Abstract
Increased particulate air pollutant concentrations (PM) have been associated with platelet activation. It was postulated that increased air pollutant concentrations would be associated with increases in measures of platelet function and that responses would be blunted when taking aspirin and/or fish oil. Data from a sequential therapy trial (30 subjects with type 2 diabetes mellitus), with 4 clinic visits (1st: No supplements, 2nd: aspirin, 3rd: omega-3 fatty acid supplements, 4th: aspirin and omega-3 fatty acids) per subject, were utilized. Using linear mixed models, adjusted for relative humidity, temperature, visit number, and season, changes in 3 platelet function measures including (1) aggregation induced by adenosine diphosphate [ADP], (2) aggregation induced by collagen, and (3) thromboxane B2 production were associated with interquartile range (IQR) increases in mean concentrations of ambient PM2.5, black carbon, ultrafine particle (UFP; 10–100nm), and accumulation mode particles (AMP; 100–500nm) in the previous 1 – 96 hr. IQR increases in mean UFP and AMP concentrations were associated with significant decreases in platelet response with the largest being a −0.43 log(pg/ml) decrease in log(thromboxane B2; 95% CI=−0.8, −0.1) associated with each 582 particles/cm3 increase in AMP, and a −1.7 ohms reduction in collagen-induced aggregation (95% CI= −3.1, −0.3) associated with each 2097 particles/cm3 increase in UFP in the previous 72 hr. This UFP effect on thromboxane B2 was significantly muted in diabetic subjects taking aspirin (−0.01 log[pg/ml]; 95% CI = −0.4, 0.3). The reason for this finding remains unknown, and needs to be investigated in future studies.
Keywords: platelet aggregation, aspirin, fish oil, omega-3 fatty acids, particulate matter, epidemiology, air pollution
INTRODUCTION
Increased particulate air pollutant concentrations (PM) were previously associated with triggering of acute cardiovascular and cerebrovascular events including acute myocardial infarction and ischemic stroke in the previous few hr to days, in many but not all studies (Chen, et al., 2015; Chang, et al., 2015; D'Ippoliti, et al., 2003; Mustafic, et al., 2012; Gardner, et al., 2014; Nuvolone et al., 2011; Peters et al., 2001; 2005; Rich et al., 2010; Wellenius, et al., 2005, 2012; Zanobetti & Schwartz, 2005), with others reporting similar associations with long term exposures to PM (Beckerman et al 2012; Pope and Dockery, 2006; Hoek et al 2013). Potential mechanisms that may explain triggering of these acute events, by particulate matter (PM) include vascular dysfunction, inflammation, and coagulation among others (Brook, 2004; Langrish et al., 2012; Simkhovich et al, 2008). Previous studies in humans linked higher levels of ambient PM pollution with increases in systemic inflammatory markers such as C–reactive protein, fibrinogen, and IL-6 levels in the previous day (Bind et al., 2012; Rückerl et al., 2007; Ghio et al., 2012), or genes involved in inflammation (Brocata et al., 2014. Various investigators also reported increases in platelet activation markers (CD40L and p-selectin) associated with elevated ambient air pollution levels in the previous few days in healthy young subjects (Rich et al, 2012a; Strak et al., 2013; Wu et al., 2012) and patients with type II diabetes (Frampton et al., 2012; Stewart et al., 2010).
Aspirin has long been used clinically as an anti-platelet drug for patients at high risk of cardiovascular outcomes such as individuals with type 2 diabetes mellitus.. Aspirin reduces the production of thromboxane A2 by inhibiting acetylation of cyclooxygenase -1 (COX-1) and subsequent production of other pro-inflammatory and pro-thrombotic prostaglandins (Collaboration, 2002; Grundy, 2004; Gurbel et al., 2007). However, Krasopoulos et al (2008) showed that 28% of individuals displayed biochemical aspirin resistance (i.e. “nonresponse”), which attenuated the anti-platelet function of aspirin especially in patients with diabetes mellitus. Omega-3 fatty acids (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) were also found to exert potent cardioprotective effects by antagonizing metabolism of arachidonic acid via the COX-1 pathway into thromboxane A2 and pro inflammatory products, which are known to increase the risk of acute cardiovascular events (GISSI-Prevenzione Investigators, 1999; Iso et al., 2001; Kris-Etherton, 2002; Lemaitre et al., 2003). Recently Abdolahi et al (2014) reported that fish oil and aspirin + fish oil combined therapy may reduce cardiovascular risk in patients with type II diabetes mellitus by reducing plasma lypophospholipids and platelet aggregation.
Data exploring whether fish oil or aspirin therapy blunts adverse cardiovascular consequences of ambient air pollution are limited. One study in Mexico City noted decreased heart rate variability (a marker of autonomic dysfunction) associated with short term increases in PM, but no such association in subjects taking fish oil supplements (Romieu et al., 2008). Tong et al (2012) reported that fish oil supplementation attenuated fine and ultrafine concentrated ambient particles (CAP) induced changes in heart rate variability and repolarization parameters, and blunted CAP-induced elevation in plasma lipids in a controlled exposure study of type II diabetes patients. To our knowledge, no study has examined whether fish oil, aspirin, or combined fish oil and aspirin therapy modifies the effect of PM pollution on markers of platelet function in patients with type II diabetes mellitus. Data from a sequential therapy trial in patients with type II diabetes mellitus, and continuous ambient air pollution data were coupled to weather data monitoring in Rochester, New York, as used in our previous studies (Evans et al, 2014; Rich et al., 2010; 2012b;; Wasserman et al., 2014) to examine two separate hypotheses. First, it was postulated that ambient PM air pollution levels in the previous few hr and days might be associated with increased platelet aggregation and thromboxane B2 production. Second, it was proposed that aspirin and fish oil, both independently and together, might lessen or mute this response.
METHODS
Study population
The study population and protocol of the parent study from which we drew the data for the analyses described below were previously described (Block et al., 2013; Abdolahi, et al., 2014; Block, et al., 2015). In short, 30 participants aged 40–80 who had type II diabetes mellitus based upon criteria from the Executive Committee of the American Diabetes Association Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus (Expert Committee on the Diagnosis and Classification of Diabetes Mellitus, 2003) were recruited for participation in a sequential therapy trial of aspirin and fish oil. The goal of this study was to conduct a clinical trial in individuals with type 2 diabetes mellitus to investigate the effects of EPA+DHA and aspirin treatment on platelet function and lysophospholipid metabolism. Study participants were asked not to take vitamins, nutritional supplements, and herbal preparations during the trial study period. Patients were excluded with a diagnosis of coronary heart disease, congestive heart failure, peripheral vascular disease, stroke, atrial fibrillation, a history of malignancy (except subjects who have been disease-free for greater than 10 years or whose only malignancy has been basal or squamous cell skin carcinoma), peptic ulcer, or gastrointestinal bleeding in the past 5 years, a diagnosed bleeding disorder, use of antiplatelet or antithrombotic therapy (defined as clopidogrel, ticlopidine, cilostazol, dipyridamole, trapidil, warfarin, and argatroban), oral contraceptive use, or daily use of nonsteroidal anti-inflammatory drugs. Other exclusion criteria included a calculated creatinine clearance <60 mg/dl, signs of obstructive hepatic disease, any other obvious metabolic disease that would influence lipid metabolism, based upon a screening complete blood count and comprehensive metabolic profile, pregnancy, surgery within 30 days of screening, history of drug or alcohol abuse, or current weekly alcohol consumption >14 units/week (i.e. 1 unit=1 beer, 1 glass of wine, 1 mixed cocktail containing 1 ounce of alcohol), allergy to aspirin or fish/fish oil, and tobacco use. The use of any diabetes medications was permitted, including insulin.
Protocol
An 8 week sequential therapy clinical trial was conducted at the University of Rochester’s Clinical Research Center between October 2010 and March 2012, in which participants had 4 separate study visits for phlebotomy, with blood collected using a vacutainer and citrate as the anti-coagulant. Participants visited the Center for Visit 1 (prior to aspirin and fish oil supplementation) following a pre - trial 10-day aspirin-free period, and had their first phlebotomy. After the visit, participants started a single dose (81 mg/day) regimen of aspirin for 7 days. After these 7 days, participants visited the Center for Visit 2, where a blood sample was taken, and were then instructed to discontinue aspirin and begin a 28 day regimen of fish oil (4g/day). At Visit 3 (28 days after Visit 2), participants had the third phlebotomy and subsequently instructed to continue the same fish oil regimen (4g/day) combined with the aspirin (81mg) regimen for 7 days. Seven days after Visit 3, participants visited the Center for their last study visit (Visit 4) and phlebotomy. Blood levels of EPA and DHA increased as expected, as baseline plasma concentrations averaged 106±48 mg/ml for DHA and 13±7 mg/ml for EPA, which increased to 190±65 mg/ml (p<0.0001) for DHA and 61±26 mg/ml for EPA (p<0.0001) 28 days after fish oil ingestion. (Block et al 2013). Platelet function testing, as described below, was performed using blood from each phlebotomy. The University of Rochester Medical Center Research Subjects Review Board approved all study activities for both the original sequential therapy trial and this re-analysis of those data.
Platelet aggregation direct measures
Whole blood electrical impedance platelet aggregation was performed using a Chronolog Whole Blood Lumi-Aggregometer® (Model 560VS) with reagents from Chronolog Corporation (Havertown, PA-USA) within 2 hr of blood collection via atraumatic phlebotomy using sodium citrate tubes using ADP at 2.5 µM and collagen at 1 µg/ml (ADP-10). These concentrations of platelet agonists are known to induce reproducible platelet aggregation (Shattil et al 1985; Block et al 2013). For each concentration of agonist, a 500 µl aliquot of fresh whole blood anticoagulated with 0.105 M sodium citrate was incubated with 500 µl normal physiological saline at 37°C for 5 min in the presence of a stir bar. A clean electrode was introduced into the cuvette and a baseline measurement of electrical impedance at 20 Ωv was recorded. The aggregation of platelets was measured as a change in impedance over a period of 5 min. The characteristics of the resultant platelet function curve, using Aggro/link software (version 5.1), was used to measure the characteristics of the resultant platelet function curve from Chronolog Corporation. The change in aggregation, measured in ohms, was collected via this method. I Impedance-based whole blood platelet aggregation methods were used rather than optical-based platelet-rich plasma methods. This evaluates platelets in a physiologic milieu in the presence of red and white blood cells, which are known to modulate platelet function, display higher sensitivity, and do not require centrifugation, thereby avoiding injuring platelets (Dyszkiewicz-Korpanty et al 2005). To confirm that each participant ingested aspirin, inhibition of the traditionally robust response to arachidonic acid served as verification that subjects were taking daily aspirin (Block et al 2013).
Thromboxane B2 (TXB2)
A competitive enzyme linked immunosorbent assay (EIA) was utilized according to manufacturer's instructions (TXB2 EIA Kit, Cayman Chemical Co., Ann Arbor, MI) in order to analyze thromboxane B2 (TXB2) in citrated plasma. All reagents were prepared fresh using ultra-pure water, and samples diluted as necessary in sample buffer to fall within the standard curve (typically 1:2). No apparent evidence for interference was found in the assay using serially diluted plasma (i.e. <20% difference in the final calculated TXB2 concentration), and therefore did not further purify the plasma samples. The limit of detection (LOD) was 7.8 pg/ml. Stimulated blood was not employed for these analyses as it was the goal of the original sequential therapy trial (Block et al 2013), from which this study used data, to determine effects of aspirin and fish oil ingestion alone without additional complication(s) of if and how artificially stimulating them impacted this. Further, a thrombin control was not used since each subject served as their own control in the original analysis (i.e. comparison of TXB2 levels while taking aspirin and/or fish oil compared to TXB2 level while taking neither). All TXB2 assays were analyzed in duplicate, and fell within the standard curve.
Air Pollution and Meteorology Measurements
Hourly PM air pollutant concentrations were measured at the New York State Department of Environmental Protection site in Rochester, New York. The number concentration of ultrafine particles (10–100 nm) and accumulation mode particles (100–500 nm) were measured using a Scanning Mobility Particle Sizer (SMPS, TSI, Inc., Shoreview, MN) for each hr between October, 2010 and March 2013. Particulate matter (PM2.5) was measured continuously using a Tapered Element Oscillating Microbalance (TEOM; ThermoFisher, Franklin, MA). Carbon black (CB) was measured using an AE-22 aethalometer (Magee Scientific, Berkeley, CA) with two wavelengths of measurements (370 and 880 nm). Hourly temperature and relative humidity were also measured at this site.
Statistical Analysis
Descriptive statistics were calculated for each pollutant including PM2.5, BC, UFP, and AMP, as well as each platelet aggregation marker including ADP -10 induced aggregation, collagen induced aggregation, and TXB2 production for all visits together and each visit separately. TXB2 data were log transformed in order to fulfill the assumption of residual normality. Pearson correlation coefficients were calculated for all air pollutant and platelet measure combinations.
Next, linear mixed effect regression models with a random intercept were used to estimate the change (and 95% confidence intervals) in each platelet measure associated with interquartile range (IQR) increases in the 1, 12, 24, 48, 72, and 96 hr mean concentrations of each pollutant. A compound symmetry structure was selected for the covariance matrix to model the correlation between repeated measures for each participant. A separate confounder model was built for each outcome. For those models estimating the change in log (TXB2) associated with each IQR increase in pollutant concentration, the mean relative humidity in the past 72 hr, mean temperature in the past 72 hr, and indicator variables for study visit were included. For those models estimating the change in ADP-10 induced aggregation associated with each IQR increase in pollutant concentration, the 72 hr mean relative humidity using a natural spline (2 degrees of freedom [df]), 72 hr mean temperature using a natural spline (2 df), and indicator variables for study visit were included. For those models estimating the change in collagen induced aggregation associated with each IQR increase in pollutant concentration, the 72 hr mean relative humidity using a natural spline (3 df), the 72 hr mean temperature, indicator variables for study visit, and indicator variables for season were again included. For all models/splines, relative humidity, temperature, and the best functional form (i.e. df =1, 2, 3 or 4) and averaging time (i.e. previous 1, 12, 24, 48, 72, or 96 hr) of relative humidity and temperature was selected that minimized the Akaike Information Criteria (AIC) for each outcome. Data were adjusted for season only for collagen models since it was not predictive of the other two platelet outcomes and did not result in a lower AIC.
Subsequently, linear regression models including interaction terms between study visit and each pollutant (PM*Visit-2; PM*Visit-3; PM*Visit-4) were used to estimate the change in each platelet aggregation marker associated with each IQR increase in mean pollutant concentration 1, 12, 24, 48, 72, and 96 hr before the clinic visit, separately by study visit (Visit 1 – 4). The same variables used in the outcome specific linear mixed models described above were included again. An F-test compared each interaction model to a nested model without the interaction term in order to judge the global significance of effect modification by study visit. The criterion for significance was set at p<0.05.
RESULTS
Participant characteristics (N=30) were described in detail previously (Block et al, 2013). Briefly, the average age at baseline was 56.5 years, and half of the participants were male. Participants were predominantly Caucasian (57%) and African American (30%), never smokers (63%), with 60% having at least a college degree. Thirty % reported eating fish at least once a week, while 37% noted doing so less than once a month. Only 13% of participants consumed alcohol at least once per week, while 77% consumed alcohol less than once a month. Sixteen (53%) were taking statins, with 4 subjects (13%) taking other lipid-lowering medications. Distributions of systolic and diastolic blood pressure, high density lipoprotein, and heart rate at each visit are shown in Table 1. All 30 subjects completed the study and were able to tolerate aspirin and fish oil regimens.
Table 1.
Distribution of blood pressure, high density lipoprotein, and pulse at each of 4 visits (n=30 at each visit)
| Mean ± SD |
Min. | 50th Percentile |
Max. | |
|---|---|---|---|---|
| Systolic Blood Pressure (mm Hg) | ||||
| Visit 1: Control | 124.6±15.0 | 91.0 | 125.0 | 157.0 |
| Visit 2: Aspirin Only | 130.2±17.0 | 95.0 | 130.5 | 165.0 |
| Visit 3: Fish Oil Only | 125.7±12.0 | 94.0 | 126.5 | 149.0 |
| Visit 4: Aspirin & Fish Oil | 129.9±15.6 | 100.0 | 131.0 | 172.0 |
| Diastolic Blood Pressure (mm Hg) | ||||
| Visit 1: Control | 70.5±10.0 | 50.0 | 70.5 | 92.0 |
| Visit 2: Aspirin Only | 72.0±12.1 | 39.0 | 74.5 | 93.0 |
| Visit 3: Fish Oil Only | 70.6±7.6 | 55.0 | 71.0 | 85.0 |
| Visit 4: Aspirin & Fish Oil | 73.9±12.5 | 50.0 | 71.0 | 97.0 |
| High Density Lipoprotein (mg/dl) | ||||
| Visit 1: Control | 32.5±12.6 | 14.6 | 30.1 | 72.9 |
| Visit 2: Aspirin Only | 42.0±27.2 | 12.8 | 33.2 | 123.3 |
| Visit 3: Fish Oil Only | 35.6±13.5 | 17.3 | 32.3 | 76.3 |
| Visit 4: Aspirin & Fish Oil | 40.5±23.0 | 16.4 | 34.0 | 121.3 |
| Heart Rate (bpm) | ||||
| Visit 1: Control | 73.9±12.1 | 54.0 | 75.0 | 95.0 |
| Visit 2: Aspirin Only | 72.5±12.2 | 51.0 | 71.5 | 95.0 |
| Visit 3: Fish Oil Only | 72.5±10.6 | 56.0 | 73.0 | 87.0 |
| Visit 4: Aspirin & Fish Oil | 70.4±10.9 | 52.0 | 71.5 | 91.0 |
Descriptive statistics for each mean 1 hr and 24 hr pollutant concentration used in the study are shown in Table 2. Each was similar across visits. Table 3 shows descriptive statistics for the three platelet markers by visit. Log (TXB2) was highest at Visit 3 after 28 days of fish oil supplementation (median = 7.1 pg/ml), while ADP induced aggregation was highest at Visit 1 (median =13.0 ohms) when subjects were not ingesting aspirin or fish oil. Collagen induced aggregation was highest at Visit 1 (median = 19 ohms) and Visit 3 (median = 21 ohms), both visits when participants were not taking aspirin (Table 3).
Table 2.
Distribution of 1 hour and 24 hour mean pollutant concentrations for each subject.
| Pollutant | Averaging Time |
N | Mean ± SD | Min. | 5th Percentile |
25th Percentile |
50th Percentile |
75th Percentile |
95th Percentile |
Max. |
|---|---|---|---|---|---|---|---|---|---|---|
| PM2.5 (µg/m3) |
1 hour | 108 | 7.2 ± 4.3 | 0.0 | 1.1 | 4.2 | 6.9 | 10.4 | 15.8 | 17.7 |
| 24 hour | 102 | 7.0 ± 3.5 | 2.1 | 2.9 | 4.8 | 6.7 | 9.0 | 12.8 | 22.2 | |
| Black Carbon (µg/m3) |
1 hour | 118 | 1.2 ± 0.6 | 0.2 | 0.3 | 0.6 | 1.1 | 1.5 | 2.8 | 3.2 |
| 24 hour | 118 | 0.9 ± 0.8 | 0.3 | 0.5 | 1.0 | 1.3 | 1.5 | 2.4 | 3.9 | |
| Ultrafine particles (p/cm3) |
1 hour | 108 | 3,872 ± 3,823 | 310 | 615 | 1,782 | 3,125 | 4,697 | 9,545 | 23,118 |
| 24 hour | 108 | 3,102 ± 2,541 | 645 | 784 | 1,745 | 2,489 | 3,660 | 6,801 | 11,721 | |
| Accumulation mode particles (p/cm3) |
1 hour | 108 | 698 ± 572 | 78 | 162 | 305 | 572 | 844 | 1,855 | 2,811 |
| 24 hour | 108 | 691± 468 | 201 | 278 | 391 | 512 | 962 | 1,399 | 1,747 | |
Table 3.
Distribution of each platelet function measure by study visit
| Platelet Function Measure |
Visit | Mean±SD | Minimum | 5th Percentile |
25th Percentile |
50th Percentile |
75th Percentile |
95th Percentile |
Maximum |
|---|---|---|---|---|---|---|---|---|---|
| Log(Thromboxane B2) log(pg/ml) |
1 | 6.4 ± 1.1 | 4.1 | 4.8 | 5.6 | 6.4 | 7.2 | 8.2 | 8.5 |
| 2 | 4.0 ± 1.1 | 0.9 | 2.2 | 3.6 | 3.8 | 4.8 | 5.8 | 6.0 | |
| 3 | 7.0 ± 1.1 | 4.0 | 4.7 | 6.6 | 7.1 | 7.5 | 8.4 | 9.0 | |
| 4 | 4.4 ± 0.8 | 3.1 | 3.2 | 3.8 | 4.5 | 5.1 | 5.8 | 5.8 | |
| Collagen (ohms) | 1 | 20.0±4.7 | 10.0 | 11.8 | 18.0 | 19.0 | 23.0 | 27.6 | 28.0 |
| 2 | 12.1±5.4 | 6.0 | 6.0 | 8.0 | 10.5 | 15.5 | 20.6 | 28.0 | |
| 3 | 19.8±4.8 | 7.0 | 9.8 | 18.0 | 21.0 | 23.5 | 25.6 | 26.0 | |
| 4 | 12.7±5.2 | 4.0 | 5.9 | 9.3 | 12.0 | 15.5 | 22.1 | 25.0 | |
| ADP-10 (ohms) | 1 | 13.0±5.5 | 4.0 | 4.0 | 9.0 | 13.0 | 16.0 | 21.1 | 25.0 |
| 2 | 12.2±5.8 | 1.0 | 2.0 | 8.3 | 12.0 | 16.0 | 20.6 | 24.0 | |
| 3 | 12.3±5.1 | 4.0 | 4.5 | 8.3 | 12.5 | 14.0 | 21.0 | 23.0 | |
| 4 | 12.0±4.9 | 1.0 | 3.5 | 10.0 | 12.0 | 15.0 | 19.1 | 20.0 | |
TXB2 was uncorrelated with ADP -10 induced aggregation (r = 0.07) and moderately correlated with collagen induced aggregation (r=0.59) across visits. ADP-10 induced aggregation and collagen induced aggregation were weakly correlated (r = 0.35).
Supplemental Table 1 provides the unadjusted change in each platelet outcome associated with each IQR increase in mean pollutant concentrations in the previous 1, 12, 24, 48, 72, and 96 hr, across all visits. In adjusted analyses shown in Table 4, IQR increases in mean PM2.5, BC, UFP, and AMP concentrations were generally associated with small (0.16 ohms – 1.55 ohms) increases in ADP-10 induced aggregation, although the effects were all not statistically significant. Unexpectedly, IQR increases in mean UFP and AMP concentrations were generally associated with decreased log TXB2 production and collagen induced aggregation, although the results were statistically significant for only a subset of estimates. The largest changes were associated with 72 hr mean pollutant concentrations, including a 1.6 ohm reduction in collagen-induced aggregation (95% CI= −2.96, −0.20) associated with each 2,097 particles/cm3 increase in UFP concentration, and a −0.43 decrease in log(TXB2; pg/mL) (95% CI=−0.79, −0.07) associated with each 582 particles/cm3 increase in AMP concentration. Increases in mean PM2.5 concentrations were associated with small increases in log(thromboxane B2) production and collagen induced aggregation, although none were statistically significant. BC was associated with increases in collagen and ADP-10 induced aggregation, and small decreases in log(thromboxane B2), but the results were again not statistically significant.
Table 4.
Adjusted Change in ADP-10 induced aggregation (Ohms), Log(thromboxane B2) (pg/ml), and Collagen 1 induced aggregation (Ohms) associated with each interquartile range (IQR) increase in air pollutant concentration, by averaging time (All Visits combined; N=120).
| LOG(THROMBOXANE B2 PRODUCTION log[pg/ml]) |
COLLAGEN 1 INDUCED AGGREGATION [ohms] |
ADP-10 INDUCED AGGREGATION [ohms] |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pollutant | Lag time (Hours) |
IQR | N | Change | 95% CI | p- value |
N | Change | 95% CI | p-value | N | Change | 95% CI | p-value |
| PM2.5 | 1 h | 6.20 | 93 | 0.03 | (−0.28, 0.34) | 0.85 | 94 | 0.24 | (−1.46, 1.94) | 0.79 | 94 | 0.67 | (−0.69, 2.02) | 0.35 |
| 12 h | 5.20 | 90 | 0.03 | (−0.27,0.31) | 0.88 | 91 | 0.18 | (−1.34, 1.70) | 0.82 | 91 | 1.02 | (−0.17, 2.21) | 0.11 | |
| 24h | 4.20 | 90 | 0.13 | (−0.12, 0.38) | 0.33 | 91 | 0.48 | (−0.93, 1.90) | 0.52 | 91 | 0.26 | (−0.93, 1.45) | 0.68 | |
| 48h | 3.67 | 90 | 0.07 | (−0.17, 0.31) | 0.57 | 91 | 0.57 | (−0.82, 1.96) | 0.44 | 91 | 0.16 | (−0.93,1.25) | 0.78 | |
| 72h | 3.75 | 85 | 0.02 | (−0.27, 0.30) | 0.93 | 86 | 0.58 | (−0.97, 2.13) | 0.48 | 91 | 0.38 | (−0.75, 1.51) | 0.53 | |
| 96h | 3.72 | 76 | 0.05 | (−0.30, 0.39) | 0.80 | 76 | 0.43 | (−1.46, 2.33) | 0.67 | 76 | 0.48 | (−0.91, 1.89) | 0.52 | |
| Black Carbon | 1 h | 0.94 | 96 | −0.25 | (−0.51, 0.01) | 0.07 | 98 | 0.32 | (−1.05, 1.69) | 0.91 | 98 | 0.60 | (−0.41, 1.61) | 0.26 |
| 12 h | 0.60 | 96 | −0.10 | (−0.37, 0.18) | 0.50 | 98 | 0.43 | (−1.01, 1.86) | 0.58 | 98 | 0.64 | (−0.43, 1.71) | 0.26 | |
| 24h | 0.45 | 96 | −0.10 | (−0.37, 0.16) | 0.50 | 98 | 0.06 | (−1.31, 1.44) | 0.91 | 98 | 0.29 | (−0.77, 1.35) | 0.61 | |
| 48h | 0.43 | 96 | −0.05 | (−0.30, 0.20) | 0.72 | 98 | 0.14 | (−1.16, 1.44) | 0.84 | 98 | 0.56 | (−0.44, 1.56) | 0.29 | |
| 72h | 0.37 | 96 | −0.07 | (−0.30, 0.16) | 0.57 | 98 | 0.30 | (−0.91, 1.51) | 0.64 | 98 | 0.23 | (−0.72,1.18) | 0.65 | |
| 96h | 0.33 | 96 | −0.01 | (−0.25, 0.24) | 0.96 | 98 | 0.76 | (−0.51, 2.02) | 0.26 | 98 | 0.31 | (−0.70, 1.31) | 0.57 | |
| Ultrafine Particles |
1h | 2915 | 92 | −0.14 | (−0.34, 0.05) | 0.12 | 93 | −0.53 | (−1.57, 0.50) | 0.34 | 93 | 0.50 | (−0.34, 1.45) | 0.26 |
| 12h | 2722 | 92 | −0.30 | (−0.59, −0.01) | 0.06 | 93 | −0.96 | (−2.58, 0.65) | 0.27 | 93 | 0.53 | (−071, 1.77) | 0.68 | |
| 24h | 1915 | 92 | −0.26 | (−0.44, −0.08) | 0.01 | 93 | −0.68 | (−1.67, 0.32) | 0.17 | 93 | 0.33 | (−0.58, 1.24) | 0.32 | |
| 48h | 1918 | 92 | −0.23 | (−0.42, −0.01) | 0.05 | 93 | −1.15 | (−2.31,0.01) | 0.06 | 93 | 0.71 | (−0.33, 1.75) | 0.20 | |
| 72h | 2097 | 92 | −0.28 | (−0.55,−0.01) | 0.04 | 93 | −1.58 | (−2.96, −0.20) | 0.03 | 93 | 0.32 | (−0.91, 1.56) | 0.62 | |
| 96h | 1999 | 92 | −0.31 | (−0.61,0.01) | 0.05 | 93 | −1.50 | (−3.05, 0.06) | 0.07 | 93 | 0.68 | (−0.75, 2.10) | 0.37 | |
| Accumulation Mode Particles |
1h | 539 | 92 | −0.29 | (−0.52, −0.06) | 0.02 | 93 | −0.33 | (−1.55, 0.89) | 0.61 | 93 | 0.72 | (−0.25, 1.69) | 0.17 |
| 12h | 557 | 92 | −0.27 | (−0.52, −0.01) | 0.05 | 93 | −0.39 | (−1.72, 0.94) | 0.58 | 93 | 0.76 | (−0.32, 2.77) | 0.18 | |
| 24h | 571 | 92 | −0.30 | (−0.60, 0.01) | 0.06 | 93 | −1.07 | (−2.66, 0.51) | 0.34 | 93 | 0.46 | (−0.90, 1.83) | 0.52 | |
| 48h | 509 | 92 | −0.23 | (−0.51, 0.06) | 0.09 | 93 | −1.21 | (−2.70, 0.27) | 0.13 | 93 | 0.59 | (−0.66, 1.84) | 0.37 | |
| 72h | 582 | 92 | −0.43 | (−0.79, −0.07) | 0.02 | 93 | −1.58 | (−3.50, 0.34) | 0.13 | 93 | 0.68 | (−0.93, 1.42) | 0.42 | |
| 96h | 534 | 92 | −0.33 | (−0.71, 0.05) | 0.10 | 93 | −0.49 | (−2.45, 1.47) | 0.72 | 93 | 1.55 | (−0.15, 3.24) | 0.09 | |
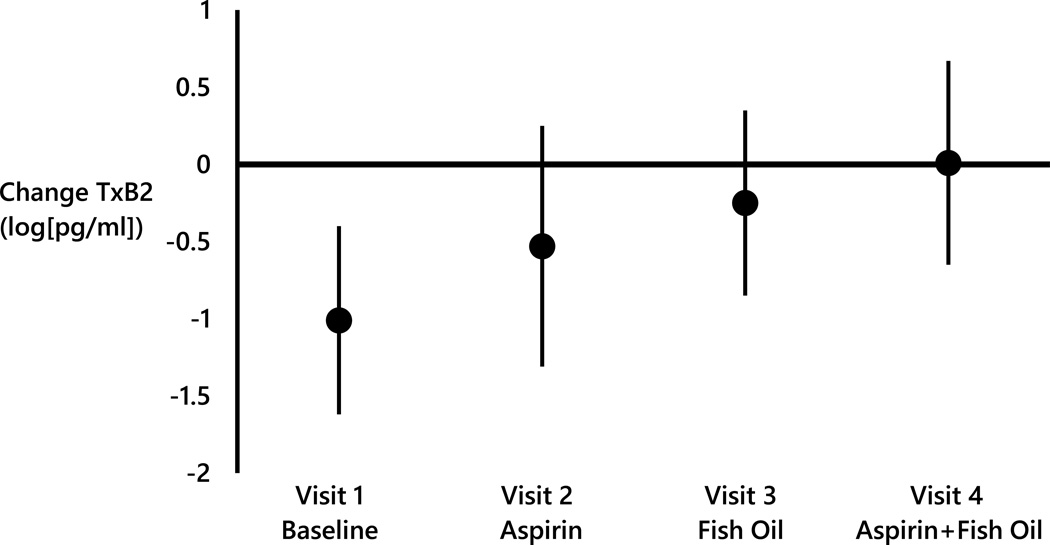
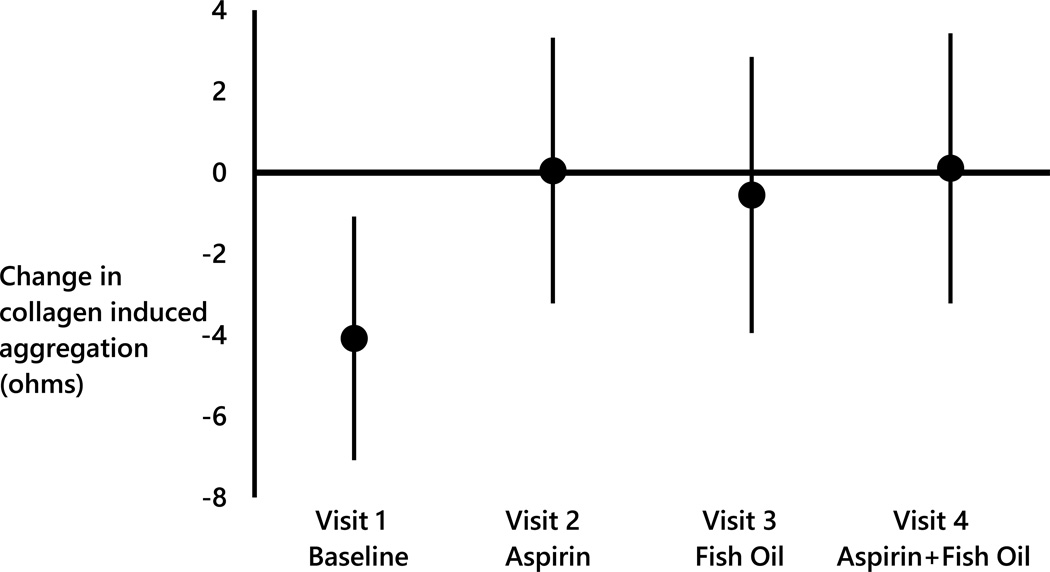
Since the largest changes in platelet aggregation and thromboxane TXB2 associated with pollutant concentrations were in the previous 72 hr, change in log(TXB2) and collagen induced aggregation associated with IQR increases in the 72 hr mean concentrations of AMP, by study visit, are illustrated in Figure 1. In Visit 1, a 1.01 log(pg/mL) decrease in log(TXB2) (95% CI= −1.62, −0.40) was associated with each 582 particles/cm3 increase in the mean concentration of AMP in the previous 72 hr. However, there was little change in log(TXB2) associated with the same increase in AMP when taking aspirin (Visit 2: Beta = −0.53 log[pg/mL]; 95% CI = −1.31, 0.25), when taking fish oil (Visit 3: Beta = −0.25 log[pg/mL]; 95% CI = −0.85, 0.35), or when taking both aspirin and fish oil (Visit 4: Beta = 0.01 log[pg/mL]; 95% CI = −0.65, 0.67). A similar pattern was noted for collagen induced aggregation associated with each IQR increase in the mean UFP concentration in the previous 72 hr (Figure 2). In Visit 1, a 4.08 ohms decrease in collagen induced aggregation (95% CI= −7.08, 1.08) was associated with each 2,097 particles/cm3 increase in the mean UFP concentration in the previous 72 hr However, no marked change in collagen induced aggregation associated with the same increase in UFP concentration when taking aspirin (Visit 2: 0.05 ohms; 95% CI = −3.22, 3.32), fish oil (Visit 3: −0.55 ohms 95% CI = −3.95, 2.85), or both aspirin and fish oil (Visit 4: 0.11 ohms 95% CI = −3.22, 3.43)(Figure 2) was observed.
Figure 1.
Change in log(thromboxane B2) associated with every 582 particles/cm3 increase in the 72 hour mean concentration of AMP stratified by study visit.
Figure 2.
Change in collagen induced aggregation (ohms) associated with every 2,100 particles/cm3 increase in the 72 hour mean concentration of UFP stratified by study visit.
DISCUSSION
Previous studies examining effects of air pollution on markers of platelet function have been inconsistent, with some reporting increases in markers of platelet activation including CD40L, p-selectin and vWF and poor vascular function associated with increased PM pollutant concentrations in the previous few hr and days (Richet al., 2012a; Strak et al., 2013; Wu et al., 2012), whereas other studies reported no association, or even decreased platelet activation (Frampton et al., 2012). Zhang et al (2013) assessed cardiorespiratory biomarker responses due to changes in ambient air pollutant concentrations, and found that as levels of ambient particles decreased from the pre-Olympic to during-Olympic time period in Beijing, China in 2008, there were significant reductions in some markers of platelet activation (e.g. −34% decrease in p-selectin, −13% decrease in vWF, and −6% decrease in CD40L), but unexpected increases in markers of platelet aggregation (7%; Rich et al., 2012a; Zhang, et al., 2013). Additionally, Frampton et al (2012) found that a 2 hr controlled exposure to ambient UFP resulted in decreased surface expression of platelet activation markers in patients with type II diabetes. In contrast, another clinical study demonstrated elevated platelet-neutrophil (absolute change 6%, 95% CI 2–10%) and platelet-monocyte (absolute change 3%, 95% CI 0.2–7%) aggregates associated with diesel exhaust inhalation (Lucking et al., 2008). Consequently, exactly how ambient exposure affects platelet function and aggregation is complex, likely context-dependent, and requires further study.
It is well known that TXB2 is generated by the COX1 enzyme pathway from its precursor, arachidonic acid (Gurbel et al., 2007). It is also known that EPA is metabolized via the COX 1 enzyme pathway and thus, can exert competitive effects on arachidonic acid metabolism (Wasserman et al., 2014). Aspirin has been shown to be less effective as a cardiovascular disease prevention agent in subjects with diabetes mellitus, compared to subjects without diabetes mellitus, although the reasons for this observation are not known (Krasopoulos et al., 2008). Aspirin, EPA, and DHA interact with leukocytes and endothelial cells to generate potent tissue protective and anti-inflammatory lipid mediator byproducts of EPA and DHA (Serhan et al, 2008). In our study, some of the effects of increased concentrations of ambient particles on platelet function were mitigated when subjects were taking aspirin. Further research is needed to understand the mechanisms by which aspirin alone, or in combination with omega-3 fatty acids, may alter the hemostatic milieu created by air pollutants, especially in subjects with type II diabetes mellitus. Surprisingly, small increases in systolic and diastolic blood pressure, at visits when subjects had been taking aspirin, were observed. This is an interesting finding because chronic aspirin use is generally not associated with major effects on blood pressure (Bautista et al., 2010; Bonten et al., 2015).
Our study had several strengths. First, our study design allowed assessment of associations between increased ambient pollutant concentrations and platelet markers with repeated measures within a subject. This enabled a within person assessment of effect measure modification by aspirin and fish oil supplements, reducing opportunities for residual confounding by subject characteristics. Second, well validated measures of platelet aggregation and TXB2 production, that have been shown to be causal factors in thrombus formation, were used. By using an NIH-sponsored clinical research center, our study protocol was standardized, enhancing consistency in how phlebotomy, clinic visit procedures, and supplement distribution occurred.
Some weaknesses also need to be considered. First, there was a relatively small sample size of patients with type II diabetes mellitus (n = 30) available for this study. This sample size, which is generally smaller than many other panel studies that examined associations between ambient PM air pollution and cardiovascular biomarkers (Brook et al., 2004, 2010; Rich et al., 2012a, 2012b; Zhang et al., 2013), likely resulted in limited statistical power to see significant changes in platelet aggregation measures associated with increased air pollutant concentrations in the previous few hours and days, as well as reduced precision in those effect estimates. Further, there was not a group of participants without type II diabetes mellitus, and thus it could not be evaluated whether these same findings occurred in this group as well. Second, pollutant concentrations were assigned from one central monitoring station to represent each study participant’s exposure to PM of outdoor origin, regardless of how close a study participant lived, worked, or spent time to that monitoring location. Since this was a secondary data analysis of a completed trial, we do not have subject addresses to know exactly how far each subject lived from the monitoring station, or what they were doing immediately before each study visit. This exposure error is most likely non-differential with respect to platelet aggregation levels in the blood, resulting in biases toward the null and underestimation of effect sizes. Third, addresses were not geocoded, and thus it was not possible to assess impacts of other factors (e.g. distance to roadway, neighborhood level socioeconomic status) on these same outcomes. Thus, it is also possible that our findings are due to residual confounding by an unmeasured confounder that is both correlated with temporal variations in UFP and AMP concentrations measured at the central monitoring site, and is a predictor of platelet aggregation and thromboxane B2 production. Fourth, only a few markers of platelet function were measured in this study and no other components of the coagulation/hemostatic process. For example, effects of increased UFP and AMP concentrations on p-selectin and CD40 ligand may be opposite to those known for TXB production since their regulation may be COX-independent. Last, it was not possible to assess whether particle surface area or particle composition impacted these platelet measures, as these were either not measured at all at the monitoring station, or only measured every third day. Therefore, there was not sufficient statistical power to evaluate associations between these particle measures and these platelet measures.
In summary, inconsistent with our first a priori hypothesis, decreased platelet aggregation and diminished TBX production associated with short-term increases in PM air pollution concentrations were observed. Although these increases in ultrafine particles represent only a small increase in particle mass, it may be that the number of ultrafine particles is an important determinant of whether PM is associated with changes in platelet aggregation. However, consistent with our second a priori hypothesis, these associations were blunted when taking aspirin and/or fish oil. Given the known adverse effects of PM air pollution exposure and well-established cardioprotective effects of aspirin and fish oil, it is conceivable that reductions in platelet function observed may still be detrimental in part due to the disrupted lipid milieu of patients with type II diabetes mellitus. It may be that these decreases are in response to a compensatory mechanism triggered by air pollution exposure that produces increases in another cardiometabolic substrate that contributes to enhanced risk of acute cardiovascular and cerebrovascular events. The prevalence of diabetes mellitus is increasing globally (Hu 2011). Excess TBX (a product of arachidonic acid) release occurs in those with this major cause of morbidity and mortality and coronary artery disease (Colwell and American Diabetes 2004). As aspirin and EPA and DHA in fish oil reduce production of TBX as well as pro-inflammatory and pro-thrombotic prostaglandins from arachidonic acid (Terano, et al. 1983, Ohmori, et al. 2006, Gurbel et al. 2007), their use in those with diabetes mellitus needs to be further investigated. How these inexpensive and safe agents alter effects of ambient PM air pollution on metabolic pathways that influence cardiovascular disease risk in those with diabetes mellitus is also a potentially rich area of future research.
Supplementary Material
Acknowledgments
The authors would like to thank the study participants. This publication was supported by National Heart Lung and Blood Institute (NHLBI) Grant # 5R21HL102582-02, the University of Rochester CTSA award number KL2 RR024136 from the National Center for Research Resources, and the National Center for Advancing Translational Sciences of the National Institutes of Health. Other support from the NIH was provided by R01 HL071933, R21 ES023032 and P30 ES001247. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI, NIEHS, or NIH.
REFERENCES
- Abdolahi A, Georas SN, Thomas Brenna J, Cai X, Thevenet-Morrison K, Phipps RP, Block RC. The effects of aspirin and fish oil consumption on lysophosphatidylcholines and lysophosphatidic acids and their correlates with platelet aggregation in adults with diabetes mellitus. Prostaglandins, Leukot Essent Fatty Acids (PLEFA) 2014;90:61–68. doi: 10.1016/j.plefa.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antithrombotic Trialists' Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. Br Med J. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista LE, Vera LM. Antihypertensive effects of aspirin: what is the evidence? Curr Hyperten Rep. 2010;12:282–289. doi: 10.1007/s11906-010-0115-5. [DOI] [PubMed] [Google Scholar]
- Beckerman BS, Jerrett M, Finkelstein M, Kanaroglou P, Brook JR, Arain MA, Sears MR, Stieb D, Balmes J, Chapman K. The association between chronic exposure to traffic-related air pollution and ischemic heart disease. J Toxicol Environ Health A. 2012;75(7):402–411. doi: 10.1080/15287394.2012.670899. [DOI] [PubMed] [Google Scholar]
- Bind M-A, Baccarelli A, Zanobetti A, Tarantini L, Suh H, Vokonas P, Schwartz J. Air pollution and markers of coagulation, inflammation, and endothelial function: associations and epigene-environment interactions in an elderly cohort. Epidemiology. 2012;23:332–340. doi: 10.1097/EDE.0b013e31824523f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block RC, Abdolahi A, Smith B, Meednu N, Thevenet-Morrison K, Cai X, Cui H, Mousa S, Brenna JT, Georas S. Effects of low-dose aspirin and fish oil on platelet function and NF-kappaB in adults with diabetes mellitus. Prostaglandins, Leukot Essent Fatty Acids (PLEFA) 2013;89:9–18. doi: 10.1016/j.plefa.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block RC, Abdolahi A, Tu X, Georas SN, Brenna JT, Phipps RP, Lawrence P, Mousa SA. The effects of aspirin on platelet function and lysophosphatidic acids depend on plasma concentrations of EPA and DHA. Prostaglandins, Leukot Essent Fatty Acids (PLEFA) 2015;96:17–24. doi: 10.1016/j.plefa.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonten TN, Snoep JD, Assendelft WJ, Zwaginga JJ, Eikenboom J, Huisman MV, Rosendaal FR, van der Bom JG. Time-dependent effects of aspirin on blood pressure and morning platelet reactivity: a randomized cross-over trial. Hypertension. 2015;65:743–750. doi: 10.1161/HYPERTENSIONAHA.114.04980. [DOI] [PubMed] [Google Scholar]
- Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC, Jr, Tager I. Air pollution and cardiovascular disease: A statement for healthcare professionals from the expert panel on population and prevention science of the American Heart Association. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA, III, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC, Jr, Whitsel L, Kaufman JD on behalf of the American Heart Association Council on Epidemiology and Prevention, Council on the Kidney in Cardiovascular Disease, and Council on Nutrition, Physical Activity and Metabolism. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Chen WC, Weng YH, Chiu YW, Yang CY. Short-term effects of coarse particulate matter on hospital admissions for cardiovascular diseases: a case-crossover study in a tropical city. J Toxicol Environ Health A. 2015;25:1–13. doi: 10.1080/15287394.2015.1083520. [DOI] [PubMed] [Google Scholar]
- Colwell JA American Diabetes Association. Aspirin therapy in diabetes. Diabetes Care. 2004;27(Suppl 1):S72–S73. doi: 10.2337/diacare.27.2007.s72. [DOI] [PubMed] [Google Scholar]
- Dyszkiewicz-Korpanty AM, Frenkel EP, Sarode R. Approach to the assessment of platelet function: comparison between optical-based platelet-rich plasma and impedance-based whole blood platelet aggregation methods. Clin Appl Thromb/Hemost. 2005;11:25–35. doi: 10.1177/107602960501100103. [DOI] [PubMed] [Google Scholar]
- Evans KA, Halterman JS, Hopke PK, Fagnano M, Rich DQ. Increased ultrafine particles and carbon monoxide concentrations are associated with asthma exacerbation among urban children. Environ Res. 2014;129:11–19. doi: 10.1016/j.envres.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(Suppl 1):S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- Frampton MW, Bausch J, Chalupa D, Hopke PK, Little EL, Oakes D, Stewart JC, Utell MJ. Effects of outdoor air pollutants on platelet activation in people with type 2 diabetes. Inhal Toxicol. 2012;24:831–838. doi: 10.3109/08958378.2012.724117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner B, Ling F, Hopke PK, Frampton MW, Utell MJ, Zareba W, Cameron SJ, Chalupa D, Kane C, Kulandhaisamy S, Topf MC, Rich DQ. Ambient fine particulate air pollution triggers ST-elevation myocardial infarction, but not non-ST elevation myocardial infarction: a case-crossover study. Part Fibre Toxicol. 2014;11:1. doi: 10.1186/1743-8977-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GISSI-Prevenzione Investigators. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- Ghio AJ, Carraway MS, Madden MC. Composition of air pollution particles and oxidative stress in cells, tissues, and living systems. J Toxicol Environ Health B Crit Rev. 2012;15:1–21. doi: 10.1080/10937404.2012.632359. [DOI] [PubMed] [Google Scholar]
- Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C National Heart, Lung, and Blood Institute. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on Scientific Issues Related to Definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- Gurbel PA, Bliden KP, DiChiara J, Newcomer J, Weng W, Neerchal NK, Gesheff T, Chaganti SK, Etherington A, Tantry US. Evaluation of dose-related effects of aspirin on platelet function: results from the Aspirin-Induced Platelet Effect (ASPECT) study. Circulation. 2007;115:3156–3164. doi: 10.1161/CIRCULATIONAHA.106.675587. [DOI] [PubMed] [Google Scholar]
- Hoek G, Krishnan RM, Beelen R, Peters A, Ostro B, Brunekreef B, Kaufman JD. Long-term air pollution exposure and cardio-respiratory mortality: a review. Environ Health. 2013;12:43. doi: 10.1186/1476-069X-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu FB. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care. 2011;34(6):1249–1257. doi: 10.2337/dc11-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iso H, Rexrode KM, Stampfer MJ, Manson JE, Colditz GA, Speizer FE, Hennekens CH, Willett WC. Intake of fish and omega-3 fatty acids and risk of stroke in women. JAMA. 2001;285:304–312. doi: 10.1001/jama.285.3.304. [DOI] [PubMed] [Google Scholar]
- Krasopoulos G, Brister SJ, Beattie WS, Buchanan MR. Aspirin “resistance” and risk of cardiovascular morbidity: Systematic review and meta-analysis. Br Med J. 2008;336:195–198. doi: 10.1136/bmj.39430.529549.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kris-Etherton PM, Harris WS, Appel LJ American Heart Association. Nutrition Committee. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- Langrish JP, Bosson J, Unosson J, Muala A, Newby DE, Mills NL, Blomberg A, Sandström T. Cardiovascular effects of particulate air pollution exposure: Time course and underlying mechanisms. J Intern Med. 2012;272:224–239. doi: 10.1111/j.1365-2796.2012.02566.x. [DOI] [PubMed] [Google Scholar]
- Lemaitre RN, King IB, Mozaffarian D, Kuller LH, Tracy RP, Siscovick DS. n-3 Polyunsaturated fatty acids, fatal ischemic heart disease, and nonfatal myocardial infarction in older adults: The Cardiovascular Health Study. Am J Clin Nutr. 2003;77:319–325. doi: 10.1093/ajcn/77.2.319. [DOI] [PubMed] [Google Scholar]
- Lucking AJ, Lundback M, Mills NL, Faratian D, Barath SL, Pourazar J, Badimon JJ, Gerlofs-Nijland ME, Cassee FR, Boman C, Donaldson K, Sandstrom T, Newby DE, Blomberg A. Diesel exhaust inhalation increases thrombus formation in man. Eur Heart J. 2008;29:3043–3051. doi: 10.1093/eurheartj/ehn464. [DOI] [PubMed] [Google Scholar]
- Nuvolone D, Balzi D, Chini M, Scala D, Giovannini F, Barchielli A. Short-term association between ambient air pollution and risk of hospitalization for acute myocardial infarction: results of the Cardiovascular Risk and Air Pollution in Tuscany (RISCAT) Study. Am J Epidemiol. 2011;174:63–71. doi: 10.1093/aje/kwr046. [DOI] [PubMed] [Google Scholar]
- Ohmori T, Yatomi Y, Nonaka T, Kobayashi Y, Madoiwa S, Mimuro J, Ozaki Y, Sakata Y. Aspirin resistance detected with aggregometry cannot be explained by cyclooxygenase activity: involvement of other signaling pathway(s) in cardiovascular events of aspirin-treated patients. J Thromb Haemost. 2006;4:1271–1278. doi: 10.1111/j.1538-7836.2006.01958.x. [DOI] [PubMed] [Google Scholar]
- Peters A, Dockery DW, Muller JE, Mittleman MA. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001;103:2810–2815. doi: 10.1161/01.cir.103.23.2810. [DOI] [PubMed] [Google Scholar]
- Peters A, von Klot S, Heier M, Trentinaglia I, Cyrys J, Hörmann A, Hauptmann M, Wichmann HE, Löwel H. Particulate air pollution and nonfatal cardiac events Part I. Air pollution, personal activities, and onset of myocardial infarction in a case-crossover study. Research Report (Health Effects Institute) 2005;124:1–66. discussion 67–82, 141–148. [PubMed] [Google Scholar]
- Pope CA, III, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc. 2006;56:709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- Rich DQ, Kipen HM, Huang W, Wang G, Wang Y, Zhu P, Ohman-Strickland P, Hu M, Philipp C, Diehl SR, Lu SE, Tong J, Gong J, Thomas D, Zhu T, Zhang J. Association between changes in air pollution levels during the Beijing Olympics and biomarkers of inflammation and thrombosis in healthy young adults. JAMA. 2012a;307:2068–2078. doi: 10.1001/jama.2012.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich DQ, Kipen HM, Zhang J, Kamat L, Wilson AC, Kostis JB for the Myocardial Infarction Data Acquisition System Study Group (MIDAS) Triggering of transmural infarctions, but not nontransmural infarctions, by ambient fine particles. Environ Health Persp. 2010;118:1229–1234. doi: 10.1289/ehp.0901624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich DQ, Zareba W, Beckett W, Hopke PK, Oakes D, Frampton MW, Bisognano J, Chalupa D, Bausch J, O'Shea K, Wang Y, Utell MJ. Are ambient ultrafine, accumulation mode, and fine particles associated with adverse cardiac responses in patients undergoing cardiac rehabilitation? Environ Health Persp. 2012b;120:1162–1169. doi: 10.1289/ehp.1104262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romieu I, Garcia-Esteban R, Sunyer J, Rios C, Alcaraz-Zubeldia M, Velasco SR, Holguin F. The effect of supplementation with omega-3 polyunsaturated fatty acids on markers of oxidative stress in elderly exposed to PM2.5. Environ Health Persp. 2008;116:1237–1242. doi: 10.1289/ehp.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rückerl R, Greven S, Ljungman P, Aalto P, Antoniades C, Bellander T, Berglind N, Chrysohoou C, Forastiere F, Jacquemin B, von Klot S, Koenig W, Küchenhoff H, Lanki T, Pekkanen J, Perucci CA, Schneider A, Sunyer J, Peters A AIRGENE Study Group. Air pollution and inflammation (interleukin-6, c-reactive protein, fibrinogen) in myocardial infarction survivors. Environ Health Persp. 2007;115:1072–1080. doi: 10.1289/ehp.10021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattil SJ, Hoxie JA, Cunningham M, Brass LF. Changes in the platelet membrane glycoprotein IIb.IIIa complex during platelet activation. J Biol Chem. 1985;260:11107–11114. [PubMed] [Google Scholar]
- Simkhovich BZ, Kleinman MT, Kloner RA. Air pollution and cardiovascular injury. J Am Coll Cardiol. 2008;52:719–726. doi: 10.1016/j.jacc.2008.05.029. [DOI] [PubMed] [Google Scholar]
- Stewart JC, Chalupa DC, Devlin RB, Frasier LM, Huang L-S, Little EL, Lee SM, Phipps RP, Pietropaoli AP, Taubman MB, Utell MJ, Frampton MW. Vascular effects of ultrafine particles in persons with type 2 diabetes. Environ Health Persp. 2010;118:1692–1698. doi: 10.1289/ehp.1002237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strak M, Hoek G, Godri KJ, Gosens I, Mudway IS, van Oerle R, Spronk HM, Cassee FR, Lebret E, Kelly FJ, Harrison RM, Brunekreef B, Steenhof M, Janssen NAH. Composition of PM affects acute vascular inflammatory and coagulative markers - The RAPTES Project. PLoS ONE. 2013;8:e58944. doi: 10.1371/journal.pone.0058944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terano T, Hirai A, Hamazaki T, Kobayashi S, Fujita T, Tamura Y, Kumagai A. Effect of oral administration of highly purified eicosapentaenoic acid on platelet function, blood viscosity and red cell deformability in healthy human subjects. Atherosclerosis. 1983;46:321–331. doi: 10.1016/0021-9150(83)90181-8. [DOI] [PubMed] [Google Scholar]
- Tong H, Rappold AG, Diaz-Sanchez D, Steck SE, Berntsen J, Cascio WE, Devlin RB, Samet JM. Omega-3 fatty acid supplementation appears to attenuate particulate air pollution–induced cardiac effects and lipid changes in healthy middle-aged adults. Environ Health Persp. 2012;120:952–957. doi: 10.1289/ehp.1104472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman EB, Zareba W, Utell MJ, Oakes D, Hopke PK, Frampton M, Chalupa D, Beckett W, Rich DQ. Acute changes in ambient temperature are associated with adverse changes in cardiac rhythm. Air Qual Atmos Health. 2014;7:357–367. doi: 10.1007/s11869-014-0244-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellenius GA, Schwartz J, Mittleman MA. air pollution and hospital admissions for ischemic and hemorrhagic stroke among Medicare beneficiaries. Stroke. 2005;36:2549–2553. doi: 10.1161/01.STR.0000189687.78760.47. [DOI] [PubMed] [Google Scholar]
- Wellenius GA, Burger MR, Coull BA, Schwartz J, Suh HH, Koutrakis P, Schlaug G, Gold DR, Mittleman MA. Ambient air pollution and the risk of acute ischemic stroke. Arch Intern Med. 2012;172:229–234. doi: 10.1001/archinternmed.2011.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Deng F, Wei H, Huang J, Wang H, Shima M, Wang X, Qin Y, Zheng C, Hao Y, Guo X. Chemical constituents of ambient particulate air pollution and biomarkers of inflammation, coagulation and homocysteine in healthy adults: a prospective panel study. Part Fibre Toxicol. 2012;9:49. doi: 10.1186/1743-8977-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanobetti A, Schwartz J. The effect of particulate air pollution on emergency admissions for myocardial infarction: a multicity case-crossover analysis. Environ Health Persp. 2005;113:978–982. doi: 10.1289/ehp.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhu T, Kipen H, Wang G, Huang W, Rich D, Zhu P, Wang Y, Lu SE, Ohman-Strickland P, Diehl S, Hu M, Tong J, Gong J, Thomas D HEI Health Review Committee. Cardiorespiratory biomarker responses in healthy young adults to drastic air quality changes surrounding the 2008 Beijing Olympics. Res Rep (Health Effects Institute) 2013;174:5–174. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.