Abstract
Cholesterol 7α-hydroxylase (CYP7A1) is the first and rate-limiting enzyme in the conversion of cholesterol to bile acids in the liver. In addition to absorption and digestion of nutrients, bile acids play a critical role in the regulation of lipid, glucose, and energy homeostasis. We have backcrossed Cyp7a1−/− mice in a mixed B6/129Sv genetic background to C57BL/6J mice to generate Cyp7a1−/− mice in a near-pure C57BL/6J background. These mice survive well and have normal growth and a bile acid pool size ∼60% of WT mice. The expression of the genes in the alternative bile acid synthesis pathway are upregulated, resulting in a more hydrophilic bile acid composition with reduced cholic acid (CA). Surprisingly, Cyp7a1−/− mice have improved glucose sensitivity with reduced liver triglycerides and fecal bile acid excretion, but increased fecal fatty acid excretion and respiratory exchange ratio (RER) when fed a high-fat/high-cholesterol diet. Supplementing chow and Western diets with CA restored bile acid composition, reversed the glucose tolerant phenotype, and reduced the RER. Our current study points to a critical role of bile acid composition, rather than bile acid pool size, in regulation of glucose, lipid, and energy metabolism to improve glucose and insulin tolerance, maintain metabolic homeostasis, and prevent high-fat diet-induced metabolic disorders.
Keywords: bile acids and salt/metabolism, cholesterol/diet, liver, lipids
Bile acids are amphipathic molecules that aid in the absorption of dietary fats, nutrients, and vitamins. Bile acids are also endogenous ligands that activate the nuclear receptor, farnesoid X receptor (FXR), and the membrane G protein-coupled bile acid receptor, Gpbar-1 (aka TGR5). Bile acids are synthesized from cholesterol exclusively in the liver by cholesterol 7α-hydroxylase (CYP7A1), the first and rate-limiting enzyme in the classic bile acid biosynthetic pathway. Sterol 12α-hydroxylase (CYP8B1) catalyzes the synthesis of cholic acid (CA) and determines the ratio of CA to chenodeoxycholic acid (CDCA) and its derivatives, and thus, the hydrophobicity of the bile acid pool (1). In mouse liver, CDCA is converted to α- and β-muricholic acid (MCA), which are highly soluble and are FXR antagonists (2). Bile acids can also be synthesized via the alternative pathway, which is initiated by mitochondrial sterol 27-hydroxylase (CYP27A1) and oxysterol 7α-hydroxylase (CYP7B1) to synthesize mainly CDCA in the liver. The alternative pathway contributes to ∼18% of the total bile acids in humans and as much as 50% in rodents (3, 4). Bile acids are conjugated to the amino acids taurine or glycine in humans, but predominantly taurine in mice, and are secreted into bile and stored in the gallbladder until they are released into the small intestine after the ingestion of food. Within the intestinal track, bile acids emulsify dietary fats and form mixed micelles with fatty acids and 2-monoacylglycerols, which are absorbed into the enterocyte. Here, bacterial bile salt hydrolase deconjugates the conjugated bile acids and 7α-dehydroxylase converts CA and CDCA to deoxycholic acid (DCA) and lithocholic acid, respectively. These secondary bile acids are toxic and excreted into feces. Approximately 95% of bile acids, consisting of mainly CA, DCA, and CDCA in humans and CA and α/β-MCA in mice, are reabsorbed in the ileum and transported back to the liver via portal circulation. This enterohepatic circulation occurs multiple times per day to regulate bile acid synthesis and aid in distribution of nutrients, drugs, and xenobiotics to the liver. The small amount of bile acids lost in feces is replenished by de novo synthesis in the liver [reviewed in (5)]. Bile acids inhibit bile acid synthesis by two mechanisms. In the liver, bile acids activate FXR, which induces the inhibitory nuclear receptor, small heterodimer partner (SHP), to inhibit Cyp7a1 gene transcription (6). In the intestine, FXR induces fibroblast growth factor 15 (FGF15; or human ortholog FGF19), which is circulated to hepatocytes to activate fibroblast growth factor receptor 4 (FGFR4) signaling to suppress Cyp7a1 gene expression via activation of mitogen-activated protein kinases, including the extracellular signal-related kinase and cJun (7).
Bile acid synthesis represents the main catabolic pathway for disposal of cholesterol and maintenance of whole body cholesterol homeostasis. More recently studies have unveiled critical roles of bile acids in the regulation of lipid, glucose, and energy metabolism (8–10). Manipulations of the bile acid pool size by bile acid supplementation, bile acid sequestrants, or pharmacological activation of FXR and TGR5 signaling have been shown to improve insulin sensitivity and glucose tolerance in mouse models of diabetes and obesity and in nonalcoholic steatohepatitis patients (11–13). Bile acids also have been implicated in rapidly improving insulin resistance in type 2 diabetic patients after bariatric surgery and in animal models (14–16).
Genetic ablation of the Cyp7a1 gene in mice (Cyp7a1−/−) resulted in high postnatal mortality and malnutrition phenotypes that were prevented by bile acid and vitamin supplementation (17). The original adult Cyp7a1−/− mice in the mixed genetic background (B6/129Sv) had a reduced bile acid pool (∼30% of WT mice) and a normal lipid profile, while a later study of the same Cyp7a1−/− mice (B6/129Sv) in a different colony demonstrated improved survival and a milder phenotype (18), though females in this colony were hypercholesterolemic. Two recent studies using the original Cyp7a1−/− mice (B6/129Sv) demonstrated that even low dose bile acid feeding could rescue the knockout phenotype (19, 20). These studies also reported that Cyp8b1 gene expression was upregulated in Cyp7a1−/− mice (B6/129Sv), while genes involved in the alternative bile acid synthesis pathway were not, despite increased oxidized sterol content. We reported recently that transgenic overexpression of CYP7A1 in C57BL/6J mice resulted in increased bile acid pool with altered bile acid composition consisting of reduced CA and increased CDCA, resulting in reduced fat absorption and activation of FXR signaling to improve glucose and insulin tolerance. Interestingly, these mice were resistant to high-fat/high-cholesterol diet-induced insulin resistance and obesity (21). In Cyp7a1-transgenic mice, liver de novo cholesterol synthesis was stimulated but fatty acid synthesis was decreased, coupled with increased biliary bile acid and cholesterol secretion to maintain bile acid and cholesterol homeostasis (22). To further study bile acid synthesis in liver metabolism and disease, we generated Cyp7a1−/− mice in a C57BL/6J genetic background to test to determine whether Cyp7a1−/− mice develop symptoms of metabolic syndrome expected from high-fat/high-cholesterol diet feeding. To our surprise, the Cyp7a1−/− mice we generated had improved glucose tolerance; these mice also had a larger bile acid pool size than Cyp7a1−/− (B6/129Sv) mice and had altered bile acid composition consistent with induction of the genes in the alternative bile acid synthesis pathway, which may play a critical role in maintaining liver metabolic homeostasis and preventing diet-induced obesity and diabetes.
MATERIALS AND METHODS
Animals
Cyp7a1−/− mice originally obtained from Jackson Laboratory (Bar Harbor, ME) (Cyp7a1tm1Rus/6J) were backcrossed with C57BL/6J mice for seven generations to produce founder Cyp7a1−/− mice in an approximately 99.6% pure C57BL/6J background. Founder mice were pair-bred in the Comparative Medicine Unit at Northeast Ohio Medical University (NEOMED). Male WT C57BL/6J mice (originally obtained from Jackson Laboratory) were bred at NEOMED to serve as controls. All animal protocols were approved by the Institutional Animal Care and Use Committee of NEOMED.
Six-week-old male WT C57BL/6J and Cyp7a1−/− mice (hereafter referred to as C57BL/6J genetic background) were maintained on a standard chow diet (LabDiet #5008; St. Louis, MO) with water ad libitum in a temperature-controlled facility with a 12 h light/12 h dark schedule (6:00 PM = lights off). Additional age-matched cohorts of male C57BL/6J and Cyp7a1−/− mice were maintained on a high-fat/high-cholesterol Western diet (42% kcal from fat, 0.2% cholesterol; TD.88137; Harlan Laboratories, Indianapolis, IN) for 18 weeks (n = 6). To determine whether bile acid supplementation could reverse the knockout phenotype, 8-week-old C57BL/6J and Cyp7a1−/− male mice were maintained on a standard chow diet or a Western diet supplemented with 0.03% CA (19) (w/w; Sigma-Aldrich, St. Louis, MO) for 10 weeks.
Metabolic analysis
At 5–6 months of age, metabolic analysis of male WT and Cyp7a1−/− mice fed chow or Western diet with or without CA was performed using a comprehensive lab animal monitoring system (CLAMS) (Columbus Instruments, Columbus, OH). The CLAMS utilizes indirect calorimetry to determine metabolic performance continuously. Briefly, mice were housed individually in sealed clear Plexiglas cages through which fresh room air was continuously passed at 0.5 l/min. Via O2 and CO2 sensors, exhaust air was sampled in each cage in succession at 2 min intervals over 24 h. O2 consumption (VO2), CO2 production (VCO2), respiratory exchange ratio [RER (VCO2/VO2), an estimate of fuel usage], and energy (heat) production were calculated and recorded electronically over 24 h for each mouse (following a 48 h acclimation period). Total locomotor activity (measured by x, y, and z axis infrared beam breaks) and diet consumption were also recorded electronically for each mouse. Weekly body weight was recorded in all mice. An EchoMRI 3-in-1 body composition analyzer (EchoMRI; Houston TX) was used to record measurements of fat and lean tissue mass in live mice before metabolic analysis. After completion of recording, mice were fasted overnight before euthanization and tissues were then collected for analysis.
Glucose, insulin, and fat tolerance tests
Glucose tolerance tests were performed in separate cohorts of 5-month-old male WT and Cyp7a1−/− mice maintained on chow or Western diet with or without 0.03% CA supplementation (n = 5–7). Mice were ip injected with D-glucose dosed at 2 g/kg body weight following a 16 h fast. Insulin tolerance was tested by ip injection of insulin (Humulin R; Eli Lilly, Indianapolis, IN) dosed at 0.75 U/kg body weight following a 5 h fast. Blood samples were collected via tail vein and blood glucose was measured using a OneTouch Ultra Mini glucometer (LifeScan, Milpitas, CA). Oral fat tolerance was determined in chow-fed male WT and Cyp7a1−/− mice. Mice were gavaged with 15 ml/kg corn oil (Sigma-Aldrich) and blood was collected hourly via the tail vein for serum analysis of triglycerides.
Real-time PCR analysis
Total RNA was isolated from mouse tissue using TRI-Reagent (Sigma-Aldrich). Quantitative real-time PCR was performed using TaqMan gene expression assays according to the manufacturer’s instructions (ThermoFisher Scientific Inc., Waltham, MA). Relative mRNA levels were calculated using the comparative Ct method and experimental values were normalized to Gapdh as an internal control.
Lipid analysis
Lipids were extracted from homogenized liver tissue and dried feces with 2:1 chloroform:isopropanol followed by evaporation at 60°C. The resulting lipids were dissolved in water with 2% Triton X-100. Liver and serum cholesterol, triglycerides (ThermoFisher), and free fatty acids (Wako Diagnostics, Richmond, VA) were analyzed using commercially available kits.
Bile acid analysis
Bile acids were isolated from 100 mg liver, whole intestine, whole gallbladder, and 200 mg feces by a series of ethanol and methanol extractions overnight at 65°C. Bile acid content was quantified by kit (Genzyme Diagnostic, Cambridge, MA) and bile acid pool size was determined by totaling bile acids in liver, gallbladder, and intestine. Content measured in liver was back-calculated to whole liver weight to determine total liver bile acid content. Bile acid composition was determined by ultra (U)HPLC-quadrupole (Q)TOFMS (23). Gallbladder bile (25 μl) was incubated in 500 μl isopropanol overnight in a 55°C water bath. After vortexing for 30 s, the resulting mixture was centrifuged at 15,000 rcf for 15 min. Twenty microliters of the supernatant was diluted with 980 μl of water:methanol (1:1). Following centrifugation at 14,000 rcf for 20 min, the supernatant was transferred to a sample vial for analysis. Five microliters of the supernatant was injected to a UHPLC-QTOFMS setup for analysis. Bile acid separation was achieved using a 1260 Infinity Binary LC System (Agilent Technologies, Santa Clara, CA) equipped with a 100 × 2.7 mm (Agilent XDB C18) column. The column temperature was maintained at 45°C. The flow rate was 0.3 ml/min with a gradient ranging from 2% to 98% aqueous acetonitrile containing ammonia acetate (pH 8.6) in a 25 min run. QTOFMS was operated in negative mode with electrospray ionization. Ultra-highly pure nitrogen was applied as the drying gas (12 l/min) and the collision gas. The drying gas temperature was set at 325°C and nebulizer pressure was kept at 35 psi. During the analysis of samples, real-time mass correction and accurate mass were achieved by continuously measuring standard reference ions at m/z 119.0363 (m/z 966.0072 for the negative mode). Bile acid hydrophobicity indices of mouse gallbladder bile composition was calculated using the following values (24): tauro-α-MCA (T-α-MCA) = −0.84, tauro-β-MCA (T-β-MCA) = −0.78, tauro-cholic acid (TCA) = 0, tauro-CDCA (TCDCA) = +0.46, tauro-DCA (TDCA) = +0.59, tauro-lithocolic acid = +1.
RESULTS
Bile acid pool size is reduced and composition is altered in Cyp7a1−/− mice
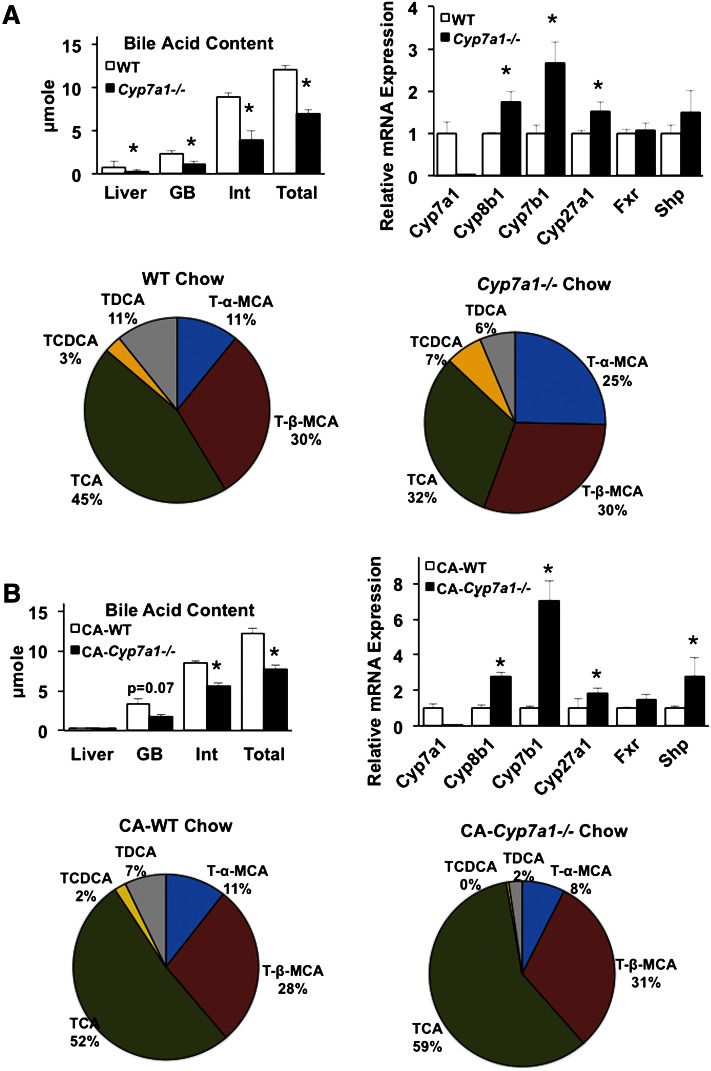
Bile acid pool size, defined as total liver, gallbladder, and intestinal bile acids, was determined. Figure 1A (upper left panel) demonstrates that bile acid content in liver, gallbladder, and intestine was reduced and total bile acid pool size in Cyp7a1−/− mice was significantly reduced to approximately 50–60% of WT mice, which is ∼2-fold larger than the previously reported pool size in Cyp7a1−/− mice (B6/129Sv). After supplementation with 0.03% CA, intestinal bile acid content and overall bile acid pool size in Cyp7a1−/− mice remained significantly reduced compared with chow-fed mice (Fig. 1B, upper left panel). Gallbladder bile acid composition analysis (Fig. 1A, lower panels) revealed Cyp7a1−/− mice have markedly reduced TCA (32%) and TDCA (6%) compared with WT mice (45% and 11%, respectively) and roughly doubled content of T-α-MCA (25%) and TCDCA (7%) compared with WT (11% and 3%, respectively), while T-β-MCA was not changed. As a result, the bile acid hydrophobicity index was reduced to −0.3764 in Cyp7a1−/− mice compared with −0.2477 in WT mice. Real-time PCR analysis of liver gene expression demonstrated that mRNA expression levels of Cyp8b1 in the classic pathway and Cyp7b1 in the alternative bile acid synthesis pathway were significantly increased, while Fxr and Shp mRNA levels were not changed (Fig. 1A, upper right panel). Increasing Cyp8b1, Cyp7b1, and Cyp27a1 expression is due to reduced bile acid pool and, thus, reduced feedback inhibition in Cyp7a1−/− mice. Surprisingly, feeding Cyp7a1−/− mice with 0.03% CA resulted in further induction of Cyp8b1 and Cyp7b1, while Cyp27a1 and Shp gene expression were moderately increased compared with WT mice (Fig. 1B, upper right panel). This may be because CA reduced Cyp8b1 and Cyp7b1 mRNA levels in WT mice. Supplementation with CA increased TCA (from 32% to 59%) and decreased T-α-MCA (from 25% to 8%) in Cyp7a1−/− mice, while composition in WT mice indicates a slight increase in TCA (from 45% to 52%), reduced TCDCA (from 7% to ∼1%), and no change in T-β-MCA. Thus, CA supplementation largely restored bile acid composition in Cyp7a1−/− mice to that of WT mice. Breeding Cyp7a1−/− mice into a C57/BL6J background may increase the bile acid pool by stimulating the alternative pathway, resulting in reduced TCA and TDCA and increased TCDCA and T-α-MCA in Cyp7a1−/− mice.
Fig. 1.
Bile acid pool size is reduced and composition is altered in Cyp7a1−/− mice. A (upper left panel): Bile acid pool size [summed bile acids from liver, gallbladder (GB), and intestine (Int)] is significantly reduced in Cyp7a1−/− mice compared with WT mice (n = 5). A (upper right panel): Liver bile acid synthesis and nuclear receptor gene expression in WT and Cyp7a1−/− mice fed chow diet. A (lower panel): Bile acid composition, expressed as a percentage of total detected bile acids, in chow-fed WT and Cyp7a1−/− mice. B (upper left panel): Bile acid pool size in Cyp7a1−/− mice remained significantly suppressed compared with WT mice after supplementation with 0.03% CA. B (upper right panel): Bile acid and nuclear receptor gene expression in mice fed 0.03% CA. B (lower panel): Bile acid composition in WT and Cyp7a1−/− mice fed CA. WT (white bar), Cyp7a1−/− (black bar). Data were analyzed by Student’s t-test; *P < 0.05.
Cyp7a1−/− mice in a C57BL/6J genetic background exhibit normal physiological characteristics with improved glucose tolerance that is reversed with CA supplementation
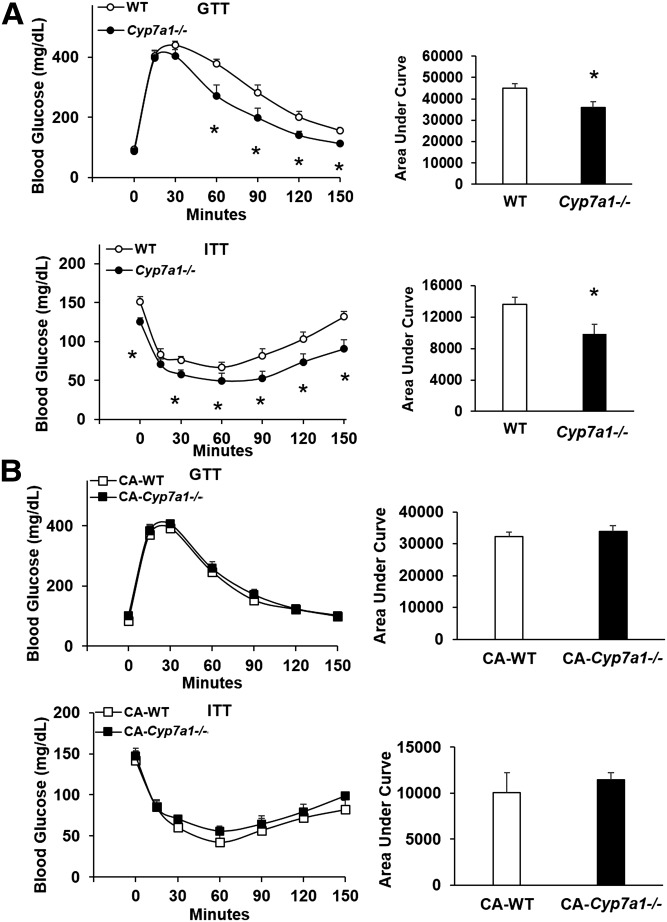
The original Cyp7a1-deficient mice (B6/129Sv) had high mortality unless pups were nutritionally supplemented with vitamins or bile acids (17). The Cyp7a1−/− mice we generated by backcrossing to a nearly pure C57BL/6J (99.6%) genetic background exhibit normal pup survival rate, as well as normal body weight/growth, liver weight, white adipose weight, and food intake comparable to WT controls maintained on normal rodent chow diet (supplementary Fig. 1). Following an ip injection of glucose after an overnight fast, Cyp7a1−/− mice responded with reduced blood glucose levels within 60 min, and blood glucose remained significantly lower than WT counterparts through 150 min after injection, indicating increased glucose tolerance (Fig. 2A, upper panel). When ip injected with insulin after a 5 h fast, blood glucose levels in Cyp7a1−/− mice were significantly reduced compared with WT mice (Fig. 2A, lower panel), though fasting glucose levels prior to the test were reduced in Cyp7a1−/− mice. These data are somewhat surprising, as we previously reported that Cyp7a1-transgenic mice also have improved insulin sensitivity and glucose tolerance (21, 22). When fed 0.03% CA for 10 weeks, the improved glucose tolerance in Cyp7a1−/− mice was attenuated (Fig. 2B). These data support that CA levels are positively linked to insulin resistance in diabetes, and reducing CA in both Cyp7a1-transgenic mice and Cyp7a1−/− mice improved glucose and insulin sensitivity.
Fig. 2.
Glucose and insulin tolerance is significantly improved in Cyp7a1−/− mice and is reversed by CA feeding. A: Male Cyp7a1−/− mice maintained on chow diet have significantly reduced blood glucose levels following glucose (upper panel) and insulin (lower panel) challenge (n = 5). B: After 10 week supplementation with 0.03% CA, glucose (upper panel) and insulin (lower panel) tolerance was reduced in Cyp7a1−/− mice. GTT, glucose tolerance test; ITT, insulin tolerance test. WT (white circle or bar), Cyp7a1−/− (black circle or bar), WT + CA (white square), Cyp7a1−/− + CA (black square). Data were analyzed by Student’s t-test; *P < 0.05.
Cyp7a1−/− mice fed Western diet have reduced and altered bile acid composition
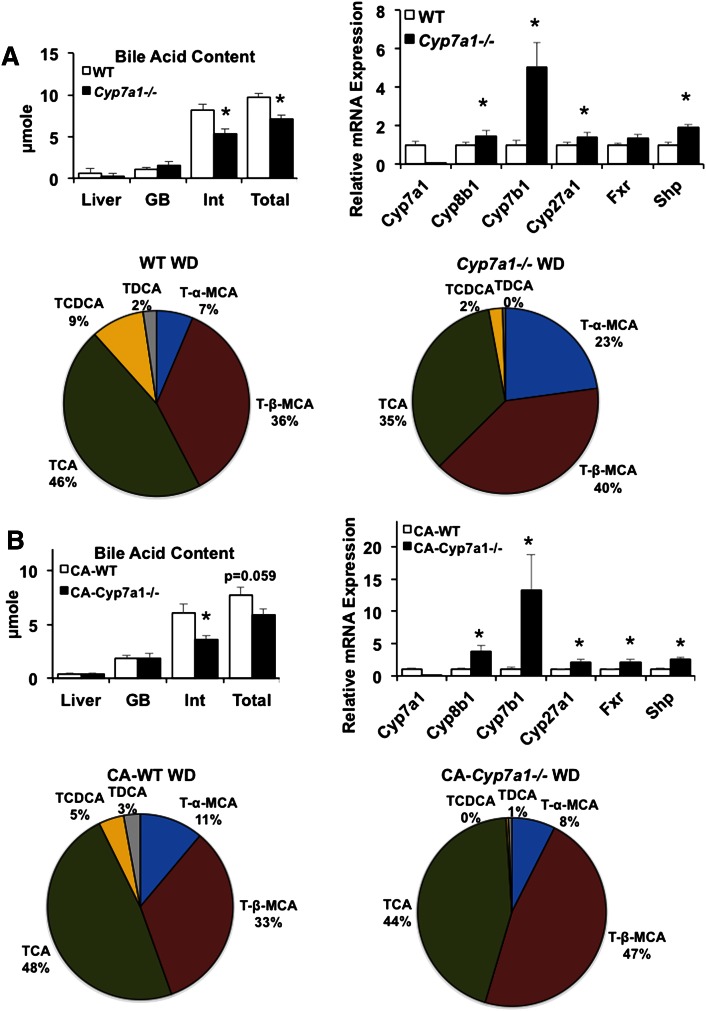
Under Western diet feeding, Cyp7a1−/− mice had significantly reduced intestinal bile acid content, which resulted in approximately 30% reduction in pool size, largely due to reduced bile acid content in the intestine (Fig. 3A, upper left panel). Real-time PCR analysis indicated that Cyp7b1 mRNA levels were markedly increased by ∼5-fold and Cyp27a1 mRNA levels were also significantly elevated in Cyp7a1−/− mice compared with WT mice; additionally, liver Fxr mRNA expression remained unchanged, but Shp mRNA expression was significantly elevated in Cyp7a1−/− mice fed a Western diet (Fig. 3A, upper right panel). In Western diet-fed WT mice, TCA content was similar to chow-fed controls, while TCDCA content tripled with Western diet feeding (9%) compared with chow diet (3%; compare Fig. 1A and Fig. 3A). TDCA decreased from 11% in chow-fed WT mice to 2% in Western diet-fed WT mice, possibly indicating a high-fat diet-induced dysbiosis in the microbial communities responsible for the conversion of CA to DCA in the intestine, or reduced reabsorption of DCA into the bile acid pool (Fig. 3A). Western diet feeding in Cyp7a1−/− mice resulted in an increase of T-β-MCA to 40% compared with chow-fed Cyp7a1−/− mice. TCDCA was reduced in Western diet-fed versus chow-fed Cyp7a1−/− mice (2% vs. 7%, respectively). Within Western diet feeding, T-α-MCA content tripled and TCA was reduced by 11% in Cyp7a1−/− mice compared with WT mice. Remarkably, these changes in bile acid composition resulted in reduced bile acid hydrophobicity in Western diet-fed Cyp7a1−/− mice (−0.496) compared with Western diet-fed WT mice (−0.2864), chow-fed Cyp7a1−/− mice (−0. 3764), and chow-fed WT mice (−0.2477). With 0.03% CA supplementation, intestinal bile acid content in Cyp7a1−/− mice was reduced and overall pool size trended toward reduction (Fig. 3B, lower left panel). Bile acid synthesis gene expression was again induced, notably Cyp8b1 and Cyp7b1 (Fig. 3B, upper right panel). Hepatic expression of Cyp27a1, Fxr, and Shp was also significantly increased compared with WT mice. CA supplementation decreased T-α-MCA content from 23% to 8% and increased TCA and T-β-MCA in Cyp7a1−/− mice, while bile acid composition in WT mice fed Western diet plus CA remained unchanged (Fig. 3B, lower panel). Thus, CA supplementation only partially restored bile acid composition in Cyp7a1−/− mice, and it is surprising that CA supplementation did not increase CA content in both Western diet-fed WT and Cyp7a1−/− mice. These data suggest that the high cholesterol content in Western diet may further stimulate the alternative bile acid synthesis pathway to produce more T-β-MCA and increase bile acid hydrophobicity in Cyp7a1−/− mice.
Fig. 3.
Cyp7a1−/− mice fed Western diet have reduced and altered bile acid composition. A (upper left panel): Bile acid pool size is significantly reduced in Western diet-fed Cyp7a1−/− mice compared with WT mice (n = 6). GB, gallbladder; Int, intestine. A (upper right panel): Bile acid synthesis and nuclear receptor gene expression in WT and Cyp7a1−/− mice maintained on Western diet. A (lower panel): Bile acid composition, expressed as a percentage of total detected bile acids, in Western diet-fed WT and Cyp7a1−/− mice. B (upper left panel): Bile acid pool size in Western diet-fed mice supplemented with 0.03% CA. B (upper right panel): Bile acid synthesis and nuclear receptor gene expression in WT and Cyp7a1−/− mice maintained on Western diet + 0.03% CA. B (lower panel): Bile acid composition in Western diet + CA-fed WT and Cyp7a1−/− mice. WT (white bar), Cyp7a1−/− (black bar). Data were analyzed by Student’s t-test; *P < 0.05.
Cyp7a1−/− mice fed Western diet gain weight but maintain improved glucose tolerance
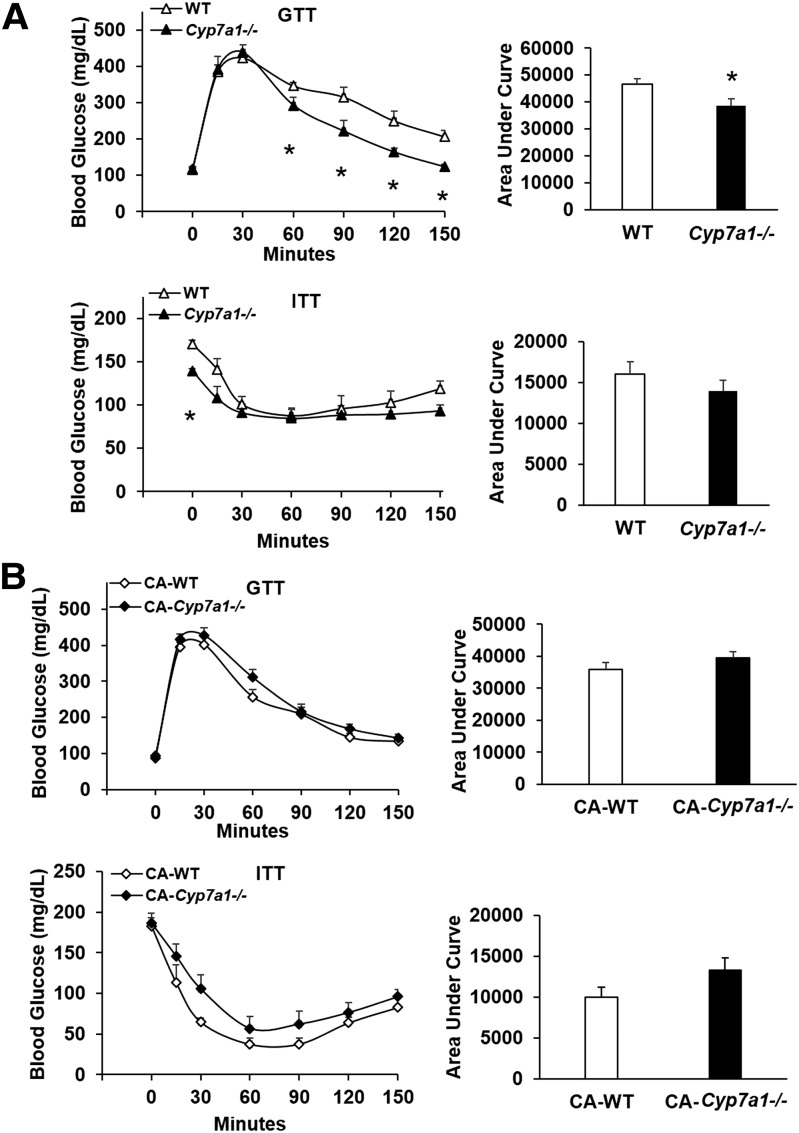
When fed a high-fat/high-cholesterol Western diet for 18 weeks, body weight increased in Cyp7a1−/− mice, which was statistically insignificant from WT controls. Liver and adipose tissue weight did not significantly differ between genotypes; though interestingly, Cyp7a1−/− mice ate significantly more food compared with WT controls (supplementary Fig. 2). Glucose tolerance was significantly improved in Western diet-induced obese Cyp7a1−/− mice beginning 60 min after glucose injection and remained lower than WT controls up to 150 min after injection (Fig. 4A, upper panel). Insulin tolerance test results did not differ between Cyp7a1−/− and WT mice, though 5 h fasting blood glucose levels in Cyp7a1−/− mice prior to insulin injection were significantly lower than WT mice (Fig. 4A, lower panel). After 10 weeks of 0.03% CA supplementation, glucose and insulin tolerance (Fig. 4B) were not different statistically between WT and Cyp7a1−/− mice.
Fig. 4.
Cyp7a1−/− mice fed Western diet maintain improved glucose tolerance, which is reversed with CA supplementation. A: Cyp7a1−/− mice fed Western diet have significantly improved response to glucose (upper panel) but not insulin (lower panel), though the baseline 5 h fasting blood glucose levels were significantly reduced in Cyp7a1−/− mice (n = 6–7). GTT, glucose tolerance test; ITT, insulin tolerance test. B: Following feeding a Western diet supplemented with 0.03% CA for 10 weeks, glucose (upper panel) and insulin (lower panel) tolerance was worsened in Cyp7a1−/− mice. WT (white triangle or bar), Cyp7a1−/− (black triangle or bar), WT + CA (white diamond), Cyp7a1−/− + CA (black diamond). Data were analyzed by Student’s t-test; *P < 0.05.
Lipid profiles in Cyp7a1−/− mice are altered and results in increased fecal lipid excretion
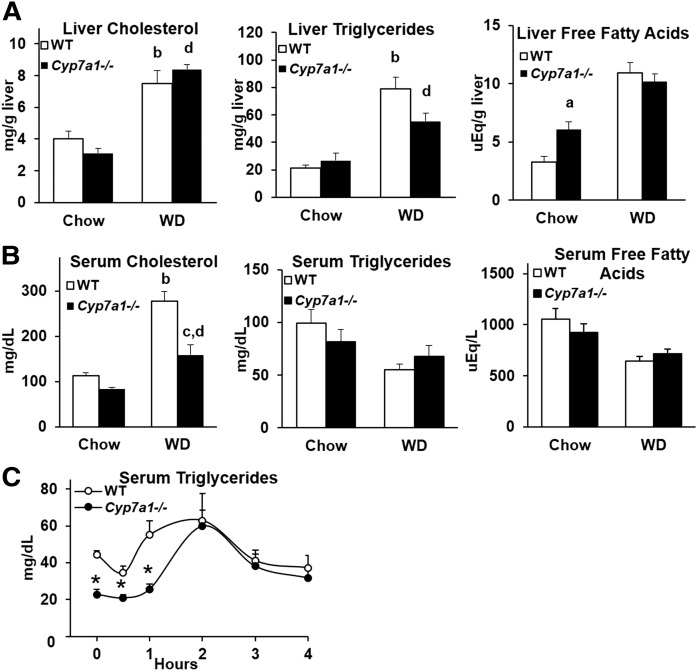
Liver and serum lipids were analyzed in chow- and Western diet-fed WT and Cyp7a1−/− mice. On chow diet, liver cholesterol and liver triglycerides were unaltered, while liver free fatty acid content was increased in Cyp7a1−/− mice (Fig. 5A), and serum cholesterol, triglycerides, and free fatty acids were unaltered, consistent with previous studies done in Cyp7a1−/− (6J/129Sv) mice (18) (Fig. 5B). When fed a Western diet, liver cholesterol, triglycerides, and free fatty acids increased in both WT and Cyp7a1−/− mice (Fig. 5A), but Cyp7a1−/− mice had significantly reduced serum cholesterol. An oral fat tolerance test was conducted in chow-fed mice, which resulted in significantly reduced serum triglyceride levels in Cyp7a1−/− mice for 2 h indicating reduced postprandial fat absorption in the intestine or increased fecal fat excretion in Cyp7a1−/− mice (Fig. 5C).
Fig. 5.
Lipid profiles in Cyp7a1−/− mice are mildly altered. A: Liver cholesterol (left), triglycerides (middle), and free fatty acids (right). B: Serum cholesterol (left), triglycerides (middle), and free fatty acids (right). C: Cyp7a1−/− mice had significantly reduced serum triglyceride levels during oral fat tolerance testing. WT (white bar or circle), Cyp7a1−/− (black bar or circle). A, B: Analyzed by one-way ANOVA followed by post hoc Bonferroni-Holm test where appropriate; a = genotype effect (P < 0.05) within chow diet; b = diet effect (P < 0.05) within WT genotype; c = genotype effect (P < 0.05) within Western diet; d = diet effect (P < 0.05) within Cyp7a1−/− genotype. C: Analyzed by Student’s t-test; *P < 0.05.
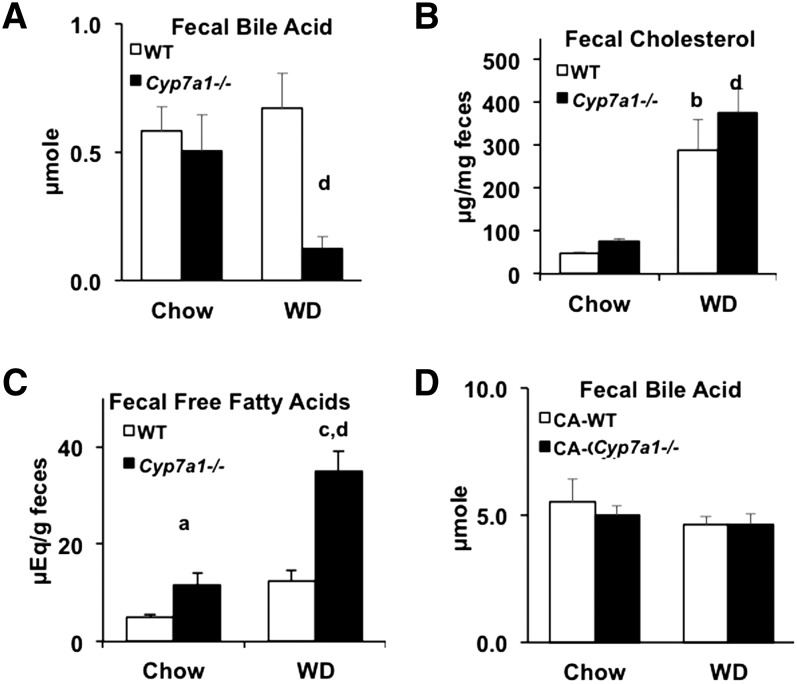
Fecal bile acid and cholesterol contents were similar in Cyp7a1−/− mice and WT mice (Fig. 6A, B). Western diet feeding significantly increased fecal cholesterol secretion in both WT and Cyp7a1−/− mice. However, Western diet markedly increased fecal fatty acid secretion in Cyp7a1−/− mice (Fig. 6C). CA supplementation markedly increased fecal bile acid excretion, though there was no significant difference between genotypes (Fig. 6D). Thus, Cyp7a1−/− mice in a C57BL/6J background may reabsorb more bile acids to maintain a larger bile acid pool size compared with Cyp7a1−/− mice in a mixed genetic background and increased bile acid excretion after CA feeding may explain the slightly reduced bile acid pool size and similar CA levels in bile after Western diet feeding (Fig. 3A, B).
Fig. 6.
Fecal bile acid, cholesterol, and free fatty acid profiles. Male WT and Cyp7a1−/− mice (n = 5–7) were maintained on standard rodent chow diet or Western diet for 18 weeks. Feces were collected and analyzed for bile acids (A), cholesterol (B), free fatty acids (C), and bile acids after dietary supplementation with 0.03% CA (D). WT (white bar), Cyp7a1−/− (black bar). Data were analyzed by one-way ANOVA followed by post hoc Bonferroni-Holm test where appropriate; a = genotype effect (P < 0.05) within chow diet; b = diet effect (P < 0.05) within WT genotype; c = genotype effect (P < 0.05) within Western diet; d = diet effect (P < 0.05) within Cyp7a1−/− genotype.
Resting metabolic rate is increased in Cyp7a1−/− mice maintained on Western diet
Next, we used CLAMS equipment to determine metabolic phenotype in chow-fed and Western diet-fed mice. When mice were fed a normal chow diet, resting RER, energy expenditure (heat), and locomotor activity did not differ between Cyp7a1−/− mice and WT controls (supplementary Fig. 3), indicating that lack of the Cyp7a1 gene does not produce a lethal metabolic phenotype in mice and their overt physiology is similar to WT mice.
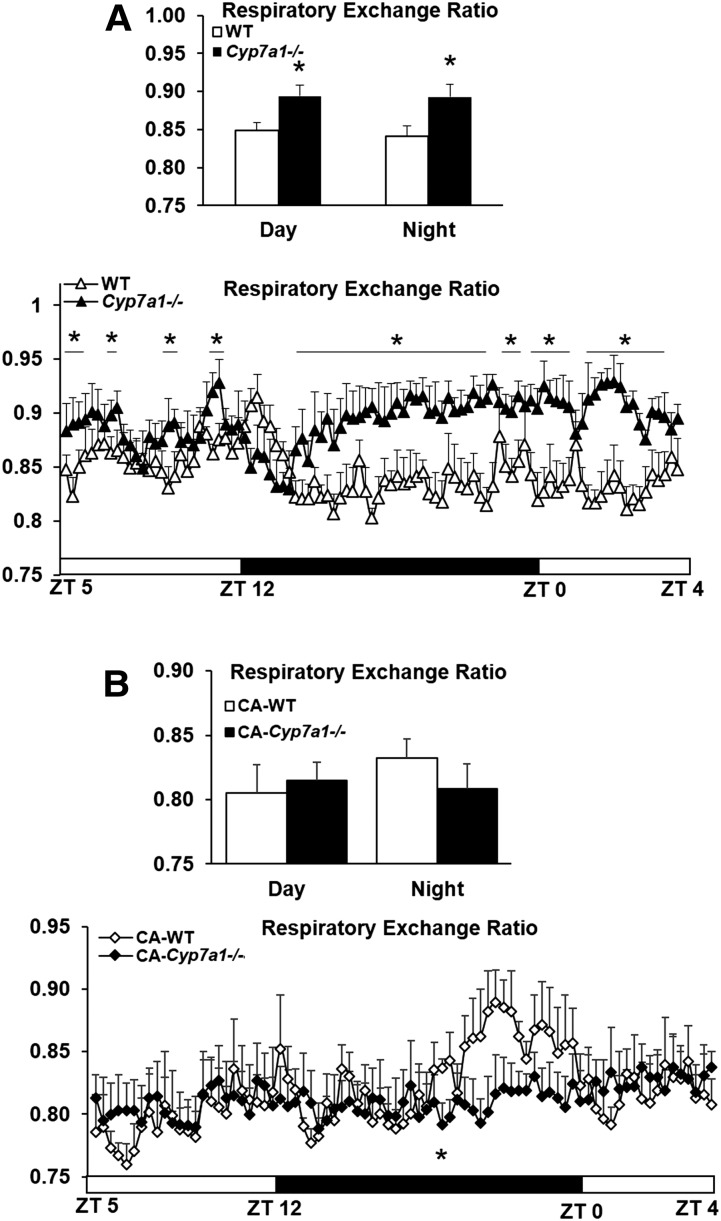
After Western diet feeding, 24 h RER was significantly elevated in Cyp7a1−/− mice (∼0.9) compared with WT controls (∼0.84); it was determined that RER was increased during both the day (inactive period) and night (active period) (Fig. 7A), indicating that the elevation in RER was not an artifact due to the normal diurnal increase in nighttime locomotor activity and was not associated with energy expenditure (supplementary Fig. 4). Following dietary supplementation with 0.03% CA, RER of Cyp7a1−/− mice was reduced to ∼0.81 and that of WT mice was reduced to ∼0.82 (Fig. 7B).
Fig. 7.
Resting metabolic rate is increased in Cyp7a1−/− mice maintained on Western diet and is reduced after CA supplementation. Male WT and Cyp7a1−/− mice (n = 6) were maintained on Western diet for 18 weeks. A: Cyp7a1−/− mice have increased RER over both day (inactive period) and night (active period). B: After 10 weeks feeding with Western diet + 0.03% CA, RER in Cyp7a1−/− mice was not significantly different from WT mice. WT (white triangle, diamond, or bar), Cyp7a1−/− (black triangle, diamond, or bar), black horizontal bar indicates the dark phase. Data were analyzed by Student’s t-test or one-way ANOVA followed by post hoc Bonferroni-Holm test where appropriate; *P < 0.05Q4. ZT, Zeitgeber time.
EchoMRI was used to measure body composition (percentage of fat and lean mass normalized to body weight) in live mice. Under chow-fed or Western diet-fed conditions, there were no differences in fat or lean mass between WT and Cyp7a1−/− mice; when normalized to total body weight, Western diet significantly increased fat mass and reduced total lean mass (supplementary Fig. 5).
Expression of genes involved in lipid and glucose metabolism is altered in Cyp7a1−/− mice
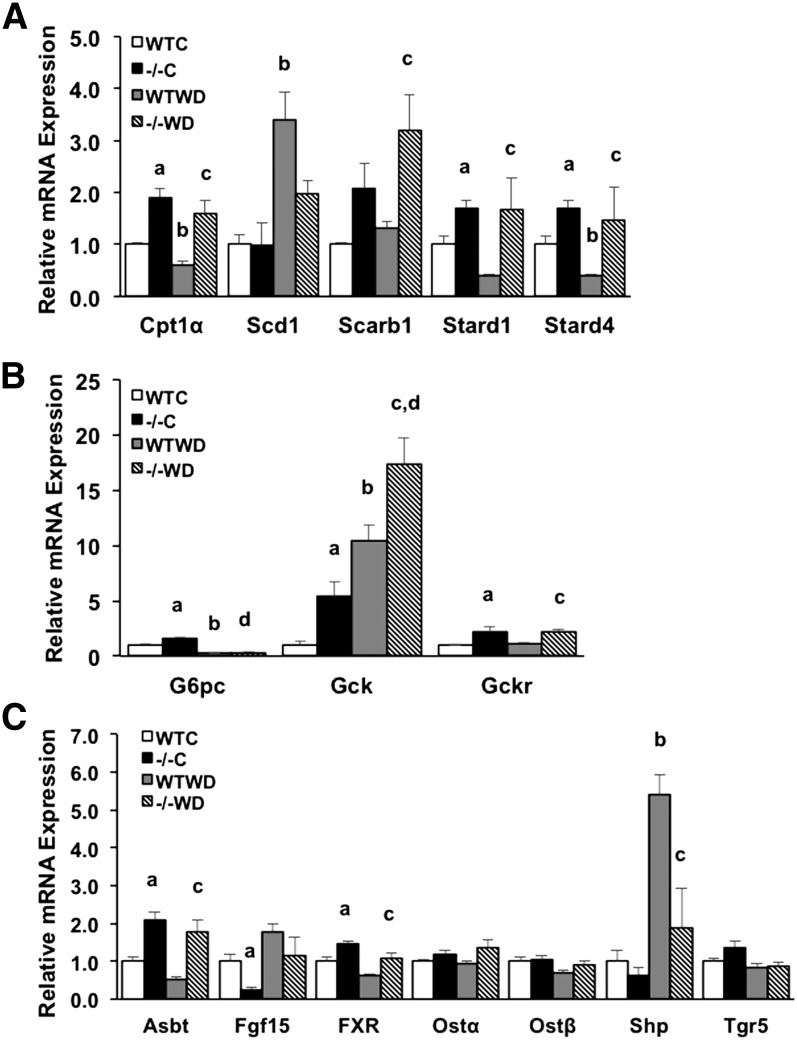
Next, we measured mRNA expression levels of several genes involved in lipid metabolism. In the liver, mRNA expression levels of scavenger receptor class B member 1 (Scarb1 or Srb1), steroidogenic acute regulatory protein (StAR, Stard1), StAR-related lipid transfer domain containing 4 (Stard4), and carnitine palmityltransferase 1α (Cpt1α) genes were all significantly increased or trending toward increased in Cyp7a1−/− mice while expression of sterol-CoA desaturase (Scd1) was reduced in Cyp7a1−/− mouse liver (Fig. 8A). Expression of glucose-6-phosphatase (G6pc) was significantly upregulated in chow-fed Cyp7a1−/− mice and Western diet feeding significantly suppressed its expression in both WT and Cyp7a1−/− mice, while glucokinase (Gck) and glucokinase regulatory protein (Gckr) mRNA expression was significantly elevated under both chow and Western diet feeding compared with WT mice (Fig. 8B), suggesting glycolysis may be stimulated to reduce serum glucose level in Cyp7a1−/− mice. In the ileum, mRNA expression levels of apical sodium-dependent bile acid transporter (Asbt) and Fxr were significantly elevated in Cyp7a1−/− mice independent of diet, while Fgf15 was reduced under chow-fed conditions in Cyp7a1−/− mice (Fig. 8C). Increased Asbt expression is correlated to increased bile acid reabsorption in the ileum (25). Decreasing Fgf15 may reduce activation of hepatic FGFR4 signaling to induce Cyp8b1 expression (Fig. 1A). Expression of Shp in the ileum was highly upregulated by Western diet in WT mice and was suppressed in Cyp7a1−/− mice, while expression of sinusoidal bile acid efflux transporters organic solute transporter α (Ostα) and β (Ostβ) and Tgr5 was not differentially regulated in Cyp7a1−/− mice or by Western diet (Fig. 8C).
Fig. 8.
Genes involved in lipid and glucose metabolism are altered in Cyp7a1−/− mice. mRNA gene expression in WT and Cyp7a1−/− mice (n = 5–7) maintained on chow and Western diet. Liver lipid metabolism genes (A), liver glucose metabolism genes (B), and ileum bile acid transporter genes and nuclear receptor genes (C). WTC, WT chow; −/−C, Cyp7a1−/− chow; WTWD, WT Western diet; −/−WD, Cyp7a1−/− Western diet. Data were analyzed by one-way ANOVA followed by post hoc Bonferroni-Holm test where appropriate; a = genotype effect (P < 0.05) within chow diet; b = diet effect (P < 0.05) within WT genotype; c = genotype effect (P < 0.05) within Western diet; d = diet effect (P < 0.05) within Cyp7a1−/− genotype.
We examined canonical genes involved in bile acid, lipid, and energy metabolism in liver and brown adipose tissue (supplementary Fig. 6). Bile acid transport genes in the liver of Cyp7a1−/− and WT mice remained relatively unchanged, though sinusoidal bile acid uptake transporter Na+-taurocholate cotransport polypeptide (Ntcp) mRNA was reduced in Western diet-fed WT mice, while Ostβ mRNA was significantly increased by Western diet in Cyp7a1−/− mice (supplementary Fig. 6A). HMG-CoA reductase (Hmgcr) and LDL receptor (Ldlr) gene expression were unchanged, while proprotein convertase subtilisin/kexin type 9 (Pcsk9) was suppressed by Western diet (supplementary Fig. 6B). Pcsk9 is an inhibitor of Ldlr (26); decreased Pcsk9 may increase Ldlr-mediated uptake of serum cholesterol in Cyp7a1−/− mice. Acetyl-CoA carboxylase (Acc) mRNA expression was upregulated in Western diet-fed WT mice and was further increased in Western diet-fed Cyp7a1−/− mice, while Fasn was not altered. Genes involved in energy metabolism in the liver were mildly altered, including increased expression of Pparα and sirtuin (silent mating type information regulation 2 homolog) 1 (Sirt1) in Western diet-fed Cyp7a1−/− mice (supplementary Fig. 6C). Increased expression of Pparα and Sirt1 by Western diet may reduce liver triglyceride content and increase fecal fatty acid secretion in Cyp7a1−/− mice (27, 28). Lastly, we measured the expression of energy metabolism genes in brown adipose tissue, and found no significant changes in the expression in Pparγ coactivator 1α (Pgc1α), Pparα, Sirt1, or uncoupling protein (Ucp), supporting our data that the phenotype demonstrated in Cyp7a1−/− mice is not due to changes in energy metabolism (supplementary Fig. 6D). Together, these data indicate that lack of the Cyp7a1 gene may produce significant gene-level changes to increase mitochondrial cholesterol uptake and transport to stimulate the alternative bile acid synthesis pathway, resulting in increased intestinal bile acid reabsorption to compensate for the reduced classic bile acid biosynthesis pathway, thus avoiding a severe metabolic phenotype.
DISCUSSION
There is sparse information regarding the physiology of Cyp7a1−/− mice, which were first characterized in a mixed genetic background. These mice had low survival rates and required vitamin supplementation to reach adulthood, and had reduced bile acid pool size, reduced fecal excretion of bile acids, and reduced fecal sterol and intestinal cholesterol absorption. It was also more recently reported that Cyp7a1−/− mice exhibited induction of Cyp8b1 gene expression and repression of genes involved in the alternative synthesis pathway, and that these mice were physiologically responsive to low-dose CA and CDCA supplementation (19, 20). The mice used in the present study were obtained by backcrossing the mixed background B6/129Sv strain with C57BL/6J mice for seven generations to obtain a nearly pure C57 background and represent a model suitable for use in dietary studies. Here, we report an alternative phenotype in which both Cyp8b1 and alternative bile acid synthesis genes (Cyp7b1, Cyp27a1) are upregulated under both chow- and Western diet-fed conditions, which resulted in a 2-fold increase in bile acid pool size compared with the original colony of Cyp7a1−/− mice in the mixed genetic background, altered gallbladder bile acid composition, and reduced bile acid hydrophobicity. The mice also have improved glucose and/or insulin sensitivity when maintained on either normal chow or Western diet and increased RER when maintained on Western diet.
Several studies demonstrate a causative link between increased 12α-hydroxylated bile acid (CA+DCA) levels and insulin insensitivity in rodents (9) and humans (29). Cyp7a1−/− mice have a reduced CA+DCA:CDCA+MCA ratio under both chow (0.61 compared with 1.27 in WT mice) and Western diet (0.54 compared with 0.92 in WT mice) feeding conditions. Therefore, the increased glucose sensitivity inherent to Cyp7a1−/− mice may be due to the dramatically altered bile acid composition, despite having a reduced bile acid pool. Increased TCDCA and T-α-MCA in Cyp7a1−/− mice indicates an induction of the alternative pathway of bile acid synthesis, which produces mainly CDCA that is then converted to α- and β-MCA. This is consistent with the significantly increased Cyp7b1 and Cyp27a1 mRNA expression we observed in the liver. We also observed an increase in Cyp8b1 gene expression in Cyp7a1−/− mice, possibly due to a reduction in feedback inhibition via FGF15/FGFR4 signaling, which inhibits Cyp7a1 and Cyp8b1 (30). Reduced ileal Fgf15 and Shp gene expression may result in derepression of Cyp8b1. In addition, MCAs were recently identified as potent FXR antagonists (2); therefore, increasing TMCAs may antagonize feedback inhibition of Cyp8b1 expression in both chow- and Western diet-fed Cyp7a1−/− mice. Despite stimulation of Cyp8b1 gene expression, the composition of CA in the bile acid pool was reduced 10% in Cyp7a1−/− mice, indicating that upregulation of the alternative bile acid synthesis pathway may dominate the classic pathway in these mice. Supplementation of CA (0.03%) reversed the glucose tolerant phenotype in Cyp7a1−/− mice, and altered bile acid composition to resemble that of WT mice. CA has low critical micellar concentration that enhances mixed micelle formation with phospholipids and cholesterol (31) and may increase fat absorption and facilitate the reduction in glucose tolerance after CA feeding. These data support our hypothesis that reducing CA, a 12α-hydroxylated bile acid, in both Cyp7a1−/− mice in this study and in Cyp7a1-transgenic mice in our previous study may improve glucose tolerance and insulin sensitivity to protect against Western diet-induced metabolic syndrome. In Cyp7a1−/− mice, TCDCA content in bile is increased and FXR signaling may be activated to improve glucose tolerance. TCDCA is the most efficacious endogenous FXR ligand and activation of FXR signaling is known to improve hyperglycemia and hyperlipidemia in diabetic mice (32). Thus, bile acid composition, not bile acid pool size, plays a critical role in the regulation of hepatic metabolic homeostasis.
Western diet-fed Cyp7a1−/− mice have increased RER (i.e., the ratio of CO2 produced/O2 consumed) that is not due to typical diurnal variation or night-time induction of locomotor activity. RER is used as a tool to estimate respiratory quotient, the proportion of carbohydrate-to-fat utilization of metabolic oxygen. Accordingly, an RER of ∼0.84 in WT mice maintained on Western diet indicates a near-equal utilization of carbohydrates and fats, such that approximately 49% of O2 is consumed by carbohydrate catabolism while 51% of O2 is consumed by fat (33), while an RER of ∼0.9 in Cyp7a1−/− mice shifts O2 utilization to approximately 66% for carbohydrate catabolism and 34% for fat catabolism. This shift was only observed in Western diet-fed Cyp7a1−/− mice, which ate significantly more food despite an equal gain in body weight compared with control mice. This may indicate a reduced ability to absorb dietary fat due to a smaller bile acid pool with lower CA. CA is the most efficacious bile acid in mixed micelle formation for emulsification of fats and cholesterol absorption (34). Indeed, RER was reduced in Western diet-fed Cyp7a1−/− mice from ∼0.90 to 0.81 after CA supplementation, indicating increasing fat catabolism for energy production.
We also observed differential regulation of several key genes involved in cholesterol and lipid metabolism. Expression of Stard1 and Stard4 were increased in Cyp7a1−/− mice, independent of diet. Overexpression of Cyp27a1 was shown to increase StAR protein, while overexpression of Stard1 in HepG2 cells increased the rate of bile acid synthesis and the production of 27-hydroxycholesterol (35). Likewise, overexpression of Stard4 in primary mouse hepatocytes increased bile acid synthesis (36). CYP27A1 is located in the mitochondrial inner membrane, which does not contain cholesterol. STARD1 facilitates the transport of cholesterol to mitochondria to provide substrate for CYP27A1. Increased expression of Stard1 and Stard4 may contribute to stimulation of the alternative bile acid synthesis pathway in Cyp7a1−/− mice.
In the ileum of Cyp7a1−/− mice, Asbt gene expression was upregulated. Reduced FXR/FGF15 in the ileum may contribute to an increase in intestinal Asbt in Cyp7a1−/− mice (37). ASBT is a key bile acid reabsorption transporter known to be inhibited by FXR and increased intestinal ASBT has been linked to increased bile acid pool and reduced fecal excretion of bile acids (25, 38). Therefore, increased ileum Asbt expression could account for the relative increase in bile acid pool size in our Cyp7a1−/− mice compared with the 70% reduction in bile acid pool size in Cyp7a1 (B6/129Sv) mice (17).
Interestingly, we previously reported that Cyp7a1-transgenic mice (with 2-fold increase in CYP7A1 enzyme activity and 2.5-fold increase in bile acid pool size) have a hydrophobic bile acid pool consisting of markedly increased CDCA and ursodeoxycholic acid and reduced CA (22). These mice are protected against diet-induced obesity and have improved glucose sensitivity. Here, we demonstrate that Cyp7a1−/− mice have a reduced bile acid pool with reduced hydrophobicity coupled with improved glucose tolerance. Both bile acid sequestrants (reducing bile acid pool size) and bile acid supplementation (increasing bile acid pool size) have beneficial effects in improving insulin sensitivity and glucose tolerance in human studies (39, 40). Our current study points to a critical role of bile acid composition, rather than bile acid pool size, in the regulation of bile acid signaling in control of glucose, lipid, and energy metabolism.
We conclude that the Cyp7a1−/− mice characterized here represent a useful mouse model suitable for dietary studies and the study of bile acid and lipid metabolism. They present with a unique phenotype, such that survivability, body weight, and body composition are comparable to that of normal WT mice, but when challenged with a physiological stimulus (glucose or insulin bolus, Western diet) have an improved response that we speculate is due to a uniquely altered bile acid composition, and may be used to study the interactions of bile acids and lipid homeostasis.
Supplementary Material
Footnotes
Abbreviations:
- ASBT
- apical sodium-dependent bile acid transporter
- CA
- cholic acid
- CDCA
- chenodeoxycholic acid
- CLAMS
- comprehensive lab animal monitoring system
- CYP7A1
- cholesterol 7α-hydroxylase
- CYP7B1
- oxysterol 7α-hydroxylase
- CYP8B1
- sterol 12α-hydroxylase
- CYP27A1
- sterol 27-hydroxylase
- DCA
- deoxycholic acid
- FGF15
- fibroblast growth factor 15
- FGFR4
- fibroblast growth factor receptor 4
- FXR
- farnesoid X receptor
- Ldlr
- LDL receptor
- MCA
- muricholic acid
- NEOMED
- Northeast Ohio Medical University
- Pcsk9
- proprotein convertase subtilisin/kexin type 9
- QTOFMS
- quadrupole TOFMS
- RER
- respiratory exchange ratio
- SHP
- small heterodimer partner
- Sirt1
- sirtuin
- StAR
- steroidogenic acute regulatory protein
- Stard4
- steroidogenic acute regulatory protein-related lipid transfer domain containing 4
- TCA
- tauro-cholic acid
- TCDCA
- tauro-chenodeoxycholic acid
- TDCA
- tauro-deoxycholic acid
- T-α-MCA
- tauro-α-muricholic acid
- T-β-MCA
- tauro-β-muricholic acid
- UHPLC
- ultra HPLC
This study was supported by Foundation for the National Institutes of Health Ruth L. Kirschstein National Research Service Award DK096784 to J.M.F and National Institutes of Health Grants DK44442 and DK58379 to J.Y.L.C. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Chiang J. Y. L. 2009. Bile acids: regulation of synthesis. J. Lipid Res. 50: 1955–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sayin S.I., Wahlström A., Felin J., Jäntti S., Marschall H. U., Bamberg K., Angelin B., Hyötyläinen T., Orešič M., and Bäckhed F.. 2013. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 17: 225–235. [DOI] [PubMed] [Google Scholar]
- 3.Vlahcevic Z. R., Stravitz R. T., Heuman D. M., Hylemon P. B., and Pandak W. M.. 1997. Quantitative estimations of the contribution of different bile acid pathways to total bile acid synthesis in the rat. Gastroenterology. 113: 1949–1957. [DOI] [PubMed] [Google Scholar]
- 4.Duane W. C., and Javitt N. B.. 1999. 27-Hydroxycholesterol: production rates in normal human subjects. J. Lipid Res. 40: 1194–1199. [PubMed] [Google Scholar]
- 5.Li T., and Chiang J. Y. L.. 2014. Bile acid signaling in metabolic disease and drug therapy. Pharmacol. Rev. 66: 948–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu T. T., Makishima M., Repa J. J., Schoonjans K., Kerr T. A., Auwerx J., and Mangelsdorf D. J.. 2000. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol. Cell. 6: 507–515. [DOI] [PubMed] [Google Scholar]
- 7.Song K-H., Li T., Owsley E., Strom S., and Chiang J. Y. L.. 2009. Bile acids activate fibroblast growth factor 19 signaling in human hepatocytes to inhibit cholesterol 7α-hydroxylase gene expression. Hepatology. 49: 297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li T., Francl J. M., Boehme S., Ochoa A., Zhang Y., Klaassen C. D., Erickson S. K., and Chiang J. Y. L.. 2012. Glucose and insulin induction of bile acid synthesis: mechanisms and implications in diabetes and obesity. J. Biol. Chem. 287: 1861–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qi Y., Jiang C., Cheng J., Krausz K. W., Li T., Ferrell J. M., Gonzalez F. J., and Chiang J. Y. L.. 2015. Bile acid signaling in lipid metabolism: metabolomic and lipidomic analysis of lipid and bile acid markers linked to anti-obesity and anti-diabetes in mice. Biochim. Biophys. Acta. 1851: 19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broeders E.P., Nascimento E.B. , Havekes B., Brans B., Roumans K.H., Tailleux A., Schaart G., Kouach M., Charton J., Deprez B., et al. . 2015. The bile acid chenodeoxycholic acid increases human brown adipose tissue activity. Cell Metab. 22: 418–426. [DOI] [PubMed] [Google Scholar]
- 11.Prawitt J., Caron S., and Staels B.. 2014. Glucose-lowering effects of intestinal bile acid sequestration through enhancement of splanchnic glucose utilization. Trends Endocrinol. Metab. 25: 235–244. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi M., Ikegami H., Fujisawa T., Nojima K., Kawabata Y., Noso S., Babaya N., Itoi-Babaya M., Yamaji K., Hiromine Y., et al. . 2007. Prevention and treatment of obesity, insulin resistance, and diabetes by bile acid–binding resin. Diabetes. 56: 239–247. [DOI] [PubMed] [Google Scholar]
- 13.Ma K., Saha P. K., Chan L., and Moore D. D.. 2006. Farnesoid X receptor is essential for normal glucose homeostasis. J. Clin. Invest. 116: 1102–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pournaras D. J., Glicksman C., Vincent R. P., Kuganolipava S., Alaghband-Zadeh J., Mahon D., Bekker J. H., Ghatei M. A., Bloom S. R., Walters J. R., et al. . 2012. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology. 153: 3613–3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albaugh V. L., Flynn C. R., Cai S., Xiao Y., Tamboli R. A., and Abumrad N. N.. 2015. Early increases in bile acids post Roux-en-Y gastric bypass are driven by insulin-sensitizing, secondary bile acids. J. Clin. Endocrinol. Metab. 100: E1225–E1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flynn C. R., Albaugh V. L., Cai S., Cheung-Flynn J., Williams P. E., Brucker R. M., Bordenstein S. R., Guo Y., Wasserman D. H., and Abumrad N. N.. 2015. Bile diversion to the distal small intestine has comparable metabolic benefits to bariatric surgery. Nat. Commun. 6: 7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishibashi S., Schwarz M., Frykman P. K., Herz J., and Russell D. W.. 1996. Disruption of cholesterol 7α-hydroxylase gene in mice: I. Postnatal lethality reversed by bile acid and vitamin supplementation. J. Biol. Chem. 271: 18017–18023. [DOI] [PubMed] [Google Scholar]
- 18.Erickson S. K., Lear S. R., Deane S., Dubrac S., Huling S. L., Nguyen L., Bollineni J. S., Shefer S., Hyogo H., Cohen D. E., et al. . 2003. Hypercholesterolemia and changes in lipid and bile acid metabolism in male and female cyp7a1-deficient mice. J. Lipid Res. 44: 1001–1009. [DOI] [PubMed] [Google Scholar]
- 19.Jones R. D., Repa J. J., Russell D. W., Dietschy J. M., and Turley S. D.. 2012. Delineation of biochemical, molecular, and physiological changes accompanying bile acid pool size restoration in Cyp7a1-/- mice fed low levels of cholic acid. Am. J. Physiol. Gastrointest. Liver Physiol. 303: G263–G274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones R. D., Lopez A. M., Tong E. Y., Posey K. S., Chuang J. C., Repa J. J., and Turley S. D.. 2015. Impact of physiological levels of chenodeoxycholic acid supplementation on intestinal and hepatic bile acid and cholesterol metabolism in Cyp7a1-deficient mice. Steroids. 93: 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li T., Owsley E., Matozel M., Hsu P., Novak C. M., and Chiang J. Y. L.. 2010. Transgenic expression of cholesterol 7α-hydroxylase in the liver prevents high-fat diet-induced obesity and insulin resistance in mice. Hepatology. 52: 678–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li T., Matozel M., Boehme S., Kong B., Nilsson L-M., Guo G., Ellis E., and Chiang J. Y. L.. 2011. Overexpression of cholesterol 7α-hydroxylase promotes hepatic bile acid synthesis and secretion and maintains cholesterol homeostasis. Hepatology. 53: 996–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woolbright B. L., Dorko K., Antoine D. J., Clarke J. I., Gholami P., Li F., Kumer S. C., Schmitt T. M., Forster J., Fan F., et al. . 2015. Bile acid-induced necrosis in primary human hepatocytes and in patients with obstructive cholestasis. Toxicol. Appl. Pharmacol. 283: 168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heuman D. M. 1989. Quantitative estimation of the hydrophilic-hydrophobic balance of mixed bile salt solutions. J. Lipid Res. 30: 719–730. [PubMed] [Google Scholar]
- 25.Xu G., Shneider B. L., Shefer S., Nguyen L. B., Batta A. K., Tint G. S., Arrese M., Thevananther S., Ma L., Stengelin S., et al. . 2000. Ileal bile acid transport regulates bile acid pool, synthesis, and plasma cholesterol levels differently in cholesterol-fed rats and rabbits. J. Lipid Res. 41: 298–304. [PubMed] [Google Scholar]
- 26.Horton J. D., Cohen J. C., and Hobbs H. H.. 2009. PCSK9: a convertase that coordinates LDL catabolism. J. Lipid Res. 50: S172–S177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kersten S. 2014. Integrated physiology and systems biology of PPARα. Mol. Metab. 3: 354–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hou X., Xu S., Maitland-Toolan K. A., Sato K., Jiang B., Ido Y., Lan F., Walsh K., Wierzbicki M., Verbeuren T. J., et al. . 2008. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J. Biol. Chem. 283: 20015–20026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haeusler R. A., Astiarraga B., Camastra S., Accili D., and Ferrannini E.. 2013. Human insulin resistance is associated with increased plasma levels of 12α-hydroxylated bile acids. Diabetes. 62: 4184–4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kong B., Wang L., Chiang J. Y., Zhang Y., Klaassen C. D., and Guo G. L.. 2012. Mechanism of tissue-specific farnesoid X receptor in suppressing the expression of genes in bile-acid synthesis in mice. Hepatology. 56: 1034–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang D. Q-H., Tazuma S., Cohen D. E., and Carey M. C.. 2003. Feeding natural hydrophilic bile acids inhibits intestinal cholesterol absorption: studies in the gallstone-susceptible mouse. Am. J. Physiol. Gastrointest. Liver Physiol. 285: G494–G502. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y., Lee F. Y., Barrera G., Lee H., Vales C., Gonzalez F. J., Willson T. M., and Edwards P. A.. 2006. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc. Natl. Acad. Sci. USA. 103: 1006–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McLean J. A., and Tobin G.. 1987. Cambridge University Press, Cambridge, UK. [Google Scholar]
- 34.Wang D. Q-H., Lammert F., Cohen D. E., Paigen B., and Carey M. C.. 1999. Cholic acid aids absorption, biliary secretion, and phase transitions of cholesterol in murine cholelithogenesis. Am. J. Physiol. 276: G751–G760. [DOI] [PubMed] [Google Scholar]
- 35.Hall E. A., Ren S., Hylemon P. B., Rodriguez-Agudo D., Redford K., Marques D., Kang D., Gil G., and Pandak W. M.. 2005. Detection of the steroidogenic acute regulatory protein, StAR, in human liver cells. Biochim. Biophys. Acta. 1733: 111–119. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez-Agudo D., Ren S., Wong E., Marques D., Redford K., Gil G., Hylemon P., and Pandak W. M.. 2008. Intracellular cholesterol transporter StarD4 binds free cholesterol and increases cholesteryl ester formation. J. Lipid Res. 49: 1409–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sinha J., Chen F., Miloh T., Burns R. C., Yu Z., and Shneider B. L.. 2008. beta-Klotho and FGF-15/19 inhibit the apical sodium-dependent bile acid transporter in enterocytes and cholangiocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 295: G996–G1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li H., Chen F., Shang Q., Pan L., Shneider B. L., Chiang J. Y. L., Forman B. M., Ananthanarayanan M., Tint G. S., Salen G., et al. . 2005. FXR-activating ligands inhibit rabbit ASBT expression via FXR-SHP-FTF cascade. Am. J. Physiol. Gastrointest. Liver Physiol. 288: G60–G66. [DOI] [PubMed] [Google Scholar]
- 39.Ratziu V., de Ledinghen V., Oberti F., Mathurin P., Wartelle-Bladou C., Renou C., Sogni P., Maynard M., Larrey D., Serfaty L., et al. . 2011. A randomized controlled trial of high-dose ursodesoxycholic acid for nonalcoholic steatohepatitis. J. Hepatol. 54: 1011–1019. [DOI] [PubMed] [Google Scholar]
- 40.Staels B. 2009. A review of bile acid sequestrants: potential mechanism(s) for glucose-lowering effects in type 2 diabetes mellitus. Postgrad. Med. 121: 25–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.