Abstract
Background. The relevance of antibodies (Abs) in the defense against Mycobacterium tuberculosis infection remains uncertain. We investigated the role of Abs to the mycobacterial capsular polysaccharide arabinomannan (AM) and its oligosaccharide (OS) fragments in humans.
Methods. Sera obtained from 29 healthy adults before and after primary or secondary bacillus Calmette-Guerin (BCG) vaccination were assessed for Ab responses to AM via enzyme-linked immunosorbent assays, and to AM OS epitopes via novel glycan microarrays. Effects of prevaccination and postvaccination sera on BCG phagocytosis and intracellular survival were assessed in human macrophages.
Results. Immunoglobulin G (IgG) responses to AM increased significantly 4–8 weeks after vaccination (P < .01), and sera were able to opsonize BCG and M. tuberculosis grown in both the absence and the presence of detergent. Phagocytosis and intracellular growth inhibition were significantly enhanced when BCG was opsonized with postvaccination sera (P < .01), and these enhancements correlated significantly with IgG titers to AM (P < .05), particularly with reactivity to 3 AM OS epitopes (P < .05). Furthermore, increased phagolysosomal fusion was observed with postvaccination sera.
Conclusions. Our results provide further evidence for a role of Ab-mediated immunity to tuberculosis and suggest that IgG to AM, especially to some of its OS epitopes, could contribute to the defense against mycobacterial infection in humans.
Keywords: tuberculosis, Mycobacterium tuberculosis, Mycobacterium bovis BCG, immunoglobulin, human antibodies, antibody-mediated immunity, immune response, polysaccharide, oligosaccharide, bacterial capsule
Active tuberculosis remains a major public health problem, with around 9 million new cases and 1.5 million associated deaths globally [1]. The only licensed vaccine, bacillus Calmette-Guerin (BCG), confers limited protection against pulmonary tuberculosis, and does not prevent Mycobacterium tuberculosis infection [2]. Tuberculosis vaccines under development aim to enhance cell-mediated immunity but, as of today, have not demonstrated sufficient protective efficacy in humans [2]. Thus, further exploration of other arms of the immune system may inform new vaccine development strategies.
Increasing evidence suggests that antibodies (Abs) could contribute to the defense against M. tuberculosis [3, 4]. However, their role is insufficiently studied because of the general perception that M. tuberculosis, a largely intracellular pathogen, is outside the reach of extracellularly located Abs. Abs against capsular and noncapsular surface polysaccharides are protective against a variety of microbial pathogens, including those with intracellular location [4, 5]. Mycobacteria have a capsule, an important virulence factor, that consists largely of polysaccharides, proteins, and, to a smaller extent, glycolipids [6, 7]. α-glucan and arabinomannan (AM) account for 70%–80% and 10%–20% of the polysaccharide content, respectively [8, 9]. We, as well as others, have previously shown that AM is more immunogenic than α-glucan in patients with tuberculosis [10, 11]. We have further demonstrated that Ab responses to capsular AM and the related cell wall glycolipid lipoarabinomannan (LAM) correlate strongly and significantly in patients with tuberculosis [10], suggesting, in accordance with other studies [12], that AM is the immunogenic portion of LAM. Of note, LAM is not a component of the mycobacterial capsule [8, 9]. Importantly, we and others have shown that passive administration of monoclonal Abs (mAbs) to AM and the AM portion of LAM improve the outcome of M. tuberculosis infection in mice [12, 13] and that AM-containing conjugate vaccines result in high immunoglobulin G (IgG) titers to AM and protection of mice against M. tuberculosis infection (unpublished data) [14, 15]. In humans, an increase in LAM-specific Abs after BCG vaccination has been associated with enhanced innate and cell-mediated immune responses against BCG [16]. Furthermore, a lack of Abs to LAM was associated with tuberculosis dissemination in children [17]. These experimental and clinical data support the notion that Abs to AM might play a protective role against M. tuberculosis infection in both animal models and humans.
In this study, we tested Ab responses to capsular AM in sera obtained before and after BCG vaccination from adult subjects undergoing a clinical study to assess mycobacterial growth inhibition assays (MGIAs) as correlates for vaccine efficacy [18]. We hypothesized that BCG vaccination would lead to increased Ab titers to capsular AM, particularly to some of its oligosaccharide (OS) epitopes, and that such Ab responses would be associated with defensive effects against mycobacterial infection.
MATERIALS AND METHODS
Subjects
Sera were obtained from 30 healthy, M. tuberculosis–uninfected adults before and after (weeks 4, 8, and 24) primary or secondary BCG vaccination at the University of Oxford (Oxford, United Kingdom) [18]. Participants were recruited under a protocol approved by the Oxfordshire Research Ethics Committee [18], and peripheral blood mononuclear cells (PBMCs) from independent healthy BCG vaccinated subjects were collected under a protocol approved by the Institutional Review Board of the Albert Einstein College of Medicine. Written informed consent was obtained from all individuals prior to enrollment.
Enzyme-Linked Immunosorbent Assays (ELISAs)
Capsular AM from BCG (Pasteur) or M. tuberculosis (H37Rv) was isolated, purified, and lyophilized as described [19]. LAM, isolated from the cell wall of M. tuberculosis H37Rv, was obtained from BEI Resources (NR-14848). Maxisorp plates were coated with AM or LAM at 10 μg/mL, sera were added at 1:50 dilution, and ELISAs were performed as described elsewhere [10], with further details in the Supplementary Methods.
AM Microarrays
A panel of 12 AM fragments (corresponding to motifs at the nonreducing terminus of the molecule, which have previously been shown to be recognized by anti-AM/LAM Abs) [20–22] were synthesized, coupled to bovine serum albumin via a squarate linker [23], and printed on epoxy-coated glass slides [24]. After blocking, the slides were incubated with sera (1:100), the murine mAb CS35 (a positive control known to recognize AM and LAM from various mycobacterial strains [15]), or the murine IgG2a mAb 9d8 (known to recognize only M. tuberculosis AM) [13, 15, 19] and processed essentially as reported elsewhere [24], with details described in the Supplementary Methods. We note that we used IgG2a 9d8, a switch variant of the protective IgG3 mAb 9d8, which binds to M. tuberculosis AM in a similar manner as the parent mAb [19], for technical reasons, because it does not clump like the IgG3 isotype and is easier to purify.
Phagocytosis Assay
BCG bacteria were grown in Middlebrook 7H9 and conjugated with fluorescein isothiocyanate (FITC). Human monocytic cells (THP-1) were differentiated into adherent macrophages and then incubated for 2 hours with heat-inactivated sera (10% in Roswell Park Memorial Institute 1640 medium). The FITC-labeled BCG was then added at a multiplicity of infection (MOI) of 20. Phagocytosis was evaluated by flow cytometry, and fluorescence from the noninternalized FITC-labeled BCG was quenched by treating the cells with trypan blue [25]. We coincubated macrophages and sera prior to BCG infection to allow simultaneous processing of many samples, which we considered important for data comparison. To ensure that our method was comparable to the standard approach, we performed experiments with BCG preopsonized with sera from 3 subjects. To study any potential additive effects of complement on phagocytosis, sera from 2 subjects were either not heat inactivated or were supplemented with 1% non–heat-inactivated serum from a healthy volunteer. We further obtained primary macrophages from PBMCs of 2 BCG-vaccinated and M. tuberculosis–uninfected donors as described previously [26], coincubated those with paired sera from 2 representative subjects, and infected them with FITC-BCG at MOI of 10. Further details are described in the Supplementary Methods.
Mycobacterial Growth Assay
THP-1 cells coincubated with paired sera and infected with BCG at MOI of 10 were harvested, lysed, and plated on days 1 and 4. Colony-forming units were counted after incubation at 37°C for 3 and 4 weeks. Further details are described in the Supplementary Methods. Ab responses were further correlated to PBMC-based MGIAs performed at Oxford University because whole-blood assays, owing to variability in mycobacterial stocks and week-to-week performances of the assays, showed considerably more variation than PBMC-based assays, which were run in only 2 batches [18].
Transmission Electron Microscopy (TEM) and Immunofluorescence
BCG (Pasteur) or M. tuberculosis (H37Rv) was grown in the presence and absence of 0.05% tyloxapol. For TEM studies, BCG strains were fixed as described previously [7]. For immunofluorescence studies, fixed BCG or M. tuberculosis was incubated with the murine mAbs IgG1 24C5 to glucan [27], IgG3 CS35 to AM/LAM [15], isotype-matched controls, or human sera (1:100). Secondary Abs and further details are described in the Supplementary Methods.
Assessment of Phagosome-Lysosome (P-L) Fusion
THP-1 macrophages were coincubated with 10% heat-inactivated paired sera from 2 subjects (chosen on the basis of high intracellular growth inhibition rates with postvaccination sera) and infected with Alexa 488–conjugated BCG [28] at a MOI of 20 for 1 hour, followed by incubation with 100 nM LysoTracker Red DND-99 (Life Technologies) for 1 hour as described elsewhere [29]. After washing and fixing, the cells were examined with a Leica SP5 confocal microscope, 14–18 images were taken for each sample, and a minimum of 50 BCG phagosomes were counted to quantify the percentage of phagosomes co-localizing with the LysoTracker. Further details are described in the Supplementary Methods.
Statistical Analysis
Statistical analysis was performed using Prism software, version 6.04 (GraphPad). Comparisons were made using the nonparametric Wilcoxon matched-pairs signed rank test or the Mann–Whitney U test. Correlations were assessed by Spearman rank correlation. Correlations involving Ab responses to the 12 AM epitopes were adjusted for multiple comparisons, using the Holm-Bonferroni method [30].
RESULTS
Ab Responses Before and After BCG Receipt
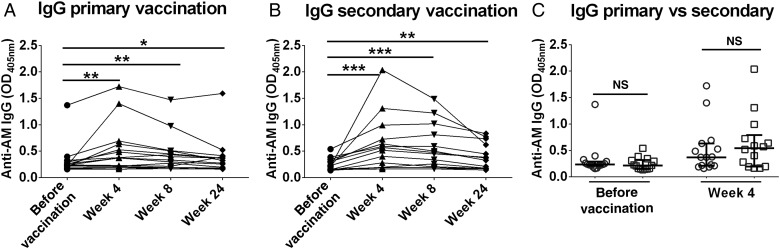
In both vaccination groups, compared with sera obtained before vaccination, sera obtained 4 and 8 weeks after vaccination had significantly higher IgG titers to AM (P < .01), which declined at 24 weeks (Figure 1A and 1B). Similar increases were observed for IgG2 responses (Supplementary Figure 1A and 1B), with a weak correlation between total IgG and IgG2 responses (r = 0.36, P = .05). While no significant differences were observed between the primary and secondary vaccination groups' total IgG responses before and 4 weeks after vaccination (Figure 1C), the secondary BCG vaccination group had significantly higher IgG2 titers at both time points (Supplementary Figure 1C), although the overall magnitude of IgG2 increase was similar between the groups. Increases in IgA and IgM titers to AM were only significant after secondary vaccination (Supplementary Figures 2 and 3). Of note, 1 subject (in the secondary vaccination group) with a prevaccination IgG reactivity of 4.9 SDs greater than the average was excluded as an outlier, while another subject (primary vaccination group) with a prevaccination value of 1.8 SDs above average was included, because we used an inclusion threshold of 2 SDs from the mean prevaccination value.
Figure 1.
Immunoglobulin G (IgG) antibody (Ab) responses to arabinomannan (AM) before and after bacillus Calmette-Guerin (BCG) vaccination. A and B, IgG responses after primary vaccination (A) and after secondary BCG vaccination (B). The Wilcoxon matched-pairs signed rank test was used for analysis. C, Comparison of IgG responses between primary and secondary vaccination group, using the Mann–Whitney U test. Circles represent the primary vaccination group, and the squares represent the secondary vaccination group. Lines and error bars represent medians with interquartile ranges. *P < .05, **P < .01, ***P < .001, and ****P < .0001. Abbreviation: NS, not significant (P ≥ .05).
Because knowledge about the similarity in AM and LAM epitope recognition might have important implications, we tested IgG reactivities to AM isolated from the capsule of both BCG and M. tuberculosis H37Rv and correlated those to LAM isolated from the cell wall of M. tuberculosis H37Rv in postvaccination sera from 18 randomly chosen subjects (Supplementary Figure 4). The responses to LAM and AM correlated strongly (P < .0001), with correlations between LAM and AM isolated from the same strain (H37Rv) slightly stronger (r = 0.95) than those between AM and AM or LAM isolated from different strains (r = 0.88).
Ab Responses to AM OS Epitopes
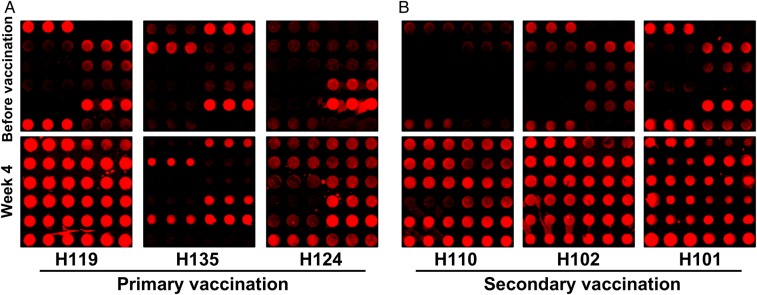
Regardless of vaccination group, subjects' sera reacted with AM epitopes in heterogeneous patterns (Figure 2). Overall, secondary vaccination appeared to induce more-pronounced IgG responses to AM OS epitopes than did primary vaccination (Supplementary Figure 5).
Figure 2.
Immunoglobulin G (IgG) reactivity with a 12-member arabinomannan (AM) oligosaccharide (OS) microarray. Representative microarray images of sera obtained from 3 subjects in the primary (A) and secondary (B) vaccination groups before and 4 weeks after bacillus Calmette-Guerin (BCG) vaccination, demonstrating the heterogeneous patterns of reactivity to AM OS epitopes (printed in triplicates). For each subject, the brightness and contrast of the images were adjusted with the same settings, using GenePixPro6.1.
Enhanced BCG Macrophage Phagocytosis With Postvaccination Sera
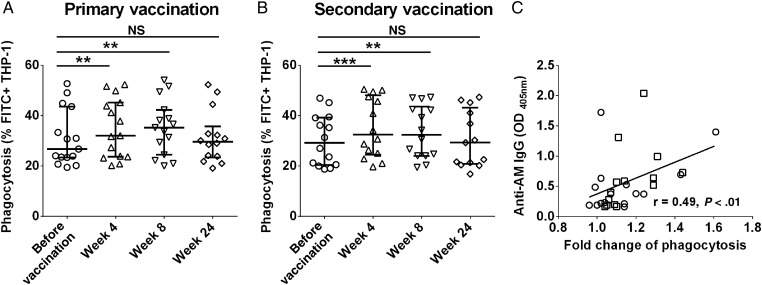
For both vaccination groups, the THP-1 BCG phagocytosis increased significantly upon coincubation with sera obtained 4 and 8 weeks after vaccination (P < .01) but declined with sera obtained 24 weeks after vaccination (Figure 3A and 3B). Similar proportional enhancements of BCG phagocytosis were observed when comparing the standard method of preopsonizing BCG to our method of coincubating macrophages with sera before BCG infection (Supplementary Figure 6) and when using PBMC-derived macrophages (Supplementary Figure 7). However, with the addition of complement we found no considerable differences in phagocytosis between prevaccination and postvaccination sera (Supplementary Figure 8).
Figure 3.
Effects of postvaccination sera on bacillus Calmette-Guerin (BCG) phagocytosis by human macrophages. Phagocytosis rates increased significantly upon coincubation of THP-1 cells with sera obtained 4 and 8 weeks after vaccination, compared with sera obtained before vaccination, but decreased to prevaccination levels upon coincubation with sera obtained 24 weeks after vaccination in both the primary (A) and secondary (B) vaccination groups. Lines and error bars represent medians with interquartile ranges. The Wilcoxon matched-pairs signed rank test was used for analysis. *P < .05, **P < .01, ***P < .001, and ****P < .0001. Of note, 2 subjects, one from the primary vaccination group and one from the secondary vaccination group, had insufficient serum volume 24 weeks after vaccination and thus have no phagocytosis data for this time point. C, Significant correlation between immunoglobulin G (IgG) responses to arabinomannan (AM) 4 weeks after vaccination and enhanced BCG phagocytosis (in fold change compared to coincubation with prevaccination sera) by THP-1 cells coincubated with corresponding sera obtained 4 weeks after vaccination, using Spearman rank correlation test. Circles represent the primary vaccination group, and squares represent the secondary vaccination group. Abbreviations: FITC, fluorescein isothiocyanate; NS, not significant (P ≥ .05).
Correlations Between BCG Phagocytosis and IgG Responses to AM and AM OS Epitopes
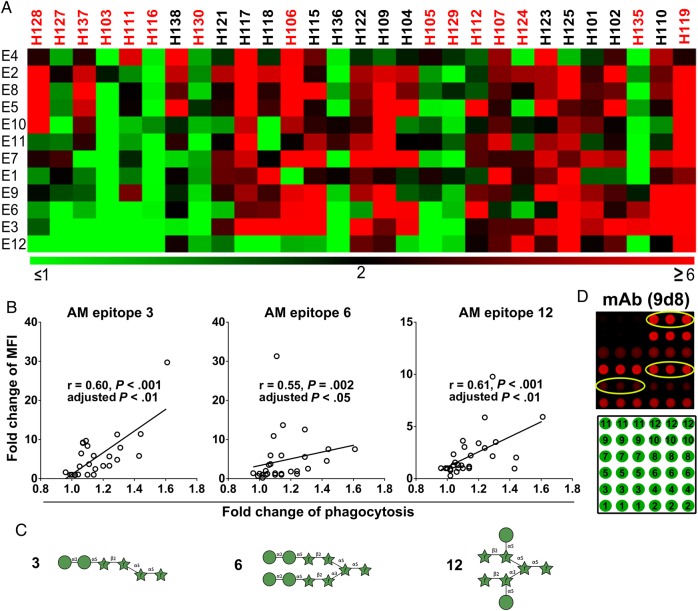
Increases in IgG reactivity to native AM by sera obtained 4 weeks after vaccination correlated significantly with increases in BCG phagocytosis when incubating THP-1 cells with the corresponding sera (P < .01; Figure 3C). The enhanced phagocytosis was strongly associated with increased IgG reactivity to certain AM OS epitopes, namely fragments 3, 6, and 12 (P < .05; Figure 4A and 4B). Interestingly, all 3 AM OS contained at least 2 residues of mannose (Figure 4C), and 2 of 3 epitopes (fragments 6 and 12) were also recognized by the murine IgG2a mAb 9d8 to M. tuberculosis AM (Figure 4D) [13, 19]. Of note, several other segments not associated with phagocytosis also contained mannose (Supplementary Figure 9).
Figure 4.
Enhancement of bacillus Calmette-Guerin (BCG) phagocytosis correlated with arabinomannan (AM)–epitope specific immunoglobulin G (IgG) responses. A, Heat map showing enhanced phagocytosis associated with antibody (Ab) responses to certain AM epitopes. The heat map was generated using the software MultiExperiment Viewer (available at: http://www.tm4.org/mev.html). The color feature represents fold change of median fluorescence intensity (MFI; from ≤1 to ≥6) with sera obtained 4 weeks after vaccination, compared with prevaccination sera. The subjects lined from left to right showed enhancement of BCG phagocytosis by THP-1 cells when coincubated with sera obtained 4 weeks after vaccination, compared with prevaccination sera (from low to high). Primary vaccination subjects' identifiers are listed in red, and those for subjects in the secondary vaccination group are in black fonts. B, Significant correlations between increased IgG reactivity to certain AM epitopes 4 weeks after vaccination and enhanced BCG phagocytosis by human macrophages coincubated with corresponding sera. Analysis was performed by the Spearman rank correlation test and the Holm-Bonferroni correction for multiple comparisons. C, Structures of AM epitopes 3, 6, and 12. Of note, all 3 segments contained at least 2 residues of mannose (green circles). Symbols used are those developed by the Consortium for Functional Glycomics, with green circles denoting D-mannose and green stars denoting D-arabinose. D, AM oligosaccharide fragments (printed in triplicates) recognized by switch-variant IgG2a of the protective IgG3 monoclonal Ab (mAb) 9d8 against Mycobacterium tuberculosis AM. Positions of fragments on the microarray are shown below.
Effects of Postvaccination Sera on Intracellular Mycobacterial Growth Reduction
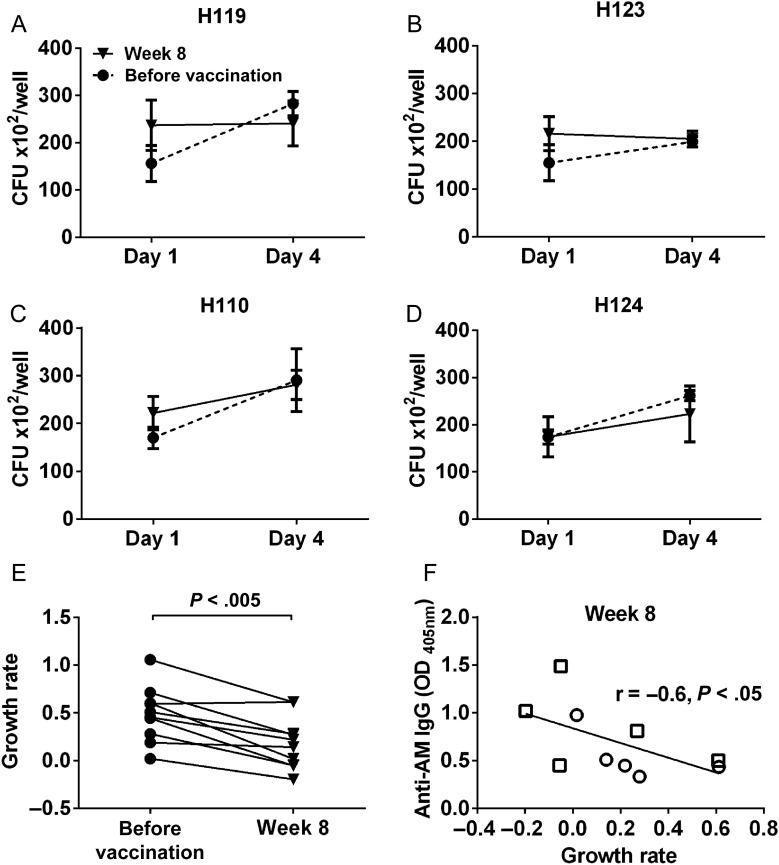
Significantly lower BCG growth rates were seen in THP-1 cells treated with postvaccination sera as compared to prevaccination sera (P < .01; Figure 5A–E). Moreover, BCG growth reduction correlated significantly with postvaccination IgG titers to AM (P < .05; Figure 5F). Because the number of paired sera tested was limited to 10 for practical reasons, correlations of IgG reactivities to AM OS epitopes lacked statistical power and were thus nonsignificant.
Figure 5.
Bacillus Calmette-Guerin (BCG) growth in human macrophages incubated with postvaccination sera compared to prevaccination sera. THP-1 cells incubated with heat-inactivated sera obtained before and 8 weeks after vaccination from 10 subjects were infected with BCG (multiplicity of infection, 10) on day 1 and harvested for colony-forming unit (CFU) counting on days 1 (3 hours after infection) and 4. Of note, sera obtained 8 weeks after vaccination were used because insufficient quantity was left of sera obtained 4 weeks after vaccination. The 10 subjects were selected on the basis of the highest increase of BCG phagocytosis with THP-1 cells incubated with postvaccination sera relative to those incubated with prevaccination sera. A–D, Representative BCG growth in THP-1 cells incubated with paired sera (subjects H119 and H124 are from the primary vaccination group, and subjects H110 and H123 are from the secondary vaccination group). E, Significant BCG growth reduction in THP-1 cells incubated with postvaccination sera as compared to prevaccination sera. Because the initial amount of internalized BCG was different between most paired prevaccination and postvaccination sera, the growth rate was assessed by the proportion of increased bacteria relative to day 1 and was calculated as [CFU at day 4 – CFU at day 1]/CFU at day 1. Comparisons were made using the Wilcoxon matched-pairs signed rank test. The n value was limited to the top 10 phagocytosis-enhancing sera to allow for the processing of all sera in 1 experiment, which was performed at 2 separate time points. F, Correlation between immunoglobulin G (IgG) titers 8 weeks after vaccination and mycobacterial growth reduction, using the Spearman rank correlation test. Circles represent the primary vaccination group, and squares represent the secondary vaccination group. Abbreviation: AM, arabinomannan.
Ab responses were further correlated to PBMC-based MGIAs, for which data were available for 17 subjects, 8 from the primary and 9 from the secondary vaccination group [18]. At 4 weeks after vaccination, mycobacterial growth inhibition showed a significant correlation with IgG responses to AM (P < .01; Supplementary Figure 10).
TEM and Immunofluorescence Studies
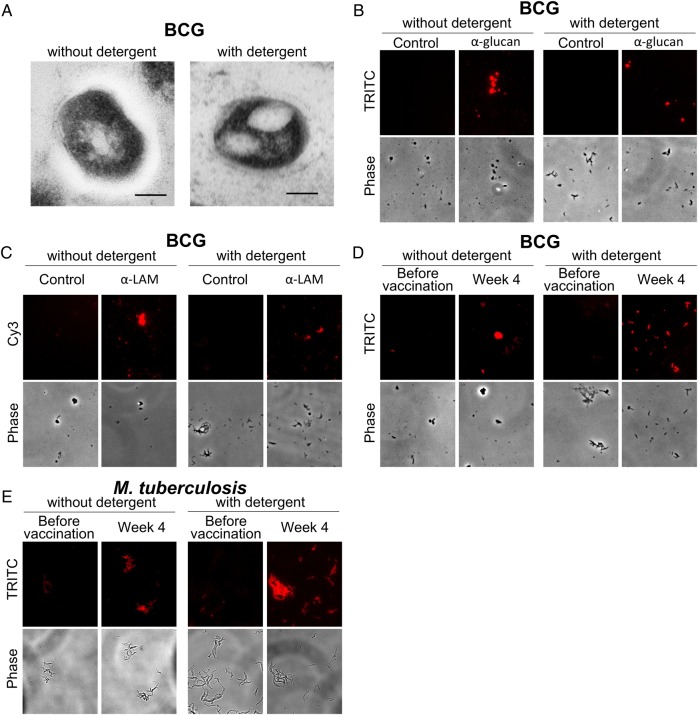
Consistent with other studies, the capsule was partially stripped off the bacteria when detergent was added to the culture medium [27, 31], and a thinner but still visible zone was observed surrounding the detergent treated bacteria (Figure 6A). Immunofluorescence microscopy showed that mAbs to α-glucan and AM/LAM bound to both BCG grown with and BCG grown without detergent, while an irrelevant isotype-matched control mAb did not (Figure 6B and 6C). Importantly, opsonization of BCG and M. tuberculosis grown under both culture conditions was considerably stronger with postvaccination serum as compared to prevaccination serum (Figure 6 D and 6E).
Figure 6.
Transmission electron microscopy and immunofluorescence studies of mycobacteria grown without and with detergent. A, A visible electron-transparent zone is surrounding bacillus Calmette-Guerin (BCG) grown without detergent but not with detergent, indicating reduced capsule formation in the presence of detergent. The scale bar denotes 100 nm. B–D, Immunofluorescence microscopy of BCG incubated with control monoclonal antibody (mAb) 2D10 (immunoglobulin G1 [IgG1] against the Cryptococcus neoformans capsular polysaccharide glucuronoxylomannan) and anti-glucan IgG1 mAb 24C5 (B), with control mAb (IgG3, clone B10 of unknown specificity) and anti-LAM IgG3 mAb CS35 (C), and with prevaccination and postvaccination sera from subject H119 (D). E, Immunofluorescence microscopy of Mycobacterium tuberculosis (H37Rv) incubated with prevaccination and postvaccination sera from subject H119. Paired sera from this subject were selected on the basis of a high postvaccination IgG titer to arabinomannan, combined with enhanced postvaccination phagocytosis. In each panel of A–D, and in each sub panel of E, the brightness and contrast of the images were adjusted with the same settings, using ImageJ 1.48. Abbreviation: M. tuberculosis, Mycobacterium tuberculosis.
P-L Fusion
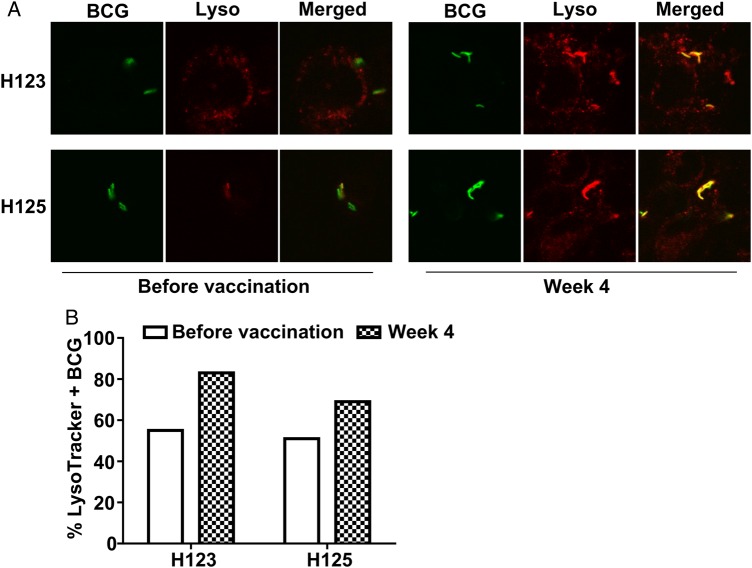
To determine Ab effects on P-L fusion, we assessed paired sera from 2 representative subjects and found that postvaccination sera as compared to prevaccination sera enhanced P-L fusion (Figure 7).
Figure 7.
Effect of postvaccination sera on phagosome-lysosome (P-L) fusion in bacillus Calmette-Guerin (BCG)–infected THP-1 cells. A, THP-1 cells coincubated with sera obtained from 2 subjects (H123 and H125) before and 4 weeks after vaccination, followed by infection with Alexa 488–conjugated BCG at a multiplicity of infection of 20, were labeled with LysoTracker probes and analyzed by confocal microscopy. Images show BCG (green) localized within phagosomes, lysosomes (red), and colocalization of BCG/phagosomes and lysosomes (yellow) indicating P-L fusion. B, The percentage of LysoTracker + BCG/phagosomes (yellow) was considerably higher with postvaccination sera, relative to that with prevaccination sera, suggesting that the increased antibody reactivity to arabinomannan could reduce intracellular mycobacterial growth via FcɣR-mediated increase in P-L fusion.
DISCUSSION
Our studies show that primary and secondary BCG vaccination in humans elicit serum IgG responses to the mycobacterial capsular polysaccharide AM that correlate strongly and significantly with responses to the cell wall glycolipid LAM. In accordance with prior studies investigating Ab reactivities to LAM [16], we demonstrate enhanced BCG opsonophagocytosis with postvaccination sera and show that the increased IgG reactivities to AM are significantly associated with enhanced BCG phagocytosis and growth reduction by human macrophages when incubated with the corresponding sera. Our data suggest that these associations might be driven by IgG responses targeting 3 specific AM epitopes. Interestingly, 2 of these epitopes are also strongly recognized by the murine mAb 9d8, which targets M. tuberculosis AM. These results are important in view of our previous results demonstrating that mAb 9d8 (IgG3) was protective against M. tuberculosis infection in mice [13], and contribute further supportive evidence for a role of Ab-mediated immunity in tuberculosis.
AM and LAM are major components of the mycobacterial cell envelope, and both have critical roles in tuberculosis pathogenesis [32]. Using a variety of AM-binding murine mAbs, data from our prior studies suggest that human and murine Abs against AM are diverse and heterogeneous [33]. We now provide additional data, showing that sera from M. tuberculosis uninfected adults recognize AM OS epitopes in heterogeneous patterns before and after primary or secondary BCG vaccination. These results suggest that individual prior exposure to environmental mycobacteria and BCG vaccination, and possibly also genetic influences on immune responses [34], could influence the repertoire of AM epitopes recognized. They further indicate the importance of using well-defined, homogenous glycans in preference to heterogeneous polysaccharides isolated from nature in observational, mechanistic, and vaccination studies.
The low IgG levels to AM prior to BCG vaccination in both primary and secondary vaccination groups, as well as their decline to near prevaccination levels at 24 weeks in both vaccination groups, are consistent with the often less robust and short-lived Ab responses to carbohydrate antigens and the need for conjugate vaccines to elicit lasting Abs [35]. Subjects in the revaccination group had significantly higher IgG2 titers before and after secondary BCG vaccination than subjects in the primary vaccination group but overall similar magnitudes in IgG2 responses. These results indicate that prior BCG vaccination could lead to ultimately higher titers of IgG2 to AM. The relevance of this finding remains to be explored because there is little evidence that BCG revaccination confers additional protection against tuberculosis [36, 37]. The weak correlation between total IgG and IgG2 responses to AM suggests that other IgG subclass responses against AM are also elicited, consistent with our prior human data [10], and the observation that IgG subclass responses to carbohydrates are not restricted to IgG2 [38]. Although not the focus of this study, we note that the induction of mucosal in addition to systemic AM-specific Abs could have important implications for vaccine efficacy. It is, consequently, noteworthy that oral BCG vaccination was shown to induce LAM-reactive secretory IgA [39]. We found a broad range of increases in AM-specific Ab titers after BCG vaccination, which, along with the epitope-specific heterogeneity, is in line with the frequently observed individual variation of Ab responses to mycobacterial antigens [40]. Whether the heterogeneity in Ab titers and epitope-specific responses, and/or the rapid decline of vaccine induced Abs, could be associated with the limitations of BCG vaccine effectiveness warrants further exploration.
The significant correlation between the postvaccination AM-specific IgG titer and the enhanced BCG phagocytosis and mycobacterial growth reduction in macrophages when coincubated with the corresponding sera suggest that these associations are FcγR mediated. These results are consistent with data from de Valliere et al, who demonstrated that Abs to LAM induced by BCG vaccination were associated with enhanced mycobacterial internalization and growth inhibition by primary human macrophages and neutrophils [16]. The increase in P-L fusion in macrophages infected with BCG preopsonized with postvaccination sera supports that our observed enhanced effects are FcɣR mediated. These data are in accordance with other studies, such as that by Armstrong et al, who demonstrated that rabbit tuberculosis-immune sera opsonize M. tuberculosis and promote macrophage phagocytosis, as well as P-L fusion [41]; that by Kang et al, who showed that entry of ManLAM beads into macrophages via mannose receptors inhibited P-L fusion while entry of anti-LAM mAb coated beads via FcɣRs did not [42]; and that by Kumar et al, who recently demonstrated that human natural opsonizing Abs promote phagosome maturation and restrict M. tuberculosis survival in human macrophages [43].
We anticipated that some of our observed Ab-associated effects would have been further increased in the presence of complement because human IgG can enhance complement activation and increase phagocytosis of BCG and M. tuberculosis by macrophages [44, 45]. Interestingly, in the presence of complement, phagocytosis was similarly and considerably enhanced with both prevaccination and postvaccination sera. Prior studies have shown enhanced phagocytosis via complement receptors with nonimmune sera [44, 46], but to our knowledge this has not been associated with reduced intracellular growth or enhanced P-L fusion. Further studies to elucidate the effects of combined activation of complement and FcɣRs on intracellular M. tuberculosis survival are needed but were beyond the scope of this study. We also made the interesting observation that PBMC MGIA results 4 weeks after vaccination [18] correlated significantly with the IgG titers to AM in the corresponding sera. A possible explanation could be that the PBMCs may have been coated with AM-specific Abs, leading to better uptake and killing of mycobacteria by phagocytic cells, although the portion of such Abs would almost certainly be small. Another possibility is that BCG induces both cell-mediated and humoral immunity.
Glycan microarrays, a recently developed technology [24, 47], have enabled the identification of Abs that recognize immunologically relevant OS fragments from organisms such as Salmonella and malaria parasites [47, 48]. Here, the use of novel mycobacterial glycan microarrays has enabled us to delineate the spectrum of Ab responses elicited to AM OS fragments. Our data suggest that Abs targeting specific AM OS epitopes could be more protective than Abs targeting other AM epitopes—a hypothesis supported by the observation that 2 of 3 epitopes recognized by human sera overlap with those recognized by the switch variant of the murine protective mAb 9d8 [13, 19]. Further characterization of human Abs recognizing specific AM OSs and the generation of mAbs against these epitopes are now warranted, to elucidate the specific mechanisms of such Abs in vitro and in vivo.
In accordance with other experimental studies, we cultured BCG in medium supplemented with detergent to avoid heavy bacterial clumping—an approach required for the feasibility of our experiments, with the downside of removing the mycobacterial capsule to a considerable extent [27, 31]. We attempted to investigate whether subjects' Abs targeted AM of any potentially remaining capsular fraction or the related motifs present in cell wall–associated LAM [32]. Consistent with other EM studies, we saw a clear reduction of the capsule from BCG grown with detergent as compared to without detergent [31]. Nevertheless, BCG grown under both conditions was reactive with a mAb directed against capsular α-glucan [9, 27], suggesting that some components of the capsule remained. This observation was consistent with our prior data showing that the anti-glucan mAb binds to M. tuberculosis strains grown under both conditions with only slightly greater apparent affinity for M. tuberculosis grown without detergent [27]. Thus, the subjects' Abs could have bound to capsular AM and/or the cell wall–associated AM portion of LAM, which is supported by the observation that IgG responses to capsular AM and cell wall LAM correlated strongly and significantly. Of note, when isolated from the same strain, correlations between reactivities to AM and LAM were even stronger than correlations between AM isolated from different strains, supporting that the surface expression of AM is strain dependent [19, 49, 50]. We further showed that postvaccination but not prevaccination sera were reactive with BCG and with M. tuberculosis grown in both the presence and absence of detergent, confirming that Abs elicited through BCG vaccination can opsonize both strains, regardless of culture conditions.
In summary, our studies show that BCG vaccination induces Abs to the mycobacterial capsular polysaccharide AM in humans and that these are strongly correlated with reactivities to the cell wall glycolipid LAM. In accordance with prior studies, we show that these Ab responses are associated with defense mechanisms against mycobacterial infection and provide further evidence indicating that they are FcɣR mediated. Importantly, this work is the first to demonstrate that such Ab responses display a heterogeneous recognition pattern of specific AM OS epitopes. Our results demonstrate that these Abs are biologically active in that they can promote mycobacterial opsonization, phagocytosis, and intracellular growth reduction, and enhance P-L fusion. Our data support and complement previous experimental and clinical studies demonstrating the relevance of Abs targeting AM, specifically some of its OS epitopes, and highlight the importance of further mechanistic studies with mAbs against specific AM glycan structures.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank Dr Neeraj Saini from the Albert Einstein College of Medicine for providing us with the THP-1 cells.
Financial support. This work was supported by the Food and Drug Administration (grant1U18 FD004012/01 to H. A. F., H. M., M. J. B., and J. M. A.), the Aeras TB Vaccine Foundation (to A. C. and J. M. A.), the Bill and Melinda Gates Foundation (to T. L. L., A. C., and J. M. A.); the Alberta Glycomics Centre (to T. L. L.), the Canadian Institutes for Health Research (T. L. L.); the National Institutes of Health (NIH) National Institute of Allergy and Infectious Diseases (grant AI105684 to J. M. A.), the Wellcome Trust (senior clinical research fellowship to H. M.), the National Center for Advancing Translational Sciences (grants UL1 TR001073 and TL1 TR001072 to the Center for Clinical and Translational Research, Albert Einstein College of Medicine), and the NIH National Cancer Institute (grant P30CA013330 to the Analytical Imaging Facility, Albert Einstein College of Medicine).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization (WHO). Global tuberculosis report 2014. Geneva, Switzerland: WHO, 2014. [Google Scholar]
- 2.Andersen P, Kaufmann SH. Novel vaccination strategies against tuberculosis. Cold Spring Harb Perspectives Med 2014; 4:a018523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Achkar JM, Casadevall A. Antibody-mediated immunity against tuberculosis: implications for vaccine development. Cell Host Microbe 2013; 13:250–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Achkar JM, Chan J, Casadevall A. B cells and antibodies in the defense against Mycobacterium tuberculosis infection. Immunol Rev 2015; 264:167–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casadevall A, Pirofski LA. A reappraisal of humoral immunity based on mechanisms of antibody-mediated protection against intracellular pathogens. Adv Immunol 2006; 91:1–44. [DOI] [PubMed] [Google Scholar]
- 6.Daffe M, Etienne G. The capsule of Mycobacterium tuberculosis and its implications for pathogenicity. Tuber Lung Dis 1999; 79:153–69. [DOI] [PubMed] [Google Scholar]
- 7.Stokes RW, Norris-Jones R, Brooks DE, Beveridge TJ, Doxsee D, Thorson LM. The glycan-rich outer layer of the cell wall of Mycobacterium tuberculosis acts as an antiphagocytic capsule limiting the association of the bacterium with macrophages. Infect Immun 2004; 72:5676–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lemassu A, Daffe M. Structural features of the exocellular polysaccharides of Mycobacterium tuberculosis. Biochem J 1994; 297(Pt 2):351–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ortalo-Magne A, Dupont MA, Lemassu A, Andersen AB, Gounon P, Daffe M. Molecular composition of the outermost capsular material of the tubercle bacillus. Microbiology 1995; 141(Pt 7):1609–20. [DOI] [PubMed] [Google Scholar]
- 10.Yu X, Prados-Rosales R, Jenny-Avital ER, Sosa K, Casadevall A, Achkar JM. Comparative evaluation of profiles of antibodies to mycobacterial capsular polysaccharides in tuberculosis patients and controls stratified by HIV status. Clin Vaccine Immunol 2012; 19:198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keitel WA, Dai Z, Awe RW et al. . Effects of infection and disease with Mycobacterium tuberculosis on serum antibody to glucan and arabinomannan: two surface polysaccharides of this pathogen. Bmc Infect Dis 2013; 13:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamasur B, Haile M, Pawlowski A, Schroder U, Kallenius G, Svenson SB. A mycobacterial lipoarabinomannan specific monoclonal antibody and its F(ab’) fragment prolong survival of mice infected with Mycobacterium tuberculosis. Clin Exp Immunol 2004; 138:30–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teitelbaum R, Glatman-Freedman A, Chen B et al. . A mAb recognizing a surface antigen of Mycobacterium tuberculosis enhances host survival. Proc Natl Acad Sci U S A 1998; 95:15688–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamasur B, Haile M, Pawlowski A et al. . Mycobacterium tuberculosis arabinomannan-protein conjugates protect against tuberculosis. Vaccine 2003; 21:4081–93. [DOI] [PubMed] [Google Scholar]
- 15.Glatman-Freedman A, Casadevall A, Dai Z et al. . Antigenic evidence of prevalence and diversity of Mycobacterium tuberculosis arabinomannan. J Clin Microbiol 2004; 42:3225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Valliere S, Abate G, Blazevic A, Heuertz RM, Hoft DF. Enhancement of innate and cell-mediated immunity by antimycobacterial antibodies. Infect Immun 2005; 73:6711–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costello AMD, Kumar A, Narayan V et al. . Does antibody to mycobacterial antigens, including lipoarabinomannan, limit dissemination in childhood tuberculosis. T Roy Soc Trop Med H 1992; 86:686–92. [DOI] [PubMed] [Google Scholar]
- 18.Fletcher HA, Tanner R, Wallis RS et al. . Inhibition of mycobacterial growth in vitro following primary but not secondary vaccination with Mycobacterium bovis BCG. Clin Vaccine Immunol 2013; 20:1683–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwebach JR, Casadevall A, Schneerson R et al. . Expression of a Mycobacterium tuberculosis arabinomannan antigen in vitro and in vivo. Infect Immun 2001; 69:5671–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaur D, Lowary TL, Vissa VD, Crick DC, Brennan PJ. Characterization of the epitope of anti-lipoarabinomannan antibodies as the terminal hexaarabinofuranosyl motif of mycobacterial arabinans. Microbiol-Sgm 2002; 148:3049–57. [DOI] [PubMed] [Google Scholar]
- 21.Rademacher C, Shoemaker GK, Kim HS et al. . Ligand specificity of CS-35, a monoclonal antibody that recognizes mycobacterial lipoarabinomannan: a model system for oligofuranoside-protein recognition. J Am Chem Soc 2007; 129:10489–502. [DOI] [PubMed] [Google Scholar]
- 22.Murase T, Zheng RB, Joe M et al. . Structural insights into antibody recognition of mycobacterial polysaccharides. J Mol Biol 2009; 392:381–92. [DOI] [PubMed] [Google Scholar]
- 23.Kamath VP, Diedrich P, Hindsgaul O. Use of diethyl squarate for the coupling of oligosaccharide amines to carrier proteins and characterization of the resulting neoglycoproteins by MALDI-TOF mass spectrometry. Glycoconj J 1996; 13:315–9. [DOI] [PubMed] [Google Scholar]
- 24.Campbell CT, Zhang Y, Gildersleeve JC. Construction and use of glycan microarrays. Curr Protoc Chem Biol 2010; 2:37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hed J, Hallden G, Johansson SG, Larsson P. The use of fluorescence quenching in flow cytofluorometry to measure the attachment and ingestion phases in phagocytosis in peripheral blood without prior cell separation. J Immunol Methods 1987; 101:119–25. [DOI] [PubMed] [Google Scholar]
- 26.Fuss IJ, Kanof ME, Smith PD, Zola H. Isolation of whole mononuclear cells from peripheral blood and cord blood. Curr Protoc Immunol 2009; doi:10.1002/0471142735.im0701s85. [DOI] [PubMed] [Google Scholar]
- 27.Schwebach JR, Glatman-Freedman A, Gunther-Cummins L et al. . Glucan is a component of the Mycobacterium tuberculosis surface that is expressed in vitro and in vivo. Infect Immun 2002; 70:2566–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ponpuak M, Delgado MA, Elmaoued RA, Deretic V. Monitoring autophagy during Mycobacterium tuberculosis infection. Methods Enzymol 2009; 452:345–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris J, Hope JC, Keane J. Tumor necrosis factor blockers influence macrophage responses to Mycobacterium tuberculosis. J Infect Dis 2008; 198:1842–50. [DOI] [PubMed] [Google Scholar]
- 30.Holm S. A simple sequentially rejective multiple test procedure. Scand Stat 1979; 6:65–70. [Google Scholar]
- 31.Sani M, Houben EN, Geurtsen J et al. . Direct visualization by cryo-EM of the mycobacterial capsular layer: a labile structure containing ESX-1-secreted proteins. PLoS Pathog 2010; 6:e1000794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Angala SK, Belardinelli JM, Huc-Claustre E, Wheat WH, Jackson M. The cell envelope glycoconjugates of Mycobacterium tuberculosis. Crit Rev Biochem Mol Biol 2014; 49:361–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Navoa JA, Laal S, Pirofski LA et al. . Specificity and diversity of antibodies to Mycobacterium tuberculosis arabinomannan. Clin Diagn Lab Immunol 2003; 10:88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haralambieva IH, Ovsyannikova IG, Pankratz VS, Kennedy RB, Jacobson RM, Poland GA. The genetic basis for interindividual immune response variation to measles vaccine: new understanding and new vaccine approaches. Expert Rev Vaccines 2013; 12:57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Astronomo RD, Burton DR. Carbohydrate vaccines: developing sweet solutions to sticky situations? Nat Rev Drug Discov 2010; 9:308–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodrigues LC, Pereira SM, Cunha SS et al. . Effect of BCG revaccination on incidence of tuberculosis in school-aged children in Brazil: the BCG-REVAC cluster-randomised trial. Lancet 2005; 366:1290–5. [DOI] [PubMed] [Google Scholar]
- 37.Randomised controlled trial of single BCG, repeated BCG, or combined BCG and killed Mycobacterium leprae vaccine for prevention of leprosy and tuberculosis in Malawi. Karonga Prevention Trial Group. Lancet 1996; 348:17–24. [PubMed] [Google Scholar]
- 38.von Gunten S, Smith DF, Cummings RD et al. . Intravenous immunoglobulin contains a broad repertoire of anticarbohydrate antibodies that is not restricted to the IgG2 subclass. J Allergy Clin Immunol 2009; 123:1268–76 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown RM, Cruz O, Brennan M et al. . Lipoarabinomannan-reactive human secretory immunoglobulin A responses induced by mucosal bacille Calmette-Guerin vaccination. J Infect Dis 2003; 187:513–7. [DOI] [PubMed] [Google Scholar]
- 40.Kunnath-Velayudhan S, Salamon H, Wang HY et al. . Dynamic antibody responses to the Mycobacterium tuberculosis proteome. Proc Natl Acad Sci U S A 2010; 107:14703–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Armstrong JA, Hart PD. Phagosome-lysosome interactions in cultured macrophages infected with virulent tubercle bacilli. Reversal of the usual nonfusion pattern and observations on bacterial survival. J Exp Med 1975; 142:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang PB, Azad AK, Torrelles JB et al. . The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J Exp Med 2005; 202:987–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar SK, Singh P, Sinha S. Naturally produced opsonizing antibodies restrict the survival of Mycobacterium tuberculosis in human macrophages by augmenting phagosome maturation. Open Biol 2015; 5:150171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schlesinger LS, Bellingerkawahara CG, Payne NR, Horwitz MA. Phagocytosis of mycobacterium-Tuberculosis is mediated by human monocyte complement receptors and complement component-C3. J Immunol 1990; 144:2771–80. [PubMed] [Google Scholar]
- 45.Hetland G, Wiker HG, Hogasen K, Hamasur B, Svenson SB, Harboe M. Involvement of antilipoarabinomannan antibodies in classical complement activation in tuberculosis. Clin Diagn Lab Immunol 1998; 5:211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferguson JS, Weis JJ, Martin JL, Schlesinger LS. Complement protein C3 binding to Mycobacterium tuberculosis is initiated by the classical pathway in human bronchoalveolar lavage fluid. Infect Immun 2004; 72:2564–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park S, Lee MR, Shin I. Fabrication of carbohydrate chips and their use to probe protein-carbohydrate interactions. Nat Protoc 2007; 2:2747–58. [DOI] [PubMed] [Google Scholar]
- 48.Blixt O, Hoffmann J, Svenson S, Norberg T. Pathogen specific carbohydrate antigen microarrays: a chip for detection of Salmonella O-antigen specific antibodies. Glycoconj J 2008; 25:27–36. [DOI] [PubMed] [Google Scholar]
- 49.Wittkowski M, Mittelstadt J, Brandau S et al. . Capsular arabinomannans from Mycobacterium avium with morphotype-specific structural differences but identical biological activity. J Biol Chem 2007; 282:19103–12. [DOI] [PubMed] [Google Scholar]
- 50.Misaki A, Azuma I, Yamamura Y. Structural and immunochemical studies on D-arabino-D-mannans and D-mannans of Mycobacterium tuberculosis and other Mycobacterium species. J Biochem 1977; 82:1759–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.