Abstract
Natural products have served humankind as drug leads for thousands of years. In the last century natural products have not only served as drugs but have inspired the generation of countless synthetic drugs and drug-leads around natural product pharmacophores. There are no disease targets for which natural products have played a more significant role than in the case of malaria and other parasitic diseases. In this review the significance of the manzamine class of marine alkaloids is presented as an example of the future utility of the oceans in the development of antiparasitics. The manzamines represent one of the few new structural classes identified in recent decades with potential for the control of malaria and tuberculosis. While considerable work remains to successfully optimize this class of drug-leads the novel pharmacophore and significant metabolic stability combined with a rapid onset of action and long half-life all strongly support further investigations of this group of potential drug candidates.
Keywords: Manzamine, Malaria, Marine Natural Products, Infectious Diseases
1. INTRODUCTION
Recent decades have revealed an array of unique and bioactive structural classes from the world’s Oceans. A number of recent reviews provide a general discussion of the promise of marine natural products against an array of disease targets [1–3] as well as specific infectious diseases such as for antiparasitic drugs, [4] antinematodal drugs, [5] antituberculosis agents, [6] and antifungal agents [7]. In addition there are a number of extensive reviews focusing on marine natural products such as “The influence of natural products upon drug discovery” by Newman, Cragg and Snader, [8] “Marine natural products” by Faulkner [9] and “Indole alkaloid marine natural products: An established source of cancer drug leads with considerable promise for the control of parasitic, neurological and other diseases” by Gul and Hamann [10]. Therefore, the primary focus of this review is regarding the use of the manzamine class of marine natural products for the control of malaria. Manzamines are a specific example of how a unique group of highly active marine natural products can be optimized to yield promising candidates for the control of parasitic diseases with a clearly new but still undefined mechanism of action.
From a historical point of view there are few examples in which natural products have played a more vital role in therapeutic intervention than in the treatment of malaria. This is due in part to the significant level of effort utilizing traditional medicines for a disease target which also frequently occurs in regions rich in diverse terrestrial natural products. Malaria also represents one of the oldest examples involving the use of a natural product as chemotherapy. The use of quinine for the control of malaria dates back greater than 350 years [11, 12]. One of the greatest historical challenges for the control of malaria which still remains an obstacle is providing inexpensive drugs to individuals who are in need of treatment. Past demands for quinine have resulted in shortages of supply and promoted the eventual development of synthetic aminoquinolines in the early 19th century. These drugs represented significant advances in less expensive and readily available treatments and prophylactics for the control of malaria. Unfortunately, in recent decades most of the malaria endemic regions are now faced with chloroquine and multidrug resistant forms of the parasite. As a result there remains a significant need for novel antimalarial chemotypes to help overcome the challenge of resistance while providing cost-effective alternatives to current drugs. The least explored of the planet’s naturally occurring drug leads are the chemically defended organisms of the oceans and particularly fruitful have been the invertebrates, algae and microbial communities. Typically overlooked and the focus of this short review is the value that small molecules from the ocean offer in regard to unique opportunities for lead optimization and pharmaceutical product development. In addition there is a significant and unexplored potential for the creative application of semisynthetic and biocatalytic transformations to the generation of countless analogs from marine natural product leads for evaluation in malaria and other neglected disease lead discovery and development programs.
The marine animal species available for evaluation as antimalarial drug leads number in excess of several million and the combination of an animal rather than plant dominated environment with the availability of elements such as bromine has led to the biosynthesis of classes of metabolites unique to marine environments [13]. In addition the oceans encompass 70 percent of the earth’s surface and 95 percent of its tropical biosphere creating a tremendous under utilized resource for the characterization of novel antimalarial structural classes. A particularly important aspect of the marine environment in regard to sourcing novel structural classes is the diversity at higher taxonomic levels, which generally yields greater diversity in chemotypes. This biological diversity has already resulted in a vast array of small molecule diversity and an incorporation of elements not readily available to terrestrial species. Combing the most accessible levels of the oceans during the last thirty years has provided an unparalleled succession of unique new compounds exhibiting pharmacologically useful activities in which malaria has only become a significant focus in recent years [14]. A small group of marine researchers has isolated over 12,000 compounds from marine invertebrates (phyla: Annelida, Arthropoda, Brachiopoda, Bryozoa, Chordata, Cnidaria, Echinodermata, Hemichordata, Mollusca, Nematoda, Platyhelminthes, Porifera), from algae, (phyla: Chlorophycota, Chromophycota, Chromophyta, Cyanophycota, Euglenophycota, Rhodophycota) and from microorganisms (bacteria, fungi and protozoa) [15]. It has been estimated that less than 0.5% of the marine animals have received even a cursory effort to discover agents against malaria and other neglected diseases.
2. MALARIA DRUG RESISTANCE AND METHODS FOR REDUCING COST OF TREATMENT
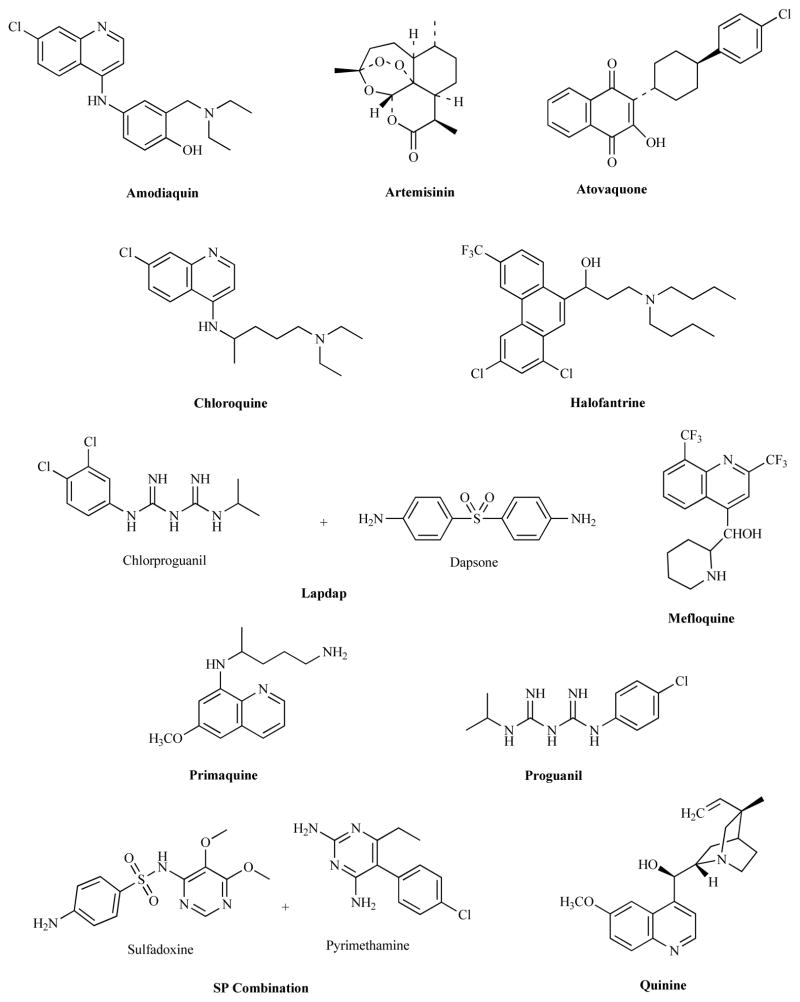
The failure rate for current antimalarial drugs now exceeds 25% in many countries [16]. Drug resistance for one of the drugs in a combination treatment compromises the effectiveness of a new drug like artemisinin and as a result new effective classes of antimalarial agents are desperately needed. The optimum scenario for the prevention of drug resistance in malaria is the use of two or more drugs in combination for which no resistance currently exist for either drug. Unfortunately due to the added cost of combination therapies policymakers often elect to recommend single drug regimens despite the certainty for the early development of resistance. There are now many artemisinin-based combination treatments capable of 90% cure rates which include a fixed dosage of mefloquine, piperaquine, lumefantrine, pyronaridine or chlorproguanil-dapsone (Fig. 1). Unfortunately, despite the added cure rates new combination therapies increase costs. The price for a treatment course of chloroquine is 0.15US$ and 0.25 for sulfadoxine-pyrimethamine. A treatment course with artesunate is around 1.0US$ and combination therapies with artesunate are in the range of 1.2–1.8US$. As a result the cost of goods for producing a marine derived natural product has been a tremendous liability in the practical application of marine natural products to diseases like malaria.
Fig. (1).
Currently available antimalarial drugs and new drug combinations.
The most compelling reason for the development of antimalarial combination therapies is improved efficacy and efficiency. Combination therapies are effective in reducing duration of therapy and increasing compliance but certainly most important is the reduction in the rate of developing resistance. Drugs with unrelated targets and in combination can decrease the rate of resistance to a point at which a resistant parasite may only occur once in millions of individuals. This is based on the principle that if the frequency of resistance to drug A is 108 and drug B is 1012 that multidrug resistance will only occur in one in 1020 parasites. Since the maximum concentration per individual is 108 parasites a multidrug resistant parasite should only occur once in many millions of infected individuals [1]. Although deviation from this model is certain to occur, it is clear from the treatment of HIV, Mycobacterium tuberculosis and cancer that multidrug combination therapies with unique targets are much more effective in disease control while reducing the rate and likelihood of resistance. As a result there is a significant unmet need for novel drug leads with new mechanisms of action to control malaria.
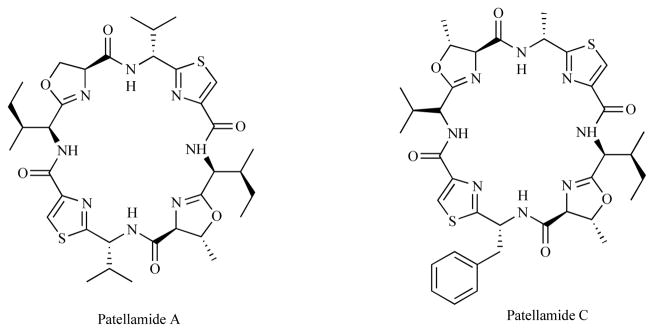
While the oceans are now a well established resource for novel drug leads their application to malaria has been hampered by methods for cost-effective production [17]. Recent efforts by the Schmidt and Nelson group [18] involving the successful heterologous expression of the biosynthetic pathways of marine invertebrate products in Escherichia coli indicates that the production of these drug leads through fermentation will eventually be practical. In this example the cyclic peptides know as the patellamides (Fig. 2) were successfully expressed from the biosynthetic gene cluster isolated from an obligate cyanobacterial symbiont.
Fig. (2).
The chemical structures of Patellamide A and C.
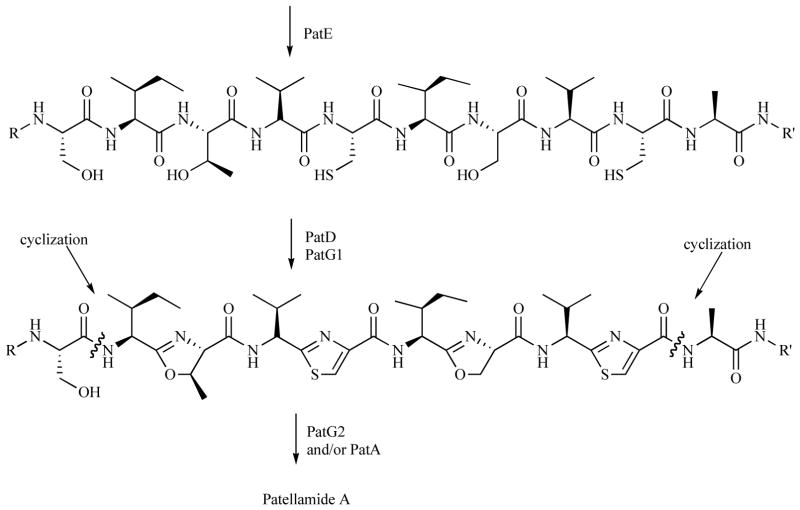
Although the reported yields of patellamide produced through heterologous expression were quite small, (20 μg/L) the successful expression of a complicated marine natural product in E. coli represents a remarkable achievement and promising step toward the discovery and development of novel marine natural products as treatments for malarial and other neglected diseases. The patellamide (pat) pathway was successfully amplified from DNA using a producing reef strain while no products were amplified from a closely related but non-producing strain. The point of epimerization of amino acids from L to D still remains unclear. The proposed biogenesis for the thiazole and oxazolines are presented in Scheme 1 along with the points of cyclization.
Scheme 1.
Proposed biosynthetic pathway to the Patellamides [18].
3. SEMISYNTHETIC LIBRARIES USING NATURAL PRODUCT TEMPLATES
The combined application of combinatorial chemistry and high-throughput screening has yielded significant opportunities to utilize novel marine natural products as starting materials for lead optimization. In addition it has been suggested that combinatorial libraries may be only as effective as the scaffold or template that is utilized to prepare them, hence the added value for novel marine natural products [19]. While natural products clearly remain unrivalled in their ability to produce sophisticated molecular architecture with outstanding biological activity, their utility as drug leads is significantly improved through synthetic modifications. Integrating the rational selection of natural products templates with combinatorial chemistry is clearly the next logical step to expand the diversity space occupied by natural products. In addition the selection of a natural product scaffold with preexisting biological activity allows for the rational selection of high-throughput assays for the evaluation of new analogs. There are numerous interesting and highly successful examples of SAR studies that begin with the total synthesis of a template (i.e. the diterpene in sarcodictyins), which is then followed by functional modifications to the core structure which could also be produced through biosynthesis [20]. In other cases remarkable numbers of compounds reminiscent of natural products have been created through a carefully designed template. Schreiber’s group [21] has reported well over 2 million compounds prepared from a single scaffold providing just three reactive sites. This approach can clearly be applied to readily available natural product scaffolds provided in abundance from marine invertebrates, algae and bacteria. Bioactive leads available in >50mg quantities are extremely common and provide the opportunity for the preparation of numerous analogs utilizing the bioactive marine natural products as startingproducts as starting materials. By applying parallel synthesis to a marine derived natural product we have shown that it is possible to generate libraries of compounds and provide preliminary SAR quickly after the identification of an initial lead [22]. In addition a focus on natural product structures that are exceptionally sophisticated but yet readily available eliminates the concerns regarding shortages in supply during preclinical and clinical development.
Our selection of marine natural product scaffolds or templates is based on several basic criteria including the drug-like properties of the class, the functional group characteristics for the compound, its bioactivity and the capacity of the source organism to provide a sustainable supply of the starting material for semisynthesis with negligible impact on the environment. In the example of the manzamine class the starting materials can be isolated in kg or greater quantities and this molecule was selected due to its activity against malaria and other infectious diseases [23].
Statistically it has been shown that when it comes to diversity, natural products remain an unrivaled source of new lead molecules and novel pharmacophores [24]. Unfortunately natural products do not yield certain key functional groups (arene, amine, amide) as frequently as they are found in drug products [9]. For example natural products have been shown to produce hydroxyl functionality >65% of the time, while this functional group is only found in 40% of successful drugs.
Rifampicin (Fig. 3) is one of many examples resulting from a hybrid fermentation-synthetic approach to the generation of drugs or drug leads. Rifampicin is a mycobactericidal (MIC 0.25 μg/mL) semisynthetic derivative of rifamycin which inhibits DNA-dependent RNA polymerase in prokaryotic but not in eukaryotic cells. Rifampicin successfully enters phagocytic cells and can kill intracellular, intracavitary or even dormant Tuberculosis bacilli [25]. This drug is noteworthy since it exemplifies the value that semisynthetics from a bacteria scaffold can have on the development of clinically relevant drugs.
Fig. (3).
Chemical structure of Rifampicin.
4. BIOTRANSFORMATIONS AND BIOCATALYSIS [26]
In addition to semisynthesis, biotransformation studies provide access to metabolites for SAR and lead optimization. Metabolism studies are also an essential part of the process leading to the approval of any clinically useful drug. Historically microorganisms have been utilized as in vitro models for the prediction of mammalian drug metabolism due to the significant similarity of microbial enzyme systems, specifically fungi, with mammalian liver enzyme systems [27]. An additional and significant application of biocatalysis is in the area of lead generation and optimization of second-generation analogs with greater activity and less toxicity than the parent marine natural product. Biocatalysis has a distinct advantage in that it provides access to a broad spectrum of enzymatic activity and specificity [28]. The advantages of applying enzymes to structural modifications lie in their high selectivity and their ability to work under mild reaction conditions. These characteristics make them particularly suitable for the modification of natural products, which may easily rearrange or decompose with the application of inorganic catalysis [29]. The details of the microbial metabolism studies utilized in the study of the manzamines as well as the microorganisms (>30) utilized in screening have been previously reported [30–32].
5. MANZAMINE ALKALOIDS
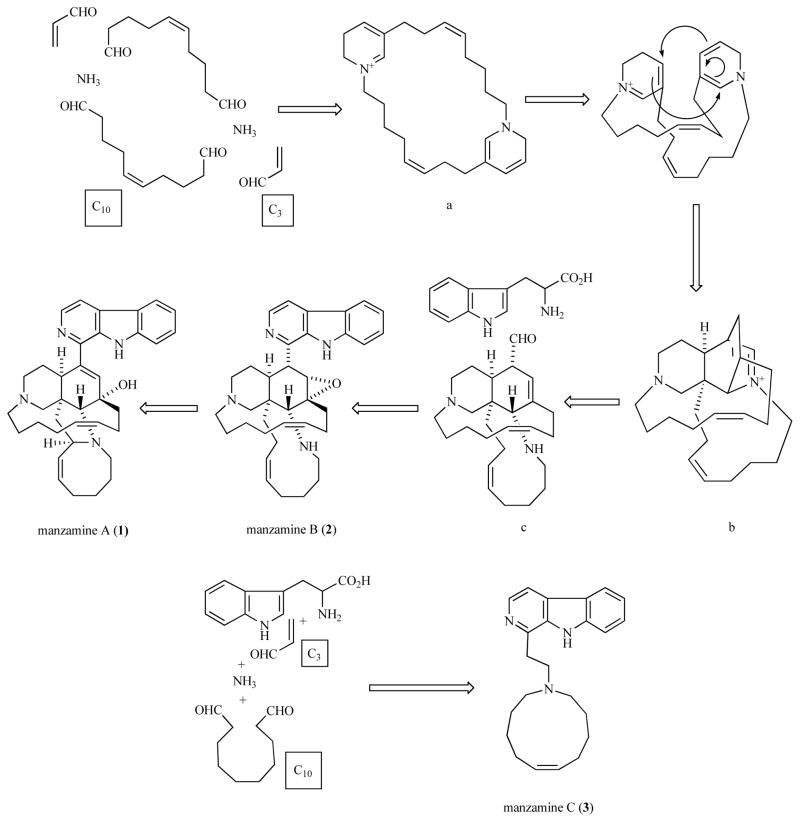
The manzamines are a unique group of polycyclic marine-derived alkaloids first reported in 1986 from the Okinawan sponge genus Haliclona [33]. These compounds possess a fused and bridged tetra- or pentacyclic ring system, which is attached to a β-carboline moiety through an apparent Pictet-Spengler reaction with the aldehyde known as ircinal A shown in the proposed biogenetic pathway in Scheme 2 [34]. Since the first report of manzamine A (Fig. 4), an additional forty manzamine-type alkaloids have been identified from nine different sponge genera (four orders) [35, 36]. The occurrence of the manzamine alkaloids from a diverse group of unrelated sponges has generated speculation that these metabolites maybe of microbial origin. A microbial source for these compounds would clearly facilitate their development as drug leads. The most impressive characteristic of this unusual group of alkaloids is their potent activity against several infectious diseases. The need to discover new lead compounds with improved activity against infectious diseases and less toxicity has become increasingly urgent. Malaria accounts for over a million deaths each year worldwide with 273 million cases reported in 1998 [37]. The most dangerous malaria parasite, Plasmodium falciparum which causes cerebral malaria, is expected to spread in the central or northern regions of Europe and North America within a few decades.
Scheme 2.
Biogenetic path of manzamines A, B, and C proposed by Baldwin and Whitehead [34].
Fig. (4).
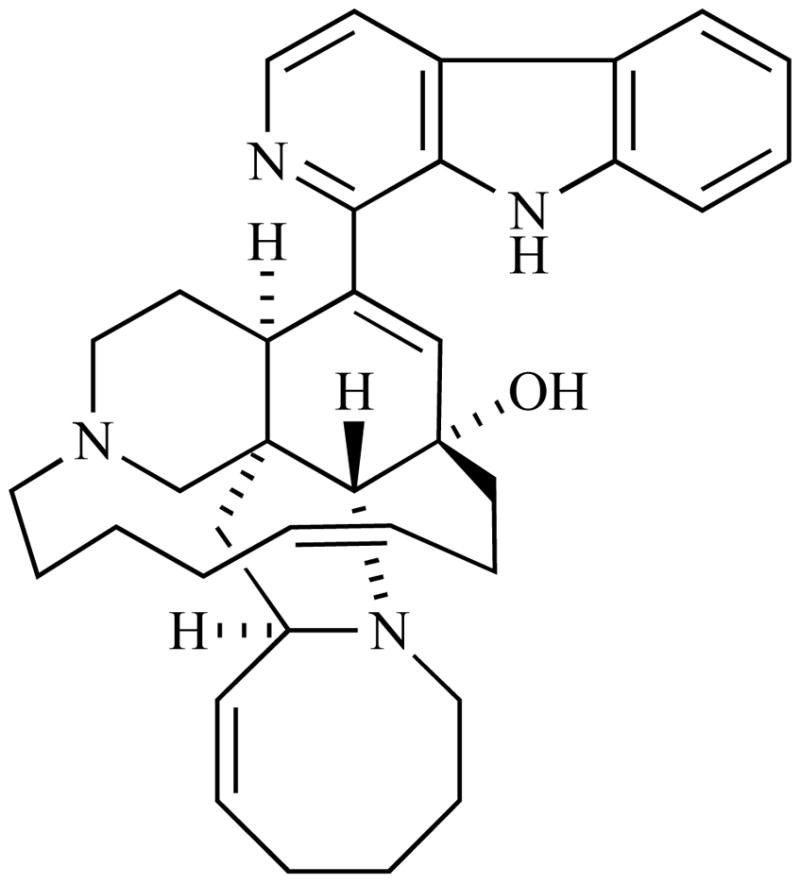
Chemical structure of Manzamine A.
Several other important opportunistic infections such as those caused by Mycobacterium tuberculosis and Toxoplasma gondii were also shown to be inhibited by the manzamine alkaloids [38]. The pathogenic synergy of these infectious diseases with HIV has increased significantly [39]. For example, the incidence of tuberculosis infection in HIV positive cases is 50 fold over HIV negative ones and the same holds true in the case of T. gondii [40]. One-third of the global human population conceals latent tuberculosis, which generates more than three million mortality cases annually. The increasing incidence of tuberculosis and toxoplasmosis is also confounded by the emergence of drug-resistance and multidrug resistance to M. tuberculosis as well as the lack of any current chemotherapy that targets the latent form of T. gondii. Therefore, it is quite important to continue to support the evaluation of manzamine alkaloids as a novel class of antiinfectives for these diseases [38]. An array of manzamine related molecules along with the unprecedented manzamine dimer, neo-kauluamine, have been identified from an extremely common genus of Indo-Pacific sponge called Acanthostrongylophora which has provided a very high-yielding source of this class of compounds for biological evaluation [41, 42].
In vitro analysis of several manzamine analogs against T. gondii indicated significant activity. Manzamine A displayed 70% inhibition of the parasite at 0.1 μM concentrations without host cell toxicity. The activity is significantly increased at concentrations of 1 and 10 μM even though it was accompanied by an increase in host-cell toxicity. As a result manzamine A was selected for in vivo analysis since it was the most active and least toxic in vitro. A daily intraperitoneal (i.p.) dose of 8 mg/kg of manzamine A, for 8 consecutive days, beginning on day 1 following the infection, prolonged the survival of SW mice to 20 days, as compared with 16 days for the untreated control. Additional manzamine isolations, SAR and optimized dosing studies will be of significant value to improve the in vivo efficacy of the manzamines against T. gondii. All new and known manzamines, with the exception of neo-kauluamine, induced 98–99% inhibition of Mycobacterium tuberculosis (H37Rv) with an MIC <12.5 μg/mL. Manzamine A, E and 8-hydroxymanzamine A exhibit MIC endpoints of 1.56, 3.13 and 3.13 μg/mL, respectively.
Manzamine A and 8-hydroxymanzamine A were assayed in vivo against Plasmodium berghei with a single i.p. dose of 100 μmoles/kg and exhibited no apparent toxicity. 8-Hydroxymanzamine A efficiently reduced parasitemia with an increase in the average survival days of P. berghei-infected mice (9–12 days), as compared with: untreated controls (2–3 days), mice treated with artemisinin (2 days) and chloroquine (6 days). Three 50 μmoles/kg i.p. doses of manzamine A were found to be curative and totally cleared the parasite and two oral doses (100 μmoles/kg) provided a notable reduction of parasitemia. The pharmacokinetic properties of manzamine A and possibly the rest of the manzamines with its rapid onset of action (2 hours) followed by continuous sustainable bioavailability provides effective parasite eradication in rodents [43]. These data indicate that manzamines are more active than the currently available antimalarial drugs artemisinin and chloroquine. A continuing challenge of the manzamine class is cytotoxicity. The manzamine dimer neo-kauluamine [44] is cytotoxic with an IC50 of 1.0 μg/mL, against human lung and colon carcinoma cells as compared with the first manzamine dimer kauluamine, which also showed significant cytotoxicity [45].
The initial evaluation of the manzamine alkaloids in murine models proved that these antimalarial leads are more effective than the currently used drugs, artemisinin and chloroquine. The in vivo improvement in potency despite similar in vitro activity is certain to be due in part to the longer half-life [46]. The manzamines are clearly valuable candidates for further investigation and development as promising leads against malaria and perhaps other serious infectious diseases. The need for developing antimalarials from novel structural classes and with unique mechanisms of action is extremely important for the long term and sustainable control of drug resistant malaria. These compounds are currently under investigation in our laboratory as scaffolds for the generation of optimized leads by semisynthesis and biocatalysis.
6. CONCLUSIONS
The oceans clearly hold a tremendous potential for both the discovery of new leads as well as the creation of novel or optimized chemotypes with a variety of potential therapeutic applications. The fact that natural products are responsible for the genesis of 60% of our cancer drugs and 75% of our infectious disease treatments attests to the significant role natural products have played historically [47]. Within the vast resource of marine flora and fauna are novel and bioactive structural classes to stem the tide of drug resistant parasites as well as controlling chronic illnesses including cancer, cardiovascular, neurological and metabolic diseases. The rational and efficient application of this biological and chemical reservoir depends on the technology to collect, rapidly recognize, characterize and modify these secondary metabolites. The recent advances in life support systems combined with analytical and synthetic instrumentation have made it possible to utilize these resources for the creation of tremendous numbers of unusual and bioactive molecules. The manzamine class stands out as a unique group of metabolites with significant potential for the control of parasitic diseases. Although considerable effort remains to provide an optimized and safe drug from the manzamine class, their novel mechanism of action, chemical and metabolic stability as well as their significant activity in animals makes them an outstanding lead class for the control of malaria.
Acknowledgments
I would likely to gratefully acknowledge the dedicated students, postdoctoral fellows and numerous collaborators for their contributions to the information presented in this review of our research related to the manzamine alkaloids and their activity for the control of infectious diseases. Jennifer Allman is gratefully acknowledged for assistance with the preparation of this manuscript. Financial support for this work was provided by the National Institutes of Health (NI-AID), The World Health Organization, The Medicines for Malaria Venture and NOAA including The Mississippi-Alabama Sea Grant Consortium.
ABBREVIATIONS
- i.p
Intraperitoneal
- SAR
Structure-activity relationships
References
References 48–50 are related articles recently published in Current Pharmaceutical Design.
- 1.Mayer AMS, Hamann MT. Marine pharmacology in 1999: compounds with antibacterial, anticoagulant, antifungal, antihelmintic, antiinflammatory, antiplatelet, antiprotozoal and antiviral activities; affecting the cardiovascular, endocrine, immune and nervous systems and other miscellaneous mechanisms of action. Comp Biochem Physiol C Toxicol Pharmacol. 2002;132C:315–39. doi: 10.1016/s1532-0456(02)00094-7. [DOI] [PubMed] [Google Scholar]
- 2.Mayer AMS, Hamann MT. Marine pharmacology in 2000: compounds with antibacterial, anticoagulant, antifungal, antihelmintic, antiinflammatory, antiplatelet, antiprotozoal and antiviral activities; affecting the cardiovascular, endocrine, immune and nervous systems and other miscellaneous mechanisms of action. Mar Biotechnol. 2004;6:37–52. doi: 10.1007/s10126-003-0007-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayer AMS, Hamann MT. Marine pharmacology in 2001–2002: compounds with antihelmintic, antibacterial, anticoagulant, antidiabetic, antifungal, antiinflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis and antiviral activities; affecting the cardiovascular, endocrine, immune and nervous systems and other miscellaneous mechanisms of action. Comp Biochem Physiol C Toxicol Pharmacol. 2005;140:265–86. doi: 10.1016/j.cca.2005.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kayser O, Kiderlen AF, Croft SL. Natural products as potential antiparasitic drugs. In: Atta-ur-Rahman, editor. Studies in Natural Products Chemistry (Bioactive Natural Products, Part G) Vol. 26. Elsevier Science; Amsterdam, The Netherlands: 2002. pp. 779–48. [Google Scholar]
- 5.Ghisalberti EL. Secondary metabolites with antinematodal activity. In: Atta-ur-Rahman, editor. Studies in Natural Products Chemistry (Bioactive Natural Products, Part G) Vol. 26. Elsevier Science; Amsterdam, The Netherlands: 2002. pp. 425–506. [Google Scholar]
- 6.El Sayed KA, Bartyzel P, Shen X, Perry TL, Zjawiony JK, Hamann MT. Marine natural products as antituberculosis agents. Tetrahedron. 2000;56:949–53. [Google Scholar]
- 7.Hong-yu L, Matsunaga S, Fusetani N. Antifungal metabolites from marine sponges. Curr Org Chem. 1998;2:649–82. [Google Scholar]
- 8.Newman DJ, Cragg GM, Snader KM. The influence of natural products upon drug discovery. Nat Prod Rep. 2000;17:215–34. doi: 10.1039/a902202c. [DOI] [PubMed] [Google Scholar]
- 9.Faulkner DJ. Marine natural products. Nat Prod Rep. 2002;19:1–48. doi: 10.1039/b009029h. [DOI] [PubMed] [Google Scholar]
- 10.Gul W, Hamann MT. Indole alkaloid marine natural products: an established source of cancer drug leads with considerable promise for the control of parasitic, neurological and other diseases. Life Sci. 2005;78:442–53. doi: 10.1016/j.lfs.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kremsner PG, Krishna S. Antimalarial combinations. Lancet. 2004;364:285–94. doi: 10.1016/S0140-6736(04)16680-4. [DOI] [PubMed] [Google Scholar]
- 12.Honigsbaum M. The fever trail: in search for the cure for malaria. Farrar, Straus and Giroux. 2001:1–307. [Google Scholar]
- 13.Bradner WT. In: Cancer and chemotherapy. Crooke ST, Prestayko AW, editors. Vol. 1. New York: Academic Press; 1980. p. 313. [Google Scholar]
- 14.Donia M, Hamann MT. Marine natural products and their potential applications as antiinfective agents. Lancet Infect Dis. 2003;3:338–48. doi: 10.1016/S1473-3099(03)00655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blunt JW, Munro MHG. Marinlit: a database of the literature on marine natural products. University of Canterbury; 2005. [Google Scholar]
- 16.Adjuik M, Agnamey P, Babiker A, Baptista J, Borrmann S, Brasseur P, et al. Artesunate combinations for treatment of malaria: meta-analysis. Lancet. 2004;363:9–17. doi: 10.1016/s0140-6736(03)15162-8. [DOI] [PubMed] [Google Scholar]
- 17.Enticknap JJ, Thompson R, Peraud O, Lohr JE, Hamann MT, Hill RT. Molecular analysis of a Florida Keys sponge: implications for natural products discovery. Mar Biotechnol. 2004;6:288–93. [Google Scholar]
- 18.Schmidt EW, Nelson JT, Rasko DA, Sudek S, Eisen JA, Haygood MG, et al. Patellamide A and C biosynthesis by a microcin-like pathway in Prochloron didemnin, the cyanobacterial symbiont of Lissoclinum patella. Proc Natl Acad Sci USA. 2005;102(20):7315–20. doi: 10.1073/pnas.0501424102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.For medicinal purposes Petsko GA. Nature. 1996;384(6604):7–10. doi: 10.1038/384007a0.Verdine GL. The combinatorial chemistry of nature. Nature. 1996;384(6604):11–13. doi: 10.1038/384011a0.
- 20.Nicolaou KC, Sanghee K, Pfefferkorn J, Xu J, Ohshima T, Hosokawa S, et al. Synthesis and biological activity of sarcodictyins. Angew Chem Int Edit. 1998;37:1418–21. doi: 10.1002/(SICI)1521-3773(19980605)37:10<1418::AID-ANIE1418>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 21.Tan DS, Foley MA, Shair MD, Schreiber SL. Stereoselective synthesis of over two million compounds having structural features both reminiscent of natural products and compatible with miniaturized cell based assays. J Am Chem Soc. 1998;120:8565–66. [Google Scholar]
- 22.Wilkins S. PhD Dissertation. Department of Pharmacognosy, University of Mississippi; 2000. High-throughput approaches to the identification and optimization of marine natural product leads. [Google Scholar]
- 23.Pennaka HK, Yousaf M, Rao KV, Kelly M, Hill RT, Franzblau SG, et al. Solving limited supplies of marine pharmaceuticals through the rational and high-throughput modification of high-yielding marine natural product scaffolds. Mar Biotechnol. 2004;6:S273–80. [Google Scholar]
- 24.Henkel T, Brunne RM, Muller H, Reichel F. Statistical investigation into the structural complimentarity of natural products and synthetic compounds. Angew Chem Int Edit. 1999;38:643–47. doi: 10.1002/(SICI)1521-3773(19990301)38:5<643::AID-ANIE643>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 25.Rang HP, Dale MM, Ritter JM, Gardner R. Antimycobacterial agents. 3. New York: Churchill Livingstone; 1995. Pharmacology; pp. 738–43. [Google Scholar]
- 26.Kasanah N, Rao KV, Yousaf M, Wedge DE, Hill RT, Hamann MT. Biotransformation studies of the manzamine alkaloids. Mar Biotechnol. 2004;6:S268–72. [Google Scholar]
- 27.Clark AM, McChesney JD, Hufford CD. The use of microorganisms for the study of drug metabolism. Med Res Rev. 1985;5:231–53. doi: 10.1002/med.2610050203. [DOI] [PubMed] [Google Scholar]
- 28.Holland HL. Microbial transformations. Curr Opin Chem Biol. 1998;2(1):77–84. doi: 10.1016/s1367-5931(98)80039-2. [DOI] [PubMed] [Google Scholar]
- 29.Riva S. Biocatalytic modification of natural products. Curr Opin Chem Biol. 2001;5:106–11. doi: 10.1016/s1367-5931(00)00178-2. [DOI] [PubMed] [Google Scholar]
- 30.El Sayed KA, Mayer AMS, Kelly M, Hamann MT. The biocatalytic transformation of furan to amide in the bioactive marine natural product palinurin. J Org Chem. 1999;64:9258–60. [Google Scholar]
- 31.El Sayed KA, Hamann MT, Waddling CA, Jensen C, Lee SK, Dunstan CA, et al. Structurally novel bioconversion products of the marine natural product sarcophine effectively inhibit JB6 cell transformation. J Org Chem. 1998;63:7449–55. doi: 10.1021/jo9813134. [DOI] [PubMed] [Google Scholar]
- 32.Kasanah N, Yousaf M, Rao KV, Wedge DE, Hamann MT. The biocatalytic conversion of 8-hydroxymanzemine A to manzamine A. Tetrahedron Lett. 2002;44:1291–93. [Google Scholar]
- 33.Sakai R, Higa T, Jefford CW, Bernardinelli G. Manzamine A, a novel antitumor alkaloid from a sponge. J Am Chem Soc. 1986;108:6404–05. [Google Scholar]
- 34.Baldwin JE, Whitehead RC. On the biosynthesis of the manzamines. Tetrahedron Lett. 1992;33(15):2059–62. [Google Scholar]
- 35.Tsuda M, Kobayashi J. Structures and biogenesis of manzamines and related alkaloids. Heterocycles. 1997;46:765–94. [Google Scholar]
- 36.Magnier E, Langlois Y. Manzamine alkaloids, syntheses and synthetic approaches. Tetrahedron. 1998;54:6201–58. [Google Scholar]
- 37.Rogers DJ, Randolph SE. The global spread of malaria in a future, warmer world. Science. 2000;289:1763–66. doi: 10.1126/science.289.5485.1763. [DOI] [PubMed] [Google Scholar]
- 38.El Sayed KA, Kelly M, Kara UAK, Ang KKH, Hamann MT. New manzamine alkaloids with potent activity against infectious diseases. J Am Chem Soc. 2001;123:1804–08. doi: 10.1021/ja002073o. [DOI] [PubMed] [Google Scholar]
- 39.Jones PB, Parrish NM, Houston TA, Stapon A, Niharika PB, Dick JD, et al. A new class of antituberculosis agents. J Med Chem. 2000;43:3304–14. doi: 10.1021/jm000149l. [DOI] [PubMed] [Google Scholar]
- 40.Khan AA, Slifer T, Araujo FG, Remington JS. Trovafloxacin is active against Toxoplasma gondii. Antimicrob Agents Chemo. 1996;40:1855–59. doi: 10.1128/aac.40.8.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rao KV, Kasanah N, Wahyuono S, Tekwani BL, Schinazi RF, Hamann MT. New manzamine alkaloids from a common Indonesian sponge and their activity against infectious and tropical parasitic diseases. J Nat Prod. 2003;67:1314–18. doi: 10.1021/np0400095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rao KV, Santarsiero BD, Mesecar AD, Schinazi RF, Tekwani BL, Hamann MT. New manzamine alkaloids with activity against infectious and tropical parasitic diseases from an Indonesian sponge. J Nat Prod. 2003;66:823–28. doi: 10.1021/np020592u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yousaf M, Hammond NL, Peng J, Wahyuono S, McIntosh KA, Charman WN, et al. New manzamine alkaloids from an Indo-Pacific sponge; pharmacokinetics, oral availability and the significant activity of several manzamines against HIV-I, AIDS opportunistic infections and inflammatory diseases. J Med Chem. 2004;47:3512–17. doi: 10.1021/jm030475b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peng J, Hu JF, Kazi AB, Li Z, Avery M, Peraud O, et al. Manadomanzamines A and B, a novel alkaloid ring system with potent activity against mycobacteria and HIV-1. J Am Chem Soc. 2003;125:13382–86. doi: 10.1021/ja030087z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohtani II, Ichiba T, Isobe M, Kelly-Borges M, Scheuer PJ. Kauluamine, an unprecedented manzamine dimer from an Indonesian marine sponge, Prianos sp. J Am Chem Soc. 1995;117:10743–44. [Google Scholar]
- 46.Ang KKH, Michael JH, Higa T, Hamann MT, Kara UAK. In vivo antimalarial activity of the beta-carboline alkaloid manzamine A. Antimicrob Agents Chem. 2000;44:1645–49. doi: 10.1128/aac.44.6.1645-1649.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period of 1981–2002. J Nat Prod. 2003;66:1022–37. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 48.Cortes-Selva F, Jimenez IA, Munoz-Martinez F, Campillo M, Bazzocchi IL, Pardo L, et al. Dihydro-beta-agarofuran sesquiterpenes: a new class of reversal agents of the multidrug resistance phenotype mediated by P-glycoprotein in the protozoan parasite Leishmania. Curr Pharm Des. 2005;11(24):3125–39. doi: 10.2174/1381612054864920. [DOI] [PubMed] [Google Scholar]
- 49.Rodriguez-Concepcion M. The MEP pathway: a new target for the development of herbicides, antibiotics and antimalarial drugs. Curr Pharm Des. 2004;10(19):2391–400. doi: 10.2174/1381612043384006. [DOI] [PubMed] [Google Scholar]
- 50.Seeber F, Aliverti A, Zanetti G. The plant-type ferredoxin-NADP+ reductase/ferredoxin redox system as a possible drug target against apicomplexan human parasites. Curr Pharm Des. 2005;11(24):3159–72. doi: 10.2174/1381612054864957. [DOI] [PubMed] [Google Scholar]