Abstract
Study Objectives:
Polysomnographic investigation of sleep architecture in children presenting with pediatric acute-onset neuropsychiatric syndrome (PANS).
Methods:
Fifteen consecutive subjects meeting criteria for PANS (mean age = 7.2 y; range 3–10 y) underwent single-night full polysomnography (PSG) read by a pediatric neurologist.
Results:
Thirteen of 15 subjects (87%) had abnormalities detected with PSG. Twelve of 15 had evidence of rapid eye movement (REM) sleep motor disinhibition, as characterized by excessive movement, laughing, hand stereotypies, moaning, or the continuation of periodic limb movements during sleep (PLMS) into REM sleep.
Conclusions:
This study shows various forms of REM sleep motor disinhibition present in a population of children with PANS.
Citation:
Gaughan T, Buckley A, Hommer R, Grant P; Williams K, Leckman JF, Swedo SE. Rapid eye movement sleep abnormalities in children with pediatric acute-onset neuropsychiatric syndrome (PANS). J Clin Sleep Med 2016;12(7):1027–1032.
Keywords: obsessive compulsive disorder, PANS, polysomnography, REM sleep behavior disorder
INTRODUCTION
Schenck et al.1 were the first to suggest that rapid eye movement (REM) sleep neurobehavioral disorders were a separate category of parasomnia in a 1986 case series of four males aged 67–72 y. Polysomnography (PSG) of these four subjects showed a loss of chin atonia and high limb-twitch activity among other REM sleep pathology, whereas videography showed various REM sleep behaviors including punching, kicking, and dream enactment.1 Since then, REM sleep behavior disorder (RBD) has been recognized across a wide range of ages, sometimes decades prior to the onset of other neurobehavioral changes and often heralding serious neurodegenerative conditions affecting synuclein.2 A recent retrospective study of patients showing REM sleep without atonia on PSG found a majority (73%) of patients had idiopathic RBD, defined as RBD in the absence of known neurological or sleep disorders.3 Recent reports suggest that clinical and subclinical forms of RBD occur in children and adolescents.4 Although pediatric RBD is considered to be a rare occurrence, its prevalence may be underestimated due to limited awareness, overlap with other parasomnias, and the requirement of nocturnal PSG for diagnosis.4,5 Additionally, it is unclear whether or not the adult clinical definitions should pertain to pediatric presentations due to a current lack of neuropathologic studies of childhood RBD.4 Differentiating RBD from other parasomnias in the younger population is significant clinically because of the potential to inform on neuropsychiatric state and therefore guide treatment choices, as evidenced in conditions such as narcolepsy where 60% of patients will have RBD.5,6
BRIEF SUMMARY
Current Knowledge/Study Rationale: REM sleep behavior disorder (RBD) has been demonstrated across all ages, but has been reported to occur only rarely in childhood. Among adults, RBD is associated with neurodegenerative conditions; its significance in pediatric patient populations is unknown. Demonstrating abnormalities of sleep architecture in children with PANS may provide additional insights into the etiopathogenesis of the neuropsychiatric syndrome.
Study Impact: This case series reports on sleep-related motor disturbances among children with PANS, particularly in the REM state. Pediatric RBD is likely not generated by the same mechanisms that often predict the onset of a neurodegenerative disorder among adults, and may instead be a useful marker of transient central nervous system disturbance in select cohorts.
In adult psychiatric populations, RBD has been associated with the use of psychotropic medications and particularly with serotonin reuptake-blocking antidepressants.3,7 However, a recent study suggests that antidepressants cannot fully explain RBD symptomatology in their psychiatric patients, as matched psychiatric controls with comparable antidepressant courses did not show REM behaviors upon PSG evaluation. The psychiatric-RBD (pRBD) group in this study also differed from the typical idiopathic (without psychiatric diagnoses) RBD group, with pRBD patients tending to be younger and with more females than males.7 A review of PSG records from an academic sleep center found similar shifts in RBD demographics, additionally finding a high rate of autoimmune disease in female patients (20%).3 This corroborates earlier findings of RBD and lack of atonia in REM sleep among patients with a variety of autoimmune disorders, including among others, voltage-gated potassium channel antibody-associated limbic encephalitis (VGCK-LE), Guillain-Barré syndrome, and anti-N-methyl D-aspartate receptor encephalitis.8–10 A recent case presentation of two female patients with breast cancer and paraneoplastic cerebellar disease also presenting with RBD showed clinical resolution and restoration of atonia during REM sleep after immunotherapy with intravenous immunoglobulin.11 These studies hint toward a potential autoimmune target for intervention in RBD.
PSG in specific conditions such as Tourette syndrome and posttraumatic stress disorder in adults in both populations have found varying REM abnormalities and REM and nonrapid eye movement (NREM) motor aberrations, but little has been shown with regard to REM behavior disorder specifically.12–14 There is a similar dearth of pediatric PSG studies in neuropsychiatric disorders, particularly with regard to documenting motor-related NREM/REM abnormalities and RBD symptoms. However, the literature suggests various REM abnormalities are present across many disorders. In a review of sleep in children and adolescents with obsessive-compulsive disorder (OCD), Reynolds et al. found only one PSG study of children with OCD.15 Nine adolescents with OCD showed significantly decreased total REM sleep time and latency compared to sex-matched controls.16 Pediatric depression mirrors some of the REM disturbances seen in the more expansive adult literature, albeit with less consistent findings. The most frequent sleep marker in adult populations, shortened REM latency, was found in less than one-half (11 of 23) of the studies in children and adolescents.17 A more recent meta-analysis of early-onset depression found that among children and adolescents with major depressive disorder, 23% had shorter REM latency and 37% has increased REM density compared to healthy controls.18 Objective studies of pediatric sleep in Tourette syndrome are lacking, with a single PSG study failing to note differences in REM sleep variables. In that small study, patients with Tourette syndrome and healthy controls had similar nocturnal movements.19 Sleep in tic disorders has been studied alongside attention deficit hyperactivity disorder (ADHD) because of their frequent comorbidity. Children with ADHD (both with and without comorbid tic disorder) had shorter REM sleep latency and an increased REM sleep percentage than those with tic disorder alone, including shorter REM sleep latency and an increased REM sleep percentage. In the ADHD/tic disorder (TD) study, children with tic disorders showed significantly more microarousals in REM sleep than those without TD.20 The ADHD-only literature, however, shows a lack of significant differences in objective sleep measurements for children with ADHD versus controls, with a meta-analysis of 16 studies showing no difference in REM variables21 and a more recent study showing no differences on any sleep variables.22 In total, objective studies of pediatric sleep in neuropsychiatric disorders are scarce and the absence of PSG is particularly notable. Furthermore, no studies were found that prospectively evaluated pediatric subjects with specific disorders for REM sleep motor abnormalities.
Pediatric acute-onset neuropsychiatric syndrome (PANS) was defined in 2010 by the unusually abrupt onset of OCD or eating restrictions, accompanied by comorbid symptoms in at least two of seven categories: anxiety; emotional lability or depression; irritability, aggression and/or severe oppositional behaviors; behavioral (developmental) regression; deterioration in school performance; sensory or motor abnormalities; and somatic signs and symptoms, including sleep disturbances, enuresis, urinary frequency, mydriasis, and others.23 Sleep complaints are reported to affect as many as 84% of children with PANS, and include initial, middle, and/or terminal insomnia and various parasomnias.23,24 However, there is a complete lack of data from polysomnographic studies in this population.24 With this case series, we describe observed polysomnographic abnormalities that may contribute to the symptomatology or illuminate underlying mechanisms involved in the syndrome's etiologies.
METHODS
Subjects
The consecutive case series includes 15 children, (7 males and 8 females), aged 3–10 y with a mean age of 7.2 y presenting to the National Institutes of Health (NIH) Clinical Center between May 2013 and April 2014. All children had acute onset or exacerbation of neuropsychiatric symptoms and met diagnostic criteria for PANS.23 Eleven children also met criteria for pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection (PANDAS), due to evidence of a preceding group A streptococcal infection.25,26 Duration of illness at the time of sleep study ranged from 1–25 mo with a mean duration of 6 mo. At the time of PSG, 14 of 15 children were taking antibiotics, but none was taking another medication. Ten patients were participants in a natural history investigation of childhood neuropsychiatric disorders (NCT01778504). Seven of the 15 were participants in a controlled trial of intravenous immunoglobulin, with three receiving it as part of that investigation (NCT01281969). Two subjects were participants in both studies. Both investigations were approved by the Combined Neuroscience (CNS) Institutional Review Board and all parents of all subjects gave written consent for study participation (protocols 11-M-0058 and 13-M-0028).
Recordings
All children completed a single-night full PSG with extended array and audiovisual recording. Outputs were read by a pediatric neurologist with a specialization in sleep disorders (A.B.). Parasomnias, periodic limb movements (PLM), and other PSG abnormalities were defined according to the American Academy of Sleep Medicine (AASM) criteria.27 REM sleep behavior disorder (RBD) was defined by the AASM scoring rules or by the continuation of PLM into REM sleep. REM sleep motor disinhibition was used to refer to the presence of moaning, laughing, excessive aperiodic limb movements, or stereotypies of the hands and fingers clearly evident in REM sleep.
RESULTS
Summary of Cases
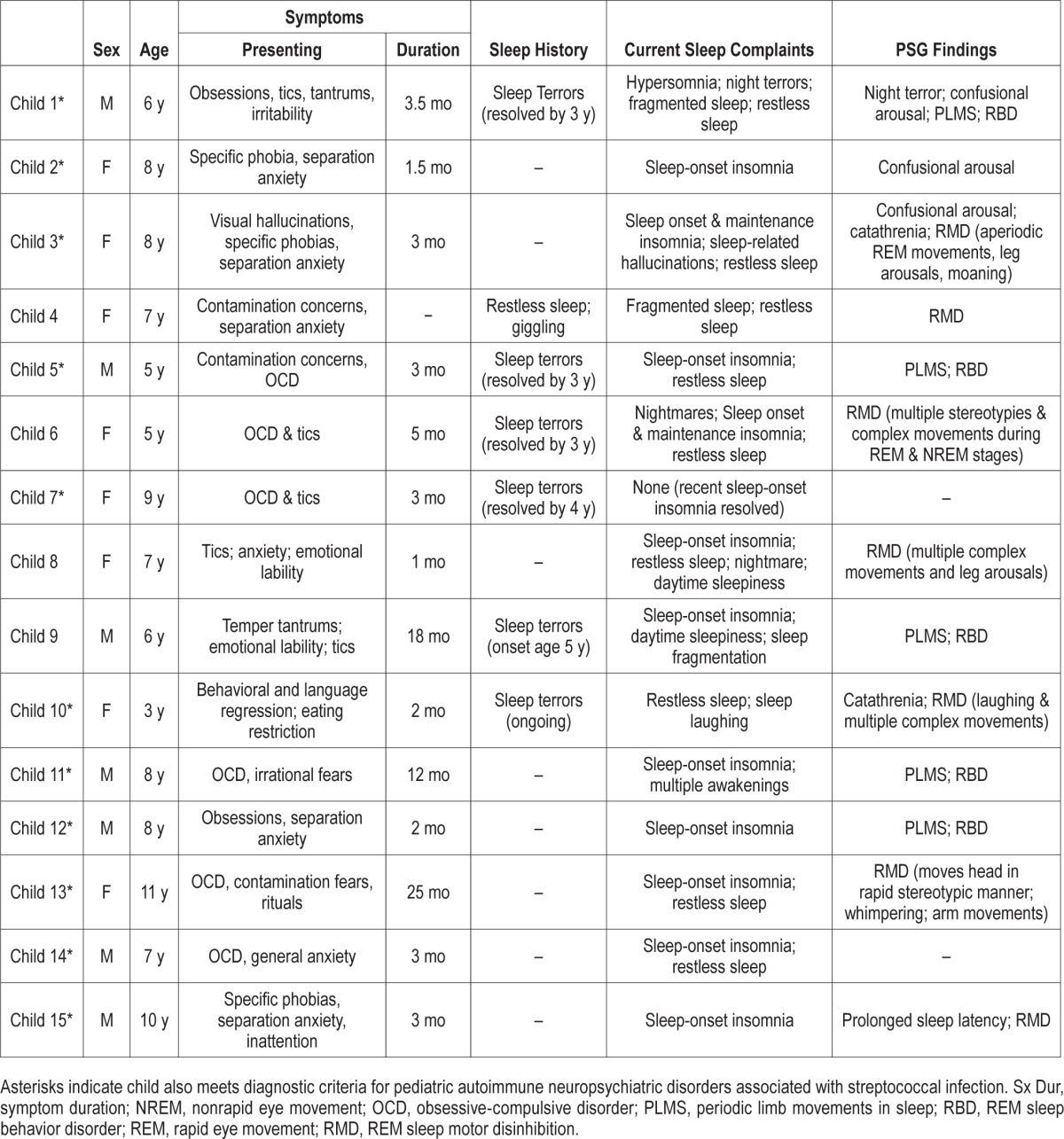
Fourteen of 15 patients had at least one sleep complaint at the time of PSG, with 11 patients complaining of sleep-onset insomnia and 9 of restless sleep. One child (#7) had no current sleep complaints, but had experienced sleep-onset insomnia at the time of acute illness; her PSG was one of only two without abnormalities. The other child with a normal PSG was a 7-y-old boy with current complaints of sleep-onset insomnia and restless sleep. Thirteen patients (87%) had at least one PSG finding. PSG identified three patients with confusional arousals and eight patients with REM motor disinhibition; four patients met criteria for RBD as currently defined by AASM criteria.27 Five patients had a clinically significant periodic leg movement index and one patient presented with catathrenia. One patient was noted to have night terrors and one patient showed prolonged sleep latency on examination. Table 1 contains clinical presentations and PSG results for all 15 patients and three illustrative case histories are included in the next paragraphs.
Table 1.
Clinical presentations and polysomnogram findings for 15 children with pediatric acute-onset neuropsychiatric syndrome.
Patient 5
A 5-y-old boy presents with acute-onset urinary urgency followed by severe and impairing neuropsychiatric symptoms. The child's prior medical/developmental history is notable only for undescended testes and orchiopexy, and parental report of night terrors between the ages of 18 – 42 mo. His current illness was characterized by contamination obsessions, handwashing and cleaning compulsions, repeating rituals, generalized and separation anxiety, emotional lability, and sleep disturbances, specifically, sleep-onset insomnia and restless sleep with frequent awakenings. Family history is significant for hypnopompic hallucinations and sleep paralysis, as well as possible slow wave sleep (SWS) parasomnias in the patient's father. Both the patient's father and his younger sister experienced night terrors as toddlers.
Outside medical evaluation revealed no organic etiology for urinary disturbance, but 6 w after symptom onset, a throat culture requested by the patient's parents to assess for possible PANDAS revealed active strep infection. A 10-day course of antibiotic treatment was prescribed, followed by ongoing antibiotics at a prophylactic dose.
At the time of NIH evaluation, approximately 3 mo after symptom onset, obsessive-compulsive symptoms remained prominent while sleep was noted to be somewhat improved. Physical examination was notable for pectus excavatum. No tics or movement disturbance were evident. Brain magnetic resonance imaging (MRI) without contrast revealed very faint diffuse T2/fluid-attenuated inversion recovery signal hyperintensity in the periatrial white matter bilaterally, with unknown clinical significance. Routine electroencephalography (EEG) revealed frequent moderate amplitude posterior central spike and wave epileptiform abnormalities in the left parietal region and lower amplitude spike and wave discharges in the left central and temporal regions. Epileptiform activity was noted during awake, drowsy, and sleep states, but no seizures were recorded.
PSG revealed a total sleep time of 556 min during a recording time of 590.4 min (sleep efficiency 94.2%). All stages of sleep showed grossly appropriate architecture with normal progression through sleep stages. Eleven apneas were noted, and 10 were central in origin (2 REM, 8 NREM); the overall apnea-hypopnea index was 1.2 events/h, with an average SpO2 of 96.5% and a minimum of 90%. Frequent limb movements were noted, with a limb related arousal index of 1.2 events/h, and a clinically signifi-cant periodic leg movement index of 22 events/h. PLMs persisted into REM sleep and other more complex movements were noted during REM sleep as well, including fine finger movements and forceful kicks. No epileptiform correlates were observed during the episodes of abnormal movements during sleep.
Patient 6
A 5-year-old girl with medical/developmental history notable only for stuttering, which was described as a normal developmental speech dysfluency, had mild anxiety symptoms for approximately 6–8 w prior to the explosive onset of numerous neuropsychiatric symptoms. The impairing symptoms began approximately 1 mo following a febrile illness accompanied by a rash on the trunk and abdomen, and included the following symptoms: obsessive-compulsive symptoms characterized by contamination fears, handwashing and counting compulsions, confessing, and intrusive violent thoughts; emotional lability; new-onset movement disorder manifesting as facial twitches and grimacing, fist clenching, posturing of arms, and erratic gait; and new-onset sleep disturbance (described mainly as difficulty falling and staying asleep and frequent nightmares). Family history was significant for anxiety and impulse control disorders.
A comprehensive outside medical evaluation yielded diagnoses of unspecified tic and anxiety disorders. A diagnosis of PANDAS was also considered, and a 10-day course of amoxicillin was prescribed followed by a 14-day course of cephalexin.
At the time of NIH evaluation, approximately 3.5 mo after symptom onset, the child was significantly improved. Physical examination was notable for shotty cervical lymphadenopathy. No tics were noted on examination, but fist clenching and occasional posturing of the arms were noted. Brain MRI without contrast was normal and EEG was within the broad range of normal limits for age in the awake and asleep states. No focal, paroxysmal, or epileptiform abnormalities were seen.
PSG revealed a total sleep time of 523.5 min during a recording time of 593.4 min (sleep efficiency, 88.2%). All stages of sleep showed grossly appropriate architecture with normal progression through sleep stages. No apneas were noted, but spontaneous arousals were quite frequent at 11.9 events/h. Multiple upper and lower limb movements were documented during all stages of sleep and the majority of these movements did not result in awakening. Movements included odd arm posturing (e.g., arms raised above the head with clenched fists) in the drowsy state, and a 2 Hz “tremor-like” movement of the right forearm in slow wave sleep. Movements documented during REM sleep included the following: hand stereotypies (left and right hands individually, as well as bilateral movements, sometimes resulting in arousal), head shakes, facial grimaces, complex arm and hand movements, and large body jerks. No epileptiform correlates were observed during the episodes of abnormal movements during sleep.
Patient 12
An 8-year-old boy with no significant medical/developmental history presents with acute onset of moderately severe neuro-psychiatric symptoms that interfered with functioning at home and with peers, as well as school attendance and performance. His symptoms included restricted eating, contamination fears, intrusive images, extreme perfectionism, symmetry concerns, and ordering/arranging and repeating compulsions. In addition, he had separation and generalized anxiety, impulsivity, rage episodes, motoric hyperactivity, dysgraphia, simple tics of the arms and head, and initial insomnia. Family history was significant for depressive disorder and ADHD.
Outside medical evaluation was conducted 4 days after symptom onset. PANDAS was suspected by the child's pediatrician, who did a rapid strep test, which was positive. A 9-day course of amoxicillin was prescribed but symptoms failed to remit. The child returned to his pediatrician 3 w later; a rapid strep test was negative at that time, but amoxicillin was restarted.
At the time of NIH evaluation, approximately 2 mo after symptom onset, neuropsychiatric symptoms remained prominent and impairing. Physical examination was unremarkable. Brain MRI without contrast was normal. EEG was notable for a single right frontal spike-wave discharge during stage II sleep, but otherwise normal.
PSG revealed a total sleep time of 513.9 min during a recording time of 586.4 min (sleep efficiency, 87.6%). All stages of sleep showed grossly appropriate architecture with normal progression through sleep stages. Mild snoring was noted but the apnea-hypopnea index was minimal at 0.1 events/h, and average saturation of oxygen, or SpO2, was 97.6%, with a low of 94%. Spontaneous arousals were frequent at 10.1 events/h. Periodic limb movements were noted mostly during N3 sleep at a rate of 6.9 events/h. Multiple episodes of abrupt lower extremity movement (vigorous kicks) occurred during REM sleep, not resulting in arousal. No EEG correlates were observed during the episodes of abnormal movements during sleep.
DISCUSSION
RBD has been reported to occur only rarely in children. However, in this consecutive case series of 15 children with PANS, we observed 4 children (27%) met RBD criteria and noted evidence of REM sleep motor disinhibition in 8 others (53%). Only two patients (13%) had a normal PSG, and PANS symptoms had remitted for one of those children.
Stores' 2008 review5 found only 24 published cases of child and adolescent patients meeting RBD criteria, and a more recent case series identified 13 additional subjects.29 Most of the cases presented in the review were associated with narcolepsy, medication use, or anatomical abnormalities of the hindbrain (e.g., tumors or trauma).5 A handful of cases were attributed to a neurological condition or the neuropsychiatric state of the child, including one case each of juvenile Parkinson disease, autism, Tourette syndrome, Smith-Magenis syndrome, and Moebius syndrome. One of the most substantial case series of RBD reported on clinical features and PSG in 15 children aged 3–17 y with overlapping comorbid diagnoses including: anxiety (n = 8); ADHD/attention deficit disorder or inattentiveness (n = 10); nonspecific developmental or language delay (n = 6); and OCD (n = 2).28 The disparate etiologies speak to the complexity of the REM sleep state, which may be disrupted by mechanical, chemical, or combinations of abnormalities. Only one pilot study to date has explored quantitative analysis of pediatric REM sleep without atonia (RSWA), comparing 12 patients with REM motor abnormalities to age and gender matched controls. The authors found that phasic muscle density increased with age in their cohort and that the earliest sign of RSWA found was an increase in phasic burst duration in the anterior tibialis.29 This approach takes an important step toward rigorous study of muscle activity during REM sleep across normal and abnormal development, and future longitudinal examination of these types of measures will further illuminate how abnormalities may relate to pathology.
REM sleep is an active process that requires activation of a population of REM active neurons in the upper pons. These glutamatergic neurons provide descending inputs to the medulla and spinal cord, where they activate gamma-aminobutyric acidergic and glycinergic inhibitory interneurons that hyperpolarize motor neurons and cause muscle atonia during REM sleep.30 Other ascending projections from the sublaterodorsal nucleus, parabrachial nucleus, precoeruleus area, and pedunculopontine and laterodorsal tegmental nuclei are thought to activate forebrain components of REM sleep, such as EEG desynchronization, hippocampal theta activity, and dreaming.31 REM sleep represents an enhanced state of peripheral motor inhibition in conjunction with cortical motor activity overdrive. The prevailing balance is one of atonia with momentary changes in the inhibition/excitation that are observable as muscle twitches. Deviations from expected behavior secondary to incomplete transitions between REM/NREM and between REM/awake states include RBD, RSWA, sleep onset and offset hallucinations and cataplexy. Although there are strong associations between some neurodegenerative disorders (e.g., the synucleopathies) and RBD, the etiologic factors have not been conclusively demonstrated, which suggests that we should take a very cautious approach to our interpretation of increased tone during sleep in our psychiatric pediatric population. This is particularly important, given that muscle tone during REM sleep is likely to be higher in children than in adults.32,33 Thus, the presence of higher than adult tone or even movement during REM sleep in children may be developmentally normal. This seems an unlikely explanation of the findings in this case series, however, because ages ranged from 5 to 12 y. Further, 12 of 14 currently symptomatic children (86%) were noted to have REM sleep abnormalities. Longitudinal investigations may provide important information about the nature and course of these abnormalities.
In summary, much more needs to be known about normal pediatric movement during sleep. Our literature review suggests that children with various neuropsychiatric presentations may have motor-based sleep disturbance concomitant with their behavioral and cognitive symptomatology. In this case series we found eight patients with REM motor disinhibition, and four patients meeting criteria for RBD, indicating that PSG abnormalities may be common in this population. Therefore, further investigation into sleep disorders in PANS populations is warranted. The diagnostic evaluation of children presenting with acute-onset psychopathology would benefit from including polysomnographic evaluation with special emphasis on electromyogram (EMG). In addition, longitudinal studies utilizing PSG in various pediatric populations will greatly contribute to our understanding of the neurodevelopmental role of pediatric sleep and the implications of sleep disturbances in childhood neuropsychiatric syndromes.
DISCLOSURE STATEMENT
This was not an industry supported study. Support for this project was provided by the Intramural Research Program of the National Institute of Mental Health MH002666 (NCT01281969, protocol 11-M-0058 and NCT01778504, protocol 13-M-0028). Dr. Leckman has received support from the NIH (salary and research funding), Tourette Syndrome Association (research funding), Grifols LLC (research funding) and Klingenstein Third Generation Foundation (medical student fellowship program); he receives book royalties from John Wiley and Sons, McGraw-Hill, and Oxford University Press. The other authors have indicated no financial conflicts of interest. The remaining authors indicate no financial conflicts of interest.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- ADHD
attention deficit hyperactivity disorder
- EMG
electromyogram
- NIH
National Institutes of Health
- NREM
non-rapid eye movement
- OCD
obsessive-compulsive disorder
- PANDAS
pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection
- PANS
pediatric acute-onset neuropsychiatric syndrome
- PLM
periodic limb movements
- PLMS
periodic limb movements during sleep
- pRBD
psychiatric rapid eye movement sleep behavior disorder
- PSG
polysomnography
- RBD
rapid eye movement sleep behavior disorder
- REM
rapid eye movement
- RSWA
REM sleep without atonia
- SpO2
average saturation of oxygen
- TD
tic disorder
- VGCK-LE
voltage-gated potassium channel antibody-associated limbic encephalitis
REFERENCES
- 1.Schenck CH, Bundlie SR, Ettinger MG, Mahowald MW. Chronic behavioral disorders of human REM sleep: a new category of parasomnia. Sleep. 1986;9:293–308. doi: 10.1093/sleep/9.2.293. [DOI] [PubMed] [Google Scholar]
- 2.Boeve BF, Silber MH, Parisi JE, et al. Synucleinopathy pathology and REM sleep behavior disorder plus dementia or parkinsonism. Neurology. 2003;61:40–5. doi: 10.1212/01.wnl.0000073619.94467.b0. [DOI] [PubMed] [Google Scholar]
- 3.Ju Y-E, Larson-Prior L, Duntley S. Changing demographics in REM sleep behavior disorder: possible effect of autoimmunity and antidepressants. Sleep Med. 2011;12:278–83. doi: 10.1016/j.sleep.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 4.Kotagal S. Rapid eye movement sleep behavior disorder during childhood. Sleep Med Clin. 2015;10:163–67. doi: 10.1016/j.jsmc.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Stores G. Rapid eye movement sleep behaviour disorder in children and adolescents. Dev Med Child Neurol. 2008;50:728–32. doi: 10.1111/j.1469-8749.2008.03071.x. [DOI] [PubMed] [Google Scholar]
- 6.Dauvilliers Y, Jennum P, Plazzi G. Rapid eye movement sleep behavior disorder and rapid eye movement sleep without atonia in narcolepsy. Sleep Medicine. 2013;14:775–81. doi: 10.1016/j.sleep.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Lam SP, Li SX, Chan JW, et al. Does rapid eye movement sleep behavior disorder exist in psychiatric populations? A clinical and polysomnographic case-control study. Sleep Med. 2013;14:788–94. doi: 10.1016/j.sleep.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 8.Coban A, Ismail Kucukali C, Bilgic B, et al. Evaluation of incidence and clinical features of antibody-associated autoimmune encephalitis mimicking dementia. Behav Neurol. 2014;2014:935379. doi: 10.1155/2014/935379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cochen V, Arnulf I, Demeret S, et al. Vivid dreams, hallucinations, psychosis and REM sleep in Guillain-Barre syndrome. Brain. 2005;128:2535–45. doi: 10.1093/brain/awh585. [DOI] [PubMed] [Google Scholar]
- 10.Iranzo A, Graus F, Clover L, et al. Rapid eye movement sleep behavior disorder and potassium channel antibody-associated limbic encephalitis. Ann Neurol. 2006;59:178–81. doi: 10.1002/ana.20693. [DOI] [PubMed] [Google Scholar]
- 11.Vale TC, do Prado LB, do Prado GF, Barsottini OG, Pedroso JL. Rapid eye movement-sleep behavior disorder in paraneoplastic cerebellar degeneration: improvement with immunotherapy. Sleep. 2016;39:117–20. doi: 10.5665/sleep.5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohrs S, Rasch T, Altmeyer S, et al. Decreased sleep quality and increased sleep related movements in patients with Tourette's syndrome. J Neurol Neurosurg Psychiatry. 2001;70:192–7. doi: 10.1136/jnnp.70.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mellman TA, Kobayashi I, Lavela J, Wilson B, Hall Brown TS. A relationship between REM sleep measures and the duration of posttraumatic stress disorder in a young adult urban minority population. Sleep. 2014;37:1321–6. doi: 10.5665/sleep.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobayashi I, Boarts JM, Delahanty DL. Polysomnographically measured sleep abnormalities in PTSD: a meta-analytic review. Psychophysiology. 2007;44:660–9. doi: 10.1111/j.1469-8986.2007.537.x. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds KC, Gradisar M, Alfano CA. Sleep in children and adolescents with obsessive-compulsive disorder. Sleep Med Clin. 2015;10:133–41. doi: 10.1016/j.jsmc.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Rapoport J, Elkins R, Langer DH, et al. Childhood obsessive-compulsive disorder. Am J Psychiatry. 1981;138:1545–54. doi: 10.1176/ajp.138.12.1545. [DOI] [PubMed] [Google Scholar]
- 17.Rao U. Sleep disturbances in pediatric depression. Asian J Psychiatr. 2011;4:234–47. doi: 10.1016/j.ajp.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Augustinavicius JL, Zanjani A, Zakzanis KK, Shapiro CM. Polysomnographic features of early-onset depression: a meta-analysis. J Affect Disord. 2014;158:11–8. doi: 10.1016/j.jad.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Kostanecka-Endress T, Banaschewski T, Kinkelbur J, et al. Disturbed sleep in children with Tourette syndrome: a polysomnographic study. J Psychosomatic Res. 2003;55:23–9. doi: 10.1016/s0022-3999(02)00602-5. [DOI] [PubMed] [Google Scholar]
- 20.Kirov R, Kinkelbur J, Banaschewski T, Rothenberger A. Sleep patterns in children with attention-deficit/hyperactivity disorder, tic disorder, and comorbidity. J Child Psychol Psychiatry. 2007;48:561–70. doi: 10.1111/j.1469-7610.2007.01729.x. [DOI] [PubMed] [Google Scholar]
- 21.Cortese S, Faraone SV, Konofal E, Lecendreux M. Sleep in children with attention-deficit/hyperactivity disorder: meta-analysis of subjective and objective studies. J Am Acad Child Adolesc Psychiatry. 2009;48:894–908. doi: 10.1097/CHI.0b013e3181ac09c9. [DOI] [PubMed] [Google Scholar]
- 22.Wiebe S, Carrier J, Frenette S, Gruber R. Sleep and sleepiness in children with attention deficit / hyperactivity disorder and controls. J Sleep Res. 2013;22:41–9. doi: 10.1111/j.1365-2869.2012.01033.x. [DOI] [PubMed] [Google Scholar]
- 23.Swedo SE, Leckman JF, Rose NR. From research subgroup to clinical syndrome: modifying the PANDAS criteria to describe PANS (pediatric acute-onset neuropsychiatric syndrome) Pediatr Therapeut. 2012;2:113. [Google Scholar]
- 24.Chang K, Frankovich J, Cooperstock M, et al. Clinical evaluation of youth with pediatric acute-onset neuropsychiatric syndrome (PANS): recommendations from the 2013 PANS Consensus Conference. J Child Adolesc Psychopharmacol. 2015;25:3–13. doi: 10.1089/cap.2014.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swedo SE, Leonard HL, Garvey M, et al. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: clinical description of the first 50 cases. Am J Psychiatry. 1998;155:264–71. doi: 10.1176/ajp.155.2.264. [DOI] [PubMed] [Google Scholar]
- 26.Swedo SE, Leonard HL, Rapoport JL. The pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection (PANDAS) subgroup: separating fact from fiction. Pediatrics. 2004;113:907–11. doi: 10.1542/peds.113.4.907. [DOI] [PubMed] [Google Scholar]
- 27.Iber C, Ancoli-Israel S, Chesson ALJ, Quan SF. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, 1st ed. [Google Scholar]
- 28.Lloyd R, Tippmann-Peikert M, Slocumb N, Kotagal S. Characteristics of REM sleep behavior disorder in childhood. J Clin Sleep Med. 2012;8:127–31. doi: 10.5664/jcsm.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hancock KL, St Louis EK, McCarter SJ, et al. Quantitative analyses of REM sleep without atonia in children and adolescents with REM sleep behavior disorder. Minn Med. 2014;97:43. [PubMed] [Google Scholar]
- 30.Luppi PH, Clement O, Sapin E, et al. Brainstem mechanisms of paradoxical (REM) sleep generation. Pflugers Arch. 2012;463:43–52. doi: 10.1007/s00424-011-1054-y. [DOI] [PubMed] [Google Scholar]
- 31.Saper CB, Scammell TE. Emerging therapeutics in sleep. Ann Neurol. 2013;74:435–40. doi: 10.1002/ana.24000. [DOI] [PubMed] [Google Scholar]
- 32.Ferri R, Bruni O, Fulda S, Zucconi M, Plazzi G. A quantitative analysis of the submentalis muscle electromyographic amplitude during rapid eye movement sleep across the lifespan. J Sleep Res. 2012;21:257–63. doi: 10.1111/j.1365-2869.2011.00958.x. [DOI] [PubMed] [Google Scholar]
- 33.Kohyama J. REM sleep atonia: from basic background to clinical application. J Med Dent Sci. 2001;48:29–39. [PubMed] [Google Scholar]


