Abstract
CD4+ T follicular helper cells (TFH) have been identified as the T-cell subset specialized in providing help to B cells for optimal activation and production of high affinity antibody. We recently demonstrated that the expansion of peripheral blood influenza-specific CD4+IL-21+ICOS1+ T helper (TH) cells, three weeks after vaccination, associated with and predicted the rise of protective neutralizing antibodies to avian H5N1. In this study, healthy adults were vaccinated with plain seasonal trivalent inactivated influenza vaccine (TIIV), MF59®-adjuvanted TIIV (ATIIV), or saline placebo. Frequencies of circulating CD4+ TFH1 ICOS+ TFH cells and H1N1-specific CD4+IL-21+ICOS+ CXCR5+ TFH and CXCR5- TH cell subsets were determined at various time points after vaccination and were then correlated with hemagglutination inhibition (HI) titers. All three CD4+ T cell subsets expanded in response to TIIV and ATIIV, and peaked 7 days after vaccination. To demonstrate that these TFH cell subsets correlated with functional antibody titers, we defined an alternative endpoint metric, decorrelated HI (DHI), which removed any correlation between day 28/day 168 and day 0 HI titers, to control for the effect of preexisting immunity to influenza vaccine strains. The numbers of total circulating CD4+ TFH1 ICOS+ cells and of H1N1-specific CD4+IL-21+ICOS+ CXCR5+, measured at day 7, were significantly associated with day 28, and day 28 and 168 DHI titers, respectively. Altogether, our results show that CD4+ TFH subsets may represent valuable biomarkers of vaccine-induced long-term functional immunity.
Trial Registration
ClinicalTrials.gov NCT01771367
Introduction
Protein-based vaccines confer protection against pathogens mainly through the induction of T cell-dependent high affinity functional antibody responses. In this context a specialized subset of T helper cells (TH), identified as T follicular helper cells (TFH), differentiate and provide help to B cells in the germinal centers (GC) of secondary lymphoid organs, leading to B-cell proliferation and differentiation, and reshaping of the B-cell repertoire and Ig affinity maturation [1–5]. Thus, TFH cells play a critical role in the generation of long-lived humoral responses to antigens [3].
TFH cells were first isolated and identified in human tonsils, and were characterized by the expression of B cell follicle homing chemokine receptor CXCR5 and the inducible costimulatory molecule ICOS [6, 7]. TFH cells efficiently provide help to B cells and promote IgM to IgG immunoglobulin class switching through the production of interleukin-21 (IL-21) [8]. Studies in animal models have shown that, once differentiated and activated, TFH cells can exit GC, developing into memory TFH cells [9–12]. However, the origin of human blood circulating TFH cells remains to be established.
CD4+ TH cells expressing the chemokine receptor CXCR5 are currently termed blood memory or peripheral TFH cells and are long-lived memory cells [7, 13–15]. Recently, some human studies have contributed to a deeper characterization of blood TFH cells on the basis of the expression of additional chemokine receptors such as CXCR3, CCR6, and CCR7, the costimulatory molecule ICOS, and the immunomodulatory molecule PD-1 [13, 16, 17]. TFH cells defined as CXCR3+CCR6- share properties with TH1 cells (hereafter called TFH1 cells), while CXCR3-CCR6- and CXCR3-CCR6+ cells share properties of TH2 cells (TFH2) and of TH17 cells (TFH17), respectively [13]. TFH2 and TFH17 have a more efficient T helper activity on naive B cells, while TFH1 ICOS+ cells have a higher propensity to provide help to memory B cells [17]. In addition, we previously demonstrated that antigen-specific TFH can be identified by flow cytometry by intracelluar staining of IL-21 upon in vitro antigen stimulation [18].
The identification of early biomarkers predicting vaccine efficacy may contribute to accelerate the development of novel vaccine candidates. These biomarkers should be easy to test in large clinical trials and have a clear mechanistic relationship with the correlates or surrogates of protection taken as study’s endpoints. Recent studies showed that immunization with influenza A/California/2009 (H1N1) vaccine led to an expansion of peripheral TFH subsets in humans [13, 17, 19–21]. Moreover, ex vivo frequencies of peripheral TFH1 cells at day 7 correlated with the frequency of circulating plasmablasts and with increased levels of neutralizing antibodies to H1N1 at day 21 [13, 17]. In a previous study, we showed that a single dose of an avian H5N1 influenza vaccine induced the expansion of H5N1-specific CD4+ICOS+IL-21+ TH cells in the blood three weeks after vaccination, and that the increased frequency of these cells predicted the protective antibody titers found after the second dose of the vaccine [18].
The goal of the present study was to identify, in human peripheral blood, early TFH cells subset(s) predicting not only the rise but also the long term persistence of functional antibody titers after seasonal influenza vaccination. For this purpose, we had access to human PBMCs collected in the framework of the European Innovative Medicine Initiative funded public-private project BIOVACSAFE [22]. PBMCs from healthy subjects immunized with one dose of seasonal adjuvanted or non-adjuvanted trivalent inactivated influenza vaccine (ATIIV and TIIV, respectively) were analyzed both directly ex vivo or after in vitro antigen stimulation. Frequencies of TFH cells were determined and then correlated with HI antibody titers measured at days 28 and 168 post-vaccination. Both antigen-specific CD4+IL-21+ICOS+CXCR5+ TFH cells and ex vivo TFH1 ICOS+ cells expanded seven days after vaccination and returned to baseline levels by day 28. After accounting for the effect of baseline HI titers, we showed that the magnitude of the response of these TFH cell subsets correlated with functional antibody responses measured up to 6 months after vaccination.
Materials and Methods
Clinical samples and vaccines
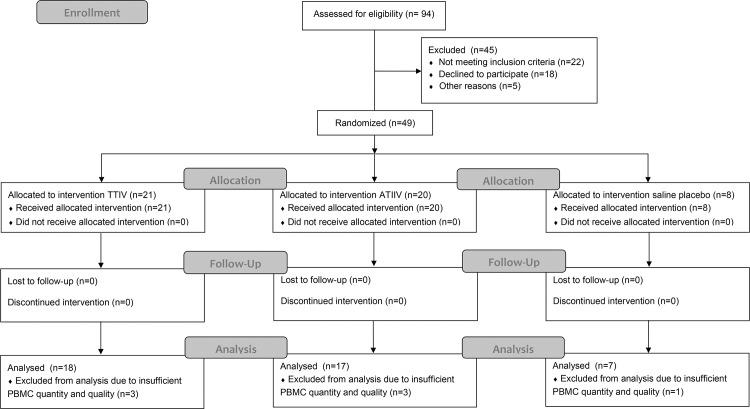
The study received ethical approval from London—Surrey Borders Research Ethics Committee (REC Ref: 13/LO/0044), and was registered on ClinicalTrials.gov prior to enrolment (NCT01771367). Forty-nine healthy adults (18–43 years old) were enrolled at the Surrey Clinical Research Centre, University of Surrey, Guildford, UK, as part of the BIOVACSAFE Consortium-funded clinical trial protocol CRC305C sponsored by the University of Surrey. The study was a partial-blind (participant and laboratory), randomised, placebo controlled exploratory study. The full study protocol is described in supplementary document S1 Text. All participants provided written informed consent. In the 2012-2013 winter season, 49 participants were randomized to allow 48 to complete with the full required reportable data in three arms and received one dose of A/California/7/2009 (H1N1), A/Victoria/361/2011 (H3N2), B/Wisconsin/1/2010-like TIIV (Agrippal®; n=21), ATIIV (Fluad®, n=20), or saline placebo (n=8). Recruitment for the study began on February 7th 2013 and ended with the last follow-up visit on November 25th 2013. See CONSORT diagram and checklist (Fig 1 and S2 Text, respectively). PBMCs were collected from each group at baseline, day 7 and day 28 after immunization, and analyzed for plasmablasts and CD4+ T cell responses. Sera were collected at day 0, day 7, day 28 and day 168 after immunization for the analyses of antibody responses.
Fig 1. CONSORT Flow diagram.
Hemagglutination Inhibition Assay (HI)
HI titers were measured for A/California/7/2009 (H1N1), A/Victoria/361/2011 (H3N2), and B/Wisconsin/1/2010-like vaccine strains (Novartis Vaccines & Diagnostics) as described elsewhere [23, 24].
Polychromatic flow cytometry analyses
Frozen PBMCs from vaccinated participants were thawed and then analyzed by flow cytometry both ex vivo and after 18 h of stimulation at 37°C with anti-CD28 and anti-CD49d (1 μg/ml each, BD Biosciences), A/California/7/2009 (H1N1) subunit vaccine antigen (1 μg/ml, Novartis Vaccines & Diagnostics), or Staphylococcus enterotoxin B (SEB) (1 μg/ml, Sigma), in the presence of Brefeldin A (5 μg/ml, Sigma) as previously described [18, 25]. Cells were stained ex vivo with Live/Dead Yellow (Invitrogen), fluorochrome-conjugated antibodies: CD3-PE-Texas Red (SK7), CD4-APC-Horizon 7 (SK3), ICOS-PE (ISA-3), CXCR5-FITC (RF8B2), CXCR3-PE-Cy5 (1C6), CCR6-Brillant Violet 421 (11A9), PD1-Brilliant Violet 785 (EH12.2H7), CD19-APC (SJ25C1), CD20-PerCP-Cy5.5 (L27), CD27-PE (L128), CD38-Alexa fluor 700 (HIT2) (BD Biosciences), CD8-Horizon V500 (RPA-T8) (Biolegend), CD45RA-PE-Cy7 (HI100) (eBioscience). H1N1-specific CD4+ T cells were analyzed for intracellular production of IL-21 with anti-IL-21-APC (3A3-N2.1) (BD Biosciences) [18, 25]. Stained cells were acquired on a BD LSR Fortessa special order flow cytometer (BD Biosciences).
Analysis of H1N1-specific CD4+IL-21+ ICOS+ TH cells
Frequency, phenotype, and cytokine profile of H1N1-specific CD4+IL-21+ICOS+ TH cells were determined by polychromatic flow cytometry following the gating strategy described in S1C Fig. The response to medium was subtracted for each subject at each time point. Data are expressed as number of antigen-specific CD4+ T cells per million of total CD4+ T cells. Data were analyzed using FlowJo (version 9.6, Tree Star) [18].
Statistical analyses
Median HI titers and frequencies of CD4+ T cells measured across different time points were compared using Wilcoxon’s signed rank test. Analyses were performed with SAS JMP 8.0.1 software. Associations between day 7 cellular responses and day 28 and 168 functional antibody responses were evaluated using the Pearson product-moment correlation metric. Data from TIIV and ATIIV vaccine cohorts were merged and analyzed as a single dataset. The potential effect of vaccine formulation as well as the negative correlation between HI responses to vaccination and baseline titers were removed, for each antigen, by fitting a mixed model and obtaining the residuals, based on a method adapted from Bucasas et al. [26]. The R package lme4 was used to perform a linear mixed effects analysis of the relationship between day 28, or day 168, HI titers fold-increase and baseline HI titers [27]. The baseline HI titer was used as only fixed effect, while random slopes and intercepts for the two vaccine cohorts were included as random effects. The model was formulated as follows: HIDayX/Day0 ~ HIDay0 + (1 + HIDay0|Cohort) + ε. Where HIDayX/Day0 represents the HI fold-increase measured either at day 28 or 168 post-vaccination, HIDay0 corresponds to the baseline HI titer, Cohort is a 2-level factor indicating which vaccine was used and ε is the residual error. Given the lack of antigen specificity, cell responses derived from the ex vivo staining were correlated to the maximun response observed across the three strains represented in the vaccine, while H1N1-specific TFH cells were correlated to H1N1-specific HI responses.
Results
Of the participants who were randomised, PBMCs of sufficient quantity and quality were obtained for 18 participants who received TIIV, 17 participants who received ATIIV and 7 who received placebo. Therefore the results reported below are only for 42 participants.
Influenza vaccination induces fast and long-lasting functional antibody responses
In this study, 42 healthy adults received a single intramuscular immunization with 2012-2013 TIIV, ATIIV, or saline placebo. B-cell responses were characterized as (i) frequencies of blood CD19+CD20-CD38+ plasmablasts measured by flow cytometry at day 0, 7 and 28, and (ii) as vaccine-specific HI antibody titers at day 0, 7, 28, and 168.
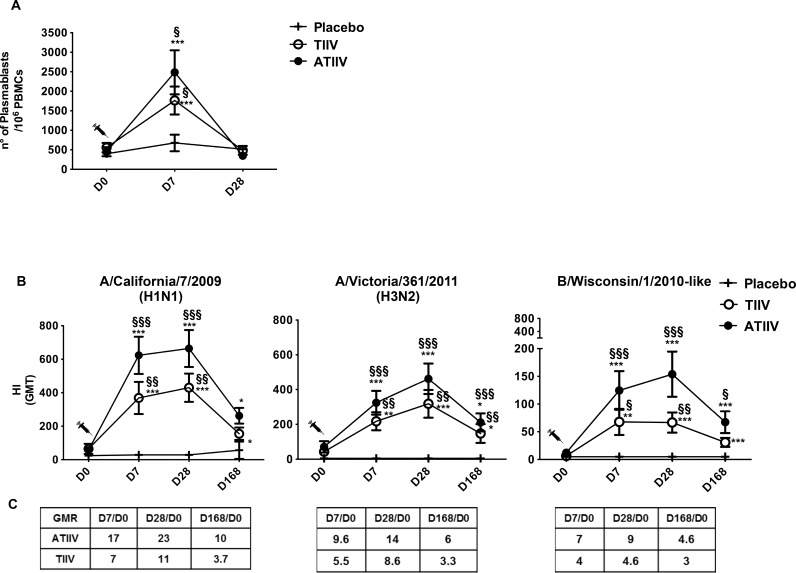
Consistent with previous studies [28–30], TIIV and ATIIV increased the frequencies of plasmablasts at day 7 compared to placebo (Fig 2A and S1A Fig for subset identification). HI geometric mean antibody titers (GMT) against the three influenza virus strains significantly increased 7 days after immunization compared to placebo. HI antibody titers peaked at day 28, and peristed up to six months later at titers which were significantly higher than placebo for the H3N2 and B strains following ATIIV (Fig 2B and 2C). These higher titers at 6 months post-ATIIV administration paralleled seroprotection rates which were higher after ATIIV than TIIV (S1 Table).
Fig 2. B-cell and functional antibody responses after seasonal influenza vaccination.
(A) Absolute number of plasmablasts (CD19+CD20- CD38+) in 106 live PBMCs acquired. (B) HI Geometric mean titers (GMT) for A H1N1 and H3N2, and B influenza strains at baseline (D0), 7 days (D7), 28 days (D28), and 168 days (D168) after a single dose of influenza vaccine. Data show three cohorts: saline placebo (n=7), TIIV (n=18), and ATIIV (n=17). (C) Geometric mean ratio (GMR) for all vaccine strains. Non-parametric Wilcoxon’s signed rank test was used for statistical analyses: *p < 0.05, **p < 0.01, and ***p < 0.001 compared to day 0; §p < 0.05, §§p < 0.01 and §§§p < 0.001 compared to saline placebo.
Circulating TFH1 cells expressing ICOS expand after both TIIV and ATIIV influenza vaccination
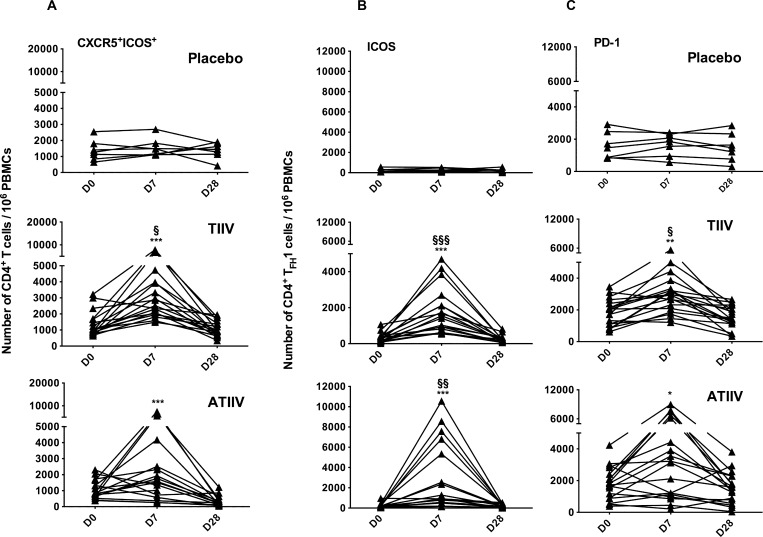
The number of CD4+ICOS+ TH cells expressing CXCR5 (CXCR5+ICOS+) increased at day 7 after vaccination with TIIV and ATIIV and returned to baseline levels by day 28 (Fig 3A). Vaccination did not affect the number of CXCR5+ICOS- nor of CXCR5-ICOS+ CD4+ TH cells (S2 Fig), in agreement with previous observations. [17]. We then determined the expression of ICOS in different TFH subsets in PBMC at day 0, 7, and 28 after administration of TIIV or ATIIV (S1B Fig for subset identification) [17]. The number of CD4+ TFH1 ICOS+ and PD-1+ cells peaked at day 7 after vaccination with TIIV and ATIIV compared to day 0, returned to baseline levels by day 28, and were also significantly higher compared to saline placebo controls (Fig 3B and 3C). Vaccination did not modify the frequencies of ICOS+ cells in the TFH17 and TFH2 cell subsets (S1B and S3 Figs). No changes in numbers of CD4+ TFH cells were observed in the placebo controls at any time point. Thus, these data show that peripheral blood TFH1 cells, expressing ICOS and PD-1, transiently expand at day 7 after TIIV, as shown previously [17], and also after ATIIV.
Fig 3. Expansion of ICOS+ and PD-1+ TFH1 cells after TIIV and ATIIV vaccination.
(A) Number of CD4+ T cells expressing CXCR5 and ICOS in human PBMCs after seasonal influenza vaccination. (B and C) Number of TFH1 cells expressing ICOS and PD-1. Data show three cohorts: saline placebo (n=7), TIIV (n=18) and ATIIV (n=17) at baseline (D0), day 7 (D7) and day 28 (D28) after a single dose of influenza vaccine. Data are shown for each participant and expressed as number of cells in 106 live PBMCs acquired. Non-parametric Wilcoxon’s signed rank test was used for statistical analyses: *p < 0.05, **p < 0.01, and ***p < 0.001 compared to day 0; §p < 0.05, §§p < 0.01 and §§§p < 0.001 compared to saline placebo.
Antigen-specific CD4+IL-21+ICOS+ TH cells expand at day 7 after vaccination and show a mixed CXCR5+/CXCR5- phenotype
We previously showed that antigen-specific blood CD4+IL-21+ICOS+ TH cells expand three weeks after influenza vaccination [17]. In the present study, we extended these observations by measuring the frequency of circulating H1N1-specific CD4+IL-21+ICOS+ TH cells as early as 7 days after vaccination. PBMCs were stimulated overnight with A/California/7/2009 (H1N1) subunit antigen, and IL-21-producing cells were identified by flow cytometry (S1C Fig for subsets identification).
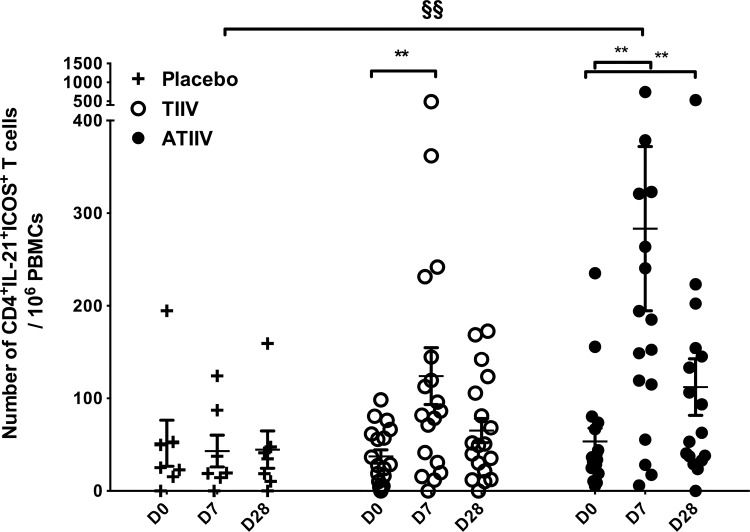
H1N1-specific CD4+IL-21+ICOS+ TH cells were already detectable on day 0 (mean values of 51 ± 25, 37 ± 7, and 53 ± 14 cells/106 CD4+ T cells in the placebo, TIIV, and ATIIV groups, respectively) suggesting that most of the participants had memory T cells due to prior vaccination or to natural exposure to circulating influenza viruses (Fig 4). Following vaccination with TIIV and ATIIV, the number of H1N1-specific CD4+IL-21+ICOS+ TH cells significantly increased at day 7 compared to day 0, and decreased by day 28 in both groups, although remaining significantly higher than baseline in the ATIIV group (Fig 4). Finally, the increased frequency of CD4+IL-21+ICOS+ TH cells at day 7 in response to ATIIV was significantly higher than in placebo controls, and persisted up to four weeks after vaccination.
Fig 4. H1N1-specific CD4+IL-21+ICOS+ TH cells expand 7 days after seasonal influenza vaccination.
Numbers of CD4+IL-21+ICOS+ TH cells in PBMCs stimulated overnight with A/California/7/2009 (H1N1) antigen. Data show three cohorts: saline placebo (n=7), TIIV (n=18) and ATIIV (n=17) at baseline (D0), day 7 (D7) and day 28 (D28) after a single dose of influenza vaccine. Data are shown for each subject and expressed as number of cells in 106 live CD4+ T cells acquired; mean ± SEM is shown. Non-parametric Wilcoxon’s signed rank test was used for statistical analyses: *p < 0.05, **p < 0.01, and ***p < 0.001 compared to day 0; §p < 0.05, §§p < 0.01 and §§§p < 0.001 compared to saline placebo.
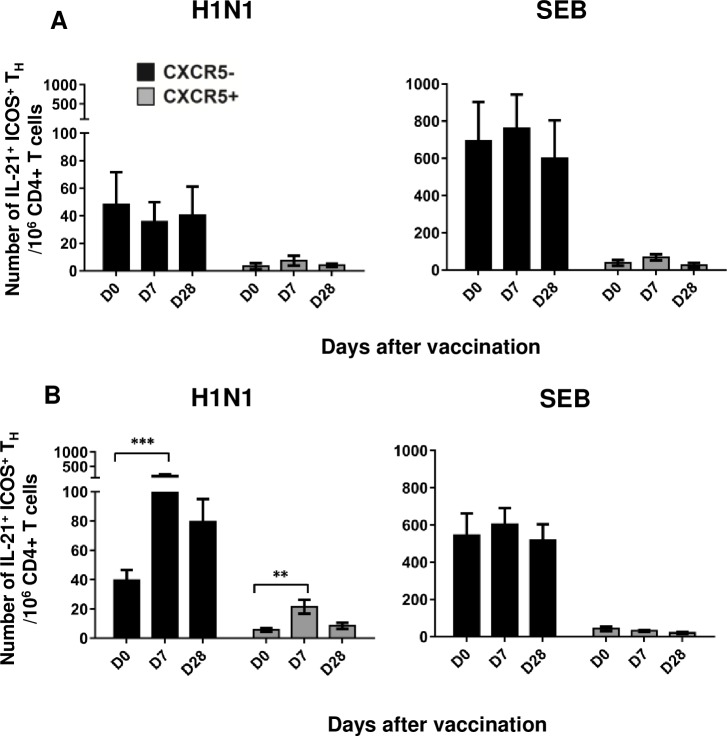
We further characterized H1N1-specific CD4+IL-21+ICOS+ TH cells at day 7 based on the level of CXCR5 expression. Due to the intrinsic variability of the single participants and due to the limited number of participants tested, we investigated this parameter by pooling together the results from the TIIV and ATIIV groups. At baseline, H1N1-specific CD4+IL-21+ICOS+ CXCR5- TH cells were more abundant than CXCR5+ TFH cells with 40 ± 7 cells/ 106 CD4+ T cells and 6 ± 1 cells/ 106 CD4+ T cells, respectively (Fig 5), in agreement with our previous results (18). Both subsets expanded at day 7, to 180 ± 44 CD4+IL-21+ICOS+CXCR5- TH cells/ 106 CD4+ T cells and 21 ± 5 CD4+IL-21+ICOS+CXCR5+ TFH cells/ 106 CD4+ T cells, respectively. At day 28, the frequency of H1N1-specific CD4+IL-21+ICOS+CXCR5+ TFH cells had returned to baseline levels, while CD4+IL-21+ICOS+CXCR5- TH cells were still high (80 ± 16 cells/ 106 CD4+ T cells). In the placebo group, the frequency of H1N1-specific CD4+IL-21+ICOS+ TH cells CXCR5- or CXCR5+ did not change over time, suggesting that the expansion of these subsets in TIIV and ATIIV groups was specifically driven by the vaccines.
Fig 5. H1N1-specific CD4+IL-21+ICOS+ TH cells subsets expressing or not CXCR5 expand after influenza vaccination.
Number of CD4+IL-21+ICOS+ TH cells, showing a CXCR5+ (black) or CXCR5- (gray) phenotype, in vaccinated participants after overnight stimulation with A/California/7/2009 (H1N1) antigen or SEB. Data show saline placebo (n=7), and merged TIIV (n=18) and ATIIV (n=17) cohorts at baseline (D0), day 7 (D7) and day 28 (D28) after a single dose of influenza vaccine. Data are expressed as number of cells in 106 live CD4+ T cells; mean ± SEM is shown. Non-parametric Wilcoxon’s signed rank test was used for statistical analyses: *p < 0.05, **p < 0.01, and ***p < 0.001 compared to day 0.
Frequencies of TFH cells at day 7 correlate with functional antibody responses after influenza vaccination
We further asked whether the expansion, at day 7, of total CD4+ TFH1 ICOS+ cells or H1N1-specific CD4+IL-21+ICOS+CXCR5+ TFH cells correlated with the rise of HI titers measured at day 28 and 168 post-immunization. In contrast to our previous study in which the vaccinees had no appreciable preexposure to avian A/Vietnam/1194/2004 (H5N1) influenza strain [18], most participants in this study had detectable levels of preexsisitng antibodies specific to one or more strains present in the 2012-2013 seasonal influenza vaccine. Baseline HI titers were inversely associated with HI titers measured at day 28 and 168 after vaccination and reached statistical significance for A/California/7/2009 (H1N1) and A/Victoria/361/2011 (H3N2) strains (S4 Fig). Therefore, in order to determine correlates of vaccine immunogenicity that were independent from vaccinees’ baseline preexisting immunity, we defined an alternative endpoint metric: decorrelated hemagglutination inhibition (DHI), which removed any linear correlation between the antibody expansion after vaccination and day 0 HI titers (S5 Fig). Total CD4+ TFH1 ICOS+ cells were correlated to the maximun HI response observed across the three strains represented in the vaccine, while H1N1-specific CD4+IL-21+ICOS+CXCR5+ TFH cells were correlated to H1N1-specific HI titers.
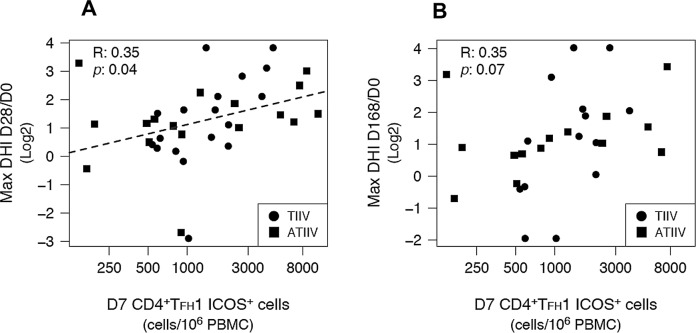
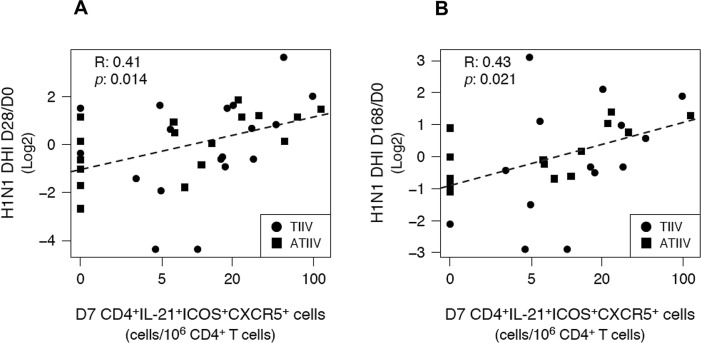
The number of CD4+ TFH1 ICOS+ cells measured at day 7 after immunization was weakly correlated with DHI responses at day 28 (R = 0.35; n = 35; p = 0.04), but not at day 168 (R = 0.35; n = 28; p = 0.07) after vaccination (Table 1 and Fig 6). In contrast, the number of H1N1-specific CD4+IL-21+ICOS+CXCR5+ TFH cells, measured at day 7, was significanly correlated with both day 28 (R = 0.41; n = 35; p = 0.01) and day 168 (R = 0.43; n = 28; p = 0.02) DHI responses (Table 1 and Fig 7). H1N1-specific CD4+IL-21+ICOS+ and CD4+IL-21+ICOS+CXCR5- TH cells were not significantly associated with DHI responses at any time point. Interestingly, plasmablasts did not show any significant association with DHI responses at any time point (Table 1), while the frequency of plasmablasts observed at day 7 post-vaccination was negatively correlated with day 0 HI titers (R = -0.46; n = 31; p = 0.01) (S6 Fig), suggesting that plasmablast responses to vaccination were also affected by the preexisting immunity status of the vaccinees.
Table 1. Correlation between cell subsets and DHI titers.
| aPredictor | Pearson R | |
|---|---|---|
| Day7 | DHI Day28/Day0 | DHI Day168/Day0 |
| Plasmablasts | b0.20 | b0.23 |
| CD4+TFH1 ICOS+ cells | b0.35* | b0.35 |
| H1N1-specific CD4+IL-21+ICOS+ TH cells | c0.23 | c0.30 |
| H1N1-specific CD4+IL-21+ICOS+CXCR5+ TFH cells | c0.41* | c0.43* |
| H1N1-specific CD4+IL-21+ICOS+CXCR5- TH cells | c0.20 | c0.25 |
Levels of correlation between cell frequencies, measured at day 7, and DHI responses measured 28 and 168 days after vaccination.
* p ≤ 0.05.
a All predictors are expressed as number of cells / 106 PBMCs
b Maximum DHI response observed across A/California/7/2009 (H1N1), A/Victoria/361/2011 (H3N2) and B/Wisconsin/1/2010-like vaccine strains.
c A/California/7/2009 (H1N1)-specific DHI responses
Fig 6. TFH1 ICOS+ cells predict functional antibody responses.
Correlations between the number of total circulating CD4+ TFH1 ICOS+ cells and the maximum DHI responses observed across the three influenza strains represented in the vaccine, measured at (A) day 28 and (B) day 168 after immunizzation. Dashed lines represent the least squares regressions fit to the data. R: Pearson product-moment correlation coefficient. p: correlation-associated p value.
Fig 7. H1N1-specific CD4+IL-21+ICOS+CXCR5+ TFH cells predict functional antibody responses.
Correlations between the number of H1N1-specific CD4+IL-21+ICOS+CXCR5+ TFH cells and H1N1-specific DHI responses measured at (A) day 28 and (B) day 168 after immunizzation. Dashed lines represent the least squares regressions fit to the data. R: Pearson product-moment correlation coefficient. p: correlation-associated p value.
In conclusion, our data suggest that the frequencies of both ex vivo TFH1 ICOS+ cells and H1N1-specific CD4+IL-21+ICOS+CXCR5+ TFH cells is associated with vaccine-induced functional antibody responses, independently of the vaccinees’ pre-immune status.
Discussion
In the present study, we have shown that various subpopulations of T cells sharing phenotypic and functional properties with TFH cells are present in the peripheral blood after vaccination with adjuvanted or non-adjuvanted influenza vaccines. The frequency of these blood T cell subpopulations peaks 7 days post-vaccination, then declines to baseline levels, although some (i.e. H1N1-specific CD4+IL-21+ICOS+CXCR5- TH cells) persist for at least four weeks. Frequencies of some TFH cell subsets, either total or antigen-specific, predicted HI titers, not only four weeks after vaccination, but also six months later.
TFH cells are acquiring an increasing interest because of their ability to provide help to B cells in the GC of secondary lymphoid organs, leading to B-cell differentiation, reshaping of the B-cell repertoire, and Ig affinity maturation [1–5]. The role of TFH cells in the generation of long-lived humoral responses to antigens gives them an ideal profile as potential early biomarkers of effective take of vaccines and possibly of long-lasting protective antibody responses. Peripheral TFH subsets in whole blood or fresh human PBMCs of healthy participants have been identified by ex vivo staining following vaccination against influenza, describing memory CD4+CXCR5+ TH cells as the most abundant population [13, 17, 31, 32]. In this study performed on frozen PBMCs, a peak expansion of CD4+CXCR5+ TFH1 cells expressing ICOS and PD-1 was detected 7 days after TIIV vaccination, while TFH2 and TFH17 cell subsets were not modulated, confirming previous observations by Bentebibel et al. [17]. Similar responses were also observed after ATIIV vaccination, suggesting that the presence of the adjuvant did not alter the profile of these TFH subsets.
We recently reported that H5N1-specific CD4+IL-21+ICOS+ TH cells expanded 21 days after vaccination, showed a CXCR5- phenotype, and positively correlated with the H5N1-specific antibody response after the second vaccine dose [18]. Here, we extended these observations by showing that the frequency of CD4+IL-21+ICOS+ TH cells, specific for the seasonal influenza A/California/7/2009 (H1N1) antigen, increased seven days after influenza vaccination and decreased by day 28. Both CXCR5- and CXCR5+ subsets expanded seven days after influenza vaccination, while at day 28, most CD4+IL-21+ICOS+ TH cells were CXCR5-, suggesting that antigen-specific memory TFH cells might be in a resting state in the periphery and transiently express CXCR5 homing receptor upon antigen encounter.
A number of independent studies have reported that preexisting antigen-specific antibodies affect responsiveness to influenza vaccination, with higher baseline levels associated with lower HI responses [33]. Therefore, we defined a new endpoint metric, DHI, that decorralated antibody expansion after vaccination from day 0 HI titers. This new endpoint enabled us to determine cellular correlates of vaccine immunogenicity that were independent from the vaccinees’ preexisting immunity to influenza vaccine strains. With this approach, we showed that frequencies of plasmablasts at day 7 did not correlate with later DHI responses, suggesting that previously described associations between plasmablasts and antibody responses [17] might not be based on a causal effect but rather linked to preexisting immunity and, as such, appear correlated when directly compared. In contrast, TFH1 ICOS+ cells were shown to have some predictive power for day 28 DHI responses, confirming previous observations [17], but not for day 168 DHI responses. In addition, we also showed that day 7 H1N1-specific CD4+IL-21+ICOS+CXCR5+ TFH cell responses were significantly correlated with both early and late H1N1-specific antibody responses, with higher cell frequencies being associated with higher DHI responses. These results highlight the complex interplay between different factors, both subject- and vaccine-related, occurring during an immunological response to vaccination, leading to the production and persistence of functional antibodies.
In the present study, we identified two discrete H1N1-specific TH populations, one being CD4+IL-21+ICOS+CXCR5+ and the other lacking the CXCR5 marker. Interestingly, CD4+IL-21+ICOS+CXCR5+ TFH cells expanded transiently after vaccination. These cells may represent a transitional state of a subset of cells belonging to the larger CD4+IL-21+ICOS+CXCR5- TH cell population that persisted at higher frequency in the blood for at least 4 weeks, maintained their antigen-specificity as demonstrated by the ability to produce IL-21 and, as shown previously, by their capacity to provide help to B cells. Nonetheless, the possibility that the antigen-specific CD4+IL-21+ICOS+CXCR5+ TFH cells identified in our study represent a small subset of TFH1 ICOS+ cells cannot be excluded. In any case, these findings provide evidence that both TFH1 ICOS+ and antigen-specific CD4+IL-21+ICOS+CXCR5+ TFH cell subsets are associated with functional antibody responses to influenza vaccination. Further experimentation is needed to test whether the early response of these TFH subsets can be used as biomarkers of later functional immune responses for vaccines other than influenza or for vaccines for which participants have no immunological memory.
Taken together, our data give new insights on TFH cell subset responses after influenza vaccination and on their potential involvement in the persistence of protective antibody levels. We envision that applying similar approaches in future clinical trials will provide new insights into the mechanisms underlying the immunological response to vaccination and advance our ability to prospectively evaluate vaccine responsiveness based on early cellular and molecular signatures. This will represent an important advance in the development of novel or improved vaccines.
Supporting Information
(A) Plasmablasts (B) Ex-vivo TFH subsets. (C) H1N1-specific CD4+IL-21+ICOS+ TH cells.
(ZIP)
Data show three cohorts: saline placebo (n=7), TIIV (n=18) and ATIIV (n=17) at day 0, day 7 and day 28 after a single dose of influenza vaccine. Data are shown for each participant and expressed as number of cells in 106 live PBMCs acquired. Non-parametric Wilcoxon’s signed rank test was used for statistical analyses. p > 0.05 compared to day 0 and to saline placebo.
(TIF)
TIIV and ATIIV vaccination did not change ICOS expression in blood TFH2 and TFH17 subsets. Data show three cohorts: saline placebo (n=7), TIIV (n=18) and ATIIV (n=17) at day 0, day 7 and day 28 after a single dose of influenza vaccine. Data are shown for each participant and expressed as number of cells in 106 live PBMCs acquired. Non-parametric Wilcoxon’s signed rank test was used for statistical analyses. p > 0.05 compared to day 0 and to saline placebo.
(TIF)
HI titers were determined for A/California/7/2009 (H1N1), A/Victoria/361/2011 (H3N2) and B/Wisconsin/1/2010-like vaccine strains.
(TIF)
HI titers were determined for A/California/7/2009 (H1N1), A/Victoria/361/2011 (H3N2) and B/Wisconsin/1/2010-like vaccine strains.
(TIF)
Baseline HI titers refer to the maximun value observed across A/California/7/2009 (H1N1), A/Victoria/361/2011 (H3N2) and B/Wisconsin/1/2010-like vaccine strains. Dashed lines represent the least squares regressions fit to the data. R: Pearson product-moment correlation coefficient. p: correlation associated p value.
(TIF)
(DOCX)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(DOCX)
Acknowledgments
We thank Sandra Nuti, Simona Tavarini, and Chiara Sammicheli from the Novartis Flow-Cy-TOF Core Facility, for technical assistance; Giulia Lapini and Simona Piccirella at VisMederi; Julia Boyle, Aldona Greenwood, Kat Pizzoferro and the Staff at the Surrey Clinical Research Centre, University of Surrey; and Claus Alauddin Andersen at GSK Vaccines for input on the statistical analyses.
DL and CB conducted the clinical trial; FSp, FC, EM designed the experiments; FSp, EB, LZ, FSc, RC, NC, DR, performed the experiments; FSp and EB analyzed the data; FSp, ES and DM performed statistical analyses; FSp, ES, SB, DL, GDG wrote the paper.
Data Availability
Data are available within the paper and its Supporting Information files.
Funding Statement
These studies were funded by the Innovative Medicines Initiative Joint Undertaking under grant agreement no. 115308, resources of which are composed of financial contribution from the European Union's Seventh Framework Programme (FP7/2007-2013) and EFPIA companies’ in kind contribution. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annual review of immunology. 2008;26:741–66. 10.1146/annurev.immunol.26.021607.090344 . [DOI] [PubMed] [Google Scholar]
- 2.Chan TD, Brink R. Affinity-based selection and the germinal center response. Immunological reviews. 2012;247(1):11–23. 10.1111/j.1600-065X.2012.01118.x . [DOI] [PubMed] [Google Scholar]
- 3.Crotty S. Follicular helper CD4 T cells (TFH). Annual review of immunology. 2011;29:621–63. Epub 2011/02/15. 10.1146/annurev-immunol-031210-101400 . [DOI] [PubMed] [Google Scholar]
- 4.Goodnow CC, Vinuesa CG, Randall KL, Mackay F, Brink R. Control systems and decision making for antibody production. Nature immunology. 2010;11(8):681–8. 10.1038/ni.1900 . [DOI] [PubMed] [Google Scholar]
- 5.Shulman Z, Gitlin AD, Targ S, Jankovic M, Pasqual G, Nussenzweig MC, et al. T follicular helper cell dynamics in germinal centers. Science. 2013;341(6146):673–7. 10.1126/science.1241680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. The Journal of experimental medicine. 2000;192(11):1545–52. Epub 2000/12/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. The Journal of experimental medicine. 2000;192(11):1553–62. Epub 2000/12/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, et al. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. The Journal of experimental medicine. 2010;207(2):353–63. Epub 2010/02/10. 10.1084/jem.20091738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi YS, Yang JA, Yusuf I, Johnston RJ, Greenbaum J, Peters B, et al. Bcl6 expressing follicular helper CD4 T cells are fate committed early and have the capacity to form memory. Journal of immunology. 2013;190(8):4014–26. 10.4049/jimmunol.1202963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X, Yan X, Zhong B, Nurieva RI, Wang A, Wang X, et al. Bcl6 expression specifies the T follicular helper cell program in vivo. The Journal of experimental medicine. 2012;209(10):1841–52, S1-24. 10.1084/jem.20120219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacLeod MK, David A, McKee AS, Crawford F, Kappler JW, Marrack P. Memory CD4 T cells that express CXCR5 provide accelerated help to B cells. Journal of immunology. 2011;186(5):2889–96. 10.4049/jimmunol.1002955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber JP, Fuhrmann F, Hutloff A. T-follicular helper cells survive as long-term memory cells. European journal of immunology. 2012;42(8):1981–8. 10.1002/eji.201242540 . [DOI] [PubMed] [Google Scholar]
- 13.Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34(1):108–21. Epub 2011/01/11. S1074-7613(10)00491-7 [pii] 10.1016/j.immuni.2010.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmitt N, Bentebibel SE, Ueno H. Phenotype and functions of memory Tfh cells in human blood. Trends Immunol. 2014;35(9):436–42. Epub 2014/07/08. 10.1016/j.it.2014.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsai LM, Yu D. Follicular helper T-cell memory: establishing new frontiers during antibody response. Immunology and cell biology. 2014;92(1):57–63. 10.1038/icb.2013.68 . [DOI] [PubMed] [Google Scholar]
- 16.Bentebibel SE, Jacquemin C, Schmitt N, Ueno H. Analysis of human blood memory T follicular helper subsets. Methods in molecular biology. 2015;1291:187–97. 10.1007/978-1-4939-2498-1_16 . [DOI] [PubMed] [Google Scholar]
- 17.Bentebibel SE, Lopez S, Obermoser G, Schmitt N, Mueller C, Harrod C, et al. Induction of ICOS+CXCR3+CXCR5+ TH Cells Correlates with Antibody Responses to Influenza Vaccination. Sci Transl Med. 2013;5(176):176ra32 Epub 2013/03/15. 10.1126/scitranslmed.3005191 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spensieri F, Borgogni E, Zedda L, Bardelli M, Buricchi F, Volpini G, et al. Human circulating influenza-CD4+ ICOS1+IL-21+ T cells expand after vaccination, exert helper function, and predict antibody responses. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(35):14330–5. Epub 2013/08/14. 10.1073/pnas.1311998110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bentebibel SE, Schmitt N, Banchereau J, Ueno H. Human tonsil B-cell lymphoma 6 (BCL6)-expressing CD4+ T-cell subset specialized for B-cell help outside germinal centers. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(33):E488–97. Epub 2011/08/03. 1100898108 [pii] 10.1073/pnas.1100898108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pallikkuth S, Parmigiani A, Silva SY, George VK, Fischl M, Pahwa R, et al. Impaired peripheral blood T-follicular helper cell function in HIV-infected nonresponders to the 2009 H1N1/09 vaccine. Blood. 2012;120(5):985–93. Epub 2012/06/14. 10.1182/blood-2011-12-396648 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pallikkuth S, Pilakka Kanthikeel S, Silva SY, Fischl M, Pahwa R, Pahwa S. Upregulation of IL-21 receptor on B cells and IL-21 secretion distinguishes novel 2009 H1N1 vaccine responders from nonresponders among HIV-infected persons on combination antiretroviral therapy. Journal of immunology. 2011;186(11):6173–81. 10.4049/jimmunol.1100264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis DJ, Lythgoe MP. Application of "Systems Vaccinology" to Evaluate Inflammation and Reactogenicity of Adjuvanted Preventative Vaccines. J Immunol Res. 2015;2015:909406 10.1155/2015/909406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ansaldi F, Zancolli M, Durando P, Montomoli E, Sticchi L, Del Giudice G, et al. Antibody response against heterogeneous circulating influenza virus strains elicited by MF59- and non-adjuvanted vaccines during seasons with good or partial matching between vaccine strain and clinical isolates. Vaccine. 2010;28(25):4123–9. Epub 2010/05/04. 10.1016/j.vaccine.2010.04.030 . [DOI] [PubMed] [Google Scholar]
- 24.Nicholson KG, Colegate AE, Podda A, Stephenson I, Wood J, Ypma E, et al. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet. 2001;357(9272):1937–43. Epub 2001/06/27. S0140-6736(00)05066-2 [pii] 10.1016/S0140-6736(00)05066-2 . [DOI] [PubMed] [Google Scholar]
- 25.Galli G, Medini D, Borgogni E, Zedda L, Bardelli M, Malzone C, et al. Adjuvanted H5N1 vaccine induces early CD4+ T cell response that predicts long-term persistence of protective antibody levels. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(10):3877–82. Epub 2009/02/25. 0813390106 [pii] 10.1073/pnas.0813390106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bucasas KL, Franco LM, Shaw CA, Bray MS, Wells JM, Nino D, et al. Early patterns of gene expression correlate with the humoral immune response to influenza vaccination in humans. J Infect Dis. 2011;203(7):921–9. 10.1093/infdis/jiq156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bates D, Maechler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using {lme4}. Journal of Statistical Software. 2015;67(1):1–48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- 28.Clark TW, Pareek M, Hoschler K, Dillon H, Nicholson KG, Groth N, et al. Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. N Engl J Med. 2009;361(25):2424–35. Epub 2009/09/12. 10.1056/NEJMoa0907650 . [DOI] [PubMed] [Google Scholar]
- 29.Faenzi E, Zedda L, Bardelli M, Spensieri F, Borgogni E, Volpini G, et al. One dose of an MF59-adjuvanted pandemic A/H1N1 vaccine recruits pre-existing immune memory and induces the rapid rise of neutralizing antibodies. Vaccine. 2012;30(27):4086–94. 10.1016/j.vaccine.2012.04.020 [DOI] [PubMed] [Google Scholar]
- 30.Greenberg ME, Lai MH, Hartel GF, Wichems CH, Gittleson C, Bennet J, et al. Response to a monovalent 2009 influenza A (H1N1) vaccine. N Engl J Med. 2009;361(25):2405–13. Epub 2009/09/12. 10.1056/NEJMoa0907413 . [DOI] [PubMed] [Google Scholar]
- 31.Locci M, Havenar-Daughton C, Landais E, Wu J, Kroenke MA, Arlehamn CL, et al. Human circulating PD-1+CXCR3-CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity. 2013;39(4):758–69. 10.1016/j.immuni.2013.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ueno H, Banchereau J, Vinuesa CG. Pathophysiology of T follicular helper cells in humans and mice. Nature immunology. 2015;16(2):142–52. 10.1038/ni.3054. 10.1038/ni.3054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsang JS, Schwartzberg PL, Kotliarov Y, Biancotto A, Xie Z, Germain RN, et al. Global analyses of human immune variation reveal baseline predictors of postvaccination responses. Cell. 2014;157(2):499–513. 10.1016/j.cell.2014.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Plasmablasts (B) Ex-vivo TFH subsets. (C) H1N1-specific CD4+IL-21+ICOS+ TH cells.
(ZIP)
Data show three cohorts: saline placebo (n=7), TIIV (n=18) and ATIIV (n=17) at day 0, day 7 and day 28 after a single dose of influenza vaccine. Data are shown for each participant and expressed as number of cells in 106 live PBMCs acquired. Non-parametric Wilcoxon’s signed rank test was used for statistical analyses. p > 0.05 compared to day 0 and to saline placebo.
(TIF)
TIIV and ATIIV vaccination did not change ICOS expression in blood TFH2 and TFH17 subsets. Data show three cohorts: saline placebo (n=7), TIIV (n=18) and ATIIV (n=17) at day 0, day 7 and day 28 after a single dose of influenza vaccine. Data are shown for each participant and expressed as number of cells in 106 live PBMCs acquired. Non-parametric Wilcoxon’s signed rank test was used for statistical analyses. p > 0.05 compared to day 0 and to saline placebo.
(TIF)
HI titers were determined for A/California/7/2009 (H1N1), A/Victoria/361/2011 (H3N2) and B/Wisconsin/1/2010-like vaccine strains.
(TIF)
HI titers were determined for A/California/7/2009 (H1N1), A/Victoria/361/2011 (H3N2) and B/Wisconsin/1/2010-like vaccine strains.
(TIF)
Baseline HI titers refer to the maximun value observed across A/California/7/2009 (H1N1), A/Victoria/361/2011 (H3N2) and B/Wisconsin/1/2010-like vaccine strains. Dashed lines represent the least squares regressions fit to the data. R: Pearson product-moment correlation coefficient. p: correlation associated p value.
(TIF)
(DOCX)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(DOCX)
Data Availability Statement
Data are available within the paper and its Supporting Information files.