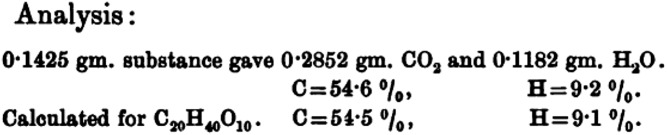Abstract
Arachidonic acid and esterified arachidonate are ubiquitous components of every mammalian cell. This polyunsaturated fatty acid serves very important biochemical roles, including being the direct precursor of bioactive lipid mediators such as prostaglandin and leukotrienes. This 20 carbon fatty acid with four double bonds was first isolated and identified from mammalian tissues in 1909 by Percival Hartley. This was accomplished prior to the advent of chromatography or any spectroscopic methodology (MS, infrared, UV, or NMR). The name, arachidonic, was suggested in 1913 based on its relationship to the well-known arachidic acid (C20:0). It took until 1940 before the positions of the four double bonds were defined at 5,8,11,14 of the 20-carbon chain. Total synthesis was reported in 1961 and, finally, the configuration of the double bonds was confirmed as all-cis-5,8,11,14. By the 1930s, the relationship of arachidonic acid within the family of essential fatty acids helped cue an understanding of its structure and the biosynthetic pathway. Herein, we review the findings leading up to the discovery of arachidonic acid and the progress toward its complete structural elucidation.
Keywords: total synthesis, linoleic acid, ozonolysis, potassium permanganate, Hazura’s rule, chemical analysis, fractional crystallization, octobromoarachidic acid, essential fatty acid
Arachidonic acid is present in all mammalian cells, typically esterified to membrane phospholipids, and is one of the most abundant polyunsaturated fatty acids present in human tissue. Our understanding of the importance of this fatty acid and its role in normal cell biology and tissue homeostasis continues to grow and cannot be overstated. Not only is this tetra-unsaturated fatty acid important for normal cellular membrane fluidity, but it also is a substrate for numerous enzymatic transformations that form biologically active lipid mediators, such as prostaglandins, leukotrienes, epoxyeicosatetraenoic acids, and endocannabinoids. Yet, this seemingly simple lipid took over 50 years for complete structure elucidation in the first half of the 20th century.
The tools available for lipid biochemists in the 21st century to carry out structural characterization are immensely powerful and, in some cases, taken for granted. This was not the case at the turn of the last century when structural characterization of lipids was exceedingly difficult. In large part, this was due to the absence of any modern techniques based upon physical chemical properties such as NMR, infrared (IR) absorption, UV absorption, and even mass spectrometric behavior, including exact mass measurements for elemental compositions calculations. Even X-ray crystallography was in its infancy and stearic acid X-ray crystallography was still challenging in 1927 (1). Equally important was the absence of powerful separation technology that enabled relatively pure molecules to be isolated by straightforward chromatographic strategies. These facts are particularly relevant to the discovery and structural characterization of arachidonic acid.
This polyunsaturated fatty acid is largely present in tissues esterified as glycerolipids or glycerophospholipids. To make it even more challenging, it could only be isolated from animal tissues along with a large number of closely related fatty acids, which made pure arachidonic acid exceedingly difficult to obtain. Finally, and perhaps one of the most difficult aspects of the structural characterization of this important fatty acid was that even when pure, this organic compound was an oil rather than the crystalline substance at virtually any temperature available in laboratories of the early 1900s. Yet, it is with these features in the difficulty of studying such a fatty acid, that the origin of the structure of arachidonic acid emerged.
At the end of the 19th century, there were indications that polyunsaturated fatty acids were present in seed oils and fish oil, but there was very little information as to their exact structure (2, 3). The structure of the monounsaturated oleic acid (4) and dienoic linoleic acid (5) had been characterized by this time in large part because of the more favorable feature of being present in very high abundance in certain biological systems, such as plant oils. Early studies of these octadecenyl and octadecadienyl fatty acids revealed that bromination yielded crystalline derivatives by addition of two and four bromine atoms, respectively. This ability to use crystallization as well as distillation as a means to purify these unsaturated fatty acids was a central strategy for purification.
The strategy of lipid structural characterization in the first half of the 20th century was to compare specific properties of the unknown with those of a known compound. This was largely based upon crystallization behavior and experiments involving mixing crystals of the unknown with crystals of the suspected known compound to carry out a mixed melting point determination. Sharp melting points that were not depressed for identical compounds were the critical signature of identity. The other important tool was that of combustion analysis of the purified lipid, where it was possible to experimentally determine exact quantities of elements, such as carbon, hydrogen, and bromine, and calculate empirical formulas based upon the weight atom percent. One of the challenges of this era in chemistry, however, was that there was no convenient way to determine oxygen content in the purified organic molecule.
DISCOVERY OF ARACHIDONIC ACID
The existence of polyunsaturated fatty acids containing a very high iodine number was first described in the late 1800s in studies of fish oils by Bull (6) and Tsujimoto (7). However, these investigators did not characterize in chemical detail these polyunsaturated fatty acids except to state that they were of the series CnH2n-8O2, in which arachidonate belongs. However, measurement of the iodine value (grams of iodine that reacts with 100 g of fatty acid) of somewhat purified polyunsaturated fatty acids was found to be above 300 for fatty acid components in herring oil (6).
The first isolation and characterization of a tetraunsaturated 20-carbon fatty acid was reported by a young investigator at the Lister Institute, Percival Hartley, who was working on his D.Sc. degree from the University of London (8). Hartley (Fig. 1) was interested in the fact that fats and fatty acids present in adipose tissue had a very low number of double bonds, as judged by the reaction with iodine (low iodine number), and that these fatty acids were fairly stable to heating and exposure to air (9). This was not true of fatty acids isolated from mammalian tissues and organs, in particular the kidney, liver, and heart, which had very high iodine values and underwent chemical reactions when exposed to air. Hartley noted this behavior was similar to that observed with drying oils such as linseed oil. He carried out the initial studies starting with 200 g of fresh liver tissue from which he extracted glycerophosphocholines (lecithin) using a method of selective solubility in ethanol over acetone (10). The lecithin was saponified to free fatty acids, which he noted had a very high iodine value, suggesting the presence of unsaturated fatty acids, certainly more unsaturated than oleic acid. These fatty acids were then precipitated as lead salts and dissolved in ether because it was known that lead salts of unsaturated fatty acids were more soluble in ether than the lead salts of saturated fatty acids. A solution of these partially purified fatty acids from liver was chemically titrated with 1N NaOH and found to have a molecular mass between 308 and 312 Da in two different preparations. These lead salts were then methylated, vacuum distilled, and collected as four different fractions. The two fractions that boiled at the highest temperature (180°C and over 190°C) had an iodine value of 249 and a mean molecular mass (by titration) of 313 Da. It was noted that the molecular mass of these polyunsaturated fatty acids from the liver was sufficiently high that they must contain at least 20 carbon atoms in their molecular structure.
Fig. 1.

Photograph of Percival Hartley, the first individual to isolate and purify arachidonic acid and determine that it was an eicosatetraenoic acid (9). With permission of the Royal Society.
In order to further characterize these purified fatty acids, reaction with bromine was carried out (9). It was already well-established that bromine addition products of polyunsaturated fatty acids would yield bromides that often could be crystalized. The previous noted work of Tsujimoto (7) described the formation of octobromostearic acid from an unsaturated fatty acid isolated from sardines (C18H28O2Br8). However, it was found that the reaction of bromine with the purified liver polyunsaturated fatty acid yielded an insoluble bromination product that had a very high melting point of 222°C. No solvent was found that could dissolve this precipitant, but analysis for bromine content (as AgBr) led to a calculation of an empirical formula of C20H32O2Br8 (Fig. 2). This insoluble octobromoarachidic acid was similar to previously described bromination reactions from fish oils. The content of this insoluble octobromoarachidic acid corresponded to between 8 and 9% of the total liver fatty acids, and thus this novel fatty acid was a fairly abundant component of liver.
Fig. 2.
Chemical analysis of the octobromoarachidic acid by Hartley (9).
The oxidation of unsaturated fatty acids with mild potassium permanganate was used in these early years of polyunsaturated fatty acid chemistry as an additional chemical technique to define the number of double bonds in fatty acids according to what was termed Hazura’s rule (5). This rule indicated that two hydroxyl groups are added for every double bond present in the fatty acid. Following the method of Hazura (5), purified liver polyunsaturated fatty acids (vacuum distillate) were oxidized using alkaline permanganate, which yielded a white precipitant that could be recrystallized from hot water. By chemical analysis, this product was found to very nicely correspond to octahydroxyarachidic acid (C20H40O10) (Fig. 3). These properties convinced Hartley that this polyunsaturated 20-carbon fatty acid had four double bonds (9).
Fig. 3.
Chemical analysis of the octahydroxyarachidic acid by Hartley (9).
It was very difficult for Hartley to understand the biosynthetic origin of this unusual polyunsaturated fatty acid, but considering the reasonably high amount of the fatty acid present in liver, he felt that this was a product of important biochemical reactions.
Interestingly, Percival Hartley did not name this compound, but rather it was left up to J. Lewkowitsch (11), who recorded these findings in the compilation of physical and chemical data of oils, fats, and waxes published in 1913 (Fig. 4). He noted that Hartley did not name this acid, so, based on the established name of arachidic acid (C20:0), he suggested “arachidonic acid” for its C20:4 analog. The methods employed by Hartley to purify arachidonic acid, namely formation of the insoluble octobromoarachidic acid, were used by subsequent investigators for many years as a means for isolation of arachidonic acid from tissues.
Fig. 4.
Arachidonic acid is named. The lower paragraph on page 215 of the Lewkowitsch compendium marks the origin of “arachidonic” acid (11).
DOUBLE BOND LOCATION
The determination of the exact positions of double bonds along the 20-carbon chain of arachidonic acid was the result of efforts of several investigators and proved to be a significant challenge. Part of the challenge was the difficulty in preparing pure arachidonic acid and the potential isomerization of double bonds that occurred during the purification protocols widely employed. For example, arachidonic acid was isolated from tissues such as brain (12) by the protocol, essentially as described by Hartley, involving preparation of octobromoarachidic acid that could be exhaustively extracted by organic solvents followed by retroconversion to eicosatetraenoic acid by debromination using preparations of zinc dust. The major unsaturated fatty acids present in tissues were oleic and linoleic acid and their organic solvent-soluble dibromo- and tetrabromooctadecanoic acid, were readily removed. This step of isolation of the octobromoarachidic acid was on solid grounds, but the debromination step to generate eicosatetraenoic acid assumed that the loss of Br2 to form a carbon-carbon double bond took place at the same carbon atoms and with the same geometry (cis) of arachidonic acid. One important contribution during this time was confirmation of the unbranched structure of arachidonic acid because normal chain arachidic acid (C20:0) was formed by catalytic hydrogenation (12).
Already in the early 1900s, it was appreciated from the work of Goldsobel (13) and Erdmann, Bedford, and Raspe (14) that linoleic acid was 9,12-octadecadienoic acid with a methylene group interrupting the two double bonds in the fatty acyl chain. Permanganate oxidation to the tetrahydroxyoctadecanoate (sativic acid), which was further degraded by permanganate (13) or treatment with ozone to yield the end products, hexanoic acid (methyl terminus) and azelaic acid (carboxy terminus) (14), confirmed the position of the double bonds at carbon-9 and carbon-12. These early studies, in large part, were possible due to the abundance of 18:2 in seed oils and ease of crystallization of the fatty acid itself. These observations led to a general acceptance of Δ9,12 double bond positions in linoleic acid.
Interest in a more complete structural characterization of arachidonic was stimulated by the studies of Burr and Burr (15) and the emerging theme of the biochemistry of essential fatty acids. This was a recent topic of a historical review for the Journal of Lipid Research (16). Two investigators, one in the United States, James B. Brown (Ohio State University) and the other in Great Britain, Ida Smedley-Maclean (Lister Institute, London), became interested in a more complete structural characterization of arachidonic acid. Brown had been working with unsaturated fatty acids since his thesis work at the University of Illinois (17). Smedley-Maclean was already a respected lipid biochemist and was, in fact, the first female to be Chairman of the Biochemical Society (Fig. 5) (18). In 1938, her laboratory published work on the requirement of linoleic acid to synthesize arachidonic acid present in tissue (19). Interestingly, her studies of the biochemistry and structure of arachidonic acid were being published as World War II was underway and the Battle of Britain was raging in London.
Fig. 5.

Photograph of Ida Smedley-Maclean. With permission from the British Federation of University Women.
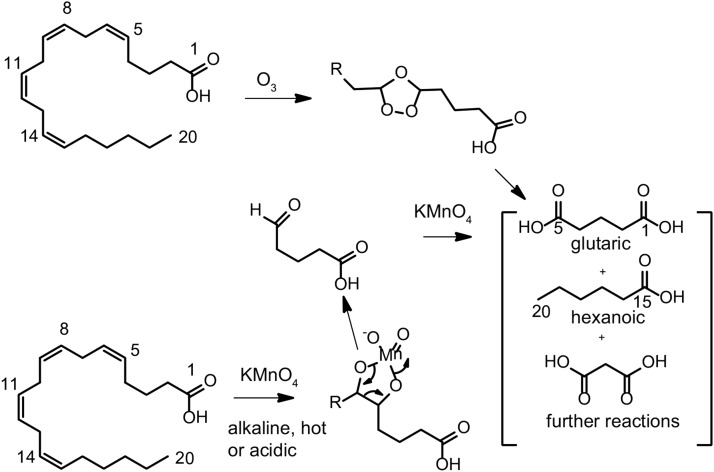
The first publication suggesting the double bond positions of arachidonic acid came from the laboratory of Brown (20) where ozonolysis of arachidonic acid was employed to specifically cleave double bonds and then characterize the resulting products (Fig. 6). Interestingly, the major topic of this paper dealt with the isolation of 95% pure arachidonic acid by fractional crystallization and vacuum distillation, avoiding the intermediate formation of the bromo adduct. Despite this apparent advance, his findings of various ozonolysis products, including acetaldehyde and succinic acid as well as adipic acid (hexadienoic acid), suggested that the first double bond of arachidonic acid was at carbon-6, predicting a structure of 6,10,14,18-eicosatetraenoic acid and not in agreement with the origin of arachidonic acid from linoleic acid. However, Brown suggested this work was tentative and needed further investigation.
Fig. 6.
Outline of the chemical reactions of arachidonic acid with ozone and alkaline permanganate to yield degradation products indicative of positions of double bonds in arachidonic acid. The reaction at the first double bond of arachidonic acid at carbon-5 is indicated.
Smedley-Maclean read this paper and was concerned that publication of her studies of the oxidative degradation of arachidonic acid, which yielded different conclusions, could not be postponed because of the conditions in London at that time (21). Rather than ozonolysis, she used oxidation by alkaline potassium permanganate to cleave the double bonds and form carboxylic acids (Fig. 6). Her studies led to the identification of glutaric acid (1,5-pentanedioic acid), oxalic acid, and succinic acid, which likely were derived from malonic acid as well as hexanoic acid. These products suggested that the first double bond was at carbon-5 (hence the dicarboxylic acid, glutaric acid) and formation of the small amount of hexanoic acid located the terminal double bond at carbons 14-15. She thus characterized arachidonate as 5,8,11,14-eicosatetraenoic acid, a structure consistent with a biochemical origin involving linoleic acid (21).
About 1 year later, the group at Ohio State University published more complete studies of the ozonolysis and permanganate oxidation of their highly purified arachidonic acid and confirmed the results of Smedley-Maclean and the formation of the identical products of glutaric acid, as well as some hexanoic and malonic acid, and thus the structure of arachidonic acid was confirmed to be 5,8,11,14-eicosatetraenoic acid (22).
GEOMETRY OF DOUBLE BONDS
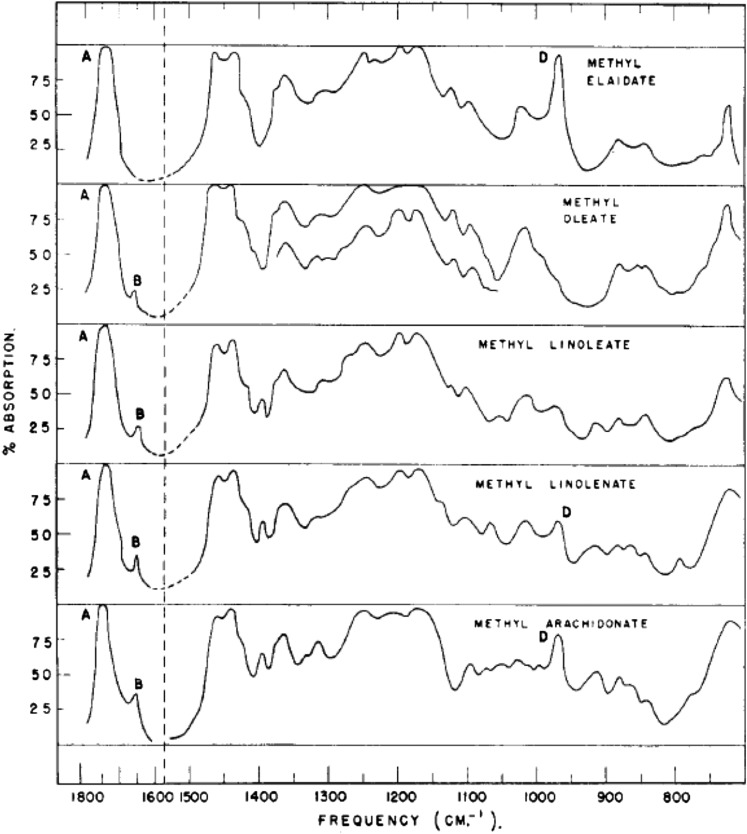
By the end of the 1940s, it was well-accepted that arachidonic acid had the double bond positions that we recognize today, and furthermore, with the compelling experiments that had been carried out on essential fatty acids, it was generally assumed that double bonds were of all cis configuration (23). However, experimental evidence for the cis double bonds had to await the development of spectroscopic techniques, such as IR spectroscopy and NMR spectrometry. IR spectroscopy was becoming available in chemical laboratories in the 1950s with the development of instrumentation that could measure the IR spectra of organic molecules. This technique was applied to the saturated and polyunsaturated fatty acids in order to assess the absorption characteristics of this class of molecules (24, 25). The presence of a cis or trans double bond could be ascertained from the bending vibration at 965–975 cm−1 for trans configuration, as appears in elaidic acid (trans-9-octadecaenoic acid). Interestingly, this same study did reveal this band in arachidonic acid (24), suggesting it could have a trans double bond (Fig. 7). However, the source of arachidonate was not stated and, if the octobromoarachidic acid/debromination step was involved in the purification, this might explain a chemical cis-to-trans rearrangement. Indeed, this paper did describe the IR spectra of octobromoarachidic acid.
Fig. 7.
IR spectroscopy of arachidonic acid and other unsaturated fatty acids. The trans bending vibration at 965–975 cm−1 is indicated by the letter “D” in the figure. Reprinted with permission from (24). Copyright 1952 American Chemical Society.
CHEMICAL SYNTHESIS OF ARACHIDONIC ACID
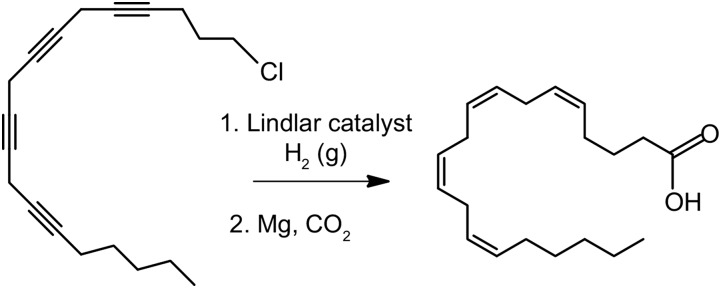
By the end of the 1950s, it was generally accepted that arachidonic acid had an all cis arrangement of the double bonds at carbon atoms 5,8,11,14 in an unbranched 20-carbon chain. This was based largely on the previously described work as well as integration of the biochemical studies of arachidonic acid as an essential fatty acid. In order to firmly prove this structure, chemical synthesis of arachidonic acid in an unambiguous manner was needed. Such a synthesis was reported in 1961 by two independent groups, one at Hoffman LaRoche Laboratories (26) and the other from Boston University (27). Both prepared the identical triple-bonded intermediate (Fig. 8). This tetraalkyne was then reduced by the well-known Lindlar catalyst, which rather effectively produces cis double bonds from triple bonds (26). The tetra-cis product (Fig. 8) was then carbonylated to yield arachidonic acid (25, 26). The resulting synthetic product was compared with arachidonic acid from natural sources. The comparisons included gas chromatographic retention times of the methyl esters. Both the natural and the synthetic had a retention time of 57 min, using an eight foot glass gas chromatographic column, in one of the first reported uses of GC to determine arachidonate purity and identity. The synthetic arachidonic acid was also compared with arachidonate purified by normal phase chromatography, which had now come into the tool box of the lipid chemist (28).
Fig. 8.
Final chemical steps employed in the synthesis of arachidonic acid that define the cis configurations of all double bonds (26, 27).
Thus, it took approximately 50 years to carry out the full structural characterization of arachidonic acid in terms of carbon chain length, positions of double bonds, and geometry of the double bonds. Using modern technology, this is a rather trivial experiment using techniques such as MS and NMR spectroscopy. Remarkably, and to the best of the present authors’ knowledge, the complete structural analysis employing uniformly-labeled [13C]arachidonic acid by NMR has not been reported. Nonetheless, a wealth of current methods supersede the hurdles overcome by Hartley and his successors in defining one of the taken-for-granted fundamentals of lipid biochemistry, the structures of arachidonic acid and the essential fatty acids.
Acknowledgments
The authors wish to acknowledge Professor Robert Freedman (Warwick University, UK) and Walter Shaw, a former student in the Department of Physiological Chemistry at Ohio State University, for their kind assistance with this manuscript. This historical review was made possible, in part, through contributions of photographs from The Royal Society, The Royal Society of Chemistry, and the British Federation of Women Graduates and their kind permission to reproduce them in this review. The photo of Dr. Ida Smedley-Maclean included in this manuscript originally appeared in History of the British Federation of University Women, 1907–1957 by J. H. Sondheimer (1958), a book published by the British Federation of University Women, now known as the British Federation of Women Graduates. The authors wish to acknowledge the British Federation of Women Graduates’ kind permission to reproduce the photo here.
Footnotes
This work was supported by Office of Extramural Research, National Institutes of Health Grants U54 HL117798 and GM15431. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Müller A. 1927. An x-ray investigation of certain long-chain compounds. Proc. R. Soc. A. 114: 542–562. [Google Scholar]
- 2.Hofstaedter P. G. 1854. Untersuchung des Fettes des Kopfes des Pottwalls (Physeter macrocephalus Shaw). Annalen der Chemie Pharmacie. 91: 177–185. [Google Scholar]
- 3.Hazura K. 1898. Über Trochnende Ölsäuren. Monatsh. Chem. 9: 180–197. [Google Scholar]
- 4.Edmed F. G. 1898. LXV.—Constitution of oleic acid and its derivatives. Part I. J. Chem. Soc. Trans. 73: 627–634. [Google Scholar]
- 5.Hazura K., and Grüssner A.. 1888. Über die Oxydation ungesättigter Fettsäuren mit Kaliumpermanganat. Monatsh. Chem. 9: 944–955. [Google Scholar]
- 6.Bull H. 1899. Über die Bestimmung Stark Ungesättigter Fettsäuren in den Thranen. Chemiker-Zeitung. 23: 1043–1044. [Google Scholar]
- 7.Tsujimoto M. 1906. On a new unsaturated fatty acid in japanese sardine oil. J. College Engineering Tokyo. 4: 1–10. [Google Scholar]
- 8.Dale H. H. 1957. Percival Hartley 1881–1957. Biogr. Mem. Fellows R. Soc. 3: 80–100. [Google Scholar]
- 9.Hartley P. 1909. On the nature of the fat contained in the liver, kidney, and heart. Part II. J. Physiol. 38: 353–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roaf H. E., and Edie E. S.. 1905. Williams and Norgate, London. [Google Scholar]
- 11.Lewkowitsch J. 1913. Chemical Technology and Analysis of Oils, Fats and Waxes. 5th edition. Macmillan, London. [Google Scholar]
- 12.Wesson L. G. 1924. The isolation of arachidonc acid from brain tissue. J. Biol. Chem. 60: 183–187. [Google Scholar]
- 13.Goldsobel G. 1910. Structure of drying oils. J. Russ Phys. Chem. Soc. 42: 55–57. [Google Scholar]
- 14.Erdmann E., Bedford F., and Raspe F.. 1909. Konstitution der Linolensäure. Ber. Dtsch. Chem. Ges. 42: 1334–1346. [Google Scholar]
- 15.Burr G. O., and Burr M. M.. 1930. On the nature and role of the fatty acids essential in nutrition. J. Biol. Chem. 86: 587–621. [Google Scholar]
- 16.Spector A. A., and Kim H-Y.. 2015. Discovery of essential fatty acids. J. Lipid Res. 56: 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown J. B., and Beal G. D.. 1923. The highly unsaturated fatty acids of fish oils. J. Am. Chem. Soc. 45: 1289–1303. [Google Scholar]
- 18.Rayner-Canham M. F., and Rayner-Canham G.. 2008. Chemistry was Their Life: Pioneer British Women Chemists, 1880–1949. Imperial College Press, London. [Google Scholar]
- 19.Nunn L. C., and Smedley-Maclean I.. 1938. The nature of the fatty acids stored by the liver in the fat-deficiency disease of rats. Biochem. J. 32: 2178–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shinowara G. Y., and Brown J. B.. 1940. Studies on the chemistry of the fatty acids: VI. The application of crystallization methods to the isolation of arachidonic acid, with a comparison of the properties of the acid prepared by crystallization and by debromination, observations on the structure of arachidonic acid. J. Biol. Chem. 134: 331–340. [Google Scholar]
- 21.Dolby D. E., Nunn L. C. A., and Smedley-Maclean I.. 1940. The constitution of arachidonic acid (preliminary communication). Biochem. J. 34: 1422–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mowry D. T., Brode W. R., and Brown J. B.. 1942. Studies on the chemistry of the fatty acids. X. The structure of arachidonic acid as evidenced by oxidative degradation and selective hydrogenation. J. Biol. Chem. 142: 679–691. [Google Scholar]
- 23.Holman R. T. 1951. Metabolism of isomers of linoleic and linolenic acids. Proc. Soc. Exp. Biol. Med. 76: 100–102. [DOI] [PubMed] [Google Scholar]
- 24.Sinclair R. G., McKay A. F., Myers G. S., and Jones R. N.. 1952. The infrared absorption spectra of unsaturated fatty acids and esters. J. Am. Chem. Soc. 74: 2578–2585. [Google Scholar]
- 25.Holman R. T., and Edmondson P. R.. 1956. Near-infrared spectra of fatty acids and some related substances. Anal. Chem. 28: 1533–1538. [Google Scholar]
- 26.Rachlin A. J., Wasyliw N., and Goldberg M. W.. 1961. Synthesis of arachidonic acid. J. Org. Chem. 26: 2688–2693. [Google Scholar]
- 27.Ege S. N., Wolovsky R., and Gensler W. J.. 1961. Synthesis of methyl 5,8,11,14-eicosatetraenoate (methyl arachidonate). J. Am. Chem. Soc. 83: 3080–3085. [Google Scholar]
- 28.Herb S. F., Riemenschneider R. W., and Donaldson J.. 1951. Isolation of natural arachidonic acid as its methyl ester. J. Am. Oil Chem. Soc. 28: 55–58. [Google Scholar]