Abstract
The glucocorticoid-induced leucine zipper (GILZ), a primary target of glucocorticoids, is expressed in human adipocytes, but its importance in adipocyte function is unknown. Because TNFα is increased in obese adipose tissue and antagonizes a number of glucocorticoid actions, we investigated the interplay of these pathways. GILZ knockdown increased and GILZ overexpression decreased interleukin-6 (IL-6) and leptin mRNA and protein secretion. GILZ knockdown increased the magnitude of the glucocorticoid effect on leptin secretion, but did not affect the glucocorticoid suppression of IL-6. Although GILZ silencing decreased adiponectin mRNA levels, it did not affect the amount of adiponectin secreted. GILZ negatively modulated pro-inflammatory signaling pathways, blocking basal and TNFα-stimulated (1 h) p65 nuclear factor κB nuclear translocation and transcriptional activity by binding to p65 in the cytoplasm. GILZ silencing increased basal ERK1/2 and JNK phosphorylation, and decreased MAPK phosphatase-1 protein levels. Longer term TNFα (4 h or 24 h) treatment decreased GILZ expression in human adipocytes. Furthermore, adipose tissue GILZ mRNA levels were reduced in proportion to the degree of obesity and expression of inflammatory markers. Overall, these results suggest that GILZ antagonizes the pro-inflammatory effects of TNFα in human adipocytes, and its downregulation in obesity may contribute to adipose inflammation and dysregulated adipokine production, and thereby systemic metabolism.
Keywords: leptin, interleukin-6, nuclear factor κB, mitogen-activated protein kinase, mitogen-activated protein kinase phosphatase-1, glucocorticoid-induced leucine zipper
Glucocorticoids modulate many aspects of adipose tissue biology. They are required for the full differentiation of adipocyte precursors (1, 2) and the maintenance of adipogenic gene expression (3, 4). Glucocorticoids regulate adipose metabolic and endocrine function (4–8), and play an important role in restraining adipose tissue inflammation (9). Previously, we showed that about 20% of adipose-expressed genes are affected by long-term glucocorticoid treatment in human adipose tissue in vitro (4). After binding to the glucocorticoid receptor (GR), glucocorticoids can directly activate or repress target gene expression. The pleiotropic effects of GR activation are amplified as secondary targets are also affected (3). Obesity is associated with higher cortisol production within adipose tissue (10), so it is critical to understand the mechanisms that regulate responses to this hormone.
The glucocorticoid-induced leucine zipper (GILZ, TSC22D3) is a primary target of glucocorticoids/GR and known to mediate the anti-inflammatory, immunosuppressive, and anti-proliferative actions of glucocorticoids in many cell types (11–16). We previously found that GILZ is expressed in human adipose tissue and newly differentiated human adipocytes in culture, and its expression is upregulated by dexamethasone (Dex) through GR (4, 17). The role of GILZ in adipocyte function, however, has not been addressed. Using both loss- and gain-of-function approaches, we investigated the role of GILZ on adipokine production, focusing on interleukin-6 (IL-6) and leptin, both of which can have pro-inflammatory actions, and adiponectin, which is an anti-inflammatory and insulin-sensitizing adipokine (18).
In some cell types, GILZ mainly exerts anti-inflammatory effects by binding to p65 nuclear factor κB (NF-κB) and decreasing its nuclear translocation and transcriptional activity, as well as by suppressing MAPKs (11, 14, 19, 20). We therefore investigated the influence of manipulating GILZ expression on the activity these signaling pathways. Because TNFα antagonizes glucocorticoid action and plays a critical role in regulating adipocyte inflammation, we tested to determine whether TNFα treatment affected GILZ expression and action in human adipocytes, and assessed the influence of obesity on GILZ expression in human adipose tissue. Overall, our data indicate that GILZ exerts anti-inflammatory effects in human adipocytes, and that the downregulation of GILZ may contribute to inflammation and adipocyte dysfunction in human obesity.
MATERIALS AND METHODS
Materials
All chemicals were purchased from Sigma (St. Louis, MO), except rosiglitazone (Enzo, Farmingdale, NY). Recombinant human insulin was obtained from Lilly (Indianapolis, IN), collagenase type I from Worthington Biochemical (Lakewood, NJ), and recombinant human TNFα from PeproTech (Rocky Hill, NJ). Cell culture media and fetal bovine serum were obtained from Life Technologies (Carlsbad, CA). GILZ and control siRNA were purchased from Qiagen (Germantown, MD) and Lipofectamine and PLUS reagents were from Life Technologies.
GILZ expression in human adipose tissue
Abdominal subcutaneous adipose tissue samples were obtained by needle aspiration from 33 healthy volunteers as previously described and reported (21) (20 male, 13 female; BMI 27.3 ± 5.0 kg/m2; age 28.8 ± 7.2 years). Samples were snap-frozen in liquid nitrogen and stored at −80°C until RNA extraction.
Isolation and culture of human adipose-derived stromal/stem cells
Abdominal subcutaneous adipose tissues were obtained from six subjects (two male, four female; BMI 33.5 ± 3.9 kg/m2; age 45.6 ± 4.1 years) during elective surgeries. The study protocol was approved by the Institutional Review Board of Boston University Medical Center (17, 22) and all subjects provided signed informed consent. Adipose-derived stromal/stem cells were obtained with collagenase digestion and cultured as previously described (24, 25). Cells from individual subjects were used without pooling.
GILZ knockdown in differentiated human adipocytes
Adipose-derived stromal/stem cells were plated and differentiated (23, 24). On day 9 of differentiation, adipocytes were transfected with control or GILZ siRNA (10 nM, final concentration) using Lipofectamine and PLUS reagents (25). After overnight transfection, medium was replenished with the maintenance medium (DMEM/F12 with 10 nM insulin and 10 nM Dex) and cultured for an additional 4–5 days for knockdown. Cells were deprived of glucocorticoids overnight, treated with Dex (10 nM) for 24 h in the presence of 10 nM insulin, and then harvested for RNA and protein analysis. Culture media during the 24 h treatment were collected and stored at −80°C for adipokine measurement. To test the effects of GILZ knockdown on ERK1/2, JNK, MAPK phosphatase-1 (MKP-1), and NF-κB signaling, control or GILZ knockdown adipocytes were treated with TNFα (3 ng/ml) for 1 h and harvested to get total cell lysates and cytoplasmic and nuclear extracts.
Adenovirus-mediated overexpression of GILZ
To generate adenovirus to drive the expression of GILZ, the full-length GILZ cDNA fragment was subcloned into KpnI/XbaI cut pAdTrack-CMV shuttle vector (26), which contains an internal EGFP reference marker. The resultant shuttle vector was then cut with PmeI and used for transformation into AdEasy BJ5183 cells. Correct recombination of the resulting viral vector was confirmed by restriction enzyme digestion and partial sequencing of the GILZ insert. Finally, the PacI-digested viral DNA was transfected into HEK 293 cells for virus production and amplification (26). Adenoviruses with only EGFP reference marker were used as control. On day 11–12 of differentiation, adipocytes were infected with control or GILZ adenovirus for 6 h and cultured for an additional 3 days in the maintenance medium for overexpression. Dex effects were tested by treating cells with Dex (10 nM) for 24 h after overnight glucocorticoid removal. The effects of GILZ overexpression on NF-κB signaling were also tested after treating with TNFα, as previously described.
NF-κB reporter activity
To directly test whether GILZ overexpression affected transcriptional activity of NF-κB, control or GILZ-overexpressing adipocytes were transfected with p65 NF-κB reporter plasmid (1 μg; gift from Dr. Ido, Boston University) and pRL-TK plasmid (Renilla luciferase, 250 ng; Promega, Madison, WI) using Lipofectamine and PLUS reagents (27). After overnight transfection, cells were treated with TNFα (3 ng/ml) for 3 h and harvested in passive lysis buffer. Firefly and Renilla luciferase activities were measured with the Dual-Luciferase assay kit (Promega) in a plate reader (Tecan Infinite 1000 PRO; Tecan, San Jose, CA). The reporter activity was expressed as the ratio of firefly to Renilla luciferase.
GILZ coimmunoprecipitation with p65 NF-κB
To investigate whether GILZ physically interacts with NF-κB in vivo, we performed coimmunoprecipitation experiments. Control or GILZ-overexpressing adipocytes were treated with Dex for 6 h, and cytoplasmic extracts were prepared and used for immunoprecipitation (IP) with GILZ antibody (2 μg per IP) bound to protein G using Dynabeads protein G IP kit (Life Technologies). After IP and washing, IP materials were mixed with sample loading dye and used for Western blotting for p65 NF-κB.
RNA extraction and gene expression
Total RNA was extracted from adipose tissue samples or cells with Qiazol (Qiagen). RNA quantity and quality were assessed using a Nano Drop (Thermo Scientific, Rockford, IL). Total RNA (0.5–1 μg) was reverse transcribed (Transcriptor first strand synthesis kits; Roche, Indianapolis, IN), followed by quantitative (q)PCR with TaqMan probes (Life Technologies) using Light Cycler 480 II (Roche). Expression levels relative to cyclophilin A (PPIA) are presented.
Measurement of leptin, adiponectin, and IL-6 secretion
Leptin, adiponectin, and IL-6 concentrations in culture media were measured using ELISA kits (R&D, Minneapolis, MN) as previously described (18).
Immunoblotting
Cells were harvested into cell lysis buffer (Cell Signaling, Beverly, MA) supplemented with 5% SDS and protease inhibitors and processed (24). Nuclear and cytoplasmic proteins were isolated using the NE-PER nuclear and cytoplasmic extraction reagents (Thermo Scientific) following the manufacturer’s instructions. Protein was resolved in 10 or 15% SDS-PAGE gels and transferred to PVDF membranes, followed by blocking in 5% nonfat dry milk. Membranes were probed with primary antibodies overnight at 4°C for GILZ (Santa Cruz Biotechnology, Santa Cruz, CA), total and phospho-ERK1/2 (Tyr202/204), total and phospho-JNK (Thr183/Tyr185), total and phospho-p65 NF-κB (Ser536) (all from Cell Signaling), adipose tissue triglyceride lipase (ATGL) (28), MKP-1 (Santa Cruz Biotechnology), and loading controls (HSP90 from Santa Cruz Biotechnology and RNA POLII from Millipore, Darmstadt, Germany). After incubating with HRP-linked secondary antibodies, chemiluminescence images were captured and band intensities were quantified using Multi Gauge software (Fuji Film, Japan).
Statistical analysis
Data are expressed as mean ± SEM and analyzed using GraphPad Prism. After log-transformation of the data, effects of GILZ silencing and overexpression, Dex treatment, TNF treatment, and their interactions were tested using a two-way repeated measures ANOVA followed by post hoc Student’s paired t-tests when main effects or interactions were significant, as indicated in figure legends. Differences between means were considered statistically different when P < 0.05.
RESULTS
Knockdown of GILZ increased IL-6 and leptin expression, while decreasing adiponectin mRNA levels
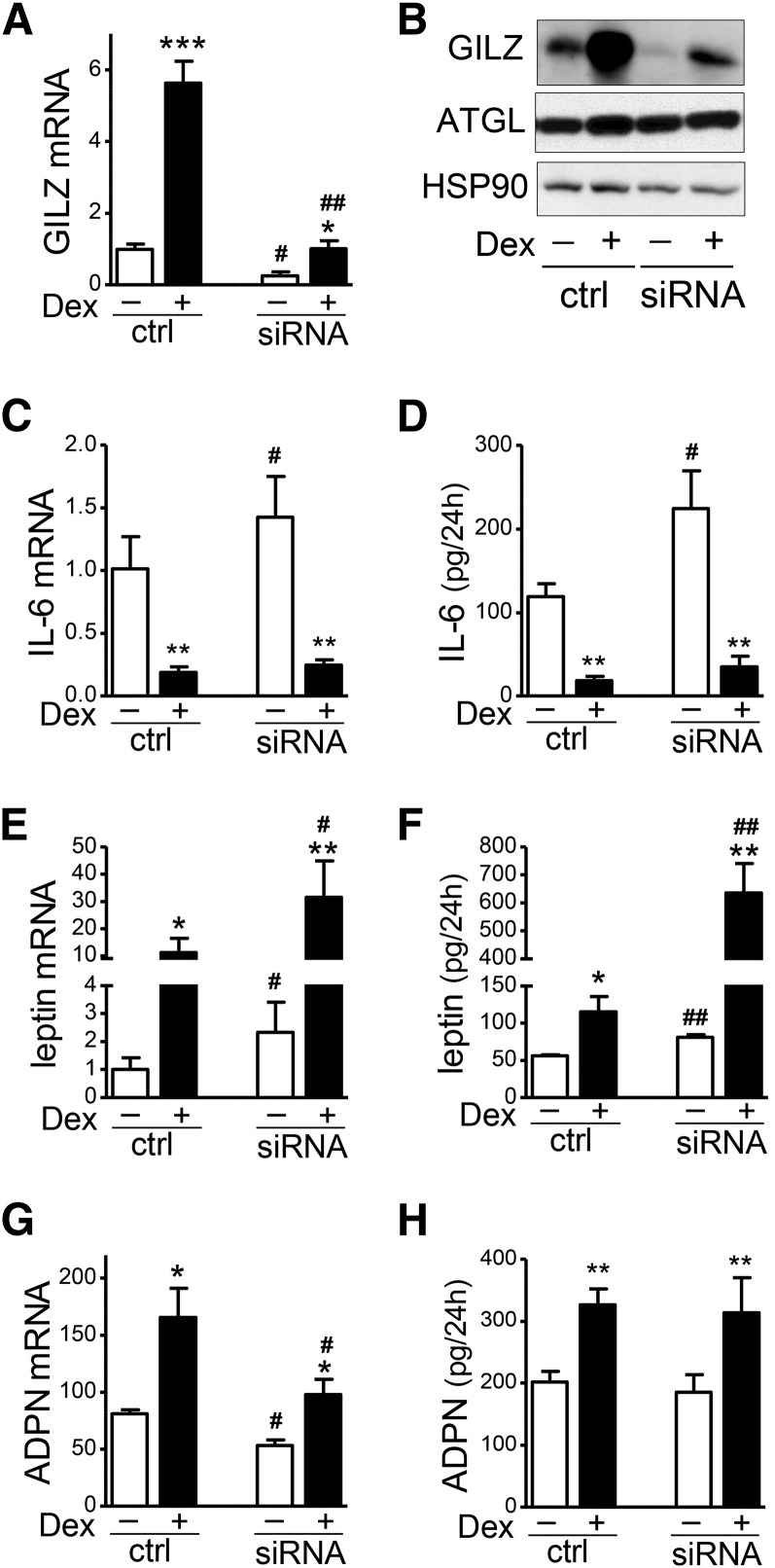
To test the functional role of GILZ in human adipocytes, GILZ expression levels were reduced with RNAi. An ∼80% reduction in GILZ mRNA and protein expression levels was achieved (Fig. 1A, B). The effects of GILZ silencing on adipokine expression was tested in the presence or absence of Dex (10 nM, 24 h). Similar to our previous report (18), Dex suppressed IL-6 mRNA levels and secretion by ∼80% in control human adipocytes. GILZ knockdown significantly increased IL-6 mRNA levels by 58 ± 23% in the basal and 65 ± 39% in the Dex-suppressed condition (Fig. 1C). In parallel, GILZ knockdown induced IL-6 protein secretion, 80 ± 16% in the basal and 85 ± 35% in the Dex-suppressed condition (Fig. 1D). The ability of Dex to suppress IL-6 expression was not affected by GILZ knockdown (mRNA: −81.0 ±2.0% vs. −81.6 ± 3.3% and secretion: −85.0 ± 3.1% vs. −85.4 ± 4.3% in control and knockdown, respectively; n = 4–5, NS).
Fig. 1.
GILZ knockdown increased IL-6 and leptin expression in human adipocytes. Differentiated adipocytes were transfected with GILZ siRNA and treated with Dex (10 nM, 24 h) and harvested for mRNA and protein analysis. Culture media were collected during the 24 h treatment and used to measure secreted adipokines. GILZ knockdown levels were confirmed at the mRNA (A) and protein (B) levels with RT-qPCR and Western analysis. Effects of GILZ silencing on IL-6 mRNA levels (C) and secretion (D), leptin mRNA (E) and secretion (F), and adiponectin mRNA (G) and secretion (H) were determined with qPCR and ELISA, respectively. A two-way repeated measures ANOVA was used to test the effects of the main effect of GILZ silencing and Dex treatment and their interaction. When main effects or their interactions were significant, effects of Dex treatment or GILZ silencing within condition were tested with paired t-test; *P < 0.05, **P < 0.01, ***P < 0.001, Dex effects; #P < 0.05, ##P < 0.01, GILZ silencing effects; n = 4–6. ctrl, control.
GILZ knockdown increased leptin mRNA levels by 145% in the basal and 200% in the Dex-induced condition (Fig. 1E), and increased leptin secretion by 45% in the basal and 500% in the presence of Dex (Fig. 1F). Although Dex increased leptin secretion both in the control and GILZ-silenced cells, Dex stimulation of leptin secretion was greater when GILZ was silenced (GILZ siRNA × Dex interaction by ANOVA significant with P < 0.01, n = 5). The effect of Dex was 7.7 ± 1.1-fold compared with 2.0 ± 0.3-fold after GILZ silencing (P < 0.05, paired t-test).
In contrast to the stimulatory effects of GILZ silencing on IL-6 and leptin, GILZ silencing reduced mRNA levels of adiponectin, an anti-inflammatory adipokine (by 34% in the basal and 38% in the Dex-stimulated condition, Fig. 1G), but did not significantly affect the total amount of adiponectin secreted during 24 h (Fig. 1H). GILZ silencing did not affect the protein levels of ATGL, used as a marker of adipocyte differentiation (Fig. 1B).
Overexpression of GILZ decreased IL-6 and leptin expression
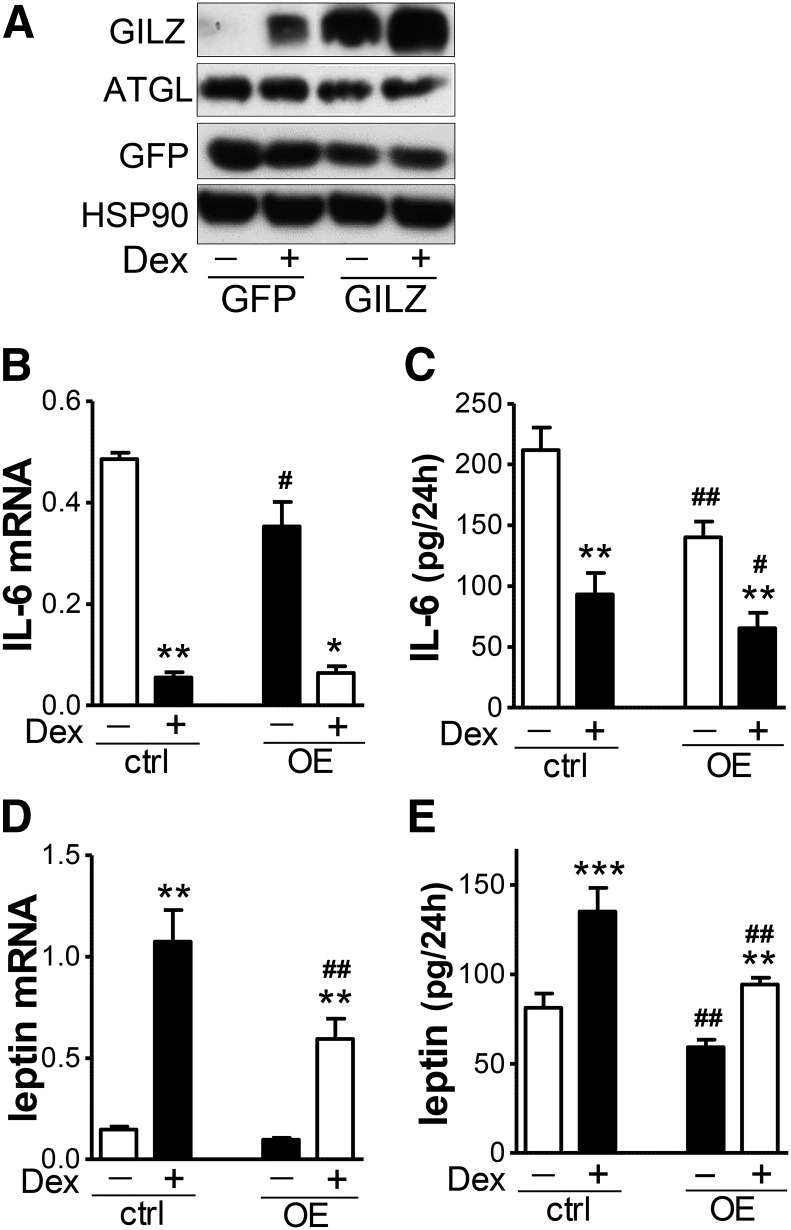
To complement the knockdown studies, we overexpressed GILZ in differentiated human adipocytes; adenovirus-mediated overexpression increased its protein levels by 5- to 10-fold without affecting ATGL levels (Fig. 2A). Overexpression of GILZ decreased IL-6 and leptin mRNAs and secreted proteins by 30–55%, both in the basal and Dex-treated conditions (Fig. 2B–E), without significantly affecting adiponectin mRNA levels and secretion during 24 h (data not shown).
Fig. 2.
GILZ overexpression decreased IL-6 and leptin expression and secretion in human adipocytes. GILZ was overexpressed in human adipocytes using adenovirus. Cells were then treated with Dex (10 nM) for 24 h and harvested and medium during the 24 h treatment was collected. GILZ expression levels were measured with Western blotting (A). IL-6 (B) and leptin (D) mRNA was measured with RT-qPCR. IL-6 (C) and leptin (E) protein in secretion media was measured by ELISA. Effects of GILZ overexpression and Dex treatment were tested with a two-way repeated measures ANOVA followed by post hoc paired t-tests to test effects of GILZ and Dex (*P < 0.05, **P < 0.01, ***P < 0.001, Dex effects; #P < 0.05, ##P < 0.01, GILZ overexpression effects; n = 4–6). GFP, green fluorescent protein; ctrl, control; OE, overexpression.
GILZ suppressed inflammatory signaling in human adipocytes
GILZ silencing increased ERK1/2 and JNK phosphorylation and nuclear translocation of p65 NF-κB.
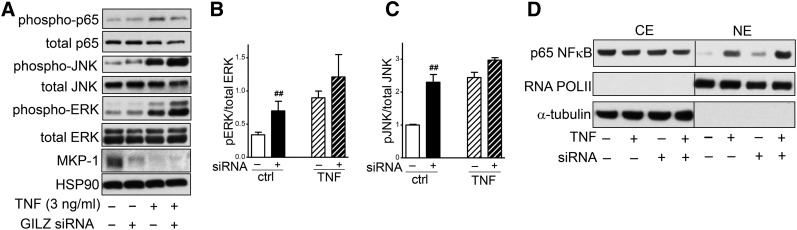
We next examined whether GILZ regulated inflammatory signaling by TNFα in human adipocytes. GILZ silencing significantly increased basal ERK1/2 phosphorylation (Tyr202/204) by 3.0 ± 0.8-fold and JNK phosphorylation (Thr183/Tyr185) by 2.4 ± 0.4-fold, and decreased MKP-1 expression by ∼70% (Fig. 3A–C). TNFα (3 ng/ml, 1 h) increased ERK1/2 and JNK phosphorylation as expected, but GILZ silencing did not significantly affect TNFα-stimulated levels, although there was a trend to increase JNK phosphorylation (P = 0.07 with paired t-test, n = 5). Although GILZ knockdown did not affect the phosphorylation (Ser 536) of p65 NF-κB to total, it increased p65 nuclear translocation under both basal and TNFα-stimulated conditions (Fig. 3A, D).
Fig. 3.
GILZ knockdown increased basal phosphorylation of ERK1/2 and JNK, and p65 NF-κB nuclear translocation. A: Control or GILZ-silenced human adipocytes were treated with TNFα (3 ng/ml) for 1 h, and ERK1/2, JNK, and p65 NF-κB phosphorylation and MKP-1 expression levels were determined with Western blotting. HSP90 was used as a loading control. B: Quantification of ERK1/2 phosphorylation relative to total ERK1/2. C: Quantification of JNK phosphorylation relative to total JNK. ##P < 0.01 compared with control siRNA, n = 5. D: After treating with TNFα (3 ng/ml, 1 h), nuclear extracts (NE) and cytoplasmic extracts (CE) were prepared and used for measurement of p65 NF-κB. α-Tubulin and RNA POLII were used as markers of cytoplasmic and nuclear extracts, respectively. Representative blots of two (for MKP-1) or three experiments are shown. ctrl, control.
GILZ overexpression decreased nuclear translocation and transcriptional activity of p65 NF-κB.
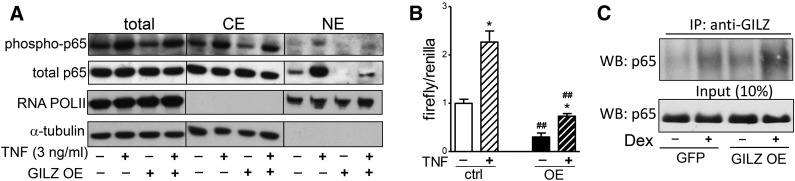
GILZ overexpression decreased p65 NF-κB translocation into the nucleus in both basal and TNFα-stimulated conditions (3 ng/ml, 1 h), without significantly affecting the ratio of phospho (Ser 536) to total p65 levels (Fig. 4A). To directly test whether GILZ affected NF-κB transcriptional activity, control or GILZ-overexpressing human adipocytes were transfected with a NF-κB luciferase reporter. TNFα (3 ng/ml, 3 h) increased NF-κB activity by 2- to 3-fold in control cells. Overexpression of GILZ decreased NF-κB luciferase reporter activity by ∼70% both in the basal and TNFα-stimulated conditions (Fig. 4B).
Fig. 4.
GILZ overexpression decreased the nuclear translocation and transcriptional activity of p65 NF-κB. A: Control or GILZ-overexpressing human adipocytes were treated with TNFα (3 ng/ml, 1 h) and phospho (Ser536) and total p65 NF-κB along with loading controls (RNA POLII and α-tubulin) in total cell lysates, and cytoplasmic extracts (CE) and nuclear extracts (NE) were determined with Western blotting. Representative blots of three independent experiments are shown. B: Control (ctrl) or GILZ-overexpressing (OE) adipocytes were transfected with NF-κB reporter plasmid. After overnight transfection, cells were treated with TNFα (3 ng/ml, 3 h) and NF-κB activity was determined by measuring luciferase activity. Data are presented as relative ratio of firefly (NF-κB) to Renilla (transfection control) luciferase activity. The effects of GILZ overexpression or TNF treatment were determined with a two-way repeated measures ANOVA (P < 0.001); *P < 0.05, TNFα effects; ##P < 0.01, GILZ overexpression effects; n = 4. C: Control or GILZ-overexpressing adipocytes were treated with Dex for 6 h and cytoplasm was prepared and used for IP of GILZ. The p65 NF-κB in IP material and cytosolic extracts were determined with Western blotting (WB). Representative blots of three independent experiments are shown. GFP, green fluorescent protein.
GILZ coimmunoprecipitated with p65 NF-κB in human adipocytes.
To investigate whether GILZ directly interacts with NF-κB in human adipocytes, control or GILZ-overexpressing cells were treated with Dex for 6 h to induce GILZ protein levels (∼4- to 6-fold, not shown), and GILZ was immunoprecipitated from cytoplasmic extracts followed by Western blotting of p65. Coimmunoprecipitation results demonstrated that GILZ physically interacted with p65 in the cytoplasm (Fig. 4C). Furthermore, induction of GILZ expression by Dex treatment or adenovirus-mediated overexpression increased this interaction by 2- to 3-fold. Together, these results suggest that, in human adipocytes, GILZ physically interacts with p65 in the cytoplasm, blocking its translocation into the nucleus and transcriptional activity.
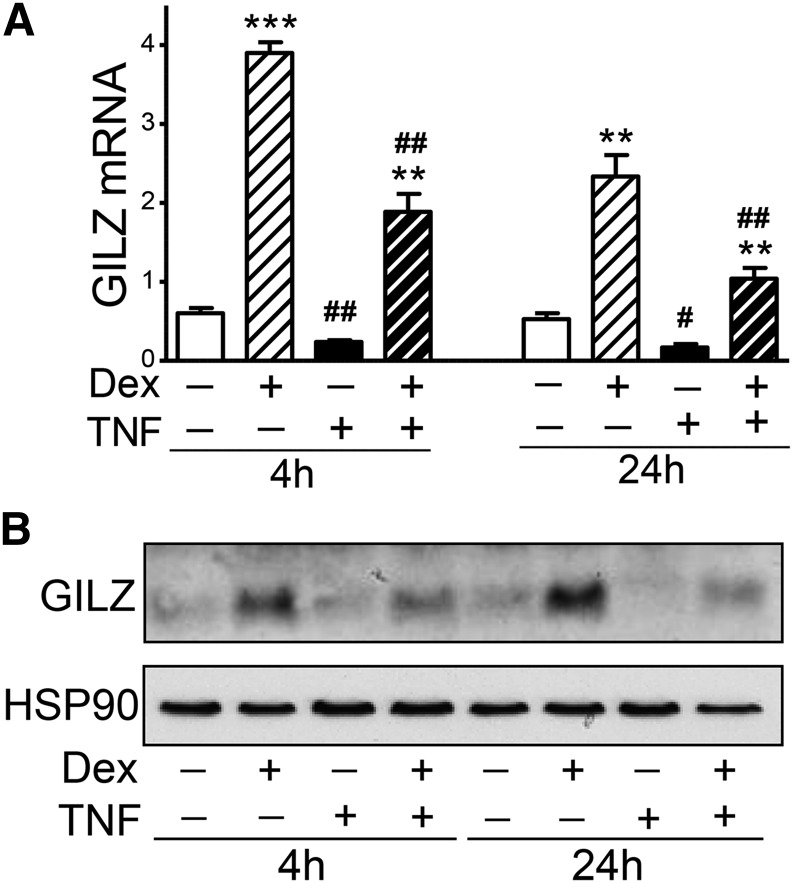
TNFα decreased GILZ expression in human adipocytes
TNFα is known to antagonize glucocorticoid actions (29) and decrease GILZ expression levels in other cells (15, 29). We therefore tested the interplay of these two pathways in the regulation of GILZ expression in newly differentiated human adipocytes. Dex (10 nM) significantly induced GILZ mRNA levels, ∼6-fold after 4 h and ∼3.5-fold after 24 h treatment (Fig. 5A). Dex-induction of GILZ mRNA and protein was detectable within 1 h of treatment, and cortisol (200 nM) increased GILZ expression levels with similar magnitude with 10 nM Dex (data not shown). TNFα (3 ng/ml) decreased GILZ mRNA levels by 60% after 4 h and 65% after 24 h treatment. In addition, TNFα antagonized the Dex-induction of GILZ mRNA expression. Similar effects of Dex and TNFα on GILZ protein levels were observed (Fig. 5B).
Fig. 5.
TNFα suppression of GILZ expression in human adipocytes. A: After overnight Dex removal, differentiated human adipocytes (d14) were treated without or with Dex (10 nM), TNFα (3 ng/ml), or Dex + TNFα for 4 h and 24 h. TNFα effects were significant with a two-way repeated measures ANOVA. **P < 0.01, ***P < 0.001, Dex effects; #P < 0.05, ##P < 0.01, TNF effects; n = 4. B: GILZ protein levels were measured with immunoblotting and representative blots of n = 3 are presented. HSP90 is shown as a loading control.
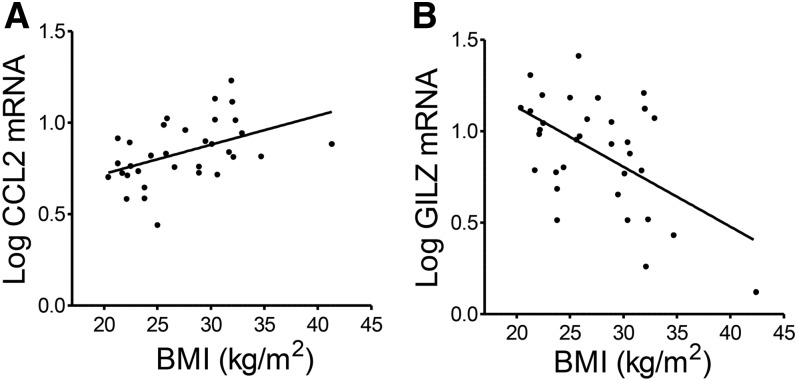
GILZ expression in adipose tissue was decreased in human obesity
GILZ expression is known to be downregulated in inflammatory conditions (12, 15, 30). We therefore reasoned that adipose tissue GILZ expression levels might be reduced with the chronic inflammation observed in human obesity. In a sample of abdominal subcutaneous adipose tissue samples from healthy young men and women (32), CCL2 (r = 0.46, P = 0.007, Fig. 6A) and CD68 (r = 0.33, P = 0.05, not shown) mRNA expression levels were positively correlated with BMI, as expected. GILZ mRNA levels negatively correlated with BMI (r = −0.54, P = 0.001, Fig. 6B). These associations remained significant after statistical adjustment for sex or age of the tissue donors (not shown).
Fig. 6.
GILZ expression in adipose tissue is decreased in human obesity. GILZ and CCL2 mRNA levels were measured with RT-qPCR in human adipose tissue obtained through a needle biopsy, n = 33. A: Correlation between CCL2 mRNA levels and BMI; r = 0.46, P = 0.007. B: Correlation between GILZ mRNA levels and BMI; r = −0.54, P = 0.001.
DISCUSSION
This study identifies a previously unrecognized role of GILZ as an anti-inflammatory factor in human adipocytes. Using knockdown and overexpression studies in cultured human adipocytes, we demonstrated that GILZ regulates mRNA expression and production of two adipokines, IL-6 and leptin, as well as basal and TNFα-stimulated (1 h) inflammatory signaling through the ERK1/2, JNK, and NF-κB pathways. Furthermore, we found that longer-term (4 h and 24 h) TNFα treatment suppressed GILZ mRNA and protein levels in human adipocytes, and that GILZ mRNA levels in human adipose tissue were decreased in proportion to increasing BMI and expression of markers of inflammation, CCL-2 and CD68. Thus, chronically high TNFα within obese adipose tissue (32) resulting from the accumulation of activated macrophages may decrease levels of GILZ, which in turn further increases adipose inflammation by decreasing the anti-inflammatory effect of glucocorticoids.
GILZ expression, at both mRNA and protein levels, was rapidly induced by Dex within 1 h treatment in human adipocytes, as expected from the fact that GILZ is a primary target of the GR and GR response elements have been identified in its promoter region (33). Accordingly, we showed that GR is required for glucocorticoid induction of GILZ in human adipocytes (17). Consistent with this in vitro result, preliminary data show that GILZ mRNA levels are also increased by 2- to 3-fold in human adipose tissue in vivo 3.5 h after cortisol administration (K. Karastergiou, M-J. Lee, and S. K. Fried, unpublished observation), similar to a recent study showing that glucocorticoids induce GILZ mRNA levels in rat adipose tissue in vivo (34), and supporting the physiological relevance of our findings in cultured human adipocytes.
GILZ expression in vivo is known to be downregulated in inflammatory diseases and conditions (12, 15, 30). Our study is, however, the first to demonstrate that GILZ expression is reduced in human adipose tissue in obesity. Obese adipose tissue is subjected to chronic inflammation (36) with elevated levels of TNFα (32), which is known to decrease GILZ expression (15, 29). Thus, our data suggest that the high TNFα produced by activated macrophages within obese human adipose tissue may downregulate GILZ expression and contribute to the lower expression levels of GILZ expression in human obesity. This downregulation may initiate a vicious cycle in which the downregulation of GILZ in both adipocytes and macrophages decreases the ability of glucocorticoid to suppress adipose inflammation.
GILZ may also influence systemic energy homeostasis and metabolism by downregulating adipocyte production of leptin and IL-6, while possibly enhancing the expression of an anti-inflammatory and insulin-sensitizing adipokine, adiponectin (19). GILZ knockdown decreased adiponectin mRNA levels, but did not decrease its secretion during 24 h, perhaps due to high levels of cellular stores. Careful time course studies are needed to test this possibility. GILZ appears to have a selective role in mediating glucocorticoid effects on adipokines, especially leptin, as its knockdown and overexpression did not affect the expression levels of ATGL, used here as a marker of adipogenic differentiation.
The anti-inflammatory actions of GILZ are known to be mediated by its effects on a key pro-inflammatory signaling mediator, NF-κB; however, the exact mechanism is cell-type dependent. In endothelial cells, GILZ decreases NF-κB transcriptional activity without affecting nuclear translocation of p65 NF-κB (29), but in other cells types, GILZ directly binds to p65 NF-κB and decreases its nuclear translocation and, therefore, transcriptional activity (14, 19, 20). Our data indicate that in human adipocytes, GILZ decreases basal NF-κB activity by this latter mechanism. GILZ also antagonized TNFα stimulation of nuclear translocation and transcriptional activity of NF-κB. Thus, GILZ may reduce cytokine expression under both basal and TNFα-stimulated conditions. Unfortunately, we could not use NF-κB reporter assays in GILZ knockdown experiments because double transfection in differentiated human adipocytes significantly reduced cell viability.
GILZ signaling through MAPKs may also contribute to its anti-inflammatory effect. GILZ knockdown increased basal ERK1/2 and JNK phosphorylation, but did not significantly further increase TNFα-stimulated levels, although there was a trend to increase pJNK (P = 0.07, n = 5). GILZ has been shown to decrease MAPK phosphorylation by increasing expression of MKP-1 in bone marrow-derived macrophages (36). Accordingly, we found that GILZ silencing decreased MKP-1 protein expression in parallel to the increased ERK1/2 and JNK phosphorylation. Thus, part of the stimulatory effects of GILZ silencing on ERK and JNK phosphorylation and activity may be mediated via effects on MKP-1. Further investigation of GILZ effects on other signaling pathways, including p38 MAPK and Ras/Raf-1 (11, 14), are warranted.
Although the anti-inflammatory role of GILZ is well-accepted in the context of different cell types and conditions (11, 14, 19, 20), whether GILZ is required for glucocorticoid suppression of inflammatory response is less clear (14, 37). We found that Dex, a GR agonist, suppressed IL-6 to the same extent after GILZ knockdown or overexpression in human adipocytes, suggesting GILZ-independent actions of Dex on IL-6 expression. On the other hand, the magnitude of the Dex-induction of leptin protein was much greater in GILZ-silenced cells (2.0-fold in control vs. 7.7-fold in GILZ knockdown), suggesting that the ability of Dex to induce production of this key adipokine is in part mediated by GILZ. The lower levels of GILZ that result from low grade chronic inflammation in obesity and endotoxemia may therefore contribute to the hyperleptinemia associated with these conditions (38). TNFα also decreases sensitivity to the anti-lipolytic effect of insulin in human adipocytes, and activation of GR antagonizes this effect (28); whether GILZ is required for these effects is not known. Further studies are needed to define the GILZ-dependent and -independent actions of glucocorticoids in the regulation of endocrine and metabolic functions, and inflammation in human adipocytes.
In summary, we demonstrate that GILZ exerts anti-inflammatory actions and regulates adipokine production in human adipocytes. Our data document lower adipose expression of GILZ in human obesity and show that this downregulation may be mediated by TNFα. Low GILZ levels in obesity may directly limit anti-inflammatory signaling and impair some of the anti-inflammatory effects of glucocorticoids. Together with the ability of TNFα to phosphorylate GR and directly impair its signaling (39), TNFα-induced reductions in GILZ expression may escalate the inflammatory response and perpetuate the chronic inflammation and hyperleptinemia that is typical of obesity. Enhancing GILZ expression in adipose tissue could be a novel approach to reduce adipose inflammation and its consequences for metabolic risk in obesity, while avoiding their undesirable metabolic effects of glucocorticoids (40, 41). In vivo modulation of GILZ has been effective in rodent models of inflammatory diseases (42, 43), so this strategy merits further study in the context of obesity.
Footnotes
Abbreviations:
- ATGL
- adipose tissue triglyceride lipase
- Dex
- dexamethasone
- GILZ
- glucocorticoid-induced leucine zipper
- GR
- glucocorticoid receptor
- IL-6
- interleukin-6
- MKP-1
- MAPK phosphatase-1
- NF-κB
- nuclear factor κB
This work supported by a start-up fund from the Department of Medicine, Boston University School of Medicine and a Pilot and Feasibility grant from Joslin Diabetes Center (M-J.L.), National Institute of Diabetes and Digestive and Kidney Diseases Grants R01DK080448 and P30DK046200 (S.K.F.), and the Society for Women’s Health Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors declare no conflicts of interest.
REFERENCES
- 1.Hauner H., Schmid P., and Pfeiffer E. F.. 1987. Glucocorticoids and insulin promote the differentiation of human adipocyte precursor cells into fat cells. J. Clin. Endocrinol. Metab. 64: 832–835. [DOI] [PubMed] [Google Scholar]
- 2.Pantoja C., Huff J. T., and Yamamoto K. R.. 2008. Glucocorticoid signaling defines a novel commitment state during adipogenesis in vitro. Mol. Biol. Cell. 19: 4032–4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu C. Y., Mayba O., Lee J. V., Tran J., Harris C., Speed T. P., and Wang J. C.. 2010. Genome-wide analysis of glucocorticoid receptor binding regions in adipocytes reveal gene network involved in triglyceride homeostasis. PLoS One. 5: e15188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee M. J., Gong D. W., Burkey B. F., and Fried S. K.. 2011. Pathways regulated by glucocorticoids in omental and subcutaneous human adipose tissues: a microarray study. Am. J. Physiol. Endocrinol. Metab. 300: E571–E580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee M. J., and Fried S. K.. 2009. Integration of hormonal and nutrient signals that regulate leptin synthesis and secretion. Am. J. Physiol. Endocrinol. Metab. 296: E1230–E1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fried S. K., Bunkin D. A., and Greenberg A. S.. 1998. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J. Clin. Endocrinol. Metab. 83: 847–850. [DOI] [PubMed] [Google Scholar]
- 7.Bruun J. M., Lihn A. S., Madan A. K., Pedersen S. B., Schiott K. M., Fain J. N., and Richelsen B.. 2004. Higher production of IL-8 in visceral vs. subcutaneous adipose tissue. Implication of nonadipose cells in adipose tissue. Am. J. Physiol. Endocrinol. Metab. 286: E8–E13. [DOI] [PubMed] [Google Scholar]
- 8.Peckett A. J., Wright D. C., and Riddell M. C.. 2011. The effects of glucocorticoids on adipose tissue lipid metabolism. Metabolism. 60: 1500–1510. [DOI] [PubMed] [Google Scholar]
- 9.Patsouris D., Neels J. G., Fan W., Li P. P., Nguyen M. T., and Olefsky J. M.. 2009. Glucocorticoids and thiazolidinediones interfere with adipocyte-mediated macrophage chemotaxis and recruitment. J. Biol. Chem. 284: 31223–31235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee M. J., Pramyothin P., Karastergiou K., and Fried S. K.. 2014. Deconstructing the roles of glucocorticoids in adipose tissue biology and the development of central obesity. Biochim. Biophys. Acta. 1842: 473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ayroldi E., Zollo O., Macchiarulo A., Di M. B., Marchetti C., and Riccardi C.. 2002. Glucocorticoid-induced leucine zipper inhibits the Raf-extracellular signal-regulated kinase pathway by binding to Raf-1. Mol. Cell. Biol. 22: 7929–7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berrebi D., Bruscoli S., Cohen N., Foussat A., Migliorati G., Bouchet-Delbos L., Maillot M. C., Portier A., Couderc J., Galanaud P., et al. . 2003. Synthesis of glucocorticoid-induced leucine zipper (GILZ) by macrophages: an anti-inflammatory and immunosuppressive mechanism shared by glucocorticoids and IL-10. Blood. 101: 729–738. [DOI] [PubMed] [Google Scholar]
- 13.Liu J., Zhang M., Niu C., Luo Z., Dai J., Wang L., Liu E., and Fu Z.. 2013. Dexamethasone inhibits repair of human airway epithelial cells mediated by glucocorticoid-induced leucine zipper (GILZ). PLoS One. 8: e60705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng Q., Fan H., Ngo D., Beaulieu E., Leung P., Lo C. Y., Burgess R., van der Zwan Y. G., White S. J., Khachigian L. M., et al. . 2013. GILZ overexpression inhibits endothelial cell adhesive function through regulation of NF-kappaB and MAPK activity. J. Immunol. 191: 424–433. [DOI] [PubMed] [Google Scholar]
- 15.Hahn R. T., Hoppstadter J., Hirschfelder K., Hachenthal N., Diesel B., Kessler S. M., Huwer H., and Kiemer A. K.. 2014. Downregulation of the glucocorticoid-induced leucine zipper (GILZ) promotes vascular inflammation. Atherosclerosis. 234: 391–400. [DOI] [PubMed] [Google Scholar]
- 16.Ayroldi E., Zollo O., Bastianelli A., Marchetti C., Agostini M., Di V. R., and Riccardi C.. 2007. GILZ mediates the antiproliferative activity of glucocorticoids by negative regulation of Ras signaling. J. Clin. Invest. 117: 1605–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee M. J., and Fried S. K.. 2014. The glucocorticoid receptor, not the mineralocorticoid receptor, plays the dominant role in adipogenesis and adipokine production in human adipocytes. Int. J. Obes. (Lond). 38: 1228–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trujillo M. E., and Scherer P. E.. 2006. Adipose tissue-derived factors: impact on health and disease. Endocr. Rev. 27: 762–778. [DOI] [PubMed] [Google Scholar]
- 19.Ayroldi E., Migliorati G., Bruscoli S., Marchetti C., Zollo O., Cannarile L., D’Adamio F., and Riccardi C.. 2001. Modulation of T-cell activation by the glucocorticoid-induced leucine zipper factor via inhibition of nuclear factor kappaB. Blood. 98: 743–753. [DOI] [PubMed] [Google Scholar]
- 20.Di Marco B., Massetti M., Bruscoli S., Macchiarulo A., Di V. R., Velardi E., Donato V., Migliorati G., and Riccardi C.. 2007. Glucocorticoid-induced leucine zipper (GILZ)/NF-kappaB interaction: role of GILZ homo-dimerization and C-terminal domain. Nucleic Acids Res. 35: 517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karastergiou K., Fried S. K., Xie H., Lee M. J., Divoux A., Rosencrantz M. A., Chang R. J., and Smith S. R.. 2013. Distinct developmental signatures of human abdominal and gluteal subcutaneous adipose tissue depots. J. Clin. Endocrinol. Metab. 98: 362–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee M. J., and Fried S. K.. 2014. Optimal protocol for the differentiation and metabolic analysis of human adipose stromal cells. Methods Enzymol. 538: 49–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hauner H., Skurk T., and Wabitsch M.. 2001. Cultures of human adipose precursor cells. Methods Mol. Biol. 155: 239–247. [DOI] [PubMed] [Google Scholar]
- 24.Lee M. J., Wu Y., and Fried S. K.. 2012. A modified protocol to maximize differentiation of human preadipocytes and improve metabolic phenotypes. Obesity (Silver Spring). 20: 2334–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee M. J., Pickering R. T., and Puri V.. 2014. Prolonged efficiency of siRNA-mediated gene silencing in primary cultures of human preadipocytes and adipocytes. Obesity (Silver Spring). 22: 1064–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo J., Deng Z. L., Luo X., Tang N., Song W. X., Chen J., Sharff K. A., Luu H. H., Haydon R. C., Kinzler K. W., et al. . 2007. A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat. Protoc. 2: 1236–1247. [DOI] [PubMed] [Google Scholar]
- 27.Lee M. J., Yang R. Z., Gong D. W., and Fried S. K.. 2007. Feeding and insulin increase leptin translation. Importance of the leptin mRNA untranslated regions. J. Biol. Chem. 282: 72–80. [DOI] [PubMed] [Google Scholar]
- 28.Lee M. J., and Fried S. K.. 2012. Glucocorticoids antagonize tumor necrosis factor-alpha-stimulated lipolysis and resistance to the antilipolytic effect of insulin in human adipocytes. Am. J. Physiol. Endocrinol. Metab. 303: E1126–E1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eddleston J., Herschbach J., Wagelie-Steffen A. L., Christiansen S. C., and Zuraw B. L.. 2007. The anti-inflammatory effect of glucocorticoids is mediated by glucocorticoid-induced leucine zipper in epithelial cells. J. Allergy Clin. Immunol. 119: 115–122. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X. H., Lu X., Long X. B., You X. J., Gao Q. X., Cui Y. H., and Liu Z.. 2009. Chronic rhinosinusitis with and without nasal polyps is associated with decreased expression of glucocorticoid-induced leucine zipper. Clin. Exp. Allergy. 39: 647–654. [DOI] [PubMed] [Google Scholar]
- 31.Divoux A., Karastergiou K., Xie H., Guo W., Perera R. J., Fried S. K., and Smith S. R.. 2014. Identification of a novel lncRNA in gluteal adipose tissue and evidence for its positive effect on preadipocyte differentiation. Obesity (Silver Spring). 22: 1781–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hotamisligil G. S., Arner P., Caro J. F., Atkinson R. L., and Spiegelman B. M.. 1995. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J. Clin. Invest. 95: 2409–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J. C., Derynck M. K., Nonaka D. F., Khodabakhsh D. B., Haqq C., and Yamamoto K. R.. 2004. Chromatin immunoprecipitation (ChIP) scanning identifies primary glucocorticoid receptor target genes. Proc. Natl. Acad. Sci. USA. 101: 15603–15608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ayyar V. S., Almon R. R., Jusko W. J., and Dubois D. C.. 2015. Quantitative tissue-specific dynamics of in vivo GILZ mRNA expression and regulation by endogenous and exogenous glucocorticoids. Physiol. Rep. 3: e12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalupahana N. S., Moustaid-Moussa N., and Claycombe K. J.. 2012. Immunity as a link between obesity and insulin resistance. Mol. Aspects Med. 33: 26–34. [DOI] [PubMed] [Google Scholar]
- 36.Fan H., Kao W., Yang Y. H., Gu R., Harris J., Fingerle-Rowson G., Bucala R., Ngo D., Beaulieu E., and Morand E. F.. 2014. Macrophage migration inhibitory factor inhibits the antiinflammatory effects of glucocorticoids via glucocorticoid-induced leucine zipper. Arthritis Rheumatol. 66: 2059–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen N., Mouly E., Hamdi H., Maillot M. C., Pallardy M., Godot V., Capel F., Balian A., Naveau S., Galanaud P., et al. . 2006. GILZ expression in human dendritic cells redirects their maturation and prevents antigen-specific T lymphocyte response. Blood. 107: 2037–2044. [DOI] [PubMed] [Google Scholar]
- 38.Anderson P. D., Mehta N. N., Wolfe M. L., Hinkle C. C., Pruscino L., Comiskey L. L., Tabita-Martinez J., Sellers K. F., Rickels M. R., Ahima R. S., et al. . 2007. Innate immunity modulates adipokines in humans. J. Clin. Endocrinol. Metab. 92: 2272–2279. [DOI] [PubMed] [Google Scholar]
- 39.Szatmáry Z., Garabedian M. J., and Vilcek J.. 2004. Inhibition of glucocorticoid receptor-mediated transcriptional activation by p38 mitogen-activated protein (MAP) kinase. J. Biol. Chem. 279: 43708–43715. [DOI] [PubMed] [Google Scholar]
- 40.Cheng Q., Morand E., and Yang Y. H.. 2014. Development of novel treatment strategies for inflammatory diseases-similarities and divergence between glucocorticoids and GILZ. Front. Pharmacol. 5: 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ayroldi E., Macchiarulo A., and Riccardi C.. 2014. Targeting glucocorticoid side effects: selective glucocorticoid receptor modulator or glucocorticoid-induced leucine zipper? A perspective. FASEB J. 28: 5055–5070. [DOI] [PubMed] [Google Scholar]
- 42.Beaulieu E., Ngo D., Santos L., Yang Y. H., Smith M., Jorgensen C., Escriou V., Scherman D., Courties G., Apparailly F., et al. . 2010. Glucocorticoid-induced leucine zipper is an endogenous antiinflammatory mediator in arthritis. Arthritis Rheum. 62: 2651–2661. [DOI] [PubMed] [Google Scholar]
- 43.Srinivasan M., and Janardhanam S.. 2011. Novel p65 binding glucocorticoid-induced leucine zipper peptide suppresses experimental autoimmune encephalomyelitis. J. Biol. Chem. 286: 44799–44810. [DOI] [PMC free article] [PubMed] [Google Scholar]