Abstract
The marine environment produces natural products from a variety of structural classes exhibiting activity against numerous disease targets. Historically marine natural products have largely been explored as anticancer agents. The indole alkaloids are a class of marine natural products that show unique promise in the development of new drug leads. This report reviews the literature on indole alkaloids of marine origin and also highlights our own research. Specific biological activities of indole alkaloids presented here include: cytotoxicity, antiviral, antiparasitic, anti-inflammatory, serotonin antagonism, Ca-releasing, calmodulin antagonism, and other pharmacological activities.
Keywords: Indole alkaloids, Bioactive marine natural products, New drug leads
Introduction
The marine environment, covering 70% of the earth's surface and 95% of its tropical biosphere represents 34 of the 36 phyla of life and provides a fascinating variety of biodiversity exceeding that of the terrestrial environment. Not surprising is that marine organisms produce an unprecedented molecular diversity by the incorporation of elements like bromine that are not readily available to terrestrial species. Partially responsible for the unique secondary metabolism of marine life are the ecological pressures in the marine ecosystem including significant competition for space, deterrence of predation and a high level of symbiosis between different species (Konig et al., 1994). Due to the biogenetic origin, marine organism secondary metabolites possess a number of structural differences as compared to terrestrial natural products. In addition, marine plants, mostly of the algal class (nonvascular), are unrelated to the majority of the terrestrial flora; similarly, the marine invertebrates such as sponges (Porifera), coelenterates (Cnidaria) and mollusks (Mollusca) are also not closely related to their terrestrial counterparts (Pelletier, 1986). Over 12,000 compounds from marine invertebrates (phyla: Annelida, Arthropoda, Brachiopoda, Bryozoa, Chordata, Cnidaria, Echinodermata, Hemichordata, Mollusca, Nematoda, Platyhelminthes, Porifera), algae (phyla: Chlorophycota, Chromophycota, Chromophyta, Cyanophycota, Euglenophycota, Rhodophycota), and microorganisms (bacteria, fungi and protozoa), have been discovered by a relative few marine research groups (Blunt and Munro, 2001). Natural products from the oceans have been reviewed by infectious disease such as for antiparasitic drugs (Kayser et al., 2002), antituberculosis agents (El Sayed et al., 2000), and antiHIV agents (Gochfeld et al., 2003). Furthermore, there are some excellent general and extensive reviews focusing on marine natural products such as “Marine bioprospecting—trawling for treasure and pleasure” (Capon, 2001), “The influence of natural products upon drug discovery” (Newman et al., 2000), “Marine natural products” (Faulkner, 2002), and Marine Pharmacology 1999 and 2000 (Mayer and Hamann, 2002, Mayer and Hamann, 2004). The application of synthetic modifications and biocatalysis on the production of marine natural product libraries has been reviewed in: “Enhancing marine natural product structural diversity and bioactivity through semisynthesis and biocatalysis” in Current Pharmaceutical Design (Hamann, 2003).
In this paper we have focused on the marine indole alkaloids due to the significant activity that they have elicited in cancer or cytotoxicity assays and would also like to draw attention to the tremendous unexplored potential this class of secondary metabolites may have on neurological targets and behavioral diseases.
A variety of marine sources including sponges, tunicates, red alga, acorn worms, and symbiotic bacteria have been shown to generate indole alkaloids, which represent the largest number and most complicated of the marine alkaloids (1 / 4 of total alkaloids) (Kobayashi et al., 1990). The alkaloids obtained from marine organisms frequently possess novel frameworks while in other cases terrestrially related compounds clearly exist. Marine metabolites often possess complexities such as halogen substituents. Their structure elucidation, chemical modification, stereochemistry, synthesis, and pharmacology have received a great deal of interdisciplinary attention from areas of research other than chemistry and include pharmacology, physiology, and medicine.
In this report we have focused on the pharmacologically active indole alkaloid marine natural products that have been shown to have biological activity, including cytotoxic, antiviral, antimicrobial, antiparasitics, anti-inflammatory, antiserotonin, Ca2+, calmodulin-antagonistic activity and antitopoisomerase-I activity, along with in vivo activity when available.
Cytotoxic indole alkaloids
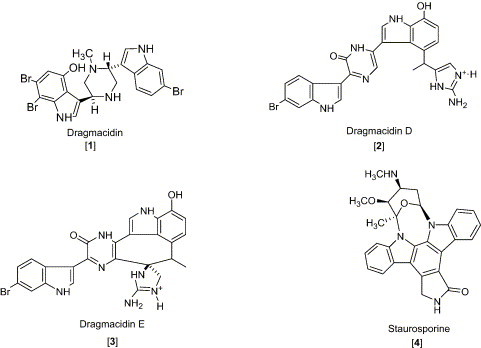
Dragmacidin [1], is a bisindole alkaloid isolated from a deep water marine sponge Dragmacidin sp. and showed cytotoxicity with an IC50 of 15 μg/mL against P-388 cell lines and 1–10 μg/mL against A-549 (human lung), HCT-8 (human colon), and MDAMB (human mammary) cancer cell lines in vitro (Kohmoto et al., 1988). Dragmacidin [1] and a number of marine natural products including 2 and 3 contain two indole groups joined by a piperazine ring system.
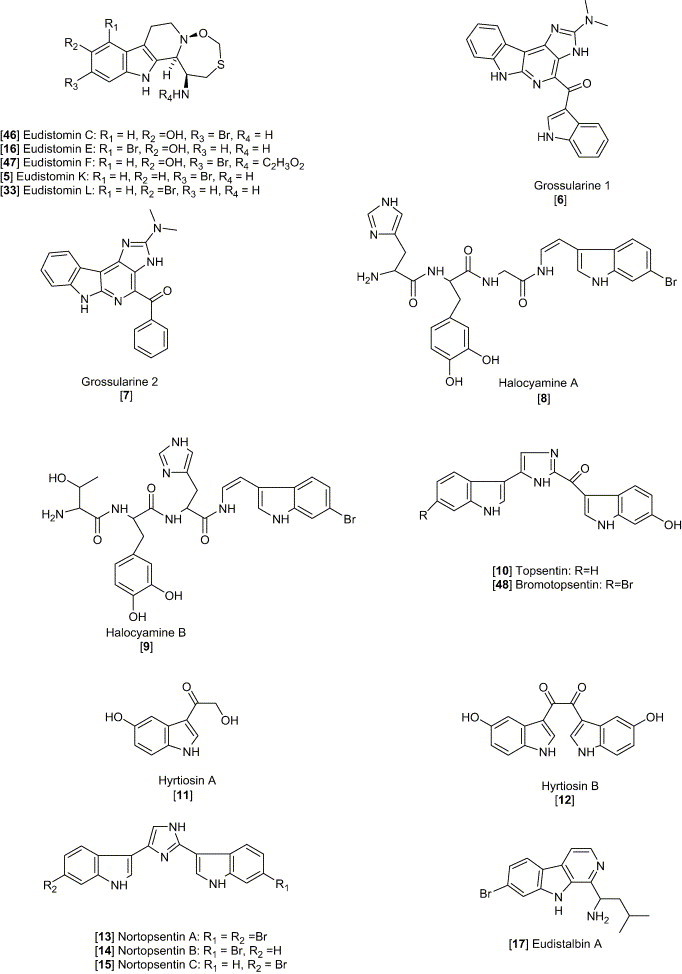
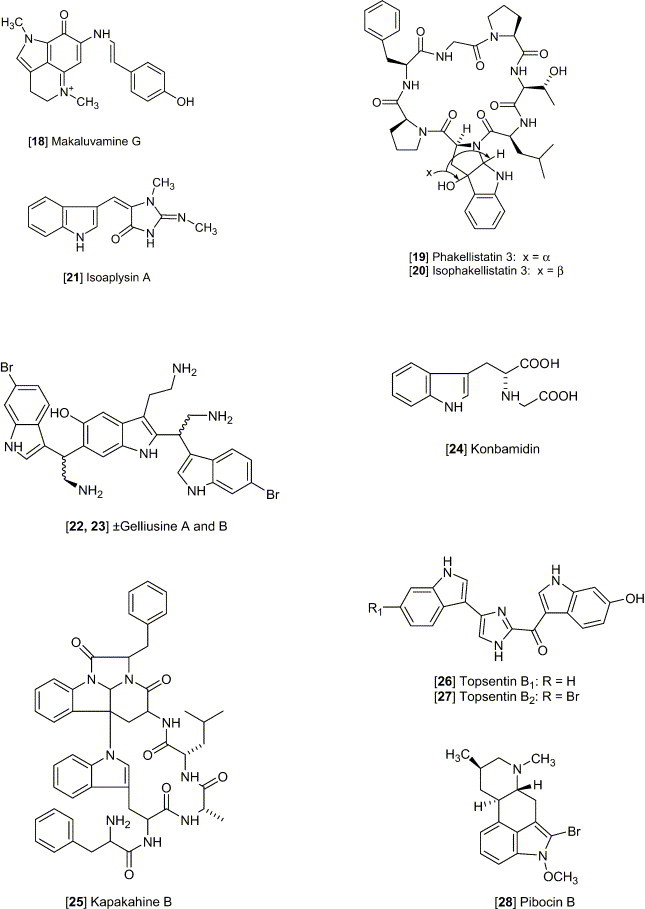
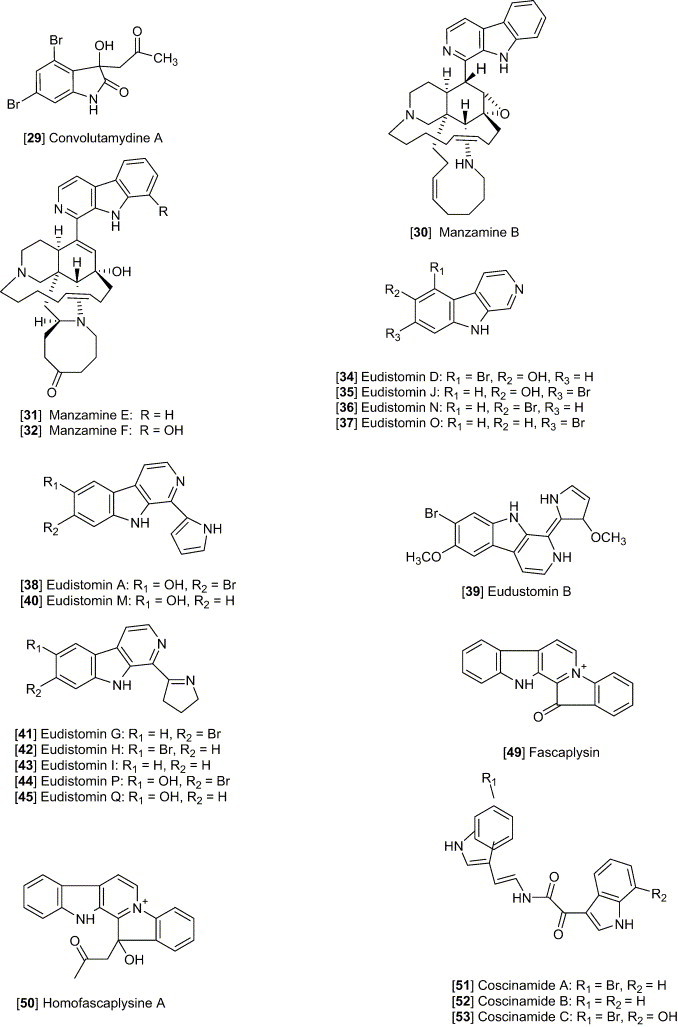
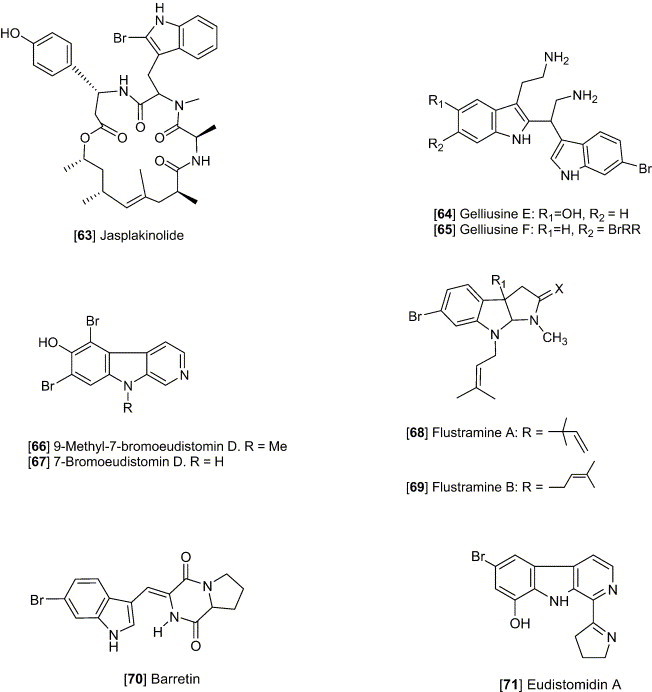
Staurosporine [4], an indolo [2,3-α] carbazole alkaloid, possesses cytotoxicity against a number of experimental tumor cell lines (Meksuriyen and Cordell, 1988). Staurosporine was first identified from Streptomyces staurosporeus Awaya (AM-2282) and other actinomycetes, including Streptomyces actuosus and Streptomyces strain M-193. The ED50 values of staurosporine were shown to be 0.0024 and < 0.08 μg/mL against KB and P-388, respectively. This study also revealed that staurosporine did not inhibit tubulin polymerization or disrupt micro tubular function at concentrations of 6 and 32 μg/mL, whereas the other antitumor agents, vincristine, vinblastine, and colchicine, exhibit a characteristic inhibition of cell mitosis in metaphase and prevents assembly of microtubules. Eudistomin K [5] isolated from the Caribbean ascidian Eudistoma olivaceum, was described as an antitumor lead against L-1210, A-549, HCT-8 and P-388 cell lines, with an in vitro IC50 of P-388 reported as 0.01 μg/mL (Lake et al., 1989). Grossularine 1 and 2 [6, 7], isolated from the tunicate Dendrodoa grossularia, possess cytotoxic properties, against L-1210 (ID50 6 and 4 μg/mL, respectively), WiDr (colon) and MCF7 (breast) (both are < 0.01 μg /mL) (Moquin-Pattey and Guyot, 1989), and also appear to act as a monointercalating agent of DNA. Halocyamine A and B [8, 9] are tetrapeptide-like metabolites isolated from the solitary ascidian Halocynthia roretzi and exhibited cytotoxic activity against neuronal cells cultured from fetal rat brain, mouse neuroblastoma N-18 cells, and human hepatoma Hep-G2 cells. The distribution of halocyamine A and B was only in the “morula”-like cells, the most plentiful cell type (more than 50%) among the H. roretzi hemocytes (Azumi et al., 1990). From the Caribbean deep-sea sponge Spongosorites ruetzleri, topsentin [10] was shown to possess a bis (indolyl) imidazole structure and exhibits in vitro activity against P-388 (IC50 3.0 μg/mL) and human tumor cells (HCT-8, A-549, T47D, 20 μg/mL) and in vivo activity against P-388 (T/C137%, 150 mg/kg) and B16 melanoma (T/C 144%, 37.5 mg/kg) (Tsujii et al., 1988). The evaluation of topsentin analogues for biological activity showed that the introduction of a hydroxyl group enhances the cytotoxicity while bromination diminishes activity. Hyrtiosins A and B [11, 12], isolated from the Okinawan sponge Hyrtios erecta exhibited cytotoxic activities greater than 5-hydroxyindole-3-aldehyde, which was reported to be active in vitro against human epidermoid carcinoma KB cells (IC50 4.3 μg/mL) (Kobayashi et al., 1990). Nortopsentins A, B, and C [13, 14, 15], isolated from the Caribbean deep-sea sponge S. ruetzleri, possess the unique imidazolediylbis[indole] skeleton and inhibits activity against P-388 cells with IC50 values of 7.6, 7.8, and 1.7 μg/mL, respectively (Sakemi and Sun, 1991). Methylated derivatives of the nortopsentins had improved activity against P-388 cells when compared to that of the parent compound. Eudistomin E and Eudistalbin A [16, 17] were shown to possess cytotoxic activities with ED50 < 0.005 and 3.2 μg/mL, respectively, in vitro against KB cells (Adesanya et al., 1992) and were extracted from the marine tunicate Eudistoma album with EtOH. Dragmacidin D [2], a new bis (indole)-derived sponge metabolite, was isolated from the sponge Spongosorites sp. and exhibits anticancer activity in vitro against P-388 and A-549 with IC50 values of 1.4 and 4.4 μg/mL, respectively (Wright et al., 1992). Makaluvamine G [18] is structurally related to the discorhabdins and was isolated from the Indonesian sponge Histodermella sp. and was shown to be cytotoxic against P-388, A-549, HT-29, MCF-7 (human breast cancer), and KB with IC50 values of 0.5, 0.5, 0.5, 0.5, and 0.4 μg/mL, respectively (Carney et al., 1993). Two isomeric cyclo-heptapeptides named phakellistatin 3 and isophakellistatin 3 [19, 20] were identified from the Western Indian marine sponge Phakellia carteri (Pettit et al., 1994). A significant difference in the activity was observed with the photo-Trp indole ring juncture, phakellistatin (trans-ring juncture) showing inhibition of P-388, while isophakellistatin (cis-ring juncture) showed no significant effects. Isoaplysin A [21] is a tryptophan-derived marine natural product, isolated from the Okinawan marine sponge Aplysina sp. (Kondo et al., 1994, Hu et al., 2002) and was shown to be weakly cytotoxic against murine lymphoma L-1210 (IC50 11.5 μg/mL) and KB (31% inhibition at 20 μg/mL) cells. The d-6-bromopypaphorine exhibited no cytotoxic activity. Aplysinopsin and methylaplysinopsin showed comparative cytotoxicity, with an IC50 of 2.3 and 6.4 μg/mL, respectively, against L-1210, and 3.5 μg/mL (aplysinopsin) and 6.7 μg/mL (methylaplysinopsin) against KB. Gelliusines A and B [22, 23] are two diastereometric brominated tris-indole alkaloids occurring as enantiomeric pairs, isolated from a deep water New Caledonian sponge Gellius or Orina sp. (Bifulco et al., 1994). The inhibition of KB, P-388, P-388/dox, HT-29 and NSCLCN-6 cell lines for 22 and 23 was evaluated and shown to yield IC50 values of between 10 and 20μg/mL. The Okinawan marine sponge Ircinia sp. yielded an indole alkaloid named konbamidin [24], which exhibited cytotoxicity against Hela cells in vitro with an IC50 value of 5.4 μg/mL (Shinonaga et al., 1994). Kapakahine B [25], a cyclic hexapeptide with an α-carboline ring system, was isolated from the marine sponge Cribrochalina olemda (Nakao et al., 1995). This peptide showed moderate cytotoxicity against P-388 murine leukemia cells with an IC50 value of 5.0 μg/mL. Styelin D, a 32-residue peptide isolated from the blood cells (hemocytes) of the solitary ascidian Styela clava, contains two novel amino acids, dihydroxy-arginine and dihydroxy-lysine, and two distinctly unusual amino acids including, 6-bromotryptophan and 3,4-dihydroxyphenylalanine (Taylor et al., 2000). Styelin D exhibited cytotoxicity against HCT-116 cells with an IC50 value of 10.1 μg/mL, and human ME-180 cervical epithelial cells with an ED50 value of 50 μg/mL. Topsentin B1 and B2 [26, 27] are bisindole alkaloids, isolated from the Mediterranean marine sponge Rhaphisia lacazei, which inhibit human broncopulmonary cancer cells NSCLC-N6 (IC50 12.0 and 6.3 μg/mL, respectively) (Casapullo et al., 2000). Pibocin B [28], isolated from the ascidian Eudistoma sp. obtained in the Sea of Japan, exhibited cytotoxicity against mouse Ehrlich carcinoma cells with an ED50 value of 25 μg/mL (Makarieva et al., 2001). Convolutamydine A [29], extracted from Amathia convoluta collected in the Gulf of Mexico near Florida, was shown to have significant activity against HL-60 human plomyelocytic leukemia cells, including a change in culture plate adhesion, growth arrest, and phagocytosis of particles at 0.1–25 μg/mL (Kamano et al., 1995). Manzamine B, E, and F, [30, 31, 32] collected from various sponges including an Amphimedon sp. throughout the world, have been shown to have IC50 values of 6.0, 5.0, and 5.0 μg/mL, respectively, against P-388 murine leukemia cells. These metabolites have recently been discussed in our detailed review included in the series the “Alkaloids” 2003 which discusses their biological activity against cancer, infectious diseases and in particular the highly promising activity against malaria and tuberculosis (Hu et al., 2003).
Antiviral activity
Eudistomin K and L [5, 33], contain a novel oxathiazepine ring and were isolated from the Caribbean tunicate E. olivaceum (Rinehart et al., 1984). They inhibit HSV-1 growth at 0.25 and 0.10 μg/disk, respectively. In further investigations, seventeen eudistomins (A–Q) from E. olivaceum were divided into four groups (group 1: simple β-carbolines, including eudistomins D [34], J [35], N [36], and O [37]; group 2: pyrrolyl-β-carbolines, including eudistomins A [38], B [39] (Benson et al., 2000), and M [40]; group 3: pyrrolinyl-β-carbolines, including eudistomins G [41], H [42], I [43], P [44], and Q [45]; and group 4: tetrahydro-β-carbolines with an oxathiazepine ring, including eudistomins C [46], E [16], F [47], K [5], and L [33]) (Rinehart et al., 1987). The eudistomins containing the oxathiazepine ring (C, E, F, K, and L) showing the most significant antiviral activity against HSV-1, of which C and E, with a phenolic group were active down at 0.005–0.01 μg/disk. The trend of antiviral potency of eudistomins was in the order of group 4 (C, E, K, and L) ≫ group 3 (H and P) = group 1 [D and N (O)] > group 2 (A and B). The substituent (Br and/or OH) on the β-carboline also affects the antiviral activity of eudistomins, which can be expressed as E (5-Br, 6-OH) > C (6-OH) > L(6-Br) = K (7-Br) and P (6-OH, 7-Br) = H (6-Br) > C (7-Br) ≈ Q (6-OH) ≈ I (no substitution). Eudistomins C and E possess the activities against RNA viruses such as Coxsachie A-21 virus and equine rhinovirus and against DNA viruses such as HSV-1, HSV-2, and Vaccinia virus. The acetylation on the phenol and primary amine functional groups of eudistomin C reduces its activity by 100-fold. Eudistomins were also isolated from the New Zealand ascidian Ritterella sigillinoides (Lake et al., 1989) and showed antiviral activity in vitro against Herpes simplex Type I and Polio vaccine Type I viruses. The similar activity trend was also reported as eudistomin C and K (0.04–0.05 μg/disc) > debromoeudistomin K and eudistomin K sulfoxide (0.4 μg/disc) > eudistomin O (0.5 μg/disc) > β-carboline (2 μg/disc). Topsentin and bromotopsentin [10, 48] were isolated from the Caribbean deep-sea sponge S. ruetzleri collected in the Bahamas and possess Bis (indolyl) imidazole structures (Tsujii et al., 1988). They exhibited antiviral activities in vitro against HSV-1, Vesivular stomatitis virus (VSV), and the corona virus A-59. Fascaplysin and homofascaplysin A cation [49, 50], isolated from the Benga Lagoon sponge Fascaplysinopis reticulata, possess antiviral properties, with the inhibitions against reverse transcriptase (at 1 mg/mL): 81%, 58%, and 94%, respectively (Jimenez et al., 1991). Dragmacidin D (2), a bis (indole)-derived sponge metabolite, isolated from sponge Spongosorites sp. inhibited in vitro replication of feline leukemia virus (FeLV) with an IC50 of 6.25 μg/mL (Wright et al., 1992). Coscinamide A, B, and C [51, 52, 53], isolated from the organic extract of Coscinoderma sp. collected in Papua, New Guinea, showed activity in the NCI's XTT-based anti-HIV assay (Bokesch et al., 2000). Extractions that were isolated from Flustra foliacea using crude petroleum ether were shown to strongly inhibit influenza virus plaque growth (Morales-Rios et al., 2001).
Antimicrobial/antiparasitic activity
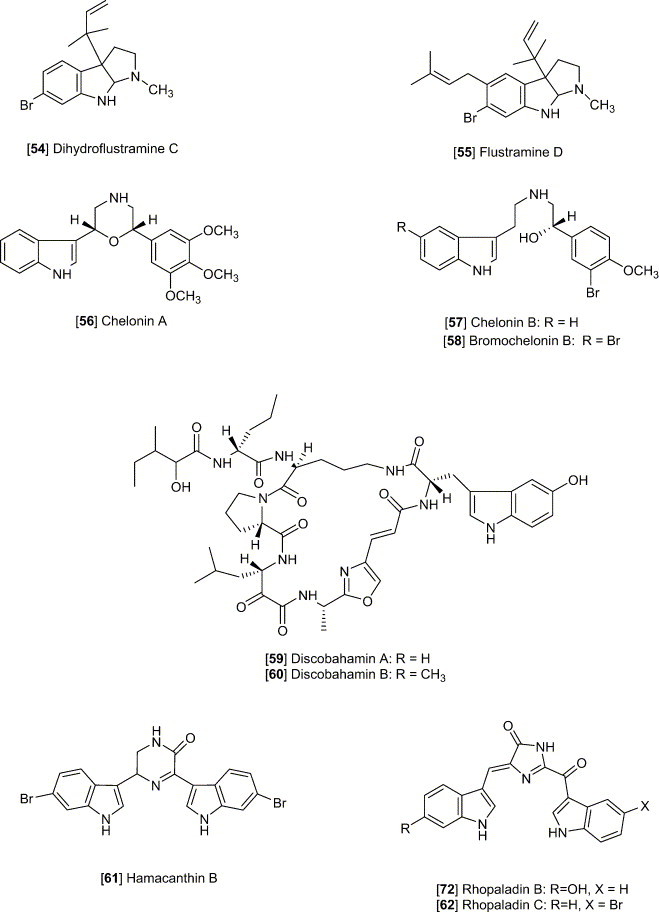
Dihydroflustramine C and Flustramine D [54, 55], are interesting in regard to the marine origin and belonging to the physostigmine structural class of alkaloids, reported from the marine bryozoan F. foliacea (Laycock et al., 1986). The alkaloid fraction mainly containing these two compounds exhibited a wide antibacterial spectrum including activity against Enterobacter cloacae, Escherichia coli, Klebsiella pneumoniae, Proteus vulgaris, Pseudomonas aeruginosa, Salmonella typhimurium, Serratia marcescens, Staphylococcus aureus and Staphylococcus epidermidis in disc diffusion assays (0.5 mg/disc). Eudistomins A-Q, isolated from the Caribbean tunicate E. olivaceum, displayed a widely differing degree of antimicrobial activities (Rinehart et al., 1987). The eudistomins containing the oxathiazepine rings are generally most active. Some eudistomins are active against Bacillus subtilis (D, I, and Q) or Saccharomyces cerevisiae (H) or both (P), while others are active against E. coli and Penicillium atrovenetum [C, K, L, and N(O)]. Fascaplysin [49], biogenetically derivable from tryptamine and structurally belonging to the pentacyclic quaternary salt, was isolated from the Fijian sponge Fascaplysinopsis sp. (Roll et al., 1988). Fascaplysin showed activities against the growth of several microbes, with a 15-mm zone at 0.1 μg/disk against S. aureus, 8-mm zone at 5 μg/disk against E. coli, 11-mm zone at 1 μg/disk against Candida albicans, and 20-mm zone at 0.1 μg/disk against S. cerevisiae. Halocyamine A and B [8–9], modified tetrapeptides, were isolated from the solitary ascidian H. roretzi from Japan (Azumi et al., 1990). Halocyamine A inhibited the growth of several microbes, including the Gram-positive bacteria B. subtilis (MIC 150 μg/mL), Bacillus megaterium (MIC 50 μg/mL), Bacillus cereus (MIC 100 μg/mL), and the yeast Candida neoformans (MIC 100 μg/mL). Halocyamine B displayed the comparative antimicrobial activities as A. Chelonin A, B and bromochelonin B [56, 57, 58], isolated from the marine sponge Chelonaplysilla sp., are another example of antimicrobial tryptophan derivatives (Bobzin and Faulkner, 1991). They exhibited activity against B. subtilis at a concentration of 100 μg/disk. Dragmacidin D [2], a new bis (indole)-derived sponge metabolite, isolated from the sponge Spongosorites sp. (Wright et al., 1992) inhibited the growth of several microbes, including E. coli (MIC 15.6 μg/mL), B. subtilis (MIC 3.1 μg/mL), Candida aeruginosa (MIC 62.5 μg/mL), C. albicans (MIC 15.6 μg/mL), and C. neoformans (3.9 μg/mL). Styelin D, a peptide isolated from the blood cells (hemocytes) of the solitary ascidian S. clava, displayed antimicrobial activity against methicillin-resistant and susceptible strain of S. aureus (Taylor et al., 2000). Styelin D has an effect on the outer and inner membranes on E. coli, making the outer membranes permeable to nitrocefin (a β-lactamase substrate) and the inner membranes permeable to o-nitrophenyl β-d-galactopyranoside (a β-galactosidase substrate). Nortopsentins A, B and C [13–15], isolated from the Caribbean deep-sea sponge S. ruetzleri, exhibited antifungal activity against C. albicans (Sakemi and Sun, 1991). From the Bahamian deep water marine sponge Discodermia sp., antifungal alkaloids discobahamin A and B [59, 60] were isolated (Gunasekera et al., 1994). They showed weak activity against the yeast C. albicans. Pibocin B [28], isolated from the ascidian Eudistoma sp. obtained from the Sea of Japan, was shown to have antimicrobial activity against B. subtilis, C. albicans, and S. aureus (Makarieva et al., 2001). Hamacanthin B [61] was obtained from Hamacantha sp, a deep-water marine sponge, and was shown to inhibit activity of C. albicans and C. neoformans (Jiang et al., 2002). Dichloromethane extractions from the cheilostome bryozoan F. foliacea exhibited antimicrobial activity against B. subtilis (Morales-Rios et al., 2001). Rhopaladin C [62], isolated from the Okinawan tunicate Rhopalaea sp., was shown to have antimicrobial activity against Corynebacterium xerosis and Sarcina lutea (MIC, 16 μg/mL each) (Sato et al., 1998). The manzamine alkaloids are now represented by approximately 40 different reported structures which can be divided into those which contain an indole moiety (as part of a beta-carboline system and represented by 30–32) and those without a beta-carboline system. The β-carboline system has been shown to play a prominent role in the very promising antimalarial activity for this class of alkaloids (reviewed in Hu et al., 2003). Jasplakinolide [63], obtained from a sponge collected in Fiji, was shown to have an MIC of 25 μg/mL against C. albicans. For a review of marine natural products with significant activity against infectious diseases please see our review “Marine natural products and their potential applications as anti-infective agents” in Lancet Infectious Diseases 2003 by Donia and Hamann (2003).
Anti-inflammatory indole alkaloids
Chelonin A [56], isolated from the sponge Chelonaplysilla sp. found in a marine lake in Palau exhibited antiinflammatory activity in addition to the antimicrobial activity discussed earlier (Bobzin and Faulkner, 1991). Chelonin A showed a 60% inhibition against PMA-induced inflammation in a mouse ear model (50 μg/ear), but shows no in vitro inhibition against bee venom phosopholipase A2. Dragmacidin [1], isolated from the sponges Dragmacidin sp. and Hexadella sp. has been shown to have anti-inflammatory activity against bee venom PLA2 and mouse ear edema in vitro and in vivo assays, respectively (Jiang et al., 1994).
Activity of indole alkaloids on serotonin receptors
Gelliusine A [22], is a brominated tris-indole alkaloid occurring as enantiomeric pairs, isolated from a deep water New Caledonian sponge Gellius or Orina sp. (Bifulco et al., 1994). At higher concentrations (5–70 μg/mL), gelliusine A causes a serotonin-like and methysergide-sensitive contraction; while at low concentration, it is able to antagonize serotonin-induced contraction, indicating the ability to block the serotoninergic receptor. In a later investigation, gelliusines A, B, E and F [22–23, 64–65] were submitted to a series of receptor binding assays at a concentration of 5 μg/mL (Bifulco et al., 1995), including the somatostatin receptor site (the inhibition of specific ligand binding were 100%, 100%, 87% and 91%, respectively), the neuropeptide Y receptor (90%, 62%, 63% and 67%, respectively), and the human B2 bradykinin receptor site (100%, 93%, 63% and 89%, respectively) (Bifulco et al., 1995). A number of halogenated indoles related to isoaplysin A [21] have shown interesting selectivity for serotonin receptors when evaluated for affinity toward the 5-HT2A and 5-HT2C receptors. Clearly one of the more interesting and unexplored application for marine natural products is their potential selective affinity for various neurological targets like the 5-HT subtypes and the resulting impact on behavior in whole animal studies (see our discussion in Hu et al., 2002).
Ca-releasing activity
9-Methyl-7-bromoeudistomin D [66], isolated from the Caribbean tunicate E. olivaceum, was found to induce Ca2+ release from the sarcoplasmic reticulum (Kobayashi et al., 1989). The comparison of several eudistomin analogues suggested that the 9-methyl group is quite important to the activity, and the bromine substituent in the β-carboline is better than chlorine or iodine. The SAR also indicated that the alkyl substitution to N-2 in the β-carboline lead to decrease the activity. Compared to caffeine, 9-methyl-7-bromoeudistomin was approximately 1000 time more potent in causing Ca2+ release from the sarcoplasmic reticulum of skeletal muscle. 7-Bromoeudistomin D [67], obtained by demethylation of 7-bromo-O-methyleudistomin D, was found to be 400 times more potent than caffeine in Ca2+ releasing action (Rinehart et al., 1987). Isolated from F. foliacea, flustramines A and B [68–69], were found to cause relaxation of both skeletal and smooth muscle (Morales-Rios et al., 2001). Barretin [70], isolated from the Swedish cold-water sponge Geodia baretti, was shown to inhibit contractions of a Guinea-pig ileum (Lidgren et al., 1986).
Calmodulin-antagonistic activity
The Okinawan tunicate Eudistoma glaucus afforded eudistomidin A [71] and was shown to be the first calmodulin antagonist from marine origin (Kobayashi et al., 1986). IC50 (2 × 10− 5M) against calmodulin-activated brain phosphodiesterase was 15 times more potent than W-7 (3 × 10− 4M), a well-known calmodulin antagonist.
Enzyme inhibitors
Isolated from the Okinawan tunicate Rhopalaea sp., Rhopaladin B [72], was the only compound from the series Rhopaladin A–D that was shown to inhibit cyclin dependent kinase 4 and c-erbB-2 kinase with an IC50 of 12.5 and 7.4, respectively (Sato et al., 1998). Dragmacidin D and E [2, 3], isolated from Dragmacidin sp., were shown to inhibit serine-threonine protein phosphatases. Dragmacidin D (2) was also shown to inhibit neural nitric oxide synthase (βNOS), which may prove to help in the treatment of Huntington's, Parkinson's, and Alzheimer's diseases (Yang et al., 2002).
Anti-topoisomerase-I activity
The evaluation of an Indonesian sponge Histodermella sp., collected from Manado Bay, afforded makaluvamine G [18], a structural analog to the discorhabdin compounds, and exhibited moderate inhibition of topoisomerase-I (IC50 3.0 μM) (Carney et al., 1993). The activity of inhibition of RNA (IC50 15 μM), DNA (IC50 15 μM), and protein (IC50 21 μM) synthesis was also reported.
Discussion
A wide diversity of biologically active indole alkaloids have been reported from marine sources. Indole alkaloids derived from Dragmacidin sp. have been shown to exhibit cytotoxic activity, anti-inflammatory activity, and to inhibit enzyme activity. The Fijian sponge Fascaplysinopsis sp. produced cytotoxic and antiviral compounds. S. staurosporeus and D. grossularia, which yielded staurosporine and grossularine 1 and 2, respectively, both were shown to possess cytotoxic properties. Eudistomins A-Q were obtained from the ascidian E. olivaceum. Cytotoxic activity was characteristic of these alkaloids, as well as antiviral, Ca2+, and antimicrobial. Indole alkaloids isolated from H. roretzi were shown to exhibit cytotoxicity and antimicrobial activity. S. ruetzleri afforded compounds that possessed cytotoxic, antiviral, and antimicrobial activity. The Okinawan sponges H. erecta and Aplysina sp. gave compounds that exhibited cytotoxic activity. The Indonesian sponge Histodermella sp. yielded makaluvamine G, which was shown to have cytotoxic and anti-topoisomerase-1 activity. Several indole alkaloids were isolated from Eudistoma sp. and were shown to exhibit cytotoxic, antimicrobial, and calmodulin-antagonistic activities. P. carteri, Ircinia sp., and Cribochalina olemda all possessed indole alkaloids that had cytotoxic activity. Indole alkaloids isolated from the New Caledonian sponge Gellius or Orina sp. was shown to have cytotoxic activity, as well as activity against serotonin receptors.
Conclusion
The biological activity of marine indole alkaloids is clearly a product of the unique functionality and elements involved in the biosynthesis of marine natural products. For instance, the bromination of many of the mentioned natural products has the potential to increase the biological activity significantly. The cytotoxic activity of grossularine 1 is increased as compared to grossularine 2 due to the extra indole group in place of the benzene ring. As seen with the nortopsentin compounds, bromination does not always result in better activity. Nortopsentin B with only one R group brominated is cytotoxic at 0.2 μg/mL and more active than nortopsentin A with two brominated R groups and nortopsentin C which also has one bromine opposite that of nortopsentin B and this change decreases its activity to 1.7 μg/mL (against P-388 cells.) The decrease in cytotoxic activity can also be observed with topsentin B1 and B2 where the addition of bromine reduces the activity by half. In antiviral activity, the specific placement of substituents is vital as can be seen with eudistomin K and L. In eudistomin K, with the bromine placed in the R3 position, as opposed to the R2 position as in L, the growth inhibition was shown to be 0.25 μg/mL, 0.15μg/mL more active than L. Eudistomin K was also shown to be active against Herpes simplex Type I and Polio vaccine Type I viruses, while L was not. Concerning the activity of indole alkaloids on serotonin receptors, the addition of an indole group, as seen in gelliusine A and B, to the main Gelliusine structure causes a significant increase in the inhibition of ligand binding, the neuropeptide Y receptor, and the human B2 bradykinin receptor site. Gelliusine E shows the poorest activity, which may be due to the lack of the extra indole group of gelliusine A and B and the extra bromine carried by gelliusine F. In the case of the isoaplysin related alkaloids the addition of bromine to the indole system elicited very significant improvements in binding selectivity to the various 5-HT subtypes. This clearly indicates that these halogenated marine indole alkaloids are certain to help define the function of the various 5-HT subtypes in addition to the identification of drug leads with potential clinical applications.
Acknowledgements
We are grateful to Tammy Lee, Dr. Muhammad Yousaf and Dian Shen for their help in the preparation of this paper. This work was support by NIH (NIAID) and the Medicines for Malaria Venture.
Appendix.
References
- Adesanya S.A., Chbani M., Pais M., Debitus C. Brominated β-carbolines from the marine tunicate Eudistoma album. Journal of Natural Products. 1992;55(4):525–527. doi: 10.1021/np50082a025. [DOI] [PubMed] [Google Scholar]
- Azumi K., Yokosawa H., Ishii S. Halocyamines: novel antimicrobial tetrapeptide-like substances isolated from the hemocytes of the solitary ascidian Halocynthia roretzi. Biochemistry. 1990;29:159–165. doi: 10.1021/bi00453a021. [DOI] [PubMed] [Google Scholar]
- Benson D.A., Karsch-Mizrachi I., Lipman D.J., Ostell J., Rapp B.A., Wheeler D.L. Genbank Nucleic Acids Research. 2000;28(1):15–18. doi: 10.1093/nar/28.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bifulco G., Bruno I., Minale L., Riccio R., Calignano A., Debitus C. (±)-Gelliusines A and B, two diastereomeric brominated tris-indole alkaloids from a deep water New Caledonian marine sponge (Gellius or Orina sp.) Journal of Natural Products. 1994;57(9):1294–1299. doi: 10.1021/np50111a020. [DOI] [PubMed] [Google Scholar]
- Bifulco G., Bruno I., Riccio R., Lavayre J., Bourdy G. Further brominated bis- and tris–indole alkaloids from the deep-water New Caledonian marine sponge Orina sp. Journal of Natural Products. 1995;58(8):1254–1260. doi: 10.1021/np50122a017. [DOI] [PubMed] [Google Scholar]
- Blunt J.W., Munro M.H.G. 2001. University of Canterbury, Marinlit: a Database of the Literature on Marine Natural Product. [Google Scholar]
- Bobzin S.C., Faulkner D.J. Aromatic alkaloids from the marine sponge chelonaplysilla sp. Journal of Organic Chemistry. 1991;56:4403–4407. [Google Scholar]
- Bokesch H.R., Pannell L.K., McKee T.C., Boyd M.R. Coscinamides A, B and C, three new bis-indole alkaloids from the marine sponge Coscinoderma sp. Tetrahedron Letters. 2000;41:6305–6308. [Google Scholar]
- Capon R.J. Marine bioprospecting — trawling for treasure and pleasure. European Journal of Organic Chemistry. 2001;4:633–645. [Google Scholar]
- Carney J.R., Scheuer P.J., Kelly-Borges M. Makaluvamine G, a cytotoxic pigment from an Indonesian sponge Histodermella sp. Tetrahedron. 1993;49(38):8483–8486. [Google Scholar]
- Casapullo A., Bifulco G., Bruno I., Riccio R. New bisindole alkaloids of the topsentin and hamacanthin classes from the Mediterranean marine sponge Rhaphisia lacazei. Journal of Natural Products. 2000;63:447–451. doi: 10.1021/np9903292. [DOI] [PubMed] [Google Scholar]
- Donia M., Hamann M.T. Marine natural products and their potential applications as anti-infective agents. The Lancet Infectious Diseases. 2003;3:338–348. doi: 10.1016/S1473-3099(03)00655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Sayed K.A., Bartyzel P., Shen X., Perry T.L., Zjawiony J.K., Hamann M.T. Marine natural products as antituberculosis agents. Tetrahedron. 2000;56:949–953. [Google Scholar]
- Faulkner D.J. Marine natural products. Natural Product Reports. 2002;19:1–48. doi: 10.1039/b009029h. [DOI] [PubMed] [Google Scholar]
- Gochfeld D.J., El Sayed K.A., Yousaf M., Hu J.F., Bartyzel P., Dunbar D.C., Wilkins S.P., Zjawiony J.K., Schinazi R.F., Wirtz S.S., Tharnish P.M., Hamann M.T. Marine natural products as lead anti-HIV agents. Mini Reviews in Medicinal Chemistry. 2003;3:401–424. doi: 10.2174/1389557033487962. [DOI] [PubMed] [Google Scholar]
- Gunasekera S.P., Pomponi S.A., McCarthy P.J. Discobahamins A and B, new peptides from the Bahamian deep water marine sponge Discodermia sp. Journal of Natural Products. 1994;57(1):79–83. doi: 10.1021/np50103a011. [DOI] [PubMed] [Google Scholar]
- Hamann M.T. Enhancing marine natural product structural diversity and bioactivity through semisynthesis and biocatalysis. Current Pharmaceutical Design. 2003;9:879–889. doi: 10.2174/1381612033455297. [DOI] [PubMed] [Google Scholar]
- Hu J.F., Schetz J.A., Kelly M., Peng J.N., Ang K.K.H., Flotow H., Leong C.Y., Ng S.B., Buss A.D., Wilkins S.P., Hamann M.T. New antiinfective and human 5-HT2 receptor binding natural and semisynthetic compounds from the Jamaican sponge Smenospongia aurea. Journal of Natural Products. 2002;65(4):476–480. doi: 10.1021/np010471e. [DOI] [PubMed] [Google Scholar]
- Hu J.F., Hamann M.T., Hill R., Kelly M. The manzamine alkaloids. The Alkaloids. 2003;60:207–285. doi: 10.1016/s0099-9598(03)60004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B., Smallheer J.M., Amaral-Ly C., Wuonola M.A. Total synthesis of (±)-Dragmacidin: a cytotoxic bis (indole) alkaloid of marine origin. Journal of Organic Chemistry. 1994;59:6823–6827. [Google Scholar]
- Jiang B., Yang C.G., Wang J. Enantioselective synthesis of marine indole alkaloid Hamacanthin B. Journal of Organic Chemistry. 2002;67:1396–1398. doi: 10.1021/jo0108109. [DOI] [PubMed] [Google Scholar]
- Jimenez C., Quinoa E., Adamczeski M., Hunter L.M., Crews P. Novel sponge-derived amino acids. 12. Tryptophan-derived pigments and accompanying sesterterpenes from fascaplysinopis reticulata. Journal of Organic Chemistry. 1991;56:3403–3410. [Google Scholar]
- Kamano Y., Zhang H.P, Ichihara Y., Kizu H., Komiyama K., Pettit G.R. Convolutamydine A, a novel bioactive hydroxyoxindole alkaloid from marine bryozoan Amathia convoluta. Tetrahedron Letters. 1995;36(16):2783–2784. [Google Scholar]
- Kayser O., Kiderlen A.F., Croft S.L. Natural products as antiparasitic drugs. Parasitic Res. 2002;90:S55–S62. doi: 10.1007/s00436-002-0768-3. [DOI] [PubMed] [Google Scholar]
- Kobayashi J., Nakamura H., Ohizumi Y., Hirata Y. Eudistomidin-A, a novel calmodulin antagonist from the Okinawan tunicate Eudistoma glaucus. Tetrahedron Letters. 1986;27(10):1191–1194. [Google Scholar]
- Kobayashi J., Ishibashi M., Nagai U., Ohizumi Y. 9-Methyl-7-bromoeudistomin D, a potent inducer of calcium release from sarcoplasmic reticulum of skeletal muscle. Experientia. 1989;45:782–783. doi: 10.1007/BF01974589. [DOI] [PubMed] [Google Scholar]
- Kobayashi J., Murayama T., Ishibashi M., Kosuge S., Takamatsu M., Ohizumi Y., Kobayashi H., Ohta T., Nozoe S., Sasaki T. Hyrtiosins A and B, new indole alkaloids from the Okinawan marine sponge Hyrtios erecta. Tetrahedron. 1990;46(23):7699–7702. [Google Scholar]
- Kohmoto S., Kashman Y., McConnell O.J., Rinehart K.L., Wright A., Koehn F. Dragmacidin, a new cytotoxic bis (indole) Alkaloid from a deep water marine sponge, Dragmacidon sp. Journal of Organic Chemistry. 1988;53:3116–3118. [Google Scholar]
- Kondo K., Nishi J., Ishibashi M., Kobayashi J. Two new tryptophan-derived alkaloids from the Okinawan marine sponge Aplysina sp. Journal of Natural Products. 1994;57(7):1008–1011. doi: 10.1021/np50109a023. [DOI] [PubMed] [Google Scholar]
- Konig G.M., Wright A.D., Sticher O., Angerhofer C.K., Pezzuto J.M. Biological activities of selected marine natural products. Planta Medica. 1994;60:532–537. doi: 10.1055/s-2006-959565. [DOI] [PubMed] [Google Scholar]
- Lake R.J., Blunt J.W., Munro M.H.G. Eudistomins from the New Zealand Ascidian Ritterella sigillinoides. Australian Journal of Chemistry. 1989;42:1201–1206. [Google Scholar]
- Laycock M.V., Wright J.L.C., Findlay J.A., Patil A.D. New physostigmine related bromoalkaloids from the marine bryozoan Flustra foliacea. Canadian Journal of Chemistry. 1986;64:1312–1316. [Google Scholar]
- Lidgren G., Bohlin L., Bergman J. Studies of Swedish marine organisms VII. A novel biologically active indole alkaloid from the sponge Geodia baretti. Tetrahedron Letters. 1986;27(28):3283–3284. [Google Scholar]
- Makarieva T.N., Dmitrenok A.S., Dmitrenok P.S., Grebnev B.B., Stonik V.A. Pibocin B, the first N-O-Methylindole marine alkaloid, a metabolite from the Far-Eastern ascidian Eudistoma species. Journal of Natural Products. 2001;64:1559–1561. doi: 10.1021/np010161w. [DOI] [PubMed] [Google Scholar]
- Mayer A.M.S., Hamann M.T. Marine pharmacology in 1999: compounds with antibacterial, anticoagulant, antifungal, anthelmintic, antiinflammatory, antiplatelet, antiprotozoal and antiviral activities; affecting the cardiovascular, endocrine, immune and nervous systems and other miscellaneous mechanisms of action. Comparative Biochemistry and Physiology, Part C Toxicology and Pharmacology. 2002;132C:315–339. doi: 10.1016/s1532-0456(02)00094-7. [DOI] [PubMed] [Google Scholar]
- Mayer A.M.S., Hamann M.T. Marine pharmacology in 2000: compounds with antibacterial, anticoagulant, antifungal, anthelmintic, antiinflammatory, antiplatelet, antiprotozoal and antiviral activities; affecting the cardiovascular, endocrine, immune and nervous systems and other miscellaneous mechanisms of action. Marine Biotechnology. 2004;6:37–52. doi: 10.1007/s10126-003-0007-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meksuriyen D., Cordell G.A. Biosynthesis of staurosporine, 1. 1H- and 13C-NMR assignments. Journal of Natural Products. 1988;51(5):884–892. doi: 10.1021/np50059a012. [DOI] [PubMed] [Google Scholar]
- Moquin-Pattey C., Guyot M. Grossularine-1 and grossularine-2, cytotoxic α-carbolines from the tunicate: Dendrodoa grossularia. Tetrahedron. 1989;45(11):3445–3450. [Google Scholar]
- Morales-Rios M.S., Suarez-Castillo O.R., Trujillo-Serrato J.J., Joseph-Nathan P. Total syntheses of five indole alkaloids from the marine bryozoan Flustra foliacea. Journal of Organic Chemistry. 2001;66:1186–1192. doi: 10.1021/jo0012647. [DOI] [PubMed] [Google Scholar]
- Nakao Y., Yeung B.K.S., Yoshida W.Y., Scheuer P.J., Kelly-Borges M. Kapakahine B: a cyclic hexapeptide with an α-carboline ring system from the marine sponge Cribrochalina olemda. Journal of American Chemical Society. 1995;117:8271–8272. [Google Scholar]
- Newman D.J., Cragg G.M., Snader K.M. The influence of natural products upon drug discovery. Natural Products Reports. 2000;17:215–234. doi: 10.1039/a902202c. [DOI] [PubMed] [Google Scholar]
- Pelletier S.W. Alkaloids: chemical and biological perspectives. 1986;4:275–330. [Google Scholar]
- Pettit G.R., Tan R., Herald D.L., Cerny R.L., Williams M.D. Antineoplastic agents.277. Isolation and structure of phakellistatin 3 and isophakellistatin 3 from a republic of Comoros marine sponge. Journal of Organic Chemistry. 1994;59(7):1593–1595. [Google Scholar]
- Rinehart K.L., Kobayashi J., Harbour G.C., Hughes R.G., Mizsak S.A., Scahill T.A. Eudistomins C, E, K and L, potent antiviral compounds containing a novel oxathiazepine ring from Caribbean tunicate Eudistoma olivaceum. Journal of American Chemical Society. 1984;106:1524–1526. [Google Scholar]
- Rinehart K.L., Kobayashi J., Harbour G.C., Gilmore J., Mascal M., Holt T.G., Shield L.S., Lafargue F. Eudistomins A-Q, β-carbolines from the antiviral Caribbean tunicate Eudistoma olivaceum. Journal of American Chemical Society. 1987;109:3378–3387. [Google Scholar]
- Roll D.M., Ireland C.M., Lu H.S.M., Clardy J. Fascaplysin, an unusual antimicrobial pigment from the marine sponge Fascaplysinopsis sp. Journal of Organic Chemistry. 1988;53:3276–3278. [Google Scholar]
- Sakemi S., Sun H.H. Nortopsentins A, B, and C. Cytotoxic and antifungal imidazolediylbis [indoles] from the sponge Spongosorites ruetzleri. Journal of Organic Chemistry. 1991;56:4304–4307. [Google Scholar]
- Sato H., Tsuda M., Watanabe K., Kobayashi J. Rhopaladins A∼D, new indole alkaloids from marine tunicate Rhopalaea sp. Tetrahedron. 1998;54:8687–8690. [Google Scholar]
- Shinonaga H., Shigemori H., Kobayashi J. Konbamidin, a new indole alkaloid from the Okinawan marine sponge Ircinia sp. Journal of Natural Products. 1994;57(11):1603–1605. doi: 10.1021/np50113a026. [DOI] [PubMed] [Google Scholar]
- Taylor S.W., Craig A.G., Fischer W.H., Park M., Lehrer R.I. Styelin D, an extensively modified antimicrobial peptide from ascidian hemocytes. Journal of Biological Chemistry. 2000;275(49):38417–38426. doi: 10.1074/jbc.M006762200. [DOI] [PubMed] [Google Scholar]
- Tsujii S., Rinehart K.L., Gunasekera S.P., Kashman Y., Cross S.S., Lui M.S., Pomponi S.A., Diaz M.C. Topsentin, bromotopsentin, and dihydrodeoxybromo-topsentin: antiviral and antitumor bis-(indolyl) imidazoles from Caribbean deep-sea sponges of the family halichondriidae. Journal of Organic Chemistry. 1988;53:5446–5453. [Google Scholar]
- Wright A.E., Pomponi S.A., Cross S.S., McCarthy P. A new bis (indole) alkaloid from a deep-water marine sponge of the genus Spongosorites. Journal of Organic Chemistry. 1992;57:4772–4775. [Google Scholar]
- Yang C.G., Liu G., Jiang B. Preparing functional bis (indole) pyrazine by stepwise cross-coupling reactions: an efficient method to construct the skeleton of dragmacidin D. Journal of Organic Chemistry. 2002;67:9392–9396. doi: 10.1021/jo026450m. [DOI] [PubMed] [Google Scholar]


