, Dueck AC, Geyer HL, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: Prospective international assessment of an abbreviated symptom burden scoring system among patients with mpns. Journal of clinical oncology. 2012;30:4098–4103. doi: 10.1200/JCO.2012.42.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scherber R, Dueck AC, Johansson P, et al. The myeloproliferative neoplasm symptom assessment form (MPN-SAF): International prospective validation and reliability trial in 402 patients. Blood. 2011;118:401–408. doi: 10.1182/blood-2011-01-328955. [DOI] [PubMed] [Google Scholar]
- 6.Gangat N, Caramazza D, Vaidya R, et al. DIPSS plus: a refined dynamic international prognostic scoring system for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol. 2011;29:392–397. doi: 10.1200/JCO.2010.32.2446. [DOI] [PubMed] [Google Scholar]
- 7.Ballen KK, Shrestha S, Sobocinski KA, et al. Outcome of transplantation for myelofibrosis. Biology blood marrow transplant. 2010;16(3):358–367. doi: 10.1016/j.bbmt.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mesa RA, Silverstein MN, Jacobsen SJ, Wollan PC, Tefferi A. Population-based incidence and survival figures in essential thrombocythemia and agnogenic myeloid metaplasia: An olmsted county study, 1976–1995. American journal of hematology. 1999;61(1):10–15. doi: 10.1002/(sici)1096-8652(199905)61:1<10::aid-ajh3>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 9.Mesa RA, Mehta J, Wang H, et al. Epidemiology of myeloproliferative disorders in us - a real world analysis. ASH Annual Meeting Abstracts. 2012;120(21):2834. [Google Scholar]
- 10.Moulard O, Mehta J, Fryzek J, Olivares R, Iqbal U, Mesa RA. Epidemiology of myelofibrosis, essential thrombocythemia, and polycythemia vera in the european union. European journal of haematology. 2014;92(4):289–297. doi: 10.1111/ejh.12256. [DOI] [PubMed] [Google Scholar]
- 11.Moulard O, Mehta J, Olivares R, Iqbal U, Mesa RA. Epidemiology of myelofibrosis (mf), polycythemia vera (pv) and essential thrombocythemia (et) in the european union (eu) ASH Annual Meeting Abstracts. 2012;120(21):1744. [Google Scholar]
- 12.Anderson LA, Mcmullin MF. Epidemiology of mpn: What do we know? Current hematologic malignancy reports. 2014;9(4):340–349. doi: 10.1007/s11899-014-0228-z. [DOI] [PubMed] [Google Scholar]
- 13.Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase jak2 in human myeloproliferative disorders. Lancet. 2005;365(9464):1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 14•.James C, Ugo V, Le Couedic JP, et al. A unique clonal jak2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144–1148. doi: 10.1038/nature03546. Pivotal study demonstrating the role of the JAK tyrosine kinase mutation in myelofibrosis (MF) [DOI] [PubMed] [Google Scholar]
- 15.Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of jak2 in myeloproliferative disorders. The New England journal of medicine. 2005;352(17):1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 16.Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase jak2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer cell. 2005;7(4):387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 17.Zhao R, Xing S, Li Z, et al. Identification of an acquired jak2 mutation in polycythemia vera. The Journal of biological chemistry. 2005;280(24):22788–22792. doi: 10.1074/jbc.C500138200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furqan M, Mukhi N, Lee B, Liu D. Dysregulation of jak-stat pathway in hematological malignancies and jak inhibitors for clinical application. Biomarker research. 2013;1(1):5. doi: 10.1186/2050-7771-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gabler K, Behrmann I, Haan C. Jak2 mutants (e. G., jak2v617f) and their importance as drug targets in myeloproliferative neoplasms. Jak-Stat. 2013;2(3):e25025. doi: 10.4161/jkst.25025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hatzimichael E, Tsolas E, Briasoulis E. Profile of pacritinib and its potential in the treatment of hematologic disorders. Journal of blood medicine. 2014;5:143–152. doi: 10.2147/JBM.S51253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quintas-Cardama A, Kantarjian H, Cortes J, Verstovsek S. Janus kinase inhibitors for the treatment of myeloproliferative neoplasias and beyond. Nature reviews Drug discovery. 2011;10(2):127–140. doi: 10.1038/nrd3264. [DOI] [PubMed] [Google Scholar]
- 22.Rodig SJ, Meraz MA, White JM, et al. Disruption of the jak1 gene demonstrates obligatory and nonredundant roles of the jaks in cytokine-induced biologic responses. Cell. 1998;93(3):373–383. doi: 10.1016/s0092-8674(00)81166-6. [DOI] [PubMed] [Google Scholar]
- 23••.Neubauer H, Cumano A, Muller M, Wu H, Huffstadt U, Pfeffer K. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell. 1998;93(3):397–409. doi: 10.1016/s0092-8674(00)81168-x. Deficiency model detailing the role of JAK2 as an essential developmental checkpoint in hematopoeisis. [DOI] [PubMed] [Google Scholar]
- 24•.Parganas E, Wang D, Stravopodis D, et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93(3):385–395. doi: 10.1016/s0092-8674(00)81167-8. Study examining the essential role of JAK2 in receptor-mediated cytokine signaling. [DOI] [PubMed] [Google Scholar]
- 25.Park SY, Saijo K, Takahashi T, et al. Developmental defects of lymphoid cells in jak3 kinase-deficient mice. Immunity. 1995;3(6):771–782. doi: 10.1016/1074-7613(95)90066-7. [DOI] [PubMed] [Google Scholar]
- 26.Karaghiosoff M, Neubauer H, Lassnig C, et al. Partial impairment of cytokine responses in tyk2-deficient mice. Immunity. 2000;13(4):549–560. doi: 10.1016/s1074-7613(00)00054-6. [DOI] [PubMed] [Google Scholar]
- 27.Rochman Y, Kashyap M, Robinson GW, et al. Thymic stromal lymphopoietin-mediated stat5 phosphorylation via kinases jak1 and jak2 reveals a key difference from il-7-induced signaling. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(45):19455–19460. doi: 10.1073/pnas.1008271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griesinger F, Hennig H, Hillmer F, et al. A bcr-jak2 fusion gene as the result of a t(9;22)(p24;q11.2) translocation in a patient with a clinically typical chronic myeloid leukemia. Genes, chromosomes & cancer. 2005;44(3):329–333. doi: 10.1002/gcc.20235. [DOI] [PubMed] [Google Scholar]
- 29.Lacronique V, Boureux A, Valle VD, et al. A tel-jak2 fusion protein with constitutive kinase activity in human leukemia. Science. 1997;278(5341):1309–1312. doi: 10.1126/science.278.5341.1309. [DOI] [PubMed] [Google Scholar]
- 30.Cirmena G, Aliano S, Fugazza G, et al. A bcr-jak2 fusion gene as the result of a t(9;22)(p24;q11) in a patient with acute myeloid leukemia. Cancer genetics and cytogenetics. 2008;183(2):105–108. doi: 10.1016/j.cancergencyto.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Walz C, Ahmed W, Lazarides K, et al. Essential role for stat5a/b in myeloproliferative neoplasms induced by bcr-abl1 and jak2(v617f) in mice. Blood. 2012;119(15):3550–3560. doi: 10.1182/blood-2011-12-397554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quintas-Cardama A, Verstovsek S. Molecular pathways: Jak/stat pathway: Mutations, inhibitors, and resistance. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19(8):1933–1940. doi: 10.1158/1078-0432.CCR-12-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. The New England journal of medicine. 2013;369(25):2379–2390. doi: 10.1056/NEJMoa1311347. [DOI] [PubMed] [Google Scholar]
- 34.Nangalia J, Massie CE, Baxter EJ, et al. Somatic calr mutations in myeloproliferative neoplasms with nonmutated jak2. The New England journal of medicine. 2013;369(25):2391–2405. doi: 10.1056/NEJMoa1312542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mascarenhas J, Hoffman R. Ruxolitinib: The first fda approved therapy for the treatment of myelofibrosis. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18(11):3008–3014. doi: 10.1158/1078-0432.CCR-11-3145. [DOI] [PubMed] [Google Scholar]
- 36.Singer J, Al-Fayoumi S, Ma H, Komrokji RS, Mesa R, Verstovsek S. Comprehensive kinase profile of pacritinib, a non-myelosuppressive jak2 kinase inhibitor in phase 3 development in primary and post et/pv myelofibrosis. 2014;124 [Google Scholar]
- 37.Tefferi A. Jak inhibitors for myeloproliferative neoplasms: Clarifying facts from myths. Blood. 2012;119(12):2721–2730. doi: 10.1182/blood-2011-11-395228. [DOI] [PubMed] [Google Scholar]
- 38.Patnaik MM, Lasho TL, Finke CM, et al. Mpl mutation effect on jak2 46/1 haplotype frequency in jak2v617f-negative myeloproliferative neoplasms. Leukemia. 2010;24(4):859–860. doi: 10.1038/leu.2010.1. [DOI] [PubMed] [Google Scholar]
- 39.Tefferi A. Novel mutations and their functional and clinical relevance in myeloproliferative neoplasms: Jak2, mpl, tet2, asxl1, cbl, idh and ikzf1. Leukemia. 2010;24(6):1128–1138. doi: 10.1038/leu.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40•.Harrison C, Kiladjian JJ, Al-Ali HK, et al. Jak inhibition with ruxolitinib versus best available therapy for myelofibrosis. The New England journal of medicine. 2012;366(9):787–798. doi: 10.1056/NEJMoa1110556. Pivotal Phase III COMFORT I study of first-in-class JAK inhibitor ruxolitinib in MF. [DOI] [PubMed] [Google Scholar]
- 41•.Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. The New England journal of medicine. 2012;366(9):799–807. doi: 10.1056/NEJMoa1110557. Pivotal Phase III COMFORT II study of first-in-class JAK inhibitor ruxolitinib in MF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tefferi A, Guglielmelli P, Lasho TL, et al. CALR and ASXL1 mutations-based molecular prognostication in primary myelofibrosis: an international study of 570 patients. Leukemia. 2014;28:1494–1500. doi: 10.1038/leu.2014.57. [DOI] [PubMed] [Google Scholar]
- 43.Tefferi A, Lasho TL, Finke CM, et al. CALR vs JAK2 vs MPL-mutated or triple-negative myelofibrosis: clinical, cytogenetic and molecular comparisons. Leukemia. 2014;28:1472–1477. doi: 10.1038/leu.2014.3. [DOI] [PubMed] [Google Scholar]
- 44.Kiyoi H. Flt3 inhibitors: Recent advances and problems for clinical application. Nagoya journal of medical science. 2015;77(1–2):7–17. [PMC free article] [PubMed] [Google Scholar]
- 45.Small D. Flt3 mutations: Biology and treatment. ASH Education Program Book. 2006;2006(1):178–184. doi: 10.1182/asheducation-2006.1.178. [DOI] [PubMed] [Google Scholar]
- 46.Taylor SJ, Dagger SA, Thien CB, Wikstrom ME, Langdon WY. Flt3 inhibitor ac220 is a potent therapy in a mouse model of myeloproliferative disease driven by enhanced wild-type flt3 signaling. Blood. 2012;120(19):4049–4057. doi: 10.1182/blood-2012-06-436675. [DOI] [PubMed] [Google Scholar]
- 47.Desterke C, Bilhou-Nabera C, Guerton B, et al. Flt3-mediated p38-mapk activation participates in the control of megakaryopoiesis in primary myelofibrosis. Cancer research. 2011;71(8):2901–2915. doi: 10.1158/0008-5472.CAN-10-1731. [DOI] [PubMed] [Google Scholar]
- 48.Kuriakose E. The role of the megakaryocyte in primary myelofibrosis. 2015;2015(April 14) [Google Scholar]
- 49.Corporation I. Jakafi prescribing information. 2014. [Google Scholar]
- 50.Vannucchi AM, Kiladjian JJ, Griesshammer M, et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med. 2015;372:426–435. doi: 10.1056/NEJMoa1409002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Galli S, Mclornan D, Harrison C. Safety evaluation of ruxolitinib for treating myelofibrosis. Expert opinion on drug safety. 2014;13(7):967–976. doi: 10.1517/14740338.2014.916273. [DOI] [PubMed] [Google Scholar]
- 52.Deeg HJ, Odenike O, Scott BL, et al. Phase ii study of sb1518, an orally available novel jak2 inhibitor, in patients with myelofibrosis. ASCO Meeting Abstracts. 2011;29(15_suppl):6515. [Google Scholar]
- 53.Verstovsek S, Deeg HJ, Odenike O, et al. Phase 1/2 study of sb1518, a novel jak2/flt3 inhibitor, in the treatment of primary myelofibrosis. ASH Annual Meeting Abstracts. 2010;116(21):3082. [Google Scholar]
- 54.Verstovsek S, Odenike O, Scott B, et al. Phase i dose-escalation trial of sb1518, a novel jak2/flt3 inhibitor, in acute and chronic myeloid diseases, including primary or post-essential thrombocythemia/polycythemia vera myelofibrosis. ASH Annual Meeting Abstracts. 2009;114(22):3905. [Google Scholar]
- 55••.Komrokji RS, Seymour JF, Roberts AW, et al. Results of a phase 2 study of pacritinib (sb1518), a jak2/jak2(v617f) inhibitor, in patients with myelofibrosis. Blood. 2015 doi: 10.1182/blood-2013-02-484832. Phase II study reporting the efficacy of pacritinib in MF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Komrokji RS, Wadleigh M, Seymour JF, et al. Results of a phase 2 study of pacritinib (sb1518), a novel oral jak2 inhibitor, in patients with primary, post-polycythemia vera, and post-essential thrombocythemia myelofibrosis. ASH Annual Meeting Abstracts. 2011;118(21):282. [Google Scholar]
- 57••.Mesa RA, Egyed M, Szoke A, et al. Results of the persist-1 phase iii study of pacritinib (pac) versus best available therapy (bat) in primary myelofibrosis (pmf), post-polycythemia vera myelofibrosis (ppv-mf), or post-essential thrombocythemia-myelofibrosis (pet-mf). Abstract lba7006. Presented at. 2015 ASCO Annual Meeting; Chicago, Illinois. 2015. First results of Phase III study of pacritinib in MF. [Google Scholar]
- 58.CTI BioPharma. Oral Pacritinib Versus Best Available Therapy to Treat Myelofibrosis with Thrombocytopenia (PAC326) NCT02055781. https://clinicaltrials.gov.
- 59.William AD, Lee AC, Blanchard S, et al. Discovery of the macrocycle 11-(2-pyrrolidin-1-yl-ethoxy)-14,19-dioxa-5,7,26-triaza-tetracyclo[19.3.1.1(2,6) 1(8,12)]heptacosa-1(25),2(26),3,5,8,10,12(27),16,21,23-decaene (sb1518), a potent janus kinase 2/fms-like tyrosine kinase-3 (jak2/flt3) inhibitor for the treatment of myelofibrosis and lymphoma. Journal of medicinal chemistry. 2011;54(13):4638–4658. doi: 10.1021/jm200326p. [DOI] [PubMed] [Google Scholar]
- 60.Poulsen A, William A, Blanchard S, et al. Structure-based design of oxygen-linked macrocyclic kinase inhibitors: Discovery of sb1518 and sb1578, potent inhibitors of janus kinase 2 (jak2) and fms-like tyrosine kinase-3 (flt3) Journal of computer-aided molecular design. 2012;26(4):437–450. doi: 10.1007/s10822-012-9572-z. [DOI] [PubMed] [Google Scholar]
- 61.Hart S, Goh KC, Novotny-Diermayr V, et al. Sb1518, a novel macrocyclic pyrimidine-based jak2 inhibitor for the treatment of myeloid and lymphoid malignancies. Leukemia. 2011;25(11):1751–1759. doi: 10.1038/leu.2011.148. [DOI] [PubMed] [Google Scholar]
- 62.Hart S, Goh KC, Novotny-Diermayr V, et al. Pacritinib (sb1518), a jak2/flt3 inhibitor for the treatment of acute myeloid leukemia. Blood cancer journal. 2011;1(11):e44. doi: 10.1038/bcj.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seymour J, Scott B, Roberts A, et al. Long-term safety and efficacy analysis of the two phase 1 studies of sb1518, a novel oral jak2 inhibitor, in patients with advanced myeloid malignancies. Presented at: 16th Congress of the European Hematology Association; London, England. 2011. [Google Scholar]
- 64.Al-Fayoumi S, Wang L, Li H, Wada R, Dean JP, Verstovsek S. Exposure-response analysis for pacritinib (sb1518), a novel oral jak2/flt3 inhibitor, in patients with myelofibrosis. 2013;122 [Google Scholar]
- 65.Al-Fayoumi S, Wang L, Dean JP, Benner S. Characterization of the pharmacokinetic and pharmacodynamic properties of pacritinib, a novel oral jak2/flt3 inhibitor, in patients with myelofibrosis, aml, and lymphoma. Presented at: 18th Congress of the European Hematology Association; Stockholm, Sweden. 2013. [Google Scholar]
- 66.Verstovsek S, Cernohous P, Komrokji RS, et al. Safety overview of phase 1–2 studies of pacritinib, a non-myelosuppressive jak2/flt3 inhibitor, in patients with hematological malignancies. Presented at: European Hematology Association; Stockholm, Sweden. 2013. [Google Scholar]
- 67.Verstovsek S, Dean JP, Cernohous P, et al. Pacritinib, a dual jak2/flt3 inhibitor: An integrated efficacy and safety analysis of phase ii trial data in patients with primary and secondary myelofibrosis (mf) and platelet counts ≤ 100,000/μl. 2013;122 [Google Scholar]
- 68.Verstovsek S, Tam CS, Wadleigh M, et al. Phase i evaluation of xl019, an oral, potent, and selective jak2 inhibitor. Leukemia research. 2014;38(3):316–322. doi: 10.1016/j.leukres.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Verstovsek S, Hoffman R, Mascarenhas J, et al. A phase i, open-label, multi-center study of the jak2 inhibitor azd1480 in patients with myelofibrosis. Leukemia research. 2015;39(2):157–163. doi: 10.1016/j.leukres.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pardanani A, Laborde RR, Lasho TL, et al. Safety and efficacy of cyt387, a jak1 and jak2 inhibitor, in myelofibrosis. Leukemia. 2013;27(6):1322–1327. doi: 10.1038/leu.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abdelrahman RA, Begna K, Al-Kali A, et al. Momelotinib treatment-associated neuropathy: Prevalence, risk factors and outcome in 100 patients with myelofibrosis. 2014;124 doi: 10.1111/bjh.13262. [DOI] [PubMed] [Google Scholar]
- 72.Nakaya Y, Shide K, Naito H, et al. Effect of ns-018, a selective jak2v617f inhibitor, in a murine model of myelofibrosis. Blood cancer journal. 2014;4:e174. doi: 10.1038/bcj.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Younes A, Fanale M, Mclaughlin P, et al. Phase-i study of the novel oral jak-2 inhibitor sb1518 in patients with relapsed lymphoma: Evidence of clinical and biologic activity. ASH Annual Meeting Abstracts. 2009;114(22):588. doi: 10.1200/JCO.2012.42.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Younes A, Fanale MA, Mclaughlin P, Copeland A, Zhu J, De Castro Faria S. Phase i study of a novel oral jak-2 inhibitor sb1518 in patients with relapsed lymphoma: Evidence of clinical and biologic activity in multiple lymphoma subtypes. ASH Annual Meeting Abstracts. 2010;116(21):2830. doi: 10.1200/JCO.2012.42.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Younes A, Romaguera J, Fanale M, et al. Phase i study of a novel oral janus kinase 2 inhibitor, sb1518, in patients with relapsed lymphoma: Evidence of clinical and biologic activity in multiple lymphoma subtypes. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(33):4161–4167. doi: 10.1200/JCO.2012.42.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]