Abstract
Background
Compared to the widely-adopted 2–4 months of pre-operative therapy for patients with borderline resectable (BR) or locally advanced (LA) pancreatic ductal adenocarcinoma (PDAC), our institution tends to administer a longer duration before considering surgical resection. Using this unique approach, the aim of this study was to determine preoperative variables associated with survival.
Methods
Records from patients with BR/LA PDAC who underwent attempt at surgical resection from 1992–2014 were reviewed.
Results
After a median duration of 6 months of pre-operative treatment, 109 patients with BR/LA PDAC (BR 63, LA 46) were explored; 93 (85.3%) underwent pancreatectomy. Those who received at least 6 months of pre-operative treatment had longer median overall survival (OS) than those who received less (52.8 vs. 32.1 months, P=0.044). On multivariate analysis, pre-operative treatment duration was the strongest predictor of survival (hazard ratio (HR) 4.79, P=0.043). However, OS was similar in those whose CA19-9 normalized regardless of whether they received more or less than 6 months of chemotherapy (71.4 vs. 101.8 months, P=0.930).
Conclusions
Pre-operative CA19-9 decline can guide treatment duration in patients with BR/LA PDAC. We endorse 6 months of therapy except in those patients whose values normalize, where surgery can be considered after a shorter course.
Keywords: pancreatic ductal adenocarcinoma, neoadjuvant therapy, CA 19-9 antigen
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a highly lethal disease with a 5-year overall survival (OS) of less than 10% [1]. It is predicted to be the 2nd-leading cause of cancer-related deaths in the United States by 2020 [2]. Surgical resection provides the only chance of cure; however, only 15–20% of patients present with early stage, potentially resectable disease at the time of diagnosis. Nearly half have metastatic disease and are not candidates for an operation. The remaining 30–40% of patients present with tumors that involve the surrounding major vasculature. In recent years, this latter class has been subdivided into 2 groups based on radiographic features: borderline resectable (BR) or locally advanced (LA) [3].
Although varying definitions of BR and LA pancreatic cancer exist [4], a consensus statement co-sponsored by the Americas Hepato-Pancreato-Biliary Association (AHPBA), Society for Surgery of the Alimentary Tract (SSAT), and Society of Surgical Oncology (SSO) in 2009 was endorsed by the National Comprehensive Cancer Network (NCCN) [5]. In this classification system, tumors are considered BR if there is any superior mesenteric vein (SMV) or portal vein (PV) involvement which is reconstructable and/or limited arterial involvement, defined as abutment or less than 50% circumferential encasement of the superior mesenteric artery (SMA), abutment or short-segment encasement of the common hepatic artery (CHA), and no involvement of the celiac trunk. LA tumors are defined as having non-reconstructable SMV or PV involvement, encasement of greater than 50% of the SMA circumference, long-segment CHA encasement, or any celiac trunk abutment.
Resection of these locally progressed (BR or LA) cancers has a high likelihood of microscopic (R1) or even gross (R2) positive margins [6,7]. Patients who undergo these incomplete operations carry a similar OS to those who do not undergo resection [8,9,10]. Therefore, the current preferred approach to patients with BR/LA disease is to administer chemotherapy with or without radiation therapy prior to consideration for surgery [11]. Although most patients with BR/LA tumors are administered 2 to 4 months of preoperative treatment, both the duration and type of therapy varies widely across institutions [12–25].
We previously reported that our institution’s approach to patients with BR/LA PDAC tends to involve a longer duration (median 6 months) of pre-operative therapy than is widely adopted before evaluation for possible surgery. Using this strategy, our patients who underwent tumor resection had amongst the best reported survival [26,27]. In this study, we aimed to take advantage of this unique approach and identify pre-operative variables associated with survival to help guide treatment decisions for patients with BR/LA disease. Moreover, since 2012 we have followed all patients with BR/LA PDAC closely from the time of their diagnosis. Using this well-annotated data, we also aimed to report the percentage of patients who underwent tumor resection and the survival of those not operated on to determine if we were “over-selecting” those offered surgical treatment.
Methods
Study design
Records from 2 groups of patients who underwent treatment for BR/LA PDAC at the University of California-Los Angeles (UCLA) Hirshberg Center for Pancreatic Diseases were reviewed from a prospectively maintained database: (i) those who underwent attempt at surgical resection from 1992–2014 and (ii) those followed from the time of diagnosis from 2012–2014. Vascular involvement was determined for all patients by retrospective review of pre-operative contrast-enhanced computed tomography (CT) scans, magnetic resonance imaging (MRI), or operative reports by a single physician and defined as BR or LA based on the AHPBA/SSO/SSAT classification. Imaging was also assessed both before and after pre-operative treatment for maximum tumor diameter, specific vessel involvement, and evidence of distant metastatic disease. Hospital and clinic charts of all patients were reviewed for demographics, date of diagnosis, type and duration of pre-operative therapy, use of radiation therapy, pre- and post-treatment serum cancer antigen 19-9 (CA 19-9) levels, follow-up, and disease-specific survival. All patients had a pre-treatment histologic diagnosis of PDAC, and date of diagnosis was considered to be the date of this biopsy. OS was measured from the date of diagnosis to date of death or last follow-up.
For those who underwent resection, pathology reports were reviewed for tumor histology, size, grade, TNM stage, presence or absence of perineural (PNI) and lymphovascular invasion (LVI), margin status, and histopathologic (HP) response. Tumor size was defined as the maximum gross diameter. Margins were considered positive if tumor extended up to the inked margin. HP response was scored using the College of American Pathologists guidelines [28]. Tumor recurrence after resection was determined from imaging and defined as local if there was increased soft-tissue thickening in the resection bed. Those with any new lesions outside of the operative field were considered to have distant recurrence. Biopsies were not routinely performed to confirm recurrence.
Those patients who were followed from their time of diagnosis from 2012–2014 underwent repeat imaging and serum CA 19-9 checks every 2–4 months while receiving pre-operative treatment. They were selected for surgical exploration if the tumors were thought to be resectable by a multidisciplinary tumor board based on invasive and non-invasive tests. However, all patients taken to surgery had no evidence of disease progression on imaging, decreasing CA 19-9 levels, and good functional status. Shrinkage of the tumor or clear fat planes around the vessels was not required as previous studies have shown that response to treatment is not well-reflected by these radiographic signs [26,29].
This study was approved by the UCLA Institutional Review Board.
Statistical Analysis
SPSS version 22.0 (IBM) was used for statistical analysis and data management. Means of continuous variables with normal distributions were compared using the two-tailed t test. Non-parametric continuous variables were compared using the Mann-Whitney U test or Kruskal-Wallis test. Pearson’s chi-square test was used to analyze categorical data. Survival analysis was performed using the Kaplan-Meier method with comparisons informed by the log-rank test. Multivariate Cox regression analyses were performed using statistically significant univariate parameters with P < .10 as the initial entry criterion. Continuous variables were split at their median for these analyses. Statistical significance was defined as P < .05.
Results
Clinical characteristics of patients undergoing surgery after pre-operative therapy
From 1992–2014, a total of 109 patients were taken for surgical exploration after pre-operative treatment for BR (n=63) or LA (n=46) PDAC (Table 1). The median age was 62 years, and 63 (57.8%) were female. Most (n=54, 52.4%) resections were performed in or after 2010 (Supplemental Table 1). The median duration of pre-operative chemotherapy treatment was 6.0 months, and the most common regimen was 5-fluorouracil (5-FU) based multi-agent therapy (n=34, 43.0% of those whose regimen was known). Most did not receive pre-operative radiation therapy (n=73, 67.0%). Of the 65 patients who had oncologic follow-up at UCLA, 37 (56.9%) received adjuvant chemotherapy in addition to their pre-op treatment. The median tumor size on initial imaging was 3.0 centimeters (cm) and significantly decreased to 1.85 cm after pre-operative treatment (P=<0.001). The mean decrease in tumor size was 28.7%. Median CA 19-9 levels were 236 U/mL prior to therapy and 23.6 U/mL when selected as appropriate candidates for surgery (P=0.092).
Table 1.
Clinical Characteristics of Patients Taken for Surgical Exploration After Pre-Operative Treatment for Borderline Resectable or Locally Advanced Pancreatic Cancer
| All Patients (n=109) | Patients Who Underwent Pancreatic Resection (n=93) | Patients Found to be Unresectable at Time of Exploration (n=16) | P | |
|---|---|---|---|---|
|
| ||||
| Extent of disease (%) | 0.004 | |||
| Borderline-resectable | 63 (57.8) | 59 (63.4) | 4 (25.0) | |
| Locally-Advanced | 46 (42.2) | 34 (36.6) | 12 (75.0) | |
|
| ||||
| Gender (%) | 0.075 | |||
| Male | 46 (42.2) | 36 (38.7) | 10 (62.5) | |
| Female | 63 (57.8) | 57 (61.3) | 6 (37.5) | |
|
| ||||
| Median age, years (range) | 62 (41–86) | 61 (42–86) | 63 (41–82) | 0.980 |
|
| ||||
| Surgical exploration prior to pre-operative therapy (%) | 0.563 | |||
| Yes | 34 (31.2) | 30 (32.3) | 4 (25.0) | |
| No | 75 (68.8) | 63 (67.7) | 12 (75.0) | |
|
| ||||
| Chemotherapy treatment (% of known) | 0.112 | |||
| Single agent | 11 (14.1) | 8 (11.8) | 3 (30.0) | |
| Multi-agent | 67 (85.9) | 60 (88.2) | 7 (70.0) | |
| Unknown | 31 | 25 | 6 | |
|
| ||||
| Radiation therapy (%) | 0.324 | |||
| Yes | 36 (33.0) | 29 (31.2) | 7 (43.8) | |
| No | 73 (67.0) | 64 (68.8) | 9 (56.3) | |
|
| ||||
| Median initial tumor size on CT, cm (range) | 3.0 (0–7) | 3.0 (0–7) | 3.75 (0–5.5) | 0.149 |
|
| ||||
| Median downstaged tumor size on CT, cm (range) | 1.85 (0–6.5) | 1.8 (0–6.5) | 2.0 (0–5.1) | 0.904 |
|
| ||||
| Tumor size decrease of >30% on CT (% of known) | 0.820 | |||
| Yes | 38 (56.7) | 32 (56.1) | 6 (60.0) | |
| No | 29 (43.3) | 25 (43.9) | 4 (40.0) | |
| Unknown | 42 | 36 | 6 | |
|
| ||||
| Median initial CA19-9, U/mL (range) | 236 (3–57000) | 192.7 (3–57000) | 236.9 (3–6784) | 0.098 |
|
| ||||
| Median downstaged CA19- 9, U/mL (range) | 23.6 (3–2015) | 21 (3–295) | 78 (15–2015) | 0.011 |
|
| ||||
| Downstaged CA 19-9 ≤ 35 U/mL (% of known) | 0.034 | |||
| Yes | 43 (56.6) | 40 (61.5) | 3 (27.3) | |
| No | 33 (43.4) | 25 (38.5) | 8 (72.7) | |
| Unknown | 33 | 28 | 5 | |
CT indicates computed tomography; cm, centimeters, CA 19-9, serum cancer antigen 19-9
Table 1 compares the clinical characteristics of those whose tumors were removed (“resected”) to those whose cancers were determined to be advanced-stage during surgery (“unresected”). Ninety-three of the 109 (85.3%) patients selected for surgery underwent tumor resection. “Resected” patients were more likely to have BR (59/93, 63.4%) than LA (36.6%) disease at the time of diagnosis (P=0.004). Additionally, the post-treatment (pre-operative) CA19-9 level in “resected” patients was significantly lower (21 vs. 78 U/mL, P=0.011) and also more likely to reach a normal value (≤35 U/mL) than those who were “unresected” (61.5% vs. 27.3%, P=0.03).
Notably, the size of the tumor on pre- or post-treatment imaging was not significantly different between “resected” and “unresected” patients, and the “resected” group was also not more likely to have shown at least a partial response by the Response Evaluation Criteria in Solid Tumors (RECIST) criteria (P=0.82) [30]. Other factors that were not predictive of resectability included use of radiation therapy pre-operatively (P=0.324), the specific chemotherapy regimen administered (P=0.499), receipt of adjuvant therapy (P=0.412), or the year surgery was performed (P=0.372).
Operative and pathologic information of “resected” patients
The histopathologic findings of the 93 patients who underwent pancreatectomy are shown in Table 2. The most common operation was a pancreaticoduodenectomy (n=80, 86.0%). Only 12 (12.9%) patients underwent a vascular resection. Despite the low rate of vein resection, negative margins were obtained in 80 (86.0%) patients and were equivalent in patients who had been originally staged with BR (51/59, 86.4%) or LA (29/34, 85.3%) disease. Only 3 of the 12 (25.0%) patients who underwent a vascular resection had invasion of the venous adventitia on final pathology.
Table 2.
Clinicopathologic Characteristics of Patients Undergoing Pancreatic Resection for Borderline Resectable or Locally Advanced Pancreatic Adenocarcinoma After Pre-Operative Chemotherapy
| All Patients (n=93) | Borderline Resectable (n=59) | Locally Advanced (n=34) | P | |
|---|---|---|---|---|
|
| ||||
| Operation (%) | 0.743 | |||
| Pancreaticoduodenectomy | 80 (86.0) | 50 (84.7) | 30 (88.2) | |
| Distal pancreatectomy | 11 (11.8) | 8 (13.6) | 3 (8.8) | |
| Other pancreatectomy | 2 (2.2) | 1 (1.7) | 1 (2.9) | |
|
| ||||
| Vascular Operation (%) | 0.030 | |||
| Venous resection | 12 (12.9) | 11 (18.6) | 1 (2.9) | |
| No venous resection | 81 (87.1) | 48 (81.4) | 33 (97.1) | |
|
| ||||
| Pathologic tumor size, cm (range) | 2.0 (0–6.6) | 2.5 (0–6.6) | 1.2 (0–3.7) | 0.001 |
|
| ||||
| T stage | 0.005 | |||
| T0 | 12 (12.9) | 7 (11.9) | 5 (14.7) | |
| T1 | 13 (14.0) | 4 (6.8) | 9 (26.5) | |
| T2 | 6 (6.5) | 5 (8.5) | 1 (2.9) | |
| T3 | 58 (62.4) | 43 (72.9) | 15 (44.1) | |
| T4 | 3 (3.2) | 0 | 3 (8.8) | |
| Not assessed | 1 (1.1) | 0 | 1 (2.9) | |
|
| ||||
| Grade | 0.214 | |||
| Well-differentiated | 16 (17.2) | 8 (13.6) | 8 (23.5) | |
| Moderately-differentiated | 46 (49.5) | 35 (59.3) | 11 (32.4) | |
| Poorly-differentiated | 15 (16.1) | 9 (15.3) | 6 (17.6) | |
| Unknown, no residual tumor | 12 (12.9) | 7 (11.9) | 5 (14.7) | |
| Unknown, not assessed | 4 (4.3) | 0 | 4(11.8) | |
|
| ||||
| N stage | 0.028 | |||
| N0 | 59 (63.4) | 33 (55.9) | 26 (76.5) | |
| N1 | 33 (35.5) | 26 (44.1) | 7 (20.6) | |
| Not assessed | 1 (1.1) | 0 | 1 (2.9) | |
|
| ||||
| Perineural invasion | 0.394 | |||
| Present | 64 (68.8) | 43 (72.9) | 21 (61.8) | |
| Absent | 26 (28.0) | 15 (25.4) | 11 (32.4) | |
| Unknown | 3 (3.3) | 1 (1.7) | 2(5.9) | |
|
| ||||
| Lymphovascular invasion | 0.428 | |||
| Present | 24 (25.8) | 17 (28.8) | 7 (20.6) | |
| Absent | 60 (64.5) | 37 (62.7) | 23 (67.6) | |
| Indeterminate | 6 (6.5) | 4 (6.8) | 2 (5.9) | |
| Unknown | 3 (3.3) | 1 (1.7) | 2 (5.9) | |
|
| ||||
| Margins (%) | 0.408 | |||
| Negative | 80 (86.0) | 51 (86.4) | 29 (85.3) | |
| Positive | 13 (14.0) | 8 (13.6) | 5 (14.7) | |
|
| ||||
| Histopathologic response (%) | 0.176 | |||
| Complete | 12 (12.9) | 7 (11.9) | 5 (14.7) | |
| Marked | 11 (11.8) | 5 (8.5) | 6 (17.6) | |
| Minimal to moderate | 14 (15.1) | 11 (18.6) | 3 (8.8) | |
| Poor to no response | 23(24.7) | 18 (30.5) | 5 (14.7) | |
| Unknown | 33 (33.5) | 18 (30.5) | 19 (55.9) | |
CT indicates computed tomography; cm, centimeters, CA 19-9, serum cancer antigen 19-9
Of the 60 pathology reports where it was described, 23 (38.3%) tumors showed either a complete or marked histopathologic response to pre-operative therapy. Interestingly, most patients (59/93, 63.4%) did not have any evidence of nodal involvement, and node-negative disease was significantly more common in LA than BR patients (76.5% vs. 55.9%, P=0.028). Paradoxically, those with initially LA cancers also had smaller tumors than BR patients (1.2cm vs. 2.5cm, P=0.001) and were less likely to undergo venous resection (2.9% vs. 18.6%, P=0.030).
Survival and recurrence outcomes
The median OS for all 109 patients who were selected for surgery after pre-operative therapy was 33.6 (range 7.5–222.2) months. There were no operative deaths. As expected, those whose tumors were resected had significantly longer median OS than those found to be unresectable during surgery (41.9 (range 7.5–222.2) vs. 24.3 (range 9.0–35.3) months, P=<0.001). When grouped by initial stage, the median OS in “resected” patients was 62.2 (range 7.5–213.4) months for those with BR and 39.7 (range 11.8–222.2) months for those with LA disease (P=0.434, Figure 1).
Figure 1.
Kaplan-Meier survival curve comparing the overall survival of patients who were taken for surgical exploration after pre-operative treatment of borderline resectable or locally advanced pancreatic ductal adenocarcinoma. Those with initially borderline resectable disease who were able to be resected at the time of exploration (BR-resected) had a median overall survival of 62.2 months, which was significantly improved over those with initially borderline resectable disease who were still locally progressed (BR-unresectable) (24.3 months, P=0.003). Similarly, those who were resected after pre-operative treatment of locally advanced disease (LA-resected) had significantly better survival than those who were still locally advanced at the time of exploration (LA-unresectable) (39.7 months vs 25.0 months, P=0.044).
Surprisingly, there was no apparent benefit to having a R0 (n=80) as compared to a R1 resection (n=13), (median OS 51.8 (range 7.5–222.2) vs. 38.6 (range 12.0–70.4) months, P=0.153). At a median follow-up of 28.6 months, recurrence was documented in 45 of the 93 patients who underwent resection, with distant (n=26, 57.8%) being somewhat more common than local.
On univariate analysis, a number of significant predictors of survival were identified (Table 3). These included a normal post-treatment serum CA 19-9 level (≤35 U/mL), pre-operative treatment duration, tumor grade, T and N stage, PNI, and HP response. On multivariate analysis, only tumor grade (hazard ratio (HR) 8.70, P=0.010), post-treatment normal CA 19-9 level (HR 3.45, P=0.039), and pre-operative treatment duration (HR 4.79, P=0.043) remained statistically significant. Of note, one patient was removed from the multivariate analysis because he had undetectable CA 19-9 levels (i.e., a “non-secretor”).
Table 3.
Cox Proportional Hazard Models of Cliniocopathologic Factors on Survival of Patients After Tumor Resection
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age (<61y/≥61y) | 1.52 (0.84–2.73) | 0.163 | ||
| Sex (male/female) | 0.84 (0.46–1.53) | 0.565 | ||
| Extent of initial disease (BR/LA) | 1.39 (0.77–2.50) | 0.279 | ||
| Pre-operative therapy treatment duration (<6 months/≥6 months) | 2.16 (1.35–3.47) | 0.001 | 4.79 (1.05–21.9) | 0.043 |
| Adjuvant chemotherapy (no/yes) | 0.71 (0.32–1.61) | 0.415 | ||
| Tumor size on initial CT (<3cm/≥3cm) | 1.17 (0.57–2.38) | 0667 | ||
| Tumor size on downstaged CT (<1.8cm/≥1.8cm) | 1.45 (0.72–2.94) | 0.300 | ||
| CT size change (<30%/≥ 30%) | 1.58 (0.70–3.60) | 0.273 | ||
| Initial CA19-9 (<200 U/mL/≥200 U/mL) | 1.89 (0.91–3.94) | 0.089 | 1.02 (0.36–2.84) | 0.976 |
| Downstaged CA 19-9 (≤35 U/mL/>35 U/mL) | 2.49 (1.20–5.21) | 0.015 | 3.45 (1.06–11.2) | 0.039 |
| Ca19-9 change (<50%/≥50%) | 0.72 (0.26–1.98) | 0.520 | ||
| Chemotherapy (single agent/multiple agents) | 0.43 (0.15–1.24) | 0.12 | ||
| XRT (no/yes) | 0.971(0.72–1.32) | 0.848 | ||
| Vascular resection (no/yes) | 1.57 (0.69–3.58) | 0.285 | ||
| T stage (T0–T2/T3–T4) | 3.95 (3.44–6.90) | 0.001 | 2.53 (0.60–10.6) | 0.206 |
| Grade (Well-moderately differentiated/poorly differentiated) | 2.14 (1.00–4.55) | 0.049 | 8.70 (1.66–45.5) | 0.010 |
| LN involvement (negative/positive) | 3.07 (1.62–5.81) | 0.001 | 2.56 (0.78–8.47) | 0.123 |
| LVI (not present/present) | 1.85 (0.97–3.52) | 0.064 | 1.72 (0.16–2.06) | 0.401 |
| PNI (not present/present) | 2.79 (1.37–5.68) | 0.005 | 1.00 (0.16–6.21) | 0.998 |
| Margin (R0/R1) | 1.75 (0.80–3.82) | 0.138 | ||
| HP response (moderate-complete response/poor-partial response) | 10.1 (2.24–45.5) | 0.003 | 1.00 (0.01–2.94) | 0.925 |
HR indicates hazard ratio; CI, confidence interval; y, year; BR, borderline resectable; LA locally advanced; cm, centimeters; CA 19-9, serum cancer antigen 19-9 level; XRT, radiation therapy; LN, lymph node; LVI, lymphovascular invasion; PNI, perineural invasion; HP, histopathologic
Impact of CA19-9 Decline and Length of Therapy on Survival
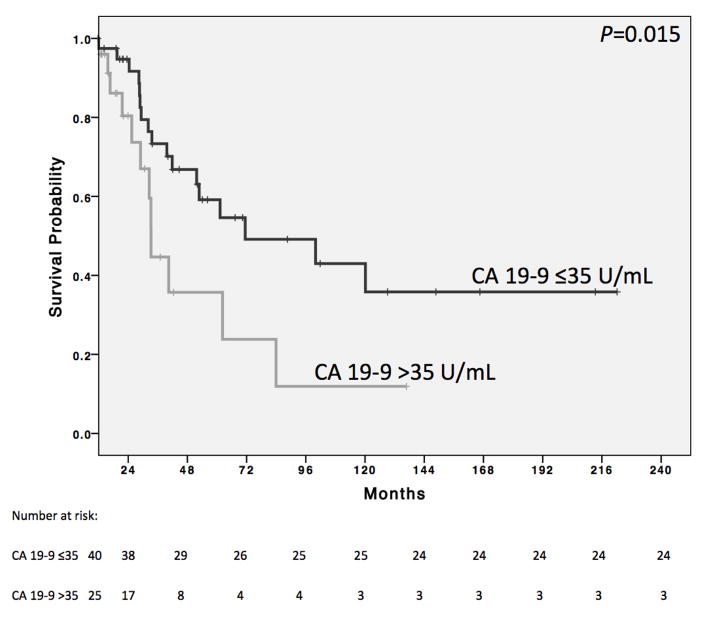
We next used the Kaplan-Meier method to determine the median OS of patients split by the clinical factors found to be predictive of survival on multivariate analysis: post-treatment CA 19-9 level and pre-operative treatment duration. Patients with a normal post-treatment CA 19-9 level had significantly longer survival than those whose level was elevated (71.4 (range 11.6–222.2) vs. 32.5 (range 7.5–136.8) months, P=0.015; Figure 2). After excluding patients who were taken for exploration after less than 3 months of chemotherapy because of their inability to tolerate it (n=6), the median OS of those who received at least 6 months of treatment (n=61) was significantly longer than those who received less (n=24), (52.8 (range (11.8–222.2) vs. 32.1 (range 7.5–129.1) months, P=0.044, Figure 3).
Figure 2.
Kaplan-Meier survival curve comparing the overall survival of patients whose tumors were resected with a normal pre-operative serum cancer antigen 19-9 level (CA 19-9 ≤35 U/mL) to those whose levels were still elevated (CA 19-9 >35 U/mL). The median overall survival was significantly longer for patients with normal levels than those with elevated (71.4 vs 32.5 months, P=0.015).
Figure 3.
a. Bar graph illustrating the length of pre-operative treatment received by patients who underwent pancreatic resection after pre-operative treatment of borderline resectable or locally advanced pancreatic cancer. b. Kaplan-Meier survival curve comparing the overall survival between patients who underwent pancreatic resection after receiving less than 6 months of pre-operative treatment to those who received 6 months or more for initially borderline resesctable or locally advanced pancreatic ductal adenocarcinoma. Overall median survival was significantly greater for those who received 6 months or more (52.8 vs 32.1 months, P=0.044).
We then performed a detailed survival analysis that stratified on a combination of both variables (Table 4). The best OS (median 101.8 (range 11.6–129.1) months) was actually in the group that received less than 6 months of treatment but whose CA19-9 normalized (n=9). Similarly, those whose CA 19-9 levels normalized who received a longer treatment course also did well (n=31, median OS 71.4 (range 12.0–222.2) months; P=0.930). Conversely, those whose CA19-9 levels remained elevated and were taken to surgery after less than 6 months (n=7) had markedly shorter survival than those who received the extended duration of treatment (n=18), (median OS 16.7 (range 7.5–83.9) vs. 33.2 (range 12.9–136.8) months; P=0.047).
Table 4.
Association Between Post-treatment CA 19-9 levels, Pre-operative Treatment Duration, and Median Overall Survival
| <6 Months Pre-operative Treatment | ≥6 Months Pre-operative Treatment | |
|---|---|---|
| CA 19-9 ≤ 35 | 101.8 months (n=9) | 71.4 months (n=31) |
| CA 19-9 > 35 | 16.7 months (n=7) | 33.2 months (n=18) |
CA 19-9 indicates serum cancer antigen 19-9
Percentage of the subset of patients with BR or LA disease followed from time of diagnosis who underwent resection
From 2012–2014, 90 patients with BR/LA PDAC were closely followed from their time of diagnosis. Of these, 9 were lost to follow-up or received treatment at another facility. Therefore, detailed clinical and treatment information was available for 81 patients in this cohort: 44 BR and 37 LA (Supplemental Table 2).
Using this subset, we determined the percentage of patients who underwent surgical resection (Figure 4) and also the survival of all who were diagnosed with BR or LA disease. Of those with BR disease, 28/44 (63.6%) were taken to the operating room for exploration after a median of 6.0 months of pre-operative treatment (Figure 4a). All but one patient underwent resection (96.4% of those surgically explored and 61.4% of all BR patients followed from diagnosis), and 23 had negative margins (82.1% of those surgically explored and 52.3% of all BR patients treated). Median survival of those who underwent resection cannot yet be calculated; however, 19 (70.4%) are alive at a median follow-up of 16.7 months. Median OS was 13.1 (range 3.3–34.6) months in those who did not have an operation.
Figure 4.
Diagram of treatment received by 81 patients diagnosed with borderline resectable (a) or locally advanced (b) pancreatic cancer from 2012–2014.
Of those diagnosed with LA disease, 13 of 37 (35.1%) were selected for surgical exploration after a median duration of 9.0 months of pre-operative treatment (Figure 4b). All received at least 6 months of pre-operative therapy. Of these 13, 8 underwent pancreatectomy (61.5% of those surgically explored and 21.6% of all LA patients followed from diagnosis), and all had a R0 resection. As with the BR patients, median survival of those who underwent resection cannot yet be calculated; however, 7 (87.5%) are alive at a median follow-up of 21.2 months. Those who did not have an operation had a median OS of 16.2 (range 2.6–35.4) months. For both BR and LA disease, patients who were selected for surgery were neither more likely to have received a specific chemotherapy regimen nor radiation therapy than those who were not considered surgical candidates.
Discussion
It has become increasingly accepted that the 30–40% of patients with PDAC who present with BR or LA disease should be treated with a period of systemic chemotherapy, with or without radiation therapy, prior to surgery. Justifications for this strategy include increased likelihood of resection and negative margins, decreased need for vascular resection, early treatment of occult metastases, better tolerance of chemotherapy pre-operatively than after surgery, and allowing assessment of tumor response to chemotherapy. However, despite these potential benefits and wide acceptance of this approach, the type and duration of pre-operative treatment has not yet been standardized. The most common schedule includes a 2–4 month course, particularly for patients with BR disease, followed by tumor restaging and consideration for surgical resection.
Since the early 1990’s, we have tended to give at least 6 months of pre-operative therapy prior to consideration for surgical resection. We have previously reported on the excellent OS of patients undergoing resection with this approach and also the histopathologic variables on surgical pathology associated with survival that can be used to help make adjuvant treatment decisions [26,27]. However, there has been criticism that our approach may be “too selective,” and there could be a subset of patients who did not undergo, but may have benefitted from, surgical resection. To address this latter issue, since 2012 we have been closely and prospectively following all patients with BR/LA PDAC from their time of diagnosis. Therefore, the aims of this study were to, using our unique approach, (i) identify pre-operative variables associated with survival that can potentially be used to refine what we believe to be the optimal patient selection for surgery, and (ii) determine the percentage of patients diagnosed with BR or LA disease who undergo surgery and the OS of those who did not.
From 1992–2014, a total of 93 (59 BR and 34 LA) patients had their tumors surgically removed. The median OS was 62.2 months for BR and 39.7 months for LA patients (P=0.434). The patients who received at least 6 months of pre-operative treatment had better OS than those who had less (52.8 vs. 32.1 months, P=0.044). The pre-operative CA19-9 values normalized (≤35 U/mL) in 40 patients, and this was a strong pre-operative predictor of survival on multivariate analysis (HR=3.45, P=0.039). Interestingly, when examining both CA19-9 and treatment duration simultaneously, patients whose CA19-9 levels did not normalize and completed at least 6 months of pre-operative therapy survived significantly longer than those who received less (33.2 vs. 16.7 months, P= 0.047). Conversely, those whose CA19-9 levels normalized did equally well regardless of the duration of therapy given (<6 vs. ≥6 months, 101.8 vs. 71.4 months, P=0.930). In the subset of patients we prospectively followed from the time of diagnosis (n=81), 28/44 (63.6%) of those with BR and 8/37 (21.6%) with LA had their tumors removed. During this same time frame, the patients who did not undergo surgery had a median OS of 13.1 months for BR and 16.2 months for LA disease.
Our finding that CA19-9 decrease to normal was a strong pre-operative predictor of survival is consistent with multiple prior studies which have also shown that CA 19-9 decline is a useful measure of the degree of response to treatment and that normalization confers a particularly favorable prognosis, even in the pre-operative setting [31–33]. There is also evidence that markedly elevated CA 19-9 levels are indicative of early metastases that are not yet detectable on imaging [34,35], and many patients initially diagnosed with locally progressed disease probably also have occult metastases. Therefore, normalization of CA 19-9 levels likely indicates response of both the primary tumor as well as subclinical micrometastases. As most (70–80%) patients with pancreatic cancer die of distant metastatic disease even after what is thought to be complete surgical resection of the primary tumor [36], early treatment of occult metastases is likely critical to improving survival.
We speculate that the early treatment of micrometastases may be one reason why longer duration (≥6 months) of pre-operative therapy was associated with improved survival in our series. Additionally, our strategy may better select patients who will benefit from surgery by allowing a greater length of time for those with locally aggressive biology or occult metastases to become apparent during the treatment period. Furthermore, those who receive a longer length of chemotherapy pre-operatively may be receiving more chemotherapy overall, as cytotoxic therapy is better tolerated prior to surgery. Receiving the bulk of chemotherapy while the tumor is still present also allows for easier assessment of treatment response, and necessary changes in regimens can be made. Lastly, there were also indicators of significant tumor downstaging in our patients receiving a longer duration of treatment. A marked or complete HP tumor response was seen in 38.3% of patients in whom HP response was recorded, and only 3/12 (25%) resected veins and 3/93 (3.2%) of all resected tumors had confirmed pathologic tumor involvement of the vessel wall. Overall, a combination of the above factors is likely responsible for the superior OS seen in our series.
To address the concern that by giving a longer duration of pre-operative therapy we may be “over-selecting” those taken for surgery and missing a subset of patients who could benefit from an operation, we compared our data with other large independent studies which also examined the rates of exploration, resection, and OS after pre-operative treatment for BR or LA PDAC (Table 5). The majority of patients in these studies were given 2–4 months of pre-operative therapy. Studies that separately examined patients with BR PDAC revealed that 50.9–77.1% of them were selected for surgical exploration and 40.0–74.3% ultimately underwent resection. The median OS ranged from 19.2–45 months. Similarly, patients with LA tumors were selected for surgery 14.3–53.3% of the time, and 10.2–49.0% underwent resection. The median OS for the LA patients ranged from 12.7 to 32 months. When comparing our study to these prior publications, we surgically explored and ultimately resected 61.4% BR and 21.6% LA PDACs, which is comparable to these studies. Importantly, the patients who were not selected for surgery had a median OS of 16.2 months, which is similar to what can be achieved with multi-agent chemotherapy for this stage of patients. Therefore, there does not appear to be a large group who could benefit from surgery that is missed with our strategy. However, comparison between patients with initially BR versus LA disease who underwent resection unexpectedly showed that LA patients had smaller, lower T stage tumors with less nodal involvement. They were also less likely to undergo a vascular resection and were more likely to have had at least a partial response to pre-operative treatment on imaging. These findings suggest that we may be more discriminatory at selecting those with LA disease for surgery than those with BR tumors. Nevertheless, a randomized controlled trial with intention to treat would be needed to definitively determine the best treatment strategy.
Table 5.
Selected Prior Studies Evaluating Pre-Operative Treatment of Patients with Borderline Resectable and Locally Advanced PDAC
| Total patients treated | Pre-operative treatment type | Duration of pre-operative treatment | No. of patients surgically explored (% of total patients) | No. of patients undergoing resection (%of total patients) | No. of R0 resections (% of total patients) | Median overall survival of patients after resection, months | |
|---|---|---|---|---|---|---|---|
| Stokes et al (2011)24 | 40 BR | Capecitabine + XRT | 12–41 weeks (median 19) | 21 (52.5%) | 16 (40.0%) | 14 (35.0%) | 23 |
| Katz et al (2012)15 | 122 BR | GEM* + XRT +/− 5-FU or Capecitabine | 2–4 months | 92 (75.4%) | 85 (69.7%) | 81 (66.4%) | 33 |
| Takahashi et al (2013)25 | 80 BR | GEM* + XRT | 3 months | 52 (65.0%) | 32 (40.0%) | --- | a |
| Cho et al (2013)26 | 30 BR | GEM* + XRT | --- | N/A | 30 (N/A) | 29 (N/A) | 45 |
| Rose et al (2014)27 | 64 BR | GEM + Docetaxel +/− other agents +/− XRT | 24 weeks | 39 (60.9%) | 31 (48.4%) | 27 (42.2%) | --- |
| Takeda et al (2014)28 | 35 BR | GEM + XRT | 1–2 months | 27 (77.1%) | 26 (74.3%) | 26 (74.3%) | 41.2 |
| Mellon et al (2015)29 | 110 BR | GTX + XRT | 2–3 months | 56 (50.9%) | 46 (41.8%) | 44 (40.0%) | 19.2 |
| Aristu et al (2003)30 | 47 LA | Cisplatin + 5- FU; Paclitaxel, 5-FU, Cisplatin GEM + Docetaxel; + XRT | Variable | 12 (25.5%) | 9 (19.1%) | --- | 23 |
| Sa Cunha et al (2005)31 | 61 LA | 5-FU + Cisplatin + XRT | Variable | 23 (37.7%) | 13 (21.3%) | 11 (18.0%) | 28 |
| Allendorf et al (2008)32 | 78 LA | GTX + XRT | 3 months | 78 (N/A) | 59 (N/A) | --- | 17.8 |
| Bickenbach et al (2011)33 | 36 LA | GEM* +/− XRT | Variable | N/A | 36 (N/A) | 30 (N/A) | 30 |
| Strobel et al (2012)34 | 257 LA | GEM* +/− XRT | Variable | 137 (53.3%) | 120 (46.7%) | 42 (16.3%) | 12.7 |
| Ben-Josef et al (2012)35 | 50 LA | GEM + XRT | 2–4 months | --- | 12 (24.0%) | 10 (20.0%) | 32 |
| Habermehl et al (2012)36 | 215 LA | GEM + XRT | Variable | 104 (48.4%) | 51 (49.0%) | 20 (39.2%) | 14.4 |
| Mellon et al (2015)29 | 49 LA | FOLFIRINOX + XRT | 2–3 months | 7 (14.3%) | 5 (10.2%) | 5 (10.2%) | 15 |
PDAC indicates pancreatic ductal adenocarcinoma; BR, borderline resectable; LA, locally advanced, XRT, radiation therapy; GEM*, gemcitabine-based multi-agent therapy; GEM, single-agent gemcitabine; 5-FU, 5-fluorouracil; GTX, gemcitabine, docetaxel and capecitabine; FOLFIRINOX, 5-fluorouracil, leucovorin, irinotecan and oxaliplatin
--- information not available
34% 5 year survival
This study is not without limitations. We were restricted by its retrospective nature, which has inherent biases. Our database only had information for all patients diagnosed with BR or LA pancreatic cancer at the time of diagnosis from 2012–2014 rather than for the entire time period of 1992–2014, so we only know the recent percentage of those treated who were eventually taken to surgery. However, the number of pancreatic operations for patients with BR/LA disease is increasing rapidly at our institution, with nearly 1/3 of them occurring over the last 3 years. We also did not find margin status to be a predictor of survival, which was surprising given that one of the goals of pre-operative treatment is to attain a R0 resection. A possible explanation is that those who underwent resections had tumors which were responsive to chemotherapy, as indicated by decreasing CA 19-9 levels, and therefore their residual microscopic disease was likely able to be treated with adjuvant treatment. Unfortunately, many patients were treated by oncologists outside of UCLA after resection, so there is a lack of data regarding adjuvant treatment. However, we generally recommend adjuvant chemotherapy when resection margins are positive. There was also significant variability in treatment regimens among patients, so we could not examine the efficacy of a specific treatment regimen (e.g., FOLFIRINOX) or the role of radiation therapy. Lastly, an inherent difficulty with using CA 19-9 as a tumor marker is the fact that approximately 5–10% of patients do not have the capacity to secrete CA 19-9 (37), and therefore, our treatment strategy is not applicable to this group of patients.
Conclusion
Although patients with BR or LA disease comprise a large portion (~40%) of those initially diagnosed with PDAC, their treatment strategy is not standardized across centers. At our institution, we tend to administer a longer duration of chemotherapy than most prior to consideration for surgical resection. Our approach is supported by the present study, as we identified longer (≥6 months) pre-operative chemotherapy as a strong predictor of patient survival after resection. However, the survival of those whose CA19-9 levels normalized during treatment and received less than 6 months was similar to those who received the longer course. Therefore, while we still endorse longer (≥ 6 months) treatment prior to surgery in patients with BR/LA disease, we conclude that surgery can be considered earlier in those whose CA19-9 declines to normal during treatment and negative margins appear achievable.
Supplementary Material
Footnotes
Disclosures and funding sources: The authors have nothing to disclose
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–21. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 3.Katz MH, Pisters PW, Evans DB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg. 2008;206:833–48. doi: 10.1016/j.jamcollsurg.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malafa MP. Defining borderline resectable pancreatic cancer: emerging consensus for an old challenge. J Natl Compr Canc Netw. 2015;13:501–4. doi: 10.6004/jnccn.2015.0068. [DOI] [PubMed] [Google Scholar]
- 5.Callery MP, Chang KJ, Fishman EK, et al. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1727–33. doi: 10.1245/s10434-009-0408-6. [DOI] [PubMed] [Google Scholar]
- 6.Capussotti L, Massucco P, Ribero D, et al. Extended lymphadenectomy and vein resection for pancreatic head cancer: outcomes and implications for therapy. Arch Surg. 2003;138:1316–22. doi: 10.1001/archsurg.138.12.1316. [DOI] [PubMed] [Google Scholar]
- 7.van Geenen RC, ten Kate FJ, de Wit LT, et al. Segmental resection and wedge excision of the portal or superior mesenteric vein during pancreatoduodenectomy. Surgery. 2001;129:158–63. doi: 10.1067/msy.2001.110221. [DOI] [PubMed] [Google Scholar]
- 8.Neoptolemos JP, Stocken DD, Dunn JA, et al. Influence of resection margins on survival for patients with pancreatic cancer treated by adjuvant chemoradiation and/or chemotherapy in the ESPAC-1 randomized controlled trial. Ann Surg. 2001;234:758–68. doi: 10.1097/00000658-200112000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg. 2006;10:1199–210. doi: 10.1016/j.gassur.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 10.Katz MH, Wang H, Fleming JB, et al. Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol. 2009;16:836–47. doi: 10.1245/s10434-008-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abrams RA, Lowy AM, O’Reilly EM, et al. Combined modality treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1751–6. doi: 10.1245/s10434-009-0413-9. [DOI] [PubMed] [Google Scholar]
- 12.Stokes JB, Nolan NJ, Stelow EB, et al. Preoperative capecitabine and concurrent radiation for borderline resectable pancreatic cancer. Ann Surg Oncol. 2011;18:619–27. doi: 10.1245/s10434-010-1456-7. [DOI] [PubMed] [Google Scholar]
- 13.Katz MH, Fleming JB, Bhosale P, et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer. 2012;118:5749–56. doi: 10.1002/cncr.27636. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi H, Ohigashi H, Gotoh K, et al. Preoperative gemcitabine-based chemoradiation therapy for resectable and borderline resectable pancreatic cancer. Ann Surg. 2013;258:1040–50. doi: 10.1097/SLA.0b013e31829b3ce4. [DOI] [PubMed] [Google Scholar]
- 15.Cho IR, Chung MJ, Bang S, et al. Gemcitabine based neoadjuvant chemoradiotherapy therapy in patients with borderline resectable pancreatic cancer. Pancreatology. 2013;13:539–43. doi: 10.1016/j.pan.2013.07.064. [DOI] [PubMed] [Google Scholar]
- 16.Rose JB, Rocha FG, Alseidi A, et al. Extended neoadjuvant chemotherapy for borderline resectable pancreatic cancer demonstrates promising postoperative outcomes and survival. Ann Surg Oncol. 2014;21:1530–7. doi: 10.1245/s10434-014-3486-z. [DOI] [PubMed] [Google Scholar]
- 17.Takeda Y, Nakamori S, Eguchi H, et al. Neoadjuvant gemcitabine-based accelerated hyperfractionation chemoradiotherapy for patients with borderline resectable pancreatic adenocarcinoma. Jpn J Clin Oncol. 2014;44:1172–80. doi: 10.1093/jjco/hyu143. [DOI] [PubMed] [Google Scholar]
- 18.Mellon EA, Hoffe SE, Springett GM, et al. Long-term outcomes of induction chemotherapy and neoadjuvant stereotactic body radiotherapy for borderline resectable and locally advanced pancreatic adenocarcinoma. Acta Oncol. 2015;54:979–85. doi: 10.3109/0284186X.2015.1004367. [DOI] [PubMed] [Google Scholar]
- 19.Aristu J, Cañón R, Pardo F, et al. Surgical resection after preoperative chemoradiotherapy benefits selected patients with unresectable pancreatic cancer. Am J Clin Oncol. 2003;26:30–6. doi: 10.1097/00000421-200302000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Sa Cunha A, Rault A, Laurent C, et al. Surgical resection after radiochemotherapy in patients with unresectable adenocarcinoma of the pancreas. J Am Coll Surg. 2005;201:359–65. doi: 10.1016/j.jamcollsurg.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Allendorf JD, Lauerman M, Bill A, et al. Neoadjuvant chemotherapy and radiation for patients with locally unresectable pancreatic adenocarcinoma: feasibility, efficacy, and survival. J Gastrointest Surg. 2008;12:91–100. doi: 10.1007/s11605-007-0296-7. [DOI] [PubMed] [Google Scholar]
- 22.Bickenbach KA, Gonen M, Tang LH, et al. Downstaging in pancreatic cancer: a matched analysis of patients resected following systemic treatment of initially locally unresectable disease. Ann Surg Oncol. 2012;19:1663–9. doi: 10.1245/s10434-011-2156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strobel O, Berens V, Hinz U, et al. Resection after neoadjuvant therapy for locally advanced, “unresectable” pancreatic cancer. Surgery. 2012;152:S33–42. doi: 10.1016/j.surg.2012.05.029. [DOI] [PubMed] [Google Scholar]
- 24.Ben-Josef E, Schipper M, Francis IR, et al. A phase I/II trial of intensity modulated radiation (IMRT) dose escalation with concurrent fixed-dose rate gemcitabine (FDR-G) in patients with unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2012;84:1166–71. doi: 10.1016/j.ijrobp.2012.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Habermehl D, Kessel K, Welzel T, et al. Neoadjuvant chemoradiation with Gemcitabine for locally advanced pancreatic cancer. Radiat Oncol. 2012;7:28. doi: 10.1186/1748-717X-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donahue TR, Isacoff WH, Hines OJ, et al. Downstaging chemotherapy and alteration in the classic computed tomography/magnetic resonance imaging signs of vascular involvement in patients with pancreaticobiliary malignant tumors: influence on patient selection for surgery. Arch Surg. 2011;146:836–43. doi: 10.1001/archsurg.2011.152. [DOI] [PubMed] [Google Scholar]
- 27.Kadera BE, Sunjaya DB, Isacoff WH, et al. Locally advanced pancreatic cancer: association between prolonged preoperative treatment and lymph-node negativity and overall survival. JAMA Surg. 2014;149:145–53. doi: 10.1001/jamasurg.2013.2690. [DOI] [PubMed] [Google Scholar]
- 28.Washington K, Berlin J, Branton P, et al. Protocol for the Examination of Specimens From Patients with Carcinoma of the Exocrine Pancreas Version 3.2.0.0. Northfield, IL: College of American Pathologists; 2012. pp. 1–18. [Google Scholar]
- 29.Dholakia AS, Hacker-Prietz A, Wild AT, et al. Resection of borderline resectable pancreatic cancer after neoadjuvant chemoradiation does not depend on improved radiographic appearance of tumor-vessel relationships. J Radiat Oncol. 2013;2:413–425. doi: 10.1007/s13566-013-0115-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1. 1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 31.Berger AC, Garcia M, Jr, Hoffman JP, et al. Postresection CA 19-9 predicts overall survival in patients with pancreatic cancer treated with adjuvant chemoradiation: a prospective validation by RTOG 9704. J Clin Oncol. 2008;26:5918–22. doi: 10.1200/JCO.2008.18.6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tzeng CW, Balachandran A, Ahmad M, et al. Serum carbohydrate antigen 19-9 represents a marker of response to neoadjuvant therapy in patients with borderline resectable pancreatic cancer. HPB (Oxford) 2014;16:430–8. doi: 10.1111/hpb.12154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Combs SE, Habermehl D, Kessel KA, et al. Prognostic impact of CA 19-9 on outcome after neoadjuvant chemoradiation in patients with locally advanced pancreatic cancer. Ann Surg Oncol. 2014;21:2801–7. doi: 10.1245/s10434-014-3607-8. [DOI] [PubMed] [Google Scholar]
- 34.Kim TH, Han SS, Park SJ, et al. CA 19-9 level as indicator of early distant metastasis and therapeutic selection in resected pancreatic cancer. Int J Radiat Oncol Biol Phys. 2011;81:743–8. doi: 10.1016/j.ijrobp.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 35.Yoo T, Lee WJ, Woo SM, et al. Pretreatment carbohydrate antigen 19-9 level indicates tumor response, early distant metastasis, overall survival, and therapeutic selection in localized and unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2011;81:623–30. doi: 10.1016/j.ijrobp.2011.02.063. [DOI] [PubMed] [Google Scholar]
- 36.Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27:1806–13. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kannagi R. Carbohydrate antigen sialyl Lewis a--its pathophysiological significance and induction mechanism in cancer progression. Chang Gung Med J. 2007;30:189–209. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.