Abstract
Background. Maternal-fetal transferred dengue virus (DENV)–specific antibodies have been implicated in the immunopathogenesis of dengue during infancy.
Methods. A prospective birth cohort was established in a dengue-endemic area in the Northeast Region of Brazil. DENV-specific immunoglobulin G (IgG) and DENV-1–4 serotype–specific neutralizing antibody (NAb) levels were assessed in 376 paired maternal and umbilical cord blood samples. The kinetics of enhancing activity by maternally acquired DENV antibodies was determined in serum samples from children enrolled in the cohort.
Results. Mothers were mostly immune to DENV-3 alone (53.7%) or combined with DENV-4 (30.6%). Levels of DENV-specific IgG, DENV-3 NAbs, and DENV-4 NAbs were significantly higher in newborns than in their respective mothers. Mothers immune to a single serotype transferred greater levels of DENV-specific IgG (P = .02) and DENV-3 NAbs (P = .04) than mothers immune to multiple DENV serotypes. Maternally acquired DENV-3 NAbs disappeared in >90% of the children by the age of 4 months. The peak enhancing activity was detected by the age of 2 months (P < .0001) and rapidly declined by the age of 4 months (P = .0035).
Conclusions. Unlike Asian infants, the enhancing activity of DENV infection by maternally transferred DENV antibodies occurs at earlier ages in Brazilian children. These findings might explain the low occurrence of severe dengue among infants in our setting.
Keywords: dengue, placental transfer, IgG, newborn, antibody dependent enhancement
Dengue is a mosquito-borne viral infection caused by 4 related but antigenically distinct dengue virus (DENV) serotypes (DENV-1–4) [1]. Currently, more than half of the human population is at risk of DENV infection [2], and severe dengue remains a leading cause of hospitalization and death among children, particularly in some Southeast Asian countries [3, 4]. In Brazil, severe cases and dengue-related hospitalization rates are usually greater among adults. Although a transient shift toward younger age groups was observed between 2006 and 2008, the incidence of severe dengue cases in infants is still much lower than in Southeast Asia [5].
The immunopathogenesis of severe dengue is still incompletely understood. One of the leading hypothesis explaining the increased risk for severe dengue in secondary infections, named antibody-dependent enhancement, suggests that nonneutralizing antibodies, elicited by a primary infection, favors virus uptake into the target cells during a subsequent exposure to a different DENV serotype, leading to increased virus load and severe disease [6, 7].
In infants, severe dengue often occurs in primary infections and is associated with the presence of antibodies transferred from their dengue-immune mothers during pregnancy [8–12]. Maternally acquired DENV-specific antibodies play a dual role in infants during the first year of life: they confer protection at birth, and then they decline to a lower level capable of increasing the risk of severe DENV infection through antibody-dependent enhancement [4, 7, 9, 10, 13].
The placental transfer of maternal immunoglobulin G (IgG) and its subclasses (IgG1–4) to the fetus starts around the thirteenth week of gestation and progressively increases throughout pregnancy, peaking during the third trimester, when the IgG concentration in fetal serum often exceeds maternal levels [14, 15]. The mechanism of transfer of maternal IgG to the fetus is mediated by the neonatal Fc receptors (FcRn) present on syncytiotrophoblast cells in the placental tissues [15]. Several factors have been shown to interfere in the placental transfer of IgG antibodies [16–18], including high levels of maternal total IgG [14]. A high level of IgG antibodies leads to a saturation of FcRn receptors present in the placental tissues and is correlated with a reduction on the transfer efficiency of antigen-specific IgG to the fetus [15], as demonstrated in some viral infectious, such as measles, herpes simplex virus infection, and varicella-zoster virus infection [19, 20].
The efficient transfer of maternal DENV-specific IgG to the fetus has been demonstrated in several studies involving paired maternal and umbilical cord blood specimens (hereafter, “mother-cord pairs”) [8, 9, 11, 12], including the placental transfer of DENV-specific IgG subclasses (IgG1 and IgG4) that have been implicated in the pathogenesis of severe dengue [12, 21, 22]. Prospective studies conducted in Asian infants have also provided evidence that the peak of enhancing activity by maternally transferred DENV-specific antibodies occurs between ages 6 and 9 months, which correlates with the age-related epidemiology of the severe dengue cases in this region [13]. In the Americas, information on the transfer of DENV-specific antibodies in mother-cord pairs and on the kinetics of DENV infection enhancing activity is still scarce. Therefore, a prospective birth cohort was established in a dengue-endemic area in the Northeast Region of Brazil between 2010 and 2014 [23].
Here, we describe the placental transfer of DENV-specific antibody and its subclasses (IgG1 and IgG4) in the mother-cord pairs enrolled in this study. Moreover, we analyze the role of maternal total IgG levels and dengue immunity in the transfer of DENV-specific antibodies to the fetus. In the cohort of children, we determine the kinetics of enhancing activity by maternally acquired DENV-specific antibodies during their first year of life.
METHODS
Study Population
The prospective cohort study was conducted in the city of Recife, a large urban area in the Northeast Region of Brazil. During recruitment (March 2011–May 2012), around 17 000 dengue cases were reported in the city [24], and all DENV serotypes cocirculated in the region, with the predominance of DENV-4 (Health Department, Pernambuco State, unpublished data).
Mother-Newborn Pairs
Study design and data collection from the mother-newborn pairs have been described in detail elsewhere [24]. In summary, healthy pregnant women were recruited at the maternity ward of the Instituto de Medicina Integral Prof Fernando Figueira, a large, publicly funded teaching hospital in Recife. Women living in Recife who had a low-risk pregnancy and agreed to have their infants followed up during the first year of life were eligible for the study. Individual information and maternal blood samples were collected at the time of admission for delivery. Umbilical cord blood samples were obtained immediately after birth. Serum samples were separated and stored at −70°C.
Healthy Infants
Children were followed during the first year of life. Details of their follow-up have been published elsewhere [25]. In summary, 415 neonates were randomly allocated into 2 groups and examined by a pediatrician at scheduled time points after birth. Group 1 comprised 212 children who were evaluated at 2, 6, and 10 months of age, and group 2 comprised 203–children who evaluated at 4, 8, and 12 months of age. Blood samples were collected at each visit, and serum samples were separated and stored at −70°C. To better understand the kinetics of DENV infection–enhancing activity by maternally transferred DENV-specific antibodies, a cross-sectional set of independent serum samples were randomly selected from the children included in cohort. The inclusion criteria for infants from whom samples were selected was as follows: (1) born to mothers with measurable levels of DENV-specific IgG, as indicated by an in-house enzyme-linked immunosorbent assay (ELISA); (2) born to mothers with immunity to monotypic infection by DENV-3, as determined by a plaque reduction neutralization test (PRNT); (3) no evidence of recent DENV infection during the cohort follow-up, as determined by immunoglobulin M (IgM) analysis and/or reverse transcription polymerase chain reaction (RT-PCR) analysis. Samples collected at birth (umbilical cord) and at 2, 4, 6, 8, 10, and 12 months of age from 210 infants (30 serum samples at each time point) were used in the experiments.
Laboratory Assays
Levels of total IgG were obtained using a quantitative sandwich ELISA. An in-house capture ELISA was used for the detection of virus-binding IgG, consisting of DENV-specific IgG and DENV-specific IgG1 and IgG4 subclasses. A PRNT was used for the detection of DENV serotype–specific neutralizing antibodies, following a modified protocol previously described elsewhere [26]. Enhancing activity was determined by flow cytometry in sera from children, using K562 FcγRII-expressing cells. Serum samples were tested at a low dilution (1:10) to mimic in vivo conditions. Enhancing activity was measured as the n-fold increase in the percentage of DENV-infected cells relative to that in DENV-naive serum. Laboratory procedures have been described in detail in the Supplementary Materials.
Ethical Considerations
Written informed consent was obtained from a parent or guardian of each parturient or healthy infant enrolled in the study. The protocol was approved by the Ethical Committee of Aggeu Magalhaes Research Center (CAAE-0061.0.095.000-10) and the Instituto de Medicina Integral Prof Fernando Figueira (no. 2744/2010).
Data Analysis
The main characteristics of the mother-cord pairs were described. Titers of DENV-specific IgG, IgG1, and IgG4 subclasses and serotype-specific neutralizing antibodies were estimated using nonlinear regression, and values obtained were log transformed (log10). Maternal and newborn antibodies mean titers were compared using the nonparametric Wilcoxon test (paired samples). We measured placental transfer as a ratio, calculated as follows: [newborn antibody titer/maternal antibody titer] × 100. We used Pearson correlation to measure the association between maternal total IgG and the antibody transfer ratio (TR). The median TRs between the 2 groups were compared using Mann–Whitney test. The proportion of children with detectable levels of DENV-specific antibodies over time was determined. Antibody levels at each time point were compared using the Kruskal–Wallis test. The level of significance was set at .05. Statistical analysis was performed using R, version 3.2.1, and Graph Pad Prism, version 6.0e.
RESULTS
Characteristics of the Mother-Newborn Pairs
A total of 376 mother-cord pairs were analyzed. Mothers were mostly young adults (age range, 13–43 years), and 10% reported smoking habits during the current gestation (Table 1). Overall, 42.2% of parturients reported infections during pregnancy (135 had urinary tract infection, 1 had hepatitis, and 5 had syphilis). Macroscopic abnormalities of the placenta were observed in 4.5% of the mothers. Among the neonates, 51.9% were female, and most had normal body weight (Table 1). Serotype-specific immunity to DENV was investigated through PRNT in all parturients enrolled in this study. Nearly 90% of the mothers presented neutralizing antibodies to at least 1 DENV serotype, mostly against DENV-3 alone (53.7%) or to the combination of DENV-3 and DENV-4 (30.6%; Table 1).
Table 1.
Main Characteristics of 376 Mother-Newborn Pairs
| Characteristic | Value |
|---|---|
| Mothers | |
| Age, y | 23.7 ± 6.2 |
| Report of smoking habits during pregnancya | 35 (10.0) |
| Infectious events during pregnancyb | 159 (42.2) |
| Use of immunosuppressive or corticosteroids during pregnancy | 17 (4.5) |
| Macroscopic placental abnormalities | 17 (4.5) |
| DENV immunityc | |
| Monotypic | |
| DENV-3 | 202 (53.7) |
| Multitypic | |
| DENV-3/DENV-4 | 115 (30.6) |
| Other serotype combinations | 20 (5.3) |
| Newborns | |
| Birth weight, g | 3 301 ± 370.0 |
| Female sex | 195 (51.9) |
Data are no. (%) of participants or mean value ± SD.
Abbreviation: DENV, dengue virus.
a Data are missing for 27 participants.
b The occurrence of infectious events during pregnancy was documented by trained nurses.
c DENV immunity was determined by a plaque reduction neutralization test.
Placental Transfer of Maternal DENV Antibodies
We investigated the levels of DENV-specific IgG, by ELISA, in both mothers and newborns. DENV-specific IgG antibody levels in newborns were statistically significantly higher than those in their corresponding mothers (TR, 103.8%; P < .05). This pattern was similar regardless of DENV serotype-specific immunity of the mother (TR, 108.2% for DENV-3 [P < .05] and 110.3% for DENV-4 [P < .05]; Table 2).
Table 2.
Antibody (Ab) Titer and Placental Transfer Ratio (TR) of Dengue Virus (DENV)–Specific Immunoglobulin G (IgG), DENV-1–4 Serotype–Specific Neutralizing Abs, and DENV-Specific IgG1 and IgG4 Subclasses in 376 Pairs of Maternal and Umbilical Cord Blood Samples
| Serologic Variable | Maternal Sera (n = 376) |
Cord Sera (n = 376) |
TR,b % | P Valuec | ||
|---|---|---|---|---|---|---|
| Positive, No. (%) | Ab Titer,a Mean ± SD | Positive, No. (%) | Ab Titer,a Mean ± SD | |||
| DENV-specific IgG | 373 (99.2) | 4.82 ± 0.61 | 372 (98.9) | 4.99 ± 0.63 | 103.8 | <.05 |
| DENV immunity | ||||||
| DENV-1 | 17 (4.5) | 1.47 ± 0.24 | 22 (5.8) | 1.48 ± 0.16 | 103.6 | .53 |
| DENV-2 | 10 (2.6) | 1.51 ± 0.31 | 9 (2.4) | 1.37 ± 0.10 | 92.7 | .23 |
| DENV-3 | 333 (88.5) | 2.47 ± 0.66 | 340 (90.4) | 2.63 ± 0.69 | 108.2 | <.05 |
| DENV-4 | 127 (33.7) | 1.56 ± 0.44 | 151 (40.1) | 1.70 ± 0.46 | 110.3 | <.05 |
| DENV-specific IgG subclass | ||||||
| IgG1 | 353 (94.6) | 4.31 ± 0.64 | 354 (95.6) | 4.40 ± 0.64 | 103.3 | <.05 |
| IgG4 | 241 (64.2) | 2.56 ± 0.51 | 213 (56.9) | 2.53 ± 0.53 | 97.0 | <.05 |
Abbreviation: SD, standard deviation.
a Log transformed (log10).
b Calculated as follows: [newborn antibody titer/maternal antibody titer] x 100.
c Wilcoxon test (paired samples).
More than 90% of the DENV IgG–positive mothers had detectable levels of DENV-specific IgG1 subclass, which was efficiently transferred to their newborn (TR, 103.3%; P < .05). On the other hand, DENV-specific IgG4 subclass was detected in nearly 65% of the DENV-specific IgG–positive mothers, and its levels were significantly lower in the newborns as compared to the mothers (TR, 97%; P < .05; Table 2), suggesting that its transfer was not as efficient as that of IgG1.
Maternal Total IgG Levels and Placental Transfer of Total IgG and DENV Antibodies
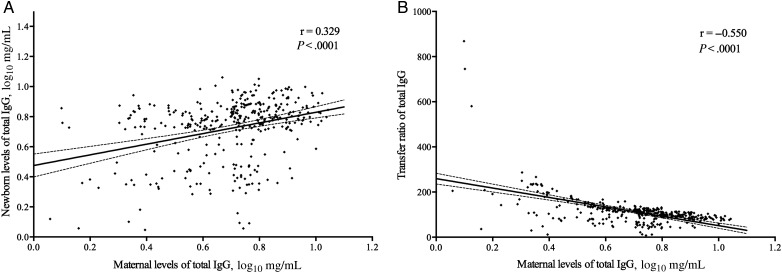
We quantified the levels of total IgG in the mother-cord pairs to assess the effect of maternal total IgG levels on the transfer of total IgG to the neonates. A positive correlation was found between levels of total IgG in the maternal and newborn samples (r = 0.329; P < .0001; Figure 1A). Moreover, higher levels of total IgG in the mothers were associated with a lower TR of these antibodies to their newborns (r = −0.550; P < .0001; Figure 1B). We also analyzed the correlation between maternal levels of total IgG and the TR of DENV-specific antibodies to the neonate. We found that higher levels of maternal total IgG antibodies was negatively correlated with the TR of DENV-3 neutralizing antibodies (r = −0.126; P = .02) but not with the TR of DENV-specific IgG (r = −0.011; P = .82). In addition, the maternal TR of total IgG was negatively correlated with TR of DENV-specific IgG4 (r = −0.135, P = .05) but not with TR of IgG1 subclass (r = −0.054, P = .34).
Figure 1.
Levels of total immunoglobulin G (IgG) in paired maternal and umbilical cord blood samples. A, There was positive correlation between levels of total IgG in the maternal and newborn samples (Pearson r = 0.329; P < .0001). B, There was a significant negative correlation between levels of maternal total IgG and the transfer ratio (TR) of total IgG to the newborn (Pearson r = −0.550; P < .0001). The TR was calculated as follows: [newborn antibody titer/maternal antibody titer] x 100. The dashed lines show the 95% confidence intervals.
Maternal DENV Immunity and Placental Transfer of DENV Antibodies
We investigated the role of maternal DENV immunity on the transfer of DENV-specific antibodies across the placenta. Interestingly, we found that the placental TRs of DENV-specific IgG (TR, 101.7% vs 103.9%; P = .02) and DENV-3 neutralizing antibodies (TR, 104.3% vs 106.8%; P = .04) were lower in mothers immune to >1 serotype (multitypic infection) as compared to those exposed to only 1 serotype (monotypic infection; Table 3). The same pattern was observed for the TRs of DENV-specific IgG1 and IgG4 subclasses, although results were not statistically significant.
Table 3.
Transfer Ratio (TR) of Dengue Virus (DENV)-Associated Antibodies (Abs) and Previous Maternal History of Dengue
| Serologic Variable | Transfer Ratio,a Median (Range) |
P Valuec | |
|---|---|---|---|
| Monotypicb | Multitypicb | ||
| DENV-specific IgG | 103.9 (68.4–141.9) | 101.7 (75.3–137.7) | .02 |
| DENV-3 neutralizing Ab | 106.8 (39.5–192.5) | 104.3 (72.1–146.5) | .04 |
| DENV-specific subclass | |||
| IgG1 | 103.5 (76.0–175.4) | 103.1 (73.8–137.3) | .36 |
| IgG4 | 98.0 (56.9–161.0) | 95.1 (60.0–127.6) | .16 |
Abbreviation: IgG, immunoglobulin G.
a Calculated as follows: [newborn antibody titer/maternal antibody titer] x 100.
b Determined by a plaque reduction neutralization test.
c By the Mann–Whitney test.
Kinetics of Maternally Derived DENV Antibodies in the Prospective Cohort
To further characterize the kinetics of decay of maternally derived DENV-specific antibodies, we randomly selected a subset of independent serum samples obtained at birth (from the umbilical cord) and at 2, 4, 6, 8, 10, and 12 months of age from healthy children with no evidence of current/previous DENV infection who were born to DENV-3–immune mothers.
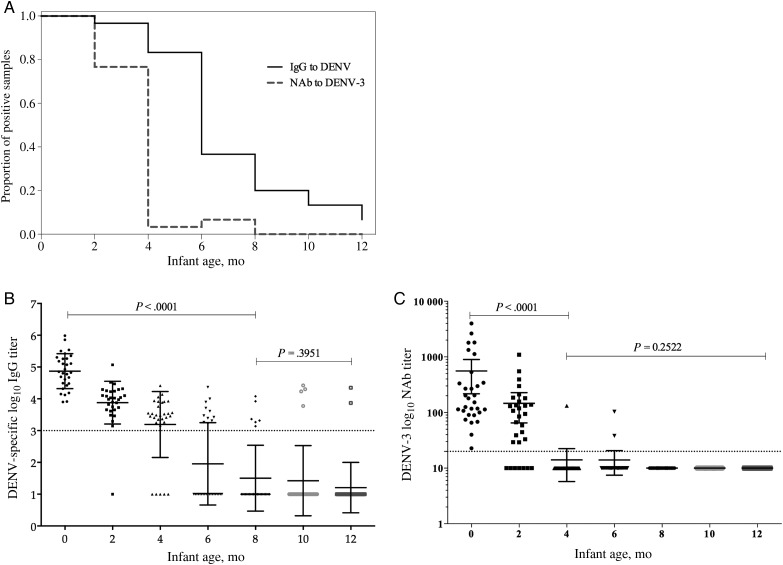
We first determined the antibody decay of DENV-specific IgG (binding IgG) and neutralizing antibodies to DENV-3. Maternally derived DENV-specific IgG was detected in 100% of cord serum samples and disappeared in 63.4%, 80%, and 94% of children by 6, 8, and 12 months of age, respectively. In contrast, neutralizing antibodies to DENV-3 were detected in only approximately 7% of the infants by the age of 6 months and were undetectable by the age of 8 months (Figure 2A).
Figure 2.
Decay of maternally transferred dengue virus (DENV)–specific antibodies in infants during the first year of life. A, The proportion of infants at each follow-up time point with measurable levels of DENV-specific immunoglobulin G (IgG) and DENV-3 neutralizing antibodies. B and C, Antibody decay for DENV-specific IgG (B) and DENV-3 (C). Abbreviation: NAb, neutralizing antibody.
Both levels of DENV-specific IgG (r = −0.777; P < .0001) and DENV-3 neutralizing antibodies (r = −0.734; P < .0001) were negatively correlated with age. Higher mean DENV-specific IgG titers (±SD) were detected at birth (4.87 ± 0.54) and declined to 3.84 ± 0.82, 1.32 ± 1.77, and 0.27 ± 1.04 at 2, 6, and 12 months of age, respectively (P < .001; Figure 2B). The mean titers (±SD) of DENV-3 neutralizing antibodies significantly dropped from birth (2.35 ± 0.55) to 2 months (1.82 ± 0.58) and 4 months (1.03 ± 0.20) of age (P < .001; Figure 2C).
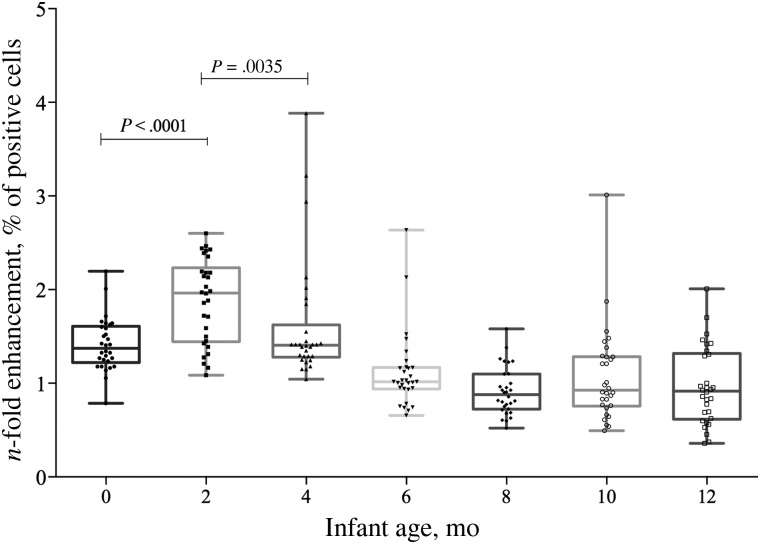
We assessed the kinetics of enhancing activity by maternally acquired DENV-specific antibodies in serum samples obtained from infants during their first year of life. Enhancing activity was measured as the n-fold increase in the percentage of DENV-infected cells relative to that in DENV-naive serum. Interestingly, the enhancing activity significantly increased by the age of 2 months (1.96; range, 1.1–2.6), compared with the value at birth (P < .0001), and rapidly declined at the age of 4 months (P = .0035). No enhancing activity was detected in children ≥6 months of age (Figure 3).
Figure 3.
Kinetics of enhancing activity. Serum samples from children born to dengue virus serotype 3 (DENV-3)–immune mothers were heat inactivated, mixed with DENV-2 16681 (prototype), and cultured with K562 cells for 2 days. Shown is the n-fold increase in the percentage of DENV-2–infected cells in children's sera, compared with that in Flavivirus-naive control serum. Sera from 2-month-old infants provided significantly more enhancement than sera collected at any other time point (P < .05, by the Mann–Whitney test).
DISCUSSION
Antibody responses play both pathogenic and protective roles during DENV infection [27]. This ambiguous function is even more evident in neonates, in whom maternally transferred DENV-specific antibodies protect them during the first months of life but can enhance DENV infection through antibody-dependent enhancement and, thus, cause severe disease as their titers drop to subneutralizing levels [4, 9, 10]. The mechanisms involved in the transfer of maternal DENV-specific antibodies to neonates are not fully understood. Here we unveiled the role of maternal antibody levels and history of DENV infection on the efficiency of DENV-specific antibody transfer to neonates, as well as the kinetics of antibody decay during their first year.
We have previously demonstrated that the seroprevalence of anti-DENV IgG among the parturients enrolled in this cohort surpasses 90% [23]. Here, we showed that the study population mostly comprised young adult mothers immune to DENV-3 alone (53.7%) or in combination with DENV-4 (30.6%). Although immunity to DENV-1 and DENV-2 was present among the DENV-immune mothers, it represented only 5% of the cohort participants. These findings probably reflect the epidemiological scenario of DENV circulation in Brazil in the last 15 years. DENV-3 circulated predominantly in Brazil between 2002 and 2006, and DENV-4 circulated after its introduction in 2010 [5, 26, 28]. However, the dengue epidemiological profile observed among our pregnant population is vastly distinct from that in studies of maternal transfer of DENV-specific antibodies performed in Asian countries, where all 4 DENV serotypes have cocirculated for >5 decades and mothers are usually immune to all serotypes [8, 10, 11, 29].
As observed by our group and corroborated by others [8, 10, 11, 29, 30], mothers efficiently transferred DENV-specific antibodies to their infants. This finding reflects the active transport of IgG across the placenta, a well-documented immune mechanism mediated by FcRn [15]. Additionally, this transfer was efficient among mothers immune to DENV-3 or DENV-4. Since, in our study, there were a small proportion of mothers immune to DENV-1 and DENV-2, it was not possible to compare the TR between all DENV serotypes.
All DENV-specific IgG subclasses are detected in DENV-immune individuals [21, 22] and cross the placental tissues [12]. Although virus-specific IgG2 and IgG3 are found in DENV-immune individuals, their roles in disease protection and pathogenesis are not clear. On the other hand, IgG1 and IgG4 subclasses have been implicated on the pathogenesis of severe dengue [21]. Moreover, these IgG subclasses efficiently cross the placenta, owing to their increased affinity to FcRn [15, 31], which is the reason why we focused on the transfer of those subclasses. Our findings demonstrated that DENV-specific IgG1 was more efficiently transferred than IgG4, confirming the results of a previous study conducted among mother-cord pairs in Thailand [12]. The abundance of DENV-specific IgG1 and increased relative affinity to FcRn over IgG4 may play a role on the increased efficiency of IgG1 to cross the placenta during pregnancy.
Maternal total IgG levels negatively influenced the transfer of antibodies to neonates: as maternal IgG levels increased, the transfer of total IgG, DENV-3 neutralizing antibodies, and DENV-specific IgG4 subclass decreased. Such mechanism is not exclusive to DENV-associated antibodies, since transplacental transfer of several virus-specific antibodies [19, 20, 32] is also influenced by maternal total IgG levels. This threshold effect is even more evident in hypergammaglobulinemic mothers [20, 33]. This reduced transfer is probably a consequence of the high competition among IgG molecules for interaction with a limited number of placental FcRn receptors [15]. Notably, maternal total IgG levels did not influence the transfer of the DENV-specific IgG1 subclass. We speculate that the higher affinity of this subclass to the FcRn receptors probably plays a role in the competition process, favoring the placental transfer of IgG1 over that of IgG4. Moreover, transfer rates of both DENV-specific IgG4 subclasses and DENV-3 neutralizing antibodies were negatively correlated with maternal total IgG levels, probably suggesting a role of IgG4 in mediating neutralization.
Interestingly, we found that previous maternal immunity to DENV influenced the transfer efficiency of DENV-specific IgG and DENV-3 neutralizing antibodies to neonates. The TR of those antibodies was significantly lower among mothers with a history of exposure to multiple DENV serotypes (multitypic infection) when compared to mothers who experienced infection by a single DENV serotype (monotypic infection). Since mothers exposed to several DENV serotypes have greater levels of DENV-associated antibodies than mothers with history of a single serotype infection, competition for the interaction with FcRn receptors was possibly involved in this reduced transfer of DENV-specific IgG observed among mothers exposed to multiple DENV serotypes.
Our analysis of the maternal dengue profile revealed that mothers from our study setting were mostly exposed to monotypic infections to DENV-3, which is why we focused our evaluation of the decay of maternally derived antibodies on a subset of serum samples obtained from healthy children who had no serological and/or virological evidence of DENV exposure and were born to DENV-3–immune mothers. We demonstrated that levels of DENV-3 neutralizing antibodies declined faster than levels of DENV-specific IgG (virus-binding IgG) during the first year, confirming the findings of a prospective birth cohort study conducted in infants from Vietnam [8]. This probably reflects the ability of the DENV-specific IgG assay to detect the binding of IgG to other antigens (eg, prM/M) exposed on the surface of the virus-coated wells of the ELISA plates, while neutralizing antibodies are mainly directed to the envelope protein [6, 8].
Of note, the loss of neutralizing antibodies among children included in our study occurred sooner (in >90% children by 4 months of age), compared with different Asian cohort studies, in which neutralizing antibodies decreased to undetectable levels in around 80%–90% of children by 9 months of age [8, 29, 34]. Panhuis et al [34] showed that higher DENV-specific antibody levels at birth are associated with faster decay rates of antibodies during the first years of life. Interestingly, FcRn receptors are the molecules responsible for both placental transfer and recycling of IgG into the blood circulation [15]. Thus, we cannot exclude the possibility that the mechanism of competition for IgG binding might play a role in the faster decay rates observed in the presence of high levels of IgG at birth. Moreover, based on our evidence that mothers exposed to monotypic infections transfer greater levels of DENV-specific antibodies to their neonates, it is reasonable to speculate that the differences in decay rates may be related to maternal DENV immunity, since Asian mothers are usually immune to all DENV serotypes [8, 10, 11, 29] and probably transfer lower levels of DENV-specific antibodies to neonates as compared to the mothers from our setting.
In the prospective cohort, DENV infection (determined by IgM analysis and/or RT-PCR analysis) varied from 7.3% to 11.5% during the first year of life [25], and none of the virologically/serologically confirmed dengue cases progressed to severe dengue (unpublished data). Unlike Brazil, in Asia severe dengue is a common problem among infants aged <1 year born to DENV-immune mothers [3–5], and the decline of maternally transferred DENV-specific antibodies to below neutralizing levels has been implicated as a risk factor for severe dengue in infants experiencing primary infection [4, 9, 10]. Consistent with the faster decay of neutralizing antibodies, we demonstrated that DENV infection–enhancing activity by maternally transferred DENV-specific antibodies occurs at earlier ages (approximately 2–4 months) in children from our study setting. This immunological window for enhancement of DENV infection probably reduces the length of time that children would be vulnerable to developing severe dengue if exposed to a primary DENV infection. In addition, infants at this age are normally swaddled in clothes, reducing their exposure to the bite of mosquitoes [8]. These factors probably contribute to the reduced incidence of severe dengue among infants in our study setting and perhaps in Brazil. We acknowledge that different DENV attack rates, virus virulence of the circulating serotypes, and/or genetic background might also play a role.
In summary, we demonstrated that mothers were mostly immune to DENV-3 alone and that this previous maternal immunity profile was associated with increased placental transfer of DENV-specific antibodies to neonates. Moreover, subneutralizing levels of DENV-specific antibodies capable of mediating antibody-dependent enhancement are reached sooner in infants from our study setting. These findings have implications in the immunopathogenesis of dengue during the first months of life in Brazilian children and might explain the lower rates of severe dengue in infants observed in the Americas as compared to those in Asian countries. Moreover, the data provide useful insights about the age-related susceptibility and dynamics of dengue transmission among infants in Brazil.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank the mothers and their infants, for participating in the study; and the nurses and clinical staff from IMIP who assisted with the study.
Financial support. This work was supported by the National Council for Scientific and Technological Development of the Brazilian government (grant 482915/2010-2 MCT/CNPq-14/2010), the Strategic Program to Support Health Research/PAPES VI (grant 407697/2012-8), and the Fogarty Training Program (grant D43TW006592 Pitt GIDRTP/NIH to P. M. S. C.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Rodenhuis-Zybert IA, Wilschut J, Smit JM. Dengue virus life cycle: viral and host factors modulating infectivity. Cell Mol Life Sci 2010; 67:2773–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatt S, Gething PW, Brady OJ et al. The global distribution and burden of dengue. Nature 2013; 496:504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Dengue and severe dengue. Fact sheet no. 117. Updated May 2015. http://www.who.int/mediacentre/factsheets/fs117/en/ Accessed 29 June 2015.
- 4.Halstead SB, Lan NT, Myint TT et al. Dengue hemorrhagic fever in infants: research opportunities ignored. Emerg Infect Dis 2002; 8:1474–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teixeira MG, Siqueira JB, Ferreira GLC, Bricks L, Joint G. Epidemiological trends of dengue disease in Brazil (2000–2010): a systematic literature search and analysis. PLoS Negl Trop Dis 2013; 7:e2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitehorn J, Simmons CP. The pathogenesis of dengue. Vaccine 2011; 29:7221–8. [DOI] [PubMed] [Google Scholar]
- 7.Guzman MG, Alvarez M, Halstead SB. Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: an historical perspective and role of antibody-dependent enhancement of infection. Arch Virol 2013; 158:1445–59. [DOI] [PubMed] [Google Scholar]
- 8.Chau TNB, Hieu NT, Anders KL et al. Dengue virus infections and maternal antibody decay in a prospective birth cohort study of Vietnamese infants. J Infect Dis 2009; 200:1893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kliks SC, Nimmanitya S, Nisalak A, Burke DS. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am J Trop Med Hyg 1988; 38:411–9. [DOI] [PubMed] [Google Scholar]
- 10.Simmons CP, Chau TNB, Thuy TT et al. Maternal antibody and viral factors in the pathogenesis of dengue virus in infants. J Infect Dis 2007; 196:416–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khamim K, Hattasingh W, Nisalak A et al. Neutralizing dengue antibody in pregnant thai women and cord blood. PLoS Negl Trop Dis 2015; 9:e0003396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanaveeradej V, Endy TP, Samakoses R et al. Transplacentally transferred maternal-infant antibodies to dengue virus. Am J Trop Med Hyg 2003; 69:123–8. [PubMed] [Google Scholar]
- 13.Chau TN, Quyen NT, Thuy TT et al. Dengue in Vietnamese infants- results of infection-enhancement assays correlate with age-related disease epidemiology, and cellular immune responses correlate with disease severity. J Infect Dis 2008; 198:516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol 2012; 2012:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol 2007; 7:715–25. [DOI] [PubMed] [Google Scholar]
- 16.Okoko JB, Wesumperuma HL, Hart CA. The influence of prematurity and low birthweight on transplacental antibody transfer in a rural West African population. Trop Med Int Heal 2001; 6:529–34. [DOI] [PubMed] [Google Scholar]
- 17.Scott S, Moss WJ, Cousens S et al. The influence of HIV-1 exposure and infection on levels of passively acquired antibodies to measles virus in Zambian infants. Clin Infect Dis 2007; 45:1417–24. [DOI] [PubMed] [Google Scholar]
- 18.Cumberland P, Shulman CE, Maple PAC et al. Maternal HIV infection and placental malaria reduce transplacental antibody transfer and tetanus antibody levels in newborns in Kenya. J Infect Dis 2007; 196:550–7. [DOI] [PubMed] [Google Scholar]
- 19.Hartter HK, Oyedele OI, Dietz K, Kreis S, Hoffman JP, Muller CP. Placental transfer and decay of maternally acquired antimeasles antibodies in Nigerian children. Pediatr Infect Dis J 2000; 19:635–41. [DOI] [PubMed] [Google Scholar]
- 20.de Moraes-Pinto MI, Almeida AC, Kenj G et al. Placental transfer and maternally acquired neonatal IgG immunity in human immunodeficiency virus infection. J Infect Dis 1996; 173:1077–84. [DOI] [PubMed] [Google Scholar]
- 21.Koraka P, Suharti C, Setiati TE et al. Kinetics of dengue virus-specific serum immunoglobulin classes and subclasses correlate with clinical outcome of infection. J Clin Microbiol 2001; 39:4332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thein S, Aaskov J, Myint TT et al. Changes in levels of anti-dengue virus IgG subclasses in patients with disease of varying severity. J Med Virol 1993; 40:102–6. [DOI] [PubMed] [Google Scholar]
- 23.Leite RC, Souza AI, Castanha PMS et al. Dengue infection in pregnancy and transplacental transfer of anti-dengue antibodies in Northeast, Brazil. J Clin Virol 2014; 60:16–21. [DOI] [PubMed] [Google Scholar]
- 24.Brazilian Ministry of Health. Sistema de Informacão sobre Agravos de Notificação(SINAN). 2012. http://dtr2004.saude.gov.br/sinanweb/tabnet/dh?sinannet/dengue/bases/denguebrnet.def Accessed 9 January 2015.
- 25.Braga C, Albuquerque MFPM, Cordeiro MT et al. Prospective birth cohort in a hyperendemic dengue area in Northeast Brazil: methods and preliminary results. Cad Saude Publica 2016; 32. [DOI] [PubMed] [Google Scholar]
- 26.Castanha PMS, Cordeiro MT, Martelli CMT, Souza WV, Marques ET, Braga C. Force of infection of dengue serotypes in a population-based study in the northeast of Brazil. Epidemiol Infect 2012; 141:1080–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wahala WMPB, de Silva AM. The human antibody response to dengue virus infection. Viruses 2011; 3:2374–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cordeiro MT, Silva AM, Brito C et al. Characterization of a dengue patient cohort in Recife, Brazil. Am J Trop Med Hyg 2007; 77:1128–34. [PubMed] [Google Scholar]
- 29.Pengsaa K, Luxemburger C, Sabchareon A et al. Dengue virus infections in the first 2 years of life and the kinetics of transplacentally transferred dengue neutralizing antibodies in Thai children. J Infect Dis 2006; 194:1570–6. [DOI] [PubMed] [Google Scholar]
- 30.Argolo AF, Féres VC, Silveira LA et al. Prevalence and incidence of dengue virus and antibody placental transfer during late pregnancy in central Brazil. BMC Infect Dis 2013; 13:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stapleton NM, Andersen JT, Stemerding AM et al. Competition for FcRn-mediated transport gives rise to short half-life of human IgG3 and offers therapeutic potential. Nat Commun 2011; 2:599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonçalves G, Cutts FT, Hills M, Rebelo-Andrade H, Trigo FA, Barros H. Transplacental transfer of measles and total IgG. Epidemiol Infect 1999; 122:273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okoko BJ, Wesumperuma LH, Ota MO et al. The influence of placental malaria infection and maternal hypergammaglobulinemia on transplacental transfer of antibodies and IgG subclasses in a rural West African population. J Infect Dis 2001; 184:627–32. [DOI] [PubMed] [Google Scholar]
- 34.van Panhuis WG, Luxemburger C, Pengsaa K et al. Decay and Persistence of Maternal Dengue Antibodies among Infants in Bangkok. Am J Trop Med Hyg 2011; 85:355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.