Abstract
Levels of lipoprotein (a) [Lp(a)], a complex between an LDL-like lipid moiety containing one copy of apoB, and apo(a), a plasminogen-derived carbohydrate-rich hydrophilic protein, are primarily genetically regulated. Although stable intra-individually, Lp(a) levels have a skewed distribution inter-individually and are strongly impacted by a size polymorphism of the LPA gene, resulting in a variable number of kringle IV (KIV) units, a key motif of apo(a). The variation in KIV units is a strong predictor of plasma Lp(a) levels resulting in stable plasma levels across the lifespan. Studies have demonstrated pronounced differences across ethnicities with regard to Lp(a) levels and some of this difference, but not all of it, can be explained by genetic variations across ethnic groups. Increasing evidence suggests that age, sex, and hormonal impact may have a modest modulatory influence on Lp(a) levels. Among clinical conditions, Lp(a) levels are reported to be affected by kidney and liver diseases.
Keywords: populations, apolipoprotein (a) size, genetics, demographic and clinical characteristics, kidney and liver disease, diabetes
ETHNICITY/RACE AND LIPOPROTEIN (a) LEVELS
Lipoprotein (a) levels in different population groups
One of the most distinctive features regarding lipoprotein (a) [Lp(a)] has been the variability in levels between different population groups. Although substantial knowledge has been obtained regarding genetic variability of apo(a) between different populations, the underlying causes for this difference in levels are still largely unresolved. Early population studies investigating the association of Lp(a) with coronary artery disease (CAD) risk were largely conducted in European populations (1–4). However, striking differences in Lp(a) concentrations and distribution were noted by several groups. As an example, Blacks have a mean Lp(a) concentration twice as high as in Whites (5–8). Beyond Blacks and Whites, a heterogeneous distribution of Lp(a) concentration across Asian populations was noted with substantially elevated Lp(a) concentrations in Indians as compared with Chinese, a finding confirmed in other studies (Table 1) (7, 9). The Lp(a) distribution pattern among Chinese was closer to Whites; while among Indians, the pattern was intermediate between those of Blacks and Whites. These findings were extended to seven ethnic groups, representing Caucasians (Tyrolean, Icelandic, Hungarian), Asians (Indian, Chinese, Malay), and Blacks (Sudanese), where the overall pattern was confirmed (10). Another study in four ethnic groups demonstrated a similar ethnic-specific difference with the mean Lp(a) concentration in Ghanaian Blacks being 1.6- to 2-fold higher than those of German, Chinese, or San populations, respectively (Table 1) (11). Also among children, mean Lp(a) levels were 1.7-fold higher in African-Americans versus Caucasians (12). Among the elderly, Lp(a) levels were higher in African-American men compared with Caucasians (13). Notably, a lower Black/White ratio was reported in many studies involving US Blacks versus non-US Blacks, possibly due to gene admixture (13). Among Mexican-Americans, men and women had significantly lower Lp(a) levels compared with their respective non-Hispanic White counterparts (14). Recent studies in a multi-ethnic population have emphasized the importance of race/ethnicity as a key variable in assigning Lp(a) cutoff values for CAD risk assessment and the need to develop the most clinically useful Lp(a) cutoff values in individual race/ethnicity groups (15).
TABLE 1.
Lp(a) concentrations in different ethnic populations
| Ethnic Population (Reference) | Lp(a) Level (mg/dl) |
| Asians | |
| Chinese (11) | 15 (6–93) |
| Chinese from Singapore (10) | 7.2 ± 13.1 |
| Chinese Americans (9) | 9 (5–19)a |
| Japanese (62) | 19.5 ± 18.5 |
| Asian Indians (9) | 15 (8–33)a |
| Indians from Singapore (10) | 20.1 ± 15.9 |
| Malaysian from Singapore (10) | 12.9 ± 17.9 |
| Caucasians | |
| Hungarians (10) | 8.3 ± 11.0 |
| Tyrolean (10) | 14.1 ± 19.4 |
| Icelandic (10) | 13.5 ± 17.7 |
| German (11) | 9 (1–105) |
| Non-Hispanic Whites (105) | 10 (3–33)a |
| Non-Hispanic Whites (9) | 12 (6–30)a |
| Non-Hispanic Whites (8) | 8 (3–28)a |
| Hispanics (14) | 5.0 ± 0.5b |
| African descent | |
| Ghanaians (11) | 26 (3–240) |
| San (Bushmen) (11) | 15 (4–105) |
| Sudanese (10) | 45.7 ± 25.9 |
| African-Americans (105) | 45 (25–75)a |
| African-Americans (8) | 31 (15–54)a |
Data are expressed as mean ± SD or as the median and interquartile range.
To facilitate comparison, Lp(a) levels given in nmol/l in the original article were converted into mg/dl by use of a conversion factor of 2.4 nmol/l = 1 mg/dl.
Data are shown as mean ± SEM.
The apo(a) size polymorphism and Lp(a) levels in Whites and Blacks
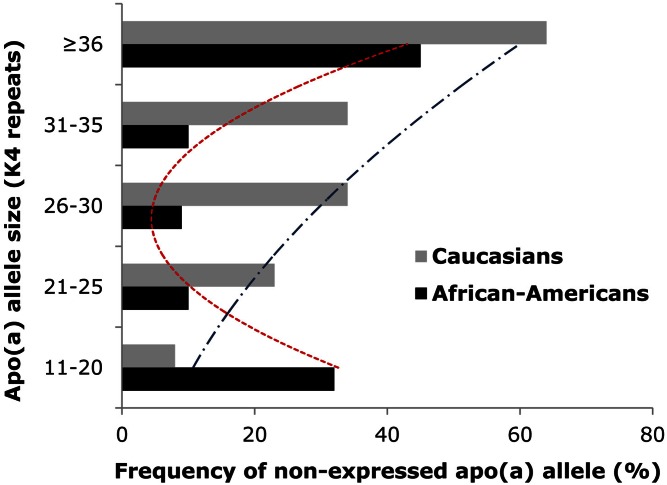
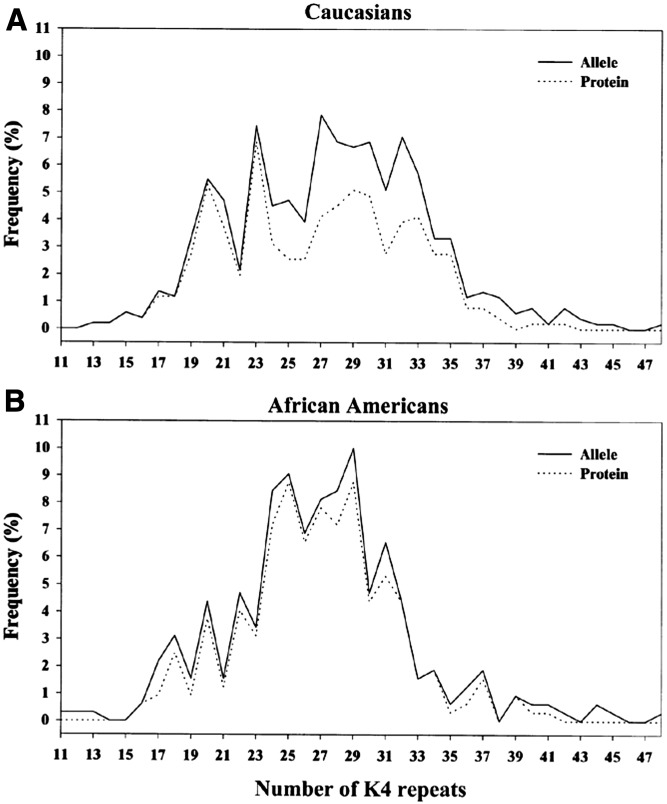
Mirroring the copy number variation in the apo(a) gene (16), there is a substantial size heterogeneity of the apo(a) protein ranging from, overall, 12 to more than 50 kringle IV (KIV) repeats, the majority being KIV type 2 (17–20). Most individuals carry two different-size apo(a) alleles, and the degree of heterozygosity at the genetic level is very high (>95%) (17). However, as not all apo(a) alleles are expressed as protein, the degree of heterozygosity at the protein level is somewhat lower (∼70%). In general, within an individual, the larger apo(a) isoform is more likely to be “nonexpressed” at the protein level than the smaller isoform. This trend is more apparent in Caucasians with a gradual rise in the nonexpressed allele frequency with an increasing number of KIV repeats. Among African-Americans, a more U-shaped distribution was seen (21) (Fig. 1). Furthermore, the smaller apo(a) size in any given individual does not always represent the quantitatively dominating Lp(a) isoform (21, 22). The larger apo(a) isoform is reported to be dominant in about one-quarter of both African-American and Caucasian heterozygotes, whereas dominance of the smaller isoform is more common in Caucasians than in African-Americans (21). An example of frequency distribution of apo(a) alleles and isoform sizes for Caucasians and African-Americans are shown in Fig. 2. Among Caucasians, nonexpressed alleles (the gap between the allele and isoform curves) were most frequent in the mid-range, whereas among African-Americans, they were fairly evenly distributed across apo(a) sizes (21).
Fig. 1.
Frequency distributions of nonexpressed apo(a) alleles across apo(a) size ranges in African-Americans and Caucasians. In general, within an individual, the larger apo(a) isoform is more likely to be nonexpressed at the protein level than the smaller isoform. This trend is more apparent in Caucasians with a gradual rise in the nonexpressed allele frequency with an increasing number of KIV repeats (21). Among African-Americans, a more U-shaped distribution was observed.
Fig. 2.
Frequency distribution of apo(a) alleles and isoforms in Caucasians (A) and African-Americans (B). Alleles are represented by the solid lines and apo(a) protein isoforms by the dashed lines (the dashed lines are not shown where they coincide with the solid lines). The isoform distribution was calculated by dividing the total number of protein bands detected by the total number of alleles, separately for each population. Homozygotes (n = 15) were excluded, as it was not possible to determine whether the single apo(a) protein band corresponded to one or two proteins. The African-American distribution had a narrower and taller peak while the Caucasian distribution was wider. Among Caucasians, nonexpressed alleles (the gap between the allele and isoform curves) were most frequent in the mid-range, whereas among African-Americans, they were fairly evenly distributed across apo(a) sizes. This figure was originally published in (21). © The American Society for Biochemistry and Molecular Biology.
Associations between LPA polymorphisms and Lp(a) levels across populations
As Lp(a) is one of the most heritable quantitative traits in humans (23, 24), efforts to understand the ethnic variability in Lp(a) concentrations have largely focused on genetics. A large proportion of variability in Lp(a) levels can be explained by variations in the LPA locus, mainly by the apo(a) gene size polymorphism, although the contribution of this variability to the overall plasma Lp(a) level varies across ethnicities (20–80%) (25). Over time, several genetic variants at the LPA locus have been identified predicting Lp(a) levels and explaining some of the variability in Lp(a) concentrations, again with a variable impact across populations. Notably, three SNPs contribute to the African-American/Caucasian difference in Lp(a) concentration (26). Two SNPs (T3888P and G+1/inKIV-8A), both suppressing Lp(a) assembly, were more common in Caucasians, whereas the third SNP (G-21A), increasing apo(a) promoter activity, was more common in African-Americans. In addition, a pentanucleotide repeat (PNR), [TTTTA]n (8–11 repeats), in the promoter region of LPA explained up to 14% of the variation in Lp(a) concentration among Caucasians, independent of KIV repeats (27). Furthermore, the PNR influenced allele-specific apo(a) levels in Caucasians, but not in African-Americans, with a stepwise decrease with increasing PNR number (28).
Recently, two SNPs at the LPA locus (rs10455872, which maps to intron 25, and rs3798220, located in the protease-like domain) have been associated with high Lp(a) levels and smaller size apo(a) (29–34). Demonstrating the variability in effect across three ethnic groups (non-Hispanic Whites, Mexican-Americans, non-Hispanic Blacks), a total of 15 LPA SNPs were associated with Lp(a) concentrations at least in one studied population, six in two populations, but none in three populations (35). Among non-Hispanic Whites, three SNPs together explained 7% of the variation in Lp(a) concentrations; in Mexican-Americans, six SNPs together explained 11%; and among non-Hispanic Blacks, 12 SNPs explained 9% of this variation. These findings point to a variability in the association between SNPs and Lp(a) concentrations across populations, and further, that individual LPA variants may contribute to interethnic differences in Lp(a) levels.
A substantial heterogeneity in estimating the proportion of Lp(a) variance explained by SNPs alone or in conjunction with the size polymorphism has been observed (29, 31, 32). Ronald et al. (32) identified a set of nine SNPs accounting for 30% of the variation in Lp(a) concentration, five of which overlapped with a set of seven SNPs identified by others (31). Six of these nine SNPs, of which four had previously been reported (29, 30), predicted Lp(a) concentrations conditional on the number of KIV repeats. Accounting for apo(a) size, SNPs rs3798220 and rs10455872 were associated with Lp(a) concentrations, and together explained 22% of Lp(a) variance. It has been proposed that rs3798220 may affect protein stability (30), whereas rs10455872 may be in linkage disequilibrium (LD) with regulatory variants (36). In an Old Order Amish population, the LPA mRNA level was higher in carriers compared with noncarriers of rs10455872, but was not different between carriers and noncarriers of rs3798220 (37). Further, the apo(a) protein level was higher in carriers compared with noncarriers of both rs10455872 and rs3798220 (37). The question arises, to what extent does this pattern vary across ethnicity? Studies in South Asians, Chinese, and Caucasians revealed that SNP rs10455872 was prevalent only in the latter (29). In addition, SNP rs6415084 within the same haplotype block as the KIV repeat polymorphism was associated with both Lp(a) concentrations and the size polymorphism in all three ethnic groups. SNPs and apo(a) size polymorphism together explained a greater proportion of variation in Lp(a) concentration in Caucasians (36%) than in Chinese (27%) or South Asians (21%).
Two additional SNPs at the LPA locus (rs6919346 in intron 37 and rs1853021 (+93C/T) in the 5′ untranslated region) were associated with a modestly elevated Lp(a) level, independent of apo(a) size, in Hutterites (a founder population), and for the former SNP, this was replicated in Caucasians (38). In African-Americans, the association between LPA +93C/T SNP and Lp(a) concentrations was opposite to that seen in Hutterites (39). A report from the Jackson Heart Study identified multiple common SNPs associated with Lp(a), accounting for up to 7% of Lp(a) level variation, as well as >70% of the African-American/Caucasian interethnic difference (40). In contrast to Caucasians, no single common SNP has been found to explain a large portion of variation in Lp(a) concentrations in African-Americans (29, 31). A limited LD between the apo(a) size polymorphism and common SNPs on the African ancestral background could account for this contrasting result (29). This extensive variability across populations illustrates the complexity of the relationship between Lp(a) concentrations and apo(a) polymorphisms, as well as methodological difficulties in accurately assessing this relationship.
The role of genetic factors beyond the LPA gene in regulating Lp(a) levels across ethnicity
Recent genome-wide association studies have reported an impact on Lp(a) by other genes. In Hutterites, eight genes on chromosome 6q26-q27 significantly impacted Lp(a) concentrations (38). An association of one SNP in the plasminogen (PLG) gene, rs14224, with Lp(a) concentrations was replicated in Caucasian males, and the SNP was in LD with the number of KIV repeats (38). In addition, two other SNPs in the PLG gene, rs783147 and rs6935921, were associated with Lp(a) levels in Caucasians, explaining 12% and ∼4.5% of Lp(a) variation, respectively (41, 42). A locus influencing Lp(a) concentrations on chromosome 2 with several candidate genes, including the TFPI gene, was reported from a study of Spanish families (43). Others have shown a positive association of the C-allele at the −174 locus of the human interleukin (IL)-6 gene with elevated Lp(a) concentrations (>60 mg/dl) (44). A meta-analysis by Zabaneh et al. (33) has identified a number of variants in four other loci, in addition to the LPA locus, that have a significant impact on Lp(a) levels. However, the findings could only be replicated for one locus (TNFRSFF11A). At present, numerous challenges, including use of specialized candidate gene chips with a restricted scope, small sample sizes, lack of replication cohorts, variability across cohorts, and focus on specific subpopulations (population isolates, end stage renal disease, or diabetes mellitus), present limitations that are further complicated by ethnic-specific differences in Lp(a) levels. Future studies addressing such limitations should bring more insights into the nature of Lp(a) heritability.
ENVIRONMENTAL FACTORS AND Lp(a) LEVELS
Age
Studies in newborns report an increase in Lp(a) levels from birth to the 7th postnatal day with a continued increase up to eight months (45). Among adolescents, Lp(a) concentrations increased in White children aged 11–17 years (12). Among adults, results have been somewhat variable. Lp(a) was associated with age in White women, but not in Black and White men (46, 47). Others report that age was significantly associated with increasing Lp(a) levels in both White men and women (48). An apparent age-related increase in Lp(a) levels was reported in Black, but not in White, female twins (49). Among Japanese, Lp(a) concentration was positively correlated with age for both men and women. Further, an association between Lp(a) and longevity has been suggested among centenarians (50–54). On the other hand, a substantial number of studies report no association between age and Lp(a) concentration (5, 14, 55–59), and the issue of to what extent age per se influences Lp(a) levels remains unresolved.
Sex
Although many studies across population groups have indicated a lack of difference in Lp(a) concentration between males and females (5, 11, 46, 58–61), the effect of sex on Lp(a) levels remains to be established. A small, but significant, elevation in Lp(a) concentration was observed in girls compared with boys for both African-Americans and Caucasians (12). A recent study also reported significantly higher Lp(a) levels in girls than in boys among healthy Arabian adolescents (56). Furthermore, the median Lp(a) concentration was higher in women than men among Whites, but not in Blacks (47). In a Japanese study, Lp(a) concentration was significantly elevated in women compared with men (62). The question has been raised whether such results might be explained by a potential confounding effect of CAD familial predisposition. Addressing this issue, no significant differences in Lp(a) concentrations between brothers and sisters were seen among healthy teenagers with a positive parental history of premature myocardial infarction (63). A study by Frohlich et al. (64) conducted in subjects with European background, found no significant difference in Lp(a) concentrations between men and women without CAD, but 2-fold increased Lp(a) concentrations in women compared with men, both with CAD. These associations remained significant after adjustments for covariates, including age. A recent study by the same group conducted in subjects with familial hypercholesterolemia reported a similar median Lp(a) concentration between men and women, although in a subgroup of subjects with CVD, Lp(a) levels were significantly higher in women (65). Among women with familial hypercholesterolemia, Nenseter et al. (66) found higher Lp(a) levels in CVD-susceptible versus CVD-resistant subjects. Thus, while a number of studies indicate higher Lp(a) levels among women, in particular under CVD-positive conditions, there are many potential confounders, such as ethnicity/race, apo(a) size distribution, menopause status, and differences in assay methodology.
Diet, normal food intake, and fasting/nonfasting state
Although there is a strong genetic impact on Lp(a) concentrations, a number of studies have reported differences in Lp(a) levels due to variations in the intake of dietary trans-fatty acids and saturated fat. Beyond this observation, many studies focusing on dietary interventions have failed to detect any significant effects on Lp(a) levels (67–69). Mensink et al. (70) reported an Lp(a)-increasing effect from diets rich in trans-monounsaturated fatty acids, and a similar result was obtained by Nestel et al. (71) with a diet enriched in elaidic acid. Reduction of saturated fat was associated with an increase in Lp(a) levels, whereas addition of saturated fat was associated with a decrease in Lp(a) levels (72, 73). In nonhuman primates, the substitution of dietary saturated fat with either mono- or polyunsaturated fatty acids resulted in a significant reduction in Lp(a) concentrations (74). In a double-blind cross-over study of moderately hypercholesterolemic postmenopausal women, compared with a partially-hydrogenated soybean oil-enriched diet, a corn oil-enriched diet lowered Lp(a) by 5% (75). Consumption of a low-fat high-carbohydrate diet for 4 weeks significantly increased Lp(a) concentration compared with a high-fat low-carbohydrate diet, and changes in Lp(a) were strongly correlated with changes in the oxidized phospholipid (OxPL)/apoB ratio (76). Among overweight/obese postmenopausal women, a 6 month hypocaloric dietary intervention significantly decreased Lp(a) concentrations by 4% (77). In the Omni Heart Trial, a randomized, three-period crossover feeding study, participants were given DASH-type healthy diets rich in carbohydrates, protein, or unsaturated fat for 6 weeks each (78). Compared with baseline, all interventional diets increased mean Lp(a) levels by 2–5 mg/dl. A diet rich in unsaturated fat increased Lp(a) levels less than a protein-rich diet, with a difference of 1.0 mg/dl in Whites and 3.7 mg/dl in Blacks. A diet rich in unsaturated fat increased Lp(a) levels less than a carbohydrate-rich diet, with a difference of −0.6 mg/dl in Whites and −1.5 mg/dl in Blacks, while a protein-rich diet increased Lp(a) levels more than a carbohydrate-rich diet, with a difference of 0.4 mg/dl in Whites and 2.2 mg/dl in Blacks. Generally, diets high in unsaturated fat increased Lp(a) levels less than diets rich in carbohydrate or protein, with greater changes in Blacks than Whites. These results suggest that substitutions of saturated fat with dietary mono- and polyunsaturated fatty acids may be preferable over protein or carbohydrates with regard to Lp(a). Overall, the magnitude of the observed changes in Lp(a) concentrations due to dietary interventions has been relatively modest. Presently, clinical guidelines do not specify whether Lp(a) concentrations should be measured in the fasting or nonfasting state. In the Copenhagen General Population Study and the Copenhagen City Heart Study participants, Lp(a) concentrations were minimally affected in response to normal food intake (17 mg/dl at fasting versus 19 mg/dl at 3–4 h since the last meal) (79).
Exercise and BMI
Many population-based and cross-sectional studies have been unable to detect an association between Lp(a) and physical activity level (49, 80–86). However, in a large multicenter study of Finnish children and young adults, an inverse correlation was seen between Lp(a) concentration and physical activity with a dose-response relationship (87). In line with these findings, physical fitness was inversely associated with Lp(a) concentration in young children and adolescents with diabetes mellitus (88). In addition, Lp(a) levels were higher in experienced distance runners and in body builders who exercised regularly, suggesting a possible effect of prolonged high-intensity exercise training on Lp(a) levels (89, 90). Overall, the magnitude of exercise-induced changes in Lp(a) levels was modest, and any impact related to specific apo(a) size isoforms has not been addressed. Despite improvements in fitness and other plasma lipoprotein concentrations, intervention studies extending from a few weeks to 4 years have not reported any changes in median Lp(a) concentration in response to moderate exercise training (83, 91–94). In a large number of Japanese subjects, Lp(a) concentrations were significantly lower in subjects with a BMI of >26 kg/m2 than in subjects with a BMI of ≤26 kg/m2 in both sexes, and BMI in females was a significant independent variable (62). On the other hand, many studies have not found any impact of BMI on Lp(a) concentrations across gender groups (5, 46, 47, 49, 59).
Inflammation
The inflammatory response is mediated by systemic acute phase reactants, such as C-reactive protein (CRP), fibrinogen, and serum amyloid A (95–97), as well as vascular inflammatory biomarkers, such as pentraxin 3 (PTX-3) and lipoprotein-associated phospholipase A2 (98, 99). The apo(a) gene contains response elements to inflammatory factors such as IL-6, and Lp(a) stimulates release of proinflammatory cytokines from vascular endothelial and smooth muscle cells, as well as from monocytes and macrophages (100, 101). A recent study reported increased Lp(a) levels in individuals with elevated IL-6 levels, and that an IL-6 blockade by tocilizumab reduced Lp(a) levels (102). Furthermore, expression of IL-6 response genes in human liver biopsies was correlated with LPA gene expression in vivo, and treatment with tocilizumab inhibited IL-6-induced LPA mRNA and protein expression in human hepatocytes (102). Importantly, OxPLs, which possess strong proinflammatory potentials, are preferentially carried on Lp(a) particles (103). Of note, the correlation between OxPL and Lp(a) concentration was stronger in individuals with smaller apo(a) isoforms than in individuals with larger apo(a) isoforms (104). These findings suggest a synergy between inflammation and Lp(a), and the magnitude of this relationship may differ across different ethnic/racial populations.
The impact of an inflammatory burden, as detected by elevated concentrations of biomarkers for systemic and vascular inflammation on Lp(a) levels associated with a defined apo(a) size, has been explored in several studies (105–107). These levels have been characterized as isoform- or allele-specific apo(a) levels (Fig. 3). Increased CRP and fibrinogen concentrations were significantly associated with higher allele-specific Lp(a) levels for smaller apo(a) size in African-Americans, while a higher plasma lipoprotein-associated phospholipase A2 activity was associated with an elevated allele-specific Lp(a) level for smaller apo(a) size in both African-Americans and Caucasians (105, 106). Further, a significant association between elevated serum amyloid A, an HDL-associated systemic inflammatory biomarker, and a higher allele-specific Lp(a) level for smaller apo(a) size was found in African-Americans (107). Taken together, these findings suggest a potential for an additive effect between molecular properties of Lp(a), in particular small size apo(a), and inflammation in promoting Lp(a)-associated CVD risk. Furthermore, fibrinogen was positively correlated with Lp(a) levels in Japanese and Whites and independently predicted levels (48, 62). Consistent with this finding, fibrinogen was also significantly associated with Lp(a) levels in older Italian subjects (60). An inflammatory score summarizing the intensity of the proinflammatory state based on four different biomarkers (CRP, fibrinogen, IL-6, and IL-1 receptor antagonist) was significantly correlated with Lp(a) concentration in this study. Among Spanish White subjects with metabolic syndrome, the CRP concentration was 2-fold greater in subjects with high Lp(a) concentrations (≥30 mg/dl) (108). In the latter group, many other inflammatory cytokines were elevated as well. In a recent study in the Copenhagen General Population Study and the Copenhagen City Heart Study participants, median Lp(a) concentrations were higher (21 mg/dl) in subjects with CRP levels >10 mg/l than among subjects with CRP levels <1 mg/l (18 mg/dl) (79). Collectively, findings to date suggest that the presence of a proinflammatory state may contribute to higher Lp(a) levels. At present, however, data on the extent to which systemic or vascular inflammation might affect Lp(a) concentrations across various ethnic/racial or geographical groups is scarce, and further studies are warranted to explore these associations. Overall, beyond underscoring an impact of inflammation on Lp(a) concentrations, these findings reinforce the concept that inflammation-associated events may contribute to the ethnic-specific or age-related differences in Lp(a) concentrations.
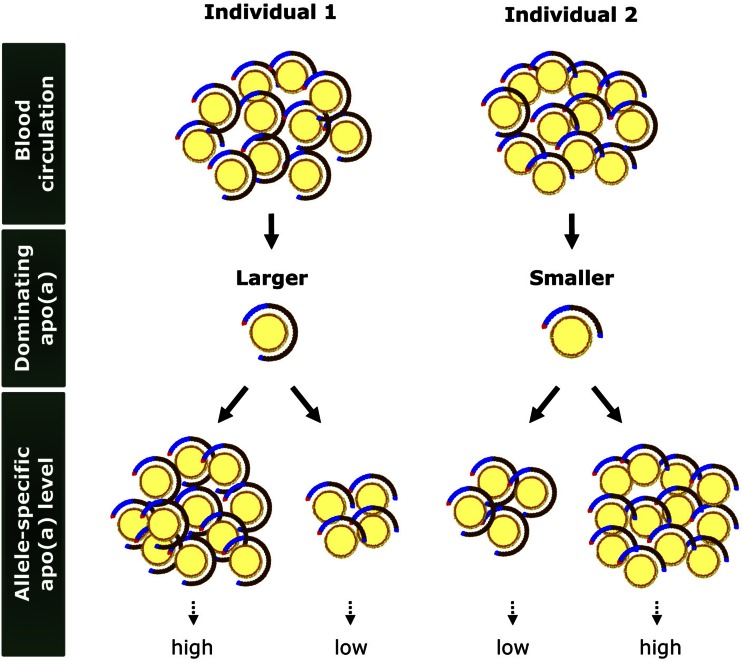
Fig. 3.
Lp(a) and allele-specific apo(a) levels. Due to a high heterozygosity index at the genetic level, the total plasma Lp(a) level represents two particle populations, one carrying smaller size apo(a) and the other carrying larger size apo(a), in the majority of individuals. Depending on the dominance pattern of apo(a), some individuals have higher allele-specific apo(a) levels with larger apo(a) sizes (Individual 1) and others have higher allele-specific apo(a) levels with smaller apo(a) sizes (Individual 2).
Menopause
Selby et al. (49) reported no association of menopausal status with Lp(a) concentration for either Whites or Blacks, but found a significantly lower Lp(a) concentration in postmenopausal women receiving hormone replacement therapy (HRT). A meta-analysis of studies conducted between 1966 and 2004 quantifying the effects of HRT in postmenopausal women reported an average of 25% reduction in Lp(a) levels (109). In a Japanese study, Lp(a) levels were significantly higher in postmenopausal than in premenopausal or perimenopausal women. After six months of HRT, Lp(a) levels decreased by 19% and remained stable for four years (110). Also, in the Framingham Offspring Study, the mean plasma Lp(a) concentration in postmenopausal subjects was higher than in premenopausal subjects, although no significant difference was found after adjustment for age (46). In the Women’s Health Study, Lp(a) concentrations were lower among women taking HRT versus those not taking HRT (111). In a recent double-blind placebo-controlled trial among postmenopausal women, treatment with letrozole, an oral nonsteroidal aromatase inhibitor, resulted in more than a doubling of mean Lp(a) levels (112). Treatment with tibolone, a synthetic steroid drug, at a dose of 2.5 mg/day for a year in postmenopausal women significantly decreased Lp(a) concentration by 28% (113).
Pregnancy
An early case report by Berg, Roald, and Sande (114) reporting on a woman with a high Lp(a) level that had given birth to three children with very low birth weights, suggested that Lp(a) may interfere with the placental circulation and cause fetal growth retardation. Subsequently, a high Lp(a) concentration was seen in a family with severe preeclampsia (115), focusing attention on the relation between Lp(a) and pregnancy. A longitudinal study reported an increase in Lp(a) levels during the first trimester, reaching its maximum in the middle of the second trimester, approximately 3-fold higher than levels at eight weeks, before returning to baseline levels at birth (116). Similar findings have been reported by others (117–123). However, other studies have not reported changes in Lp(a) levels during normal pregnancy (124–127). In pregnancies complicated with preeclampsia or hypertension, a significantly elevated Lp(a) concentration has been observed in some studies (128–136), but not consistently (120, 121, 137–143). Of note, the majority of these studies did not assess apo(a) isoform sizes. One longitudinal study reported no change in Lp(a) concentration during pregnancy in women carrying small apo(a) isoforms versus an increase in women carrying large apo(a) isoforms (119). In two studies, apo(a) isoform distribution was similar in women with preeclampsia and normal controls (137, 144).
MEDICAL CONDITIONS AND Lp(a) LEVELS
Kidney disease
Due to the strong genetic control of the LPA gene, Lp(a) levels remain unaffected by most clinical conditions. Kidney disease represents an exception as one of the few clinical conditions shown to impact Lp(a) levels, with increased levels reported in patients with nephrotic syndrome, as well as in end-stage renal disease (ESRD) or during dialysis treatment (145–156). In patients with nephrotic syndrome, a decrease in Lp(a) levels has been seen after remission of the syndrome or after antiproteinuric treatment (151–153, 155, 157). In a larger study, Kronenberg et al. (158) reported a more pronounced increase in Lp(a) among carriers of larger apo(a) sizes. Similarly in patients with mild and moderate nephrotic syndrome, an increase in Lp(a) concentration was seen among carriers of large, but not small, apo(a) isoforms (159). Underlying reasons for the increase remain unresolved, but it has been proposed that a decreased plasma albumin level and reduced oncotic pressure may contribute (160). These results underscore the value of assessing Lp(a) concentrations contributed by particles carrying specific apo(a) sizes, i.e., allele-specific apo(a) levels (21, 105).
A large number of studies have investigated Lp(a) in ESRD patients (145, 161–171), and elevated Lp(a) levels are seen both in patients undergoing hemodialysis (HD) or continuous ambulatory peritoneal dialysis (145, 161–169). Further, a mild glomerular filtration rate impairment was associated with a higher Lp(a) level in a recent study of diabetic patients (172). In other studies, however, Lp(a) concentrations did not differ from those of controls (173–177). In a large multicenter study, Kronenberg et al. (145) reported higher Lp(a) levels in patients undergoing continuous ambulatory peritoneal dialysis compared with HD. Also in this case, patients with large apo(a) isoforms showed an elevation in Lp(a) levels. A similar apo(a) phenotype-specific elevation of Lp(a) was also found in some other studies in HD patients (178–181), but not uniformly (166, 182), raising the possibility that the differences observed could be due to sample size, use of different methodologies, or the definition of what constitutes small versus large apo(a) size. As for the nephrotic syndrome, the underlying pathogenic mechanism for the elevation of Lp(a) in ESRD is unknown. Increased hepatic synthesis has been suggested (183), but an impact on catabolism cannot be ruled out. Further studies are necessary to clarify whether the elevation of Lp(a) concentration and/or apo(a) isoform size contribute to elevated CVD risk in ESRD patients.
The influence of renal transplantation on Lp(a) concentrations has also been investigated and prospective studies generally demonstrate a decrease of Lp(a) levels following kidney transplantation (169, 184–193), although the follow-up period has been limited. In contrast, mixed results regarding Lp(a) have been reported from cross-sectional studies (163, 169, 174, 185, 194–204). Notably, the decrease of Lp(a) in renal transplant patients was independent of the modality of immunosuppressive therapy (169, 184–191), arguing against a contribution by an inflammatory component.
Liver disease
The liver plays a key role in lipid metabolism (205, 206). As plasma Lp(a) originates from the liver and the concentration of Lp(a) is mainly related to the hepatic apo(a) synthetic rate (17, 207, 208), pathophysiological processes affecting liver function have the potential to influence Lp(a) levels. In general, hepatocellular damage has been associated with reduced Lp(a) levels, where the decrease in levels has been in parallel with disease progression (209–212). Thus, patients with liver cirrhosis and hepatitis have lower Lp(a) concentrations compared with healthy controls (209–215). Geiss et al. (216) reported a 41% reduction in Lp(a) concentration in patients with acute hepatitis A, B, and C, and the decrease was independent of apo(a) isoform size. Notably, a significant increase in Lp(a) concentrations was seen in those patients with chronic active hepatitis C that responded completely to a 6 month interferon treatment regimen, raising the possibility of an inflammation-mediated effect or improved liver function (210). It has been suggested that a change in Lp(a) levels, together with ferritin and α-fetoprotein levels, could constitute a sensitive and early index of liver damage or an index of liver function (209, 210, 214, 217).
The effect of alcohol consumption on Lp(a) concentration has been investigated in a number of studies. In two studies, male alcohol drinkers exhibited reduced values of Lp(a) (218, 219). On the other hand, no significant differences in Lp(a) concentration between different types of alcohol consumption were found among healthy French men (220), in postmenopausal women (221), and in Spanish men and women (222). Two recent randomized controlled trials reported conflicting results on the effect of red wine on plasma Lp(a) concentrations. Thus, in one study Lp(a) decreased after regular daily ingestion of red wine (30 g alcohol/day) (223), while no effect of red wine was seen in another study (224).
Diabetes mellitus
To date, the role of Lp(a) in diabetes mellitus remains unclear. There are many contradictory reports in the literature regarding the role of Lp(a) in diabetes mellitus, while some studies found no impact of diabetes mellitus on Lp(a) concentrations, others reported an elevation or a decrease of Lp(a) concentrations. Early studies reported elevated Lp(a) concentrations in patients with type 1 diabetes mellitus (IDDM) (225–230), as well as in patients with type 2 diabetes mellitus (NIDDM) (231, 232). Furthermore, some recent studies in Asian populations report an association between an elevated Lp(a) concentration and incident NIDDM (233–235). Rainwater et al. (236) reported significantly lower Lp(a) concentrations in NIDDM patients compared with matched nondiabetic controls in the San Antonio Heart Study. An inverse relationship between Lp(a) concentrations and NIDDM has been reported, as well as a negative correlation between Lp(a) and triglyceride levels in diabetic patients, including IDDM and NIDDM (236–239). In a recent report from the ERIC-Norfolk study, a strong inverse correlation between the Lp(a) level and new-onset NIDDM was observed (240). However, a genetic variant associated with an elevated Lp(a) level (i.e., rs10455872) was not associated with the risk of NIDDM, suggesting that elevated Lp(a) levels were not causally related to a lower risk of diabetes. On the other hand, a number of large case-control studies report similar Lp(a) concentrations in patients with IDDM (241–243) or NIDDM (242, 244, 245) compared with controls.
In a prospective Women’s Health Study of healthy women with a 13 year follow up, Mora et al. (246) found an inverse association of Lp(a) concentration with risk of NIDDM with a 20–50% lower relative risk in quintiles 2–5 compared with quintile 1. A similar inverse association was reported from a general population of Danish men and women (246). The pathophysiological mechanism that may underlie the role of Lp(a) in diabetes remains unclear. Rainwater and Haffner (247) reported that Lp(a) concentrations were inversely correlated with insulin and 2 h glucose levels in both diabetics and nondiabetics. A recent in vitro study demonstrated that insulin suppresses apo(a) production in primary cynomolgus monkey hepatocytes, which may account for the lower Lp(a) levels found in NIDDM (248).
Regarding the relationship between apo(a) isoforms and diabetes, one study has reported comparable distributions of apo(a) sizes for patients with NIDDM and controls (249). In contrast, several studies reported a higher prevalence of low molecular weight isoforms in NIDDM (250–252). Findings to date underscore that larger studies using more defined populations are required to better understand the relationship of Lp(a) concentrations and apo(a) phenotypes with diabetes mellitus.
CONCLUSIONS
Much progress has recently been made in understanding the genetic regulation of Lp(a) and the role of genetic variability predicting Lp(a) levels in different ethnic groups. Many studies have confirmed ethnic differences in Lp(a) levels where subjects of African descent have about twice as high levels as Caucasians, Hispanics, and many Asian populations, while intermediate levels are reported for South Asians. This interesting, but so far unresolved, variability might provide a possibility to assess any potential evolutionary advantage associated with Lp(a); however, as suggested in previous studies, the observed interethnic difference could also be due to the apo(a) allele distribution in the subset of the population that presumably left Africa and subsequently gave rise to other population groups. Initially, few, if any, environmental conditions were found to impact Lp(a) levels, considered as stable over the lifespan in any given individual. Although more recent studies support an impact by inflammation and some chronic disease conditions (Fig. 4), this fascinating lipoprotein still presents many challenges to be unlocked. During the last few years, advances have been made in the development of specific therapeutic options to lower Lp(a) levels. This has opened opportunities to carefully assess the role of Lp(a) in promoting CVD across ethnic/racial populations with differences in Lp(a) levels, as well as in a range of clinical conditions. In particular, the ability to lower Lp(a) levels by up to 78% with a new antisense drug designed to reduce hepatic apo(a) synthesis (253) offers possibilities to assess a role of Lp(a) in a variety of disorders, such as liver and kidney disease.
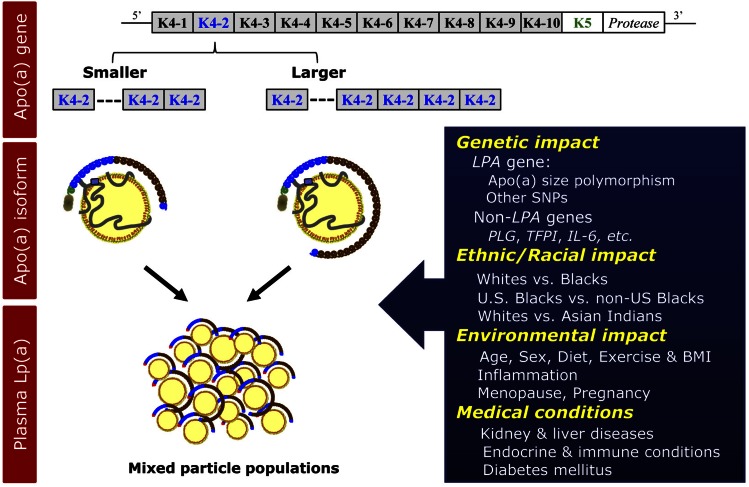
Fig. 4.
Regulation of plasma Lp(a) levels. Lp(a) levels are primarily regulated by the apo(a) gene size polymorphism, i.e., copy number of KIV repeats. In addition, recent evidence suggests a role for other SNPs in the LPA, as well as non-LPA genes in the regulation of plasma Lp(a) levels. As noted in the Fig. 3, in the majority of individuals, two different populations of Lp(a) particles carrying different-sized apo(a) contribute to the overall plasma Lp(a) level. Lp(a) levels differ substantially between various ethnic/racial groups with higher levels in Blacks than in Whites. Evidence also supports a modulatory effect of age, sex, and hormones, although with a modest size on plasma Lp(a) levels. Among clinical conditions, Lp(a) levels are reported to be affected by kidney and liver diseases. K, kringle.
Footnotes
Abbreviations:
- CAD
- coronary artery disease
- CRP
- C-reactive protein
- ESRD
- end-stage renal disease
- HD
- hemodialysis
- HRT
- hormone replacement therapy
- IDDM
- type 1 diabetes mellitus
- IL
- interleukin, KIV, kringle IV
- LD
- linkage disequilibrium
- Lp(a)
- lipoprotein (a)
- NIDDM
- type 2 diabetes mellitus
- NIH
- National Institutes of Health
- OxPL
- oxidized phospholipid
- PNR
- pentanucleotide repeat
This study was supported in part by Grant 62705 (L.B.) from the National Institutes of Health (NIH), National Heart, Lung, and Blood Institute and the University of California, Davis, NIH-funded Clinical and Translational Science Center (Grants TR000002 and RR024146). B.E. is a recipient of the University of California, Davis, Clinical and Translational Science Center Highly Innovative Pilot Award and the Mentored Clinical and Population Research Program Award from the American Heart Association (14CRP17930014), and a current Building Interdisciplinary Research Careers in Women’s Health/K12 Training Program scholar (NIH Grant 2K12HD051958). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Dahlén G., Berg K., Gillnäs T., and Ericson C.. 1975. Lp(a) lipoprotein/pre-beta1-lipoprotein in Swedish middle-aged males and in patients with coronary heart disease. Clin. Genet. 7: 334–341. [DOI] [PubMed] [Google Scholar]
- 2.Frick M. H., Dahlen G., Berg K., Valle M., and Hekali P.. 1978. Serum lipids in angiographically assessed coronary atherosclerosis. Chest. 73: 62–65. [DOI] [PubMed] [Google Scholar]
- 3.Berg K., Dahlen G., and Borresen A. L.. 1979. Lp(a) phenotypes, other lipoprotein parameters, and a family history of coronary heart disease in middle-aged males. Clin. Genet. 16: 347–352. [DOI] [PubMed] [Google Scholar]
- 4.Kostner G. M., Avogaro P., Cazzolato G., Marth E., Bittolo-Bon G., and Qunici G. B.. 1981. Lipoprotein Lp(a) and the risk for myocardial infarction. Atherosclerosis. 38: 51–61. [DOI] [PubMed] [Google Scholar]
- 5.Guyton J. R., Dahlen G. H., Patsch W., Kautz J. A., and Gotto A. M. Jr. 1985. Relationship of plasma lipoprotein Lp(a) levels to race and to apolipoprotein B. Arteriosclerosis. 5: 265–272. [DOI] [PubMed] [Google Scholar]
- 6.Parra H. J., Luyeye I., Bouramoue C., Demarquilly C., and Fruchart J. C.. 1987. Black-white differences in serum Lp(a) lipoprotein levels. Clin. Chim. Acta. 168: 27–31. [DOI] [PubMed] [Google Scholar]
- 7.Utermann G. 1989. The mysteries of lipoprotein(a). Science. 246: 904–910. [DOI] [PubMed] [Google Scholar]
- 8.Marcovina S. M., Albers J. J., Wijsman E., Zhang Z., Chapman N. H., and Kennedy H.. 1996. Differences in Lp[a] concentrations and apo[a] polymorphs between black and white Americans. J. Lipid Res. 37: 2569–2585. [PubMed] [Google Scholar]
- 9.Banerjee D., Wong E. C., Shin J., Fortmann S. P., and Palaniappan L.. 2011. Racial and ethnic variation in lipoprotein (a) levels among Asian Indian and Chinese patients. J. Lipids. 2011: 291954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandholzer C., Hallman D. M., Saha N., Sigurdsson G., Lackner C., Csaszar A., Boerwinkle E., and Utermann G.. 1991. Effects of the apolipoprotein(a) size polymorphism on the lipoprotein(a) concentration in 7 ethnic groups. Hum. Genet. 86: 607–614. [DOI] [PubMed] [Google Scholar]
- 11.Helmhold M., Bigge J., Muche R., Mainoo J., Thiery J., Seidel D., and Armstrong V. W.. 1991. Contribution of the apo[a] phenotype to plasma Lp[a] concentrations shows considerable ethnic variation. J. Lipid Res. 32: 1919–1928. [PubMed] [Google Scholar]
- 12.Srinivasan S. R., Dahlen G. H., Jarpa R. A., Webber L. S., and Berenson G. S.. 1991. Racial (black-white) differences in serum lipoprotein (a) distribution and its relation to parental myocardial infarction in children. Bogalusa Heart Study. Circulation. 84: 160–167. [DOI] [PubMed] [Google Scholar]
- 13.Knapp R. G., Schreiner P. J., Sutherland S. E., Keil J. E., Gilbert G. E., Klein R. L., Hames C., and Tyroler H. A.. 1993. Serum lipoprotein(a) levels in elderly black and white men in the Charleston Heart Study. Clin. Genet. 44: 225–231. [DOI] [PubMed] [Google Scholar]
- 14.Haffner S. M., Gruber K. K., Morales P. A., Hazuda H. P., Valdez R. A., Mitchell B. D., and Stern M. P.. 1992. Lipoprotein(a) concentrations in Mexican Americans and non-Hispanic whites: the San Antonio Heart Study. Am. J. Epidemiol. 136: 1060–1068. [DOI] [PubMed] [Google Scholar]
- 15.Guan W., Cao J., Steffen B. T., Post W. S., Stein J. H., Tattersall M. C., Kaufman J. D., McConnell J. P., Hoefner D. M., Warnick R., et al. 2015. Race is a key variable in assigning lipoprotein(a) cutoff values for coronary heart disease risk assessment: the Multi-Ethnic Study of Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 35: 996–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McLean J. W., Tomlinson J. E., Kuang W. J., Eaton D. L., Chen E. Y., Fless G. M., Scanu A. M., and Lawn R. M.. 1987. cDNA sequence of human apolipoprotein(a) is homologous to plasminogen. Nature. 330: 132–137. [DOI] [PubMed] [Google Scholar]
- 17.Berglund L., and Ramakrishnan R.. 2004. Lipoprotein(a): an elusive cardiovascular risk factor. Arterioscler. Thromb. Vasc. Biol. 24: 2219–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lackner C., Boerwinkle E., Leffert C. C., Rahmig T., and Hobbs H. H.. 1991. Molecular basis of apolipoprotein (a) isoform size heterogeneity as revealed by pulsed-field gel electrophoresis. J. Clin. Invest. 87: 2153–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lackner C., Cohen J. C., and Hobbs H. H.. 1993. Molecular definition of the extreme size polymorphism in apolipoprotein(a). Hum. Mol. Genet. 2: 933–940. [DOI] [PubMed] [Google Scholar]
- 20.Marcovina S. M., Zhang Z. H., Gaur V. P., and Albers J. J.. 1993. Identification of 34 apolipoprotein(a) isoforms: differential expression of apolipoprotein(a) alleles between American blacks and whites. Biochem. Biophys. Res. Commun. 191: 1192–1196. [DOI] [PubMed] [Google Scholar]
- 21.Rubin J., Paultre F., Tuck C. H., Holleran S., Reed R. G., Pearson T. A., Thomas C. M., Ramakrishnan R., and Berglund L.. 2002. Apolipoprotein [a] genotype influences isoform dominance pattern differently in African Americans and Caucasians. J. Lipid Res. 43: 234–244. [PubMed] [Google Scholar]
- 22.Marcovina S. M., Albers J. J., Gabel B., Koschinsky M. L., and Gaur V. P.. 1995. Effect of the number of apolipoprotein(a) kringle 4 domains on immunochemical measurements of lipoprotein(a). Clin. Chem. 41: 246–255. [PubMed] [Google Scholar]
- 23.Boerwinkle E., Leffert C. C., Lin J., Lackner C., Chiesa G., and Hobbs H. H.. 1992. Apolipoprotein(a) gene accounts for greater than 90% of the variation in plasma lipoprotein(a) concentrations. J. Clin. Invest. 90: 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rao F., Schork A. J., Maihofer A. X., Nievergelt C. M., Marcovina S. M., Miller E. R., Witztum J. L., O’Connor D. T., and Tsimikas S.. 2015. Heritability of biomarkers of oxidized lipoproteins: twin pair study. Arterioscler. Thromb. Vasc. Biol. 35: 1704–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scholz M., Kraft H. G., Lingenhel A., Delport R., Vorster E. H., Bickeboller H., and Utermann G.. 1999. Genetic control of lipoprotein(a) concentrations is different in Africans and Caucasians. Eur. J. Hum. Genet. 7: 169–178. [DOI] [PubMed] [Google Scholar]
- 26.Chretien J. P., Coresh J., Berthier-Schaad Y., Kao W. H., Fink N. E., Klag M. J., Marcovina S. M., Giaculli F., and Smith M. W.. 2006. Three single-nucleotide polymorphisms in LPA account for most of the increase in lipoprotein(a) level elevation in African Americans compared with European Americans. J. Med. Genet. 43: 917–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trommsdorff M., Kochl S., Lingenhel A., Kronenberg F., Delport R., Vermaak H., Lemming L., Klausen I. C., Faergeman O., Utermann G., et al. 1995. A pentanucleotide repeat polymorphism in the 5′ control region of the apolipoprotein(a) gene is associated with lipoprotein(a) plasma concentrations in Caucasians. J. Clin. Invest. 96: 150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubin J., Kim H. J., Pearson T. A., Holleran S., Ramakrishnan R., and Berglund L.. 2006. Apo[a] size and PNR explain African American-Caucasian differences in allele-specific apo[a] levels for small but not large apo. [a] J. Lipid Res. 47: 982–989. [DOI] [PubMed] [Google Scholar]
- 29.Lanktree M. B., Anand S. S., Yusuf S., and Hegele R. A.. 2010. Comprehensive analysis of genomic variation in the LPA locus and its relationship to plasma lipoprotein(a) in South Asians, Chinese, and European Caucasians. Circ Cardiovasc Genet. 3: 39–46. [DOI] [PubMed] [Google Scholar]
- 30.Luke M. M., Kane J. P., Liu D. M., Rowland C. M., Shiffman D., Cassano J., Catanese J. J., Pullinger C. R., Leong D. U., Arellano A. R., et al. 2007. A polymorphism in the protease-like domain of apolipoprotein(a) is associated with severe coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 27: 2030–2036. [DOI] [PubMed] [Google Scholar]
- 31.Clarke R., Peden J. F., Hopewell J. C., Kyriakou T., Goel A., Heath S. C., Parish S., Barlera S., Franzosi M. G., Rust S., et al. 2009. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N. Engl. J. Med. 361: 2518–2528. [DOI] [PubMed] [Google Scholar]
- 32.Ronald J., Rajagopalan R., Cerrato F., Nord A. S., Hatsukami T., Kohler T., Marcovina S., Heagerty P., and Jarvik G. P.. 2011. Genetic variation in LPAL2, LPA, and PLG predicts plasma lipoprotein(a) level and carotid artery disease risk. Stroke. 42: 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zabaneh D., Kumari M., Sandhu M., Wareham N., Wainwright N., Papamarkou T., Hopewell J., Clarke R., Li K., Palmen J., et al. 2011. Meta analysis of candidate gene variants outside the LPA locus with Lp(a) plasma levels in 14,500 participants of six White European cohorts. Atherosclerosis. 217: 447–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thanassoulis G., Campbell C. Y., Owens D. S., Smith J. G., Smith A. V., Peloso G. M., Kerr K. F., Pechlivanis S., Budoff M. J., Harris T. B., et al. 2013. Genetic associations with valvular calcification and aortic stenosis. N. Engl. J. Med. 368: 503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dumitrescu L., Glenn K., Brown-Gentry K., Shephard C., Wong M., Rieder M. J., Smith J. D., Nickerson D. A., and Crawford D. C.. 2011. Variation in LPA is associated with Lp(a) levels in three populations from the Third National Health and Nutrition Examination Survey. PLoS One. 6: e16604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schadt E. E., Molony C., Chudin E., Hao K., Yang X., Lum P. Y., Kasarskis A., Zhang B., Wang S., Suver C., et al. 2008. Mapping the genetic architecture of gene expression in human liver. PLoS Biol. 6: e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu W., Cheng Y. C., Chen K., Wang H., Gerhard G. S., Still C. D., Chu X., Yang R., Parihar A., O’Connell J. R., et al. 2015. Evidence for several independent genetic variants affecting lipoprotein (a) cholesterol levels. Hum. Mol. Genet. 24: 2390–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ober C., Nord A. S., Thompson E. E., Pan L., Tan Z., Cusanovich D., Sun Y., Nicolae R., Edelstein C., Schneider D. H., et al. 2009. Genome-wide association study of plasma lipoprotein(a) levels identifies multiple genes on chromosome 6q. J. Lipid Res. 50: 798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Newman D. L., Hoffjan S., Bourgain C., Abney M., Nicolae R. I., Profits E. T., Grow M. A., Walker K., Steiner L., Parry R., et al. 2004. Are common disease susceptibility alleles the same in outbred and founder populations? Eur. J. Hum. Genet. 12: 584–590. [DOI] [PubMed] [Google Scholar]
- 40.Deo R. C., Wilson J. G., Xing C., Lawson K., Kao W. H., Reich D., Tandon A., Akylbekova E., Patterson N., Mosley T. H. Jr., et al. 2011. Single-nucleotide polymorphisms in LPA explain most of the ancestry-specific variation in Lp(a) levels in African Americans. PLoS One. 6: e14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kivimäki M., Magnussen C. G., Juonala M., Kähönen M., Kettunen J., Loo B. M., Lehtimäki T., Viikari J., and Raitakari O. T.. 2011. Conventional and Mendelian randomization analyses suggest no association between lipoprotein(a) and early atherosclerosis: the Young Finns Study. Int. J. Epidemiol. 40: 470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qi Q., Workalemahu T., Zhang C., Hu F. B., and Qi L.. 2012. Genetic variants, plasma lipoprotein(a) levels, and risk of cardiovascular morbidity and mortality among two prospective cohorts of type 2 diabetes. Eur. Heart J. 33: 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.López S., Buil A., Ordoñez J., Souto J. C., Almasy L., Lathrop M., Blangero J., Blanco-Vaca F., Fontcuberta J., and Soria J. M.. 2008. Genome-wide linkage analysis for identifying quantitative trait loci involved in the regulation of lipoprotein a (Lpa) levels. Eur. J. Hum. Genet. 16: 1372–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berthold H. K., Laudes M., Krone W., and Gouni-Berthold I.. 2011. Association between the interleukin-6 promoter polymorphism -174G/C and serum lipoprotein(a) concentrations in humans. PLoS One. 6: e24719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Labeur C., Michiels G., Bury J., Usher D. C., and Rosseneu M.. 1989. Lipoprotein(a) quantified by an enzyme-linked immunosorbent assay with monoclonal antibodies. Clin. Chem. 35: 1380–1384. [PubMed] [Google Scholar]
- 46.Jenner J. L., Ordovas J. M., Lamon-Fava S., Schaefer M. M., Wilson P. W., Castelli W. P., and Schaefer E. J.. 1993. Effects of age, sex, and menopausal status on plasma lipoprotein(a) levels. The Framingham Offspring Study. Circulation. 87: 1135–1141. [DOI] [PubMed] [Google Scholar]
- 47.Bovet P., Rickenbach M., Wietlisbach V., Riesen W., Shamlaye C., Darioli R., and Burnand B.. 1994. Comparison of serum lipoprotein(a) distribution and its correlates among black and white populations. Int. J. Epidemiol. 23: 20–27. [DOI] [PubMed] [Google Scholar]
- 48.Slunga L., Asplund K., Johnson O., and Dahlen G. H.. 1993. Lipoprotein (a) in a randomly selected 25-64 year old population: the Northern Sweden Monica Study. J. Clin. Epidemiol. 46: 617–624. [DOI] [PubMed] [Google Scholar]
- 49.Selby J. V., Austin M. A., Sandholzer C., Quesenberry C. P. Jr., Zhang D., Mayer E., and Utermann G.. 1994. Environmental and behavioral influences on plasma lipoprotein(a) concentration in women twins. Prev. Med. 23: 345–353. [DOI] [PubMed] [Google Scholar]
- 50.Berg K., and Ro O. C.. 1991. Lp(a) lipoprotein level and longevity. Ann. Genet. 34: 264–269. [PubMed] [Google Scholar]
- 51.Thillet J., Doucet C., Chapman J., Herbeth B., Cohen D., and Faure-Delanef L.. 1998. Elevated lipoprotein(a) levels and small apo(a) isoforms are compatible with longevity: evidence from a large population of French centenarians. Atherosclerosis. 136: 389–394. [DOI] [PubMed] [Google Scholar]
- 52.Matsubara M., Akita H., Shibuya H., and Chiba H.. 2001. Decreased longevity in Japanese men, associated with low-molecular-weight apolipoprotein(a) phenotypes. Ann. Clin. Biochem. 38: 120–123. [DOI] [PubMed] [Google Scholar]
- 53.Panza F., D’Introno A., Capurso C., Colacicco A. M., Seripa D., Pilotto A., Santamato A., Capurso A., and Solfrizzi V.. 2007. Lipoproteins, vascular-related genetic factors, and human longevity. Rejuvenation Res. 10: 441–458. [DOI] [PubMed] [Google Scholar]
- 54.Lippi G., Targher G., Salvagno G. L., Montagnana M., Franchini M., and Guidi G. C.. 2010. Lipoprotein(a) and ageing. Clin. Lab. 56: 463–466. [PubMed] [Google Scholar]
- 55.Gaw A., Docherty G., Brown E. A., and Ford I.. 1999. Predictors of plasma lipoprotein(a) concentration in the West of Scotland Coronary Prevention Study cohort. Atherosclerosis. 143: 445–450. [DOI] [PubMed] [Google Scholar]
- 56.Akanji A. O., Al-Isa A. N., and Thalib L.. 2011. Determinants of blood levels of some thrombogenic biomarkers in healthy Arab adolescent subjects. Clin. Chem. Lab. Med. 49: 1681–1690. [DOI] [PubMed] [Google Scholar]
- 57.Zlatohlávek L., Zidková K., Vrablik M., Haas T., Prusiková M., Svobodová H., and Ceska R.. 2008. Lipoprotein(a) and its position among other risk factors of atherosclerosis. Physiol. Res. 57: 777–783. [DOI] [PubMed] [Google Scholar]
- 58.Albers J. J., and Hazzard W. R.. 1974. Immunochemical quantification of human plasma Lp(a) lipoprotein. Lipids. 9: 15–26. [DOI] [PubMed] [Google Scholar]
- 59.Cobbaert C., and Kesteloot H.. 1992. Serum lipoprotein(a) levels in racially different populations. Am. J. Epidemiol. 136: 441–449. [DOI] [PubMed] [Google Scholar]
- 60.Volpato S., Vigna G. B., McDermott M. M., Cavalieri M., Maraldi C., Lauretani F., Bandinelli S., Zuliani G., Guralnik J. M., Fellin R., et al. 2010. Lipoprotein(a), inflammation, and peripheral arterial disease in a community-based sample of older men and women (the InCHIANTI study). Am. J. Cardiol. 105: 1825–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Császár A., Romics L., Lackner C., Dieplinger H., Karádi I., and Utermann G.. 1990. Plasma concentration of lipoprotein(a) and distribution of its subtypes in the healthy population of Hungary [Article in Hungarian]. Orv. Hetil. 131: 2071–2075. [PubMed] [Google Scholar]
- 62.Nago N., Kayaba K., Hiraoka J., Matsuo H., Goto T., Kario K., Tsutsumi A., Nakamura Y., and Igarashi M.. 1995. Lipoprotein(a) levels in the Japanese population: influence of age and sex, and relation to atherosclerotic risk factors. The Jichi Medical School Cohort Study. Am. J. Epidemiol. 141: 815–821. [DOI] [PubMed] [Google Scholar]
- 63.Barra S., Cuomo V., Silvestri N., Materazzi C., Vitagliano G., Capozzi G., Caruso S., Gaeta G., and Trevisan M.. 2011. Lipoprotein(a) concentration does not differ between sexes in healthy offspring of patients with premature myocardial infarction. J. Cardiovasc. Med. (Hagerstown). 12: 482–486. [DOI] [PubMed] [Google Scholar]
- 64.Frohlich J., Dobiasova M., Adler L., and Francis M.. 2004. Gender differences in plasma levels of lipoprotein (a) in patients with angiographically proven coronary artery disease. Physiol. Res. 53: 481–486. [PubMed] [Google Scholar]
- 65.Allard M. D., Saeedi R., Yousefi M., and Frohlich J.. 2014. Risk stratification of patients with familial hypercholesterolemia in a multi-ethnic cohort. Lipids Health Dis. 13: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nenseter M. S., Lindvig H. W., Ueland T., Langslet G., Ose L., Holven K. B., and Retterstol K.. 2011. Lipoprotein(a) levels in coronary heart disease-susceptible and -resistant patients with familial hypercholesterolemia. Atherosclerosis. 216: 426–432. [DOI] [PubMed] [Google Scholar]
- 67.Masarei J. R., Rouse I. L., Lynch W. J., Robertson K., Vandongen R., and Beilin L. J.. 1984. Effects of a lacto-ovo vegetarian diet on serum concentrations of cholesterol, triglyceride, HDL-C, HDL2-C, HDL3-C, apoprotein-B, and Lp(a). Am. J. Clin. Nutr. 40: 468–478. [DOI] [PubMed] [Google Scholar]
- 68.Brown S. A., Morrisett J., Patsch J. R., Reeves R., Gotto A. M. Jr., and Patsch W.. 1991. Influence of short term dietary cholesterol and fat on human plasma Lp[a] and LDL levels. J. Lipid Res. 32: 1281–1289. [PubMed] [Google Scholar]
- 69.Berglund L. 1995. Diet and drug therapy for lipoprotein (a). Curr. Opin. Lipidol. 6: 48–56. [DOI] [PubMed] [Google Scholar]
- 70.Mensink R. P., Zock P. L., Katan M. B., and Hornstra G.. 1992. Effect of dietary cis and trans fatty acids on serum lipoprotein[a] levels in humans. J. Lipid Res. 33: 1493–1501. [PubMed] [Google Scholar]
- 71.Nestel P., Noakes M., Belling B., McArthur R., Clifton P., Janus E., and Abbey M.. 1992. Plasma lipoprotein lipid and Lp[a] changes with substitution of elaidic acid for oleic acid in the diet. J. Lipid Res. 33: 1029–1036. [PubMed] [Google Scholar]
- 72.Ginsberg H. N., Kris-Etherton P., Dennis B., Elmer P. J., Ershow A., Lefevre M., Pearson T., Roheim P., Ramakrishnan R., Reed R., et al. 1998. Effects of reducing dietary saturated fatty acids on plasma lipids and lipoproteins in healthy subjects: the DELTA Study, protocol 1. Arterioscler. Thromb. Vasc. Biol. 18: 441–449. [DOI] [PubMed] [Google Scholar]
- 73.Silaste M. L., Rantala M., Alfthan G., Aro A., Witztum J. L., Kesaniemi Y. A., and Horkko S.. 2004. Changes in dietary fat intake alter plasma levels of oxidized low-density lipoprotein and lipoprotein(a). Arterioscler. Thromb. Vasc. Biol. 24: 498–503. [DOI] [PubMed] [Google Scholar]
- 74.Brousseau M. E., Ordovas J. M., Nicolosi R. J., and Schaefer E. J.. 1994. Effects of dietary fat saturation on plasma lipoprotein(a) and hepatic apolipoprotein(a) mRNA concentrations in cynomolgus monkeys. Atherosclerosis. 106: 109–118. [DOI] [PubMed] [Google Scholar]
- 75.Vega-López S., Matthan N. R., Ausman L. M., Ai M., Otokozawa S., Schaefer E. J., and Lichtenstein A. H.. 2009. Substitution of vegetable oil for a partially-hydrogenated fat favorably alters cardiovascular disease risk factors in moderately hypercholesterolemic postmenopausal women. Atherosclerosis. 207: 208–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Faghihnia N., Tsimikas S., Miller E. R., Witztum J. L., and Krauss R. M.. 2010. Changes in lipoprotein(a), oxidized phospholipids, and LDL subclasses with a low-fat high-carbohydrate diet. J. Lipid Res. 51: 3324–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Faraj M., Lavoie M. E., Messier L., Bastard J. P., and Prud’homme D.. 2010. Reduction in serum apoB is associated with reduced inflammation and insulin resistance in post-menopausal women: A MONET study. Atherosclerosis. 211: 682–688. [DOI] [PubMed] [Google Scholar]
- 78.Haring B., von Ballmoos M. C., Appel L. J., and Sacks F. M.. 2014. Healthy dietary interventions and lipoprotein (a) plasma levels: results from the Omni Heart Trial. PLoS One. 9: e114859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Langsted A., Kamstrup P. R., and Nordestgaard B. G.. 2014. Lipoprotein(a): Fasting and nonfasting levels, inflammation, and cardiovascular risk. Atherosclerosis. 234: 95–101. [DOI] [PubMed] [Google Scholar]
- 80.Lamon-Fava S., Jimenez D., Christian J. C., Fabsitz R. R., Reed T., Carmelli D., Castelli W. P., Ordovas J. M., Wilson P. W., and Schaefer E. J.. 1991. The NHLBI Twin Study: heritability of apolipoprotein A-I, B, and low density lipoprotein subclasses and concordance for lipoprotein(a). Atherosclerosis. 91: 97–106. [DOI] [PubMed] [Google Scholar]
- 81.MacAuley D., McCrum E. E., Stott G., Evans A. E., Duly E., Trinick T., Sweeney K., and Boreham C. A.. 1996. Physical activity, lipids, apolipoproteins, and Lp(a) in the Northern Ireland Health and Activity Survey. Med. Sci. Sports Exerc. 28: 720–736. [DOI] [PubMed] [Google Scholar]
- 82.Israel R. G., Sullivan M. J., Marks R. H., Cayton R. S., and Chenier T. C.. 1994. Relationship between cardiorespiratory fitness and lipoprotein(a) in men and women. Med. Sci. Sports Exerc. 26: 425–431. [PubMed] [Google Scholar]
- 83.Hubinger L., Mackinnon L. T., and Lepre F.. 1995. Lipoprotein(a) [Lp(a)] levels in middle-aged male runners and sedentary controls. Med. Sci. Sports Exerc. 27: 490–496. [PubMed] [Google Scholar]
- 84.Szymanski L. M., Durstine J. L., Davis P. G., Dowda M., and Pate R. R.. 1996. Factors affecting fibrinolytic potential: cardiovascular fitness, body composition, and lipoprotein(a). Metabolism. 45: 1427–1433. [DOI] [PubMed] [Google Scholar]
- 85.Halle M., Berg A., von Stein T., Baumstark M. W., Konig D., and Keul J.. 1996. Lipoprotein(a) in endurance athletes, power athletes, and sedentary controls. Med. Sci. Sports Exerc. 28: 962–966. [DOI] [PubMed] [Google Scholar]
- 86.Oyelola O. O., and Rufai M. A.. 1993. Plasma lipid, lipoprotein and apolipoprotein profiles in Nigerian university athletes and non-athletes. Br. J. Sports Med. 27: 271–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Taimela S., Viikari J. S., Porkka K. V., and Dahlen G. H.. 1994. Lipoprotein (a) levels in children and young adults: the influence of physical activity. The Cardiovascular Risk in Young Finns Study. Acta Paediatr. 83: 1258–1263. [DOI] [PubMed] [Google Scholar]
- 88.Austin A., Warty V., Janosky J., and Arslanian S.. 1993. The relationship of physical fitness to lipid and lipoprotein(a) levels in adolescents with IDDM. Diabetes Care. 16: 421–425. [DOI] [PubMed] [Google Scholar]
- 89.Cardoso G. C., Posadas C., Orvananos O. O., Peniche C., Zamora J., Aguilar R., Holguin J. A., Raynaud A. S., Morrisett J. D., and Guevara J. Jr. 1994. Long distance runners and body-builders exhibit elevated plasma levels of lipoprotein(a). Chem. Phys. Lipids. 67–68: 207–221. [DOI] [PubMed] [Google Scholar]
- 90.Cohen L. I., Hartford C. G., and Rogers G. G.. 1996. Lipoprotein (a) and cholesterol in body builders using anabolic androgenic steroids. Med. Sci. Sports Exerc. 28: 176–179. [DOI] [PubMed] [Google Scholar]
- 91.Hellsten G., Boman K., Hallmans G., and Dahlen G.. 1989. Lipids and endurance physical activity. Atherosclerosis. 75: 93–94. [DOI] [PubMed] [Google Scholar]
- 92.Hubinger L., and Mackinnon L. T.. 1996. The effect of endurance training on lipoprotein(a) [Lp(a)] levels in middle-aged males. Med. Sci. Sports Exerc. 28: 757–764. [DOI] [PubMed] [Google Scholar]
- 93.Lobo R. A., Notelovitz M., Bernstein L., Khan F. Y., Ross R. K., and Paul W. L.. 1992. Lp(a) lipoprotein: relationship to cardiovascular disease risk factors, exercise, and estrogen. Am. J. Obstet. Gynecol. 166: 1182–1188, discussion 1188–1190. [PubMed] [Google Scholar]
- 94.Haskell W. L., Alderman E. L., Fair J. M., Maron D. J., Mackey S. F., Superko H. R., Williams P. T., Johnstone I. M., Champagne M. A., Krauss R. M., et al. 1994. Effects of intensive multiple risk factor reduction on coronary atherosclerosis and clinical cardiac events in men and women with coronary artery disease. The Stanford Coronary Risk Intervention Project (SCRIP). Circulation. 89: 975–990. [DOI] [PubMed] [Google Scholar]
- 95.Danesh J., Wheeler J. G., Hirschfield G. M., Eda S., Eiriksdottir G., Rumley A., Lowe G. D., Pepys M. B., and Gudnason V.. 2004. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N. Engl. J. Med. 350: 1387–1397. [DOI] [PubMed] [Google Scholar]
- 96.Danesh J., Lewington S., Thompson S. G., Lowe G. D., Collins R., Kostis J. B., Wilson A. C., Folsom A. R., Wu K., Benderly M., et al. 2005. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA. 294: 1799–1809. [DOI] [PubMed] [Google Scholar]
- 97.Uhlar C. M., and Whitehead A. S.. 1999. Serum amyloid A, the major vertebrate acute-phase reactant. Eur. J. Biochem. 265: 501–523. [DOI] [PubMed] [Google Scholar]
- 98.Rolph M. S., Zimmer S., Bottazzi B., Garlanda C., Mantovani A., and Hansson G. K.. 2002. Production of the long pentraxin PTX3 in advanced atherosclerotic plaques. Arterioscler. Thromb. Vasc. Biol. 22: e10–e14. [DOI] [PubMed] [Google Scholar]
- 99.Lavi S., McConnell J. P., Rihal C. S., Prasad A., Mathew V., Lerman L. O., and Lerman A.. 2007. Local production of lipoprotein-associated phospholipase A2 and lysophosphatidylcholine in the coronary circulation: association with early coronary atherosclerosis and endothelial dysfunction in humans. Circulation. 115: 2715–2721. [DOI] [PubMed] [Google Scholar]
- 100.Klezovitch O., Edelstein C., and Scanu A. M.. 2001. Stimulation of interleukin-8 production in human THP-1 macrophages by apolipoprotein(a). Evidence for a critical involvement of elements in its C-terminal domain. J. Biol. Chem. 276: 46864–46869. [DOI] [PubMed] [Google Scholar]
- 101.Ramharack R., Barkalow D., and Spahr M. A.. 1998. Dominant negative effect of TGF-beta1 and TNF-alpha on basal and IL-6-induced lipoprotein(a) and apolipoprotein(a) mRNA expression in primary monkey hepatocyte cultures. Arterioscler. Thromb. Vasc. Biol. 18: 984–990. [DOI] [PubMed] [Google Scholar]
- 102.Müller N., Schulte D. M., Türk K., Freitag-Wolf S., Hampe J., Zeuner R., Schröder J. O., Gouni-Berthold I., Berthold H. K., Krone W., et al. 2015. IL-6 blockade by monoclonal antibodies inhibits apolipoprotein (a) expression and lipoprotein (a) synthesis in humans. J. Lipid Res. 56: 1034–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bergmark C., Dewan A., Orsoni A., Merki E., Miller E. R., Shin M. J., Binder C. J., Horkko S., Krauss R. M., Chapman M. J., et al. 2008. A novel function of lipoprotein [a] as a preferential carrier of oxidized phospholipids in human plasma. J. Lipid Res. 49: 2230–2239. [DOI] [PubMed] [Google Scholar]
- 104.Tsimikas S., and Witztum J. L.. 2008. The role of oxidized phospholipids in mediating lipoprotein(a) atherogenicity. Curr. Opin. Lipidol. 19: 369–377. [DOI] [PubMed] [Google Scholar]
- 105.Anuurad E., Rubin J., Chiem A., Tracy R. P., Pearson T. A., and Berglund L.. 2008. High levels of inflammatory biomarkers are associated with increased allele-specific apolipoprotein(a) levels in African-Americans. J. Clin. Endocrinol. Metab. 93: 1482–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Enkhmaa B., Anuurad E., Zhang W., Pearson T. A., and Berglund L.. 2010. Association of Lp-PLA(2) activity with allele-specific Lp(a) levels in a bi-ethnic population. Atherosclerosis. 211: 526–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Enkhmaa B., Anuurad E., Ozturk Z., Zhang W., Pearson T. A., and Berglund L.. 2011. Differential associations of serum amyloid A and pentraxin-3 with allele-specific lipoprotein(a) levels in African Americans and Caucasians. Transl. Res. 158: 92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Muñoz-Torrero J. F., Rivas D., Alonso R., Crespo L., Costo A., Roman M., Martín C., and Zamorano J.. 2012. Influence of lipoprotein (a) on inflammatory biomarkers in metabolic syndrome. South. Med. J. 105: 339–343. [DOI] [PubMed] [Google Scholar]
- 109.Salpeter S. R., Walsh J. M., Ormiston T. M., Greyber E., Buckley N. S., and Salpeter E. E.. 2006. Meta-analysis: effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes. Metab. 8: 538–554. [DOI] [PubMed] [Google Scholar]
- 110.Ushioda M., Makita K., Takamatsu K., Horiguchi F., and Aoki D.. 2006. Serum lipoprotein(a) dynamics before/after menopause and long-term effects of hormone replacement therapy on lipoprotein(a) levels in middle-aged and older Japanese women. Horm. Metab. Res. 38: 581–586. [DOI] [PubMed] [Google Scholar]
- 111.Suk Danik J., Rifai N., Buring J. E., and Ridker P. M.. 2008. Lipoprotein(a), hormone replacement therapy, and risk of future cardiovascular events. J. Am. Coll. Cardiol. 52: 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wasan K. M., Goss P. E., Pritchard P. H., Shepherd L., Tu D., and Ingle J. N.. 2012. Lipid concentrations in postmenopausal women on letrozole after 5 years of tamoxifen: an NCIC CTG MA.17 sub-study. Breast Cancer Res. Treat. 136: 769–776. [DOI] [PubMed] [Google Scholar]
- 113.Perrone G., Capri O., Galoppi P., Brunelli R., Bevilacqua E., Ceci F., Ciarla M. V., and Strom R.. 2009. Effects of either tibolone or continuous combined transdermal estradiol with medroxyprogesterone acetate on coagulatory factors and lipoprotein(a) in menopause. Gynecol. Obstet. Invest. 68: 33–39. [DOI] [PubMed] [Google Scholar]
- 114.Berg K., Roald B., and Sande H.. 1994. High Lp(a) lipoprotein level in maternal serum may interfere with placental circulation and cause fetal growth retardation. Clin. Genet. 46: 52–56. [DOI] [PubMed] [Google Scholar]
- 115.Husby H., Roald B., Schjetlein R., Nesheim B. I., and Berg K.. 1996. High levels of Lp(a) lipoprotein in a family with cases of severe pre-eclampsia. Clin. Genet. 50: 47–49. [DOI] [PubMed] [Google Scholar]
- 116.Zechner R., Desoye G., Schweditsch M. O., Pfeiffer K. P., and Kostner G. M.. 1986. Fluctuations of plasma lipoprotein-A concentrations during pregnancy and post partum. Metabolism. 35: 333–336. [DOI] [PubMed] [Google Scholar]
- 117.Panteghini M., and Pagani F.. 1991. Serum concentrations of lipoprotein(a) during normal pregnancy and postpartum. Clin. Chem. 37: 2009–2010. [PubMed] [Google Scholar]
- 118.Wersch J. W., van Mackelenbergh B. A., and Ubachs J. M.. 1994. Lipoprotein(a) in smoking and non-smoking pregnant women. Scand. J. Clin. Lab. Invest. 54: 361–364. [DOI] [PubMed] [Google Scholar]
- 119.Brizzi P., Tonolo G., Esposito F., Puddu L., Dessole S., Maioli M., and Milia S.. 1999. Lipoprotein metabolism during normal pregnancy. Am. J. Obstet. Gynecol. 181: 430–434. [DOI] [PubMed] [Google Scholar]
- 120.Sattar N., Clark P., Greer I. A., Shepherd J., and Packard C. J.. 2000. Lipoprotein (a) levels in normal pregnancy and in pregnancy complicated with pre-eclampsia. Atherosclerosis. 148: 407–411. [DOI] [PubMed] [Google Scholar]
- 121.Belo L., Caslake M., Santos-Silva A., Pereira-Leite L., Quintanilha A., and Rebelo I.. 2002. Lipoprotein(a): a longitudinal versus a cross-sectional study in normal pregnancy and its levels in preeclampsia. Atherosclerosis. 165: 393–395. [DOI] [PubMed] [Google Scholar]
- 122.Manten G. T., Franx A., van der Hoek Y. Y., Hameeteman T. M., Voorbij H. A., Smolders H. C., Westers P., and Visser G. H.. 2003. Changes of plasma lipoprotein(a) during and after normal pregnancy in Caucasians. J. Matern. Fetal Neonatal Med. 14: 91–95. [DOI] [PubMed] [Google Scholar]
- 123.Lippi G., Albiero A., Montagnana M., Salvagno G. L., Scevarolli S., Franchi M., and Guidi G. C.. 2007. Lipid and lipoprotein profile in physiological pregnancy. Clin. Lab. 53: 173–177. [PubMed] [Google Scholar]
- 124.Mazurkiewicz J. C., Watts G. F., Warburton F. G., Slavin B. M., Lowy C., and Koukkou E.. 1994. Serum lipids, lipoproteins and apolipoproteins in pregnant non-diabetic patients. J. Clin. Pathol. 47: 728–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Silliman K., Shore V., and Forte T. M.. 1994. Hypertriglyceridemia during late pregnancy is associated with the formation of small dense low-density lipoproteins and the presence of large buoyant high-density lipoproteins. Metabolism. 43: 1035–1041. [DOI] [PubMed] [Google Scholar]
- 126.Chiang A. N., Yang M. L., Hung J. H., Chou P., Shyn S. K., and Ng H. T.. 1995. Alterations of serum lipid levels and their biological relevances during and after pregnancy. Life Sci. 56: 2367–2375. [DOI] [PubMed] [Google Scholar]
- 127.Rymer J., Constable S., Lumb P., and Crook M.. 2002. Serum lipoprotein (A) and apolipoproteins during pregnancy and postpartum in normal women. J. Obstet. Gynaecol. 22: 256–259. [DOI] [PubMed] [Google Scholar]
- 128.Uslu A., Uslu T., Bingol F., and Aydin S.. 1996. Lipoprotein levels in patients with pregnancy induced hypertension. Arch. Gynecol. Obstet. 258: 21–24. [DOI] [PubMed] [Google Scholar]
- 129.Wang J., Mimuro S., Lahoud R., Trudinger B., and Wang X. L.. 1998. Elevated levels of lipoprotein(a) in women with preeclampsia. Am. J. Obstet. Gynecol. 178: 146–149. [DOI] [PubMed] [Google Scholar]
- 130.Bar J., Harell D., Bardin R., Pardo J., Chen R., Hod M., and Sullivan M.. 2002. The elevated plasma lipoprotein(a) concentrations in preeclampsia do not precede the development of the disorder. Thromb. Res. 105: 19–23. [DOI] [PubMed] [Google Scholar]
- 131.Ogunyemi D., Ku W., and Arkel Y.. 2002. The association between inherited thrombophilia, antiphospholipid antibodies and lipoprotein A levels with obstetrical complications in pregnancy. J. Thromb. Thrombolysis. 14: 157–162. [DOI] [PubMed] [Google Scholar]
- 132.Aksoy H., Kumtepe Y., Akcay F., and Yildirim A. K.. 2002. Correlation of P-selectin and lipoprotein(a), and other lipid parameters in preeclampsia. Clin. Exp. Med. 2: 39–43. [DOI] [PubMed] [Google Scholar]
- 133.Mori M., Mori A., Saburi Y., Sida M., and Ohta H.. 2003. Levels of lipoprotein(a) in normal and compromised pregnancy. J. Perinat. Med. 31: 23–28. [DOI] [PubMed] [Google Scholar]
- 134.Demir B., Demir S., Atamer Y., Guven S., Atamer A., Kocyigit Y., Hekimoglu A., and Toprak G.. 2011. Serum levels of lipids, lipoproteins and paraoxonase activity in pre-eclampsia. J. Int. Med. Res. 39: 1427–1431. [DOI] [PubMed] [Google Scholar]
- 135.Bayhan G., Kocyigit Y., Atamer A., Atamer Y., and Akkus Z.. 2005. Potential atherogenic roles of lipids, lipoprotein(a) and lipid peroxidation in preeclampsia. Gynecol. Endocrinol. 21: 1–6. [DOI] [PubMed] [Google Scholar]
- 136.Parvin S., Samsuddin L., Ali A., Chowdhury S. A., and Siddique I.. 2010. Lipoprotein (a) level in pre-eclampsia patients. Bangladesh Med. Res. Counc. Bull. 36: 97–99. [DOI] [PubMed] [Google Scholar]
- 137.Nagy B., Rigo J. Jr., Fintor L., Romics L., Papp Z., and Karadi I.. 1999. Distribution of apolipoprotein(a) isoforms in normotensive and severe preeclamptic women. J. Matern. Fetal Med. 8: 270–274. [DOI] [PubMed] [Google Scholar]
- 138.Var A., Kuscu N. K., Koyuncu F., Uyanik B. S., Onur E., Yildirim Y., and Oruc S.. 2003. Atherogenic profile in preeclampsia. Arch. Gynecol. Obstet. 268: 45–47. [DOI] [PubMed] [Google Scholar]
- 139.Baksu B., Baksu A., Davas I., Akyol A., and Gulbaba G.. 2005. Lipoprotein(a) levels in women with pre-eclampsia and in normotensive pregnant women. J. Obstet. Gynaecol. Res. 31: 277–282. [DOI] [PubMed] [Google Scholar]
- 140.Wang Y., Walli A. K., Schulze A., Blessing F., Fraunberger P., Thaler C., Seidel D., and Hasbargen U.. 2006. Heparin-mediated extracorporeal low density lipoprotein precipitation as a possible therapeutic approach in preeclampsia. Transfus. Apher. Sci. 35: 103–110. [DOI] [PubMed] [Google Scholar]
- 141.Manten G. T., Sikkema M. J., Voorbij H. A., Visser G. H., Bruinse H. W., and Franx A.. 2007. Risk factors for cardiovascular disease in women with a history of pregnancy complicated by preeclampsia or intrauterine growth restriction. Hypertens. Pregnancy. 26: 39–50. [DOI] [PubMed] [Google Scholar]
- 142.Catarino C., Rebelo I., Belo L., Rocha-Pereira P., Rocha S., Castro E. B., Patricio B., Quintanilha A., and Santos-Silva A.. 2008. Fetal lipoprotein changes in pre-eclampsia. Acta Obstet. Gynecol. Scand. 87: 628–634. [DOI] [PubMed] [Google Scholar]
- 143.Bukan N., Kandemir O., Nas T., Gulbahar O., Unal A., and Cayci B.. 2012. Maternal cardiac risks in pre-eclamptic patients. J. Matern. Fetal Neonatal Med. 25: 912–914. [DOI] [PubMed] [Google Scholar]
- 144.Manten G. T., van der Hoek Y. Y., Marko Sikkema J., Voorbij H. A., Hameeteman T. M., Visser G. H., and Franx A.. 2005. The role of lipoprotein (a) in pregnancies complicated by pre-eclampsia. Med. Hypotheses. 64: 162–169. [DOI] [PubMed] [Google Scholar]
- 145.Kronenberg F., Konig P., Neyer U., Auinger M., Pribasnig A., Lang U., Reitinger J., Pinter G., Utermann G., and Dieplinger H.. 1995. Multicenter study of lipoprotein(a) and apolipoprotein(a) phenotypes in patients with end-stage renal disease treated by hemodialysis or continuous ambulatory peritoneal dialysis. J. Am. Soc. Nephrol. 6: 110–120. [DOI] [PubMed] [Google Scholar]
- 146.Heimbürger O., Stenvinkel P., Berglund L., Tranoeus A., and Lindholm B.. 1996. Increased plasma lipoprotein(a) in continuous ambulatory peritoneal dialysis is related to peritoneal transport of proteins and glucose. Nephron. 72: 135–144. [DOI] [PubMed] [Google Scholar]
- 147.Kronenberg F., Utermann G., and Dieplinger H.. 1996. Lipoprotein(a) in renal disease. Am. J. Kidney Dis. 27: 1–25. [DOI] [PubMed] [Google Scholar]
- 148.Stenvinkel P., Heimburger O., Paultre F., Diczfalusy U., Wang T., Berglund L., and Jogestrand T.. 1999. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 55: 1899–1911. [DOI] [PubMed] [Google Scholar]
- 149.Kronenberg F. 2014. Causes and consequences of lipoprotein(a) abnormalities in kidney disease. Clin. Exp. Nephrol. 18: 234–237. [DOI] [PubMed] [Google Scholar]
- 150.Thomas M. E., Freestone A., Varghese Z., Persaud J. W., and Moorhead J. F.. 1992. Lipoprotein(a) in patients with proteinuria. Nephrol. Dial. Transplant. 7: 597–601. [DOI] [PubMed] [Google Scholar]
- 151.Faucher C., Doucet C., Baumelou A., Chapman J., Jacobs C., and Thillet J.. 1993. Elevated lipoprotein (a) levels in primary nephrotic syndrome. Am. J. Kidney Dis. 22: 808–813. [DOI] [PubMed] [Google Scholar]
- 152.Wanner C., Rader D., Bartens W., Kramer J., Brewer H. B., Schollmeyer P., and Wieland H.. 1993. Elevated plasma lipoprotein(a) in patients with the nephrotic syndrome. Ann. Intern. Med. 119: 263–269. [DOI] [PubMed] [Google Scholar]
- 153.Stenvinkel P., Berglund L., Heimburger O., Pettersson E., and Alvestrand A.. 1993. Lipoprotein(a) in nephrotic syndrome. Kidney Int. 44: 1116–1123. [DOI] [PubMed] [Google Scholar]
- 154.Doucet C., Mooser V., Gonbert S., Raymond F., Chapman J., Jacobs C., and Thillet J.. 2000. Lipoprotein(a) in the nephrotic syndrome: molecular analysis of lipoprotein(a) and apolipoprotein(a) fragments in plasma and urine. J. Am. Soc. Nephrol. 11: 507–513. [DOI] [PubMed] [Google Scholar]
- 155.Kronenberg F., Lingenhel A., Lhotta K., Rantner B., Kronenberg M. F., Konig P., Thiery J., Koch M., von Eckardstein A., and Dieplinger H.. 2004. Lipoprotein(a)- and low-density lipoprotein-derived cholesterol in nephrotic syndrome: Impact on lipid-lowering therapy? Kidney Int. 66: 348–354. [DOI] [PubMed] [Google Scholar]
- 156.Li H. Q., Wu J., Niu D. M., Shi Y. H., Zhang C. N., and Wang J. J.. 2012. The level of native and oxidized lipoprotein(a) in children with nephrotic syndrome. Clin. Biochem. 45: 101–105. [DOI] [PubMed] [Google Scholar]
- 157.Gansevoort R. T., Heeg J. E., Dikkeschei F. D., de Zeeuw D., de Jong P. E., and Dullaart R. P.. 1994. Symptomatic antiproteinuric treatment decreases serum lipoprotein (a) concentration in patients with glomerular proteinuria. Nephrol. Dial. Transplant. 9: 244–250. [PubMed] [Google Scholar]
- 158.Kronenberg F., Lingenhel A., Lhotta K., Rantner B., Kronenberg M. F., Konig P., Thiery J., Koch M., von Eckardstein A., and Dieplinger H.. 2004. The apolipoprotein(a) size polymorphism is associated with nephrotic syndrome. Kidney Int. 65: 606–612. [DOI] [PubMed] [Google Scholar]
- 159.Kronenberg F., Kuen E., Ritz E., Junker R., Konig P., Kraatz G., Lhotta K., Mann J. F., Muller G. A., Neyer U., et al. 2000. Lipoprotein(a) serum concentrations and apolipoprotein(a) phenotypes in mild and moderate renal failure. J. Am. Soc. Nephrol. 11: 105–115. [DOI] [PubMed] [Google Scholar]
- 160.Appel G. B., Blum C. B., Chien S., Kunis C. L., and Appel A. S.. 1985. The hyperlipidemia of the nephrotic syndrome. Relation to plasma albumin concentration, oncotic pressure, and viscosity. N. Engl. J. Med. 312: 1544–1548. [DOI] [PubMed] [Google Scholar]
- 161.Cressman M. D., Heyka R. J., Paganini E. P., O’Neil J., Skibinski C. I., and Hoff H. F.. 1992. Lipoprotein(a) is an independent risk factor for cardiovascular disease in hemodialysis patients. Circulation. 86: 475–482. [DOI] [PubMed] [Google Scholar]
- 162.Dieplinger H., Schoenfeld P. Y., and Fielding C. J.. 1986. Plasma cholesterol metabolism in end-stage renal disease. Difference between treatment by hemodialysis or peritoneal dialysis. J. Clin. Invest. 77: 1071–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Heimann P., Josephson M. A., Fellner S. K., Thistlethwaite J. R. Jr., Stuart F. P., and Dasgupta A.. 1991. Elevated lipoprotein(a) levels in renal transplantation and hemodialysis patients. Am. J. Nephrol. 11: 470–474. [DOI] [PubMed] [Google Scholar]
- 164.Barbagallo C. M., Averna M. R., Scafidi V., Galione A., and Notarbartolo A.. 1992. Increased lipoprotein (a) levels in subjects with chronic renal failure on hemodialysis. Nephron. 62: 471–472. [DOI] [PubMed] [Google Scholar]
- 165.Shoji T., Nishizawa Y., Nishitani H., Yamakawa M., and Morii H.. 1992. High serum lipoprotein(a) concentrations in uremic patients treated with continuous ambulatory peritoneal dialysis. Clin. Nephrol. 38: 271–276. [PubMed] [Google Scholar]
- 166.Hirata K., Kikuchi S., Saku K., Jimi S., Zhang B., Naito S., Hamaguchi H., and Arakawa K.. 1993. Apolipoprotein(a) phenotypes and serum lipoprotein(a) levels in maintenance hemodialysis patients with/without diabetes mellitus. Kidney Int. 44: 1062–1070. [DOI] [PubMed] [Google Scholar]
- 167.Webb A. T., Reaveley D. A., O’Donnell M., O’Connor B., Seed M., and Brown E. A.. 1993. Lipoprotein (a) in patients on maintenance haemodialysis and continuous ambulatory peritoneal dialysis. Nephrol. Dial. Transplant. 8: 609–613. [PubMed] [Google Scholar]
- 168.Fiorini F., Masturzo P., Mij M., and Bertolini S.. 1995. Lipoprotein(a) levels in hemodialysis patients: relation to glucose intolerance and hemodialysis duration. Nephron. 70: 500–501. [DOI] [PubMed] [Google Scholar]
- 169.Gault M. H., Longerich L. L., Purchase L., Harnett J., and Breckenridge C.. 1995. Comparison of Lp(a) concentrations and some potential effects in hemodialysis, CAPD, transplantation, and control groups, and review of the literature. Nephron. 70: 155–170. [DOI] [PubMed] [Google Scholar]
- 170.Aggarwal H. K., Jain D., Lathar M., Yadav R. K., and Sawhney A.. 2010. Lipoprotein-A and carotid intima media thickness as cardiovascular risk factors in patients of chronic kidney disease. Ren. Fail. 32: 647–652. [DOI] [PubMed] [Google Scholar]
- 171.Parsons D. S., Reaveley D. A., Pavitt D. V., Misra M., and Brown E. A.. 2003. Lipoprotein (a) levels in those with high molecular weight apo (a) isoforms may remain low in a significant proportion of patients with end-stage renal disease. Nephrol. Dial. Transplant. 18: 1848–1853. [DOI] [PubMed] [Google Scholar]
- 172.Lin J., Reilly M. P., Terembula K., and Wilson F. P.. 2014. Plasma lipoprotein(a) levels are associated with mild renal impairment in type 2 diabetics independent of albuminuria. PLoS One. 9: e114397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Querfeld U., Lang M., Friedrich J. B., Kohl B., Fiehn W., and Scharer K.. 1993. Lipoprotein(a) serum levels and apolipoprotein(a) phenotypes in children with chronic renal disease. Pediatr. Res. 34: 772–776. [DOI] [PubMed] [Google Scholar]
- 174.Irish A. B., Simons L. A., Savdie E., Hayes J. M., and Simons J.. 1992. Lipoprotein(a) levels in chronic renal disease states, dialysis and transplantation. Aust. N. Z. J. Med. 22: 243–248. [DOI] [PubMed] [Google Scholar]
- 175.Buggy D., Breathnach A., Keogh B., Cooke T., and Feely J.. 1993. Lipoprotein(a) and treatment of chronic renal disease. J. Intern. Med. 234: 453–455. [DOI] [PubMed] [Google Scholar]
- 176.Docci D., Baldrati L., Capponcini C., and Feletti C.. 1994. Serum lipoprotein(a) (Lpa(a)) in haemodialysis patients. Nephrol. Dial. Transplant. 9: 733–734. [DOI] [PubMed] [Google Scholar]
- 177.Rahman M., Yang W., Akkina S., Alper A., Anderson A. H., Appel L. J., He J., Raj D. S., Schelling J., Strauss L., et al. 2014. Relation of serum lipids and lipoproteins with progression of CKD: The CRIC study. Clin. J. Am. Soc. Nephrol. 9: 1190–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Dieplinger H., Lackner C., Kronenberg F., Sandholzer C., Lhotta K., Hoppichler F., Graf H., and Konig P.. 1993. Elevated plasma concentrations of lipoprotein(a) in patients with end-stage renal disease are not related to the size polymorphism of apolipoprotein(a). J. Clin. Invest. 91: 397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Milionis H. J., Elisaf M. S., Tselepis A., Bairaktari E., Karabina S. A., and Siamopoulos K. C.. 1999. Apolipoprotein(a) phenotypes and lipoprotein(a) concentrations in patients with renal failure. Am. J. Kidney Dis. 33: 1100–1106. [DOI] [PubMed] [Google Scholar]
- 180.Zimmermann J., Herrlinger S., Pruy A., Metzger T., and Wanner C.. 1999. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 55: 648–658. [DOI] [PubMed] [Google Scholar]
- 181.Gambhir J. K., Kalra O. P., Khaira A., and Kaur H.. 2013. Association between high molecular weight apolipoprotein isoforms and lipoprotein levels in advanced chronic kidney disease and the effect of hemodialysis. Indian J. Nephrol. 23: 18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Auguet T., Senti M., Rubies-Prat J., Pelegri A., Pedro-Botet J., Nogues X., and Romero R.. 1993. Serum lipoprotein(a) concentration in patients with chronic renal failure receiving haemodialysis: influence of apolipoprotein (a) genetic polymorphism. Nephrol. Dial. Transplant. 8: 1099–1103. [PubMed] [Google Scholar]
- 183.De Sain-Van Der Velden M. G., Reijngoud D. J., Kaysen G. A., Gadellaa M. M., Voorbij H., Stellaard F., Koomans H. A., and Rabelink T. J.. 1998. Evidence for increased synthesis of lipoprotein(a) in the nephrotic syndrome. J. Am. Soc. Nephrol. 9: 1474–1481. [DOI] [PubMed] [Google Scholar]
- 184.Kronenberg F., Konig P., Lhotta K., Ofner D., Sandholzer C., Margreiter R., Dosch E., Utermann G., and Dieplinger H.. 1994. Apolipoprotein(a) phenotype-associated decrease in lipoprotein(a) plasma concentrations after renal transplantation. Arterioscler. Thromb. 14: 1399–1404. [DOI] [PubMed] [Google Scholar]
- 185.Azrolan N., Brown C. D., Thomas L., Hayek T., Zhao Z. H., Roberts K. G., Scheiner C., and Friedman E. A.. 1994. Cyclosporin A has divergent effects on plasma LDL cholesterol (LDL-C) and lipoprotein(a) [Lp(a)] levels in renal transplant recipients. Evidence for renal involvement in the maintenance of LDL-C and the elevation of Lp(a) concentrations in hemodialysis patients. Arterioscler. Thromb. 14: 1393–1398. [DOI] [PubMed] [Google Scholar]
- 186.Black I. W., and Wilcken D. E.. 1992. Decreases in apolipoprotein(a) after renal transplantation: implications for lipoprotein(a) metabolism. Clin. Chem. 38: 353–357. [PubMed] [Google Scholar]
- 187.Murphy B. G., and McNamee P. T.. 1992. Apolipoprotein (a) concentration decreases following renal transplantation. Nephrol. Dial. Transplant. 7: 174–175. [DOI] [PubMed] [Google Scholar]
- 188.Kronenberg F., Konig P., Lhotta K., Konigsrainer A., Sandholzer C., Utermann G., and Dieplinger H.. 1993. Cyclosporin and serum lipids in renal transplant recipients. Lancet. 341: 765; author reply 767. [PubMed] [Google Scholar]
- 189.Yang C. W., Kim Y. S., Kim S. Y., Chang Y. S., Yoon Y. S., and Bang B. K.. 1994. Serum levels of lipoprotein (a) after renal transplantation: short-term follow-up. Nephron. 67: 364. [DOI] [PubMed] [Google Scholar]
- 190.Segarra A., Chacon P., Martin M., Vilardell M., Vila J., Cotrina M., Fort J., Olmos A., and Piera L. L.. 1995. Serum lipoprotein (a) levels in patients with chronic renal failure–evolution after renal transplantation and relationship with other parameters of lipoprotein metabolism: a prospective study. Nephron. 69: 9–13. [DOI] [PubMed] [Google Scholar]
- 191.Murphy B. G., Yong A., Brown J. H., and McNamee P. T.. 1995. Effect of immunosuppressive drug regime on cardiovascular risk profile following kidney transplantation. Atherosclerosis. 116: 241–245. [DOI] [PubMed] [Google Scholar]
- 192.von Ahsen N., Helmhold M., Eisenhauer T., Armstrong V. W., and Oellerich M.. 1996. Decrease in lipoprotein(a) after renal transplantation is related to the glucocorticoid dose. Eur. J. Clin. Invest. 26: 668–675. [DOI] [PubMed] [Google Scholar]
- 193.Kerschdorfer L., Konig P., Neyer U., Bosmuller C., Lhotta K., Auinger M., Hohenegger M., Riegler P., Margreiter R., Utermann G., et al. 1999. Lipoprotein(a) plasma concentrations after renal transplantation: a prospective evaluation after 4 years of follow-up. Atherosclerosis. 144: 381–391. [DOI] [PubMed] [Google Scholar]
- 194.Brown J. H., Anwar N., Short C. D., Bhatnager D., Mackness M. I., Hunt L. P., and Durrington P. N.. 1993. Serum lipoprotein (a) in renal transplant recipients receiving cyclosporin monotherapy. Nephrol. Dial. Transplant. 8: 863–867. [PubMed] [Google Scholar]
- 195.Süleymanlar G., Ozben T., Sapan M., Yilmaz H., Yakupoglu G., and Karpuzoglu T.. 1994. Serum lipoproteins in renal transplant patients: the effect of cyclosporine on lipoprotein(a). Transplant. Proc. 26: 2637–2638. [PubMed] [Google Scholar]
- 196.Sugahara S., Koyama I., Yoshikawa Y., Dohi Y., and Omoto R.. 1994. Lipid and lipoprotein(a) in renal transplant recipients. Transplant. Proc. 26: 2080–2081. [PubMed] [Google Scholar]
- 197.Webb A. T., Reaveley D. A., O’Donnell M., O’Connor B., Seed M., and Brown E. A.. 1993. Does cyclosporin increase lipoprotein(a) concentrations in renal transplant recipients? Lancet. 341: 268–270. [DOI] [PubMed] [Google Scholar]
- 198.Islam M. S., Bard J. M., Le Pogamp P., Leglise D., Bourbigot B., Rivalan J., and Cledes J.. 1994. Serum lipoprotein (a) in renal transplant recipients treated with cyclosporin monotherapy compared to conventional treatment. Nephrol. Dial. Transplant. 9: 332–333. [PubMed] [Google Scholar]
- 199.Lye W. C., Hughes K., Leong S. O., Tan C. C., and Lee E. J.. 1995. Abnormal lipoprotein (a) and lipid profiles in renal allograft recipients: effects of treatment with pravastatin. Transplant. Proc. 27: 977–978. [PubMed] [Google Scholar]
- 200.Barbagallo C. M., Averna M. R., Sparacino V., Galione A., Caputo F., Scafidi V., Amato S., Mancino C., Cefalu A. B., and Notarbartolo A.. 1993. Lipoprotein (a) levels in end-stage renal failure and renal transplantation. Nephron. 64: 560–564. [DOI] [PubMed] [Google Scholar]
- 201.Segarra A., Chacon P., Vilardell M., and Piera L. L.. 1993. Cyclosporin and serum lipids in renal transplant recipients. Lancet. 341: 766–; author reply 767. [PubMed] [Google Scholar]
- 202.Sentí M., Puig J. M., Lloveras J., Aubó C., Mir M., Barbosa F., Oliveras A., and Masramón J.. 1997. Effect of cyclosporine on serum lipoprotein(a) levels in renal transplant recipients. Transplant. Proc. 29: 2404–2405. [DOI] [PubMed] [Google Scholar]
- 203.Moore R., Thomas D., Morgan E., Wheeler D., Griffin P., Salaman J., and Rees A.. 1993. Abnormal lipid and lipoprotein profiles following renal transplantation. Transplant. Proc. 25: 1060–1061. [PubMed] [Google Scholar]
- 204.Wheeler D. C., Morgan R., Thomas D. M., Seed M., Rees A., and Moore R. H.. 1996. Factors influencing plasma lipid profiles including lipoprotein (a) concentrations in renal transplant recipients. Transpl. Int. 9: 221–226. [DOI] [PubMed] [Google Scholar]
- 205.Bell A. W. 1979. Lipid metabolism in liver and selected tissues and in the whole body of ruminant animals. Prog. Lipid Res. 18: 117–164. [DOI] [PubMed] [Google Scholar]
- 206.Tietge U. J., Boker K. H., Bahr M. J., Weinberg S., Pichlmayr R., Schmidt H. H., and Manns M. P.. 1998. Lipid parameters predicting liver function in patients with cirrhosis and after liver transplantation. Hepatogastroenterology. 45: 2255–2260. [PubMed] [Google Scholar]
- 207.Hoover-Plow J., and Huang M.. 2013. Lipoprotein(a) metabolism: potential sites for therapeutic targets. Metabolism. 62: 479–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Anuurad E., Enkhmaa B., and Berglund L.. 2010. Enigmatic role of lipoprotein(a) in cardiovascular disease. Clin. Transl. Sci. 3: 327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Motta M., Giugno I., Ruello P., Pistone G., Di Fazio I., and Malaguarnera M.. 2001. Lipoprotein (a) behaviour in patients with hepatocellular carcinoma. Minerva Med. 92: 301–305. [PubMed] [Google Scholar]
- 210.Malaguarnera M., Giugno I., Trovato B. A., Panebianco M. P., Siciliano R., and Ruello P.. 1995. Lipoprotein(a) concentration in patients with chronic active hepatitis C before and after interferon treatment. Clin. Ther. 17: 721–728. [DOI] [PubMed] [Google Scholar]
- 211.Gregory W. L., Game F. L., Farrer M., Idle J. R., Laker M. F., and James O. F.. 1994. Reduced serum lipoprotein(a) levels in patients with primary biliary cirrhosis. Atherosclerosis. 105: 43–50. [DOI] [PubMed] [Google Scholar]
- 212.Irshad M. 2004. Serum lipoprotein (a) levels in liver diseases caused by hepatitis. Indian J. Med. Res. 120: 542–545. [PubMed] [Google Scholar]
- 213.Jiang J., Zhang X., Wu C., Qin X., Luo G., Deng H., Lu M., Xu B., Li M., Ji M., et al. 2008. Increased plasma apoM levels in the patients suffered from hepatocellular carcinoma and other chronic liver diseases. Lipids Health Dis. 7: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Van Wersch J. W. 1994. The behaviour of lipoprotein(a) in patients with various diseases. Scand. J. Clin. Lab. Invest. 54: 559–562. [DOI] [PubMed] [Google Scholar]
- 215.Alessandri C., Basili S., Maurelli M., Andreozzi P., Violi F., and Cordova C.. 1994. Relationship between lipoprotein(a) levels in serum and some indices of protein synthesis in liver cirrhosis. Clin. Chim. Acta. 224: 125–129. [DOI] [PubMed] [Google Scholar]
- 216.Geiss H. C., Ritter M. M., Richter W. O., Schwandt P., and Zachoval R.. 1996. Low lipoprotein (a) levels during acute viral hepatitis. Hepatology. 24: 1334–1337. [DOI] [PubMed] [Google Scholar]
- 217.Selvais P., Henrion J., Schapira M., Derue G., Hondekijn J. C., Parfonry A., and Heller F. R.. 1995. Lipoprotein (a) in liver diseases. Correlation between low levels and liver function [Article in French]. Presse Med. 24: 382–386. [PubMed] [Google Scholar]
- 218.Kervinen K., Savolainen M. J., and Kesaniemi Y. A.. 1991. A rapid increase in lipoprotein (a) levels after ethanol withdrawal in alcoholic men. Life Sci. 48: 2183–2188. [DOI] [PubMed] [Google Scholar]
- 219.Marth E., Cazzolato G., Bittolo Bon G., Avogaro P., and Kostner G. M.. 1982. Serum concentrations of Lp(a) and other lipoprotein parameters in heavy alcohol consumers. Ann. Nutr. Metab. 26: 56–62. [DOI] [PubMed] [Google Scholar]
- 220.Lecomte E., Herbeth B., Paille F., Steinmetz J., Artur Y., and Siest G.. 1996. Changes in serum apolipoprotein and lipoprotein profile induced by chronic alcohol consumption and withdrawal: determinant effect on heart disease? Clin. Chem. 42: 1666–1675. [PubMed] [Google Scholar]
- 221.Clevidence B. A., Reichman M. E., Judd J. T., Muesing R. A., Schatzkin A., Schaefer E. J., Li Z., Jenner J., Brown C. C., Sunkin M., et al. 1995. Effects of alcohol consumption on lipoproteins of premenopausal women. A controlled diet study. Arterioscler. Thromb. Vasc. Biol. 15: 179–184. [DOI] [PubMed] [Google Scholar]
- 222.Schröder H., Ferrández O., Jimenez Conde J., Sánchez-Font A., and Marrugat J.. 2005. Cardiovascular risk profile and type of alcohol beverage consumption: a population-based study. Ann. Nutr. Metab. 49: 100–106. [DOI] [PubMed] [Google Scholar]
- 223.Chiva-Blanch G., Urpi-Sarda M., Ros E., Valderas-Martinez P., Casas R., Arranz S., Guillen M., Lamuela-Raventos R. M., Llorach R., Andres-Lacueva C., et al. 2013. Effects of red wine polyphenols and alcohol on glucose metabolism and the lipid profile: a randomized clinical trial. Clin. Nutr. 32: 200–206. [DOI] [PubMed] [Google Scholar]
- 224.Droste D. W., Iliescu C., Vaillant M., Gantenbein M., De Bremaeker N., Lieunard C., Velez T., Meyer M., Guth T., Kuemmerle A., et al. 2013. A daily glass of red wine associated with lifestyle changes independently improves blood lipids in patients with carotid arteriosclerosis: results from a randomized controlled trial. Nutr. J. 12: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Haffner S. M., Tuttle K. R., and Rainwater D. L.. 1991. Decrease of lipoprotein(a) with improved glycemic control in IDDM subjects. Diabetes Care. 14: 302–307. [DOI] [PubMed] [Google Scholar]
- 226.Levitsky L. L., Scanu A. M., and Gould S. H.. 1991. Lipoprotein(a) levels in black and white children and adolescents with IDDM. Diabetes Care. 14: 283–287. [DOI] [PubMed] [Google Scholar]
- 227.Jenkins A. J., Steele J. S., Janus E. D., and Best J. D.. 1991. Increased plasma apolipoprotein(a) levels in IDDM patients with microalbuminuria. Diabetes. 40: 787–790. [DOI] [PubMed] [Google Scholar]
- 228.Bruckert E., Davidoff P., Grimaldi A., Truffert J., Giral P., Doumith R., Thervet F., and De Gennes J. L.. 1990. Increased serum levels of lipoprotein(a) in diabetes mellitus and their reduction with glycemic control. JAMA. 263: 35–36. [PubMed] [Google Scholar]
- 229.Couper J. J., Bates D. J., Cocciolone R., Magarey A. M., Boulton T. J., Penfold J. L., and Ryall R. G.. 1993. Association of lipoprotein(a) with puberty in IDDM. Diabetes Care. 16: 869–873. [DOI] [PubMed] [Google Scholar]
- 230.Salzer B., Stavljenic A., Jurgens G., Dumic M., and Radica A.. 1993. Polymorphism of apolipoprotein E, lipoprotein(a), and other lipoproteins in children with type I diabetes. Clin. Chem. 39: 1427–1432. [PubMed] [Google Scholar]
- 231.Haffner S. M., Tuttle K. R., and Rainwater D. L.. 1992. Lack of change of lipoprotein (a) concentration with improved glycemic control in subjects with type II diabetes. Metabolism. 41: 116–120. [DOI] [PubMed] [Google Scholar]
- 232.Jenkins A. J., Steele J. S., Janus E. D., Santamaria J. D., and Best J. D.. 1992. Plasma apolipoprotein (a) is increased in type 2 (non-insulin-dependent) diabetic patients with microalbuminuria. Diabetologia. 35: 1055–1059. [DOI] [PubMed] [Google Scholar]
- 233.Habib S. S., and Aslam M.. 2004. Lipids and lipoprotein(a) concentrations in Pakistani patients with type 2 diabetes mellitus. Diabetes Obes. Metab. 6: 338–343. [DOI] [PubMed] [Google Scholar]
- 234.Singla S., Kaur K., Kaur G., Kaur H., Kaur J., and Jaswal S.. 2009. Lipoprotein (a) in type 2 diabetes mellitus: Relation to LDL:HDL ratio and glycemic control. Int. J. Diabetes Dev. Ctries. 29: 80–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Nakhjavani M., Morteza A., Esteghamati A., Khalilzadeh O., Zandieh A., and Safari R.. 2011. Serum lipoprotein(a) levels are greater in female than male patients with type-2 diabetes. Lipids. 46: 349–356. [DOI] [PubMed] [Google Scholar]
- 236.Rainwater D. L., MacCluer J. W., Stern M. P., VandeBerg J. L., and Haffner S. M.. 1994. Effects of NIDDM on lipoprotein(a) concentration and apolipoprotein(a) size. Diabetes. 43: 942–946. [DOI] [PubMed] [Google Scholar]
- 237.Habib S. S., Aslam M., Shah S. F., and Naveed A. K.. 2009. Lipoprotein (a) is associated with basal insulin levels in patients with type 2 diabetes mellitus. Arq. Bras. Cardiol. 93: 28–33. [DOI] [PubMed] [Google Scholar]
- 238.Boronat M., Saavedra P., Perez-Martin N., Lopez-Madrazo M. J., Rodriguez-Perez C., and Novoa F. J.. 2012. High levels of lipoprotein(a) are associated with a lower prevalence of diabetes with advancing age: results of a cross-sectional epidemiological survey in Gran Canaria, Spain. Cardiovasc. Diabetol. 11: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Ding L., Song A., Dai M., Xu M., Sun W., Xu B., Sun J., Wang T., Xu Y., Lu J., et al. 2015. Serum lipoprotein (a) concentrations are inversely associated with T2D, prediabetes, and insulin resistance in a middle-aged and elderly Chinese population. J. Lipid Res. 56: 920–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Ye Z., Haycock P. C., Gurdasani D., Pomilla C., Boekholdt S. M., Tsimikas S., Khaw K. T., Wareham N. J., Sandhu M. S., and Forouhi N. G.. 2014. The association between circulating lipoprotein(a) and type 2 diabetes: is it causal? Diabetes. 63: 332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Purnell J. Q., Marcovina S. M., Hokanson J. E., Kennedy H., Cleary P. A., Steffes M. W., and Brunzell J. D.. 1995. Levels of lipoprotein(a), apolipoprotein B, and lipoprotein cholesterol distribution in IDDM. Results from follow-up in the Diabetes Control and Complications Trial. Diabetes. 44: 1218–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Császár A., Dieplinger H., Sandholzer C., Karádi I., Juhász E., Drexel H., Halmos T., Romics L., Patsch J. R., and Utermann G.. 1993. Plasma lipoprotein (a) concentration and phenotypes in diabetes mellitus. Diabetologia. 36: 47–51. [DOI] [PubMed] [Google Scholar]
- 243.Klausen I. C., Schmidt E. B., Lervang H. H., Gerdes L. U., Ditzel J., and Faergeman O.. 1992. Normal lipoprotein(a) concentrations and apolipoprotein(a) isoforms in patients with insulin-dependent diabetes mellitus. Eur. J. Clin. Invest. 22: 538–541. [DOI] [PubMed] [Google Scholar]
- 244.Haffner S. M., Morales P. A., Stern M. P., and Gruber M. K.. 1992. Lp(a) concentrations in NIDDM. Diabetes. 41: 1267–1272. [DOI] [PubMed] [Google Scholar]
- 245.Durlach V., Gillery P., Bertin E., Taupin J. M., Grulet H., Gross A., and Leutenegger M.. 1996. Serum lipoprotein (a) concentrations in a population of 819 non-insulin-dependent diabetic patients. Diabetes Metab. 22: 319–323. [PubMed] [Google Scholar]
- 246.Mora S., Kamstrup P. R., Rifai N., Nordestgaard B. G., Buring J. E., and Ridker P. M.. 2010. Lipoprotein(a) and risk of type 2 diabetes. Clin. Chem. 56: 1252–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Rainwater D. L., and Haffner S. M.. 1998. Insulin and 2-hour glucose levels are inversely related to Lp(a) concentrations controlled for LPA genotype. Arterioscler. Thromb. Vasc. Biol. 18: 1335–1341. [DOI] [PubMed] [Google Scholar]
- 248.Neele D. M., de Wit E. C., and Princen H. M.. 1999. Insulin suppresses apolipoprotein(a) synthesis by primary cultures of cynomolgus monkey hepatocytes. Diabetologia. 42: 41–44. [DOI] [PubMed] [Google Scholar]
- 249.Calmarza P., and Vella J. C.. 1999. Lipids, lipoproteins and apolipoprotein (a) isoforms in type 2 diabetic patients. J. Cardiovasc. Risk. 6: 171–175. [DOI] [PubMed] [Google Scholar]
- 250.Ribault A., Durou M. R., Letellier C., Wojcik F., Poirier J. Y., and Ruelland A.. 2000. Determination of lipoprotein(a) concentrations and apolipoprotein(a) molecular weights in diabetic patients. Diabetes Metab. 26: 107–112. [PubMed] [Google Scholar]
- 251.Labudovic D. D., Toseska K. N., Alabakovska S. B., and Todorova B.. 2003. Apoprotein(a) phenotypes and plasma lipoprotein(a) concentration in patients with diabetes mellitus. Clin. Biochem. 36: 545–551. [DOI] [PubMed] [Google Scholar]
- 252.Labudović D. D., Toseska K. N., Alabakovska S. B., Dimitrovski C., Jurhar M., Isjanovska R., Spiroski M., and Todorova B. B.. 2003. Frequency distribution of apoprotein(a) isoforms in patients with diabetes mellitus and healthy subjects. Croat. Med. J. 44: 435–440. [PubMed] [Google Scholar]
- 253.Tsimikas S., Viney N. J., Hughes S. G., Singleton W., Graham M. J., Baker B. F., Burkey J. L., Yang Q., Marcovina S. M., Geary R. S., et al. 2015. Antisense therapy targeting apolipoprotein(a): a randomised, double-blind, placebo-controlled phase 1 study. Lancet. 386: 1472–1483. [DOI] [PubMed] [Google Scholar]