Abstract
During different stages of tumor development the immune system can either identify and destroy tumors, or promote their growth. Therapies targeting the immune system have emerged as a promising treatment modality for breast cancer, and immunotherapeutic strategies are being examined in preclinical and clinical models. However, our understanding of the complex interplay between cells of the immune system and breast cancer cells is incomplete. In this article, we review recent findings showing how the immune system plays dual host-protective and tumor-promoting roles in breast cancer initiation and progression. We then discuss estrogen receptor α (ERα)-dependent and ERα-independent mechanisms that shield breast cancers from immunosurveillance and enable breast cancer cells to evade immune cell induced apoptosis and produce an immunosuppressive tumor microenvironment. Finally, we discuss protumorigenic inflammation that is induced during tumor progression and therapy, and how inflammation promotes more aggressive phenotypes in ERα positive breast cancers.
Keywords: Breast cancer, Immunity, Inflammation, Immunosurveillance, ER-alpha
1. Introduction
Despite significant therapeutic achievements in recent years, in industrialized countries, breast cancer remains the most common cancer in women. It causes about 40,000 deaths in the United States each year (Basu et al., 2012). Estrogen receptorα (ERα) positive breast cancers represent more than 70% of breast tumors and endocrine therapies such as selective estrogen receptor modulators (SERMs) and aromatase inhibitors are still the standard adjuvant treatment for these tumors. However, the majority of patients will develop resistance to hormonal therapy and will need alternative therapies (Clarke et al., 2001; Clarke et al., 2003; Osborne and Schiff, 2011).
For over a century, the idea that the immune system can control cancer has been a subject of debate. Only very recently has it become generally accepted that the immune system has the ability not only to prevent tumor growth but also to promote it through a process called immunoediting. This process is comprised of three phases: elimination, equilibrium and escape (Schreiber et al., 2011; Vesely et al., 2011). Elimination is achieved through identification and destruction of nascent transformed cells by acute tumor-inhibiting inflammation, characterized by infiltration of effector cells of the innate and adaptive immune system as well as production of tumor-inhibiting cytokines. The escape phase is sustained by chronic tumor-promoting inflammation, which mainly involves immunosuppressive cells and soluble factors (Vesely et al., 2011). Evading immune destruction has recently been recognized as a hallmark of cancer (Hanahan and Weinberg, 2011). In general, use of immunosuppressants following organ transplantation or HIV infection increases the risk of tumors such as skin cancer, non-Hodgkin’s lymphoma or lung cancers, but not cancers of organs such as breast, brain, prostate and ovary (Kirk et al., 2007; Jiang et al., 2010). These studies suggest that breast cancer cells may be less immunogenic or simply take longer to develop (Vesely et al., 2011). Historically pre-existing inflammation or infection was not considered to be an underlying risk factor for the development of breast cancer. However, it is now clear that the infiltration of leukocytes, in the correct context, can either eliminate or promote the development of breast cancers (DeNardo and Coussens, 2007; Coussens and Pollard, 2011). Several studies have shown that immunity and inflammation-associated gene expression signatures are able to predict or classify tamoxifen-resistant breast cancers (Jansen et al., 2005; Chanrion et al., 2008; Vendrell et al., 2008). This supports the notion that endocrine resistance is associated with a dysregulated immune response and/or excessive inflammation in the tumor microenvironment (Osborne and Schiff, 2011). A recent study suggests that the immune response profile and inflammatory signature in breast cancer may provide useful information on patient prognosis and treatment (Kristensen et al., 2012). These studies suggest that research associated with inflammation and the immune system may enhance therapeutic possibilities for breast cancers, especially for those resistant to endocrine therapies. To better understand the battle and interplay between breast cancer cells and cells of the immune system, in this review we discuss following topics: (1) anti-breast cancer effector cells of the immune system, (2) mechanisms of breast cancer resistance to antitumor immunity, (3) protumorigenic inflammation in breast cancer and (4) inflammation promotion of aggressive phenotypes of ERα positive breast cancer.
2. Anti-breast cancer effector cells of the immune system
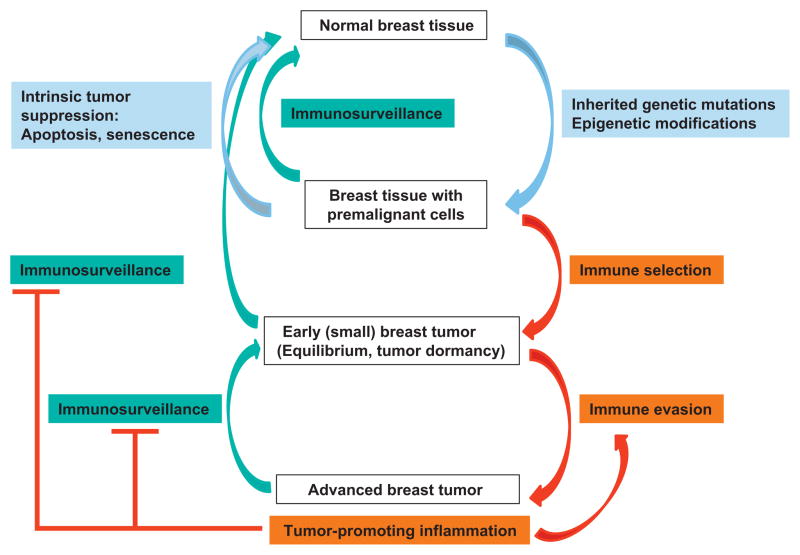
Breast cancer is often initiated by genetic and epigenetic changes in genes that regulate the function of the mammary epithelial cells (Coussens and Pollard, 2011). To prevent the development of breast cancer, diverse intrinsic tumor-suppressor mechanisms induce senescence or apoptosis of neoplastic cells (Lacroix et al., 2006; Xu et al., 2011; Nicholls et al., 2012). In parallel, the immune system is recognized as an extrinsic tumor-suppressor that can eliminate epithelial cells that have transformed to breast cancer cells and limit their growth when they have escaped intrinsic tumor suppression mechanisms (Schreiber et al., 2011; Vesely et al., 2011). The contribution of the immune system to breast cancer progression and inhibition is summarized in Fig. 1.
Fig. 1.
Immunosurveillance and inflammation in breast cancer. Inherited genetic mutation and epigenetic modifications cause premalignant transformation of mammary cells. Transformed cells can be eliminated by intrinsic or extrinsic tumor suppression mechanisms. Immune selection and immune evasion result in the development of advanced breast tumor. Immunosurveillance inhibits or reverses tumor development through killing the tumor cells. Protumorigenic inflammation accompanied advanced breast tumor promotes immune evasion and suppresses effective immunosurveillance.
2.1. Cytotoxic T lymphocytes (CTLs) as anti-breast cancer effector cells
The primary effector immune cells that eliminate breast cancer cells are CD8+ CTLs and natural killer (NK) cells. Numerous studies have revealed that CTLs can be induced to target specific antigens expressed on breast cancer cells (Disis et al., 1994; Peoples et al., 1995; Kontani et al., 2001; Neidhardt-Berard et al., 2004; Treilleux et al., 2004; Wang et al., 2006; Mine et al., 2009; Mittendorf et al., 2012). CD8+ T lymphocyte infiltration is associated with better overall patient outcomes, independent of other prognostic factors such as tumor grade, lymph node stage, size, vascular invasion and HER2 status. Morever, CD8+ T cell infiltration is associated with better breast cancer-specific survival in subgroups of patients with ERα-negative, HER2-negative or basal phenotype (Mahmoud et al., 2011). Consistent with this finding, another study showed that CD8+ T cell infiltration was associated with better patient survival in basal-like but not in non-basal triple negative breast cancers; on the contrary, CD8+ T cell infiltration was not prognostic in ER+ populations. These observations may suggest that the ER+ subgroup of breast cancer is less immunogenic than other subtypes (Liu et al., 2012). Combined chemotherapy with immunotherapy increases cytolytic activity of CTLs, which results in substantial enhancement of the antitumor effect (Ramakrishnan et al., 2010). The effect of vaccines against breast cancers is at least partly achieved through enhanced recognition and destruction of breast cancer cells by CTLs (Rech et al., 2012, Schlom, 2012, Wang et al., 2012). Together, these studies strongly suggest that CD8+ T cells have clinically significant antitumor activity against human breast cancer, and that the prognostic role of tumor infiltrated CTLs is dependent on the breast cancer subtype.
2.2. NK cells as anti-breast cancer effector cells
NK cells are cells of the innate immune system that kill tumor cells without MHC restriction (Waldhauer and Steinle, 2008). Decreased NK cell activity has been reported in patients with familial breast cancer as well as in their clinically asymptomatic first degree relatives (Strayer et al., 1986). NK cell activity was significantly reduced in different stages of breast cancers, with stage IV tumors showing reduced NK cell activity compared with stage I–III tumors (Konjevic and Spuzic, 1993). NK cell dysfunction is associated with human breast cancer progression (Mamessier et al., 2011). Unsupervised gene expression profiling of breast cancer-associated stroma revealed a gene signature that was functionally enriched in the expression of genes associated with CTLs and NK cells and was predictive of better clinical outcomes (Finak et al., 2008). Immunization against Stat3 in a mouse breast cancer xenograft model elicits strong antitumor immunity through memory CD4+ T cell dependent NK cell mediated cytotoxicity (Tkach et al., 2012). IL-2 or IL-15-activated NK cells potentiate the activity of cetuximab against triple negative breast cancer (Roberti et al., 2012). Stimulation of NK cells with a CD137-specific antibody enhances antibody-dependent cellular cytotoxicity (ADCC) and results in enhanced efficacy of trastuzumab against HER2+ breast cancer cells (Kohrt et al., 2012). These studies suggest that NK cells not only suppress human breast cancer development, but also act as an antitumor factor in breast cancers treated with chemotherapy or immunotherapy. NK cells are also critical in preventing breast cancer metastasis. When breast cancer cells are transplanted into NOD/SCID mice (which lack adaptive immunity), these mice develop non-invasive tumors. When the same cells are transplanted into NOD/SCID/γ-cnull mice (which lack both adaptive immune cells and NK cells), they develops invasive tumors that metastasize rapidly (Dewan et al., 2005). Consistent with this, the presence of NK cells in a breast tumor is negatively associated with metastasis (Olkhanud et al., 2009). Therefore, both CTL and NK cells appear to be strong antitumor effectors.
Why are CTL- and NK cell-mediated responses not effective enough to eliminate or suppress the development of overt breast cancer? Some possible mechanisms include autonomous modifications made by tumor cells that allow them to evade immune detection and destruction as well as the fact that tumor cells can induce an immunosuppressive microenvironment that diminishes the function of effector cells.
3. Tumor cell-autonomous modifications that enable breast tumors to evade immune detection and destruction
Breast cancer cell have developed several ways to avoid immune cell-mediated killing thereby allowing the development of overt tumors.
3.1. Evasion through avoiding recognition by immune cells
In a cohort of 212 patients, downregulation of HLA class I expression was found in >30% of breast cancer samples and those patients with preserved HLA class I expression had better disease-free survival (Kaneko et al., 2011). Breast cancer cells can gain resistance to NK cell-mediated killing by expressing lower levels of the NK cell receptor ligand, MICB, whose expression is regulated by the metastasis-associated microRNA, miR-10b (Tsukerman et al., 2012). These data suggest that breast cancer cells can evade immune cell-mediated cytotoxicity by avoiding tumor cell recognition by CTLs and NK cells.
3.2. Evasion through altered expression of apoptosis-associated molecules
3.2.1. Estrogen-induced proteinase inhibitor 9 (PI-9) protects breast cancer cells against immune cell-mediated killing
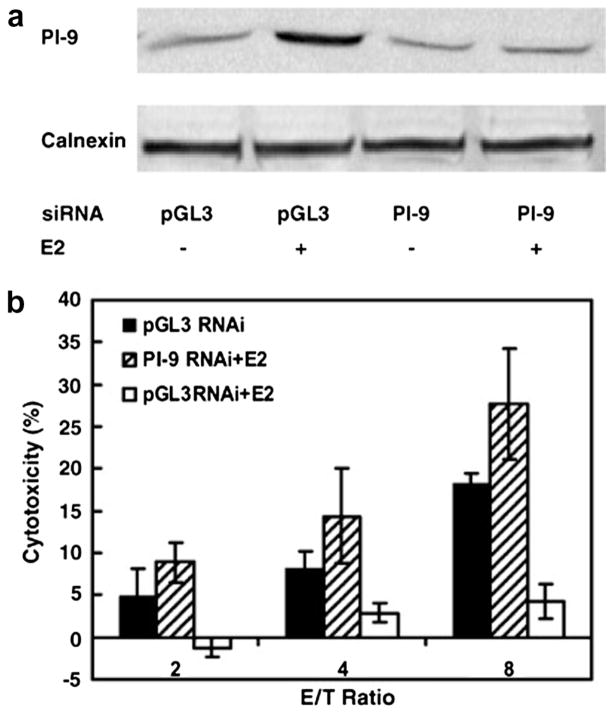
In human hepatoma cells expressing ERα, human liver biopsy specimens and breast cancer cells, we identified PI-9 as a primary estrogen-regulated gene (Kanamori et al., 2000; Jiang et al., 2007). PI-9 is the only known human intracellular inhibitor of granzyme B, the main granzyme that CTL and NK cells use to induce apoptosis of target cells (Kaiserman and Bird, 2010). Robust induction of PI-9 mRNA and protein inhibits CTL and NK cell induced apoptosis of ERα positive cells (Jiang et al., 2006, 2007). RNAi knockdown of PI-9 abolished estrogen’s ability to protect MCF-7 cells against NK cell-induced apoptosis (Fig. 2). Thus, estrogen’s ability to block NK cell-induced cytolysis derives from its ability to induce PI-9. Using a tetracycline-regulated expression system, we showed that tetracycline-induced expression of PI-9 in stably transfected cells was sufficient to protect the cells from NK cell induced apoptosis (Cunningham et al., 2007). At high levels, intracellular PI-9 shields cells from both granzyme B mediated cytolysis and Fas/Fas ligand death receptor mediated apoptosis (Cunningham et al., 2007; Kummer et al., 2007). We also identified enhancers in the promoter that are associated with PI-9 transcriptional regulation. Chromatin immunoprecipitation (ChIP) assays show that estrogen-ERα induces PI-9 transcription through direct binding of 2 ERα dimers to an unusual estrogen responsive unit containing an imperfect estrogen response element (ERE) immediately adjacent to a direct repeat of 2 ERE half sites separated by 13 nucleotides (Krieg et al., 2001, 2004). Modulators of inflammation, including IL-1β and lipopolysaccharide (LPS), also induce PI-9 expression through an upstream activator protein 1 (AP-1) site and 2 NF-κB sites (Kannan-Thulasiraman and Shapiro, 2002).
Fig. 2.
RNAi knockdown of PI-9 blocks estrogen protection against NK cell-mediated cytotoxicity. (A and B) MCF-7, human breast cancer cells were transfected with the control pGL3 luciferase siRNA, or with the PI-9 siRNA. After 24 h, ethanol vehicle or E2 was added and the cells were maintained for an additional 24 h and either harvested for Western blotting using antibodies to PI-9 and the internal standard calnexin (A) or incubated with the indicated ratios of effector NK92 cells to MCF-7 target cells and assayed for cytoxicity using the time-resolved fluorescence assay (B) (filled and open bars, pGL3 siRNA − and + E2, respectively; hatched bars, PI-9 siRNA + E2). For example, at a ratio of 8 NK cells to 1 MCF-7 cell, in MCF-7 cells in which PI-9 was not induced with E2 (E/T ratio 8, black bar), and in MCF-7 cells treated with E2 and PI-9 was knocked down with PI-9 siRNA (E/T ratio 8, hatched bar) there was substantial NK cell induced cytotoxicity (~18% and 27%, respectively). In contrast, in MCF-7 cells in which PI-9 was induced with E2 and the cells were transfected with the control siRNA (open bar), the cells were protected and NK cell induced cytotoxicity was minimal (<5%) (From Jiang et al., 2007, reprinted with permission).
A subset of breast cancers contains very high levels of ERα (Riera et al., 1999; Gritzapis et al., 2003). These tumors exhibit increased incidence of recurrence and often show a reduced response to tamoxifen (Sancho-Garnier et al., 1995; Hupperets et al., 1997; Clarke et al., 2003). In a model for these tumors in which additional ERα is expressed in MCF-7 cells under the control of a tetracycline regulated promoter (MCF-ERαHA cells), 4-hydroxytamoxifen (OHT), the active form of tamoxifen, also robustly induced PI-9 and the high levels of PI-9 in these cells effected a nearly complete inhibition of NK cell induced apoptosis, which suggests a possible mechanism for resistance to tamoxifen therapy in some ERα+ breast cancers (Jiang et al., 2007). Moreover, EGF activation of the Erk1/2 signaling pathway greatly reduces the concentration of estrogen required to induce PI-9 and shield the cells from NK cell mediated cytolysis (Jiang et al., 2007).
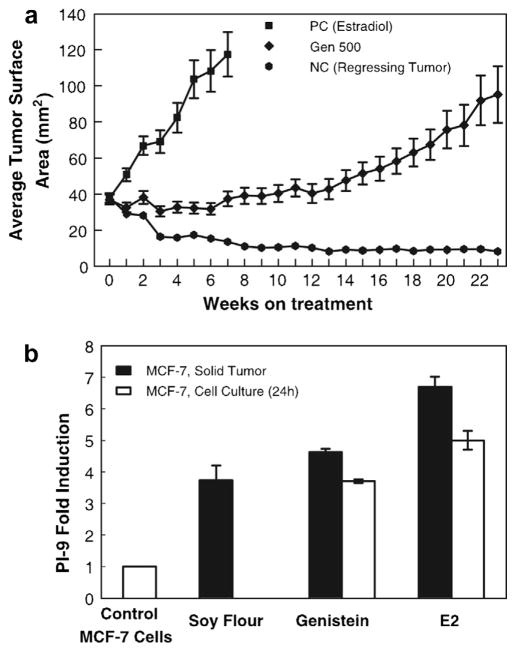
We went on to evaluate the effect of several weak xenoestrogens and the soy phytoestrogen genistein on induction of PI-9. While the xenoestrogens required high micromolar concentrations to induce even moderate levels of PI-9, genistein robustly induced PI-9 at nanomolar concentrations (Jiang et al., 2008). Genistein induction of PI-9 shielded MCF-7 cells from NK cell mediated cytolysis. The in vivo relevance of genistein induction of PI-9 was confirmed using a mouse xenograft model. In mice fed a soy-rich diet or genistein, PI-9 levels were strongly elevated in MCF-7 tumors (Fig. 3) (Jiang et al., 2008). Our studies suggest that expression of the estrogen inducible PI-9 may promote breast cancer growth by enhancing resistance to NK cell and CTL mediated immunosurveillance in vivo.
Fig. 3.
Dietary genistein induces PI-9 in MCF-7 tumors in mice. (A) Growth rates of MCF-7 tumors in ovariectomized athymic mice. At week 0, the estrogen pellets were removed, and the mice were divided into three treatment groups: PC (positive control, E2), GEN 500 (genistein 500 ppm), and NC (negative control, regressing tumor) that were fed AIN-93G diet alone. Tumor size was then measured weekly for 23 week. Data are expressed as means ± sem cross-sectional tumor area for all tumors in each group. (B) Dietary genistein and soy induce PI-9 in MCF-7 solid tumors. Mice were exposed to high (2 mg) E2 in implanted cholesterol pellets for ~11 weeks and tumors harvested (black bar) and frozen. Mice were fed diets containing genistein (500 ppm) or soy flour (diet containing 20% soy protein, ~400 ppm genistein) (black bars). Tumors were harvested at about 23 week when they reached the same size as the E2 tumors. Control MCF-7 cells were maintained in medium lacking hormones, or in 10 nm E2, or 10 nM genistein for 24 h and harvested (open bars). RNA was extracted and analyzed for PI-9 mRNA by quantitative RT-PCR. Data represent the mean ± sem for at least three samples (From Jiang et al., 2008; reprinted with permission).
3.2.2. Altered expression of other apoptosis-associated molecules in breast cancers
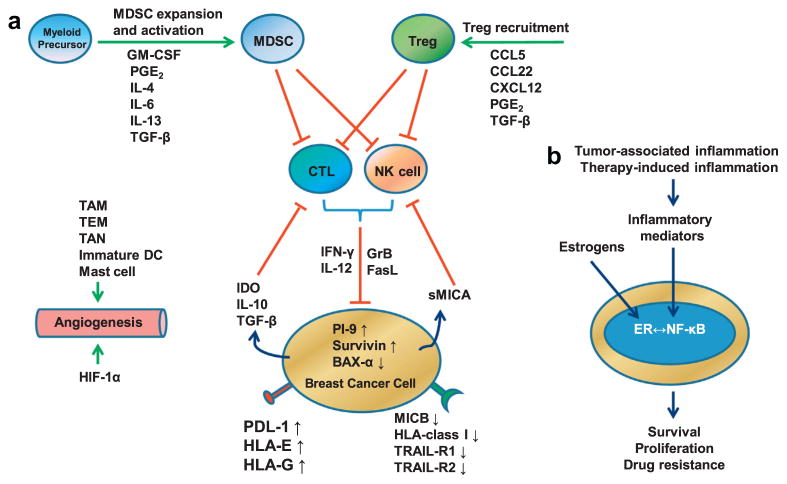
Increased expression of the anti-apoptotic molecule, survivin, is common in breast cancers, and elevated expression of survivin is associated with a poor prognosis (i.e. the presence of lymph node metastasis, higher tumor grade, HER2 overexpression and loss of hormone receptor expression) (Ryan et al., 2006). Weak or no expression of the apoptosis-promoting molecule, BAX-α, is found in breast cancer cells while this protein is expressed in high levels in normal mammary epithelia. Induction of BAX-α expression restores sensitivity of breast cancer cell to Fas/FasL induced apoptosis and reduces tumor xenograft growth in mice (Bargou et al., 1996). Mutations in the death receptors, TRAIL-R1 and TRAIL-R2, are associated with more advanced breast cancer (Shin et al., 2001). These studies suggest that tumor cells can become resistant to immune cell-mediated cytotoxicity by overexpressing anti-apoptotic or downregulating pro-apoptotic molecules (Fig. 4A).
Fig. 4.
Model showing how the immune system and inflammation eliminate or promote breast cancer. (A) Scheme showing the mechanisms by which breast cancers evade immunosurveillance. Major immunosuppressive cells including Treg and MDSCs are recruited or expanded and activated by proinflammatory mediators produced in the breast tumor microenvironment. Activated Treg and MDSCs suppress CTL and NK cells which are potent antitumor effector cells. Breast cancer cells also produce soluble factors such as IDO, IL-10, TGF-β and sMICA to suppress the activity of CTL and NK cells. Breast cancer cells evade immunosurveillance by changing the expression levels of apoptosis-associated intracellular proteins (PI-9, Survivin and BAX-α) and immune recognition- or activation-associated membranous proteins (MICB, HLA-class I, TRAIL-R1, TRAIL-R2, PDL-1, HLA-E, HLA-G). HIF-1α and proinflammatory cells such as TAM, TEM, TAN, immature DC and mast cells promote tumor angiogenesis. (B) Inflammation promotes an aggressive phenotype in ERα+ breast cancers. ERα and NF-κB activated by estrogens and proinflammatory mediators promote breast cancer cell survival, proliferation, and drug resistance. Crosstalk between ERα and NF-κB is context-dependent and can result in synergistic activation or mutual suppression. Abbreviations: Treg, regulatory T cells; MDSC; myeloid-derived suppressor cell; GM-CSF, granulocyte–macrophage colony-stimulating factor; PG, prostaglandin; CCL, CC chemokine ligand; CXCL, chemokine (C-X-C motif) ligand; CTL, cytotoxic T lymphocyte; NK, natural killer; IDO, indoleamine-pyrrole 2,3-dioxygenase; GrB, granzyme B; FasL, Fas ligand; IFN, interferon; IL, interleukin; TGF, transforming growth factor; sMICA, soluble Major Histocompatibility Complex class I related chain A; TRAIL, TNF-related apoptosis-inducing ligand; PI-9, proteinase inhibitor 9; PDL-1, programmed death ligand 1; HLA, human leukocyte antigen; TAM, tumor associated macrophage; TEM, Tie2 expressing monocyte; TAN, tumor associated neutrophil; DC, dendritic cell; HIF, hypoxia-inducible factor.
3.3. Breast cancers actively subdue immune responses
Breast cancer cells can also take a more active and direct role in subduing the immune response, through expression of immune inhibitory ligands such as B7–H1 (PD-L1), HLA-E or HLA-G (de Kruijf et al., 2010; Hasan et al., 2011) (Fig. 4A), or through actively inducing apoptosis of Fas-expressing antitumor lymphocytic cells (Gutierrez et al., 1999). Using a metastatic 4T1 breast cancer model, it was recently discovered that suppression of interferon regulatory factor (Irf7) signaling in breast cancer cells promotes tumor metastasis, and that restoration of Irf7 signaling either by over-expressing Irf7, or by treatment with type I interferon, IFN-α1, enhanced immune activity, significantly suppressed bone metastases and prolonged survival (Bidwell et al., 2012). Thus breast cancer can also evade immunosurveillance through disabling cell-autonomous IFN signaling pathways.
4. Immunosuppressive microenvironment of breast cancer
It is now widely accepted that tumor cells are able to induce a suppressive microenvironment that supports tumor growth, and that the suppressive microenvironment is comprised of immunosuppressive cells and soluble factors (DeNardo and Coussens, 2007, Coussens and Pollard, 2011, Vesely et al., 2011). Major immunosuppressive cells that are often found in breast tumors include regulatory T (Treg) cells and myeloid-derived suppressor cells (MDSCs).
4.1. Treg cells in the immunosuppressive microenvironment
FOXP3-expressing Treg cells are potent mediators of peripheral immune tolerance. Tregs can suppress a wide range of immune cells including CD4+ and CD8+ T cells, NK cells, NKT cells, B cells and antigen presenting cells (APC) through suppressing target cell activation, proliferation as well as effector functions (Shevach, 2009; Sakaguchi et al., 2010). Infiltration of FOXP3-positive Treg cells into breast cancers has been observed in numerous studies (Liyanage et al., 2002; Bohling and Allison, 2008; Ohara et al., 2009). The quantification of FOXP3-positive Treg cells has been found to be valuable in assessing breast cancer progression and prognosis. High numbers of FOXP3-positive Tregs identify patients with non-invasive ductal carcinoma in situ (DCIS) who are at an increased risk of relapse, and identify patients with invasive tumors who have shorter relapse-free and overall survival (Bates et al., 2006). FOXP3-expressing Treg cells are recruited from the circulation to primary breast cancer site through the CCL22/CCR4, CXCL12 (SDF-1)/CXCR4 and CCL5 (RANTES)/CCR1 axes (Gobert et al., 2009; Tan et al., 2011; Yan et al., 2011). Breast cancer cells can also produce PGE2 and recruit Treg cells to tumor sites through EP2 or EP4 receptors (Karavitis et al., 2012). These studies suggest that Tregs can be recruited to breast cancer sites through distinct chemotactic mechanisms (Fig. 4A). Additionally, tumor-evoked regulatory B cells can directly convert resting CD4+ T cells into FOXP3-expressing Treg cells in a TGF-β-dependent manner (Olkhanud et al., 2011). Moreover, impaired production of IFN-α by plasmacytoid dendritic cells favors expansion of Tregs infiltrated into breast tumor sites (Sisirak et al., 2012), suggesting that the tumor microenvironment may play an active role in the differentiation and expansion of Treg cells. A recent study showed that FOXP3-positive Tregs produce large amounts of receptor activator of nuclear factor-κB (RANK) ligand (RANKL), which in turn acts on RANK-expressing breast cancer cells and promotes lung metastasis (Tan et al., 2011), suggesting Tregs can promote metastasis through acting on breast cancer cells in a paracrine fashion. An ex vivo culture study showed that tamoxifen is able to induce FOXP3 expression in tumor infiltrating lymphocytes, which may represent a mechanism for endocrine resistance through Treg-mediated immunosuppression (Joffroy et al., 2010). In concert with the large body of studies showing that Tregs are a strong promoter of breast cancer development and metastasis, anti-CD25 antibody-mediated Treg depletion leads to a stronger antitumor immune response and better outcomes (Rech et al., 2012, Weiss et al., 2012), further confirming that Treg is a potent negative regulator of the antitumor immune response and represents an attractive therapeutic target in breast cancer.
4.2. Myeloid-derived suppressor cells (MDSCs) in the immunosuppressive microenvironment
MDSCs are a heterogeneous population of cells of myeloid origin that expand during cancer, inflammation and infection. MDSCs have strong T cell-suppressing function and in mice, are mainly GR1+CD11b+ cells and in humans, CD14−CD11b+ cells (Gabrilovich and Nagaraj, 2009). Factors that induce MDSC expansion include GM-CSF, PGE2, IL-6, stem cell factor (SCF) and VEGF, while IFN-γ, ligands of toll like receptors, IL-13, IL-4 and TGF-β are associated with MDSC activation (Gabrilovich and Nagaraj, 2009). Among these factors, GM-CSF is considered one of the primary breast cancer-derived soluble factors involved in the differentiation of monocytic/granulocytic progenitor cells into MDSCs (Fig. 4A). This finding suggests caution in the use of GM-CSF for ex vivo expansion of dendritic cells for cell-based immunotherapy or as an adjuvant for vaccines (Morales et al., 2010). MDSCs can suppress T cell proliferation and activity through production of reactive oxygen species (ROS), arginase and nitric oxide (NO) in an antigen-independent manner (Gabrilovich and Nagaraj, 2009). MDSCs can also directly disrupt the binding of specific peptide-MHC dimers to CD8 expressing T cells through nitration of tyrosines in the TCR-CD8 complexes. This makes CTLs unable to bind to the peptide-MHC complex and therefore inhibits antitumor activity (Nagaraj et al., 2007). MDSCs also induce nitration of MHC class I molecules on breast cancer cells, making them unable to effectively present specific peptide, and thus rendering tumor cells resistant to antigen-specific CTLs (Lu et al., 2011). In addition to acting as a potent T cell suppressor, MDSCs can reduce NK cell activity and increase breast cancer metastasis during gestation (Mauti et al., 2011). MDSCs also suppress antitumor response through induction of Treg cells (Huang et al., 2006). Effective immunosurveillance against meta-static breast cancer requires reduction of MDSC activation (Sinha et al., 2005), and altering the immunosuppressive function of MDSC is required for IL-12-induced anti-breast cancer immune response (Steding et al., 2011). These studies further demonstrate that MDSCs negatively regulate antitumor immune responses and that MDSC suppression may enhance immunosurveillance against breast cancer development and metastasis.
4.3. Immunosuppressive soluble factors
Soluble factors produced by the tumor as well as surrounding stromal cells also contribute to the formation of the immunosuppressive tumor microenvironment. Breast cancer cells can block NK cell function through secretion of soluble forms of ligand MICA (sMICA), which induces NKG2D degradation (Groh et al., 2002). Overproduction of the enzyme IDO by cancer cells suppresses CD8+ T cell activity (Uyttenhove et al., 2003), and IDO inhibition potentiates responses of breast cancer to chemotherapy (Muller et al., 2005). While TGF-β-mediated signaling in breast cancer cells may play opposite roles during different stages of tumor development (Padua et al., 2008; Araki et al., 2010; Drabsch and ten Dijke, 2011), it is widely recognized as one of the strongest immunosuppressive cytokines in the tumor microenvironment (Wrzesinski et al., 2007). TGF-β-dependent, anti-estrogen treatment-induced immunosuppressive tumor microenvironment may contribute to the development of estrogen resistance in breast cancer (Joffroy et al., 2010). The role of IL-10, which is an important immunosuppressive cytokine in breast cancer, is reviewed elsewhere (Hamidullah et al., 2012). Also, PGE2 is another important immunosuppressive mediator found in the tumor microenvironment and suppresses antitumor immune responses through various mechanisms, including enhancement of the suppressive function of Tregs and MDSCs (Chen and Smyth, 2011). In addition, tumor associated fibroblasts (TAF) often contribute to the immunosuppressive microenvironment through mechanisms such as production of tolerogenic cytokines, abrogation of antitumor function of immune effector cells and promotion of the recruitment of immunosuppressive cells (Bernhard et al., 2008; Tchou and Conejo-Garcia, 2012; Phan-Lai et al., 2013). In summary, suppressive immune cells and soluble factors act together to diminish effective antitumor immune responses and promote breast cancer progression and metastasis (Fig. 4A).
5. Protumorigenic inflammation in breast cancer
The main types of inflammation in tumorigenesis and cancer include: Chronic inflammation that precedes tumor development, tumor-associated inflammation and therapy-induced inflammation. Inflammation induces an angiogenic switch (Grivennikov et al., 2010). The roles of proinflammatory cytokines and chemokines, such as IL-1, IL-6, IL-8, TNF-α, MCP-1, CCL5 and CXCL12 in breast cancer have been reviewed previously (Ben-Baruch, 2003, Goldberg and Schwertfeger, 2010, Baumgarten and Frasor, 2012). Here we focus on tumor-associated and therapy-induced inflammation and discuss the most recent findings that may lead to novel therapeutic targets.
5.1. Protumorigenic inflammation in breast cancer development and therapy-resistance
The transient induction of IL-6 by monocyte-derived MCP-1 has recently been shown to drive a feed-forward inflammatory signaling pathway (or cascade) that leads to constitutive IL-6 production and breast cancer cell transformation and tumorigenesis (Rokavec et al., 2012), revealing a novel mechanistic link between IL-6 and breast cancer initiation. Breast cancer stem cells (CSC) represent a population of cells associated with treatment resistance and relapse following therapy (Kakarala and Wicha, 2008). IL-6 is able to enhance recruitment of bone marrow-derived mesenchymal stem cells (MSCs) to sites of growing breast tumors as well as production of CXCL7 in MSCs, which promotes the proliferation of the breast CSC population (Liu et al., 2011). Another recent study revealed that IL-6 drives a feed-forward inflammatory loop, which leads to expansion of breast CSC populations and resistance to trastuzumab in HER2+ breast cancer (Korkaya et al., 2012). These studies again suggest that IL-6 is one of the most important cytokines associated with breast cancer progression and treatment. Blockade of IL-8/CXCR1 signaling significantly reduces the breast CSC population as well as systemic metastasis, suggesting that inhibition of this signaling pathway may enhance traditional chemotherapy by simultaneously targeting the CSC population (Ginestier et al., 2010). Therefore, inflammatory cytokines and chemokines promote breast cancer development and metastasis by acting on the CSC population, and interruption of relevant signaling pathways in CSC may represent attractive therapeutic targets (Korkaya et al., 2011).
Using both syngeneic and xenograft breast cancer preclinical models, one recent study revealed that chemotherapy-induced inflammation is one of the main contributors to chemo-resistance and metastasis. Profiles of cytokines and chemokines in the tumor microenvironment further showed that chemotherapy strikingly induces endothelial cell production of TNF-α. This enhances tumor cell CXCL1/2 production through NF-κB activation, which, in turn, facilitates recruitment of CD11b+Gr1+ MDSCs. These cells release S100A8/9, an inflammatory modulator that activates the p70S6K and ERK1/2 signaling pathways and provides a survival advantage for both primary and metastatic tumor cells. Disruption of the CXCL1/2-S100A8/9 axis by CXCR2 inhibition increases the effectiveness of chemotherapy (Acharyya et al., 2012). TNF-α can also promote breast cancer metastasis by inducing the epithelial-mesenchymal transition (EMT) through the NK-κB-mediated transcriptional activation of Twist1 (Li et al., 2012). Additionally, the microRNA miR-520/373 family has been revealed to be a tumor-suppressor in ERα− breast cancers by diminishing IL-6 and IL-8 production through negatively regulating NF-κB-mediated transcription and TGF-β-activated signaling pathway (Keklikoglou et al., 2012). Lastly, IL-18 was recently identified as a cytokine that contributes to doxorubicin resistance in breast cancer treatment (Yao et al., 2011). These data together suggest that enhancing proliferation and survival of breast cancer cells as well as breast CSC by networks of cytokines and chemokines are the main mechanisms by which inflammation promotes breast tumor development, metastasis, therapy-resistance and relapse.
5.2. Inflammation-promoted angiogenesis in breast cancer progression
Inflammation also accelerates breast cancer progression through promoting tumor angiogenesis. Several types of immune cells and associated mediators are involved in this process. Among these, the tumor associated macrophage (TAM) is recognized as one of the most prominent cell types associated with increased tumor angiogenesis as well as reduced disease-free survival (Leek et al., 1996; Lin et al., 2001; Lin and Pollard, 2007; Laoui et al., 2011). Blockade of CSF1R signaling, which diminishes TAM infiltration following chemotherapy, significantly increases treatment efficacy. Another study showed that combining the inhibition of IL-1β, SDF-1 and integrin-α4β1 also enhances the efficacy of breast cancer chemotherapy by limiting infiltration of TAMs (Schmid et al., 2011). These findings suggest that altering the immune response from immunosuppressive to antitumor is a promising strategy for improving standard breast cancer treatments (De Palma and Lewis, 2011; DeNardo et al., 2011). Other cells, such as bone marrow-derived Tie2 expressing monocytes (TEMs), tumor associated neutrophils (TANs), immature dendritic cells (DCs) and mast cells all plays roles in breast cancer angiogenesis (Yu and Rak, 2003; De Palma et al., 2005; Fainaru et al., 2010; Gregory and Houghton, 2011). Tissue hypoxia and HIF-1α-induced proangiogenic factors also contribute to pathogenic angiogenesis during breast cancer progression (Kimbro and Simons, 2006). These findings suggest that the angiogenic switch during breast cancer development is regulated by proangiogenic immune cell infiltration as well as hypoxia resulting from rapid expansion of tumor mass (Fig. 4A).
6. Inflammation as a promoter for more aggressive ERα+ breast cancer
Epidemiologic studies showed that regular use of nonsteroidal anti-inflammatory drugs (NSAIDS), such as aspirin, reduce the risk of ERα+ but not ERα− breast cancers (Terry et al., 2004, Wang and Dubois, 2010). Recent research strongly suggests that a proinflammatory tumor microenvironment is an important factor contributing to resistance to endocrine therapy (Osborne and Schiff, 2011). Numerous studies have shown that ERα+ breast cancers are more responsive to proinflammatory cytokines. For instances, IL-6 promotes proliferation as well as aggressive phenotype of ERα+ breast cancer cells (Sasser et al., 2007, Sullivan et al., 2009); IL-1β reverses the suppression of estrogen target genes by SERM (Zhu et al., 2006), suggesting IL-1β may be directly associated with resistance to endocrine therapy; IL-1β also induces PI-9 expression (Kannan-Thulasiraman and Shapiro, 2002); TNF-α induces expression of genes associated with invasion, proliferation and metastasis in ERα+ breast cancer cells (Yin et al., 2009). Taken together, these works suggest that inflammation and inflammatory cytokines may induce more aggressive phenotypes of ERα+ breast cancer.
How inflammation and ERα synergistically promote aggressiveness is not completely understood. The NF-κB family transcription factors are recognized as the essential link between inflammation and cancers (Ben-Neriah and Karin, 2011). Zhou et al. linked the NF-κB signaling pathway to endocrine-resistant breast cancer (Zhou et al., 2005). More recently, Frasor et al. proposed that crosstalk between ERα and inflammation-activated NF-κB modulates gene expression in breast cancer cells and promotes this aggressive phenotype (Baumgarten and Frasor, 2012). It has long been known that ERα represses NF-κB activity (Quaedackers et al., 2007; Wang et al., 2007; Gionet et al., 2009). ERα suppression of NF-κB may act as an underlying mechanism that helps to explain why Luminal A subtype breast cancers exhibit a better prognosis. Those tumors are characterized by high ERα expression, lower NF-κB activity and low production of proinflammatory cytokines (Baumgarten and Frasor, 2012). Therefore, activation of NF-κB by inflammation may negate the effect of its suppression by ERα, which ultimately induces a more aggressive breast cancer phenotype. It has also been shown that NF-κB can suppress ERα activity in several experimental models (Bodine et al., 1999; Feldman et al., 2007; Wang et al., 2009). NF-κB and ERα can also synergistically induce expression of target genes which are involved in inflammation (Frasor et al., 2008), apoptosis (Stanculescu et al., 2010; Pradhan et al., 2012), proliferation (Tu et al., 2006) and drug-resistance (Pradhan et al., 2010). These studies in total suggest that NF-κB activation by a proinflammatory tumor microenvironment can promote an aggressive breast cancer phenotype through activating or suppressing ERα target gene expression in a context-dependent manner (Fig. 4B).
7. Concluding remarks
Breast cancer progression is not an entirely cell-autonomous process. Development and metastasis of breast tumors are influenced and even driven by cells of the immune system and associated inflammatory mediators in the tumor microenvironment. The balance between antitumor immunity and tumor-promoting inflammation determines whether the tumor will progress or be controlled or eliminated. Inflammation induced during the natural tumor progression is probably one of the main reasons that the immune system cannot effectively indefinitely restrain the expansion of breast cancer. Moreover, inflammation induced during breast cancer therapy is often protumorigenic and responsible for treatment resistance, and preliminary evidence suggests that it might also be involved in treatment-induced metastasis and relapse. Indeed, improved outcomes have been observed in patients receiving anti-inflammatory treatment in combination with standard breast cancer treatments. Therefore, the complete identification of protumorigenic inflammatory mediators and their related signaling pathways is critical for designing effective therapies to reverse the tumor-promoting microenvironment and enhance overall therapeutic efficacy. Identification of natural genetic variations that affect inflammation and immunity may also be useful for designing new preventive approaches for populations with a high risk of developing breast cancer, and provide better therapeutic targets for diagnosed breast cancer patients. Several early-phase clinical trials of immunotherapy against breast cancers have achieved safety and some demonstrate significant beneficial effects (Geller et al., 2011; Hardy et al., 2011; Clive et al., 2012; de la Torre et al., 2012; Hamilton et al., 2012; Marchini et al., 2013). More clinical trials are being carried out, including trials of therapeutic vaccines GVAX (ClinicalTrials.gov, NCT00971737) and NeuVax (ClinicalTrials.gov, NCT01479244) and anti-OX40 antibody (ClinicalTrials.gov, NCT01642290). In summary, there is compelling data showing that immunity and inflammation shape the development of breast cancer. Knowledge gained from basic and clinical researches can soon be used to implement immunotherapy as a treatment modality for many breast cancer patients.
Acknowledgments
Our research and preparation of this review is supported by NIH Grant DK 071909 (to DJS). The authors wish to gratefully acknowledge Dr. Yon Sung (Stanford University) for critically reading this manuscript.
References
- Acharyya S, Oskarsson T, Vanharanta S, Malladi S, Kim J, Morris PG, Manova-Todorova K, Leversha M, Hogg N, Seshan VE, Norton L, Brogi E, Massague J. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150:165–178. doi: 10.1016/j.cell.2012.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki S, Eitel JA, Batuello CN, Bijangi-Vishehsaraei K, Xie XJ, Danielpour D, Pollok KE, Boothman DA, Mayo LD. TGF-beta1-induced expression of human Mdm2 correlates with late-stage metastatic breast cancer. J Clin Invest. 2010;120:290–302. doi: 10.1172/JCI39194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargou RC, Wagener C, Bommert K, Mapara MY, Daniel PT, Arnold W, Dietel M, Guski H, Feller A, Royer HD, Dorken B. Overexpression of the death-promoting gene bax-alpha which is downregulated in breast cancer restores sensitivity to different apoptotic stimuli and reduces tumor growth in SCID mice. J Clin Invest. 1996;97:2651–2659. doi: 10.1172/JCI118715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Nachat-Kappes R, Caldefie-Chezet F, Vasson MP. Eicosanoids and adipokines in breast cancer: from molecular mechanisms to clinical considerations. Antioxid Redox Signal. 2012 doi: 10.1089/ars.2011.4408. [DOI] [PubMed] [Google Scholar]
- Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, Banham AH. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24:5373–5380. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- Baumgarten SC, Frasor J. Minireview: inflammation: an instigator of more aggressive estrogen receptor (ER) positive breast cancers. Mol Endocrinol. 2012;26:360–371. doi: 10.1210/me.2011-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Baruch A. Host microenvironment in breast cancer development: inflammatory cells, cytokines and chemokines in breast cancer progression: reciprocal tumor-microenvironment interactions. Breast Cancer Res. 2003;5:31–36. doi: 10.1186/bcr554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat Immunol. 2011;12:715–723. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- Bernhard H, Neudorfer J, Gebhard K, Conrad H, Hermann C, Nahrig J, Fend F, Weber W, Busch DH, Peschel C. Adoptive transfer of autologous, HER2-specific, cytotoxic T lymphocytes for the treatment of HER2-overexpressing breast cancer. Cancer Immunol Immunother. 2008;57:271–280. doi: 10.1007/s00262-007-0355-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidwell BN, Slaney CY, Withana NP, Forster S, Cao Y, Loi S, Andrews D, Mikeska T, Mangan NE, Samarajiwa SA, de Weerd NA, Gould J, Argani P, Moller A, Smyth MJ, Anderson RL, Hertzog PJ, Parker BS. Silencing of Irf7 pathways in breast cancer cells promotes bone metastasis through immune escape. Nat Med. 2012 doi: 10.1038/nm.2830. [DOI] [PubMed] [Google Scholar]
- Bodine PV, Harris HA, Komm BS. Suppression of ligand-dependent estrogen receptor activity by bone-resorbing cytokines in human osteoblasts. Endocrinology. 1999;140:2439–2451. doi: 10.1210/endo.140.6.6612. [DOI] [PubMed] [Google Scholar]
- Bohling SD, Allison KH. Immunosuppressive regulatory T cells are associated with aggressive breast cancer phenotypes: a potential therapeutic target. Mod Pathol. 2008;21:1527–1532. doi: 10.1038/modpathol.2008.160. [DOI] [PubMed] [Google Scholar]
- Chanrion M, Negre V, Fontaine H, Salvetat N, Bibeau F, Mac Grogan G, Mauriac L, Katsaros D, Molina F, Theillet C, Darbon JM. A gene expression signature that can predict the recurrence of tamoxifen-treated primary breast cancer. Clin Cancer Res. 2008;14:1744–1752. doi: 10.1158/1078-0432.CCR-07-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen EP, Smyth EM. COX-2 and PGE2-dependent immunomodulation in breast cancer. Prostaglandins Other Lipid Mediat. 2011;96:14–20. doi: 10.1016/j.prostaglandins.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke R, Leonessa F, Welch JN, Skaar TC. Cellular and molecular pharmacology of antiestrogen action and resistance. Pharmacol Rev. 2001;53:25–71. [PubMed] [Google Scholar]
- Clarke R, Liu MC, Bouker KB, Gu Z, Lee RY, Zhu Y, Skaar TC, Gomez B, O’Brien K, Wang Y, Hilakivi-Clarke LA. Antiestrogen resistance in breast cancer and the role of estrogen receptor signaling. Oncogene. 2003;22:7316–7339. doi: 10.1038/sj.onc.1206937. [DOI] [PubMed] [Google Scholar]
- Clive KS, Tyler JA, Clifton GT, Holmes JP, Ponniah S, Peoples GE, Mittendorf EA. The GP2 peptide: a HER2/neu-based breast cancer vaccine. J Surg Oncol. 2012;105:452–458. doi: 10.1002/jso.21723. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Pollard JW. Leukocytes in mammary development and cancer. Cold Spring Harb Perspect Biol. 2011:3. doi: 10.1101/cshperspect.a003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham TD, Jiang X, Shapiro DJ. Expression of high levels of human proteinase inhibitor 9 blocks both perforin/granzyme and Fas/Fas ligand-mediated cytotoxicity. Cell Immunol. 2007;245:32–41. doi: 10.1016/j.cellimm.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kruijf EM, Sajet A, van Nes JG, Natanov R, Putter H, Smit VT, Liefers GJ, van den Elsen PJ, van de Velde CJ, Kuppen PJ. HLA-E and HLA-G expression in classical HLA class I-negative tumors is of prognostic value for clinical outcome of early breast cancer patients. J Immunol. 2010;185:7452–7459. doi: 10.4049/jimmunol.1002629. [DOI] [PubMed] [Google Scholar]
- de la Torre A, Hernandez J, Ortiz R, Cepeda M, Perez K, Car A, Viada C, Toledo D, Guerra PP, Garcia E, Arbolaez M, Fernandez LE. NGlycolylGM3/VSSP vaccine in metastatic breast cancer patients: results of phase I/IIa clinical trial. Breast Cancer (Auckl) 2012;6:151–157. doi: 10.4137/BCBCR.S8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, Naldini L. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- De Palma M, Lewis CE. Cancer: macrophages limit chemotherapy. Nature. 2011;472:303–304. doi: 10.1038/472303a. [DOI] [PubMed] [Google Scholar]
- DeNardo DG, Coussens LM. Inflammation and breast cancer. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 2007;9:212. doi: 10.1186/bcr1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA, Rugo HS, Hwang ES, Jirstrom K, West BL, Coussens LM. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewan MZ, Terunuma H, Ahmed S, Ohba K, Takada M, Tanaka Y, Toi M, Yamamoto N. Natural killer cells in breast cancer cell growth and metastasis in SCID mice. Biomed Pharmacother. 2005;59(Suppl 2):S375–S379. doi: 10.1016/s0753-3322(05)80082-4. [DOI] [PubMed] [Google Scholar]
- Disis ML, Calenoff E, McLaughlin G, Murphy AE, Chen W, Groner B, Jeschke M, Lydon N, McGlynn E, Livingston RB, et al. Existent T-cell and antibody immunity to HER-2/neu protein in patients with breast cancer. Cancer Res. 1994;54:16–20. [PubMed] [Google Scholar]
- Drabsch Y, ten Dijke P. TGF-beta signaling in breast cancer cell invasion and bone metastasis. J Mammary Gland Biol Neoplasia. 2011;16:97–108. doi: 10.1007/s10911-011-9217-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fainaru O, Almog N, Yung CW, Nakai K, Montoya-Zavala M, Abdollahi A, D’Amato R, Ingber DE. Tumor growth and angiogenesis are dependent on the presence of immature dendritic cells. FASEB J. 2010;24:1411–1418. doi: 10.1096/fj.09-147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman I, Feldman GM, Mobarak C, Dunkelberg JC, Leslie KK. Identification of proteins within the nuclear factor-kappa B transcriptional complex including estrogen receptor-alpha. Am J Obstet Gynecol. 2007;196:394, e1–11. doi: 10.1016/j.ajog.2006.12.033. discussion 394 e11–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H, Chen H, Omeroglu G, Meterissian S, Omeroglu A, Hallett M, Park M. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med. 2008;14:518–527. doi: 10.1038/nm1764. [DOI] [PubMed] [Google Scholar]
- Frasor J, Weaver AE, Pradhan M, Mehta K. Synergistic up-regulation of prostaglandin E synthase expression in breast cancer cells by 17beta-estradiol and proinflammatory cytokines. Endocrinology. 2008;149:6272–6279. doi: 10.1210/en.2008-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller MA, Cooley S, Judson PL, Ghebre R, Carson LF, Argenta PA, Jonson AL, Panoskaltsis-Mortari A, Curtsinger J, McKenna D, Dusenbery K, Bliss R, Downs LS, Miller JS. A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy. 2011;13:98–107. doi: 10.3109/14653249.2010.515582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginestier C, Liu S, Diebel ME, Korkaya H, Luo M, Brown M, Wicinski J, Cabaud O, Charafe-Jauffret E, Birnbaum D, Guan JL, Dontu G, Wicha MS. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Invest. 2010;120:485–497. doi: 10.1172/JCI39397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gionet N, Jansson D, Mader S, Pratt MA. NF-kappaB and estrogen receptor alpha interactions: Differential function in estrogen receptor-negative and -positive hormone-independent breast cancer cells. J Cell Biochem. 2009;107:448–459. doi: 10.1002/jcb.22141. [DOI] [PubMed] [Google Scholar]
- Gobert M, Treilleux I, Bendriss-Vermare N, Bachelot T, Goddard-Leon S, Arfi V, Biota C, Doffin AC, Durand I, Olive D, Perez S, Pasqual N, Faure C, Ray-Coquard I, Puisieux A, Caux C, Blay JY, Menetrier-Caux C. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res. 2009;69:2000–2009. doi: 10.1158/0008-5472.CAN-08-2360. [DOI] [PubMed] [Google Scholar]
- Goldberg JE, Schwertfeger KL. Proinflammatory cytokines in breast cancer: mechanisms of action and potential targets for therapeutics. Curr Drug Targets. 2010;11:1133–1146. doi: 10.2174/138945010792006799. [DOI] [PubMed] [Google Scholar]
- Gregory AD, Houghton AM. Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res. 2011;71:2411–2416. doi: 10.1158/0008-5472.CAN-10-2583. [DOI] [PubMed] [Google Scholar]
- Gritzapis AD, Baxevanis CN, Missitzis I, Katsanou ES, Alexis MN, Yotis J, Papamichail M. Quantitative fluorescence cytometric measurement of estrogen and progesterone receptors: correlation with the hormone binding assay. Breast Cancer Res Treat. 2003;80:1–13. doi: 10.1023/A:1024462416640. [DOI] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–738. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- Gutierrez LS, Eliza M, Niven-Fairchild T, Naftolin F, Mor G. The Fas/Fas-ligand system: a mechanism for immune evasion in human breast carcinomas. Breast Cancer Res Treat. 1999;54:245–253. doi: 10.1023/a:1006102601215. [DOI] [PubMed] [Google Scholar]
- Hamidullah, Changkija B, Konwar R. Role of interleukin-10 in breast cancer. Breast Cancer Res Treat. 2012;133:11–21. doi: 10.1007/s10549-011-1855-x. [DOI] [PubMed] [Google Scholar]
- Hamilton E, Blackwell K, Hobeika AC, Clay TM, Broadwater G, Ren XR, Chen W, Castro H, Lehmann F, Spector N, Wei J, Osada T, Lyerly HK, Morse MA. Phase 1 clinical trial of HER2-specific immunotherapy with concomitant HER2 kinase inhibition [corrected] J Transl Med. 2012;10:28. doi: 10.1186/1479-5876-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hardy NM, Mossoba ME, Steinberg SM, Fellowes V, Yan XY, Hakim FT, Babb RR, Avila D, Gea-Banacloche J, Sportes C, Levine BL, June CH, Khuu HM, Carpenter AE, Krumlauf MC, Dwyer AJ, Gress RE, Fowler DH, Bishop MR. Phase I trial of adoptive cell transfer with mixed-profile type-I/type-II allogeneic T cells for metastatic breast cancer. Clin Cancer Res. 2011;17:6878–6887. doi: 10.1158/1078-0432.CCR-11-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan A, Ghebeh H, Lehe C, Ahmad R, Dermime S. Therapeutic targeting of B7–H1 in breast cancer. Expert Opin Ther Targets. 2011;15:1211–1225. doi: 10.1517/14728222.2011.613826. [DOI] [PubMed] [Google Scholar]
- Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- Hupperets PS, Volovics L, Schouten LJ, Jager JJ, Schouten HC, Hillen HF, Blijham GH. The prognostic significance of steroid receptor activity in tumor tissues of patients with primary breast cancer. Am J Clin Oncol. 1997;20:546–551. doi: 10.1097/00000421-199712000-00002. [DOI] [PubMed] [Google Scholar]
- Jansen MP, Foekens JA, van Staveren IL, Dirkzwager-Kiel MM, Ritstier K, Look MP, Meijer-van Gelder ME, Sieuwerts AM, Portengen H, Dorssers LC, Klijn JG, Berns EM. Molecular classification of tamoxifen-resistant breast carcinomas by gene expression profiling. J Clin Oncol. 2005;23:732–740. doi: 10.1200/JCO.2005.05.145. [DOI] [PubMed] [Google Scholar]
- Jiang X, Orr BA, Kranz DM, Shapiro DJ. Estrogen induction of the granzyme B inhibitor, proteinase inhibitor 9, protects cells against apoptosis mediated by cytotoxic T lymphocytes and natural killer cells. Endocrinology. 2006;147:1419–1426. doi: 10.1210/en.2005-0996. [DOI] [PubMed] [Google Scholar]
- Jiang X, Ellison SJ, Alarid ET, Shapiro DJ. Interplay between the levels of estrogen and estrogen receptor controls the level of the granzyme inhibitor, proteinase inhibitor 9 and susceptibility to immune surveillance by natural killer cells. Oncogene. 2007;26:4106–4114. doi: 10.1038/sj.onc.1210197. [DOI] [PubMed] [Google Scholar]
- Jiang X, Patterson NM, Ling Y, Xie J, Helferich WG, Shapiro DJ. Low concentrations of the soy phytoestrogen genistein induce proteinase inhibitor 9 and block killing of breast cancer cells by immune cells. Endocrinology. 2008;149:5366–5373. doi: 10.1210/en.2008-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Villeneuve PJ, Wielgosz A, Schaubel DE, Fenton SS, Mao Y. The incidence of cancer in a population-based cohort of Canadian heart transplant recipients. Am J Transplant. 2010;10:637–645. doi: 10.1111/j.1600-6143.2009.02973.x. [DOI] [PubMed] [Google Scholar]
- Joffroy CM, Buck MB, Stope MB, Popp SL, Pfizenmaier K, Knabbe C. Antiestrogens induce transforming growth factor beta-mediated immunosuppression in breast cancer. Cancer Res. 2010;70:1314–1322. doi: 10.1158/0008-5472.CAN-09-3292. [DOI] [PubMed] [Google Scholar]
- Kaiserman D, Bird PI. Control of granzymes by serpins. Cell Death Differ. 2010;17:586–595. doi: 10.1038/cdd.2009.169. [DOI] [PubMed] [Google Scholar]
- Kakarala M, Wicha MS. Implications of the cancer stem-cell hypothesis for breast cancer prevention and therapy. J Clin Oncol. 2008;26:2813–2820. doi: 10.1200/JCO.2008.16.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori H, Krieg S, Mao C, Di Pippo VA, Wang S, Zajchowski DA, Shapiro DJ. Proteinase inhibitor 9, an inhibitor of granzyme B-mediated apoptosis, is a primary estrogen-inducible gene in human liver cells. J Biol Chem. 2000;275:5867–5873. doi: 10.1074/jbc.275.8.5867. [DOI] [PubMed] [Google Scholar]
- Kaneko K, Ishigami S, Kijima Y, Funasako Y, Hirata M, Okumura H, Shinchi H, Koriyama C, Ueno S, Yoshinaka H, Natsugoe S. Clinical implication of HLA class I expression in breast cancer. BMC Cancer. 2011;11:454. doi: 10.1186/1471-2407-11-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan-Thulasiraman P, Shapiro DJ. Modulators of inflammation use nuclear factor-kappa B and activator protein-1 sites to induce the caspase-1 and granzyme B inhibitor, proteinase inhibitor 9. J Biol Chem. 2002;277:41230–41239. doi: 10.1074/jbc.M200379200. [DOI] [PubMed] [Google Scholar]
- Karavitis J, Hix LM, Shi YH, Schultz RF, Khazaie K, Zhang M. Regulation of COX2 expression in mouse mammary tumor cells controls bone metastasis and PGE2-induction of regulatory T cell migration. PLoS ONE. 2012;7:e46342. doi: 10.1371/journal.pone.0046342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keklikoglou I, Koerner C, Schmidt C, Zhang JD, Heckmann D, Shavinskaya A, Allgayer H, Guckel B, Fehm T, Schneeweiss A, Sahin O, Wiemann S, Tschulena U. MicroRNA-520/373 family functions as a tumor suppressor in estrogen receptor negative breast cancer by targeting NF-kappaB and TGF-beta signaling pathways. Oncogene. 2012;31:4150–4163. doi: 10.1038/onc.2011.571. [DOI] [PubMed] [Google Scholar]
- Kimbro KS, Simons JW. Hypoxia-inducible factor-1 in human breast and prostate cancer. Endocr Relat Cancer. 2006;13:739–749. doi: 10.1677/erc.1.00728. [DOI] [PubMed] [Google Scholar]
- Kirk GD, Merlo C, POD, Mehta SH, Galai N, Vlahov D, Samet J, Engels EA. HIV infection is associated with an increased risk for lung cancer, independent of smoking, Clin. Infect Dis. 2007;45:103–110. doi: 10.1086/518606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohrt HE, Houot R, Weiskopf K, Goldstein MJ, Scheeren F, Czerwinski D, Colevas AD, Weng WK, Clarke MF, Carlson RW, Stockdale FE, Mollick JA, Chen L, Levy R. Stimulation of natural killer cells with a CD137-specific antibody enhances trastuzumab efficacy in xenotransplant models of breast cancer. J Clin Invest. 2012;122:1066–1075. doi: 10.1172/JCI61226. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Konjevic G, Spuzic I. Stage dependence of NK cell activity and its modulation by interleukin 2 in patients with breast cancer. Neoplasma. 1993;40:81–85. [PubMed] [Google Scholar]
- Kontani K, Taguchi O, Narita T, Izawa M, Hiraiwa N, Zenita K, Takeuchi T, Murai H, Miura S, Kannagi R. Modulation of MUC1 mucin as an escape mechanism of breast cancer cells from autologous cytotoxic T-lymphocytes. Br J Cancer. 2001;84:1258–1264. doi: 10.1054/bjoc.2000.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkaya H, Liu S, Wicha MS. Breast cancer stem cells, cytokine networks, and the tumor microenvironment. J Clin Invest. 2011;121:3804–3809. doi: 10.1172/JCI57099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkaya H, Kim GI, Davis A, Malik F, Henry NL, Ithimakin S, Quraishi AA, Tawakkol N, D’Angelo R, Paulson AK, Chung S, Luther T, Paholak HJ, Liu S, Hassan KA, Zen Q, Clouthier SG, Wicha MS. Activation of an IL6 inflammatory loop mediates trastuzumab resistance in HER2+ breast cancer by expanding the cancer stem cell population. Mol Cell. 2012;47:570–584. doi: 10.1016/j.molcel.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg AJ, Krieg SA, Ahn BS, Shapiro DJ. Interplay between estrogen response element sequence and ligands controls in vivo binding of estrogen receptor to regulated genes. J Biol Chem. 2004;279:5025–5034. doi: 10.1074/jbc.M307076200. [DOI] [PubMed] [Google Scholar]
- Krieg SA, Krieg AJ, Shapiro DJ. A unique downstream estrogen responsive unit mediates estrogen induction of proteinase inhibitor-9, a cellular inhibitor of IL-1beta- converting enzyme (caspase 1) Mol Endocrinol. 2001;15:1971–1982. doi: 10.1210/mend.15.11.0719. [DOI] [PubMed] [Google Scholar]
- Kristensen VN, Vaske CJ, Ursini-Siegel J, Van Loo P, Nordgard SH, Sachidanandam R, Sorlie T, Warnberg F, Haakensen VD, Helland A, Naume B, Perou CM, Haussler D, Troyanskaya OG, Borresen-Dale AL. Integrated molecular profiles of invasive breast tumors and ductal carcinoma in situ (DCIS) reveal differential vascular and interleukin signaling. Proc Natl Acad Sci USA. 2012;109:2802–2807. doi: 10.1073/pnas.1108781108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer JA, Micheau O, Schneider P, Bovenschen N, Broekhuizen R, Quadir R, Strik MC, Hack CE, Tschopp J. Ectopic expression of the serine protease inhibitor PI9 modulates death receptor-mediated apoptosis. Cell Death Differ. 2007;14:1486–1496. doi: 10.1038/sj.cdd.4402152. [DOI] [PubMed] [Google Scholar]
- Lacroix M, Toillon RA, Leclercq G. P53 and breast cancer, an update. Endocr Relat Cancer. 2006;13:293–325. doi: 10.1677/erc.1.01172. [DOI] [PubMed] [Google Scholar]
- Laoui D, Movahedi K, Van Overmeire E, Van den Bossche J, Schouppe E, Mommer C, Nikolaou A, Morias Y, De Baetselier P, Van Ginderachter JA. Tumor-associated macrophages in breast cancer: distinct subsets, distinct functions. Int J Dev Biol. 2011;55:861–867. doi: 10.1387/ijdb.113371dl. [DOI] [PubMed] [Google Scholar]
- Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996;56:4625–4629. [PubMed] [Google Scholar]
- Li CW, Xia W, Huo L, Lim SO, Wu Y, Hsu JL, Chao CH, Yamaguchi H, Yang NK, Ding Q, Wang Y, Lai YJ, LaBaff AM, Wu TJ, Lin BR, Yang MH, Hortobagyi GN, Hung MC. Epithelial–mesenchymal transition induced by TNF-alpha requires NF-kappaB-mediated transcriptional upregulation of Twist1. Cancer Res. 2012;72:1290–1300. doi: 10.1158/0008-5472.CAN-11-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin EY, Pollard JW. Tumor-associated macrophages press the angiogenic switch in breast cancer. Cancer Res. 2007;67:5064–5066. doi: 10.1158/0008-5472.CAN-07-0912. [DOI] [PubMed] [Google Scholar]
- Liu S, Ginestier C, Ou SJ, Clouthier SG, Patel SH, Monville F, Korkaya H, Heath A, Dutcher J, Kleer CG, Jung Y, Dontu G, Taichman R, Wicha MS. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res. 2011;71:614–624. doi: 10.1158/0008-5472.CAN-10-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Lachapelle J, Leung S, Gao D, Foulkes WD, Nielsen TO. CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Res. 2012;14:R48. doi: 10.1186/bcr3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- Lu T, Ramakrishnan R, Altiok S, Youn JI, Cheng P, Celis E, Pisarev V, Sherman S, Sporn MB, Gabrilovich D. Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. J Clin Invest. 2011;121:4015–4029. doi: 10.1172/JCI45862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, Ellis IO, Green AR. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29:1949–1955. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- Mamessier E, Sylvain A, Thibult ML, Houvenaeghel G, Jacquemier J, Castellano R, Goncalves A, Andre P, Romagne F, Thibault G, Viens P, Birnbaum D, Bertucci F, Moretta A, Olive D. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Invest. 2011;121:3609–3622. doi: 10.1172/JCI45816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchini C, Kalogris C, Garulli C, Pietrella L, Gabrielli F, Curcio C, Quaglino E, Cavallo F, Amici A. Tailoring DNA Vaccines: Designing Strategies Against HER2-Positive Cancers. Front Oncol. 2013;3:122. doi: 10.3389/fonc.2013.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauti LA, Le Bitoux MA, Baumer K, Stehle JC, Golshayan D, Provero P, Stamenkovic I. Myeloid-derived suppressor cells are implicated in regulating permissiveness for tumor metastasis during mouse gestation. J Clin Invest. 2011;121:2794–2807. doi: 10.1172/JCI41936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mine T, Matsueda S, Li Y, Tokumitsu H, Gao H, Danes C, Wong KK, Wang X, Ferrone S, Ioannides CG. Breast cancer cells expressing stem cell markers CD44+ CD24 lo are eliminated by Numb-1 peptide-activated T cells. Cancer Immunol Immunother. 2009;58:1185–1194. doi: 10.1007/s00262-008-0623-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittendorf EA, Alatrash G, Qiao N, Wu Y, Sukhumalchandra P, St John LS, Philips AV, Xiao H, Zhang M, Ruisaard K, Clise-Dwyer K, Lu S, Molldrem JJ. Breast cancer cell uptake of the inflammatory mediator neutrophil elastase triggers an anticancer adaptive immune response. Cancer Res. 2012;72:3153–3162. doi: 10.1158/0008-5472.CAN-11-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales JK, Kmieciak M, Knutson KL, Bear HD, Manjili MH. GM-CSF is one of the main breast tumor-derived soluble factors involved in the differentiation of CD11b-Gr1- bone marrow progenitor cells into myeloid-derived suppressor cells. Breast Cancer Res Treat. 2010;123:39–49. doi: 10.1007/s10549-009-0622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller AJ, DuHadaway JB, Donover PS, Sutanto-Ward E, Prendergast GC. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med. 2005;11:312–319. doi: 10.1038/nm1196. [DOI] [PubMed] [Google Scholar]
- Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber DL, Schneck J, Gabrilovich DI. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt-Berard EM, Berard F, Banchereau J, Palucka AK. Dendritic cells loaded with killed breast cancer cells induce differentiation of tumor-specific cytotoxic T lymphocytes. Breast Cancer Res. 2004;6:R322–8. doi: 10.1186/bcr794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls C, Pinto AR, Li H, Li L, Wang L, Simpson R, Liu JP. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) induces cancer cell senescence by interacting with telomerase RNA component. Proc Natl Acad Sci USA. 2012;109:13308–13313. doi: 10.1073/pnas.1206672109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara M, Yamaguchi Y, Matsuura K, Murakami S, Arihiro K, Okada M. Possible involvement of regulatory T cells in tumor onset and progression in primary breast cancer. Cancer Immunol Immunother. 2009;58:441–447. doi: 10.1007/s00262-008-0570-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olkhanud PB, Baatar D, Bodogai M, Hakim F, Gress R, Anderson RL, Deng J, Xu M, Briest S, Biragyn A. Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells. Cancer Res. 2009;69:5996–6004. doi: 10.1158/0008-5472.CAN-08-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olkhanud PB, Damdinsuren B, Bodogai M, Gress RE, Sen R, Wejksza K, Malchinkhuu E, Wersto RP, Biragyn A. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4(+) T cells to T-regulatory cells. Cancer Res. 2011;71:3505–3515. doi: 10.1158/0008-5472.CAN-10-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011;62:233–247. doi: 10.1146/annurev-med-070909-182917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padua D, Zhang XH, Wang Q, Nadal C, Gerald WL, Gomis RR, Massague J. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008;133:66–77. doi: 10.1016/j.cell.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peoples GE, Goedegebuure PS, Smith R, Linehan DC, Yoshino I, Eberlein TJ. Breast and ovarian cancer-specific cytotoxic T lymphocytes recognize the same HER2/neu-derived peptide. Proc Natl Acad Sci USA. 1995;92:432–436. doi: 10.1073/pnas.92.2.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan-Lai V, Florczyk SJ, Kievit FM, Wang K, Gad E, Disis ML, Zhang M. Three-dimensional scaffolds to evaluate tumor associated fibroblast-mediated suppression of breast tumor specific T cells. Biomacromolecules. 2013;14:1330–1337. doi: 10.1021/bm301928u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan M, Bembinster LA, Baumgarten SC, Frasor J. Proinflammatory cytokines enhance estrogen-dependent expression of the multidrug transporter gene ABCG2 through estrogen receptor and NF{kappa}B cooperativity at adjacent response elements. J Biol Chem. 2010;285:31100–31106. doi: 10.1074/jbc.M110.155309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan M, Baumgarten SC, Bembinster LA, Frasor J. CBP mediates NF-kappaB-dependent histone acetylation and estrogen receptor recruitment to an estrogen response element in the BIRC3 promoter. Mol Cell Biol. 2012;32:569–575. doi: 10.1128/MCB.05869-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaedackers ME, van den Brink CE, van der Saag PT, Tertoolen LG. Direct interaction between estrogen receptor alpha and NF-kappaB in the nucleus of living cells. Mol Cell Endocrinol. 2007;273:42–50. doi: 10.1016/j.mce.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan R, Assudani D, Nagaraj S, Hunter T, Cho HI, Antonia S, Altiok S, Celis E, Gabrilovich DI. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J Clin Invest. 2010;120:1111–1124. doi: 10.1172/JCI40269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rech AJ, Mick R, Martin S, Recio A, Aqui NA, Powell DJ, Jr, Colligon TA, Trosko JA, Leinbach LI, Pletcher CH, Tweed CK, DeMichele A, Fox KR, Domchek SM, Riley JL, Vonderheide RH. CD25 blockade depletes and selectively reprograms regulatory T cells in concert with immunotherapy in cancer patients. Sci Transl Med. 2012;4:134ra62. doi: 10.1126/scitranslmed.3003330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riera J, Simpson JF, Tamayo R, Battifora H. Use of cultured cells as a control for quantitative immunocytochemical analysis of estrogen receptor in breast cancer, The Quicgel method. Am J Clin Pathol. 1999;111:329–335. doi: 10.1093/ajcp/111.3.329. [DOI] [PubMed] [Google Scholar]
- Roberti MP, Rocca YS, Amat M, Pampena MB, Loza J, Colo F, Fabiano V, Loza CM, Arriaga JM, Bianchini M, Barrio MM, Bravo AI, Domenichini E, Chacon R, Mordoh J, Levy EM. IL-2- or IL-15-activated NK cells enhance Cetuximab-mediated activity against triple-negative breast cancer in xenografts and in breast cancer patients. Breast Cancer Res Treat. 2012 doi: 10.1007/s10549-012-2287-y. [DOI] [PubMed] [Google Scholar]
- Rokavec M, Wu W, Luo JL. IL6-mediated suppression of miR-200c directs constitutive activation of inflammatory signaling circuit driving transformation and tumorigenesis. Mol Cell. 2012;45:777–789. doi: 10.1016/j.molcel.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan BM, Konecny GE, Kahlert S, Wang HJ, Untch M, Meng G, Pegram MD, Podratz KC, Crown J, Slamon DJ, Duffy MJ. Survivin expression in breast cancer predicts clinical outcome and is associated with HER2, VEGF, urokinase plasminogen activator and PAI-1. Ann Oncol. 2006;17:597–604. doi: 10.1093/annonc/mdj121. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- Sancho-Garnier H, Delarue JC, Mouriesse H, Contesso G, May-Levin F, Gotteland M, May E. Is the negative prognostic value of high oestrogen receptor (ER) levels in postmenopausal breast cancer patients due to a modified ER gene product? Eur J Cancer. 1995;31A:1851–1855. doi: 10.1016/0959-8049(95)00387-x. [DOI] [PubMed] [Google Scholar]
- Sasser AK, Sullivan NJ, Studebaker AW, Hendey LF, Axel AE, Hall BM. Interleukin-6 is a potent growth factor for ER-alpha-positive human breast cancer. FASEB J. 2007;21:3763–3770. doi: 10.1096/fj.07-8832com. [DOI] [PubMed] [Google Scholar]
- Schlom J. Therapeutic cancer vaccines: current status and moving forward. J Natl Cancer Inst. 2012;104:599–613. doi: 10.1093/jnci/djs033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid MC, Avraamides CJ, Foubert P, Shaked Y, Kang SW, Kerbel RS, Varner JA. Combined blockade of integrin-alpha4beta1 plus cytokines SDF-1alpha or IL-1beta potently inhibits tumor inflammation and growth. Cancer Res. 2011;71:6965–6975. doi: 10.1158/0008-5472.CAN-11-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Shin MS, Kim HS, Lee SH, Park WS, Kim SY, Park JY, Lee JH, Lee SK, Lee SN, Jung SS, Han JY, Kim H, Lee JY, Yoo NJ. Mutations of tumor necrosis factor-related apoptosis-inducing ligand receptor 1 (TRAIL-R1) and receptor 2 (TRAIL-R2) genes in metastatic breast cancers. Cancer Res. 2001;61:4942–4946. [PubMed] [Google Scholar]
- Sinha P, Clements VK, Ostrand-Rosenberg S. Interleukin-13-regulated M2 macrophages in combination with myeloid suppressor cells block immune surveillance against metastasis. Cancer Res. 2005;65:11743–11751. doi: 10.1158/0008-5472.CAN-05-0045. [DOI] [PubMed] [Google Scholar]
- Sisirak V, Faget J, Gobert M, Goutagny N, Vey N, Treilleux I, Renaudineau S, Poyet G, Labidi-Galy SI, Goddard-Leon S, Durand I, Le Mercier I, Bajard A, Bachelot T, Puisieux A, Puisieux I, Blay JY, Menetrier-Caux C, Caux C, Bendriss-Vermare N. Impaired IFN-alpha production by plasmacytoid dendritic cells favors regulatory T-cell expansion that may contribute to breast cancer progression. Cancer Res. 2012;72:5188–5197. doi: 10.1158/0008-5472.CAN-11-3468. [DOI] [PubMed] [Google Scholar]
- Stanculescu A, Bembinster LA, Borgen K, Bergamaschi A, Wiley E, Frasor J. Estrogen promotes breast cancer cell survival in an inhibitor of apoptosis (IAP)-dependent manner. Horm Cancer. 2010;1:127–135. doi: 10.1007/s12672-010-0018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steding CE, Wu ST, Zhang Y, Jeng MH, Elzey BD, Kao C. The role of interleukin-12 on modulating myeloid-derived suppressor cells, increasing overall survival and reducing metastasis. Immunology. 2011;133:221–238. doi: 10.1111/j.1365-2567.2011.03429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strayer DR, Carter WA, Brodsky I. Familial occurrence of breast cancer is associated with reduced natural killer cytotoxicity. Breast Cancer Res Treat. 1986;7:187–192. doi: 10.1007/BF01806249. [DOI] [PubMed] [Google Scholar]
- Sullivan NJ, Sasser AK, Axel AE, Vesuna F, Raman V, Ramirez N, Oberyszyn TM, Hall BM. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene. 2009;28:2940–2947. doi: 10.1038/onc.2009.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W, Zhang W, Strasner A, Grivennikov S, Cheng JQ, Hoffman RM, Karin M. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature. 2011;470:548–553. doi: 10.1038/nature09707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchou J, Conejo-Garcia J. Targeting the tumor stroma as a novel treatment strategy for breast cancer: shifting from the neoplastic cell-centric to a stroma-centric paradigm. Adv Pharmacol. 2012;65:45–61. doi: 10.1016/B978-0-12-397927-8.00003-8. [DOI] [PubMed] [Google Scholar]
- Terry MB, Gammon MD, Zhang FF, Tawfik H, Teitelbaum SL, Britton JA, Subbaramaiah K, Dannenberg AJ, Neugut AI. Association of frequency and duration of aspirin use and hormone receptor status with breast cancer risk. JAMA. 2004;291:2433–2440. doi: 10.1001/jama.291.20.2433. [DOI] [PubMed] [Google Scholar]
- Tkach M, Coria L, Rosemblit C, Rivas MA, Proietti CJ, Diaz Flaque MC, Beguelin W, Frahm I, Charreau EH, Cassataro J, Elizalde PV, Schillaci R. Targeting Stat3 induces senescence in tumor cells and elicits prophylactic and therapeutic immune responses against breast cancer growth mediated by NK cells and CD4+ T cells. J Immunol. 2012;189:1162–1172. doi: 10.4049/jimmunol.1102538. [DOI] [PubMed] [Google Scholar]
- Treilleux I, Blay JY, Bendriss-Vermare N, Ray-Coquard I, Bachelot T, Guastalla JP, Bremond A, Goddard S, Pin JJ, Barthelemy-Dubois C, Lebecque S. Dendritic cell infiltration and prognosis of early stage breast cancer. Clin Cancer Res. 2004;10:7466–7474. doi: 10.1158/1078-0432.CCR-04-0684. [DOI] [PubMed] [Google Scholar]
- Tsukerman P, Stern-Ginossar N, Gur C, Glasner A, Nachmani D, Bauman Y, Yamin R, Vitenshtein A, Stanietsky N, Bar-Mag T, Lankry D, Mandelboim O. MiR-10b downregulates the stress-induced cell surface molecule MICB, a critical ligand for cancer cell recognition by natural killer cells. Cancer Res. 2012 doi: 10.1158/0008-5472.CAN-11-2671. [DOI] [PubMed] [Google Scholar]
- Tu Z, Prajapati S, Park KJ, Kelly NJ, Yamamoto Y, Gaynor RB. IKK alpha regulates estrogen-induced cell cycle progression by modulating E2F1 expression. J Biol Chem. 2006;281:6699–6706. doi: 10.1074/jbc.M512439200. [DOI] [PubMed] [Google Scholar]
- Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- Vendrell JA, Robertson KE, Ravel P, Bray SE, Bajard A, Purdie CA, Nguyen C, Hadad SM, Bieche I, Chabaud S, Bachelot T, Thompson AM, Cohen PA. A candidate molecular signature associated with tamoxifen failure in primary breast cancer. Breast Cancer Res. 2008;10:R88. doi: 10.1186/bcr2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–271. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- Waldhauer I, Steinle A. NK cells and cancer immunosurveillance. Oncogene. 2008;27:5932–5943. doi: 10.1038/onc.2008.267. [DOI] [PubMed] [Google Scholar]
- Wang B, Zaidi N, He LZ, Zhang L, Kuroiwa JM, Keler T, Steinman RM. Targeting of the non-mutated tumor antigen HER2/neu to mature dendritic cells induces an integrated immune response that protects against breast cancer in mice. Breast Cancer Res. 2012;14:R39. doi: 10.1186/bcr3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10:181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Epler J, Salazar LG, Riddell SR. Recognition of breast cancer cells by CD8+ cytotoxic T-cell clones specific for NY-BR-1. Cancer Res. 2006;66:6826–6833. doi: 10.1158/0008-5472.CAN-05-3529. [DOI] [PubMed] [Google Scholar]
- Wang X, Belguise K, Kersual N, Kirsch KH, Mineva ND, Galtier F, Chalbos D, Sonenshein GE. Oestrogen signalling inhibits invasive phenotype by repressing RelB and its target BCL2. Nat Cell Biol. 2007;9:470–478. doi: 10.1038/ncb1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Belguise K, O’Neill CF, Sanchez-Morgan N, Romagnoli M, Eddy SF, Mineva ND, Yu Z, Min C, Trinkaus-Randall V, Chalbos D, Sonenshein GE. RelB NF-kappaB represses estrogen receptor alpha expression via induction of the zinc finger protein Blimp1. Mol Cell Biol. 2009;29:3832–3844. doi: 10.1128/MCB.00032-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss VL, Lee TH, Song H, Kouo TS, Black CM, Sgouros G, Jaffee EM, Armstrong TD. Trafficking of high avidity HER-2/neu-specific T cells into HER-2/neu-expressing tumors after depletion of effector/memory-like regulatory T cells. PLoS ONE. 2012;7:e31962. doi: 10.1371/journal.pone.0031962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrzesinski SH, Wan YY, Flavell RA. Transforming growth factor-beta and the immune response: implications for anticancer therapy. Clin Cancer Res. 2007;13:5262–5270. doi: 10.1158/1078-0432.CCR-07-1157. [DOI] [PubMed] [Google Scholar]
- Xu D, Takeshita F, Hino Y, Fukunaga S, Kudo Y, Tamaki A, Matsunaga J, Takahashi RU, Takata T, Shimamoto A, Ochiya T, Tahara H. MiR-22 represses cancer progression by inducing cellular senescence. J Cell Biol. 2011;193:409–424. doi: 10.1083/jcb.201010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan M, Jene N, Byrne D, Millar EK, O’Toole SA, McNeil CM, Bates GJ, Harris AL, Banham AH, Sutherland RL, Fox SB. Recruitment of regulatory T cells is correlated with hypoxia-induced CXCR4 expression, and is associated with poor prognosis in basal-like breast cancers. Breast Cancer Res. 2011;13:R47. doi: 10.1186/bcr2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Zhang Y, Chen K, Hu X, Xu LX. Discovery of IL-18 as a novel secreted protein contributing to doxorubicin resistance by comparative secretome analysis of MCF-7 and MCF-7/Dox. PLoS ONE. 2011;6:e24684. doi: 10.1371/journal.pone.0024684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Chen X, Shu Y. Gene expression of the invasive phenotype of TNF-alpha-treated MCF-7 cells. Biomed Pharmacother. 2009;63:421–428. doi: 10.1016/j.biopha.2009.04.032. [DOI] [PubMed] [Google Scholar]
- Yu JL, Rak JW. Host microenvironment in breast cancer development: inflammatory and immune cells in tumour angiogenesis and arteriogenesis. Breast Cancer Res. 2003;5:83–88. doi: 10.1186/bcr573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Eppenberger-Castori S, Eppenberger U, Benz CC. The NFkappaB pathway and endocrine-resistant breast cancer. Endocr Relat Cancer. 2005;12(Suppl 1):S37–S46. doi: 10.1677/erc.1.00977. [DOI] [PubMed] [Google Scholar]
- Zhu P, Baek SH, Bourk EM, Ohgi KA, Garcia-Bassets I, Sanjo H, Akira S, Kotol PF, Glass CK, Rosenfeld MG, Rose DW. Macrophage/cancer cell interactions mediate hormone resistance by a nuclear receptor derepression pathway. Cell. 2006;124:615–629. doi: 10.1016/j.cell.2005.12.032. [DOI] [PubMed] [Google Scholar]