Abstract
Food craving is often defined as a strong desire to eat. Much work has shown that it consistently and prospectively predicts eating and weight-related outcomes, contributing to the growing obesity epidemic. Although there are clear gender differences in the prevalence and health consequences of obesity, relatively little recent work has investigated gender differences in craving, or any sex-hormone-based differences as they relate to phases of the menstrual cycle. Here, we propose that gender-related differences in food craving contribute to gender-related differences in obesity. Drawing on findings in the addiction literature, we highlight ways to incorporate gender-based differences in food craving into treatment approaches, potentially improving the efficacy of obesity and weight loss treatment. Overall, this review aims to emphasize the importance of investigating gender differences in food craving, with a view towards informing the development of more effective treatments for obesity and weight loss.
Keywords: gender, obesity, craving, menstrual cycle, sex hormones
Popular culture often portrays gender differences in food cravings, such as the notion that men crave savory foods (e.g., burgers) whereas women crave sweet foods (e.g., chocolate), especially as they approach menses [1]. While this is not always the case, some research has supported gender differences in not only the type of food craved, but also in clinically-important characteristics of craving such as frequency, severity, and regulation of food craving [2-5]. In the sections below, we will first define craving and discuss how food craving can predict eating and weight gain [6]. We will then argue that due to this predictive relationship, craving is a likely explanatory mechanism for rapidly-increasing rates of obesity, which is the second leading cause of preventable disease and death in the United States [7,8]. Next, we will review key findings on gender differences in food craving, and explain how these differences may underlie gender differences in obesity and obesity-related health consequences. Then, we will discuss evidence that hormonal differences between men and women, and hormonal variations across women’s menstrual cycles contribute to such differences. Finally, drawing on findings in the drug addictions literature, we will highlight important implications of gender differences in food craving for obesity research and treatment.
The Importance of Food Craving
Defining and Measuring Food Craving
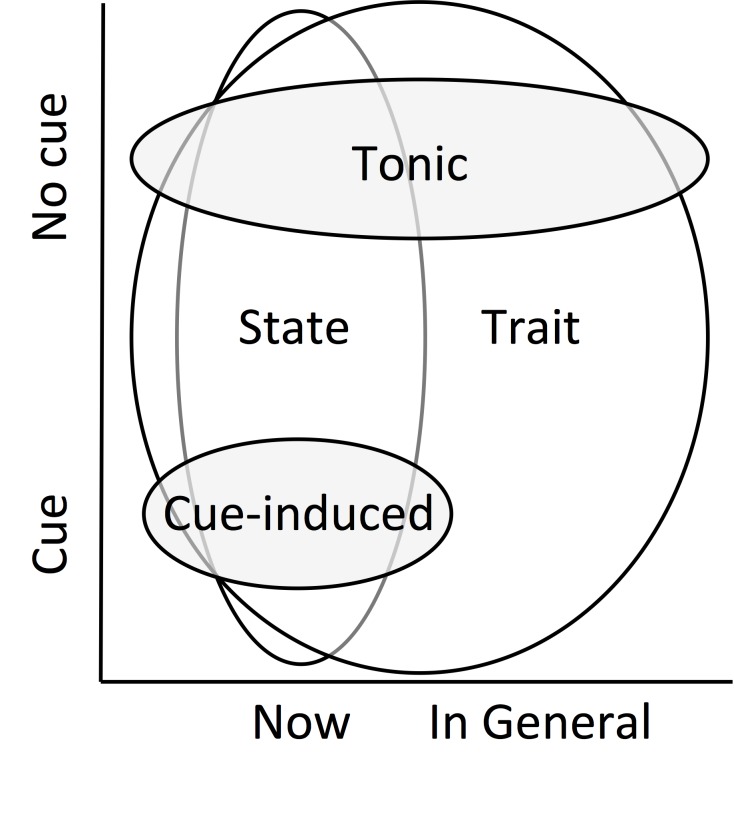
Food craving can be defined as a strong desire to eat [9-11] and is extremely common; it is experienced by more than 90 percent of the population (e.g., [2,12,13]). Several types of food craving are commonly defined and studied (see Figure 1). Tonic craving reflects a general feeling that is experienced either over time or in a particular moment, in the absence of environmental stimuli. It is often related to abstinence from a particular food (e.g., consistently craving chocolate when on a diet; [14]), and is typically measured with multi-item self-report scales/questionnaires, including the Food Craving Inventory (e.g., “I frequently desire fried chicken” [12]). Such measures of tonic craving are typically higher in restrained eaters and increase after deprivation [15]. Nevertheless, tonic craving is not the same as hunger, and can be experienced in the absence of caloric need [13,16].
Figure 1.
Conceptual overlap of commonly-defined forms of food craving. Schematic and conceptual representation of the overlap in definitions and types of craving. Tonic craving refers to a general feeling of craving that is experienced either over time or in a particular moment, in the absence of environmental stimuli. Cue-induced craving is an acute period of craving, elicited by environmental/external stimuli. State craving is a feeling of craving in a particular moment, regardless of whether a cue is present. Trait craving refers to a tendency to feel craving in general; it can refer to craving in general both in the presence of cues and without cues. Sizes of the bubbles reflect definitional overlap across Time and Cue dimensions.
On the other hand, cue-induced craving is an acute period of craving, elicited by environmental/external stimuli (e.g., craving chocolate after seeing a Godiva commercial; [14], for review, see [6]). In response to such stimuli, individuals report their current level of craving, either on a multi-item scale (e.g., [17,18]) or a single-item response (e.g., [19,20]). A large body of studies has consistently shown that exposure to visual, olfactory, or taste cues of salient food items results in cue-induced craving, along with increases in peripheral physiological signals such as heart rate, gastric activity, and salivation, known as cue reactivity [21-23]. Such cue reactivity also includes a predictable pattern of neural responses in brain systems previously associated with reward, including the ventral striatum (VS), orbitofrontal cortex (OFC), and insula (for reviews, see [24-26]). For instance, the VS is thought to play a central role in motivation and learning (e.g., [24-26]), the OFC is involved in value computation (e.g., [24-27]), and the insula has been associated with updating value based on interoceptive states (e.g., [24,28-29]). Although these brain regions have unique and varied functions, together they form part of a circuit that detects and encodes the salience of reward [24-26]. Further, activation of these regions (measured by functional neuroimaging) correlates with self-reported cue-induced craving (e.g., [30-34]; see [24-26] for reviews). As such, cue-induced craving is a conditioned response, wherein food cues present at the time of food consumption became associated with the reward of eating, and over time come to elicit conditioned physiological, neural, and craving responses [14,35].
Additional forms of craving have been discussed in the literature, which partially overlap with tonic and cue-induced craving: namely, state craving and trait craving. State craving is a feeling of craving in a particular moment, regardless of whether a cue is present; thus, it can describe either tonic or cue-induced craving assessed in a present moment. In contrast, like tonic craving, trait craving refers to a tendency to feel craving in general; however, it can also refer to craving in the presence of cues in general. For example, the Food Craving Questionnaire-Trait includes questions about the tendency to experience both tonic craving (e.g., “If I am craving something, thoughts of eating it consume me”) and cue-induced craving (e.g., “Whenever I go to a buffet I end up eating more than what I needed”; [36,37]). Conversely, the Food Craving Questionnaire-State includes questions asking about the present moment only, which could include cue-induced or tonic craving (e.g., “I have an urge for a specific food”; [37]).
Importantly, although studies can be designed to focus on a specific form of craving, the subtypes of craving are not always fully dissociable. For example, studies that measure self-reported craving across a day or week may capture some combination of these types of craving across time. For instance, subtle daily stimuli (e.g., time of day, a location associated with eating, the physical sensation of hunger) may induce craving even when individuals are unaware of the cause of the craving. However, the type of craving may be less important than the severity of the craving in contributing to craving-induced eating, and an interaction of tonic, trait, and cue-induced craving may produce the strongest overall craving in any particular moment [38]. Throughout this review, we do our best to differentiate between the types of craving where possible, based on the definitions above (see Figure 1).
Food Craving, Eating, & Weight Gain
Food craving is clinically important because it is prospectively associated with and predicts eating and weight gain [6]. Individuals who report tonic food cravings display higher caloric intake than those who report rarely experiencing food craving [39]. Tonic craving for certain foods or food groups also predicts consumption of these specific foods [40]. Tonic and trait food craving are related to long-term weight outcomes such as greater weight gain over time and lifetime high body mass index (BMI [41-43]). Similarly, food cues and cue-induced food craving are also associated with food consumption. For instance, exposure to food cues elicits a craving response that predicts subsequent eating, such that higher cue-induced craving levels are associated with greater food consumption [44]. Further, cue-induced food craving predicts overeating in overweight children [45] and adults [46]. In a nonclinical population, one study found that both neural food cue reactivity in the VS and self-reported state craving predicted subsequent eating over a period of one week [47]. We recently summarized the data on both tonic and cue-induced food craving in a quantitative meta-analysis; we found that both were prospectively predictive of eating and long term weight gain across 45 studies, with a medium effect size [6]. Importantly, these craving measures accounted for 11 percent of the variance in eating-related outcomes, which is greater than any other single predictor of eating and weight [6]. Given that food craving is strongly associated with increased food intake and weight gain, its investigation is key to understanding obesity, and may inform prevention and treatment approaches [48].
Impact of Weight Gain and Obesity
In the United States, more than two-thirds of the population (68.5 percent) is now defined as overweight (BMI > 24.5; [49]). This stands in contrast to U.S. rates of overweight 30 years ago, when only 15 percent of adults were overweight (a 400 percent increase; [49]). This is problematic because obesity has been directly and causally linked to increasing rates of obesity-related disease, including diabetes, cardiovascular disease, and hypertension [50], such that obesity is now the second leading cause of preventable disease and death in the United States [7,8]. These health outcomes illustrate that obesity is a critical clinical and public health issue, necessitating the development of effective interventions. Thus, the sections below will illustrate how understanding gender differences in food craving could lead to improved interventions for obesity.
Linking Craving and Obesity
As reviewed above, craving is a strong predictor of eating and weight gain [6]. One society-level mechanism whereby craving influences obesity is through the increase in food cues in the environment which, in turn, increases craving and eating at the population-level. Indeed, the rising rates of overweight and obesity have been associated with increases in the availability and advertisement of unhealthy/calorie-dense foods [51,52]. Accordingly, individuals who live in an environment that includes high caloric foods (e.g., fast food, such as McDonalds) eat those foods more frequently, which results in a higher BMI [53-56]. Moreover, the omnipresence of “junk food” advertisements serves as a salient food cue, leading to even more frequent unhealthy food consumption [57]. Some have termed this an “obesogenic” or “toxic” food environment, wherein ultra-prevalent food and food cues lead to increased craving, food consumption, and weight gain [52,58,59]. While this environment affects everyone to some degree, some individuals may be more sensitive to food-related cues or experience more craving, such as overweight individuals [60,61].
Gender Differences in Weight Gain and Obesity
Importantly, there are gender differences in risk for and consequences of obesity. Gender is considered a risk factor for obesity; being female doubles the chance of becoming overweight [62]. Worldwide, women are 3 percent more likely to be overweight or obese than men [63]. Similarly, in the United States, women are 3.3 percent more likely than men to be overweight [64] and are 3 percent more likely to be morbidly obese [49]. Furthermore, some work has shown that women shoulder a greater burden of obesity-related health problems, including diseases such as high blood pressure and heart disease [65,66]. In other words, greater proportions of overweight and obese women suffer from obesity-related diseases [65]. Specifically, the rate of disease is 6.6 times greater in women compared to men [66,67]. One reason for this is that health-related impairments occur at lower BMIs in women than in men [65], and this is evident on both physical [68-72] and psychological health indices [73-76]. Finally, overweight women have twice the risk of mortality compared to overweight men [66], suggesting that they are more likely to die from weight-related disease.
Gender Differences in Food Craving
Because women are disproportionately affected by obesity, there may be a gender-related mechanism that explains these differences. Given the role of craving in obesity, one mechanism underlying gender-based health disparities in obesity could be gender differences in craving. Indeed, men and women have different experiences of craving, and different behavioral responses to it. Specifically, as reviewed below, gender differences have been reported in: (1) the kinds of foods craved, (2) the intensity and frequency of craving, and (3) the ability or tendency to regulate craving.
Gender Differences in Kinds of Foods Craved
Men and women tend to crave different kinds of foods. Several studies have shown that men report more craving for savory foods (e.g., meat, fish, eggs), whereas women report more craving for sweet foods (e.g., chocolate, pastries, ice cream; [3,4]). Further, men may crave different types of sweets than women do (e.g., sugar-sweetened beverages, but not chocolate; [3]). Consistently, a few studies have shown that more than 92 percent of those who experience strong cravings for chocolate are female [77,78].
Gender Differences in Levels or Frequency of Food Craving
Men and women report different intensities and frequencies of tonic and trait food craving, although the literature on cue-induced craving is less clear. Women report experiencing stronger tonic and trait food craving overall [5,36]. For example, one study assessed baseline gender differences in trait food craving in a large sample of college students, and found that women have significantly higher trait craving scores than men, even when controlling for food deprivation and eating disorder symptoms [36]. Overweight and obese women enrolled in a weight loss treatment program also reported higher trait food craving than overweight and obese men, even when controlling for binge eating behaviors and obesity levels [79]. Further, women more frequently report experiencing craving in everyday life [3,5,80]. Indeed, women report more frequent episodes of state craving, as highlighted by a three-day food diary study, in which women reported 15.6 percent more food cravings episodes than men [5]. To our knowledge, no published studies to date specifically tested gender differences in the intensity or frequency of cue-induced food craving. However, some studies have shown gender differences in food cue reactivity, which is related to cue-induced craving. For example, in an functional magnetic resonance imaging (fMRI) study investigating neural reactivity to palatable food images, women showed greater activity in craving and taste-related brain regions such as the anterior insula in response to food cues as compared to men [81]. In another study, women also exhibited greater neural reactivity than men to high-calorie food cues under food deprivation, including in the OFC and insula [82].
Gender Differences in Regulation of Food Craving
Women may find it harder to regulate food craving compared to men. In one study, only 20 percent of women who reported craving indicated that it was “easy” for them to resist cravings, as compared to 50 percent of men; however, men and women ultimately reported equal levels of success in resisting their cravings [5]. Gender differences in the ability to reduce cue-induced craving have also been reported. Wang and colleagues (2009) found that men and women report similar cue-induced craving levels and exhibit similar levels of neural activity in craving-related brain regions after exposure to food cues [83]. However, when participants were asked to reduce craving by distracting themselves or ignoring the food cues, only men showed decreased neural activity in these regions; this is consistent with women being less able to suppress their cue-induced craving for food than men [83]. Importantly, this specific gender difference in regulation of cue-induced craving does not appear to extend more broadly to gender differences in behavioral impulsivity (see [84] for meta-analysis). Taken together, these data suggest that men and women differ in several aspects of craving and in responses to craving.
Hormonal Mechanisms Underlying Gender Differences in Food Craving and Eating
A complex interplay of biological, sociological, and environmental factors likely account for gender differences in craving and obesity. Nevertheless, sex hormones such as testosterone, progesterone, and estrogens modulate food consumption and are widely understood to be important factors driving such gender differences. First, men and women differ in their average levels of these sex hormones. Second, women experience monthly hormonal variation across the menstrual cycle while men do not, and this may contribute importantly to gender differences in food craving and consumption, as will be detailed below (see Figure 2).
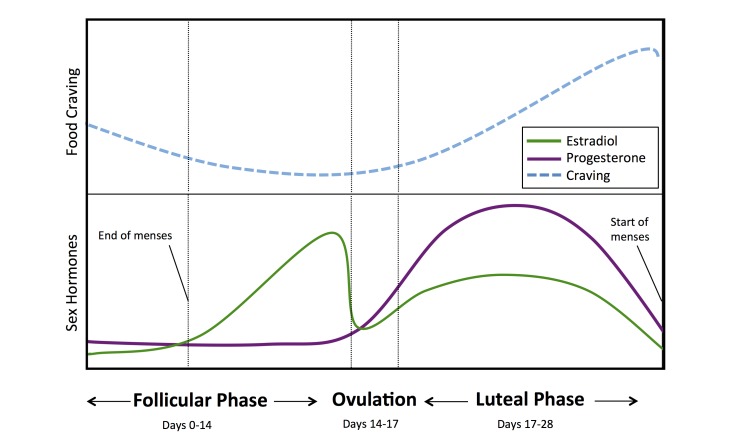
Figure 2.
Ovarian hormone levels and proposed variation in food craving for each cycle phase. Lines represent variation in sex hormones across the menstrual cycle (e.g., Allen et al., 2015; Hirschberg, 2012) and their proposed relationship to craving for food. During the follicular phase (days ~0-14), levels of estradiol increase and craving may decrease. During ovulation (days ~14-17) and the luteal phase (days ~17-28), levels of progesterone increase and then decrease before menses. Such changes may be associated with a rise in craving for food reported during this period (e.g., Hormes & Timko, 2011; Dye & Blundell, 1997; Dye, Warner & Bancroft, 1995).
Sex hormones are important modulators of food consumption, and interact with neurotransmitters and gastrointestinal systems to change energy intake and expenditure (for review, see [85-87]). Men and women are known to have different absolute levels and ratios of sex hormones, which influence eating and weight. For instance, levels of androgens (e.g., testosterone) are typically higher in men, whereas levels of estrogens (e.g. estradiol, estrone) are typically higher in women. However, there is much variability within and across sex and age, including increases in both hormones in men and women during adolescence and reductions in androgens in men and estrogens in women at older ages [88-90]. Importantly, androgens are associated with reduced risk of obesity and diabetes in men, but increased risk of obesity and diabetes in women [85,91,92]. In contrast, estrogens attenuate appetite and eating in men and women [93,94], such that reductions in estrogen increase food consumption (but c.f., tonically higher estrogen levels correlate with increased body weight and amount of body fat in women; [95,96]).
Women experience monthly variations in sex hormone levels across the menstrual cycle (for review, see [86,87]), whereas men do not have such cycles. The menstrual cycle is often described as having two primary stages based on ovarian changes: the follicular phase, when estrogen is more prominent, and the luteal phase, when progesterone is more prominent11 [97]. The follicular phase is frequently defined as starting on the first day of menses. During menses, both estrogen and progesterone levels are relatively low. After menses, estrogen levels gradually increase to prepare for ovulation. Approximately mid-cycle (~days 14-17), follicular stimulating hormone (FSH) and luteinizing hormone (LH) peak and ovulation occurs. After ovulation, the luteal phase begins, and estrogen levels decrease as progesterone levels (an estrogen antagonist) increase. At the end of the luteal phase, estrogen and progesterone levels again drop, initiating menses.
It has long been known that in females, food consumption fluctuates across the menstrual cycle due to these fluctuations of sex hormones, as observed in both animal and human studies [86,87,94,98-101]. Specifically, the reduction in estrogen and increased antagonism of estrogen by progesterone after ovulation increase eating in the luteal phase [86,87]. These effects were first causally demonstrated in animal models. For example, in rats, food consumption and weight gain increase following ovariectomy, which eliminates estrogen. These effects can be reversed by estrogen replacement, and co-administration of progesterone blocks the effects of estrogen [86,87,102-104]. These effects have also been shown in women, such that those in the luteal phase consume significantly more food and prefer sweeter foods compared to women in the follicular phase [86,87,105]. A meta-analysis across studies in women suggests that the difference in food consumption amounts to ~238 additional calories per day during the luteal phase compared to the follicular phase (and may be as high as 597 additional calories per day; [105]). Such increased caloric intake could result in a yearly weight gain of 10 to 20 pounds.
Importantly, one underlying mechanism of these cyclic variations in eating may be menstrual-phase differences in food craving. Indeed, women’s menstrual cycle variations have been shown to influence craving and food cue reactivity [106,107]. Women report increased food craving in the luteal phase, with the strongest craving occurring directly before menses and potentially continuing into menstruation [10,108-113]. In fact, it has been reported that women experience as much as 57 percent more craving in the luteal phase than in the early follicular phase [108]. In one interesting study, 28.9 percent of college age women reported chocolate cravings associated with their menstrual cycle, most frequently occurring 4 days prior to menses and continuing into menses [106]. In another study, 74.3 percent of women reported food cravings in the 7 days prior to menses, as compared to 26.9 percent reporting food cravings after menses [10].
Very little work has investigated menstrual cycle variations specifically in cue-induced food craving and associated cue reactivity, a component of cue-induced food craving. A recent study in animals found that renewal of cue-induced food consumption decreases with estradiol levels [114]. In women, neural reactivity to food cues across the menstrual cycle varies in brain regions typically associated with craving and reward (like the OFC and VS; [107]). Specifically, women showed greater neural reactivity when viewing pictures of high calorie foods during the luteal phase as compared to the follicular phase [107]. However, one behavioral study reported no significant influence of menstrual phase on cue-induced food craving [109]. In sum, both animal and human work demonstrate that food craving fluctuates along the menstrual cycle, and could explain variations in food consumption. However, there is a clear need for additional studies in this area to establish the effects of menstrual cycle variations on cue-induced food craving specifically.
Lessons from Craving in Drug Use and Addiction
Over several decades of research on addictions, it has been firmly established that craving contributes substantially to drug use (e.g., [115-118]) and relapse (e.g., [119-133]). More recently, researchers have pointed to similarities between food and drug craving. For example, the subjective experience of drug craving is thought to resemble the experience of food craving [134]. The two are also similar in their predictive utility and in their underlying mechanisms. For instance, as with drug craving, there is emerging evidence that food craving contributes substantially to and predicts eating and weight outcomes [6,48]. Mechanistically, meta-analyses have shown that cue-induced drug craving is consistently associated with neural activity in a network of regions including the VS, the OFC, and the insula [135,136], which are also consistently associated with neural activity in response to cue-induced food craving [34]. Further, it has been proposed that drugs “hijack” a hedonic reward system, which is thought to have originally evolved for food and other natural rewards (e.g., [137-147]), and that this system is responsible for craving for food and for drugs, including in addictions, obesity, and eating disorders (e.g., [141,148,149]). This suggests that parallel mechanisms contribute to drug- and food-related problems. Therefore, because of this shared circuitry, there may be (1) gender differences and (2) treatment approaches that are effective across disorders. As such, a review of gender differences in drug craving may provide useful insights into food craving and related problems.
Gender differences have been frequently reported in multiple antecedents, clinical course, subjective effects, underlying mechanisms, consequences, and outcomes of drug use and addiction (for reviews, see [150-155]). For instance, gender differences have been reported in drug craving, including cue-induced drug craving, drug cue reactivity, and drug-cue-induced neural activity in craving-related brain regions, such as the VS, OFC, and insula [156-160]. However, the precise nature of these differences remains elusive; for example, in some studies, women report more cue-induced craving, but others find no differences (for review, see [160]). Such inconsistencies may reflect variations in menstrual phase; indeed, menstrual-phase-based variations in drug craving have been reported in female drug users. For example, non-abstinent women smokers report significantly more tonic [161-164] and cue-induced craving [165] in the luteal phase than in the follicular phase of the menstrual cycle (see [166] for review). In contrast, nicotine-abstinent women have been shown to report greater state craving in the follicular phase [162,167]. Inconsistencies in findings may be due to methodological differences between studies, including in abstinence itself [97,168]. Abstinence is important especially when considering the parallel to food, as craving for food is never measured in a fully-abstinent state.
Importantly, in the addiction field, findings on menstrual-phase-based variations in craving have been applied to the realm of treatment. Specifically, several studies have shown that menstrual cycle phase at the date of smoking cessation predicts smoking status during and after treatment [166,169], such that smoking cessation quit attempts initiated during the luteal phase (when progesterone levels are dominant) are associated with better treatment outcomes than in the follicular phase (e.g., [162]; but c.f. [170,171] with nicotine replacement). These treatment effects may be due to fluctuation/reduction of perceived withdrawal symptoms, including craving [166,172] across the menstrual cycle. Ultimately, these findings suggest that treatment outcomes may be improved by considering the influence of menstrual cycle phase on cravings when beginning a substance use intervention. Although drug treatment is not a perfect model for weight control or obesity treatments (where the goal can never be complete abstinence, as it is with drugs), these findings may have applications to obesity treatments, especially because of the importance of craving for both conditions.
Implications for Gender Differences in Obesity and its Treatment
Food Craving and Treatment for Obesity
As reviewed above, food craving is related to eating and weight, and importantly, it directly interferes with weight loss treatments and dieting. One study found that trait food craving is associated with self-reported past dieting failures, such that individuals with higher trait craving report less ability to watch their weight, lose excess weight, and stay in shape [173]. Further, weight cycling in women who diet is associated with increased frequency and intensity of craving and with binge eating behavior [174,175]. In contrast, successful dieters have lower reactivity to food cues, such that exposure to food stimuli elicits lower physiological reactivity (e.g., salivation) and lower neural response in the VS as compared to unsuccessful dieters [176,177]. These findings suggest that individuals with lower levels of craving may be more likely to succeed on diets and in weight loss treatments. Indeed, longitudinal studies have observed that trait craving and cue reactivity can predict weight loss treatment outcomes. For example, Batra and colleagues (2013) found that reductions in trait craving during a weight loss intervention were associated with greater reductions in body weight [178]. Similarly, lower neural reactivity in the VS and insula to images of high-calorie foods was predictive of better short-term and long-term outcomes in weight loss treatment [179]. Thus, it may be more difficult for those with strong food cravings to successfully diet or lose weight. This may be especially relevant to women, particularly during menstrual phases when they may be more sensitive to craving and food cues.
Several treatments that directly target craving and exposure to food cues have been shown to reduce eating and improve outcomes in overweight populations. For example, pharmacotherapies such as bupropion and naltrexone that reduce craving for drugs of abuse (e.g., opiates and alcohol) are also associated with reduced self-reported craving for food and reduced BMI in weight loss trials [180-184]. Further, psychological treatments have been applied to target the associations between cue exposure, craving, and food consumption, including cue exposure and response prevention treatments (CERP), cognitive behavioral therapy (CBT), and mindfulness-based therapies (MBTs). CERP attempts to extinguish associations between a cue, conditioned responses, and a behavior by preventing the behavior from occurring. In CERP, prolonged exposure to food cues in the absence of food intake reduces self-reported state food craving [185], physiological reactivity in response to cues [186], and binge eating [185,187-189], though results are mixed [190].
In comparison, CBT aims to reduce the influence of food cues and craving on eating behavior and weight, through the use of cognitive and behavioral strategies. Cognitive strategies include the regulation of craving through cognitive reappraisal, which involves reframing a stimulus or situation with the goal of modulating its affective impact (e.g., a delicious slice of pizza might lead to long-term health consequences, including obesity; [191]). Cognitive reappraisal reduces neural reactivity in the VS and self-reported cue-induced craving for high calorie foods [19,32,192,193]. Behavioral strategies include determining the antecedents of eating behavior (e.g., food cues, craving), intervening to prevent consequences (i.e., food consumption), stimulus control (e.g., reduction of food cues in the personal environment), regular meal planning (to reduce vulnerability to food cues and craving), and exposure-based exercises (to reduce the salience of “trigger” foods and contexts; [194]). CBT is effective in the treatment of a range of food- and food craving-related conditions, including eating disorders [195,196] and obesity [197,198]. Finally, MBTs that teach individuals to notice and accept the experience of craving have demonstrated effectiveness at reducing food craving and weight in both lean and obese adults [199-202] as well as reducing episodes of binge eating in individuals with binge eating disorder [203] and bulimia nervosa [204,205], or following bariatric surgery [206]. Thus, further development of these treatment approaches, and others that target craving and/or cue exposure, may improve the effectiveness of treatments for obesity.
Gender Differences in Food Craving and Treatment for Obesity
The data summarized above links craving to eating, weight gain, and obesity, making it an important target for obesity treatment. Given gender differences reported in craving and obesity, considering gender- and sex hormone-based variations in craving and eating may further improve the efficacy of obesity treatments. However, there is a dearth of experimental and clinical work directly comparing treatment outcomes in men and women, and accounting for variation in sex hormones (e.g. across menstrual cycle, pregnancy, and age). In fact, to our knowledge, there are no gender-specific or sex hormone-sensitive interventions for obesity. Furthermore, most clinical trials for obesity do not test for (or report) gender differences in response to treatment, including in reductions in food cravings, eating, or weight-related outcomes. Importantly, recent meta-analyses of the few trials that do report gender differences found that women are less likely to complete treatment [207] and that women lose less weight than men [208], despite comprising more than 50 percent of weight loss trial participants [207]. There may also be gender differences in response to pharmacotherapy treatments; in one study, methylphenidate led to decreases in state food craving, and food consumption, in obese women, but not men [209].
As observed in the addiction literature and described above, targeting and testing treatment based on gender and sex hormone status for women is likely to improve intervention efficacy for eating-related disorders. In smoking cessation trials, it has been shown that women who quit during the follicular phase are less successful, as they are more susceptible to relapse [169]. It may be beneficial to similarly time-lock weight loss treatment so that it begins during the luteal phase of the menstrual cycle to minimize lapses. Alternatively, it may be useful to gradually introduce small dietary changes during this time window across several months; for example, by establishing “quit dates” for particular foods in the luteal phase across the course of a several months, instead of introducing several changes at once. Furthermore, given gender-based differences in food craving, it may be useful to modify the nutritional composition of foods included in weight loss plans between genders and across the menstrual cycle (for women) to adjust for variation in specific food cravings and energy needs. Indeed, as with other biological metrics such as gut microbiota, it may be possible to individualize diet plans based on hormonal profiles and other physiological variations [210]. Additionally, targeting hormonal imbalances or reducing variation across the cycle through pharmacological treatment (e.g., oral contraceptives) may change the intensity of cravings and food consumption across the cycle, influencing treatment outcomes. However, there are currently contradictory findings on this topic, with some studies suggesting that oral contraceptives decrease food craving [211,212], whereas others report no change in food craving [213,214] or eating [215,216]. Finally, providing women with psychoeducation about hormonal influences on craving and food intake may improve self-efficacy and use of coping skills, such as cognitive reappraisal or mindfulness skills, during specific times of heightened craving. Ultimately, what is needed is additional research in this area to uncover the exact effect of sex-based hormones on craving, eating, and the success of diet and obesity treatments.
Conclusion
Obesity has become an epidemic, associated with great costs to society including widespread disability, disease, and death. There are gender differences in tonic craving and cue-induced craving, which may underlie documented gender differences in obesity and obesity-related health outcomes. Furthermore, for women, there are sex hormone-related variations in craving and food cue reactivity that may hinder dieting and weight loss attempts. In the field of drug addiction, gender-informed treatments that are time-locked to menstrual phase have shown greater efficacy. We propose that there is much room for further work investigating these issues as they relate to weight loss and obesity. First, further investigation into changes in craving across the menstrual cycle is necessary to fully elucidate its timing and effects on eating. Second, current weight loss studies rarely measure craving or menstrual phase, or test for gender differences in outcomes. Reporting such information in the context of existing interventions would greatly inform our understanding of the mechanisms involved in successful treatment, and allow for the development of more targeted approaches. Third, building on treatments for addiction, studies investigating menstrual-cycle-based timing of weight loss interventions could increase our understanding of how hormonal changes can influence treatment efficacy and weight. Indeed, including assessments of hormone levels as well as craving in such investigations can increase our understanding of the role of these mechanisms in obesity and obesity treatment. Finally, testing targeted interventions to reduce hormonal variability (e.g., oral contraceptives) or to improve coping with cravings (e.g., regulation of craving, mindfulness) may bolster existing treatment approaches. Investigating these issues may improve interventions, so that gender differences in food craving and cue-reactivity are less likely to derail weight loss attempts and contribute to gender-related health disparities in obesity.
Abbreviations
- VS
ventral striatum
- OFC
orbitofrontal cortex
- BMI
Body Mass Index
- fMRI
functional magnetic resonance imaging
- CERP
Cue Exposure and Response Prevention
- CBT
Cognitive Behavioral Therapy
- MBT
Mindfulness-based Treatments
Footnotes
1Although researchers have used a variety of definitions for phases, and mechanisms for determining phase status, efforts to systematize this are under way (Allen et al, 2015).
References
- Bratskeir K. This is why women crave chocolate, men want a burger. Huffington Post [Internet] [cited 2015 Nov 5]. Available from: http://www.huffingtonpost.com/2014/11/10/chocolate-craving-pms-men-vegetables_n_6102714.html .
- Osman JL, Sobal J. Chocolate cravings in American and Spanish individuals: Biological and cultural influences. Appetite. 2006;47(3):290–301. doi: 10.1016/j.appet.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Zellner DA, Garriga-Trillo A, Rohm E. et al. Food liking and craving: A cross-cultural approach. Appetite. 1999;33(1):61–70. doi: 10.1006/appe.1999.0234. [DOI] [PubMed] [Google Scholar]
- Weingarten HP, Elston D. Food cravings in a college population. Appetite. 1991;17(3):167–175. doi: 10.1016/0195-6663(91)90019-o. [DOI] [PubMed] [Google Scholar]
- Lafay L, Thomas F, Mennen L. et al. Gender differences in the relation between food cravings and mood in an adult community: Results from the Fleurbaix Laventie Ville Sante study. Int J Eat Disord. 2001;29(2):195–204. doi: 10.1002/1098-108x(200103)29:2<195::aid-eat1009>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Boswell RG, Kober H. Food cue reactivity and craving predict eating and weight gain: A meta-analytic review. Obes Rev. 2016;17(2):159–177. doi: 10.1111/obr.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YC, McPherson k, Marsh T. et al. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378(9793):815–825. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK. et al. Prevalence of obesity among adults: United States, 2011-2012. NCHS Data Brief. 2013;131:1–8. [PubMed] [Google Scholar]
- Cherpitel CJ, Borges G, Ye Y. et al. Performance of a craving criterion in DSM Alcohol Use Disorders. J Stud Alcohol Drugs. 2010;71(1):674–684. doi: 10.15288/jsad.2010.71.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye L, Warner P, Bancroft J. Food craving during the menstrual cycle and its relationship to stress, happiness of relationship, and depression: A preliminary inquiry. J Affect Disord. 1995;34(3):157–164. doi: 10.1016/0165-0327(95)00013-d. [DOI] [PubMed] [Google Scholar]
- Pelchat ML, Johnson A, Chan R. et al. Images of desire: Food-craving activation during fMRI. Neuroimage. 2004;23(4):1486–1493. doi: 10.1016/j.neuroimage.2004.08.023. [DOI] [PubMed] [Google Scholar]
- White MA, Williamson DA, Greenway FL. et al. Development and validation of the Food Craving Inventory. Obes Res. 2002;10(2):107–114. doi: 10.1038/oby.2002.17. [DOI] [PubMed] [Google Scholar]
- Hill AJ. The psychology of food craving. PNAS. 2007;66(2):277–285. doi: 10.1017/S0029665107005502. [DOI] [PubMed] [Google Scholar]
- Jansen A. A learning model of binge eating: Cue reactivity and cue exposure. Behav Res Ther. 1998;36(3):257–272. doi: 10.1016/s0005-7967(98)00055-2. [DOI] [PubMed] [Google Scholar]
- Polivy J, Coleman J, Herman CP. The effect of deprivation on food cravings and eating behavior in restrained and unrestrained eaters. Int J Eat Disord. 2005;38(4):301–309. doi: 10.1002/eat.20195. [DOI] [PubMed] [Google Scholar]
- Kassel JA, Shiffman S. What can hunger teach us about drug craving? A comparative analysis of the two constructs. Adv Behav Res Ther. 1992;14(3):141–167. [Google Scholar]
- Berlin I, Singleton EG, Heishman SJ. Predicting smoking relapse with a multidimensional versus a single-item tobacco craving measure. Drug Alcohol Depend. 2013;132(3):513–520. doi: 10.1016/j.drugalcdep.2013.03.017. [DOI] [PubMed] [Google Scholar]
- Allen SS, Bade T, Hatsukami D. et al. Craving, withdrawal, and smoking urges on days immediately prior to smoking relapse. Nicotine Tob Res. 2008;10(1):35–45. doi: 10.1080/14622200701705076. [DOI] [PubMed] [Google Scholar]
- Kober H, Kross EF, Mischel W. et al. Regulation of craving by cognitive strategies in cigarette smokers. Drug Alcohol Depend. 2010;106(1):52–55. doi: 10.1016/j.drugalcdep.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth L, Dickinson A, Duka T. The associative basis of cue-elicited drug taking in humans. Psychopharmacology. 2010;208(3):337–351. doi: 10.1007/s00213-009-1735-9. [DOI] [PubMed] [Google Scholar]
- Nederkoorn C, Smulders FT, Jansen A. Cephalic phase responses, craving and food intake in normal subjects. Appetite. 2000;35(1):45–55. doi: 10.1006/appe.2000.0328. [DOI] [PubMed] [Google Scholar]
- Nederkoorn C, Smulders F, Havermans R. et al. Exposure to binge food in bulimia nervosa: Finger pulse amplitude as a potential measure of urge to eat and predictor of food intake. Appetite. 2004;42(2):125–130. doi: 10.1016/j.appet.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Vogele C, Florin I. Psychophysiological responses to food exposure: An experimental study in binge eaters. Int J Eat Disord. 1997;21(2):147–157. doi: 10.1002/(sici)1098-108x(199703)21:2<147::aid-eat5>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Haber SN, Behrens TE. The neural network underlying incentive-based learning: Implications for interpreting circuit disruptions in psychiatric disorders. Neuron. 2014;83(5):1019–1039. doi: 10.1016/j.neuron.2014.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML. Pleasure systems in the brain. Neuron. 2015;86(3):646–664. doi: 10.1016/j.neuron.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: Evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72(5):341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Dickinson A. The Effect of Lesions of the Insular Cortex on Instrumental Conditioning: Evidence for a Role in Incentive Memory. J Neurosci. 2000;20(23):8954–8964. doi: 10.1523/JNEUROSCI.20-23-08954.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10(1):59. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Kuhn S, Gallinat J. Common biology of craving across legal and illegal drugs - a quantitative meta-analysis of cue-reactivity brain response. Eur J Neurosci. 2011;33(7):1318–1326. doi: 10.1111/j.1460-9568.2010.07590.x. [DOI] [PubMed] [Google Scholar]
- Small DM, Zatorre RJ, Dagher A. et al. Changes in brain activity related to eating chocolate: From pleasure to aversion. Brain. 2001;124(9):1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross EF. et al. Prefrontal-striatal pathway underlies cognitive regulation of craving. PNAS. 2010;107(33):14811–14866. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H. Insula and drug cravings. Brain Struct Funct. 2010;214(5-6):593–601. doi: 10.1007/s00429-010-0259-8. [DOI] [PubMed] [Google Scholar]
- Tang DW, Fellows LK, Small DM. et al. Food and drug cues activate similar brain regions: A meta-analysis of functional MRI studies. Physiol Behav. 2012;106(3):317–324. doi: 10.1016/j.physbeh.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Martin-Soelch C, Linthicum J, Ernst M. Appetitive conditioning: Neural bases and implications for psychopathology. Neurosci Biobehav Rev. 2007;31(3):426–440. doi: 10.1016/j.neubiorev.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda-Benito A, Fernandez MC, Moreno S. Relationship of gender and eating disorder symptoms to reported cravings for food: Construct validation of State and Trait Craving Questionnaires in Spanish. Appetite. 2003;40(1):47–54. doi: 10.1016/s0195-6663(02)00145-9. [DOI] [PubMed] [Google Scholar]
- Cepeda-Benito A, Gleaves DH, Williams TL. et al. The development and validation of the State and Trait Food-Cravings Questionnaires. Behav Ther. 2000;31(1):151–173. doi: 10.1016/s0005-7967(99)00141-2. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Tiffany ST. Peak provoked craving: An alternative to smoking cue-reactivity. Addiction. 2013;108(6):1019–1025. doi: 10.1111/j.1360-0443.2012.04013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A, Weaver CFL, Blundell JE. Food craving, dietary restraint, and mood. Appetite. 1991;17(30):187–197. doi: 10.1016/0195-6663(91)90021-j. [DOI] [PubMed] [Google Scholar]
- Martin CK, O'Neil PM, Tollefson G. et al. The association between food cravings and consumption of specific foods in a laboratory taste test. Appetite. 2008;51(2):324–326. doi: 10.1016/j.appet.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilhooly CH, Das SK, Golden JK. et al. Food cravings and energy regulation: The characteristics of craved foods and their relationship with eating behaviors and weight change during 6 months of dietary energy restriction. Int J Obes. 2007;31(12):1849–1858. doi: 10.1038/sj.ijo.0803672. [DOI] [PubMed] [Google Scholar]
- Cushing CC, Benoit SC, Peugh JL. et al. Longitudinal trends in hedonic hunger after Roux-en-Y gastric bypass in adolescents. Surg Obes Relat Dis. 2014;10(1):125–130. doi: 10.1016/j.soard.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demos KE, Heatherton TF, Kelley WM. et al. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. J Neurosci. 2012;32(16):5549–5552. doi: 10.1523/JNEUROSCI.5958-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedoroff IC, Polivy J, Herman CP. The effect of pre-exposure to food cues on the eating behavior or restrained and unrestrained eaters. Appetite. 1997;28(1):33–47. doi: 10.1006/appe.1996.0057. [DOI] [PubMed] [Google Scholar]
- Jansen A, Theunissen N, Slechten K. et al. Overweight children overeat after exposure to food cues. Eat Behav. 2003;4(2):197–209. doi: 10.1016/S1471-0153(03)00011-4. [DOI] [PubMed] [Google Scholar]
- Ng L, Davis C. Cravings and food consumption in Binge Eating Disorder. Eat Behav. 2013;14(4):472–475. doi: 10.1016/j.eatbeh.2013.08.011. [DOI] [PubMed] [Google Scholar]
- Lopez RB, Hofmann W, Wagner DD. et al. Neural predictors of giving in to temptation in daily life. Psychol Sci. 2014;25(7):1337–1344. doi: 10.1177/0956797614531492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN, Grilo CM. How relevant is food craving to obesity and its treatment? Front Psychiatry. 2014;5(164):1–5. doi: 10.3389/fpsyt.2014.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK. et al. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field AE, Coakley EH, Must A. et al. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med. 2001;161(13):1581–1586. doi: 10.1001/archinte.161.13.1581. [DOI] [PubMed] [Google Scholar]
- Swinburn BA, Sacks G, Hall KD. et al. he global obesity pandemic: Shaped by global drivers and local environments. Lancet. 2011;378(9793):804–814. doi: 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]
- Brownell KD, Horgen KB. Food Fight: The inside story of the food industry, America's obesity crisis, and what we can do about it. New York: McGraw Hill, Contemporary Books; 2004. [Google Scholar]
- Prentice AM, Jebb SA. Fast foods, energy density and obesity: A possible mechanistic link. Obes Rev. 2003;4(4):187–194. doi: 10.1046/j.1467-789x.2003.00117.x. [DOI] [PubMed] [Google Scholar]
- Jeffery RW, Baxter J, McGuire M. et al. Are fast food restaurants an environmental risk factor for obesity? nt J Behav Nutr Phys Act. 2006;3(2):1–6. doi: 10.1186/1479-5868-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenheck R. Fast food consumption and increased caloric intake: A systematic review of a trajectory towards weight gain and obesity risk. Obes Rev. 2008;9(6):535–547. doi: 10.1111/j.1467-789X.2008.00477.x. [DOI] [PubMed] [Google Scholar]
- McClure AC, Tanski SE, Gilbert-Diamond D. et al. Receptivity to television fast-food restaurant marketing and obesity among U.S. Youth. Am J Prev Med. 2013;45(5):560–568. doi: 10.1016/j.amepre.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JL, Pomeranz JL, Lobstein T. et al. A crisis in the marketplace: How food marketing contributes to childhood obesity and what can be done. Annu Rev Public Health. 2009;30:211–225. doi: 10.1146/annurev.publhealth.031308.100304. [DOI] [PubMed] [Google Scholar]
- Morris MJ, Beilharz JE, Maniam J. et al. Why is obesity such a problem in the 21st century? The intersection of palatable food, cues and reward pathways, stress, and cognition. Neurosci Biobehav Rev. 2015;58:36–45. doi: 10.1016/j.neubiorev.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Wadden TA, Brownell KD, Foster GD. Obesity: Responding to the global epidemic. J Consult Clin Psychol. 2002;70(3):510–525. doi: 10.1037//0022-006x.70.3.510. [DOI] [PubMed] [Google Scholar]
- Tetley A, Brunstrom J, Griffiths P. Individual differences in food-cue reactivity. The role of BMI and everyday portion-size selections. Appetite. 2009;52(3):610–620. doi: 10.1016/j.appet.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Ferriday D, Brunstrom JM. 'I just can't help myself': Effects of food-cue exposure in overweight and lean individuals. Int J Obes. 2011;35(1):142–149. doi: 10.1038/ijo.2010.117. [DOI] [PubMed] [Google Scholar]
- Martin KS, Ferris AM. Food insecurity and gender are risk factors for obesity. J Nutr Educ Behav. 2007;39(1):31–36. doi: 10.1016/j.jneb.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Obesity and overweight. 2010. [cited 2015 Nov 5];World Health Organization. [Internet] Available from: http://www.who.int/mediacentre/factsheets/fs311/en/v .
- Flegal KM, Carroll MD, Ogden CL. et al. Prevalence and trends in obesity among US adults, 1999-2009. JAMA. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- Mond JM, Baune BT. Overweight, medical comorbidity and health-related quality of life in a community sample of women and men. Obesity. 2009;17(8):1627–1634. doi: 10.1038/oby.2009.27. [DOI] [PubMed] [Google Scholar]
- Muennig P, Lubetkin E, Jia H. et al. Gender and the burden of disease attributable to obesity. Am J Public Health. 2006;96(9):1662–1668. doi: 10.2105/AJPH.2005.068874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale EAM, Gillespie KM. Diabetes and gender. Diabetologia. 2001;44(1):3–15. doi: 10.1007/s001250051573. [DOI] [PubMed] [Google Scholar]
- Han TS, Lean MEJ, Seidell JC. Quality of life in relation to overweight and body fat distribution. Am J Public Health. 1998;88(12):1814–1820. doi: 10.2105/ajph.88.12.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford MH, Marmot M. Current obesity, steady weight change and weight fluctuation as predictors of physical functioning in middle aged office workers: The Whitehall II study. Int J Obes. 1998;22(1):23–31. doi: 10.1038/sj.ijo.0800539. [DOI] [PubMed] [Google Scholar]
- Katz DA, McHorney CA, Atkinson RL. Impact of obesity on health-related quality of life in patients with chronic illness. J Gen Intern Med. 2000;15(11):789–796. doi: 10.1046/j.1525-1497.2000.90906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Must AS, Coakley EH, Field AF. et al. The disease burden associated with overweight and obesity. JAMA. 1999;292(16):1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- Patterson RE, Frank LL, Kristal AR. et al. A comprehensive examination of health conditions associated with obesity in older adults. Am J Prev Med. 2004;27(5):385–390. doi: 10.1016/j.amepre.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Larsson U, Karlsson J, Sullivan M. mpact of overweight and obesity on health-related quality of life - a Swedish population study. Int J Obes. 2002;26(3):417–424. doi: 10.1038/sj.ijo.0801919. [DOI] [PubMed] [Google Scholar]
- Carpenter K, Allison DB, Faith MS. Relationships between obesity and DSM-IV major depressive disorder, suicide ideation, and suicide attempts: Results from a general population study. Am J Public Health. 2000;90(2):251–257. doi: 10.2105/ajph.90.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott KM, McGee MA, Wells JE. et al. Obesity and mental disorders in the adult general population. J Psychosom Res. 2008;64(1):97–105. doi: 10.1016/j.jpsychores.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Jorm AF, Korten AE, Christensen H. et al. Association of obesity with anxiety, depression and emotional well-being: A community survey. Aust N Z J Public Health. 2003;27(4):434–440. doi: 10.1111/j.1467-842x.2003.tb00423.x. [DOI] [PubMed] [Google Scholar]
- Hetherington MM, MacDiarmid JI. "Chocolate addiction": A preliminary study of its description and its relationship to problem eating. Appetite. 1993;21(3):233–246. doi: 10.1006/appe.1993.1042. [DOI] [PubMed] [Google Scholar]
- Tuomisto T, Hetherington MM, Morris M. et al. Psychological and physiological characteristics of sweet food "addiction". Int J Eat Disord. 1999;25(2):169–175. doi: 10.1002/(sici)1098-108x(199903)25:2<169::aid-eat6>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Imperatori C, Innamorati M, Tamburello S. et al. Gender differences in food craving among overweight and obese patients attending low energy diet therapy: A matched case-control study. Eat Weight Disord. 2013;18(3):297–303. doi: 10.1007/s40519-013-0054-7. [DOI] [PubMed] [Google Scholar]
- Pelchat ML. Food cravings in young and elderly adults. Appetite. 1997;28(2):103–113. doi: 10.1006/appe.1996.0063. [DOI] [PubMed] [Google Scholar]
- Uher R, Treasure J, Heining M. et al. Cerebral processing of food-related stimuli: Effects of fasting and gender. Behav Brain Res. 2006;169(1):111–119. doi: 10.1016/j.bbr.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Frank S, Laharnar N, Kullmann S. et al. Processing of food pictures: Influence of hunger, gender and calorie content. Brain Res. 2010;1350:159–166. doi: 10.1016/j.brainres.2010.04.030. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Telang F. et al. Evidence of gender differences in the ability to inhibit brain activation elicited by food stimulation. PNAS. 2009;106(4):1249–1254. doi: 10.1073/pnas.0807423106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross CP, Copping LT, Campbell A. Sex differences in impulsivity: A meta-analysis. Psychol Bull. 2011;137(1):97–130. doi: 10.1037/a0021591. [DOI] [PubMed] [Google Scholar]
- Hirschberg AL. Sex hormones, appetite and eating behaviour in women. Maturitas. 2012;71(3):248–256. doi: 10.1016/j.maturitas.2011.12.016. [DOI] [PubMed] [Google Scholar]
- Asarian L, Geary N. Sex differences in the physiology of eating. Am J Physiol Regul Integr Comp Physiol. 2013;305(11):R1215–R1267. doi: 10.1152/ajpregu.00446.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philos Trans R Soc Lond B Biol Sci. 2006;361(1471):1251–1263. doi: 10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray A, Feldmen HA, McKinlay JB. et al. Age, disease, and changing sex hormone levels in middle-aged men: Results of the Massachusetts Male Aging study. J Clin Endocrinol Metab. 1991;73(5):1016–1025. doi: 10.1210/jcem-73-5-1016. [DOI] [PubMed] [Google Scholar]
- Davison SL, Bell R, Donath S. et al. Androgen levels in adult females: Changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab. 2005;90(7):3847–3853. doi: 10.1210/jc.2005-0212. [DOI] [PubMed] [Google Scholar]
- Ferrini RL, Barrett-Connor E. Sex hormones and age: A cross-sectional study of testosterone and estradiol and their bioavailable fractions in community-dwelling men. Am J Epidemiol. 1998;147(8):750–754. doi: 10.1093/oxfordjournals.aje.a009519. [DOI] [PubMed] [Google Scholar]
- Pasquali R. Obesity and androgens: Facts and perspectives. Fertil Steril. 2006;85(5):1319–1340. doi: 10.1016/j.fertnstert.2005.10.054. [DOI] [PubMed] [Google Scholar]
- Ding EL, Song Y, Malik VS. et al. Sex differences of endogenous sex hormones and risk of Type 2 diabetes. JAMA. 2006;295(11):1288–1299. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- Brown LM, Clegg DJ. Central effects of estradiol in the regulation of food intake, body weight, and adiposity. J Steroid Biochem Mol Biol. 2010;122(1-3):65–73. doi: 10.1016/j.jsbmb.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel LA. The ovarian hormone estradiol plays a crucial role in the control of food intake in females. Physiol Behav. 2011;104(4):517–524. doi: 10.1016/j.physbeh.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LM, Gent L, Davis K. et al. Metabolic impact of sex hormones on obesity. Brain Res. 2010;1350:77–85. doi: 10.1016/j.brainres.2010.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner MA, Samojlik E, Drejka M. et al. Androgen-estrogen metabolism in women with upper body versus lower body obesity. J Clin Endocrinol Metab. 1990;70(2):473–479. doi: 10.1210/jcem-70-2-473. [DOI] [PubMed] [Google Scholar]
- Allen AM, McRae-Clark AL, Carlson S. et al. Determining menstrual phase in human biobehavioral research: A review with recommendations. Exp Clin Psychopharmacol. 2015;24(1):1–11. doi: 10.1037/pha0000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr SI, Janelle KC, Prior JC. Energy intakes are higher during the luteal phase of ovulatory menstrual cycles. Am J Clin Nutr. 1995;61(1):39–43. doi: 10.1093/ajcn/61.1.39. [DOI] [PubMed] [Google Scholar]
- Cazaja JA, Goy RW. Ovarian hormones and food intake in female guinea pigs and rhesus monkeys. Horm Behav. 1975;6(4):329–349. doi: 10.1016/0018-506x(75)90003-3. [DOI] [PubMed] [Google Scholar]
- Dalvit SP. The effect of menstrual cycle patterns of food intake. Am J Clin Nutr. 1981;34(9):1811–1815. doi: 10.1093/ajcn/34.9.1811. [DOI] [PubMed] [Google Scholar]
- Gong EJ, Garrel D, Calloway DH. Menstrual cycle and voluntary food intake. Am J Clin Nutr. 1989;49(2):252–258. doi: 10.1093/ajcn/49.2.252. [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Wade GN. Ovarian influences on the meal patterns of female rats. Physiol Behav. 1975;17(2):201–208. doi: 10.1016/0031-9384(76)90064-0. [DOI] [PubMed] [Google Scholar]
- Roy EJ, Wade GN. Role of food intake in estradiol-induced body weight changes in female rats. Horm Behav. 1977;8(3):265–274. doi: 10.1016/0018-506x(77)90001-0. [DOI] [PubMed] [Google Scholar]
- Tarttelin MF, Gorski RA. The effects of ovarian steroids on food and water intake and body weight in the female rat. Acta Endocrinol. 1973;72:551–568. doi: 10.1530/acta.0.0720551. [DOI] [PubMed] [Google Scholar]
- Buffenstein R, Poppitt SD, McDevitt RM. et al. Food intake and the menstrual cycle: A retrospective analysis, with implications for appetite research. Physiol Behav. 1995;58(6):1067–1077. doi: 10.1016/0031-9384(95)02003-9. [DOI] [PubMed] [Google Scholar]
- Hormes JM, Timko CA. All cravings are not created equal. Correlates of menstrual versus non-cyclic chocolate craving. Appetite. 2011;57(1):1–5. doi: 10.1016/j.appet.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Frank TC, Kim GL, Krzemien A. et al. Effect of menstrual cycle phase on corticolimbic brain activation by visual food cues. Brain Res. 2010;1363:81–92. doi: 10.1016/j.brainres.2010.09.071. [DOI] [PubMed] [Google Scholar]
- Dye L, Blundell JE. Menstrual cycle and appetite control: Implications for weight regulation. Hum Repro. 1997;12(6):1142–1151. doi: 10.1093/humrep/12.6.1142. [DOI] [PubMed] [Google Scholar]
- McVay MA, Copeland AL, Newman HS. et al. Food cravings and food cue responding across the menstrual cycle in a non-eating disordered sample. Appetite. 2012;59(2):591–600. doi: 10.1016/j.appet.2012.07.011. [DOI] [PubMed] [Google Scholar]
- Rozin P, Levine E, Stoess C. Chocolate craving and liking. Appetite. 1991;17(3):199–212. doi: 10.1016/0195-6663(91)90022-k. [DOI] [PubMed] [Google Scholar]
- Gallant SJ, Hamilton JA, Popiel DA. et al. Daily moods and symptoms: Effects of awareness of study focus, gender, menstrual-cycle phase, and day of the week. Health Psychol. 1991;10(3):180–189. doi: 10.1037//0278-6133.10.3.180. [DOI] [PubMed] [Google Scholar]
- Hill AJ, Heaton-Brown L. The experience of food craving: a prospective investigation in healthy women. J Psychosom Res. 1994;38(8):801–814. doi: 10.1016/0022-3999(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Cohen IT, Sherwin BB, Fleming AS. Food cravings, mood, and the menstrual cycle. Horm Behav. 1987;21(4):457–470. doi: 10.1016/0018-506x(87)90004-3. [DOI] [PubMed] [Google Scholar]
- Anderson LC, Petrovich GD. Renewal of conditioned responding to food cues in rats: Sex differences and relevance of estradiol. Physiol Behav. 2015;151:338–344. doi: 10.1016/j.physbeh.2015.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien CP, Childress AR, Ehrman R. et al. Conditioning factors in drug abuse: Can they explain compulsion? J Psychopharmacol. 1998;12(1):15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- Sinha R, Li CS. maging stress-and cue-induced drug and alcohol craving: Association with relapse and clinical implications. Drug Alc Rev. 2007;26(1):25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. et al. Dopamine in drug abuse and addiction: Results of imaging studies and treatment implications. Arch Neurol. 2007;64(11):1575–1579. doi: 10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS. et al. Association of methylphenidate-induced craving with changes in right striato-orbitofrontal metabolism in cocaine abusers: Implications in addiction. Am J Psychiatry. 1999;156(1):19–26. doi: 10.1176/ajp.156.1.19. [DOI] [PubMed] [Google Scholar]
- Catley D, O'Connell KA, Shiffman S. Absentminded lapses during smoking cessation. Psychol Addict Behav. 2000;14(1):73–76. doi: 10.1037//0893-164x.14.1.73. [DOI] [PubMed] [Google Scholar]
- Crits-Christoph P, Gibbons MBC, Barber JP. et al. Predictors of sustained abstinence during psychosocial treatments for cocaine dependence. Psychother Res. 2007;17(2):240–252. [Google Scholar]
- Doherty K, Kinnunen T, Militello FS. Urges to smoke during the first month of abstinence: Relationship to relapse and predictors. Psychopharmacology. 1995;119(2):171–178. doi: 10.1007/BF02246158. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Marrone GF, Heishman SJ. et al. Tobacco, cocaine, and heroin: Craving and use during daily life. Addict Behav. 2010;35(4):318–324. doi: 10.1016/j.addbeh.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz DT, Frederick-Osborne SL, Galloway GP. Craving predicts use during treatment for methamphetamine dependence: a prospective, repeated-measures, within-subject analysis. Drug Alcohol Depend. 2001;63(3):269–276. doi: 10.1016/s0376-8716(00)00217-9. [DOI] [PubMed] [Google Scholar]
- Galloway GP, Singleton EG. The Methamphetamine Treatment Project Corporate Authors. How long does craving predict use of methamphetamine? Assessment of use one to seven weeks after the assessment of craving. Subst Abuse. 2008;1:63–79. [PMC free article] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP. Craving is associated with smoking relapse: Findings from three prospective studies. Exp Clin Psychopharmacol. 1997;5(2):137–142. doi: 10.1037//1064-1297.5.2.137. [DOI] [PubMed] [Google Scholar]
- Litt MD, Cooney NL, Morse P. Reactivity to alcohol-related stimuli in the laboratory and in the field: Predictors of craving in treated alcoholics. Addiction. 2000;95(6):889–900. doi: 10.1046/j.1360-0443.2000.9568896.x. [DOI] [PubMed] [Google Scholar]
- O'Connell KA, Schwartz JE, Gerkovich M. et al. Playful and rebellious states vs. Negative affect in explaining the occurrence of temptations and lapses during smoking cessation. Nicotine Tob Res. 2004;6(4):661–674. doi: 10.1080/14622200410001734049. [DOI] [PubMed] [Google Scholar]
- Paliwal P, Hyman SM, Sinha R. Craving predicts time to cocaine relapse: Further validation of the Now and Brief versions of the Cocaine Craving Questionnaire. Drug Alc Depend. 2008;93(3):252–259. doi: 10.1016/j.drugalcdep.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston KL, Vahabzadeh M, Schmittner J. et al. Cocaine craving and use during daily life. Psychopharmacology. 2009;207(2):291–301. doi: 10.1007/s00213-009-1655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Martin RA, Eaton CA. et al. Cocaine craving as a predictor of treatment attrition and outcomes after residential treatment for cocaine dependence. J Stud Alcohol Drugs. 2007;68(5):641–648. doi: 10.15288/jsad.2007.68.641. [DOI] [PubMed] [Google Scholar]
- Shiffman S. The tobacco withdrawal syndrome. Cigarette Smoking as a Dependence Process. NIDA Res Monogr. 1979;23:158–184. [PubMed] [Google Scholar]
- Weiss RD, Griffin ML, Mazurick C. et al. The relationship between cocaine craving, psychosocial treatment, and subsequent cocaine use. Am J Psychiatry. 2003;160(7):1320–1325. doi: 10.1176/appi.ajp.160.7.1320. [DOI] [PubMed] [Google Scholar]
- Swan GE, Ward MM, Jack LM. Abstinence effects as predictors of 28-day relapse in smokers. Addict Behav. 1996;21(4):481–490. doi: 10.1016/0306-4603(95)00070-4. [DOI] [PubMed] [Google Scholar]
- Pelchat ML. Of human bondage: Food craving, obsession, compulsion, and addiction. Physiol Behav. 2002;76(3):347–352. doi: 10.1016/s0031-9384(02)00757-6. [DOI] [PubMed] [Google Scholar]
- Chase HW, Eickhoff SB, Laird AR. et al. The neural basis of drug stimulus processing and craving: An activation likelihood estimation meta-analysis. Biol Psychiatry. 2011;70(8):785–793. doi: 10.1016/j.biopsych.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn S, Gallinat J. Common biology of craving across legal and illegal drugs - a quantitative meta-analysis of cue-reactivity brain response. Eur J Neurosci. 2011;33(7):1318–1326. doi: 10.1111/j.1460-9568.2010.07590.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS. et al. Overlapping neuronal circuits in addiction and obesity: Evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS. et al. Addiction circuitry in the human brain. Annu Rev Pharmacol Toxicol. 2011;52(1):321–336. doi: 10.1146/annurev-pharmtox-010611-134625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS. et al. Food and drug reward: Overlapping circuits in human obesity and addiction. Curr Top Behav Neurosci. 2012;11:1–24. doi: 10.1007/7854_2011_169. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D. et al. The addictive dimensionality of obesity. Biol Psychiatry. 2013;73(9):811–818. doi: 10.1016/j.biopsych.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D. et al. Obesity and addiction: Neurobiological overlaps. Obes Rev. 2013;14(1):2–18. doi: 10.1111/j.1467-789X.2012.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D. et al. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sc. 2008;363(1507):3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nat Neurosci. 2005;8(11):1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Shifting and stopping: Fronto-striatal substrates, neurochemical modulation and clinical implications. Philos Trans R Soc Lond B Biol Sci. 2007;362(1481):917–932. doi: 10.1098/rstb.2007.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DG, Robbins TW. The neurobiological underpinnings of obesity and binge eating: A rationale for adopting the food addiction model. Biol Psychiatry. 2013;73(9):804–810. doi: 10.1016/j.biopsych.2012.08.026. [DOI] [PubMed] [Google Scholar]
- Parylak SL, Koob GF, Zorrilla EP. The dark side of food addiction. Physiol Behav. 2011;104(1):149–156. doi: 10.1016/j.physbeh.2011.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Garcia I, Horstmann A, Jurado MA. et al. Reward processing in obesity, substance addiction and non-substance addiction. Obes Rev. 2014;15(11):853–869. doi: 10.1111/obr.12221. [DOI] [PubMed] [Google Scholar]
- Wilson GT. Eating disorders, obesity and addiction. Eur Eat Disord Rev. 2010;18(5):341–351. doi: 10.1002/erv.1048. [DOI] [PubMed] [Google Scholar]
- Pogun S, Yarabas G. Sex differences in nicotine action. Handb Exp Pharmacol. 2009;192:261–291. doi: 10.1007/978-3-540-69248-5_10. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lynch WJ, Roth ME. et al. Sex and estrogen influence drug abuse. Trends Pharmacol Sci. 2004;25(5):273–279. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Lynch W. Sex differences in vulnerability to drug self-administration. Exp Clin Psychopharmacol. 2006;14(1):34–41. doi: 10.1037/1064-1297.14.1.34. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: Preclinical and clinical studies. Psychopharmacology. 2002;164(2):121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Sofuoglu M. Role of progesterone in nicotine addiction: Evidence from initiation to relapse. Exp Clin Psychopharmacol. 2010;18(6):451–461. doi: 10.1037/a0021265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29(1):36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Kozink RV, Rose JE. ndividual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacology. 2008;33(9):2148–2157. doi: 10.1038/sj.npp.1301618. [DOI] [PubMed] [Google Scholar]
- Potenza MN, Hong KA, Lacadie CM. et al. Neural correlates of stress-induced and cue-induced craving: Influences of gender and cocaine dependence. Am J Psychiatry. 2012;169(4):406–414. doi: 10.1176/appi.ajp.2011.11020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Mell MM. In: Handbook on the Cognitive Neuroscience of Addiction. Wilson SJ, editor. Oxford, UK: Wiley-Blackwell; 2015. Neural mechanisms underlying craving and the regulation of craving. [Google Scholar]
- Kilts CD, Gross RE, Ely TD. et al. The neural correlates of cue-induced craving in cocaine-dependent women. Am J Psychiatry. 2004;161(2):233–241. doi: 10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- Saladin ME, Gray KM, Carpenter MJ. et al. Gender differences in craving and cue reactivity to smoking and negative affect/stress cues. Am J Addict. 2012;21(3):210–220. doi: 10.1111/j.1521-0391.2012.00232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H, Ohashi K. Association of menstrual phase with smoking behavior, mood and menstrual phase-associated symptoms among young Japanese women smokers. BMC Womens Health. 2013;13(10):1–6. doi: 10.1186/1472-6874-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen SS, Allen AM, Lunos S. et al. Patterns of self-selected smoking cessation attempts and relapse by menstrual phase. Addict Behav. 2009;34(11):928–931. doi: 10.1016/j.addbeh.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBon M, Klesges RC, Klesges LM. Symptomatology across the menstrual cycle in smoking and nonsmoking women. Addict Behav. 1995;20(3):335–343. doi: 10.1016/0306-4603(94)00070-f. [DOI] [PubMed] [Google Scholar]
- Allen SS, Hatsukami DK, Christianson D. et al. Withdrawal and pre-menstrual symptomatology during the menstrual cycle in short-term smoking abstinence: Effects of menstrual cycle on smoking abstinence. Nicotine Tob Res. 1999;1(2):129–142. doi: 10.1080/14622299050011241. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Napier K, Ehrman R. et al. Retrospective study: Influence of menstrual cycle on cue-induced cigarette craving. Nicotine Tob Res. 2004;6(1):171–175. doi: 10.1080/14622200310001656984. [DOI] [PubMed] [Google Scholar]
- Carpenter MJ, Upadhyaya HP, LaRowe SD. et al. Menstrual cycle phase effects on nicotine withdrawal and cigarette craving: A review. Nicotine Tob Res. 2006;8(5):627–638. doi: 10.1080/14622200600910793. [DOI] [PubMed] [Google Scholar]
- DeVito EE, Herman AI, Waters AJ. et al. Subjective, physiological, and cognitive responses to intravenous nicotine: Effects of sex and menstrual cycle phase. Neuropsychopharmacology. 2014;39(6):1431–1440. doi: 10.1038/npp.2013.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Allen SS. Influence of menstrual cycle phase on smoking cessation treatment outcome: A hypothesis regarding the discordant findings in the literature. Addiction. 2009;104(11):1941–1942. doi: 10.1111/j.1360-0443.2009.02758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen SS, Bade T, Center B. et al. Menstrual phase effects on smoking relapse. Addiction. 2008;103(5):809–821. doi: 10.1111/j.1360-0443.2008.02146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Ehrman R, Lynch KG. et al. Menstrual cycle phase at quit date predicts smoking status in an NRT treatment trial: A retrospective analysis. J Women's Health. 2008;17(2):287–292. doi: 10.1089/jwh.2007.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter MJ, Saladin ME, Leinbach AS. et al. Menstrual phase effects on smoking cessation: A pilot feasibility study. J Womens Health. 2008;17(2):293–301. doi: 10.1089/jwh.2007.0415. [DOI] [PubMed] [Google Scholar]
- Allen SS, Hatuskami D, Christianson D. et al. Effects of transdermal nicotine on craving, withdrawal and premenstrual symptomatology in short-term smoking abstinence during different phases of the menstrual cycle. Nicotine Tob Res. 2000;2(3):231–241. doi: 10.1080/14622200050147493. [DOI] [PubMed] [Google Scholar]
- Meule AL, Vogele C, Kubler A. Food cravings discriminate differentially between successful and unsuccessful dieters and non-dieters. Validation of the Food Cravings Questionnaires in German. Appetite. 2012;58(1):88–97. doi: 10.1016/j.appet.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Delahantey LM, Meigs JB, Hayden D. et al. Psychological and behavioral correlates of baseline BMI in the Diabetes Prevention Program. Diabetes Care. 2002;25(11):1992–1998. doi: 10.2337/diacare.25.11.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field AE, Manson JE, Taylor CB. et al. Association of weight change, weight control practices, and weight cycling among women in the Nurses' Health Study II. Int J Obes Relat Metab Disord. 2004;28(9):1134–1142. doi: 10.1038/sj.ijo.0802728. [DOI] [PubMed] [Google Scholar]
- McCaffery JM, Haley AP, Sweet LH. et al. Differential functional magnetic resonance imaging response to food pictures in successful weight-loss maintainers relative to normal-weight and obese controls. Am J Clin Nutr. 2009;90(4):928–934. doi: 10.3945/ajcn.2009.27924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen A, Stegerman S, Roefs A. et al. Decreased salivation to food cues in formerly obese successful dieters. Psychother Psychosom. 2010;79(4):257–258. doi: 10.1159/000315131. [DOI] [PubMed] [Google Scholar]
- Batra P, Das SK, Salinardi T. et al. Relationship of cravings with weight loss and hunger. Results from a 6 month worksite weight loss intervention. Appetite. 2013;69:1–7. doi: 10.1016/j.appet.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Murdaugh DL, Cox JE, Cook EW. et al. fMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. Neuroimage. 2012;59(3):2709–2721. doi: 10.1016/j.neuroimage.2011.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JW, Greenway FL, Fujoka K. et al. Bupropion SR enhances weight loss: A 48-week double-blind placebo-controlled trial. Obes Res. 2002;10(7):633–641. doi: 10.1038/oby.2002.86. [DOI] [PubMed] [Google Scholar]
- Billes SK, Sinnayah P, Cowley MA. Naltrexone/bupropion for obesity: An investigational combination pharmacotherapy for weight loss. Pharmacol Res. 2014;84:1–11. doi: 10.1016/j.phrs.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Wadden TA, Foreyt JP, Foster GD. et al. Weight loss with Naltrexone SR/Bupropion SR combination therapy as an adjunct to behavior modification: The COR-BMOD trial. Obesity. 2011;19(1):110–120. doi: 10.1038/oby.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenway FL, Fujioka F, Pladowski RA. et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): A multicentre, randomized, double-blind, placebo-controlled, Phase 3 trial. Lancet. 2010;376(9741):595–605. doi: 10.1016/S0140-6736(10)60888-4. [DOI] [PubMed] [Google Scholar]
- Hollander P, Gupta AK, Plodkowski R. et al. Effects of naltrexone sustained-release/bupropion combination therapy on body weight and glycemic parameters in overweight and obese patients with Type 2 diabetes. Diabetes Care. 2013;36(12):4022–4029. doi: 10.2337/dc13-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen A, Broekmate J, Heymans M. Cue-exposure vs. self-control in the treatment of binge eating: A pilot study. Behav Res Ther. 1992;30(3):235–241. doi: 10.1016/0005-7967(92)90069-s. [DOI] [PubMed] [Google Scholar]
- Frankort A, Roefs A, Siep N. et al. The craving stops before you feel it: Neural correlates of chocolate craving during cue exposure with response prevention. Cereb Cortex. 2014;24(6):1589–1600. doi: 10.1093/cercor/bht016. [DOI] [PubMed] [Google Scholar]
- McIntosh VV, Carter FA, Bulik CM. et al. Five-year outcome of cognitive behavioral therapy and exposure with response prevention for bulimia nervosa. Psychol Med. 2011;41(5):1061–1071. doi: 10.1017/S0033291710001583. [DOI] [PubMed] [Google Scholar]
- Toro J, Cervera M, Feliu MH. et al. Cue exposure in the treatment of resistant bulimia nervosa. Int J Eat Disord. 2003;34(2):227–234. doi: 10.1002/eat.10186. [DOI] [PubMed] [Google Scholar]
- Martinez-Mallen E, Castro-Fornieles J, Lazaro L. et al. Cue exposure in the treatment of resistant adolescent bulimia nervosa. Int J Eat Disord. 2007;40(7):596–601. doi: 10.1002/eat.20423. [DOI] [PubMed] [Google Scholar]
- Koskina A, Campbell IC, Schmidt U. Exposure therapy in eating disorders revisited. Neurosci Biobehav Rev. 2013;37(2):193–208. doi: 10.1016/j.neubiorev.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Emotion regulation: Current status and future prospects. Psychol Inq. 2015;26(1):1–26. [Google Scholar]
- Siep N, Roefs A, Roebroeck A. et al. Fighting food temptations: The modulating effects of short-term cognitive reappraisal, suppression and up-regulation on mesocorticolimbic activity related to appetitive motivation. Neuroimage. 2012;60(1):213–220. doi: 10.1016/j.neuroimage.2011.12.067. [DOI] [PubMed] [Google Scholar]
- Giuliani NR, Calcott RD, Berkman ET. Piece of cake. Cognitive reappraisal of food craving. Appetite. 2013;64:56–61. doi: 10.1016/j.appet.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell kd. The LEARN program for weight management. Dallas, TX: American Health Publishing; 2000. [Google Scholar]
- Hay P. A systematic review of evidence for psychological treatments in eating disorders: 2005-2012. Int J Eat Disord. 2013;46(5):462–469. doi: 10.1002/eat.22103. [DOI] [PubMed] [Google Scholar]
- Wilson GT, Fairburn CG. In: A guide to treatments that work. Nathan PE, Gorman JM, editors. New York: Oxford University Press; 2007. reatments for eating disorders. [Google Scholar]
- Cooper Z, Doll HA. et al. Testing a new cognitive behavioural treatment for obesity: A randomized controlled trial with three-year follow-up. Behav Res Ther. 2010;48(8):706–713. doi: 10.1016/j.brat.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper Z, Fairburn CG. In: Handbook of obesity treatment. Wadden TA, Stunkard AJ, editors. New York: Guilford Press; 2002. Cognitive behavioral treatment of obesity. [Google Scholar]
- Alberts HJ, Mulkens S, Smeets M. et al. Coping with food cravings. Investigating the potential of a mindfulness-based intervention. Appetite. 2010;55(1):160–163. doi: 10.1016/j.appet.2010.05.044. [DOI] [PubMed] [Google Scholar]
- Dalen J, Smith BW, Shelley BM. et al. Pilot study: Mindful eating and living (MEAL): Weight, eating behavior, and psychological outcomes associated with a mindfulness-based intervention for people with obesity. Complement Ther Med. 2010;18(6):260–264. doi: 10.1016/j.ctim.2010.09.008. [DOI] [PubMed] [Google Scholar]
- O'Reilly GA, Cook L, Spruijt-Metz D. et al. Mindfulness-based interventions for obesity-related eating behaviours: A literature review. Obes Rev. 2014;15(6):453–461. doi: 10.1111/obr.12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts HJ, Thewissen R, Raes L. Dealing with problematic eating behaviour. The effects of a mindfulness-based intervention on eating behaviour, food cravings, dichotomous thinking and body image concern. Appetite. 2012;58(3):847–851. doi: 10.1016/j.appet.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Kristeller J, Wolever RQ, Sheets V. Mindfulness-based eating awareness training (mb-eat) for binge eating: A randomized clinical trial. Mindfulness. 2014;5(3):282–297. [Google Scholar]
- Katterman SN, Kleinman BM, Hood MM. et al. Mindfulness meditation as an intervention for binge eating, emotional eating, and weight loss: A systematic review. Eating Behav. 2014;15(2):197–204. doi: 10.1016/j.eatbeh.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Hill DM, Craighead LW, Safer DL. Appetite-focused dialectical behavior therapy for the treatment of binge eating with purging: A preliminary trial. Int J Eat Disord. 2011;44(3):249–261. doi: 10.1002/eat.20812. [DOI] [PubMed] [Google Scholar]
- Leahey TM, Crowther JH, Irwin SR. A cognitive-behavioral mindfulness group therapy intervention for the treatment of binge eating in bariatric surgery patients. Cogn Behav Pract. 2008;15(4):364–375. [Google Scholar]
- Robertson C, Avenell A, Boachie C. et al. Should weight loss and maintenance programmes be designed differently for men? A systematic review of long-term randomised controlled trials presenting data for men and women: The ROMEO project. Obes Res Clin Pract. 2015;10(1):70–84. doi: 10.1016/j.orcp.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Stroebele-Benschop ND, Machado AD, Milan FMP. et al. Gender differences in the outcome of obesity treatments and weight loss maintenance - a systematic review. J Obes Weight Loss Ther. 2013;3(4):1–11. [Google Scholar]
- Davis C, Fattore L, Kaplan AS. et al. The suppression of appetite and food consumption by methylphenidate: The moderating effects of gender and weight status in healthy adults. Int J Neuropsychopharmacol. 2012;15(2):181–187. doi: 10.1017/S1461145711001039. [DOI] [PubMed] [Google Scholar]
- Zeevi D, Korem T, Zmora N. et al. ersonalized nutrition by prediction of glycemic responses. Cell. 2015;163(5):1079–1094. doi: 10.1016/j.cell.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Freeman EW, Kroll R, Rapkin A. et al. Evaluation of a unique oral contraceptive in the treatment of premenstrual dysphoric disorder. J Womens Health Gend Based Med. 2001;10(6):561–569. doi: 10.1089/15246090152543148. [DOI] [PubMed] [Google Scholar]
- Marr J, Niknian M, Shulman LP. et al. Premenstrual dysphoric disorder symptom cluster improvement by cycle with the combined oral contraceptive ethinylestradiol 20 mcg plus drospirenone 3 mg administered in a 24/4 regimen. Contraception. 2011;84(1):81–86. doi: 10.1016/j.contraception.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Bancrof J, Rennie D. The impact of oral contraceptives on the experience of perimenstrual mood, clumsiness, food craving and other symptoms. J Psychosom Res. 1993;37(2):195–202. doi: 10.1016/0022-3999(93)90086-u. [DOI] [PubMed] [Google Scholar]
- McVay MA, Copeland AL, Geiselman PJ. Eating disorder pathology and menstrual cycle fluctuations in eating variables in oral contraceptive users and non-users. Eat Behav. 2011;12(1):49–55. doi: 10.1016/j.eatbeh.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Carpenter S, Neinstein LS. Weight gain in adolescent and young adult oral contraceptive users. J Adolesc Health Care. 1986;7(5):342–344. doi: 10.1016/s0197-0070(86)80163-2. [DOI] [PubMed] [Google Scholar]
- Tucci SA, Murphy LE, Boyland EJ. et al. Oral contraceptive effects on food choice during the follicular and luteal phases of the menstrual cycle. A laboratory based study. Appetite. 2010;55(3):388–392. doi: 10.1016/j.appet.2010.06.005. [DOI] [PubMed] [Google Scholar]