Abstract
Marijuana use among women is highly prevalent, but the societal conversation on marijuana rarely focuses on how marijuana affects female reproduction and endocrinology. This article reviews the current scientific literature regarding marijuana use and hypothalamic-pituitary-ovarian (HPO) axis regulation, ovarian hormone production, the menstrual cycle, and fertility. Evidence suggests that marijuana can reduce female fertility by disrupting hypothalamic release of gonadotropin releasing hormone (GnRH), leading to reduced estrogen and progesterone production and anovulatory menstrual cycles. Tolerance to these effects has been shown in rhesus monkeys, but the effects of chronic marijuana use on human female reproduction are largely unknown. Marijuana-induced analgesia, drug reinforcement properties, tolerance, and dependence are influenced by ovarian hormones, with estrogen generally increasing and progesterone decreasing sensitivity to marijuana. Carefully controlled regulation of the Endocannabinoid System (ECS) is required for successful reproduction, and the exogenous cannabinoids in marijuana may disrupt the delicate balance of the ECS in the female reproductive system.
Keywords: marijuana, endocannabinoid system, female reproduction, menstrual cycle, fertility, estrogen, progesterone, HPO axis
Introduction
Marijuana is the most commonly abused illicit drug in the world [1]. In the United States (U.S.), about 40 to 50 percent of adults have used marijuana at least once [2,3]. Data from the 2014 National Survey on Drug Use and Health (NSDUH) indicate that 8.4 percent of Americans ages 12 and older are current marijuana users (i.e., have used marijuana in the past month) [4]. Out of all Americans who currently use any illicit drug, about 80 percent use marijuana [4]. The frequency and severity of marijuana use in the U.S. are also striking. Of current marijuana users, about 40 percent are daily or near daily users [5]. In 2014, approximately 4.2 million Americans endorsed cannabis dependence or abuse, as defined by the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, or DSM-IV [4]. Cannabis use disorder, which is characterized by disruptions in daily functioning due to marijuana use, cravings for marijuana, and withdrawal upon marijuana abstinence, is endorsed by a far greater number of Americans than any other illicit drug use disorder, including prescription pain relievers (1.9 million), cocaine (0.9 million), heroin (0.6 million), or stimulants (0.5 million) [4].
Although men are more likely to use drugs [5] and have a substance use disorder [6], drug abuse seriously impacts women’s health. After alcohol and heroin, marijuana is the most common primary drug of abuse for women entering treatment for substance abuse [7]. Females appear to be more sensitive to the behavioral and physiological effects of marijuana and marijuana-like substances [8], and treatment-seeking women endorse more severe marijuana withdrawal symptoms than treatment-seeking men [9]. After tobacco and alcohol, marijuana is the most commonly abused substance by women of childbearing age [5]. According to the 2013 NSDUH, 5.4 percent of pregnant women and 11.4 percent of non-pregnant women ages 15 to 44 are current illicit drug users [5], with marijuana representing 64 to 79 percent of female drug use [5,10-12]. As marijuana becomes more widely legalized, marijuana use by women will likely increase. Although the impact of marijuana on women’s reproductive health is rarely considered in the societal conversation on marijuana, reports that marijuana use disrupts female endocrinology and reproductive function should call greater attention to this issue. This review will focus on how marijuana and its biological target, the Endocannabinoid System (ECS), interface with the female reproductive system. This discussion will cover the impact of marijuana on the menstrual cycle, fertility and pregnancy, as well as the role of the ECS in each of these processes.
Marijuana and the Female Reproductive System
Marijuana, the Menstrual Cycle, and the Hypothalamic-Pituitary-Ovarian Axis
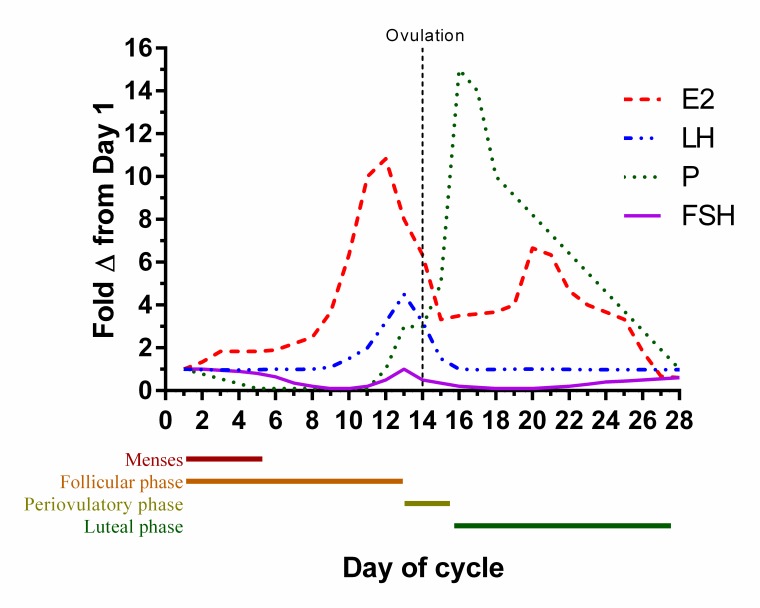
The menstrual cycle is a multiphasic process of the female reproductive system that promotes readiness to conceive and carry out a pregnancy. The ovarian phases and hormonal changes associated with the menstrual cycle are illustrated in Figure 1 and are briefly described as follows [13]. The first half of the menstrual cycle is composed of the follicular phase. This phase is characterized by the maturation of a small subset of oocyte follicles in the ovaries, which is induced by the gonadotropin follicle-stimulating hormone (FSH). Estrogen production by the follicles sharply increases as the primary follicle matures and circulating estrogen levels peak as the phase ends. Soon afterwards, the release of an oocyte from the mature follicle is triggered by a surge of the gonadotropin luteinizing hormone (LH) in a process known as ovulation. During the luteal phase, which immediately follows ovulation, the empty ovarian follicle produces and secretes relatively large concentrations of progesterone. Progesterone promotes the maintenance of the endometrial lining in a state that can support implantation and the early stages of pregnancy. The absence of a conceptus leads to decreased progesterone production, which leads to the end of the luteal phase. Menses, which is Day 1 of the new cycle, signifies the end of the cycle.
Figure 1.
Relative changes in circulating gonadotropin and ovarian hormone levels throughout the phases of the menstrual cycle. Levels of each hormone are expressed relative to their Day 1 levels. E2 = estradiol; FSH = follicle stimulating hormone; LH = luteinizing hormone; P= progesterone.
Few studies have examined the effects of marijuana on the menstrual cycle, but these studies suggest that there exists an association between marijuana use and menstrual cycle disruptions. Women who use marijuana have a slightly elevated rate of menstrual cycles that lack ovulation (anovulatory cycles) [14]. Individuals in this population are also at higher risk for decreased fertility due to ovulatory abnormalities [15]. One study found an association between occasional marijuana use (self-reported 1 to 3 times in the three months preceding the study) and prolonged follicular phase (3.5 days), resulting in delayed ovulation [14]. The authors of this study remarked that in contrast to previous findings by other investigators, they found no difference with respect to marijuana use on the length of the luteal phase. In the study they referenced, self-reported chronic moderate-to-heavy marijuana use (at least 3 times per week over the six months preceding the study) was associated with greater frequency of menstrual cycles that were anovulatory and /or had a luteal phase shorter than 11 days (38.3 percent and 12.5 percent in marijuana users and control participants, respectively) [16]. No results pertaining to the follicular phase were reported in this study. The discrepancies regarding menstrual phase length reported in these studies may be due to differences in frequency of dosing (occasional versus moderate-to-heavy). These studies had relatively small sample sizes and did not control for the amount of marijuana used at each exposure or the use of other substances, such as alcohol or tobacco use, which could affect the menstrual cycle. Therefore, the results of these studies should be interpreted with caution and more studies that rigorously examine the relationship of marijuana use and menstrual cycle irregularities in humans are needed.
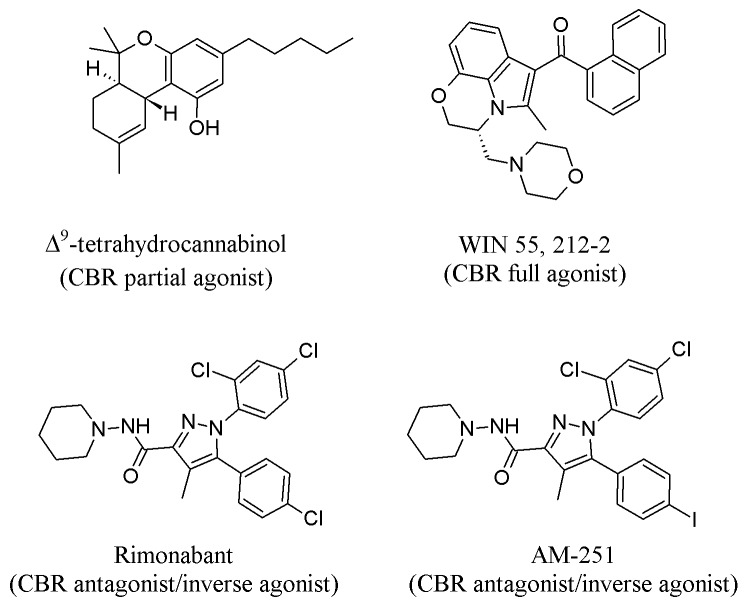
Rhesus monkeys provide one of the best animal models for the human female reproductive system because they have a 28-day menstrual cycle that is regulated by mechanisms similar to those in the human [17]; therefore, most primate studies on the effects of marijuana on the female reproductive system have been conducted in the rhesus monkey. In many of the studies cited in this review, the menstrual cycle phase-specific effects of marijuana use are determined by restricting acute or sub-chronic exposure to one phase and measuring the effects of exposure on hormonal profile, phase length and markers of phase hallmarks (e.g., menses, ovulation). Furthermore, most studies in non-human species administer the isolated psychoactive component of marijuana, Δ9-tetrahydrocannabinol (Δ9-THC, Figure 2), via injection for ease and consistency in dosing (compared to inhalation). These injections are usually intramuscular (IM) in non-human primates and intraperitoneal (IP,) or sometimes subcutaneous (SC,) in mice and rats. Almost all rhesus monkey studies cited in this review administered 2.5 mg/kg (milligrams per kilogram) Δ9-THC, IM, because this dose produces Δ9-THC blood levels comparable to those of human moderate-to-heavy marijuana users [18]. In addition to Δ9-THC, other similar drugs (called “cannabinoids”) are administered to rodent models. One such drug is WIN 55,212-2 (WIN), which is used instead of Δ9-THC for intravenous (IV) cannabinoid self-administration (SA) studies because rodents acquire and maintain WIN SA, but not Δ9-THC SA [19].
Figure 2.
Chemical structures of cannabinoids, agonists and antagonists discussed in this review. Structures obtained from The PubChem Project [108-111].
Two studies in monkeys reported the effects of Δ9-THC exposure on menstrual cycle phase length and ovulation. Daily intramuscular injections of Δ9-THC during the follicular phase of the menstrual cycle induced longer, anovulatory cycles [18]. Luteal phase length was not affected by daily Δ9-THC administration during the luteal phase at doses up to 5.0 mg/kg [20].
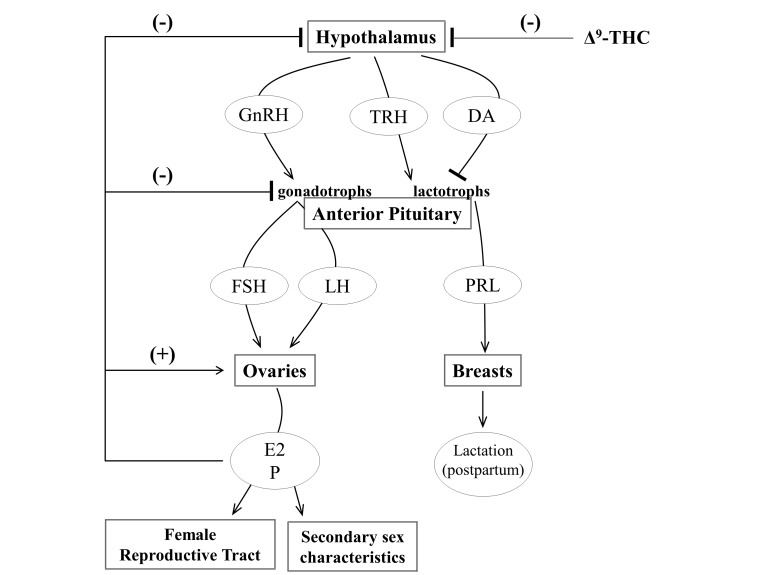
The mechanisms by which marijuana disrupts the menstrual cycle involve the hypothalamic-pituitary-ovarian (HPO) axis, which regulates female reproduction [13] (Figure 3). Overall findings from human and animal studies suggest that acute Δ9-THC suppresses the release of gonadotropin-releasing hormone (GnRH) and thyrotropin-releasing hormone (TRH) from the hypothalamus, preventing these hormones from stimulating the release of prolactin and the gonadotropins, follicle stimulating hormone (FSH) and luteinizing hormone (LH), from the anterior pituitary. The gonadotropins maintain the menstrual cycle by promoting ovarian follicle maturation, stimulating production of the ovarian steroids estradiol and progesterone, and inducing ovulation, and alterations in circulating gonadotropin can disrupt these processes. Studies examining the effects of marijuana and Δ9-THC on plasma gonadotropin, prolactin and ovarian hormone levels are summarize in Table 1. An acute inhaled dose totaling 1 gram of marijuana that was smoked in a single session was sufficient to suppress plasma LH during the luteal, but not follicular, phase of the menstrual cycle in humans [21,22]. The suppression of LH during the early luteal phase may terminate early pregnancies by reducing ovarian production of progesterone [23], a hormone that is necessary to maintain and support pregnancy. Consistent with this finding in humans, a study in rhesus monkeys found that a single, clinically relevant intramuscular dose of Δ9-THC (2.5 mg/kg) during the mid-luteal phase of the menstrual cycle decreased circulating progesterone levels in rhesus monkeys [24]. This effect was reversed by human chorionic gonadotropin (hCG) [24], suggesting that Δ9-THC-induced inhibition of progesterone was caused by suppression of gonadotropin release from the anterior pituitary gland. Studies in ovariectomized monkeys indicate that like LH and prolactin, FSH levels decrease in response to acute Δ9-THC administration [25]. Consequences of FSH suppression in cycling, intact females include reductions in ovarian follicle development, oocyte maturation, and gonadal steroid production, potentially leading to anovulatory menstrual cycles and infertility.
Figure 3.
A simplified representation of the hypothalamic-pituitary-ovarian (HPO) axis. Hypothalamic stimulation elicits the release of gonadotropin-releasing hormone (GnRH), thyrotropin-releasing hormone (TRH), and dopamine (DA) onto the anterior pituitary, which contains specialized neurons that are sensitive to these hormones. TRH stimulates and dopamine inhibits the release of prolactin (PRL) from the lactotrophs of the anterior pituitary. Prolactin promotes milk production during the postpartum period. GnRH stimulates the release follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from gonadotrophs in the anterior pituitary. FSH and LH promote the ovarian production of estrogen (primarily estradiol, E2), development of mature ovarian follicles, the release of oocytes from the mature ovarian follicles during ovulation and the production of progesterone (P) from the post-ovulatory follicle. The ovarian hormones, particularly E2, signal at the ovaries to promote follicle maturation. The ovarian hormones also exert negative feedback on the pituitary and hypothalamus to decrease release of FSH, LH and GnRH.
Table 1. Effects of marijuana or Δ9-THC on circulating gonadotropins, prolactin and ovarian hormones in female humans and rhesus monkeys.
| Treatment | Primary outcomes of treatment condition (marijuana or Δ9-THC) | |||||||||||||
| Ref | Subjects | Design | Dose | Route | Timing | FSH | LH | E2 | P | PRL | ||||
| 18 | 5 female rhesus monkeys | repeated measures | Δ9-THC (2.5 mg/kg) or vehicle | IM | daily during the follicular phase | n.d. | Supressed LH surge | Altered patters of circulating total estrogens across the cycle (supressed pre-ovulatory spike) | Suppressed luteal elevation of P | No effect | ||||
| 20 | 4 female rhesus monkeys | repeated measures | Δ9-THC (2.5 mg/kg) or vehicle | IM | daily during the luteal phase (from the day after ovulation until menses) | n.d. | n.d. | n.d. | No effect | n.d. | ||||
| 21,22 | 8 healthy human female marijuana users | repeated measures, double blind, placebo controlled | 1-gram standardized marijuana joint (1.83% Δ9-THC) or placebo joint | inh | single dose during follicular phase | n.d. | No effect | No effect | No effect | No effect | ||||
| 21,22 | 8 healthy human female marijuana users | repeated measures, double blind, placebo controlled | 1-gram standardized marijuana joint (1.83% Δ9-THC) or placebo joint | inh | single dose during luteal phase | n.d. | Decreased 60-120 mins after dose | No effect | No effect | Decreased 60-120 mins and 150-180 after dose. | ||||
| 24 | 3 female rhesus monkeys | repeated measures | Δ9-THC (2.5 mg/kg) or vehicle | IM | single dose during mid-luteal phase (day 20-22) | n.d. | n.d. | n.d. | Decreased 6-24 hours after dose; effect was prevented by HCG | n.d. | ||||
| 25 | 5 ovarectomized female rhesus monkeys | repeated measures performed at least 10 days apart | single dose of Δ9-THC (0.3125, 0.625, 1.25, 2.5, 5.0 mg/kg) or vehicle. | IM | Non-cycling animals | Decreased 6-24 hours after 5.0 mg/kg dose, 12 hours after 2.5 mg/kg dose, and 6 hours after 0.625 and 1.25 mg/kg doses. | Decreased 6-12 hours after 2.5 and 5.0 mg/kg doses, and 6 hours after 0.625 and 1.25 mg/kg doses. No effect with 0.3 mg/kg. | n.d. | n.d. | n.d. | ||||
| 26 | 5 female rhesus monkeys | repeated measures, chronic study | Δ9-THC (1.25 mg/kg in 2 monkeys or 2.5 mg/kg in 3 monkeys) or vehicle | IM | 3X per week beginning on cycle day 1 and lasting for 230 days or until 2 consecutive ovulatory cycles occurred | n.d. | Decreased for ~100 days after treatment began in 2.5 mg/kg treated group | Decreased for ~100 days after treatment began in 2.5 mg/kg treated group | Decreased for ~100 days after treatment began in 2.5 mg/kg treated group | No effect | ||||
Abbreviations: Δ9-THC, delta-9-tetrahydrocannabinol; E2, estradiol; FSH, follicle-stimulating hormone; hCG, human chorionic gonadotropin; IM, intramuscular (injection); inh, inhalation; LH, luteinizing hormone; mg/kg, milligrams (of drug) per kilogram (of body weight); n.d., not determined; P, progesterone; PRL, prolactin
Like acute dosing, chronic and sub-chronic (i.e., isolated to a single phase of the menstrual cycle) dosing can alter HPO axis function, but sub-chronic administration of Δ9-THC during the luteal phase appears to affect HPO axis function less than acute administration with the same dose. For example, daily administration of 2.5 mg/kg (IM) of Δ9-THC throughout the entire luteal phase (day after ovulation until menses) failed to alter serum progesterone levels in rhesus monkeys [20]. This discrepancy between acute and sub-chronic studies of Δ9-THC administration during the luteal phase may be caused by development of rapid tolerance to the luteum-disrupting effects of Δ9-THC, but further study is needed to confirm this possibility. In contrast to this possible rapid tolerance during the luteal phase, daily administration of Δ9-THC (2.5 mg/kg IM) throughout the follicular phase disrupted follicle development, decreased estrogen and progesterone production, blocked LH surge and prevented ovulation, which was rescued by mid-cycle gonadotropin administration [18]. This finding suggests that centrally mediated functions of the HPO axis are susceptible to disruption by sub-chronic Δ9-THC exposure during the follicular phase. Chronic Δ9-THC administration across several consecutive menstrual cycles transiently disrupts HPO axis function in the rhesus monkey. Thrice-weekly administration of Δ9-THC (2.5 mg/kg) robustly suppressed serum estradiol, progesterone, LH, and prolactin, and inhibited ovulation and menses, but the monkeys developed complete tolerance to these effects 103 to 135 days after the initial administration of Δ9-THC [26]. I could identify no comparable studies of chronic or sub-chronic marijuana or Δ9-THC administration that have been conducted in humans.
The possibility that Δ9-THC disrupts the HPO axis at the hypothalamus or pituitary gland instead of the ovary is supported by several previous findings, including Δ9-THC-induced suppression of circulating prolactin and gonadotropins, the reversal of Δ9-THC-induced ovarian steroid suppression by exogenous gonadotropins [18,24], and the lack of Δ9-THC affinity for estrogen and progesterone receptors [27]. Furthermore, suppression of gonadotropins and prolactin by Δ9-THC can be reversed by exogenous gonadotropin-releasing hormone (GnRH) and thyrotropin-releasing hormone (TRH), respectively [25,28]. This finding indicates that the anterior pituitary can be stimulated by hypothalamic hormones in the presence of Δ9-THC, providing strong evidence that the hypothalamus, not pituitary gland, is suppressed by Δ9-THC (Figure 3).
As described above, the suppression of the HPO axis and dysregulation of female reproduction by marijuana and its psychoactive component Δ9-THC is well-established in non-human primates, but evidence suggests that ovarian hormones and their neuroactive precursors (neurosteroids) also influence the subjective, behavioral and physiological effects of marijuana. The influence of ovarian hormones and neurosteroids on the effects of marijuana has been investigated in preclinical studies by directly manipulating ovarian hormones. Such manipulations include treating ovariectomized rodents with estradiol and/or progesterone replacement, or modifying the hormonal systems of intact rodents using pharmacological agents or genetic modification (e.g., knock-out rodents). Other studies have also observed how estrous cycle phase affects the behavior of intact rodents. This approach is useful because each phase (diestrus, proestrus, estrus, and metestrus) has its own distinct ovarian hormone profile, with progesterone rapidly peaking during the second half of proestrous, and estrogen gradually climbing and peaking during proestrus as well [29]. Table 2, Table 3, Table 4, and Table 5 summarize the findings of these studies by hormone. One notable recent study found that pregnenolone, the precursor for progesterone, estrogen, and all other steroid hormones, was induced in the brain by Δ9-THC administration [30]. Pregnenolone, in turn, suppressed Δ9-THC-induced effects in rodents, including hypothermia, locomotor suppression, analgesia, catalepsy, hyperphagia, and intravenous self-administration of the Δ9-THC-like drug WIN (Table 2). Pregnenolone also suppressed Δ9-THC-induced neurobiological effects in areas of the brain important in reward processing, including increased firing rates of neurons in the ventral tegmental area (VTA), increased dopamine outflow from the VTA onto the nucleus accumbens (NAc), and inhibition of excitatory post synaptic currents in nucleus accumbal neurons [30]. This study indicates that a hormone that is structurally almost identical to progesterone (Figure 4) can “dampen” a wide range of marijuana’s effects, raising the possibility that progesterone may possess similar activity. While the interaction of progesterone with marijuana has not yet been extensively investigated, preclinical and human studies of other drugs of abuse suggest that progesterone may antagonize the abuse-related effects of other drugs. For example, a state of suppressed progesterone and elevated estrogen (i.e. the follicular phase, Figure 1) was associated with enhanced ratings of drug liking, euphoria, and “high” for cocaine [31,32] and amphetamines [33,34]. Because reward processing of drugs from different classes share the common mechanism of stimulating dopamine transmission in the NAc shell and extended amygdala complex [35], the finding that ovarian hormone profile can modulate cocaine and amphetamine reward signifies that the effects of ovarian hormones on marijuana-induced reward processing should also be considered and investigated. Ovarian hormone profiles may also affect the risk of drug use relapse. In human studies of males and females, exogenous progesterone attenuated the urge to smoke in abstinent nicotine-dependent smokers [36] and reduced cue-induced drug cravings in cocaine-dependent individuals. Cocaine-dependent women with higher levels of circulating progesterone reported lower cue- and stress-induced drug craving after individualized guided script imagery compared to cocaine-dependent women with lower levels of progesterone [37], suggesting that progesterone may have protective effects against relapse for some drugs.
Table 2. Pregnenolone (PN) modulation of Δ9-THC-induced effects [30].
| Subjects | Treatment | Outcomes |
| Adult male Sprague Dawley rats | In vitro analysis of excitatory postsynaptic currents (EPSC) of NAc brain slices; pretreatment of slices with 100 nM PN, followed by 20 µM bath of Δ9-THC | PN decreased Δ9-THC-induced inhibition of EPSCs; PN decreased Δ9-THC-induced presynaptic suppression of glutamate release. |
| Adult male Sprague Dawley rats | Microdialysis probe and recording electrode placement in the right NAc and right ventral tegmental area (VTA), respectively; PN (2 mg/kg, SC) 30 minutes before Δ9-THC (IV, 0.15-1.2 mg/kg). Recording began 15 minutes after Δ9-THC administration | PN decreased Δ9-THC-induced firing rate in the VTA; PN decreased Δ9-THC-induced dopamine outflow to the NAc. |
| Adult male Wistar rats | ad libitum feeding; PN (0.5-2 mg/kg, SC), Δ9-THC (0.5 mg/kg) | PN suppressed Δ9-THC-induced hyperphagia. |
| C57BL/6N mice | Slightly food restricted; PN (2 mg/kg, SC), Δ9-THC (1 mg/kg, IP). | PN suppressed Δ9-THC-induced hyperphagia. |
| PN (6 mg/kg, SC, 30 minutes before Δ9-THC, 10 mg/kg, IP) | PN decreased Δ9-THC-induced locomotor suppression, hypothermia, catalepsy, analgesia, and memory impairment. | |
| PN-synthesis inhibitor aminogluthetimide (AMG, 50 mg/kg, IP, 30 minutes before Δ9-THC, 10 mg/kg, IP) | PN increased Δ9-THC-induced locomotor suppression, hypothermia, catalepsy and analgesia; Effect reversed by PN injection. | |
| CD1 mice trained to self-administer WIN | PN (0, 2, 4 mg/kg, SC) 30 minutes before SA session | PN decreased responses on the active nosepoke hole; PN decreased breakpoint in progressive ratio schedule of reinforcement. |
Note: Abbreviations: AMG, aminogluthetimide; Δ9-THC, delta-9-tetrahydrocannabinol; EPSCs, excitatory postsynaptic currents; IP, intraperitoneal (injection); mg/kg, milligrams per kilogram; µM, micomolar; NAc, nucleus accumbens; PN, pregnenolone; SA, self-administration; SC, subcutaneous (injection); VTA, ventral tegmental area; WIN, WIN 55, 212-2.
Table 3. Combined estrogen/progesterone modulation of Δ9-THC-induced effects.
| Subjects | Treatment | Outcomes | Ref |
| 28 adult female humans | 1-gram standardized marijuana joint (1.83% Δ9-THC) or placebo joint during the follicular, luteal, and ovulatory phases | Menstrual cycle phase had no effect on marijuana-induced changes to pulse rate or subjective ratings of intoxication and confusion. | [38] |
| 30 adult female human with moderate-to-heavy marijuana use | No treatment; participants completed marijuana use diaries and the Moos Menstrual Distress Questionnaire (MDQ) daily for 3 consecutive menstrual cycles | No covariance of marijuana use and menstrual cycle phase. | [39] |
| Adult intact and ovariectomized female and intact male Lister Hooded and Long Evans rats | Ovarian hormone depletion (ovariectomy at 9-10 weeks of age and male rats) or presence (intact female rats); WIN self-administration acquisition, maintenance, and extinction | Intact females of both strains acquired WIN SA faster, administered more drug per session, and resisted extinction of WIN SA more robustly than did males and ovariectomized females. | [40] |
| Adult intact and ovariectomized female and intact male Lister Hooded rats | WIN self-administration acquisition and extinction; drug- and cue-induced reinstatement by priming with WIN (0.15 or 0.03 mg/kg, IP) with and without visual and/or auditory cues | Intact female rats reinstated WIN SA more robustly than did intact male or ovariectomized female rats across all drug- and cue-priming conditions. | [41] |
| Adult intact male and female Sprague-Dawley rats | Intracerebroventricular (ICV) administration of Δ9-THC (100 μg) five minutes before testing session | Females had shorter latencies to withdraw in nociceptive tests than males; females in estrus had shorter latency to withdraw than those in proestrus-estrus; Females in proestrus-estrus showed greater Δ9-THC-antinocieption than females in other phases and males. | [44] |
| Four-month old intact male and female Sprague-Dawley rats | Quantification of Δ9-THC and 11-OH- Δ9-THC in brain and serum 15, 30, 60, 120, and 240 minutes after Δ9-THC (10 mg/kg, IP); SKF525A (cytochrome P450 inhibitor, 25 mg/kg, IP) thirty minutes before Δ9-THC (10 mg/kg, IP) fifteen minutes before testing session; using HPLC | Females exhibited greater brain concentrations of 11-OH- Δ9-THC than males at 120 minutes post-injection; SKF525A decreased Δ9-THC-induced antinociception in females, but not males. | [45] |
| Adult gonadectomized or sham-gonadectomized female and male Sprague-Dawley rats | E2 (females) or testosterone (males) replacement or blank capsule controls (SC implants) immediately after gonadectomy; P (500 μg, SC, females only) or vehicle every 3 days, beginning 4 days after gonadectomy; Δ9-THC (30 mg/kg, IP) or vehicle twice daily for 6.5 days, with the final dose administered 30 minutes before tolerance testing session; Rimonabant (10.0 mg/kg, IP) or vehicle 4 hours after final Δ9-THC treatment, 5 minutes before dependence testing session. | Sham-gonadectomized females developed greater tolerance to Δ9-THC-induced hypothermia than sham males; E2 and P increased rimonabant-induced chewing in chronic Δ9-THC-treated female rats. | [46] |
| Adult intact male and female Sprague-Dawley rats | ED80 dose of Δ9-THC (IP) or vehicle twice daily for 9 days; cumulative dosing of Δ9-THC (IP) on pre-chronic (1.8-32.0 mg/kg) and post-chronic (18.0-180.0 mg/kg) test days | Females developed greater tolerance to Δ9-THC-induced antinociception than males. | [47] |
| Adult gonadectomized or sham-gonadectomized female and male Sprague-Dawley rats | E2 (females) or testosterone (males) replacement or blank capsule controls (SC implants) immediately after gonadectomy; daily P (500 μg, SC, females only) or vehicle, beginning 4 days after gonadectomy; ED80 dose of Δ9-THC (IP) or vehicle twice daily for 9 days; cumulative dosing of Δ9-THC (IP) on pre-chronic (1.8-32.0 mg/kg) and post-chronic (18.0-180.0 mg/kg) test days | Females developed greater tolerance to Δ9-THC-induced antinociception than males in a non-ovarian-hormone- dependent manner. | [48] |
Note: Abbreviations: Δ9-THC, delta-9-tetrahydrocannabinol; E2, estradiol; HPLC, high performance liquid chromatography; ICV, intracerebroventricular (injection); IP, intraperitoneal (injection); μg, micrograms; MDQ, Moos Menstrual Distress Questionnaire; mg/kg, milligrams per kilogram; P, progesterone; SC, subcutaneous (injection).
Table 4. Estrogen modulation of Δ9-THC-induced effects.
| Subjects | Treatment | Outcomes | References |
| Ovariectomized female Long-Evans rats | The dose-effect of Δ9-THC (0.56-3.2 mg/kg, IP) on repeated acquisition and performance of a 4-response sequence memory and learning operant task in the presence of either E2 replacement or cholesterol control via SC implanted capsule | E2 alone improved response accuracy. E2 attenuated the Δ9-THC-induced reduction in response accuracy during acquisition and performance and reduction in response rate during acquisition. | [42] |
| Intact or ovariectomized female Sprague-Dawley rats | E2 replacement or blank capsule controls (SC implants) immediately after ovariectomy; determination of estrous cycle phase of intact females by daily vaginal lavage; acute Δ9-THC (5 or 10 mg/kg, IP) 15 minutes before testing session | E2 enhanced Δ9-THC-induced antinociception; Δ9-THC-induced antinociception was greatest during the estrus phase in intact cycling females; E2 had no effect on Δ9-THC-induced locomotor supression or catalepsy; Testosterone attenuated Δ9-THC-induced locomotor suppression. | [43] |
| Adult ovariectomized or sham- ovariectomized female Sprague-Dawley rats | E2 replacement or blank capsule controls (SC implants) immediately after ovariectomy; P (500 μg, SC) or vehicle every 3 days, beginning 4 days after ovariectomy ; Δ9-THC (30 mg/kg, IP) or vehicle twice daily for 6.5 days, with the final dose administered 30 minutes before tolerance testing session. Rimonabant (10 mg/kg, IP) or vehicle were administered 4 hours later, 5 minutes before dependence testing session | E2 increased locomotor activity in Rimonabant-treated ovariectomized female rats. | [46] |
Note: Abbreviations: Δ9-THC, delta-9-tetrahydrocannabinol; E2, estradiol; IP, intraperitoneal (injection); μg, micrograms; mg/kg, milligrams per kilogram; P, progesterone; SC, subcutaneous (injection).
Table 5. Progesterone modulation of Δ9-THC-induced effects.
| Subjects | Treatment | Outcomes | References |
| Adult ovariectomized or sham- ovariectomized female Sprague-Dawley rats | E2 replacement or blank capsule controls (SC implants) immediately after ovariectomy; P (500 μg, SC) or vehicle every 3 days, beginning 4 days after ovariectomy; Δ9-THC (30 mg/kg, IP) or vehicle twice daily for 6.5 days, with the final dose administered 30 minutes before tolerance testing session. Rimonabant (10 mg/kg, IP) or vehicle were administered 4 hours later, 5 minutes before dependence testing session | P enhanced tolerance to Δ9-THC-induced locomotor suppression in ovariectomized female rats; P enhanced Rimonabant-induced sniffing in chronic Δ9-THC-treated female rats. | [46] |
| Adult ovariectomized or sham- ovariectomized female Sprague-Dawley rats | E2 replacement or blank capsule control (SC implants) immediately after ovariectomy; daily P (500 μg, SC) or vehicle, beginning 4 days after ovariectomy; acute Δ9cumulative dosing of Δ9-THC (1.8-32.0 mg/kg, IP) 15 mins before testing session | P enhanced Δ9-THC-induced nociception in ovariectomized female rats. | [48] |
Note: Abbreviations: Δ9-THC, delta-9-tetrahydrocannabinol; E2, estradiol; IP, intraperitoneal (injection); μg, micrograms; mg/kg, milligrams per kilogram; P, progesterone; SC, subcutaneous (injection).
Figure 4.
Chemical structures of pregnenolone, progesterone and estrogen. Structures obtained from The PubChem Project [112-114].
The two studies that have examined the effects of marijuana across the menstrual cycle in humans produced null results [38,39], but several animal studies provide evidence that gonadal hormone regulation affects the abuse potential of cannabinoids (Table 3). One showed that intact female rats acquired self-administration of WIN much quicker, more often, and more robustly than male and ovariectomized female rats [40], suggesting that ovarian hormones (likely estrogen) contribute to the reinforcing effects of cannabinoid agonists like Δ9-THC. As demonstrated in another study that also compared intact females and males to ovariectomized female rats, ovarian hormones also promote cue- and drug-induced reinstatement of WIN self-administration and increase cannabinoid-seeking behavior [41].
In addition to possibly augmenting the abuse-related effects of cannabinoids, ovarian hormones may alter other Δ9-THC-related effects. Estradiol replacement treatment in ovariectomized adult rats attenuated Δ9-THC-induced decrements in learning and memory, as measured by response rate and accuracy in a nonspatial repeated acquisition and performance task [42] (Table 4). Overall, estradiol increases the sensitivity of female rats to Δ9-THC-induced antinociception, but not locomotor suppression or catalepsy [43]. Estrogen-modulated Δ9-THC-induced antinociception is largely, but not completely, due to supraspinal (central) mechanisms [44]. Peripheral mechanisms related to the metabolism of Δ9-THC likely also contribute to this effect. The induction of the CYP450 enzyme system by estrogen leads to greater metabolism of Δ9-THC to its active metabolite 11-OH-THC, which enhances antinociception in females versus males [45]. In a study examining the role of gonadal hormones on Δ9-THC tolerance and dependence, progesterone enhanced tolerance to the locomotor-suppressing effects of Δ9-THC (Table 5), and estrogen and progesterone increased precipitated withdrawal symptoms in rats chronically treated with Δ9-THC [46]. Another study found that intact female rats exhibited enhanced tolerance to Δ9-THC-induced antinociception compared to males [47]. Interestingly, this effect was not due to estrogen or progesterone [48]. Altogether, these findings indicate that there exists a bidirectional relationship between marijuana and the HPO axis, meaning that use of marijuana can alter HPO axis functionality, while ovarian hormones produced by the HPO axis impact the ultimate physiological, behavioral and subjective effects of marijuana.
Marijuana in Pregnancy Maintenance, Parturition and Lactation
Marijuana use during pregnancy is highly prevalent. Approximately 3.9 percent of pregnant women are current (past-month) users of marijuana [49], but few studies have examined the effects of marijuana on pregnancy, delivery, and lactation. Furthermore, experimental studies in which marijuana is administered to pregnant women are unethical, and observational studies in humans can be confounded by inaccurate self-reporting of marijuana use and of behavioral and sociodemographic variables that correlate with prenatal marijuana use and may impact pregnancy outcomes (e.g., age, socioeconomic status, prenatal care access, and use of tobacco, alcohol and other illicit drugs). For these reasons, in vitro and animal studies are vital for understanding how marijuana affects pregnancy. In vitro studies of early pregnancy indicate that Δ9-THC interferes with trophoblast proliferation [50] and turnover [51], which may negatively impact placentation. Marijuana can also affect hormonal regulation during pregnancy. One study showed that large doses of Δ9-THC-like drugs increased LH and estrogen in pregnant rats [52]. In contrast, a study in humans found no difference between pregnant marijuana users and gestational age-matched controls in serum concentrations of several pregnancy hormones and proteins, including human chorionic gonadotropin, pregnancy-specific beta-1-glycoprotein, placental lactogen, progesterone, 17-hydroxyprogesterone, estradiol, and estriol [53]. More studies on the effects of marijuana use on sex hormones in both pregnant and non-pregnant female humans are needed to confirm the relevance of these previous findings to human health.
Despite this null finding, marijuana use during pregnancy is associated with decreased gestational length (0.8 to 2.2 weeks earlier than non-marijuana-using sample) [10,54], and preterm birth (before 37 weeks gestation, OR = 1.5) [55]. One study suggests that cannabinoid use during pregnancy is associated with elevated risk of stillbirth (OR = 2.34), but tobacco use appears to partially confound this results, and the authors were unable to control the results for potential sociodemographic confounders [56]. A study in rhesus monkeys similarly found that administration of Δ9-THC early in pregnancy (starting on the day of pregnancy diagnosis) led to a high rate of spontaneous abortion 14 to 18 days after dosing initiation [57]. These spontaneous abortions were associated with a rapid drop in maternal chorionic gonadotropin and progesterone levels [57], an effect that is expected to terminate pregnancy. In the few pregnancies that continued to term, animals who received Δ9-THC had significantly elevated estrogen levels compared with vehicle controls [57], which is consistent with findings from a similar rat study [52]. The effect of in utero marijuana exposure on offspring outcomes is an important topic, but is outside the scope of the current discussion and is reviewed in greater depth elsewhere [58,59]
The effects of marijuana use during late pregnancy on parturition are largely unknown, but in vitro and rodent studies suggest that marijuana interferes with the production of several signaling molecules that are important in orchestrating labor and delivery, including prostaglandins [60], oxytocin [61], estrogen and progesterone.
Marijuana appears to have a suppressive effect on lactation. Acute Δ9-THC administered to lactating rats transiently suspended milk ejections and decreased ejection frequency compared to vehicle-treated controls [62]. Delta-1-THC administered to mice throughout pregnancy and lactation suppressed mammary gland lipoprotein lipase activity, delayed peak prolactin, and depressed mammary gland growth and development [63]. Marijuana use consistently suppresses prolactin levels in non-pregnant female humans [21], as does Δ9-THC administration in rhesus monkeys [18,26], but cannabinoid effects on prolactin levels, milk production and milk release in lactating primates are largely unknown. Oxytocin is a peptide hormone that is important for milk release during lactation, stimulating uterine contractions during parturition, and social bonding. In rats, chronic Δ9-THC exposure downregulates oxytocin in the nucleus accumbens and ventral tegmental area, which are two brain regions that are important for reward processing [61]. I could not identify research addressing the effects of marijuana or Δ9-THC on circulating oxytocin levels in humans or animal models.
To explore how marijuana exerts its effects on the female reproductive system, the discussion will now focus on the basic biological mechanisms of marijuana and how these mechanisms may interact with female reproduction.
Female Reproduction and the Endocannabinoid System (ECS)
The Endocannabinoid System
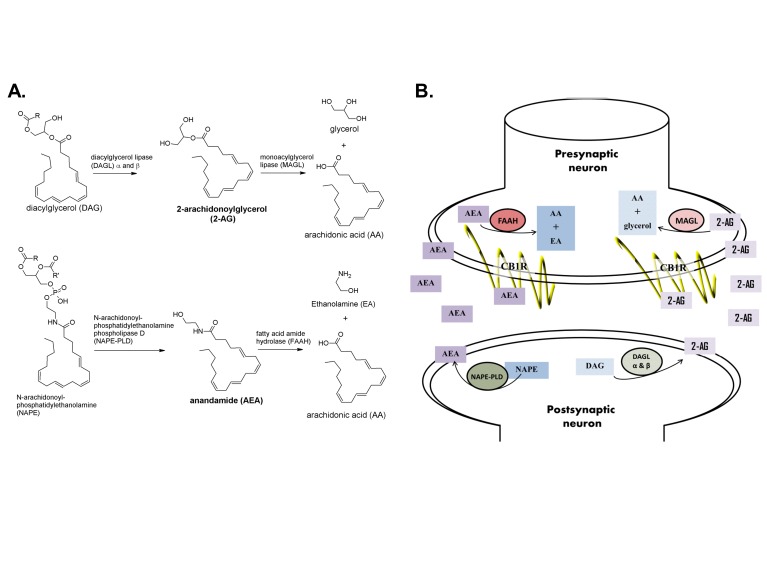
The Endocannabinoid System (ECS) is the biological system that mediates the effects of cannabinoids, including Δ9-THC, WIN, Rimonabant, AM-251 (Figure 2) and many others. The ECS is composed of the cannabinoid receptors (CB1R and CB2R), which are rhodopsin-like G-protein coupled receptors (GPCR), their endogenous ligands, and the enzymes that synthesize and degrade these endogenous ligands. Cannabinoids alter cannabinoid receptor activity by binding to the receptor and increasing its activity (i.e., acting as an agonist), decreasing its constitutive activity (i.e., acting as an inverse agonist) or blocking other ligands from accessing it (i.e., acting as an antagonist). Delta-9-THC, which is a CB1R and CB2R agonist, is one of hundreds of CBs expressed by the cannabis plant. Endogenous CBs, or endocannabinoids (eCBs), are lipophilic chemical messengers of the ECS that are produced “on demand” instead of being stored and transported in vesicles. The best-characterized eCBs are 2-arachidonylglycerol (2-AG), a full CBR agonist, and anandamide (AEA), a partial CB receptor agonist [64]. Figure 5 illustrates the formation, degradation, and retrograde signaling of these eCBs at the synapse [65-67]. In short, 2-AG is primarily synthesized by diacylglycerol lipase α and β (DAGL- α and DAGL- β) from diacylgylercerol (DAG) and is primarily broken down by monoacylglycerol lipase (MAGL). AEA is primarily synthesized by phospholipase C (PLC) and N-acyl phosphotidylethanolamine phospholipase D (NAPE-PLD) from phosphotidylethanolamine (NAPE) and is primarily degraded by fatty acid amide hydrolase (FAAH) (for more in-depth reviews of the ECS, please see [65-67]).
Figure 5.
Formation, degradation and retrograde signaling of endocannabinoids. A. The eCB 2-AG is formed primarily from DAG by DAGL-α and DAGL-β and is degraded by MAGL into AA and glycerol. The eCB AEA is formed primarily from NAPE by NAPE-PLD and degraded to AA and EA by FAAH. B. Neuronal eCB signaling occurs by retrograde processes; that is, eCBs are made in the postsynaptic neuron, passively diffuse out of the neuron, through the synaptic cleft and bind to cannabinoid receptors on pre-synaptic axonal terminals. eCBs activate CB1R, which tends to have an inhibitory, hyperpolarizing effect on the presynaptic neuron. The formation of eCBs is stimulated by increased signaling from the presynaptic neuron. Retrograde eCB signaling acts to suppress presynaptic neuronal activity by depolarization-induced suppression of inhibition (DSI, in GABAergic neurons) or depolarization-induced suppression of excitation (DSE, in glutamatergic neurons). Structures obtained from [65-67].
The ECS is distributed extensively throughout the human body and exerts influence on a multitude of biological processes. CB1R is densely expressed in the brain and modulates many CNS functions, including mood [68], appetite [69], and pain signaling [70]. Peripherally, the ECS is involved in bone remodeling [71], heavily modulates the immune system via CB2R [72], and promotes “thrifty” energy homeostasis by its CB1R-mediated actions in the liver, pancreas, gastrointestinal system, skeletal muscle and adipose tissue [73]. As described in the following sections, the ECS is also intricately involved in the female reproductive system [74], where a delicate balance of endocannabinoid production and degradation and well-regulated CBR activity are required for the reproductive tract and HPO axis to function optimally.
Interactions Between the ECS and the Female Reproductive System
ECS Expression in the Female Reproductive Tract
Components of the ECS are located throughout the reproductive tract, including in the ovary [75,76], Fallopian tubes [77], myometrium [78], and endometrium [79].
The ECS likely plays a role in the cycle of endometrial development by promoting endometrial plasticity. Methanandamide, a hydrolysis-resistant form of the eCB AEA, stimulates migration of endometrial stromal cells via ERK1/2 and PI3K/Akt activation downstream of CB1R [80]. Estradiol, but not progesterone, induces endometrial stromal cell migration by similar mechanisms [81]. Several components of the ECS, including CB1R, CB2R, the synthetic enzyme NAPE-PLD and the degradative enzyme FAAH, are highly expressed in the human endometrium, and expression levels of each protein changes throughout the menstrual cycle [79]. For example, CB1R protein expression in endometrial stroma is significantly lower during the mid-follicular phase, while CB2R in endometrial glands and stroma are robustly elevated from the mid-follicular to the late luteal phases [79]. FAAH expression in the endometrial glands is highest during menses and NAPE-PLD is lowest from the late follicular to late luteal phases [79].
The myometrium expresses several components of the ECS, including NAPE-PLD, FAAH, CB1R, but not CB2R [78]. The myometrium is also responsive to AEA stimulation of the CB1R, leading to signaling via Gαi/o-dependent inhibition of adenylate cyclase and activation of PI3K and ERK activation [78].
The ECS is active in the ovary during folliculogenesis, the process that produces mature oocytes and ovarian hormones. In both rat and human ovary, CB1R and CB2R are present in granulosa cells of follicles in several stages of maturity, from primordial tertiary, and in theca cells of secondary and tertiary follicles and corpus luteum and corpus albicans throughout the ovary [75,76]. FAAH and NAPE-PLD are detectable in theca cells and NAPE-PLD in granulosa cells [76] and AEA concentrations in follicular fluid increase as the ovarian follicle matures [76]. The functions of the ECS in the female reproductive tract remain unclear, but, as discussed below, their disruption can negatively impact reproduction and thus warrant further investigation.
ECS Activity in the Menstrual Cycle and HPO Axis
Multiple studies have determined that AEA concentrations in circulating lymphocytes are significantly elevated during the periovulatory phase of the menstrual cycle [82,83]. One study examined the relationships between AEA and HPO axis hormones and found that AEA was significantly correlated with serum LH, FSH, and estradiol, but not progesterone, in pre-menopausal women throughout the menstrual cycle [83]. Another study determined that expression of FAAH, the enzyme that degrades AEA, is the highest and AEA is the lowest during the postovulatory phase, and that FAAH is expressed at its lowest when AEA is at its highest during the periovulatory phase [82]. These findings suggest that regulation of the ECS and HPO axis are linked. More studies are needed to further uncover the mechanisms underlying this link.
Tissue concentrations of AEA in the anterior pituitary, but not hypothalamus, fluctuated significantly with estrous cycle phase in the intact female rat, with the highest concentrations expressed during estrus and the lowest during proestrous [84]. Estrogen replacement in ovariectomized rats was found to stimulate synthesis of AEA in medial basal hypothalamus neurons [85]. There were no phase-dependent changes in 2-AG tissue concentrations in the hypothalamus and pituitary. CB1R is also expressed in the anterior pituitary [84,86]. One study of intact female rabbits found that CB1R is expressed in the cytoplasm of anterior pituitary neurons where it co-localizes with estradiol-17β receptor type 1 [86]. A rat study was used in determining that gene expression of CB1R in the anterior pituitary was greatest during day 2 of diestrous and was more than double that of diestrous day 1 [84], indicating that robust changes to CB1R expression in the HPO axis can occur very rapidly. CB1R is also expressed in the hypothalamus and downregulated by estrogen [87].
In ovariectomized rats, centrally administered AEA significantly inhibited plasma LH levels relative to vehicle controls [85]. This observation is consistent with Δ9-THC-induced suppression of gonadotropins in rhesus monkeys. The AEA-induced inhibition of circulating LH was reversed by treating ovariectomized rats with estrogen, such that AEA stimulated plasma LH levels in the presence of estrogen. AEA also stimulated hypothalamic release of GnRH, but only in the presence of estrogen [85]. While this effect may signify that estrogen antagonizes the suppressive effect of AEA on LH release, these results may also be due, in part, to the difference in LH tone measured in an estrogen-deficient vs. estrogen-replacement state; ovariectomized rats had much higher plasma LH levels than did ovariectomized rats on estrogen replacement [85]. Interestingly, central administration of AEA produced equivalent levels of LH, regardless of basal LH tone; that is, AEA decreased elevated LH levels (in ovariectomized rats) and increased suppressed LH levels (in ovariectomized rats receiving estrogen replacement). This suggests that AEA is able to buffer estrogen-dependent fluctuations in circulating LH levels via mechanisms that are not well-understood, but may be induced by the downregulation of hypothalamic CB1R expression by estrogen [87]. Central administration of the CB1R antagonist/inverse agonist AM-251 in this study likewise yielded unexpected results. In ovarectomized rats, AM-251 reduced LH release even more than did AEA, and this effect appeared to be reversed by estrogen replacement treatment [85]. This paradoxical finding was also observed in intact female rabbits who were administered Rimonabant, another CB1R inverse agonist [86]. The mechanism identified in this study was Rimonabant-induced inhibition of LH secretory capacity by the pituitary, suggesting that the endocannabinoid system can signal at both the hypothalamus and pituitary to control LH release. These multiple sites of action, along with the sensitivity of hypothalamic CB1R expression to estrogen may contribute to the dependence of the LH-releasing actions of AEA and AM-251 on estrogen state.
The ECS in Conception, Implantation and Placentation
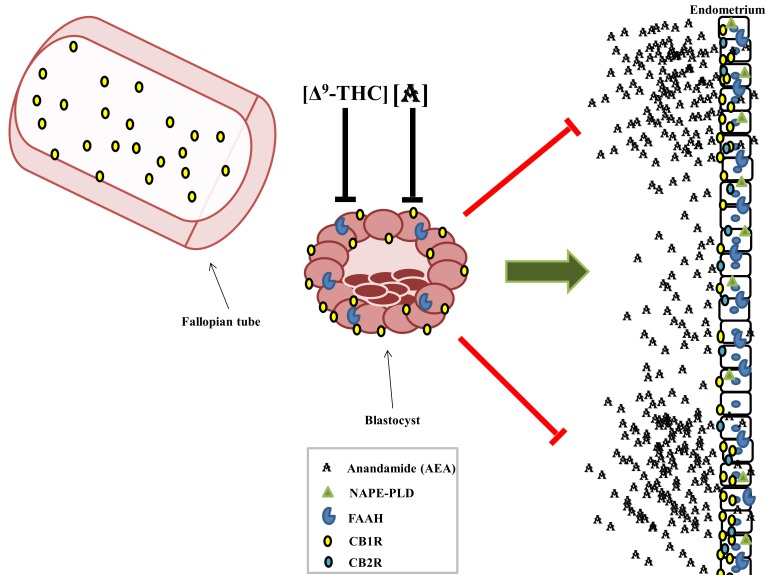
The appropriate expression of the ECS during conception and the peri-implantation period is imperative to successful implantation [88]. The CB1R is expressed extensively in the non-pregnant mouse and human uterus [79,89], and the ECS is tightly regulated in the decidua of the mouse and human endometrium [90]. Both cannabinoid receptors are expressed by the embryonic 2-cell stage [91], but activation of the embryonic CB1R by exogenous cannabinoids, such as those from marijuana, at the peri-implantation stage can result in arrested development of the embryo [92]. Studies with CB1R and CB2R knockout mice show that oviduct transport of a fertilized egg from the Fallopian tube to the uterus for implantation is CB1R-dependent, and oviducts that lack maternal CB1R retain zygotes, resulting in failed pregnancies [93]. Reduced CB1R expression in the Fallopian tube and decidua is also associated with ectopic pregnancies in humans [77]. Blastocysts that do not express CB1R or FAAH (i.e., Cnr-/- and Faah-/-) have compromised migration, attachment, and spreading capacities, which can interfere with their ability to successfully implant and invade the uterine wall [93]. Implantation can occur only in endometrial areas that express reduced levels of CB1R and the endocannabinoid AEA; the endometrial surface of the pre-receptive uterus and areas between perspective implantation sites express much more CB1R and AEA than implantation sites [88] (Figure 6). The blastocyst is rendered competent for implantation by low, but not high, concentrations of AEA in the endometrial environment, which activate blastocysts via embyronic CB1R [88]. The development of the placenta also appears to be regulated by the ECS. The metabolic and degradative enzymes of 2-AG, DAGL and MAGL, respectively, are expressed in human cytotrophoblasts [94]. AEA and 2-AG each induce apoptosis in cytotrophoblasts by CBR-mediated mechanisms [94-96]. Altogether, these reports indicate that tightly synchronized regulation of the maternal and embryonic ECS is required for successful oviduct transport, embryonic implantation, and placentation.
Figure 6.
The ECS promotes embryonic migration through the oviducts and uterine implantation. Adequate levels of CB1R expression are required in the Fallopian tube to allow passage of the embryo into the uterus and prevent ectopic implantation. Exposure to high concentrations of Δ9-THC or anandamide will arrest the development of the blastocyst, but relatively low levels of AEA are required to activate the blastocyst and promote implantation. The endometrium is spatially varied with respect to CB1R and AEA expression; implantation can only occur in areas that express low CB1R and AEA.
Pregnancy Maintenance, Parturition, Lactation and the ECS
Postimplantation, the ECS also plays a dynamic role in maintaining pregnancy, from the early 1st trimester [97] to parturition [98,99]. Deviations in the expression of one or more ECS components are associated with miscarriage. For example, in a large proportion of women with recurrent miscarriage, FAAH is aberrantly localized in the nuclei of trophoblastic cells [100], possibly reducing FAAH activity. Reduced FAAH activity, especially in circulating lymphocytes, can lead to elevated plasma AEA, which is also associated with increased risk of miscarriage [101]. Spontaneous miscarriage during the first trimester is also associated with a reduction or absence of placental FAAH with reduced placental NAPE-PLD expression and elevated placental CB1R [102]. Throughout pregnancy, placental endocannabinoid levels increase [103] and CB1R and FAAH are highly expressed in the placenta at term [104,105], suggesting that the ECS plays a role in pregnancy maintenance and preparation for parturition [105]. Prostaglandins induce cervical ripening and parturition late in pregnancy. The synthetic cannabinoid CP55,940 and endocannabinoids 2-AG and AEA increased prostaglandin PGE2 production in term amniotic and chorionic tissues in a CB1R- and COX-2 dependent manner [99]. Furthermore, plasma AEA levels increase during labor [98], but whether this effect contributes to labor, is a consequence of labor or is a correlate of labor has not yet been determined.
Conclusions and Outlook
The female reproductive system and ECS are intricately linked. Hormonal fluctuations throughout the menstrual cycle and pregnancy lead to changes in the expression of the cannabinoid receptors, endocannabinoids, and their associated synthetic and metabolic enzymes in the brain, ovaries, oviducts, uterus and in circulation. Altered ECS expression is associated with reduced fertility, ectopic pregnancy and spontaneous abortion. In addition to altering the ECS, cannabinoids found in marijuana, particularly Δ9-THC, exert inhibitory effects on hypothalamic release of GnRH and alter HPO axis regulation, potentially leading to disruption of the reproductive system. There remains much work to be done to understand how the ECS interacts with female reproduction. One intriguing consistency that should be explored further is the paradoxical effect of CB1R inverse agonists exhibiting agonist-like activity, particularly on LH release [85] and sexual motivation [106,107] in female rats. Understanding the basic mechanisms underlying these unexpected findings will provide greater insight into the functioning of the ECS in female reproduction. Of great importance, more human studies are needed to more fully understand the effects of marijuana dosing and chronicity on gonadotropin and ovarian hormone regulation, fertility, pregnancy maintenance, parturition and lactation. The changing legal status of marijuana in several US states simultaneously makes these types of studies more practical in more places and prompts their necessity in order to accurately inform the public on how marijuana affects women’s reproductive health.
Acknowledgments
This work was supported by the NIH (T32 DA022981). The author declares no conflicts of interest.
Abbreviations
- 2-AG
2-arachidonylglycerol
- AEA
anandamide
- CB1R
Cannabinoid Receptor type-1
- CB2R
Cannabinoid Receptor type-2
- CBs
cannabinoids
- Δ9-THC
Delta-9-tetrahydrocannabinol
- eCBs
endocannabinoids
- DAGL- α
diacylglycerol lipase α
- DAGL- β
diacylglycerol lipase β
- ECS
Endocannabinoid System
- E2
estradiol
- FAAH
fatty acid amide hydrolase
- FSH
follicle stimulating hormone
- GnRH
gonadotropin-releasing hormone
- GPCR
G-protein coupled receptors
- hCG
human chorionic gonadotropin
- HPO axis
hypothalamic-pituitary-ovarian axis
- inh
inhalation
- IMm
intramuscular
- IV
intravenous
- IP
intraperitoneal
- LH
luteinizing hormone
- MAGL
monoacylglycerol lipase
- mg/kg
milligrams (of drug) per kilogram (of body weight)
- NAPE-PLD
N-acyl phosphotidylethanolamine phospholipase D
- NSDUH
National Survey on Drug Use and Health
- n.d.
not determined
- PLC
phospholipase C
- P
progesterone
- PRL
prolactin
- SC
subcutaneous
- TRH
thyrotropin-releasing hormone
References
- WHO. World Health Organization Substance Abuse Facts--Cannabis. [Internet] Available from: http://www.who.int/substance_abuse/facts/cannabis/en/
- Desilver D. Nearly half of adults say they’ve tried marijuana, but not recently. Pew Reasearch [Internet] Available from: http://www.pewresearch.org/fact-tank/2013/06/03/more-than-a-third-of-adults-say-theyve-tried-pot-but-not-recently/
- Saad L. In U.S., 38% Have Tried Marijuana, Little Changed Since '80s. Princeton, NJ: Gallup [Internet]. [cited 2015 Nov 22]. Available from: http://www.gallup.com/poll/163835/tried-marijuana-little-changed-80s.aspx . English .
- Center for Behavioral Health Statistics and Quality. Behavioral health trends in the United States: Results from the 2014 National Survey on Drug Use and Health. 2015. Report No.: HHS Publication No. SMA 15-4927, NSDUB Series H-50 [Internet] Available from: http://www.samhsa.gov/data .
- Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014. Report No.: NSDUH Series H-48, HHS, Publication No. (SMA) 14-4863 [Internet]. Available from: http://www.samhsa.gov/data/sites/default/files/NSDUHresultsPDFWHTML2013/Web/NSDUHresults2013.pd .
- Grant BF, Saha TD, Ruan WJ. et al. Epidemiology of DSM-5 drug use disorder: Results from the national epidemiologic survey on alcohol and related conditions-III. JAMA Psychiatry. 2015;18:1–9. doi: 10.1001/jamapsychiatry.2015.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. Treatment Episode Data Set (TEDS): 2002-2012. National Admission to Substance Abuse Treatment Services. Report No.: BHSIS Series S-71, HHS Publication No. (SMA) 14-4850. Rockville, Maryland: SAMHSA; 2014. [Google Scholar]
- Craft RM. Sex differences in behavioral effects of cannabinoids. Life Sci. 2005;77(20):2471–2478. doi: 10.1016/j.lfs.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Herrmann ES, Weerts EM, Vandrey R. Sex differences in cannabis withdrawal symptoms among treatment-seeking cannabis users. Exp Clin Psychopharmacol. 2015;23(6):415–21. doi: 10.1037/pha0000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood RA, Keating J, Kavvadia V. et al. Substance misuse in early pregnancy and relationship to fetal outcome. Eur J Pediatr. 1999;158(6):488–92. doi: 10.1007/s004310051126. [DOI] [PubMed] [Google Scholar]
- Garcia-Serra J, Ramis J, Simo S. et al. Alternative biological materials to detect prenatal exposure to drugs of abuse in the third trimester of pregnancy. An Pediatr (Barc) 2012;77(5):323–328. doi: 10.1016/j.anpedi.2012.02.019. [DOI] [PubMed] [Google Scholar]
- Ebrahim EH, Gfroerer J. Pregnancy-related substance use in the united states during 1996-1998. Obstet Gynecol. 2003;101(2):374–379. doi: 10.1016/s0029-7844(02)02588-7. [DOI] [PubMed] [Google Scholar]
- Terranova PF. In: Medical Physiology. 2nd ed. Rhoades RA, Tanner GA, editors. Baltimore, Maryland: Lippincott Williams & Wilkins; 2003. The Female Reproductive System; pp. 667–683. [Google Scholar]
- Jukic AM, Weinberg CR, Baird DD. et al. Lifestyle and reproductive factors associated with follicular phase length. J Womens Health (Larchmt) 2007;16(9):1340–1347. doi: 10.1089/jwh.2007.0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller BA, Daling JR, Weiss NS. et al. Recreational drug use and the risk of primary infertility. Epidemiology. 1990;1(3):195–200. doi: 10.1097/00001648-199005000-00003. [DOI] [PubMed] [Google Scholar]
- Bauman J. Marihuana and the Female Reproductive System Testimony before the Subcommittee on Criminal Justice of the Committee on the Judiciary. U.S. Senate, Health Consequences of Marihuana Use [Internet] 1980. pp. 85–88. Available from: http://babel.hathitrust.org/cgi/pt?id=pur1.32754078039595;view=1up;seq=1 .
- Smith CG, Asch RH. In: NIDA Research Monograph. 44th ed. Braude MC, Ludford JP, editors. Rockville, Maryland: National Institute on Drug Abuse Office of Science; 1984. Acute, Short-Term and Chronic Effects of Marijuana on the Female Primate Reproductive Function in Marijuana Effects on the Endocrine and Reproductive Systems. [PubMed] [Google Scholar]
- Asch RH, Smith CG, Siler-Khodr TM. et al. Effects of delta 9-tetrahydrocannabinol during the follicular phase of the rhesus monkey (macaca mulatta). J Clin Endocrinol Metab. 1981;52(1):50–55. doi: 10.1210/jcem-52-1-50. [DOI] [PubMed] [Google Scholar]
- Lefever TW, Marusich JA, Antonazzo KR. et al. Evaluation of WIN 55,212-2 self-administration in rats as a potential cannabinoid abuse liability model. Pharmacol Biochem Behav. 2014;118:30–35. doi: 10.1016/j.pbb.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asch RH, Smith CG, Siler-Khodr TM. et al. Effects of delta 9-tetraphydrocannabinol administration on gonadal steroidogenic activity in vivo. Fertil Steril. 1979;32(5):576–582. doi: 10.1016/s0015-0282(16)44363-3. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK, Ellingboe J. et al. Acute effects of marihuana smoking on prolactin levels in human females. J Pharmacol Exp Ther. 1985;232(1):220–222. [PubMed] [Google Scholar]
- Mendelson JH, Mello NK, Ellingboe J. et al. Marihuana smoking suppresses luteinizing hormone in women. J Pharmacol Exp Ther. 1986;237(3):862–866. [PubMed] [Google Scholar]
- Fraser HM, Nestor JJ, Jr., Vickery BH. et al. Suppression of luteal function by a luteinizing hormone-releasing hormone antagonist during the early luteal phase in the stumptailed macaque monkey and the effects of subsequent administration of human chorionic gonadotropin. Endocrinology. 1987;121(2):612–618. doi: 10.1210/endo-121-2-612. [DOI] [PubMed] [Google Scholar]
- Almirez RG, Smith CG, Asch RH. et al. The effects of marijuana extract and delta 9-tetrahydrocannabinol on luteal function in the rhesus monkey. Fertil Steril. 1983;39(2):212–217. [PubMed] [Google Scholar]
- Smith CG, Besch NF, Smith RG. et al. Effect of tetrahydrocannabinol on the hypothalamic-pituitary axis in the ovariectomized rhesus monkey. Fertil Steril. 1979;31(3):335–339. doi: 10.1016/s0015-0282(16)43885-9. [DOI] [PubMed] [Google Scholar]
- Smith CG, Almirez RG, Berenberg J. et al. Tolerance develops to the disruptive effects of delta 9-tetrahydrocannabinol on primate menstrual cycle. Science. 1983;219(4591):1453–1455. doi: 10.1126/science.6298938. [DOI] [PubMed] [Google Scholar]
- Smith RG, Besch NF, Besch PK. et al. Inhibition of gonadotropin by delta9-tetrahydrocannabinol:Mediation by steroid receptors? Science. 1979;204(4390):325–327. doi: 10.1126/science.107589. [DOI] [PubMed] [Google Scholar]
- Asch RH, Smith CG, Siler-Khodr TM. et al. Acute decreases in serum prolactin concentrations caused by delta 9-tetrahydrocannabinol in nonhuman primates. Fertil Steril. 1979;32(5):571–575. doi: 10.1016/s0015-0282(16)44362-1. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Sofuoglu M. Role of progesterone in nicotine addiction: Evidence from initiation to relapse. Exp Clin Psychopharmacol. 2010;18(6):451–461. doi: 10.1037/a0021265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee M, Vitiello S, Bellocchio L. et al. Pregnenolone can protect the brain from cannabis intoxication. Science. 2014;343(6166):94–98. doi: 10.1126/science.1243985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 2002;159(4):397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D. et al. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol. 1999;7(3):274–283. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- Justice AJ, de Wit H. Acute effects of d-amphetamine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 1999;145(1):67–75. doi: 10.1007/s002130051033. [DOI] [PubMed] [Google Scholar]
- White TL, Justice AJ, de Wit H. et al. Differential subjective effects of D-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacol Biochem Behav. 2002;73(4):729–741. doi: 10.1016/s0091-3057(02)00818-3. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V. Reward system and addiction: What dopamine does and doesn't do. Curr Opin Pharmacol. 2007;7(1):69–76. doi: 10.1016/j.coph.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Mouratidis M, Mooney M. Progesterone improves cognitive performance and attenuates smoking urges in abstinent smokers. Psychoneuroendocrinology. 2011;36(1):123–132. doi: 10.1016/j.psyneuen.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox H, Hong KI. et al. Sex steroid hormones, stress response, and drug craving in cocaine-dependent women: Implications for relapse susceptibility. Exp Clin Psychopharmacol. 2007;15(5):445–452. doi: 10.1037/1064-1297.15.5.445. [DOI] [PubMed] [Google Scholar]
- Griffin ML, Mendelson JH, Mello NK. et al. Marihuana use across the menstrual cycle. Drug Alcohol Depend. 1986;18(2):213–224. doi: 10.1016/0376-8716(86)90053-0. [DOI] [PubMed] [Google Scholar]
- Lex BW, Mendelson JH, Bavli S. et al. Effects of acute marijuana smoking on pulse rate and mood states in women. Psychopharmacology (Berl) 1984;84(2):178–187. doi: 10.1007/BF00427443. [DOI] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Altea S. et al. Cannabinoid self-administration in rats: Sex differences and the influence of ovarian function. Br J Pharmacol. 2007;152(5):795–804. doi: 10.1038/sj.bjp.0707465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Altea S. et al. Drug- and cue-induced reinstatement of cannabinoid-seeking behaviour in male and female rats: Influence of ovarian hormones. Br J Pharmacol. 2010;160(3):724–735. doi: 10.1111/j.1476-5381.2010.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Winsauer PJ, Brauner IN. et al. Estrogen improves response accuracy and attenuates the disruptive effects of delta9-THC in ovariectomized rats responding under a multiple schedule of repeated acquisition and performance. Behav Neurosci. 2002;116(6):989–998. [PubMed] [Google Scholar]
- Craft RM, Leitl MD. Gonadal hormone modulation of the behavioral effects of Delta9-tetrahydrocannabinol in male and female rats. Eur J Pharmacol. 2008;578(1):37–42. doi: 10.1016/j.ejphar.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Wakley AA, Craft RM. Antinociception and sedation following intracerebroventricular administration of delta(9)-tetrahydrocannabinol in female vs. male rats. Behav Brain Res. 2011;216(1):200–206. doi: 10.1016/j.bbr.2010.07.037. [DOI] [PubMed] [Google Scholar]
- Tseng AH, Harding JW, Craft RM. Pharmacokinetic factors in sex differences in delta 9-tetrahydrocannabinol-induced behavioral effects in rats. Behav Brain Res. 2004;154(1):77–83. doi: 10.1016/j.bbr.2004.01.029. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Craft RM, Lefever TW. et al. The impact of gonadal hormones on cannabinoid dependence. Exp Clin Psychopharmacol. 2015;23(4):206–216. doi: 10.1037/pha0000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakley AA, Wiley JL, Craft RM. et al. Sex differences in antinociceptive tolerance to delta-9-tetrahydrocannabinol in the rat. Drug Alcohol Depend. 2014;143:22–28. doi: 10.1016/j.drugalcdep.2014.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakley AA, Wiley JL, Craft RM. et al. Gonadal hormones do not alter the development of antinociceptive tolerance to delta-9-tetrahydrocannabinol in adult rats. Pharmacol Biochem Behav. 2015;133:111–121. doi: 10.1016/j.pbb.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JY, Farr SL, Tong VT. et al. Prevalence and patterns of marijuana use among pregnant and nonpregnant women of reproductive age. Am J Obstet Gynecol. 2015;213(2):201. doi: 10.1016/j.ajog.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khare M, Taylor AH, Konje JC. et al. Delta9-tetrahydrocannabinol inhibits cytotrophoblast cell proliferation and modulates gene transcription. Mol Hum Reprod. 2006;12(5):321–333. doi: 10.1093/molehr/gal036. [DOI] [PubMed] [Google Scholar]
- Costa MA, Fonseca BM, Marques F. et al. The psychoactive compound of cannabis sativa, delta(9)-tetrahydrocannabinol (THC) inhibits the human trophoblast cell turnover. Toxicology. 2015;334:94–103. doi: 10.1016/j.tox.2015.06.005. [DOI] [PubMed] [Google Scholar]
- Rosenkrantz H, Esber HJ. Cannabinoid-induced hormone changes in monkeys and rats. J Toxicol Environ Health. 1980;6(2):297–313. doi: 10.1080/15287398009529853. [DOI] [PubMed] [Google Scholar]
- Braustein GD, Buster JE, Soares JR. et al. Pregnancy hormone concentrations in marijuana users. Life Sci. 1983;33(2):195–199. doi: 10.1016/0024-3205(83)90413-7. [DOI] [PubMed] [Google Scholar]
- Fried PA, Watkinson B, Willan A. Marijuana use during pregnancy and decreased length of gestation. Am J Obstet Gynecol. 1984;150(1):23–27. doi: 10.1016/s0002-9378(84)80103-9. [DOI] [PubMed] [Google Scholar]
- Hayatbakhsh MR, Flenady VJ, Gibbons KS. et al. Birth outcomes associated with cannabis use before and during pregnancy. Pediatr Res. 2012;71(2):215–219. doi: 10.1038/pr.2011.25. [DOI] [PubMed] [Google Scholar]
- Varner MW, Silver RM, Rowland Hogue CJ. et al. Association between stillbirth and illicit drug use and smoking during pregnancy. Obstet Gynecol. 2014;123(1):113–125. doi: 10.1097/AOG.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asch RH, Smith CG. Effects of delta 9-THC, the principal psychoactive component of marijuana, during pregnancy in the rhesus monkey. J Reprod Med. 1986;31(12):1071–1081. [PubMed] [Google Scholar]
- Fried PA, Smith AM. A literature review of the consequences of prenatal marihuana exposure. an emerging theme of a deficiency in aspects of executive function. Neurotoxicol Teratol. 2001;23(1):1–11. doi: 10.1016/s0892-0362(00)00119-7. [DOI] [PubMed] [Google Scholar]
- Brents LK. In: Handbook of Cannabis and Related Pathologies. Preedy V, editor. Cambridge, MA: Academic Press; 2017. Correlates and consequences of prenatal cannabis exposure (PCE): identifying and characterizing vulnerable maternal populations and determining outcomes for exposured offspring. [Google Scholar]
- Ruhaak LR, Felth J, Karlsson PC. et al. Evaluation of the cyclooxygenase inhibiting effects of six major cannabinoids isolated from cannabis sativa. Biol Pharm Bull. 2011;34(5):774–778. doi: 10.1248/bpb.34.774. [DOI] [PubMed] [Google Scholar]
- Butovsky E, Juknat A, Elbaz J. et al. Chronic exposure to Delta9-tetrahydrocannabinol downregulates oxytocin and oxytocin-associated neurophysin in specific brain areas. Mol Cell Neurosci. 2006;31(4):795–804. doi: 10.1016/j.mcn.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Tyrey L, Murphy LL. Inhibition of suckling-induced milk ejections in the lactating rat by delta 9-tetrahydrocannabinol. Endocrinology. 1988;123(1):469–472. doi: 10.1210/endo-123-1-469. [DOI] [PubMed] [Google Scholar]
- Raine JM, Wing DR, Paton WD. The effects of delta1-tetrahydrocannabinol on mammary gland growth, enzyme activity and plasma prolactin levels in the mouse. Eur J Pharmacol. 1978;51(1):11–17. doi: 10.1016/0014-2999(78)90056-0. [DOI] [PubMed] [Google Scholar]
- Gonsiorek W, Lunn C, Fan X. et al. Endocannabinoid 2-arachidonyl glycerol is a full agonist through human type 2 cannabinoid receptor: Antagonism by anandamide. Mol Pharmacol. 2000;57(5):1045–1050. [PubMed] [Google Scholar]
- Di Marzo V. The endocannabinoid system: Its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacol Res. 2009;60(2):77–84. doi: 10.1016/j.phrs.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Lu HC, Mackie K. An introduction to the endogenous cannabinoid system. Biol Psychiatry. 2016;19(7):516–525. doi: 10.1016/j.biopsych.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murataeva N, Straiker A, Mackie K. Parsing the players: 2-arachidonoylglycerol synthesis and degradation in the CNS. Br J Pharmacol. 2014;171(6):1379–1391. doi: 10.1111/bph.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorzalka BB, Hill MN. Putative role of endocannabinoid signaling in the etiology of depression and actions of antidepressants. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(7):1575–1585. doi: 10.1016/j.pnpbp.2010.11.021. [DOI] [PubMed] [Google Scholar]
- Li C, Jones PM, Persaud SJ. Role of the endocannabinoid system in food intake, energy homeostasis and regulation of the endocrine pancreas. Pharmacol Ther. 2011;129(3):307–320. doi: 10.1016/j.pharmthera.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Karst M, Wippermann S, Ahrens J. Role of cannabinoids in the treatment of pain and (painful) spasticity. Drugs. 2010;70(18):2409–2438. doi: 10.2165/11585260-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Rossi F, Bellini G, Luongo L. et al. The endovanilloid/endocannabinoid system: A new potential target for osteoporosis therapy. Bone. 2011;48(5):997–1007. doi: 10.1016/j.bone.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Di Marzo V. Cannabinoid receptors and endocannabinoids: Role in neuroinflammatory and neurodegenerative disorders. CNS Neurol Disord Drug Targets. 2010;9(5):564–573. doi: 10.2174/187152710793361568. [DOI] [PubMed] [Google Scholar]
- Kunos G, Tam J. The case for peripheral CB(1) receptor blockade in the treatment of visceral obesity and its cardiometabolic complications. Br J Pharmacol. 2011;163(7):1423–1431. doi: 10.1111/j.1476-5381.2011.01352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari M, Battista N, Pirazzi V. et al. he manifold actions of endocannabinoids on female and male reproductive events. Front Biosci (Landmark Ed) 2011;16:498–516. doi: 10.2741/3701. [DOI] [PubMed] [Google Scholar]
- Bagavandoss P, Grimshaw S. Temporal and spatial distribution of the cannabinoid receptors (CB1, CB2) and fatty acid amide hydroxylase in the rat ovary. . Anat Rec (Hoboken) 2010;293(8):1425–1432. doi: 10.1002/ar.21181. [DOI] [PubMed] [Google Scholar]
- El-Talatini MR, Taylor AH, Elson JC. et al. Localisation and function of the endocannabinoid system in the human ovary. PLoS One. 2009;4(2):e4579. doi: 10.1371/journal.pone.0004579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne AW, Phillips JA 3rd, Kane N. et al. CB1 expression is attenuated in fallopian tube and decidua of women with ectopic pregnancy. PLoS One. 2008;3(12):e3969. doi: 10.1371/journal.pone.0003969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brighton PJ, Marczylo TH, Rana S. et al. Characterization of the endocannabinoid system, CB(1) receptor signalling and desensitization in human myometrium. Br J Pharmacol. 2011;164(5):1479–1494. doi: 10.1111/j.1476-5381.2011.01425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AH, Abbas MS, Habiba MA. et al. Histomorphometric evaluation of cannabinoid receptor and anandamide modulating enzyme expression in the human endometrium through the menstrual cycle. Histochem Cell Biol. 2010;133(5):557–565. doi: 10.1007/s00418-010-0695-9. [DOI] [PubMed] [Google Scholar]
- Gentilini D, Besana A, Vigano P. et al. Endocannabinoid system regulates migration of endometrial stromal cells via cannabinoid receptor 1 through the activation of PI3K and ERK1/2 pathways. Fertil Steril. 2010;93(8):2588–2593. doi: 10.1016/j.fertnstert.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Gentilini D, Busacca M, Di Francesco S. et al. PI3K/akt and ERK1/2 signalling pathways are involved in endometrial cell migration induced by 17beta-estradiol and growth factors. Mol Hum Reprod. 2007;13(5):317–322. doi: 10.1093/molehr/gam001. [DOI] [PubMed] [Google Scholar]
- Lazzarin N, Valensise H, Bari M. et al. Fluctuations of fatty acid amide hydrolase and anandamide levels during the human ovulatory cycle. Gynecol Endocrinol. 2004;18(4):212–218. doi: 10.1080/09513590410001692492. [DOI] [PubMed] [Google Scholar]
- El-Talatini MR, Taylor Ah, Konje JC. et al. The relationship between plasma levels of the endocannabinoid, anandamide, sex steroids, and gonadotrophins during the menstrual cycle. . Fertil Steril. 2010;93(6):1989–1996. doi: 10.1016/j.fertnstert.2008.12.033. [DOI] [PubMed] [Google Scholar]
- Gonzalez S, Bisogno T, Wenger T. et al. Sex steroid influence on cannabinoid CB(1) receptor mRNA and endocannabinoid levels in the anterior pituitary gland. . Biochem Biophys Res Commun. 2000;270(1):260–266. doi: 10.1006/bbrc.2000.2406. [DOI] [PubMed] [Google Scholar]
- Scorticati C, Fernandez-Solari J, De Laurentiis A. et al. The inhibitory effect of anandamide on luteinizing hormone-releasing hormone secretion is reversed by estrogen. Proc Natl Acad Sci U S A. 2004;101(32):11891–11896. doi: 10.1073/pnas.0404366101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall'Aglio C, Millan P, Maranesi M. et al. Expression of the cannabinoid receptor type 1 in the pituitary of rabbits and its role in the control of LH secretion. Domest Anim Endocrinol. 2013;45(4):171–179. doi: 10.1016/j.domaniend.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Riebe CJ, Hill MN, Lee TT. et al. Estrogenic regulation of limbic cannabinoid receptor binding. . Psychoneuroendocrinology. 2010;35(8):1265–1269. doi: 10.1016/j.psyneuen.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Matsumoto H, Guo Y. et al. Differential G protein-coupled cannabinoid receptor signaling by anandamide directs blastocyst activation for implantation. Proc Natl Acad Sci U S A. 2003;100(25):14914–14919. doi: 10.1073/pnas.2436379100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SK, Paria BC, Chakraborty I. et al. Cannabinoid ligand-receptor signaling in the mouse uterus. Proc Natl Acad Sci U S A. 1995;92(10):4332–4336. doi: 10.1073/pnas.92.10.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca BM, Correia-da-Silva G, Almada M. et al. The endocannabinoid system in the postimplantation period: A role during decidualization and placentation. Int J Endocrinol. 2013;2013:510540. doi: 10.1155/2013/510540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Dey SK. Aspects of endocannabinoid signaling in periimplantation biology. Mol Cell Endocrinol. 2008;286(1-2) 1:S3–S11. doi: 10.1016/j.mce.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paria BC, Ma W, Andrenyak DM. et al. Effects of cannabinoids on preimplantation mouse embryo development and implantation are mediated by brain-type cannabinoid receptors. Biol Reprod. 1998;58(6):1490–1495. doi: 10.1095/biolreprod58.6.1490. [DOI] [PubMed] [Google Scholar]
- Xie H, Sun X, Piao Y. et al. Silencing or amplification of endocannabinoid signaling in blastocysts via CB1 compromises trophoblast cell migration. J Biol Chem. 2012;287(38):32288–32297. doi: 10.1074/jbc.M112.381145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa MA, Fonseca BM, Keating E. et al. 2-arachidonoylglycerol effects in cytotrophoblasts: Metabolic enzymes expression and apoptosis in BeWo cells. Reproduction. 2014;147(3):301–311. doi: 10.1530/REP-13-0563. [DOI] [PubMed] [Google Scholar]
- Costa MA, Keating E, Fonseca BM. et al. 2-arachidonoylglycerol impairs human cytotrophoblast cells syncytialization: Influence of endocannabinoid signalling in placental development. Mol Cell Endocrinol. 2015;399:386–394. doi: 10.1016/j.mce.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Costa MA, Fonseca BM, Teixeira NA. et al. The endocannabinoid anandamide induces apoptosis in cytotrophoblast cells: Involvement of both mitochondrial and death receptor pathways. Placenta. 2015;36(1):69–76. doi: 10.1016/j.placenta.2014.10.011. [DOI] [PubMed] [Google Scholar]
- Habayeb OM, Taylor AH, Bell SC. et al. Expression of the endocannabinoid system in human first trimester placenta and its role in trophoblast proliferation. . Endocrinology. 2008;149(10):5052–5060. doi: 10.1210/en.2007-1799. [DOI] [PubMed] [Google Scholar]
- Habayeb OM, Taylor AH, Evans MD. et al. Plasma levels of the endocannabinoid anandamide in women--a potential role in pregnancy maintenance and labor? J Clin Endocrinol Metab. 2004;89(11):5482–5487. doi: 10.1210/jc.2004-0681. [DOI] [PubMed] [Google Scholar]
- Mitchell MD, Sato TA, Wang A. et al. Cannabinoids stimulate prostaglandin production by human gestational tissues through a tissue- and CB1-receptor-specific mechanism. Am J Physiol Endocrinol Metab. 2008;294(2):E352–E356. doi: 10.1152/ajpendo.00495.2007. [DOI] [PubMed] [Google Scholar]
- Chamley LW, Bhalla A, Stone PR. et al. Nuclear localisation of the endocannabinoid metabolizing enzyme fatty acid amide hydrolase (FAAH) in invasive trophoblasts and an association with recurrent miscarriage. Placenta. 2008;29(11):970–975. doi: 10.1016/j.placenta.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Taylor AH, Finney M, Lam PM. et al. Modulation of the endocannabinoid system in viable and non-viable first trimester pregnancies by pregnancy-related hormones. Reprod Biol Endocrinol. 2011;9:152. doi: 10.1186/1477-7827-9-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabucco E, Acone G, Marenna A. et al. Endocannabinoid system in first trimester placenta: Low FAAH and high CB1 expression characterize spontaneous miscarriage. Placenta. 2009;30(6):516–522. doi: 10.1016/j.placenta.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Fonseca BM, Correia-da-Silva G, Taylor AH. et al. Characterisation of the endocannabinoid system in rat haemochorial placenta. Reprod Toxicol. 2012;34(3):347–356. doi: 10.1016/j.reprotox.2012.05.036. [DOI] [PubMed] [Google Scholar]
- Park B, Gibbons HM, Mitchell MD. et al. dentification of the CB1 cannabinoid receptor and fatty acid amide hydrolase (FAAH) in the human placenta. Placenta. 2003;24(10):990–995. doi: 10.1016/s0143-4004(03)00165-6. [DOI] [PubMed] [Google Scholar]
- Chan HW, McKirdy NC, Peiris HN. et al. The role of endocannabinoids in pregnancy. Reproduction. 2013;146(3):R101–R109. doi: 10.1530/REP-12-0508. [DOI] [PubMed] [Google Scholar]
- Lopez HH, Webb SA, Nash S. et al. Cannabinoid receptor antagonism increases female sexual motivation. Pharmacol Biochem Behav. 2009;92(1):17–24. doi: 10.1016/j.pbb.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Mani SK, Mitchell A, O'Malley BW. Progesterone receptor and dopamine receptors are required in delta 9-tetrahydrocannabinol modulation of sexual receptivity in female rats. Proc Natl Acad Sci U S A. 2001;98(3):1249–1254. doi: 10.1073/pnas.031563998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Biotechnology Information. PubChem Compound Database;CID=16078 (Δ9-THC) [cited 2016 May 3]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/16078 .
- National Center for Biotechnology Information. PubChem Compound Database; CID=5311501 (WIN 55,212-2) [cited 2016 May 3]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/5311501 .
- National Center for Biotechnology Information. PubChem Compound Database; CID=104850 (Rimonabant) [cited 2016 May 3]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/104850 .
- National Center for Biotechnology Information. PubChem Compound Database; CID=2125 (AM-251) [cited 2016 May 3]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/2125 .
- National Center for Biotechnology Information. PubChem Compound Database; CID=8955 (Pregnenolone) [cited 2016 May 3]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/8955 .
- National Center for Biotechnology Information. PubChem Compound Database; CID=5994 (Progesterone) [cited 2016 May 3]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/5994 .
- National Center for Biotechnology Information. PubChem Compound Database; CID=5757 (Estradiol) [cited 2016 May 3]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/5757 .