Abstract
A predisposing factor for development of the hyperglycaemic state of gestational diabetes mellitus (GDM) is obesity. We previously showed that increasing maternal obesity is associated with significant reductions in placental mitochondrial respiration. MicroRNA (miR)-143 has been previously shown to regulate the metabolic switch from oxidative phosphorylation to aerobic glycolysis in cancer tissues. We hypothesized that mitochondrial respiration is reduced and aerobic glycolysis is up-regulated via changes in miR-143 expression in the placenta of women with GDM. Placental tissue was collected at term from women with A1GDM (controlled by diet), A2GDM (controlled by medication) and body mass index (BMI)-matched controls (CTRL). miR-143 expression was measured by RT-PCR. Expression of mitochondrial complexes, transcription factors peroxisome proliferator-activated receptor-γ co-activator 1α (PGC1α) and peroxisome proliferator-activated receptor γ (PPARγ), components of mammalian target of rapamycin (mTOR) signalling, glucose transporter GLUT1 and glycolytic enzymes [hexokinase-2 (HK-2), phosphofructokinase (PFK) and lactate dehydrogenase (LDH)] were measured by Western blot. Trophoblast respiration was measured by XF24 Analyser. Expression of miR-143, mitochondrial complexes, and PPARγ and PGC1α, which act downstream of miR-143, were significantly decreased in A2GDM placentae compared with A1GDM and CTRL (P<0.01). Placental hPL (human placental lactogen) levels, expression of glycolytic enzymes, GLUT1 and mTOR signalling were also significantly increased by more than 2-fold in A2GDM compared with A1GDM and CTRL (P<0.05). There was a 50% reduction in mitochondrial respiration in trophoblast cells isolated from A2GDM placentae. Overexpression of miR-143 was able to increase mitochondrial respiration, increase protein expression of mitochondrial complexes and decrease expression of glycolytic enzymes by 40% compared with A2GDM. Down-regulation of miR-143 mediates the metabolic switch from oxidative phosphorylation to aerobic glycolysis in placenta of women with A2GDM.
Keywords: gestational diabetes, mammalian target of rapamycin (mTOR), miR-143, mitochondrial function, placenta
INTRODUCTION
Gestational diabetes mellitus (GDM) is a common metabolic disorder of pregnancy, manifest by impaired maternal glucose tolerance from the late second trimester of pregnancy onwards. The prevalence of GDM has been estimated at ~7% of pregnancies in the USA [1]. Gestational diabetes affects both the mother and the offspring. Elevated blood glucose levels in the mother result in elevated glucose in the fetus, leading to increased fetal insulin secretion. Insulin is a fetal growth factor leading to macrosomia and increased risk of caesarean delivery and birth trauma including vaginal tears, shoulder dystocia and asphyxia [2]. A substantial body of evidence suggests that an abnormal intra-uterine milieu elicited by maternal metabolic disturbances as diverse as malnutrition, placental insufficiency, obesity and diabetes may be able to programme susceptibility of the fetus to develop chronic diseases such as obesity, hypertension, cardiovascular diseases and type 2 diabetes in later life [3]. Studies in the Pima Indian population of the USA showed that the offspring of women with gestational diabetes developed fasting hyperglycaemia, higher rates of abnormal glucose tolerance and obesity, compared with offspring of normoglycemic women [4].
As the placenta regulates nutrient composition and supply from mother to fetus and is the source of hormonal signals that affect maternal and fetal metabolism, appropriate development of the placenta is crucial to normal fetal development [5]. The placenta itself consumes nutrients and therefore the considerable metabolic activity of the placenta provides a large and fundamentally important contribution to determining both the quality and quantity of nutrients available to fetus. Thus, consideration of placental metabolism per se is essential to complete understanding of how the placenta regulates nutrient transfer, energy balance, fetal growth and hence fetal programming [6].
Research on trophoblast over the past decades has underlined striking similarities between the proliferative, migratory and invasive properties of trophoblast and those of cancer cells [7]. Metabolic reprogramming is widely observed during cancer development to confer the ability to survive and proliferate on cancer cells. Cancer cells favour the `Warburg effect', i.e. enhanced glycolysis or aerobic glycolysis to generate energy over mitochondrial oxidative phosphorylation, even when the ambient oxygen supply is sufficient [8]. On the other hand, growth factor signalling mediated by mammalian target of rapamycin (mTOR) drives metabolism of cancer cells by regulating expression of key enzymes in metabolic pathways. Notably, mutations causing hyper-activation of mTOR are common in cancers [9].
miRNA are small non-coding RNAs that regulate gene expression either through translational repression or mRNA degradation [10]. miRNAs regulate cell proliferation, apoptosis, tumorigenesis and many other physiological or pathological processes including glucose metabolism [11]. Human miR-143, located on chromosome 5, is involved in adipogenesis and has been reported to be down-regulated in several cancers, where it inhibits hexokinase-2 (HK-2) and up-regulates aerobic glycolysis [12].
We and others have previously provided evidence for elevated oxidative stress, hypoxia and inflammation in the placenta in various pathological situations [13,14] and their relationship to altered placental function and fetal programming [15]. We have shown that mitochondrial function/oxidative phosphorylation is significantly reduced in the placenta with increasing maternal adiposity [16,17]. Recently, it was shown that mitochondrial protein expression is reduced in skeletal muscle of GDM patients [18].
Maternal obesity and GDM constitute two common, often comorbid pregnancy complications [19]. In the present study, we hypothesized that mitochondrial function and associated metabolic pathways are dysregulated in placentae from pregnancies complicated by gestational diabetes, and that miR-143 has a role in regulating mitochondrial function in the GDM placentae. We compared trophoblast respiration and mitochondrial function in placentae from women diagnosed with GDM classified as A1GDM (managed with diet to control blood glucose) and A2GDM (needs pharmacological intervention either by glyburide or insulin) with maternal BMI-matched control placentae.
MATERIALS AND METHODS
Oligomycin, FCCP [4-(trifluoromethoxy) phenyl hydrazone], rotenone and antimycin A were obtained from Sigma and dissolved in DMSO as 2.5 mM stock solutions. The human OxPhos antibody cocktail (MS601) was purchased from Abcam. AKT, AKT (Ser473), 4EBP, 4EBP (Thr389), S6K, pS6K (Thr37/46) and peroxisome proliferator-activated receptor-γ co-activator 1α (PGC1α), antibodies were purchased from Cell Signaling Technologies, peroxisome proliferator-activated receptor γ (PPARγ) antibody was purchased from Santa Cruz Biotechnology.
Collection of placental tissue
Placentas were collected from the Labor and Delivery Unit at University Hospital under a protocol approved by the Institutional Review Board of the University of Texas Health Science Center San Antonio, with informed consent from the patients. Placental villous tissue was collected at term after C-section (no labour) from women with gestational diabetes either treated with diet alone (A1GDM) or treated with glyburide or insulin (A2GDM) (n = 6/each group). The control group (CTRL) included placentae collected from healthy women with uncomplicated pregnancies, matched for pre-pregnancy BMI (within 0.5 units difference) and gestational age (n = 6). Villous tissue was randomly sampled from five sites in the placenta as described previously [20], flash frozen in liquid nitrogen and stored at −80°C or immediately used for trophoblast culture.
Placental lactogen ELISA
Human placental lactogen (hPL) was measured in placental homogenate using a sandwich ELISA from Genway (GWB-8582D2). Briefly, an aliquot of placental homogenate is incubated in wells coated with monoclonal anti-hPL antibody conjugated with horseradish peroxidase. The amount of bound peroxidase is proportional to the concentration of hPL in the homogenate.
RNA isolation and RT-PCR
Total RNA including miRNA was isolated from villous tissue and trophoblast cells using miRNAeasy kit (Qiagen). RNA concentration was measured using a Nanodrop 2000. The High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) was used for RT of 1 μg of the total RNA from villous tissue and trophoblast cells. mRNA expression of HK-2, phosphofructokinase (PFK), lactate dehydrogenase (LDH), glucose transporter 1 (GLUT1) PPARγ and PGC1α was measured (Primer sequence, listed in Supplementary Table S1). The CT values were normalized to 18S RNA. For the relative quantification of miR-143 in placental tissues, 5–10 ng of total RNA was reverse transcribed using the TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems). miR-143 expression was determined by using TaqMan assays (Applied Biosystems) and the TaqMan assay custom designed for U18 RNA was used for normalization. Reactions were performed using a Step-One Plus system (Applied Biosystems). U18 miRNA expression was used as the internal control for miR-143 expression. The changes in the cycle threshold (CT) values were calculated by the equation ΔCT = CT target − CT input. The fold differences were calculated as follows: fold difference = 2− Δ(ΔCT)CT.
Western blots
Total proteins for Western blotting were obtained from villous tissue using a standard differential centrifugation protocol and suspended in isolation medium (0.25 M sucrose and 1 mM EDTA, pH 7.4) supplemented with protease and phosphatase inhibitor cocktail (Sigma). Total protein concentrations in the fraction were determined using Bradford's reagent (Bio-Rad Laboratories). Total proteins (25 μg) were separated on 4–20% precast linear gradient gels (Bio-Rad Laboratories), transferred to nitrocellulose membranes and blocked with 5% (w/v) nonfat milk in TBST for 1 h. Membranes were incubated overnight at 4°C with primary antibody diluted in 1% nonfat milk (w/v) in TBST and detected using an appropriate peroxidase conjugated secondary antibody. Products were visualized by ECL chemiluminescence (Millipore). Band intensities were measured using the G-box system (Syngene).
Isolation and culture of primary trophoblasts
Villous cytotrophoblasts (CTs) were isolated from placental tissue of women with A2GDM and BMI matched CTLR using a trypsin/DNAse digestion and percoll gradient purification method as we have previously described [21]. CTs cells were plated in Seahorse XF24 plates, cultured in DMEM containing glucose (17 mM) and allowed to syncytialize over 72 h.
Reporter assay
Primary trophoblast cells isolated from A2GDM placentae were immediately transfected with 1 nM pre miR-143 or negative control miRNA as described previously [22]. After 24 h cells were washed and transfected with the pGL3+vector containing 3′UTR HK-2 or mutant 3′UTR HK-2 along with Renilla normalization control pRL-TK [22]. Luciferase and Renilla luminescence was measured 48 h later using the Dual–Glo luciferase kit (E2940 Promega).
Assessment of mitochondrial function
Mitochondrial function of the cultured syncytiotrophoblasts (ST) was measured using a Seahorse XF24 analyser (Seahorse Biosciences) as described [21]. Oxygen consumption rates (OCR) were normalized to total cellular protein (Bradford method). Basal respiration was calculated from four baseline OCR readings. ATP coupled, maximum respiration, spare capacity, proton leak and non-mitochondrial respiration were calculated from OCR readings following the injection of oligomycin (1 μM), FCCP (1 μM) and a mixture of rotenone (3 μM) and antimycin A (1.5 μM).
Statistical analysis
Data are reported as mean ± S.E.M. Comparisons between two groups were performed using a Student's t test. One or two-way ANOVA and Turkey's post-hoc test was used to compare data sets with more than two groups. P < 0.05 was considered significant. The OCR parameters were analysed by regression and correlation analysis against maternal BMI using Excel and GraphPad (5.0).
RESULTS
Clinical characteristics of the patients involved in the present study are shown in Table 1. The placental weight was significantly higher (P < 0.04) in A2GDM (833 ± 53 g) but not in A1GDM (720 ± 76 g, P < 0.05) compared with control group (690 ± 41 g). The fetal/placental weight ratio was also significantly lower (P < 0.03) in A2GDM (3.45 ± 0.02) but not in A1GDM (4.54 ± 0.03) compared with control group (5.26 ± 0.02). No significant difference was observed in maternal glucose during the diagnostic test for gestational diabetes between women who eventually developed A1 or A2 gestational diabetes.
Table 1.
Clinical charactercteristics of study patients
| Group | BMI (kg/m2) | Age (years) | Gest age (weeks) | Birth weight (g) | Placental weight (g) | Fetal/placental weight ratio | OGTT 0 h (glucose mg/dl) | OGTT 1 h (glucose mg/dl) | OGTT 2 h (glucose mg/dl) | OGTT 3 h (glucose mg/dl) |
|---|---|---|---|---|---|---|---|---|---|---|
| CTRL | 29.3±0.2 | 26±2 | 38.7±0.5 | 3203±354 | 690±41 | 5.26±0.02 | – | – | – | – |
| A1GDM | 27.8±1.5 | 32±1 | 39.1±0.5 | 3511±116 | 720±76 | 4.54±0.03 | 95.6±3.1 | 185.3±4.8 | 160.8±10.5 | 142.3±7.6 |
| A2GDM | 30.5±2.2 | 35±1* | 38.2±0.3 | 3399±243 | 833±53* | 3.45±0.02* | 95.7±1.1 | 188.7±11.4 | 180.0±8.7 | 141.5±13.5 |
BMI, maternal age and gestational (Gest) age of the control, A1 and A2GDM groups are shown. Placental efficiency is shown as fetal/placental weight ratio. Blood glucose levels were indicated as measured during the oral glucose tolerance test (OGTT). Values are mean ± S.E.M., n = 6 in each group.
P<0.05 compared with CTRL.
Dysregulation of placental and mitochondrial function in GDM placentae
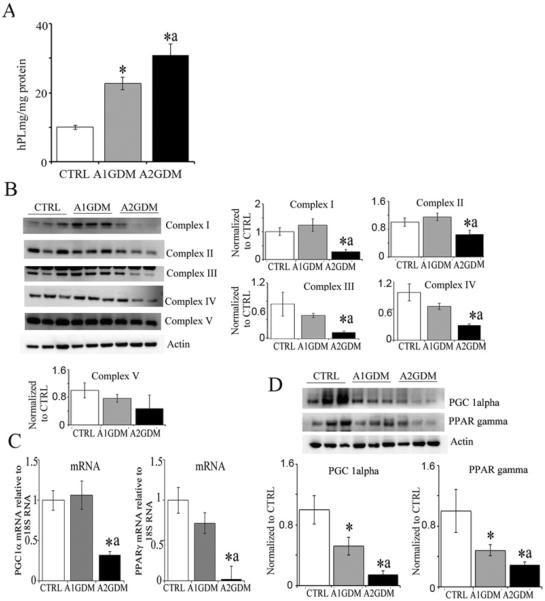
hPL production is an indicator of placental function. hPL levels in placentae from A1GDM and A2GDM were significantly higher than BMI matched CTRL (Figure 1A). Previously we have shown that mitochondrial function is compromised in the placentae with increasing maternal adiposity when compared with CTRL placentae. We found mitochondrial electron transfer complex subunits I, II, III and IV were significantly reduced in A2GDM placentae compared with CTRL and to A1GDM. However, complexes III and IV were also significantly reduced in A1GDM compared with CTRL (Figure 1B). Messenger RNA expression of PGC1α, which regulates mitochondrial biogenesis, and PPARγ, an important transcription factor regulating mitochondrial fatty acid oxidation, was significantly reduced in placental tissue of A2GDM placentae compared with CTRL but was not different between A1GDM and CTRL (Figure 1C). However at the protein level expression of both PGC1α and PPARγ was significantly reduced in A1GDM compared with CTRL and was further significantly reduced in A2GDM compared with A1GDM (Figure 1D).
Figure 1. Dysregulation of mitochondrial function in GDM placentae.
(A) hPL levels measured in placental tissue homogenates. (B) Representative Western blots and quantification of mitochondrial complex protein expression in placental tissue. (C) mRNA expression of PGC1α and PPARγ in placental villous tissue relative to 18S RNA. (D) Representative Western blot and quantification of PGC1α and PPARγ expression relative to actin. Values are mean ± S.E.M., n = 6 in each group. *P<0.05 compared with CTRL and aP<0.05 compared with A1GDM.
Glycolytic pathway is activated in GDM placentae
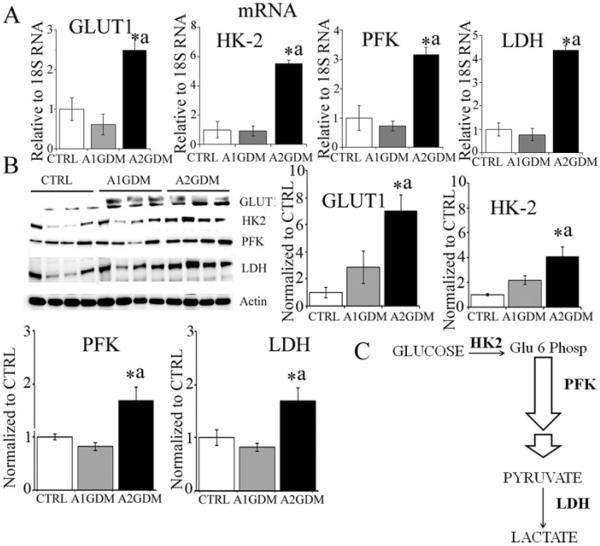
Expression of the glucose transporter GLUT1, of HK-2, the first enzyme of the glycolytic pathway, and PFK and LDH was significantly up-regulated both at mRNA and protein levels in A2GDM compared with CTRL and to A1GDM placental tissue (Figures 2A and 2B). No difference was observed between A1GDM and CTRL.
Figure 2. Expression of glucose transporter GLUT1 and glycolytic enzymes in placentae with GDM.
(A) mRNA expression of GLUT1, HK-2, PFK and LDH relative to 18S RNA. (B) Representative immunoblots and quantification of GLUT1, HK-2, PFK and LDH expression. Actin was used as the loading control. Values are mean ± S.E.M. n = 6 in each group. *P<0.05 compared with CTRL, aP<0.05 compared with A1GDM group. (C) Glycolysis pathway.
Expression of miR-143 in placentae from pregnancies with GDM
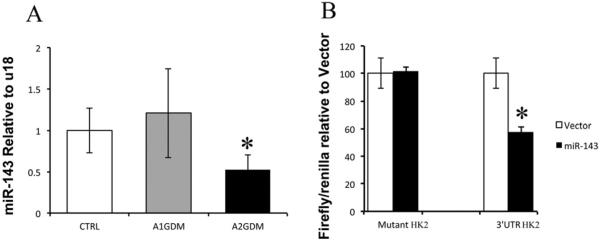
miR-143 has been previously reported to up-regulate aerobic glycolysis in several cancer tissues. In the present study, we found a significant 50% reduction of miR-143 in placental tissue from A2GDM but no such difference was observed in A1GDM compared with the BMI matched CTRL group (Figure 3A). Luciferase reporter activity was significantly down-regulated in trophoblast cells co-transfected with miR-143 and HK-2 (3′UTR) compared with those co-transfected with miR-143 and HK-2 (mutant 3′UTR). Thus, the Luciferase reporter assay confirmed that HK-2 is a direct target of miR-143 (Figure 3B).
Figure 3. Expression of miR-143 and its target gene HK-2 in placentae with GDM.
(A) miR-143 expression relative to U18 in placentas from CTRL, A1GDM and A2GDM. Expression in A1 and A2GDM were normalized to values in CTRL cells. (B) Validation of HK-2 as miR-143 target gene. Luciferase reporter assay in trophoblast cells overexpressing 3′UTR HK-2 or mutant HK-2 with or without miR-143 overexpression. Values are mean ± S.E.M. n = 6 in each group. *P<0.05 compared with CTRL.
Activation of mTOR signalling in GDM placentae
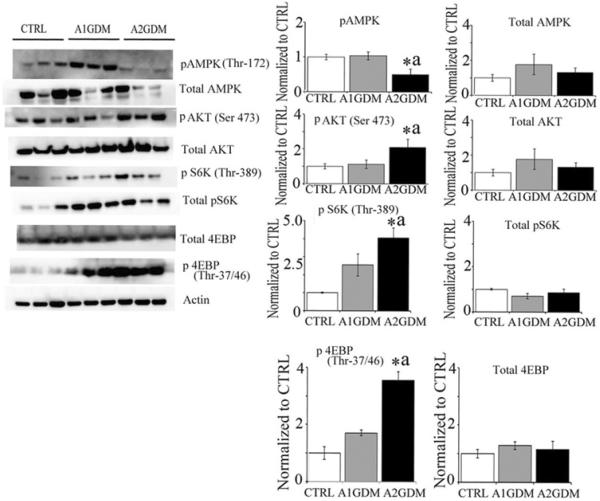
mTOR signalling constitutes a master regulator of protein translation, thereby controlling cell growth and metabolism. The energy status of the cell is signalled to mTORC1 through AMP-activated protein kinase (AMPK), a sensor of intracellular energy status. In response to energy depletion (low ATP/ADP ratio), AMPK is activated and in turn reduces mTORC1 activation. Additionally, AMPK can reduce mTORC1 activity in response to energy depletion by directly phosphorylating Raptor. In the present study, we found significantly reduced phosphorylation of AMPK and elevated mTOR activation. The downstream effects of mTORC1 are mediated by phosphorylation of eukaryotic initiation factor 4E-binding protein 1 (4EBP1) and p70 S6 kinase (S6K). We found significant up-regulation of p70 S6K and 4EBP1 in A2GDM compared with CTRL and A1GDM groups (Figure 4).
Figure 4. Activation of mTOR signalling in GDM placentae.
Representative Western blots and quantification of phospho (Thr172) and total AMPK; phospho (Ser473) and total AKT; phospho (Thr389) and total S6K; phospho (Thr37/46) and total 4EBP. Actin was used as the loading control. n = 6 in each group. *P<0.05 compared with CTRL, aP<0.05 compared with A1GDM.
Overexpression of miR-143 reduces aerobic glycolysis and rescues mitochondrial complexes in trophoblast cells
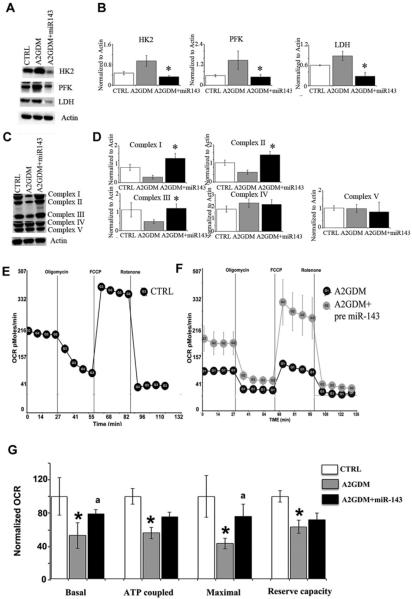
When miR-143 was overexpressed in trophoblast cells (72 h) from A2GDM placentae a significant reduction in HK-2, PFK and LDH expression was seen compared with controls (Figures 5A and 5B). On the other hand mitochondrial complexes I, II and III were significantly up-regulated when miR-143 was overexpressed in A2GDM trophoblast cells (Figures 5C and 5D). No changes were observed in complexes IV and V in trophoblast cells where miR-143 was overexpressed. Mitochondrial oxygen consumption was measured using the mitostress test to determine basal, ATP-coupled and maximal respiration and the reserve (or spare) capacity of trophoblast using the Seahorse XF24 [21]. We found no difference in the rates of trophoblast syncytialization with GDM. However, we found significant down-regulation of basal, ATP coupled, maximum respiration and spare capacity in the ST from A2GDM compared with BMI matched CTRL (Figures 5E and 5F). Transient overexpression of pre-miR-143 in the trophoblast was able to rescue the basal and maximum mitochondrial respiration to a level close to the BMI matched CTRL (Figure 5G).
Figure 5. miR-143 overexpression reduces expression of glycolytic enzymes and rescues mitochondrial function in trophoblast cells of A2GDM.
(A) Representative immunoblots and (B) quantification of glycolytic enzyme expression in trophoblast cells of CTRL and of A2GDM with and without overexpression of miR-143, n = 4. (C) Representative immunoblots and (D) quantification of mitochondrial complex expression in trophoblast cells of CTRL and of A2GDM with or without overexpression of miR-143. n = 3 for complex I and n = 4 for complexes II–V, Values are mean ± S.E.M. *P<0.05 compared with CTRL, aP<0.05 compared with A2GDM. Mitochondrial respiratory parameters were measured in ST of (E) CTRL and (F) A2GDM pregnancies with or without miR-143. OCR were measured using a Seahorse Bioscience XF24 extracellular flux analyser as described previously [21]. (G) OCR values for each respiration parameter in A2GDM with or without miR-143 were normalized to values in CTRL cells. n = 4 placentas in each group. Values are mean ± S.E.M. *P< 0.05 compared with CTRL, aP<0.05 compared with A2GDM.
DISCUSSION
The placenta has multiple roles: providing the immune interface between the mother and the fetal allograft, serving to transport nutrients and waste products between mother and fetus and being a source of many peptide and steroid hormones that influence fetal, placental and maternal metabolism and development [5]. The placenta is thought to have a critical role in the pathogenesis of GDM, as GDM-associated complications resolve following delivery of the placenta [23]. Although there was a tendency towards increased fetal weight in the A1 and A2GDM groups compared with BMI matched controls the differences were not significant. However the placental weight was higher and the fetal/placental weight ratio significantly lower in the A2GDM group suggesting that placental hypertrophy occurs to maintain fetal weight and hence the placenta is less efficient compared with other groups. The Apgar score was also not significantly different between the groups (results not shown). So fetal size is maintained by placental hypertrophy in the face of reduced placental respiration.
hPL is a major stimulus for adaptation of the maternal endocrine pancreas during gestation, thereby increasing β-cell mass-insulin production and hence helping the mother to compensate for hyperglycaemia during pregnancy [24]. Concentrations of hPL increase progressively throughout gestation and are secreted directly into both fetal and maternal circulations [25]. Increased levels of hPL in the maternal circulation have been reported, thought to be due to increased placental size [26], although our data suggest increased production per unit of tissue. Maternal hyperglycaemia impacts the production of various placental proteins and hormones including hPL, with dysregulation potentially adversely impacting fetal growth and postnatal metabolic function [25].
In healthy humans and rats, at least one-third of glucose entering the placenta is metabolized by oxidative phosphorylation through mitochondria to cover the high metabolic rate of the trophoblast [27]. In the present study, we observed significant reduction in expression of mitochondrial complexes in vivo and respiration in vitro in the A2GDM placentae. We have previously shown reduction in the expression of mitochondrial respiratory complexes and activity in placentae from pregnancies complicated by preeclampsia and maternal adiposity as well as reduced respiration in the isolated trophoblast cells of placentae from obese mothers [28,29]. Hastie et al. [17] have shown that placentae of women with pre-pregnancy obesity or pre-gestational diabetes have decreased expression of mitochondrial respiratory chain enzymes, which may have detrimental consequences on placental function. Recently a pilot study by Qiu et al. [30] found reduced mitochondrial DNA copy number and oxidative DNA damage in placental tissue from GDM women. The A2GDM subjects were being treated with either glyburide or insulin and it is conceivable that the reduction in respiration is due to the drug, however very little if any glyburide crosses the placenta [31] as the placenta actively effluxes glyburide [32], and it is bound tightly to plasma proteins making it unlikely that there is cellular uptake and effect in the placenta.
The peroxisome proliferator-activated receptors (PPARs) are a family of transcription factors that regulate energy balance by promoting either energy deposition or energy dissipation [33]. PGC1α is a co-activator of PPARγ that induces the expression of genes that promote mitochondrial biogenesis and fatty acid oxidation [34]. Down-regulation of both PGC1α and PPARγ contributes to decreasing mitochondrial function in A2GDM placentae. Our data are consistent with the recent study in high fat-fed rats showing reduced mRNA levels of genes associated with mitochondrial function, including PGC1α in the placenta as well in the liver and skeletal muscle of the offspring [34].
GLUT1 is a ubiquitous isoform of the facilitated-diffusion glucose transporter family, expressed in almost all tissues including ST of placenta [35]. GLUT1 expression and activity appear to be inversely related to extracellular glucose concentration, however, within the physiological range, GLUT1 expression is relatively refractory to glucose concentration [36]. We found significantly elevated GLUT1 expression in A2GDM placentae compared with A1GDM and CTRL, which is consistent with reports that GLUT1 transporter expression is increased in the basal membrane in diabetic pregnancies [37]. We reasoned that increased availability and consumption of glucose in GDM placentae might be reflected by an increased expression or activity of rate-limiting enzymes in the glycolytic pathway. Consistently we found significantly higher expression of HK-2, PFK (rate-limiting enzymes) and LDH (marker for aerobic glycolysis) in A2GDM placentae. It appears then that mitochondrial function is compromised and glycolysis is up-regulated in A2GDM placentae.
miRNAs are expressed in placenta and alterations in expression have been described in association with exposure to xenobiotics and cigarette smoking or with adverse pregnancy outcomes including PE and growth restriction [10]. Placenta-specific miRNAs may contribute to the pathology of GDM. miR-143 represents one of the best-characterized anti-oncomiRs [38], is located at a fragile site, often inhibited in cancers, and its expression is frequently down-regulated in cancer cell lines and in primary tumours such as colon and gastric cancers, and B-cell lymphoma [12]. Accordingly, miR-143 overexpression inhibits cell proliferation in several cancer cell lines, suggesting that the loss of miR-143 observed in human cancers maintains and supports tumour growth [39]. HK-2 is a well-studied target of miR-143 with their expression being inversely proportional to each other. Interestingly, similar to our results in A2GDM placentae, miR-143 expression was inversely associated with HK-2 expression in human lung cancer samples [40]. Peschiaroli et al. [11] have reported that miR-143 inhibits HK-2 expression via a conserved miR-143 recognition motif located in the 3′UTR of HK-2 mRNA in head and neck squamous cell carcinoma. Consistently, we found miR-143 overexpression in isolated trophoblast cells could inhibit luciferase activity in the 3′UTR of HK-2 expressing cells compared with mutant 3′UTR of HK-2. To the best of our knowledge, this is the first report describing the role of miR-143 in glucose metabolism in placenta.
AMPK is the primary cellular energy sensor and is phosphorylated at Thr172 in response to increased AMP/ATP ratio as associated with energy deprivation [41]. Down-regulation of pAMPK in A2GDM placentae might be due to the excessive nutrient availability/hyperglycaemia observed in the placentae. We also observed significantly higher levels of AKT (Ser473) phosphorylation in A2GDM placentae. The observed changes in AMPK and AKT signalling may therefore activate mTOR. mTOR is a serine/threonine kinase and represents another important nutrient-sensing pathway in mammalian cells, controlling cell growth, proliferation and metabolism in response to nutrient availability (hyperglycaemia) and growth factor signalling [42]. Activation of mTOR is known to inhibit miR-143 expression in cancer tissue [43]. mTOR has been suggested to have a very significant role in determining the energy shift away from mitochondrial oxidative phosphorylation to aerobic glycolysis [44]. In contrast reports also suggest that in rat skeletal muscle exercise-induced mTOR activation induces mitochondrial biogenesis and oxidative capacity [43]. However, activated mTOR and the shift towards aerobic glycolysis in the A2GDM placentae need to be investigated further in detail.
Interestingly, the miR-143 promoter has binding sites for PPARγ. Further studies on the regulation of miR-143 by PPARγ will reveal the relevance of miR-143 to mitochondrial function in A2GDM placentae. However, transient overexpression of pre miR-143 in trophoblast cells isolated from A2GDM placentae was able to rescue respiration compared with CTRL cells, reduce the glycolytic enzyme and improve mitochondrial complexes. These data collectively suggest that mitochondrial function is compromised in A2GDM placentae compared with A1 and BMI matched CTRL.
The placenta is a programming agent of adult health and disease [45]. Adaptations of placental phenotype in response to maternal diet and metabolic status alter fetal nutrient supply. It is therefore essential to understand how these factors can impact development and predispose individuals to metabolic disorders in the long term [45]. Hyperglycaemia during GDM results in changes in placental function, particularly with respect to the up-take, transfer and/or utilization of glucose [46]. Therefore, it is important when assessing placental handling of glucose to consider not only transport, but also placental utilization of glucose. In summary, we found significantly elevated hPL, reduced mitochondrial function, increased glycolysis, reduced miR-143 and mTOR activation in A2GDM placentae but not in A1GDM placentae. Importantly miR-143 overexpression was able to partially rescue mitochondrial function and reduce glycolytic enzymes in the trophoblast cells from A2GDM placenta. We have provided evidence for the role of miR-143 in mediating the switch to aerobic glycolysis in term A2GDM placentae. Previously we have shown sexual dimorphism in the role of miR-210 in the placental function with increasing maternal adiposity [29]. However due to small sample size we were not able to determine the fetal sex-dependent differences in miR-143 in the placentae with GDM.
Supplementary Material
CLINICAL PERSPECTIVES.
Placental efficiency is reduced in women with A2GDM com pared with BMI-matched A1GDM and non-diabetic controls. A2GDM placentae show mitochondrial dysfunction and an up-regulation of glycolysis.
Down-regulation of miR-143 might be at least partially responsible for mitochondrial dysfunction in A2GDM placentae.
Metabolic dysfunction in the A2GDM placenta may underlie altered placental nutrient transport, fetal growth and development and hence programming of the offspring for disease in later life.
ACKNOWLEDGEMENTS
We thank Dr Andres Lund, University of Copenhagen, Denmark for providing plasmids with 3′UTR and mutant 3′UTR of HK-2 and Dr Goutham Ghosh Choudary, UTHSCSA for providing pRLTk plasmid with Renilla luciferase.
FUNDING This work was supported by the National Institutes of Health [grant number HD076259 (to L.M. and A.M.)].
Abbreviations
- 4EBP
eukaryotic translation initiation factor 4E-binding protein
- A1GDM
GDM controlled by diet and exercise
- A2GDM
GDM treated with medication
- BMI
body mass index
- AMPK
5′AMP-activated protein kinase
- CT
cytotrophoblasts
- CTRL
non-diabetic control
- FCCP
p-trifluoromethoxy carbonyl cyanide phenyl hydrazone
- GDM
gestational diabetes mellitus
- GLUT1
glucose transporter 1
- HK-2
hexokinase-2
- hPL
human placental lactogen
- LDH
lactate dehydrogenase
- mTOR
mammalian target of rapamycin
- OCR
oxygen consumption rates
- PFK
phosphofructokinase
- PGC1α
peroxisome proliferator-activated receptor-γ co-activator 1α
- PPARγ
peroxisome proliferator-activated receptor γ
- pS6K
ribosomal protein S6 kinase and AKT-serine/threonine-specific protein kinase
- ST
syncytiotrophoblasts
Footnotes
AUTHOR CONTRIBUTION Sribalasubashini Muralimanoharan, Alina Maloyan and Leslie Myatt prepared the experimental design. Sribalasubashini Muralimanoharan performed all the experiments and conducted data analysis. Alina Maloyan and Leslie Myatt helped in experimental setup and oversaw data analysis. Sribalasubashini Muralimanoharan, Alina Maloyan and Leslie Myatt prepared the manuscript.
REFERENCES
- 1.Finer S, Mathews C, Lowe R, Smart M, Hillman S, Foo L, Sinha A, Williams D, Rakyan VK, Hitman GA. Maternal gestational diabetes is associated with genome-wide DNA methylation variation in placenta and cord blood of exposed offspring. Hum. Mol. Genet. 2015;24:3021–3029. doi: 10.1093/hmg/ddv013. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 2.Kjos SL, Buchanan TA. Gestational diabetes mellitus. N. Engl. J. Med. 1999;341:1749–1756. doi: 10.1056/NEJM199912023412307. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 3.Simmons R. Developmental origins of adult metabolic disease. Endocrinol. Metab. Clin. North. Am. 2006;35:193–204. doi: 10.1016/j.ecl.2005.09.006. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 4.Fetita LS, Sobngwi E, Serradas P, Calvo F, Gautier JF. Consequences of fetal exposure to maternal diabetes in offspring. J. Clin. Endocrinol. Metab. 2006;91:3718–3724. doi: 10.1210/jc.2006-0624. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 5.Myatt L. Placental adaptive responses and fetal programming. J. Physiol. 2006;572(Pt 1):25–30. doi: 10.1113/jphysiol.2006.104968. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hay WW., Jr The placenta. Not just a conduit for maternal fuels. Diabetes. 1991;40(Suppl 2):44–50. doi: 10.2337/diab.40.2.s44. CrossRef. [DOI] [PubMed] [Google Scholar]
- 7.Ferretti C, Bruni L, Dangles-Marie V, Pecking AP, Bellet D. Molecular circuits shared by placental and cancer cells, and their implications in the proliferative, invasive and migratory capacities of trophoblasts. Hum. Reprod. Update. 2007;13:121–141. doi: 10.1093/humupd/dml048. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 8.Li Z, Zhang H. Reprogramming of glucose, fatty acid and amino acid metabolism for cancer progression. Cell. Mol. Life Sci. 2015 doi: 10.1007/s00018-015-2070-4. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Majumder PK, Febbo PG, Bikoff R, Berger R, Xue Q, McMahon LM, Manola J, Brugarolas J, McDonnell TJ, Golub TR, et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat. Med. 2004;10:594–601. doi: 10.1038/nm1052. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 10.Mouillet JF, Chu T, Sadovsky Y. Expression patterns of placental microRNAs. Birth defects research Part, A., Clin. Mol. Teratol. 2011;91:737–743. doi: 10.1002/bdra.20782. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peschiaroli A, Giacobbe A, Formosa A, Markert EK, Bongiorno-Borbone L, Levine AJ, Candi E, D'Alessandro A, Zolla L, Finazzi Agrò A, Melino G. miR-143 regulates hexokinase 2 expression in cancer cells. Oncogene. 2013;32:797–802. doi: 10.1038/onc.2012.100. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Guo X, Zhang H, Xiang Y, Chen J, Yin Y, Cai X, Wang K, Wang G, Ba Y, et al. Role of miR-143 targeting KRAS in colorectal tumorigenesis. Oncogene. 2009;28:1385–1392. doi: 10.1038/onc.2008.474. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 13.Myatt L, Cui X. Oxidative stress in the placenta. Histochem. Cell Biol. 2004;122:369–382. doi: 10.1007/s00418-004-0677-x. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 14.Roberts VH, Smith J, McLea SA, Heizer AB, Richardson JL, Myatt L. Effect of increasing maternal body mass index on oxidative and nitrative stress in the human placenta. Placenta. 2009;30:169–175. doi: 10.1016/j.placenta.2008.11.019. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Myatt L, Roberts VH. Placental mechanisms and developmental origins of health and disease. In: Gluckman P, Hanson M, editors. Developmental Origins of Health and Disease. Cambridge University Press; 2006. pp. 130–142. Cambridge CrossRef. [Google Scholar]
- 16.Mele J, Muralimanoharan S, Maloyan A, Myatt L. Impaired mitochondrial function in human placenta with increased maternal adiposity. Am. J. Physiol. Endocrinol. Metab. 2014;307:E419–E425. doi: 10.1152/ajpendo.00025.2014. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hastie R, Lappas M. The effect of pre-existing maternal obesity and diabetes on placental mitochondrial content and electron transport chain activity. Placenta. 2014;35:673–683. doi: 10.1016/j.placenta.2014.06.368. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 18.Boyle KE, Hwang H, Janssen RC, DeVente JM, Barbour LA, Hernandez TL, Mandarino LJ, Lappas M, Friedman JE. Gestational diabetes is characterized by reduced mitochondrial protein expression and altered calcium signaling proteins in skeletal muscle. PLoS One. 2014;9:e106872. doi: 10.1371/journal.pone.0106872. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magee TR, Ross MG, Wedekind L, Desai M, Kjos S, Belkacemi L. Gestational diabetes mellitus alters apoptotic and inflammatory gene expression of trophobasts from human term placenta. J. Diabetes Complications. 2014;28:448–459. doi: 10.1016/j.jdiacomp.2014.03.010. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayhew TM. Taking tissue samples from the placenta: an illustration of principles and strategies. Placenta. 2008;29:1–14. doi: 10.1016/j.placenta.2007.05.010. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 21.Maloyan A, Mele J, Muralimanohara B, Myatt L. Measurement of mitochondrial respiration in trophoblast culture. Placenta. 2012;33:456–458. doi: 10.1016/j.placenta.2012.01.016. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gregersen LH, Jacobsen A, Frankel LB, Wen J, Krogh A, Lund AH. MicroRNA-143 down-regulates hexokinase 2 in colon cancer cells. BMC Cancer. 2012;12:232. doi: 10.1186/1471-2407-12-232. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuhl C. Etiology and pathogenesis of gestational diabetes. Diabetes Care. 1998;21(Suppl 2):B19–B26. CrossRef PubMed. [PubMed] [Google Scholar]
- 24.Arumugam R, Horowitz E, Lu D, Collier JJ, Ronnebaum S, Fleenor D, Freemark M. The interplay of prolactin and the glucocorticoids in the regulation of beta-cell gene expression, fatty acid oxidation, and glucose-stimulated insulin secretion: implications for carbohydrate metabolism in pregnancy. Endocrinology. 2008;149:5401–5414. doi: 10.1210/en.2008-0051. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newbern D, Freemark M. Placental hormones and the control of maternal metabolism and fetal growth. Curr. Opin. Endocrinol. Diabetes Obes. 2011;18:409–416. doi: 10.1097/MED.0b013e32834c800d. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 26.Lopez-Espinoza I, Smith RF, Gillmer M, Schidlmeir A, Hockaday TD. High levels of growth hormone and human placental lactogen in pregnancy complicated by diabetes. Diabetes Res. 1986;3:119–125. PubMed. [PubMed] [Google Scholar]
- 27.Hauguel-de Mouzon S, Shafrir E. Carbohydrate and fat metabolism and related hormonal regulation in normal and diabetic placenta. Placenta. 2001;22:619–627. doi: 10.1053/plac.2001.0698. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 28.Muralimanoharan S, Maloyan A, Mele J, Guo C, Myatt LG, Myatt L. MIR-210 modulates mitochondrial respiration in placenta with preeclampsia. Placenta. 2012;33:816–823. doi: 10.1016/j.placenta.2012.07.002. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muralimanoharan S, Guo C, Myatt L, Maloyan A. Sexual dimorphism in miR-210 expression and mitochondrial dysfunction in the placenta with maternal obesity. Int. J. Obes. (Lond) 2015;39:1274–1281. doi: 10.1038/ijo.2015.45. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiu C, Hevner K, Abetew D, Sedensky M, Morgan P, Enquobahrie DA, Williams MA. Mitochondrial DNA copy number and oxidative DNA damage in placental tissues from gestational diabetes and control pregnancies: a pilot study. Clin. Laborat. 2013;59:655–660. doi: 10.7754/clin.lab.2012.120227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elliott BD, Langer O, Schenker S, Johnson RF. Insignificant transfer of glyburide occurs across the human placenta. Am. J. Obstet. Gynecol. 1991;165(4 Pt 1):807–812. doi: 10.1016/0002-9378(91)90421-m. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 32.Kraemer J, Klein J, Lubetsky A, Koren G. Perfusion studies of glyburide transfer across the human placenta: implications for fetal safety. Am. J. Obstet. Gynecol. 2006;195:270–274. doi: 10.1016/j.ajog.2005.12.005. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 33.Holdsworth-Carson SJ, Lim R, Mitton A, Whitehead C, Rice GE, Permezel M, Lappas M. Peroxisome proliferator-activated receptors are altered in pathologies of the human placenta: gestational diabetes mellitus, intrauterine growth restriction and preeclampsia. Placenta. 2010;31:222–229. doi: 10.1016/j.placenta.2009.12.009. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 34.Borengasser SJ, Faske J, Kang P, Blackburn ML, Badger TM, Shankar K. In utero exposure to prepregnancy maternal obesity and postweaning high-fat diet impair regulators of mitochondrial dynamics in rat placenta and offspring. Physiol. Genomics. 2014;46:841–850. doi: 10.1152/physiolgenomics.00059.2014. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lager S, Powell TL. Regulation of nutrient transport across the placenta. J. Pregnancy. 2012;2012:179827. doi: 10.1155/2012/179827. doi:10.1155/2012/179827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Desforges M, Sibley CP. Placental nutrient supply and fetal growth. Int. J. Dev. Biol. 2010;54:377–390. doi: 10.1387/ijdb.082765md. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 37.Gaither K, Quraishi AN, Illsley NP. Diabetes alters the expression and activity of the human placental GLUT1 glucose transporter. J. Clin. Endocrinol. Metab. 1999;84:695–701. doi: 10.1210/jcem.84.2.5438. PubMed. [DOI] [PubMed] [Google Scholar]
- 38.Clape C, Fritz V, Henriquet C, Apparailly F, Fernandez PL, Iborra F, Avancès C, Villalba M, Culine S, Fajas L. miR-143 interferes with ERK5 signaling, and abrogates prostate cancer progression in mice. PLoS One. 2009;4:e7542. doi: 10.1371/journal.pone.0007542. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kent OA, Chivukula RR, Mullendore M, Wentzel EA, Feldmann G, Lee KH, Liu S, Leach SD, Maitra A, Mendell JT. Repression of the miR-143/145 cluster by oncogenic Ras initiates a tumor-promoting feed-forward pathway. Genes Dev. 2010;24:2754–2759. doi: 10.1101/gad.1950610. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Z, Li X, Wu S, Xue M, Chen W. Long non-coding RNA UCA1 promotes glycolysis by upregulating hexokinase 2 through the mTOR-STAT3/microRNA143 pathway. Cancer Sci. 2014;105:951–955. doi: 10.1111/cas.12461. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kavitha JV, Rosario FJ, Nijland MJ, McDonald TJ, Wu G, Kanai Y, Powell TL, Nathanielsz PW, Jansson T. Down-regulation of placental mTOR, insulin/IGF-I signaling, and nutrient transporters in response to maternal nutrient restriction in the baboon. FASEB J. 2014;28:1294–1305. doi: 10.1096/fj.13-242271. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perez-Perez A, Maymo JL, Gambino YP, Guadix P, Duenas JL, Varone CL, Sánchez-Margalet V. Activated translation signaling in placenta from pregnant women with gestational diabetes mellitus: possible role of leptin. Horm. Metab. Res. 2013;45:436–442. doi: 10.1055/s-0032-1333276. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 43.Edgett BA, Fortner ML, Bonen A, Gurd BJ. Mammalian target of rapamycin pathway is up-regulated by both acute endurance exercise and chronic muscle contraction in rat skeletal muscle. Appl. Physiol. Nutr. Metab. 2013;38:862–869. doi: 10.1139/apnm-2012-0405. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 44.Schieke SM, Phillips D, McCoy JP, Jr, Aponte AM, Shen RF, Balaban RS, Finkel T. The mammalian target of rapamycin (mTOR) pathway regulates mitochondrial oxygen consumption and oxidative capacity. J. Biol. Chem. 2006;281:27643–27652. doi: 10.1074/jbc.M603536200. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 45.Tarrade A, Panchenko P, Junien C, Gabory A. Placental contribution to nutritional programming of health and diseases: epigenetics and sexual dimorphism. J. Exp. Biol. 2015;218(Pt 1):50–58. doi: 10.1242/jeb.110320. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 46.Osmond DT, Nolan CJ, King RG, Brennecke SP, Gude NM. Effects of gestational diabetes on human placental glucose uptake, transfer, and utilisation. Diabetologia. 2000;43:576–582. doi: 10.1007/s001250051346. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.