Abstract
Study Objectives:
Obstructive sleep apnea (OSA) predicts poor functional outcome after stroke and increases the risk for recurrent stroke. Less is known about continuous positive airway pressure (CPAP) treatment on stroke recovery.
Methods:
In a pilot randomized, double-blind, sham-controlled trial, adult stroke rehabilitation patients were assigned to auto-titrating or sham CPAP without diagnostic testing for OSA. Change in Functional Independence Measure (FIM), a measure of disability, was assessed between rehabilitation admission and discharge.
Results:
Over 18 months, 40 patients were enrolled and 10 withdrew from the study: 7 from active and 3 from sham CPAP (p > 0.10). For the remaining 30 patients, median duration of CPAP use was 14 days. Average CPAP use was 3.7 h/night, with at least 4 h nightly use among 15 patients. Adherence was not influenced by treatment assignment or stroke severity. In intention-to-treat analyses (n = 40), the median change in FIM favored active CPAP over sham but did not reach statistical significance (34 versus 26, p = 0.25), except for the cognitive component (6 versus 2.5, p = 0.04). The on-treatment analyses (n = 30) yielded similar results (total FIM: 32 versus 26, p = 0.11; cognitive FIM: 6 versus 2, p = 0.06).
Conclusions:
A sham-controlled CPAP trial among stroke rehabilitation patients was feasible in terms of recruitment, treatment without diagnostic testing and adequate blinding—though was limited by study retention and CPAP adherence. Despite these limitations, a trend towards a benefit of CPAP on recovery was evident. Tolerance and adherence must be improved before the full benefits of CPAP on recovery can be assessed in larger trials.
Citation:
Khot SP, Davis AP, Crane DA, Tanzi PM, Li Lue D, Claflin ES, Becker KJ, Longstreth WT, Watson NF, Billings ME. Effect of continuous positive airway pressure on stroke rehabilitation: a pilot randomized sham-controlled trial. J Clin Sleep Med 2016;12(7):1019–1026.
Keywords: obstructive sleep apnea, rehabilitation, continuous positive airway pressure, stroke
INTRODUCTION
Stroke is the leading cause of long-term disability in the United States, yet treatments that improve function after stroke are limited. Obstructive sleep apnea (OSA) is increasingly recognized as a risk factor for ischemic and hemorrhagic stroke,1,2 with prevalence after stroke or transient ischemic attack estimated to be over 70%.3 Stroke patients with OSA compared to those without have worse functional outcome, longer hospitalization and rehabilitation stays, and higher mortality.4–9 Despite the high risk of sleep apnea among patients with stroke and the implications for both stroke recovery and recurrent stroke, few stroke survivors undergo screening, testing, or treatment for OSA.10 Barriers to evaluation and treatment involve OSA awareness among stroke survivors and clinical providers, access to in-laboratory polysomnography (PSG) testing, and the lack of consensus among stroke providers on the ideal timing for sleep testing.
Treatment with continuous positive airway pressure (CPAP) among patients diagnosed with OSA is associated with improved functional and motor outcome after stroke,11 but trials are limited by poor CPAP tolerance and adherence.12,13 Compared to the general OSA population, patients with acute stroke are typically older with more functional disability and may have more difficulty tolerating the CPAP mask, due to underlying extremity or facial paresis, dysphagia, aphasia, or neglect.14 Given these challenges, we sought to assess the feasibility of enrolling and randomizing stroke patients undergoing inpatient rehabilitation into a clinical trial of active versus sham CPAP. Secondary objectives were to examine tolerance and adherence to CPAP and the effect of auto-titrating CPAP compared to sham CPAP on functional outcome from stroke.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Obstructive sleep apnea (OSA), increasingly recognized as a risk factor for ischemic and hemorrhagic stroke, is also a predictor of poor functional outcome after stroke. We conducted a pilot study during inpatient rehabilitation after acute stroke to assess the feasibility of a sham-controlled CPAP trial, the adherence of CPAP in this setting and the effect of CPAP on functional outcomes.
Study Impact: This pilot trial demonstrates the feasibility of randomizing stroke rehabilitation patients into a trial of active versus sham CPAP and a potential benefit of active CPAP in recovery, especially for cognitive function. Future larger studies should examine if long-term CPAP use with high adherence has an impact on stroke recovery, recurrence, and mortality.
METHODS
The Human Subjects Review Committee at the University of Washington approved the study, and patients, next of kin, or both provided written informed consent. Patients were eligible for enrollment if they met all of the following criteria: older than 18 years of age; admitted to an inpatient rehabilitation unit at the University of Washington; and had a head CT or brain MRI demonstrating an ischemic or hemorrhagic stroke. Patients were excluded from enrollment if their stroke was a subarachnoid hemorrhage or due to a secondary cause (vascular malformation, vasculitis, brain tumor, head trauma, or predisposition to bleeding); they had a history of CPAP use, advanced chronic lung disease requiring supplemental oxygen, New York Heart Association class III or IV heart failure; or they needed a nasogastric feeding tube. Given the high prevalence of OSA in this population and uncertainty about an appropriate apnea-hypopnea index cutoff to utilize for the purposes of this trial, no screening diagnostic test for OSA was performed.
Randomization to active or sham CPAP was based on the assignment indicated in a sealed envelope and given to the study's sleep medicine technologist after each enrollment. The envelopes were assembled prior to the study based on a string of random numbers in balanced blocks of 4. The only people with knowledge of the treatment assignment were the study's sleep medicine technologist and respiratory therapists who were responsible for setting up and monitoring the CPAP treatment.
Philips Respironics (Murrysville, PA) provided both active and sham CPAP machines (System One REMStar Auto). Patients assigned to active CPAP were treated with auto-titrating CPAP at settings of 4 and 20 cm of water to eliminate obstructive events. The sham CPAP device in our study was designed to entail no risks beyond those with standard CPAP and provide a high level of blinding. The sham CPAP device is an auto-titrating CPAP with an internal flow restrictor and a modified elbow attached to the nasal mask. The elbow modification creates a larger than standard air leak that serves to prevent any chances of CO2 rebreathing and delivers a pressure at the mask in terface of roughly 0.75 to 1 cm H2O. The elbow modification is not noticeable when the device is fully assembled to avoid the possibility of unblinding patients, providers, or study personnel. As the elbow modification could only be used on standard nasal masks, full facemasks and nasal pillows were not an option for patients on either sham or active CPAP. The study sleep medicine technologist and respiratory therapists could make adjustments to the CPAP machine or mask so were likely unblinded to treatment allocation but would not discuss allocation with the patients, providers, or study personnel. The nurses on the rehabilitation unit, including those on night shifts, were educated about the study and the importance of CPAP adherence but remained blinded to treatment assignment. CPAP adherence was assessed by memory card that recorded mask-on time. Other information on the download, such as apnea-hypopnea index or leak, was only available on active CPAP and not assessed by or addressed by investigators in real time. To test the efficacy of blinding, 13 patients in each group completed a blinding survey at hospital discharge asking their belief on whether they had been treated with active or sham CPAP.
After discharge, all enrolled patients were referred to a sleep medicine clinic for a comprehensive evaluation, as is the routine in these units for patients with stroke given their high prevalence of OSA. The results of any in-laboratory PSG or unattended sleep study performed in an American Academy of Sleep Medicine accredited center were collected. For subjects 1–21, a total of 9 subjects did not return for their sleep clinic appointments and did not undergo any sleep testing. Due to the lower than expected rates of formal sleep testing, the study protocol was modified to include unattended sleep testing during rehabilitation. Sleep apnea testing was performed with a portable respiratory monitor (Embletta Gold; Natus, Middleton, WI) for subjects 22–40. In order to keep the study protocol consistent, the portable sleep studies were performed after initiation of treatment, typically the night prior to discharge from the rehabilitation unit, and did not impact study enrollment. The Embletta device monitors airflow by an oronasal thermistor and an oxygen desaturation probe, and respiratory effort by abdominal and thoracic wall recording. Apnea was defined as a reduction of airflow ≥ 90% for ≥ 10 s and hypopnea was defined as a reduction of airflow ≥ 30% for ≥ 10 s with an associated oxygen desaturation ≥ 4%. The apnea-hypopnea index (AHI) was defined as the mean number of apneas and hypopneas per hour of recording time. A diagnosis of sleep apnea was made in patients with an AHI ≥ 5. Oxygen desaturation index (ODI) was defined as the number of oxygen desaturations ≥ 4% events per hour. For patients who had sleep studies during inpatient rehabilitation and again after discharge from the rehabilitation unit, the results of both studies were collected though the initial study was used to calculate the time period from stroke to sleep study and the median AHI. The active CPAP machines, which had the ability to detect changes in flow auto-titration to eliminate obstructive events, were interrogated at the end of the treatment period for evidence suggestive of sleep apnea, defined as the treated download AHI ≥ 5 or mean CPAP pressure ≥ 6 cm H2O.15 This was not possible in sham devices.
Interventions to Improve CPAP Tolerance and Adherence
In this study, we defined tolerance as any continued use of CPAP at night and adherence as mean hours of CPAP use per night in those who were CPAP tolerant after enrollment until discharge from the inpatient rehabilitation unit or for a maximum of 28 days. To improve CPAP tolerance and adherence, respiratory therapists visited patients nightly to address issues arising at night. A sleep technologist met with patients at least twice weekly throughout their rehabilitation stay to reinforce CPAP adherence, monitor safety and adverse events, and make any adjustments to the CPAP mask or machine. Efforts to improve adherence to CPAP included patient education, involvement of bed partner in CPAP education when possible, desensitization of CPAP through brief periods of daytime use, and adjustments of humidity and mask, including addition of a chinstrap. For patients treated with active CPAP, changes to the device included an increase in the CPAP minimum pressure, a decrease in the CPAP maximum pressure, and use of expiratory pressure relief (A-Flex). For patients assigned to sham CPAP, sham adjustments were made to the CPAP devices. The sleep technologist also documented reasons for CPAP intolerance and poor CPAP adherence, including problems with mask fit, CPAP pressure, nasal congestion, skin or eye irritation, sleep disturbance, and anxiety associated with wearing CPAP.
Statistical Analyses
The frequency and distribution of baseline characteristics, demographics, stroke severity, comorbidities, and blinding survey results were compared by randomization assignment with bivariate analyses. We tested for a difference in our main outcome, improvement in total, cognitive and motor FIM score, by randomization assignment using Wilcox-rank sum test. We also assessed differences between the groups in the FIM efficiency, the difference between two FIM measurements divided by the rehabilitation days between those measurements. We performed intention-to-treat analyses of all study patients and then included only those who were CPAP tolerant for the on-treatment analyses. We also evaluated for differences in our secondary outcome, CPAP adherence (minutes/day), and in CPAP experience (days used, number of respiratory and sleep technologist visits) by randomization assignment using Wilcox-rank sum test. We performed post hoc exploratory analysis to evaluate for predictors of study withdrawal (CPAP intolerance) and CPAP adherence. We assessed if baseline stroke severity (either NIHSS > 5 or presence of aphasia or neglect), baseline FIM scores, randomization assignment, mask fit, anxiety with the mask after initial exposure, demographics (age, race, gender, insurance status), baseline sleepiness, and obesity differed in those who did and did not withdraw from the study using bivariate comparison. In those who were CPAP tolerant, multivariable logistic regression was used to evaluate for independent predictors of CPAP adherence, defined as a mean CPAP use ≥ 4 h/night for the study period. In our model, we included as predictors baseline demographics including age, gender, race, BMI, randomization assignment, stroke severity (NIHSS > 5), or baseline FIM.
RESULTS
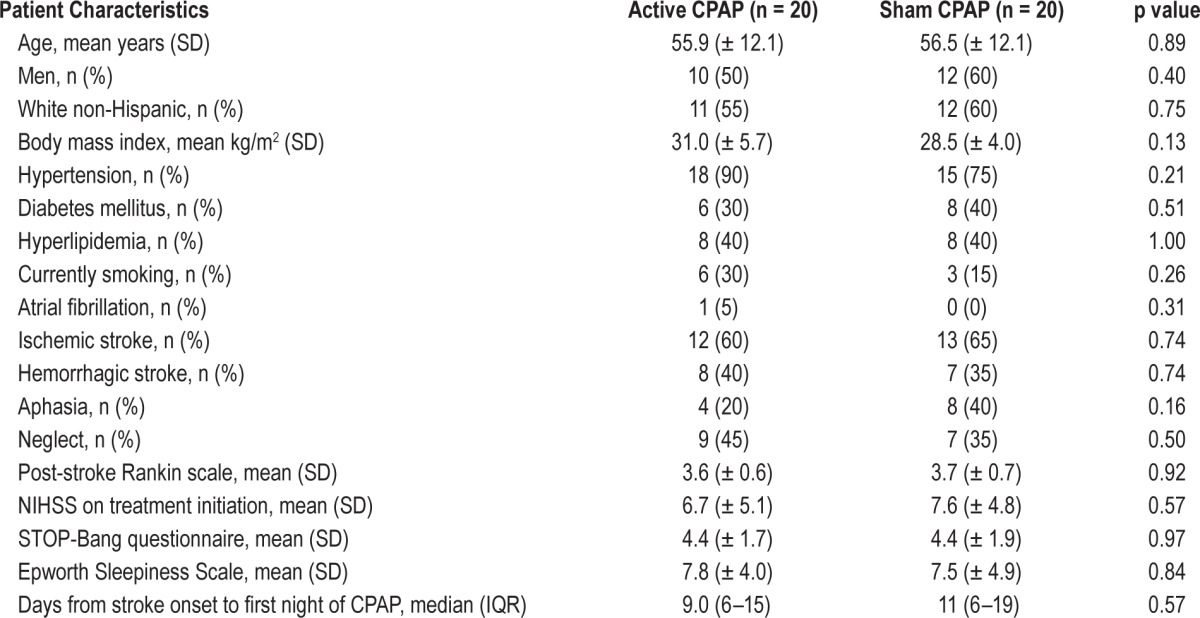
Between June 2013 and November 2014, 125 patients admitted to the inpatient rehabilitation service were assessed for eligibility, and 60 did not meet inclusion criteria (Figure 1). Among the remaining 65 patients, 25 refused to participate and 40 were randomly assigned to either active (n = 20) or sham CPAP (n = 20). Baseline characteristics were similar in the 2 groups (Table 1). Patients were predominantly white, mildly obese, and had hypertension. The majority had ischemic stroke, strokes were of moderate severity (NIHSS median 6.5), and aphasia was infrequent. The majority had markers of sleep apnea (71% with STOP-BANG > 4), and few were sleepy (22% with Epworth Sleepiness Scale > 10). The median number of days from stroke onset to first night of active or sham CPAP was 10 (IQR 6–16). Ten patients (25%) with CPAP intolerance withdrew from the study within a median of 4 days from CPAP initiation: 7 from active and 3 from sham CPAP (p > 0.10). Ten of 13 patients with sham CPAP and 9 of 13 with active CPAP believed they were using active CPAP (p = 0.32).
Figure 1. Patient flow chart.
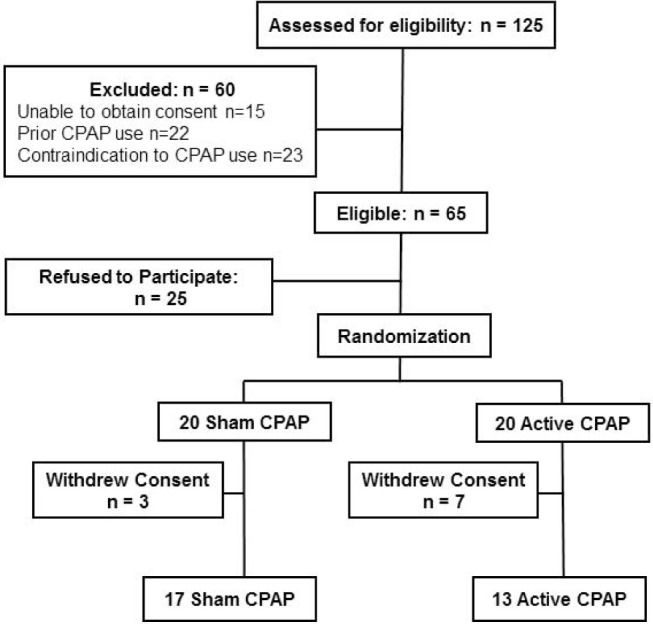
Table 1.
Main characteristics of the study population.
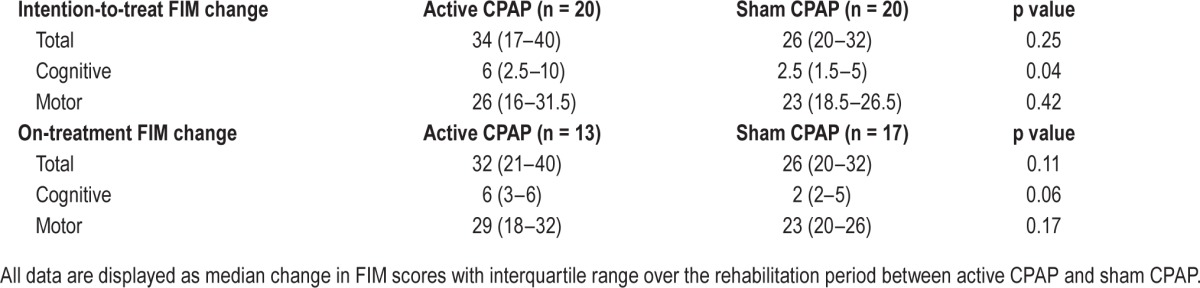
In intention-to-treat analyses (n = 40), the trend was for median change in FIM to favor active CPAP over sham (34 versus 26, p = 0.25), especially for the cognitive component (6 versus 2.5, p = 0.04) but less so for the motor component (26 versus 23, p = 0.42; Table 2). The on-treatment analyses (n = 30) yielded similar results (total FIM: 32 versus 26, p = 0.11; cognitive FIM: 6 versus 2, p = 0.06; motor FIM: 29 versus 23, p = 0.17). The analysis of the on-treatment FIM efficiency revealed a nonsignificant improvement in active CPAP over sham (total FIM efficiency: 2.1 versus 1.8, p = 0.98; cognitive FIM efficiency: 0.38 versus 0.29, p = 0.40; motor FIM efficiency: 1.7 versus 1.6, p = 0.80).
Table 2.
Change in Functional Independence Measure (FIM) during study.
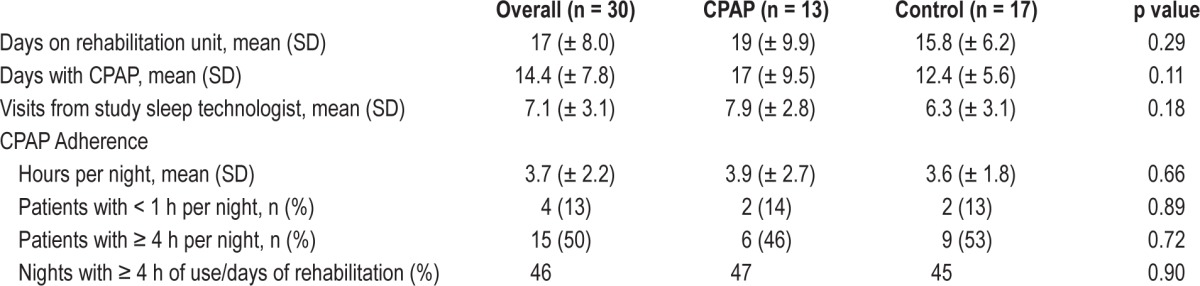
Among those who completed the trial (n = 30, 75% of those enrolled), CPAP was worn for a mean of 14 nights and for a mean of 3.7 h/night, with 50% using CPAP for an average of ≥ 4 h/night. CPAP adherence, defined as either average use or by proportion of nights with > 4 h of use, did not differ by treatment assignment (Table 3). Most patients who wore CPAP for < 4 h during the first 3 nights continued to do so over the entire study period (13/15, p < 0.001).
Table 3.
Treatment characteristics among patients who were CPAP tolerant and completed the trial.
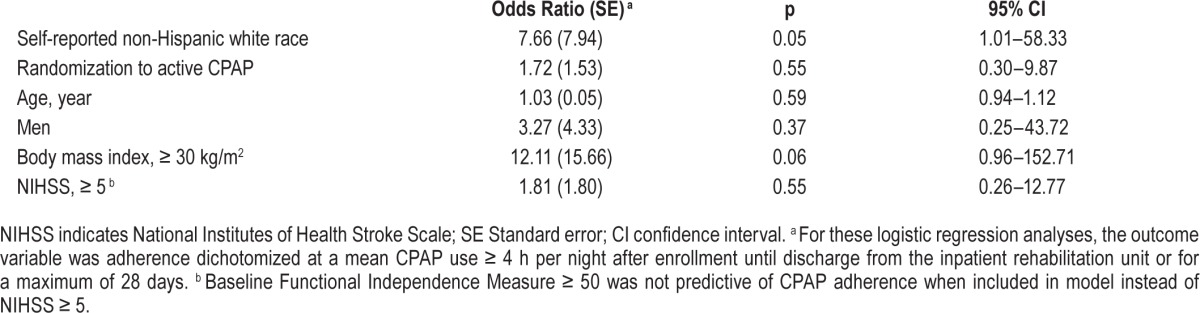
In post hoc analysis, self-reported white race compared with others was associated with better CPAP adherence (Table 4), whereas baseline FIM, stroke severity, and randomization assignment were not predictors of CPAP adherence. There was no statistical difference in baseline stroke scale, FIM score, Epworth Sleepiness Scale, demographic features, or randomization assignment in those who did and did not withdraw from the study. Compared to those without, patients with CPAP intolerance were more likely to complain of anxiety while wearing the CPAP mask (OR = 38.3, 95% CI 2.8–524.4, p = 0.006).
Table 4.
Factors independently associated with CPAP adherence among the 30 patients who completed the trial.
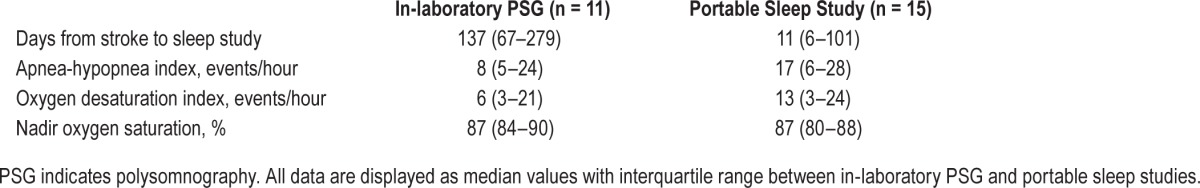
Although all study patients were referred to a sleep medicine clinic for formal evaluation of sleep apnea after discharge from the rehabilitation unit, only 18 of the 40 patients underwent testing for sleep apnea as part of routine clinical care after 14 months from completion of the study: 4 with portable unattended sleep studies and 14 with in-laboratory PSG. As part of the amended research protocol, 12 patients had portable sleep studies during the rehabilitation stay whereas 6 patients did not, either due to withdrawal from the trial prior to the sleep study (n = 4) or a non-diagnostic sleep study (n = 2). Four patients had sleep studies as part of the study procedures during rehabilitation and again, either during rehabilitation or after discharge, as part of routine clinical care. Overall, among the 26 patients who had any testing for sleep apnea (Table 5), the median time period from stroke onset to the first sleep study was 61 days (IQR 6–137), and the median AHI was 16 (IQR 5–26), with an AHI ≥ 5 in 23/26 patients (88%). There was no significant difference in stroke severity or demographic factors between those who did and did not have sleep studies. Among the 13 subjects who were assigned to active CPAP and completed the study, 12/13 patients (92%) had evidence of sleep apnea on download of data from the auto-titrating CPAP machine with either a treated AHI ≥ 5 or mean CPAP pressure ≥ 6 cm H2O.
Table 5.
Testing for sleep apnea among 26 patients who underwent portable unattended sleep studies or in-laboratory polysomnography.
DISCUSSION
This pilot trial demonstrates the feasibility of randomizing stroke patients to active and sham CPAP during inpatient rehabilitation. Among patients meeting eligibility criteria, 62% agreed to enroll, and 75% of those enrolled were retained during their rehabilitation stay. Patients were randomized without testing for OSA and were adequately blinded to treatment assignment. This pilot trial showed trends towards better FIM score improvement with active compared to sham CPAP, particularly in the cognitive component of the FIM, despite small numbers and lower than ideal adherence.
The improvement in cognitive functional recovery, but not motor recovery, with active compared to sham CPAP is worth noting. On average, the Epworth Sleepiness Scale at baseline was normal, similar to prior studies,11,16 suggesting that stroke patients with OSA do not experience the same degree of daytime sleepiness as non-stroke patients with OSA. Thus, the cognitive improvement is unlikely to be solely related to an improvement in sleepiness. A recent case-control study among stroke patients admitted to a rehabilitation unit with and without OSA found an association with OSA and lower cognitive status, based on comprehensive neuropsychological testing, as well as overall functional status.17 Yet, in a randomized trial of stroke patients diagnosed with moderately severe OSA and treated during intensive inpatient rehabilitation, CPAP had only marginal beneficial effects on cognitive testing but achieved significant effects on motor and overall functional outcome, as measured by the FIM among other tests.11 In that unblinded clinical trial, stroke patients treated with CPAP showed greater recovery one month after randomization than those not treated with CPAP, mostly in motor-related impairments.11 The relative improvement in cognitive outcome with active CPAP in our trial may have been related to multiple factors, including the initiation of CPAP without diagnostic testing for OSA or the use of sham CPAP, with the presumed elimination of a placebo effect.
Poor enrollment and retention for randomized trials in patients with stroke and OSA have led to questions about the feasibility of sham-controlled CPAP trials among this population.12,18 The enrollment rate of 62% in our study compared favorably to 40% of eligible patients in another sham-controlled CPAP trial of acute stroke patients12 and among other randomized CPAP trials of patients with stroke, where only 45% of patients without exclusionary criteria agreed to participate.18 The higher enrollment was likely related to enrollment of stroke patients participating in inpatient rehabilitation, who were already selected as motivated and with a high potential for neurologic recovery. One of the greatest challenges encountered in this trial, similar to prior CPAP trials among stroke patients,18 was CPAP intolerance. Ten of 40 patients (25%) in our study could not tolerate CPAP, commonly blaming anxiety in wearing the CPAP mask. The retention rate of 65% of patients assigned to active CPAP was comparable to three prior randomized trials of CPAP among stroke patients, where rates in the treatment group were 72%,19 71%,15 and 60%.12 In a single-arm study of CPAP during rehabilitation, 70% of stroke patients accepted CPAP after titration and a few nights on the rehabilitation ward.20 Yet the retention rate in our study was considerably lower than another trial during inpatient rehabilitation where stroke patients with moderately severe OSA were randomized to CPAP or standard physiotherapy. In this study, 88% of patients assigned to CPAP continued treatment over the study period.11 Thus 88% or higher is achievable and should be set for the goal in all future trials. The lower retention rate in our study compared to this prior trial may be related to our inclusion of patients with only mild or no OSA or possibly related to the lack of nursing involvement in administering CPAP to study patients, given our concerns about potential unblinding. CPAP intolerance was established within the first few days of treatment, as evidenced by early study withdrawal and adherence patterns. A short period of CPAP use among stroke patients with intensive support during rehabilitation to identify those patients who simply do not tolerate CPAP prior to continued treatment could result in improved retention in future CPAP trials and in a greater chance of showing CPAP to be effective in improving recovery from stroke.
The efficacy of CPAP to improve recovery from stroke cannot be fully assessed unless CPAP adherence is high. Use of CPAP for ≥ 4 hours per night was 50% in our study. Adherence to CPAP has been a challenge in numerous prior studies of CPAP among stroke patients. Among the seven randomized trials, 3 studies were limited by low or poor CPAP use.12,13,21 Two studies that were not limited by problems with retention demonstrated CPAP adherence with a median of 4.2 hours per night over one week22 and with a mean of 4.96 hours per night over a 4-week period,11 although CPAP use outside of the supportive hospital environment was not assessed in these studies. Adherence rates in our study were not influenced by treatment assignment, baseline FIM or stroke severity, and patients assigned to sham CPAP were adequately blinded to treatment allocation. The studies with CPAP initiation in the inpatient setting that have shown higher CPAP adherence have demonstrated greater improvements in stroke symptom recovery11,22 while those limited by poor adherence have been underpowered to show any significant CPAP benefit.12,13 The reasons for poor CPAP adherence in our study and other research studies remains unclear, and the definition of optimal CPAP use may be outcome specific23 with the optimal number of hours of CPAP use necessary for improved neurologic or functional outcome after stroke remaining unknown. A patient's early experiences with CPAP have been shown to predict adherence among the general OSA population24 and among patients with prior cardiovascular disease.25 Our study demonstrated a similar finding among stroke patients where the first 3 days of treatment established adherence patterns for the rest of the rehabilitation period. High rates of CPAP adherence among stroke patients, ranging from 62% to 88%, have also been demonstrated when study personnel regularly encouraged treatment adherence15,20 or when nurses were trained to administer CPAP treatment.11 The lower adherence rates in our study compared to prior rehabilitation-based studies may be explained by absence of nurses administering CPAP. Alternatively, patient-specific factors, such as poor sleep quality during stroke rehabilitation or anxiety related to wearing a CPAP mask, may have led to poor CPAP adherence. Self-reported white race compared to others was also associated with better adherence to CPAP in our study. Similar findings have been shown in other studies of at-risk populations26 and warrant further research.
The study's main limitations are the small sample size, poor adherence to treatment, and non-standardized treatment period. This feasibility trial was not powered to detect FIM differences. Thus, as expected we were not able to show a clear functional benefit of CPAP during rehabilitation after stroke. To detect a significant difference in the FIM between the groups with 80% accuracy would require a sample size of about 140 subjects in each group. Nonetheless, the degree of improvement in the cognitive component of the FIM, which was significant in the intention-to-treat analysis though not in the on-treatment analysis, did meet the cutoff for a clinically important difference for patients recovering from stroke; a cognitive FIM change score of 3 has been defined as the minimal clinically important difference.27 The follow-up FIM occurred at discharge rather than at a fixed number of days from randomization and the improvement seen in the cognitive FIM was not significant when the FIM efficiency was evaluated instead of the change in FIM. Finally, our low adherence and 25% withdrawal rate further limited our ability to evaluate differences in outcome between sham and active CPAP.
We also enrolled patients without verifying the presence or severity of OSA before initiating treatment, possibly reducing treatment benefit if no or only mild OSA were present. Our approach in this sham CPAP feasibility study seeks to shift the paradigm of post-stroke OSA testing, allowing for a more rapid initiation of therapy at a critical time of stroke recovery during the brief inpatient rehabilitation period. The logistic challenges and the subsequent delays inherent to OSA testing and interpretation would have introduced a potential barrier to the early use of CPAP in this trial, especially given the short treatment period of approximately 2 weeks. Others have also sought non-standardized ways of diagnosing OSA to provide earlier treatment without diagnostic sleep testing, including the use of flow resistance detected by auto-titrating CPAP machines.15 Current guidelines recommend that symptomatic patients with negative sleep testing on unattended portable monitors have an in-laboratory PSG to exclude the possibility of a false negative study, which may be as high as 17% among the general population,28 and presumably higher among patients with stroke undergoing testing in the hospital. Such an approach presents a challenge among patients with stroke as most are asymptomatic, as noted by the low average score on the Epworth Sleepiness Scale in this study and others,4,11,12,19 and repeating an in-laboratory PSG in the acute stroke period for patients with negative portable testing would lead to a considerable delay in treatment. Given the potential short and long-term benefits and the low risk of the intervention in a closely monitored environment, we felt that the intervention, even among those who did not have OSA evident on a single screening test, was reasonable. We confirmed the presence of possible OSA among the majority of enrolled patients through subsequent sleep studies and auto-titrating CPAP downloads. We also noted a higher AHI among patients with portable sleep studies than those with in-laboratory PSG testing, likely due to the delay in PSG testing and an improvement in underlying disease in the subacute stroke period.4
The burden of the disability following stroke remains unacceptably high, and effective treatments to improve function after stroke are limited. CPAP may provide a noninvasive, relatively low-cost intervention to decrease disability for this common and disabling disease, but access to sleep diagnostic services often limits treatment options. Our findings suggest that a sham-controlled CPAP trial among stroke patients is feasible from the standpoint of recruitment, randomization to active or sham CPAP without diagnostic testing, and adequate blinding. Our study also supports the approach of other CPAP trials that limited enrollment to stroke patients undergoing inpatient rehabilitation. Stroke rehabilitation patients were willing and able to participate in a sham-controlled CPAP trial without first obtaining a diagnostic screening test for OSA. The criteria for inpatient rehabilitation for stroke are well defined and uniformly applied in the United States. Patients must be motivated and have the potential for recovery, making them ideal candidates for early treatment with CPAP after stroke. Sham CPAP had similar tolerance compared to active CPAP and was a credible placebo treatment, allowing for successful blinding of patients and providers to treatment assignment. Our study did not demonstrate that our multiple interventions to improve CPAP tolerance conferred a benefit compared to prior trials in either improved CPAP adherence12,13,21 or improved study retention.12,15,19 These twin issues of CPAP adherence and retention will need to be successfully addressed for a definitive trail to be possible. Despite the limitations of small numbers and poor adherence, a trend towards a benefit of CPAP on recovery was evident. Given these encouraging findings in stroke recovery and the demonstration of feasibility of a sham-controlled CPAP trial, further trials of CPAP during stroke rehabilitation are warranted. The issues of early CPAP intolerance and long-term CPAP adherence, especially once outside of the supportive environment of the rehabilitation unit, will need to be addressed in any larger CPAP trial in this study population. Specifically, future larger trials could include a short run-in period to identify CPAP intolerance prior to continued CPAP treatment, a heightened focus on CPAP adherence through a structured nurse-based adherence protocol and patient questionnaires to identify early predictors of CPAP non-adherence. Interventions during inpatient rehabilitation to improve CPAP tolerance and adherence could include equipment adjustments with patient-centered mask selection,29 development of coping mechanisms to tolerate better CPAP treatment30 and counseling to improve patients' motivation to adjust and adapt to CPAP.31,32 Such factors to maximize CPAP tolerance and adherence are likely key to realize the potential short-term and long-term benefits of CPAP on stroke recovery, recurrence, and mortality.
DISCLOSURE STATEMENT
Philips Respironics (Murrysville, PA) provided all CPAP devices, placebo devices and CPAP masks but no other support or intellectual input for the trial. A pilot grant from the University of Washington Institute of Translational Health Sciences (NIH UL1TR000423) supported this work. Dr. Becker is an outcomes adjudicator for Merck and a consultant for Biogen Idec. The other authors have indicated no financial conflicts of interest. Abstract presented at the Associated Professional Sleep Societies, June 9, 2015, Seattle, WA. The work was performed at the University of Washington, Seattle, WA.
ABBREVIATIONS
- BMI
body mass index
- CPAP
continuous positive airway pressure
- FIM
Functional Independence Measure
- IQR
interquartile range
- NIHSS
National Institutes of Health Stroke Scale
- OR
odds ratio
- OSA
obstructive sleep apnea
- PSG
polysomnography
- SD
standard deviation
REFERENCES
- 1.Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–236. doi: 10.1161/STR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 2.Davis AP, Billings ME, Longstreth WT, Jr., Khot SP. Early diagnosis and treatment of obstructive sleep apnea after stroke: are we neglecting a modifiable stroke risk factor? Neurol Clin Pract. 2013;3:192–201. doi: 10.1212/CPJ.0b013e318296f274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson KG, Johnson DC. Frequency of sleep apnea in stroke and TIA patients: a meta-analysis. J Clin Sleep Med. 2010;6:131–7. [PMC free article] [PubMed] [Google Scholar]
- 4.Bassetti CL, Milanova M, Gugger M. Sleep-disordered breathing and acute ischemic stroke: diagnosis, risk factors, treatment, evolution, and long-term clinical outcome. Stroke. 2006;37:967–72. doi: 10.1161/01.STR.0000208215.49243.c3. [DOI] [PubMed] [Google Scholar]
- 5.Cherkassky T, Oksenberg A, Froom P, Ring H. Sleep-related breathing disorders and rehabilitation outcome of stroke patients: a prospective study. Am J Phys Med Rehabil. 2003;82:452–5. [PubMed] [Google Scholar]
- 6.Kaneko Y, Hajek VE, Zivanovic V, Raboud J, Bradley TD. Relationship of sleep apnea to functional capacity and length of hospitalization following stroke. Sleep. 2003;26:293–7. doi: 10.1093/sleep/26.3.293. [DOI] [PubMed] [Google Scholar]
- 7.Yan-fang S, Yu-ping W. Sleep-disordered breathing: impact on functional outcome of ischemic stroke patients. Sleep Med. 2009;10:717–9. doi: 10.1016/j.sleep.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Good DC, Henkle JQ, Gelber D, Welsh J, Verhulst S. Sleep-disordered breathing and poor functional outcome after stroke. Stroke. 1996;27:252–9. doi: 10.1161/01.str.27.2.252. [DOI] [PubMed] [Google Scholar]
- 9.Martinez-Garcia MA, Soler-Cataluna JJ, Ejarque-Martinez L, et al. Continuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: a 5-year follow-up study. Am J Respir Crit Care Med. 2009;180:36–41. doi: 10.1164/rccm.200808-1341OC. [DOI] [PubMed] [Google Scholar]
- 10.Skolarus LE, Lisabeth LD, Morgenstern LB, Burgin W, Brown DL. Sleep apnea risk among Mexican American and non-Hispanic white stroke survivors. Stroke. 2012;43:1143–5. doi: 10.1161/STROKEAHA.111.638387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryan CM, Bayley M, Green R, Murray BJ, Bradley TD. Influence of continuous positive airway pressure on outcomes of rehabilitation in stroke patients with obstructive sleep apnea. Stroke. 2011;42:1062–7. doi: 10.1161/STROKEAHA.110.597468. [DOI] [PubMed] [Google Scholar]
- 12.Brown DL, Chervin RD, Kalbfleisch JD, et al. Sleep apnea treatment after stroke (SATS) trial: is it feasible? J Stroke Cerebrovasc Dis. 2013;22:1216–24. doi: 10.1016/j.jstrokecerebrovasdis.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsu CY, Vennelle M, Li HY, Engleman HM, Dennis MS, Douglas NJ. Sleep-disordered breathing after stroke: a randomised controlled trial of continuous positive airway pressure. J Neurol Neurosurg Psychiatry. 2006;77:1143–9. doi: 10.1136/jnnp.2005.086686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dyken ME, Im KB. Obstructive sleep apnea and stroke. Chest. 2009;136:1668–77. doi: 10.1378/chest.08-1512. [DOI] [PubMed] [Google Scholar]
- 15.Bravata DM, Concato J, Fried T, et al. Continuous positive airway pressure: evaluation of a novel therapy for patients with acute ischemic stroke. Sleep. 2011;34:1271–7. doi: 10.5665/SLEEP.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172:1447–51. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aaronson JA, van Bennekom CA, Hofman WF, et al. Obstructive sleep apnea is related to impaired cognitive and functional status after stroke. Sleep. 2015;38:1431–7. doi: 10.5665/sleep.4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomfohr LM, Hemmen T, Natarajan L, et al. Continuous positive airway pressure for treatment of obstructive sleep apnea in stroke survivors: what do we really know? Stroke. 2012;43:3118–23. doi: 10.1161/STROKEAHA.112.666248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parra O, Sanchez-Armengol A, Bonnin M, et al. Early treatment of obstructive apnoea and stroke outcome: a randomised controlled trial. Eur Respir J. 2011;37:1128–36. doi: 10.1183/09031936.00034410. [DOI] [PubMed] [Google Scholar]
- 20.Wessendorf TE, Wang YM, Thilmann AF, Sorgenfrei U, Konietzko N, Teschler H. Treatment of obstructive sleep apnoea with nasal continuous positive airway pressure in stroke. Eur Respir J. 2001;18:623–9. doi: 10.1183/09031936.01.00057201. [DOI] [PubMed] [Google Scholar]
- 21.Sandberg O, Franklin KA, Bucht G, Eriksson S, Gustafson Y. Nasal continuous positive airway pressure in stroke patients with sleep apnoea: a randomized treatment study. Eur Respir J. 2001;18:630–4. doi: 10.1183/09031936.01.00070301. [DOI] [PubMed] [Google Scholar]
- 22.Minnerup J, Ritter MA, Wersching H, et al. Continuous positive airway pressure ventilation for acute ischemic stroke: a randomized feasibility study. Stroke. 2012;43:1137–9. doi: 10.1161/STROKEAHA.111.637611. [DOI] [PubMed] [Google Scholar]
- 23.Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30:711–9. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis KE, Seale L, Bartle IE, Watkins AJ, Ebden P. Early predictors of CPAP use for the treatment of obstructive sleep apnea. Sleep. 2004;27:134–8. doi: 10.1093/sleep/27.1.134. [DOI] [PubMed] [Google Scholar]
- 25.Chai-Coetzer CL, Luo YM, Antic NA, et al. Predictors of long-term adherence to continuous positive airway pressure therapy in patients with obstructive sleep apnea and cardiovascular disease in the SAVE study. Sleep. 2013;36:1929–37. doi: 10.5665/sleep.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Billings ME, Auckley D, Benca R, et al. Race and residential socioeconomics as predictors of CPAP adherence. Sleep. 2011;34:1653–8. doi: 10.5665/sleep.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beninato M, Gill-Body KM, Salles S, Stark PC, Black-Schaffer RM, Stein J. Determination of the minimal clinically important difference in the FIM instrument in patients with stroke. Arch Phys Med Rehabil. 2006;87:32–9. doi: 10.1016/j.apmr.2005.08.130. [DOI] [PubMed] [Google Scholar]
- 28.Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3:737–47. [PMC free article] [PubMed] [Google Scholar]
- 29.Wickwire EM, Lettieri CJ, Cairns AA, Collop NA. Maximizing positive airway pressure adherence in adults: a common-sense approach. Chest. 2013;144:680–93. doi: 10.1378/chest.12-2681. [DOI] [PubMed] [Google Scholar]
- 30.Stepnowsky CJ, Jr., Bardwell WA, Moore PJ, Ancoli-Israel S, Dimsdale JE. Psychologic correlates of compliance with continuous positive airway pressure. Sleep. 2002;25:758–62. doi: 10.1093/sleep/25.7.758. [DOI] [PubMed] [Google Scholar]
- 31.Aloia MS, Arnedt JT, Strand M, Millman RP, Borrelli B. Motivational enhancement to improve adherence to positive airway pressure in patients with obstructive sleep apnea: a randomized controlled trial. Sleep. 2013;36:1655–62. doi: 10.5665/sleep.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lai AY, Fong DY, Lam JC, Weaver TE, Ip MS. The efficacy of a brief motivational enhancement education program on CPAP adherence in OSA: a randomized controlled trial. Chest. 2014;146:600–10. doi: 10.1378/chest.13-2228. [DOI] [PubMed] [Google Scholar]


