Abstract
Background & Aims
Obesity is associated with an increased risk for pancreatic cancer, but it is unclear whether it affects mortality. We performed a systematic review and meta-analysis to assess the association between premorbid obesity and mortality from pancreatic cancer.
Methods
We performed a systematic search through January 2015 and identified studies of the association between premorbid obesity (at least 1 year prior to pancreatic cancer diagnosis) and pancreatic cancer–related mortality. We estimated summary adjusted hazard ratio (aHR) with 95% confidence interval (CI), comparing data from obese (body mass index [BMI] ≥30 kg/m2) and overweight subjects (BMI, 25.0–29.9 kg/m2) with those from individuals with a normal BMI (controls) by using random-effects model.
Results
We identified 13 studies (including 3 studies that pooled multiple cohorts); 5 studies included only patients with pancreatic cancer, whereas 8 studies evaluated pancreatic cancer–related mortality in cancer-free individuals at inception. In the meta-analysis, we observed increase in pancreatic cancer–related mortality among overweight (aHR, 1.06; 95% CI, 1.02–1.11; I2 = 0) and obese individuals (aHR, 1.31; 95% CI, 1.20–1.42; I2 = 43%), compared with controls; the association remained when we analyzed data from only subjects with pancreatic cancer. Each 1 kg/m2 increase in BMI was associated with 10% increase in mortality (aHR, 1.10; 95% CI, 1.05– 1.15) with minimal heterogeneity (I2 = 0). In the subgroup analysis, obesity was associated with increased mortality in Western populations (11 studies; aHR, 1.32; 95% CI, 1.22–1.42) but not in Asia-Pacific populations (2 studies; aHR, 0.98; 95% CI, 0.76–1.27).
Conclusions
In a systematic review and meta-analysis, we associated increasing level of obesity with increased mortality in patients with pancreatic cancer in Western but not Asia-Pacific populations. Strategies to reduce obesity-induced metabolic abnormalities might be developed to treat patients with pancreatic cancer.
Keywords: Survival, Adiposity, Pancreas, Malignancy
Pancreatic cancer is the fourth leading cause of cancer-related deaths in the United States.1 It carries a dismal prognosis with 5-year survival of 5%, in part because of late stage at time of diagnosis; however, even for surgically resectable pancreatic cancer, 5-year survival is only 10%–20%. Obesity, both overall and visceral, has been implicated as a risk factor for development of pancreatic cancer. In a systematic review of 23 prospective cohort studies, Aune et al2 observed a non-linear association between body mass index (BMI) and pancreatic cancer risk, with a 10% increase for each 5-unit change in BMI after adjustment for confounding variables. This may be related to induction of chronic inflammatory state in obese individuals with increased production of proinflammatory cytokines (eg, interleukin-6) and adipocytokines (eg, adiponectin, leptin), hyperinsulinemia, insulin resistance, and elevated levels of insulin-like growth factors.3–7
Recent evidence suggests that premorbid obesity may also be associated with increased mortality in patients with cancer, including colorectal,8 breast,9 prostate cancer,10 and leukemia.11 Several observational studies have evaluated the association between obesity and mortality in patients with pancreatic cancer, with variable results. In a case-control study, Li et al12 observed that obesity in adulthood was associated with reduced overall survival of pancreatic cancer, independent of disease stage and tumor resection status. In contrast, in a pooled analysis of 16 cohorts from Asian countries, Lin et al13 did not observe a significant association between increased BMI and pancreatic cancer–related mortality.
Hence, to better understand this association, we performed a systematic review with meta-analysis of observational studies that investigated the association between premorbid BMI and pancreatic cancer–related mortality.
Methods
This systematic review is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement and was conducted following a priori established protocol.14
Study Selection
We included observational studies that reported statistical measures of association (hazard ratio [HR], incidence rate ratio, or relative risk, with 95% confidence intervals [CIs]) between premorbid (ie, at least 1 year before diagnosis of pancreatic cancer) measures of obesity (BMI) and mortality (all-cause or cancer-related) in adults (relative to patients with normal BMI). Two types of patient populations were considered, studies performed in patients with established pancreatic cancer and those performed in cancer-free individuals at inception and followed for development of pancreatic cancer–related mortality. Inclusion was otherwise not restricted by study size, language, or publication type. When there were multiple publications from the same cohort, only data from the most recent comprehensive report were included.
We excluded cross-sectional studies, studies only with data on BMI changes at or after diagnosis of pancreatic cancer (confounding by underlying disease), and studies that did not provide a measure of association (precluded statistical analysis).
Data Sources and Search Strategy
First, we conducted a systematic literature search of PubMed (MEDLINE), Embase, Web of Science, Cochrane Library, and Google Scholar from inception to January 31, 2015 by using a combination of keywords or medical subject headings for cancer, obesity, mortality, and prognosis. All identified studies were combined together in a single reference manager file (EndNote; Thomson Reuters, Stamford, CT), duplicates were discarded, and the title and abstracts were reviewed by 2 authors independently to exclude studies that did not report the association between obesity and mortality, which was based on pre-specified inclusion and exclusion criteria. The full text of the remaining articles was examined to determine whether it contained relevant information. Disagreements were harmonized by consensus, in conjunction with the principal investigator. Second, the reference lists from included original articles and recent reviews and meta-analyses on obesity and cancer-related mortality were hand searched to identify any additional studies. Third, conference proceedings of major gastroenterology and oncology conferences (Digestive Diseases Week, Gastrointestinal Cancers Symposium, annual meetings of the American Society of Clinical Oncology and European Society of Medical Oncology) from 2010 to 2014 were reviewed for relevant abstracts. Figure 1 shows the schematic diagram of study selection.
Figure 1.
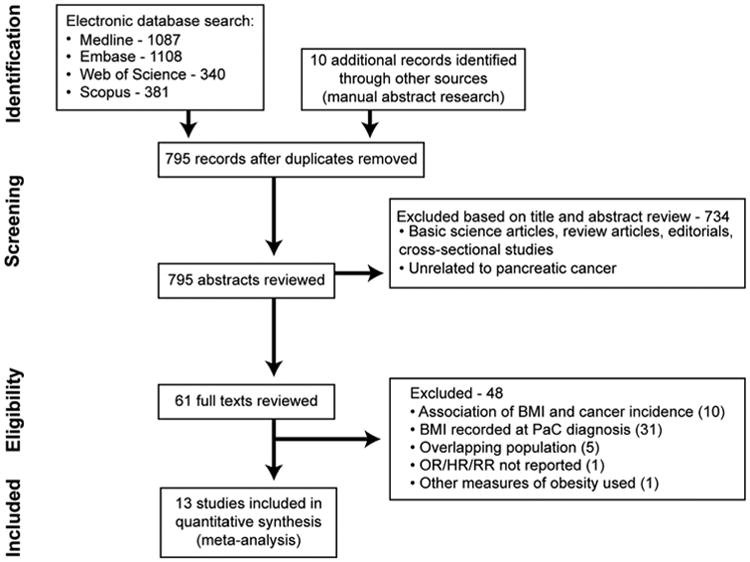
Flow sheet summarizing study identification and selection. OR, short of odds ratio; PaC, pancreatic cancer; RR, relative risk.
Data Abstraction and Quality Assessment
Two reviewers independently abstracted data on the study-related and patient-related characteristics onto a standardized form, as detailed in the Supplementary Materials.
The risk of bias in these prognostic individual studies was assessed by 2 authors independently by using the Quality In Prognosis Studies tool, which evaluates validity and bias in studies of prognostic factors across 6 domains: participation, attrition, prognostic factor measurement, confounding measurement and account, outcome measurement, and analysis and reporting.15
Outcomes Assessed
Our primary outcome was the association between obesity and pancreatic cancer–related mortality. This was assessed by using 3 approaches. First, we compared mortality risk with the highest reported BMI category in a study with the reference category and summarized these estimates. However, there were minor differences in reported categories of obesity in individual studies; hence, for standardized interpretation, we calculated mortality risk in obese patients (BMI ≥30 kg/m2) and overweight patients (BMI 25.0–29.9 kg/m2), compared with normal BMI patients. For this analysis, we pooled effects for all BMI categories of BMI ≥30 kg/m2 into a single summary estimate for obese patients and likewise pooled effects of all BMI categories for BMI 25.0–29.9 kg/m2 into a single summary estimate for overweight patients. This analysis also helped assess for the presence of a dose-response relationship (deemed to be present if the summary estimates for overweight and obese patients had an incremental association, compared with referent normal BMI). Finally, we estimated change in mortality per unit BMI by using linear trend meta-analytic statistical methodology (Supplementary Materials).16
Subgroup and Sensitivity Analyses
A priori hypotheses to assess robustness of the analysis and explain potential heterogeneity in the direction and magnitude of effect among different observational studies were assessed by using subgroup analyses. These subgroups were based on location (Western vs Asia-Pacific), design (pooled cohorts vs individual studies), and study setting (hospital-based vs population-based); during the review process, additional subgroup analyses were proposed on the basis of method of exposure ascertainment (self-reported vs measured weight and height) and time period over which study was conducted (defined on the basis of period of cohort recruitment: before 1980s vs 1980s–2000 vs beyond 2000 vs a category from 1980s to current). Because it is possible that the association between obesity and mortality in pancreatic cancer may be confounded by diabetes and stage, treatment response (especially surgical resection), and performance status, we performed subgroup analysis comparing studies that did and did not account for these variables.
We also performed a sensitivity analysis restricted only to studies reporting the association between obesity and mortality in a cohort of patients with established pancreatic cancer (excluding studies that included cancer-free individuals at inception). In addition, we performed sensitivity analysis by excluding one study at a time to assess whether a particular study significantly influenced the summary estimate and/or heterogeneity.
Statistical Analysis
We used random-effects model described by DerSimonian and Laird to calculate summary HR and 95% CI.17 Maximally adjusted HR (aHR), when reported in studies, was used for analysis to account for confounding variables. To estimate what proportion of total variation across studies was due to heterogeneity rather than chance, inconsistency index (I2 statistic) was calculated; values of <30%, 30%–59%, 60%–75%, and >75% were suggestive of low, moderate, substantial, and considerable heterogeneity, respectively.18 Subgroup analyses was performed by stratifying original estimates according to study characteristics (as described above). In this analysis, a P value < .10 for differences between subgroups was considered statistically significant.
We assessed for publication bias quantitatively by using the Egger regression test (publication bias considered present if P ≤ .10) and qualitatively by visual inspection of funnel plots.19,20 All P values were 2-tailed. All calculations and graphs were performed by using Comprehensive Meta-Analysis (CMA) version 2 (Biostat, Englewood, NJ).
Results
Of 795 unique studies identified by using our search strategy, we reviewed full texts of 61 studies, and 13 studies fulfilled our inclusion criteria and were included in the meta-analysis (10 independent cohorts and 3 studies reporting pooled data from multiple cohorts).12,13,21–31 There were no randomized controlled trials evaluating the association between obesity and mortality in patients with pancreatic cancer. Five studies32–36 from overlapping cohorts, 1 study that reported alternative measures of obesity37 (intra-abdominal fat via preoperative computed tomography imaging), and 1 study with insufficient data for estimation of statistical association were excluded.38
Characteristics and Quality of Included Studies
Thirteen studies were included that reported on 3,434,737 participants, of whom 11,626 patients with pancreatic cancer died.12,13,21–31 For the purposes of data abstraction, all studies were cohort by design (with subjects being followed over time after exposure [obesity], for development of outcome [mortality]), although some studies inherently were reported as case-control studies.12,24,25 Although 5 studies12,21,23–25 included a cohort of patients with established pancreatic cancer and reported the association between premorbid obesity and all-cause mortality, 8 studies were large cohorts of cancer-free participants at inception and followed them for development of pancreatic cancer-related mortality through record linkage with national death indices. The characteristics of the included studies are shown in Table 1. Eleven studies were based in North America or Europe,12,21–25,27–31 including 1 pooled study of 7 cohorts of black participants.27 Two studies were conducted in the Asia-Pacific region, both pooled analysis of multiple individual cohorts (30 cohorts from the Asia-Pacific Cohort Studies Collaboration26 and 16 cohorts from the Asia Cohort Consortium13). Study size ranged from 475 patients with pancreatic cancer to 1,222,630 participants (of which 8 studies had an unclear proportion of pancreatic cancer), and total number of deaths in patients with pancreatic cancer ranged from 139 to 3558. All of the included studies had an average follow-up of at least 3 years. BMI was assessed as primary measure of obesity in all studies, with premorbid evaluation performed at least 1 year before pancreatic cancer diagnosis. The association between central adiposity (using waist circumference) and mortality was assessed in only 1 study.26 The baseline characteristics of the participants in individual studies are reported in Table 2. Patients with obesity expectedly had higher rates of diabetes in included studies.
Table 1. Characteristics of Included Studies.
| First author, year of publication | Location; study design | Time period; follow-up | Patients/participants | Deaths; method of outcome assessment | BMI; % obese (BMI >30 kg/m2); timing and method of BMI ascertainment | Association between BMI categories and mortality (95% CI) | Covariates adjusted for |
|---|---|---|---|---|---|---|---|
| Ansary-Moghaddam, 200626 | Asia-Pacific; pooled cohort of 30 sites; population-based | 1961–1999; 7 y | 182,173 (all cancer-free at inception); unclear what proportion developed pancreatic cancer | 324; record linkage with death certificates | Range of mean BMI in individual cohorts: 21.2–26.9 kg/m2; NR, but 24.2% with BMI >26 kg/m2; premorbid BMI assessed at inception (measured) | Tertiles of BMI; Reference: BMI <23 kg/m2 23-26 vs reference: 1.07 (0.76–1.49) ≥26 vs reference: 1.00 (0.69–1.45) |
1, 2, 4, 6, 7 |
| Olson, 201024 | United States; single center; hospital-based | 2004–2008; 4 y | 475 (all pancreatic cancer patients) | 472; record linkage with manual chart review | NR; 23%; BMI assessed on basis of usual premorbid height and weight (self-reported) | Reference: BMI <25 kg/m2 Resected: 25-30 vs reference: 1.02 (0.56–1.88) ≥30 vs reference: 1.62 (0.76–3.44) Unresected: 25–30 vs reference: 1.05 (0.70–1.57) ≥30 vs reference: 1.38 (0.90–2.11) |
1, 2, 4, 6, 9, 10, 11, 12 |
| McWilliams, 201021 | United States; single cohort, hospital-based | 2000–2009; 3 y | 1861 (all pancreatic cancer patients) | 1527; record linkage with death indices, and periodic mailings, manual chart review and tumor registry | 28.5 ± 5.8 kg/m2; 31.9%; BMI assessed on basis of usual height and weight (66.2%) or weight at enrollment plus reported weight loss (33.8%) (combination of measured and self-reported) | Reference: BMI 18.5–24.9 kg/m2 <18.5 vs reference: 1.42 (0.76-2.68) 25–29.9 vs reference: 1.03 (0.89–1.18) 30–34.9 vs reference: 1.16 (0.98–1.37) 35–39.9 vs reference: 1.35 (1.07–1.69) ≥40 vs reference: 1.50 (1.13–1.98) |
1, 2, 6, 9 |
| Yuan, 201323 | United States; pooled analysis of 2 cohorts; population-based | 1988–2010; 4 y | 902 (all pancreatic cancer patients) | 868; record linkage with national death index, and postal contact with next of kin | 25.9 ± 4.5 kg/m2; 15.1%; premorbid BMI assessed at inception (self-reported) | Reference: BMI <25 kg/m2 25–26.99 vs reference: 0.88 (0.73–1.06) 27–29.9 vs reference: 1.04 (0.86–1.26) 30–34.9 vs reference: 1.21 (0.96–1.53) ≥35 vs reference: 1.45 (1.05–1.99) |
1, 2, 3, 4, 9, 13 |
| Gong, 201225 | United States; single cohort; population-based | 1995–1999; 10 y | 510 (all pancreatic cancer patients) | 495; record linkage with Social Security Death Index and California Death Records databases | NR; 10%; BMI assessed on basis of usual premorbid height and weight (self-reported) | Reference: BMI <25 kg/m2 25-29.9 vs reference: 1.04 (0.83–1.28) ≥30 vs reference: 1.28 (0.91–1.81) |
1, 2, 3, 4, 6, 8, 9, 14, 15, 16 |
| Li, 200912 | United States; single center; hospital-based | 2004–2008; 2 y | 841 (all pancreatic cancer patients); 609 assessable patients with at least 1 y of follow-up | 352; record linkage with Social Security Death Index and local tumor registry, manual chart review | 24.7 ± 3.8 kg/m2; 26.7%; BMI assessed on basis of usual premorbid height and weight (self-reported) | Reference: BMI <25 kg/m2 25–29.9 vs reference: 1.26 (0.94–1.69) ≥30 vs reference: 1.86 (1.35–2.56) |
6, 9, 15 |
| Reeves, 200722 | United Kingdom; single cohort; population-based | 1996–2001; >1 y | 1,222,630 (all cancer-free at inception); unclear what proportion developed pancreatic cancer | 1130; record linkage with National Health Service central registry | Median BMI: 25.4 (IQR, 23–28.6); NA; BMI assessed on basis of usual premorbid height and weight (self-reported) | Reference: 22.5–24.9 kg/m2 <22.5 vs reference: 1.11 (0.97–1.27) 25–27.4 vs reference: 1.01 (0.89–1.15) 27.5–29.9 vs reference: 1.12 (0.96–1.31) ≥30 vs reference: 1.32 (1.16–1.51) |
1, 4, 5, 17, 18, 19, 20, 21, 22 |
| Calle, 200328 | United States; single cohort; population-based | 1982–1998, 16 y | 900,053 (all cancer-free at inception); unclear what proportion developed pancreatic cancer | 3558; record linkage with national death index, and personal inquiries by volunteers | NR; NR; BMI assessed on basis of usual premorbid height and weight (self-reported) | Reference: 18.5–24.9 kg/m2 Men: 25–29.9 vs reference: 1.13 (1.03–1.25) 30–34.9 vs reference: 1.41 (1.19–1.66) 35–39.9 vs reference: 1.49 (0.99–2.22) Women: 25–29.9 vs reference: 1.11 (1.00–1.24) 30–34.9 vs reference: 1.28 (1.07–1.52) 35–39.9 vs reference: 1.41 (1.01–1.99) ≥40 vs reference: 2.76 (1.74–4.36) |
Men: 1, 3, 4, 5, 8, 20, 23, 24, 25, 26 Women: 1, 3, 4, 5, 8, 20, 22, 23, 24, 25, 26 |
| Lin, 201313 | Asia; pooled cohorts of 16 sites; population-based | 1990–2002; range of follow-up: 3–22 y | 799,452 (all cancer-free at inception); unclear what proportion developed pancreatic cancer | 1489; record linkage with death certificates | Range of mean BMI in individual cohorts: 19.9–23.7 kg/m2; NR; BMI assessed on basis of usual premorbid height and weight at inception (self-reported) | Reference: 22.5–24.9 kg/m2 <18.5 vs reference: 1.04 (0.84–1.30) 18.5–19.9 vs reference: 0.82 (0.67–1.00) 20–22.4 vs reference: 0.91 (0.80–1.05) 25–27.4 vs reference: 0.95 (0.80–1.11) 27.5–29.9 vs reference: 1.01 (0.80–1.29) ≥30 vs reference: 0.96 (0.67–1.37) |
1, 2, 4, 6, 7 |
| Bethea, 201427 | United States; pooled cohorts of 7 sites; population-based | 1982–2008; 12 y | 239,597 (all cancer-free at inception); unclear what proportion developed pancreatic cancer | 897; record linkage with national death index | NR; 33%; BMI assessed on basis of usual premorbid height and weight at inception (self-reported) | Reference: 18.5–24.9 kg/m2 25–29.9 vs reference: 1.08 (0.90–1.31) 30-34.9 vs reference: 1.25 (0.99–1.57) ≥35 vs reference: 1.31 (0.97–1.33) |
1, 2, 4, 5, 8, 20, 26 |
| Batty, 200929 | United Kingdom; single cohort; population-based | 1967–1970; 38 y | 17,898 (all cancer-free at inception); unclear what proportion developed pancreatic cancer | 163; record linkage with National Health Service central registry | NR; 33%; BMI assessed on basis of usual premorbid height and weight at inception (self-reported) | Reference: 18.5–24.9 kg/m2 25–29.9 vs reference: 1.02 (0.69–1.50) 30–34.9 vs reference: 1.18 (0.79–1.75) |
1, 4, 6, 18, 20, 26, 27, 28, 29, 30 |
| Gatspur, 200030 | United States; single cohort; population-based | 1967–1973; 25 y | 35,658 (all cancer-free at inception); unclear what proportion developed pancreatic cancer | 139; record linkage with national death index and Social Security Death Index, and postal inquiries | Men: 26.6 ± 3.7 Women: 24.0 ± 4.4; NR; premorbid BMI assessed at inception (measured) | Quartiles of BMI; Men: Reference: <24.3 kg/m2 24.2–26.3 vs reference: 1.84 (0.87–3.92) 26.3–28.6 vs reference: 1.71 (0.81–3.61) >28.6 vs reference: 3.07 (1.53–6.15) Women: Reference: <21.0 kg/m2 21.0–23.2 vs reference: 0.50 (0.18–1.41) 23.2–26.2: 1.14 (0.49–2.65) >26.2 vs reference: 0.79 (0.32–1.95) |
1, 3, 4, 6 |
| Lee, 200331 | United States; single cohort; population-based | 1962–1966; 27–31 y | 32,687 (all cancer-free at inception); unclear what proportion developed pancreatic cancer | 212; record linkage with death certificates | NR; 10.2% with BMI ≥27.5 kg/m2; BMI assessed on basis of usual premorbid height and weight at inception (self-reported) | Reference: <22.5 kg/m2 22.5-24.9 vs reference: 0.84 (0.58-1.22) 25-27.4 vs reference: 1.08 (0.74-1.57) ≥27.5 vs reference: 0.99 (0.60-1.62) |
1, 2, 4, 6, 20 |
NOTE. Variables adjusted for: 1, age; 2, sex; 3, race; 4, smoking; 5, alcohol use; 6, diabetes; 7, study (pooled cohorts); 8, education; 9, stage; 10, allergies; 11, chemo; 12, performance status; 13, year of diagnosis; 14, tumor grade; 15, primary treatment; 16, tumor site; 17, geographical region; 18, socioeconomic status; 19, reproductive history; 20, physical activity; 21, time since menopause; 22, use of hormone replacement therapy; 23, aspirin use; 24, fat consumption; 25, vegetable consumption; 26, marital status; 27, plasma cholesterol; 28, forced expiratory volume in 1 second; 29, height; 30, blood pressure. IQR, interquartile range; NR, not reported.
Table 2. Baseline Characteristics of Participants in Individual Studies.
| First author, reference | Age at pancreatic cancer diagnosis or enrollment (y) | Sex (% male) | Race (% white) | Smoking | Diabetes | Stage at diagnosis of pancreatic cancer – stage 1/2/3/4 | Cancer-related therapy (proportion of patients undergoing surgery, chemotherapy, radiation) |
|---|---|---|---|---|---|---|---|
| Ansary-Moghaddam26 | 47 | 65 | NR | 23 | 6% | NR | NR |
| Olson24 | 64 | 52 | 88 | 53 | 10 | Resected: 9% (T1/T2), 91% (T3/T4) | Resected (34%): 89% received chemotherapy as well; |
| Unresected cohort: 56% M1 | Unresected (66%): 88% received chemotherapy | ||||||
| McWilliams21 | 66 | 56 | NR | NR | 31 | Resectable (stage I/II): 30% Locally advanced: 35% Metastatic: 35% |
NR |
| Yuan23 | 71 | NA | 95 | 60 | 5 | Localized: 15% Locally advanced: 11% Metastatic: 49% Unknown: 25% |
NR |
| Gong25 | BMI <25: 66 BMI 25–29: 63 BMI ≥30: 62 |
55 | 83 | BMI <25: 69% BMI 25–<30: 72% BMI ≥30: 57% |
BMI <25: 9% BMI 25–29: 14% BMI ≥30: 39% |
Local: 12% Regional: 43% Distant: 30% Unknown: 15% |
Resection: 31% Chemo/radiation: 29% Bypass/stent: 9% Other treatment: 9% Unknown: 23% |
| Li12 | 61 | 59 | 86 | 54 | 26 | NR | Resection: 16% |
| Reeves22 | 56 | 0 | NR | 19 | NR | NR | NR |
| Calle28 | 57 | 45 | NR | 57 | NR | NR | NR |
| Lin13 | Range: 37–60 | 50 | 0 | 34 | NR | NR | NR |
| Bethea27 | 52 | 29 | 0 | NR | NR | NR | NR |
| Batty29 | NA | 100 | NR | NR | NR | NR | NR |
| Gatspur30 | 40 | 57 | 88 | 39 | NR | NR | NR |
| Lee31 | 47 | 93 | NR | 48 | 1.8 | NR | NR |
NR, not reported.
Supplementary Table 1 depicts the methodological quality of all studies. The overall risk of bias was low to moderate. Ten studies relied on self-reported weight (4 studies using self-reported “usual” body weight and height and 6 studies using self-reported “current” body weight and height at time of enrollment in cohort), and 2 studies relied on weight and height measured by research staff at enrollment; 1 study relied partly on self-reported values and partly on measured values. Mortality in most studies was assessed through record linkage with the national death index and/or other sources to obtain death certificates, and some studies complemented this with mailed inquiries to the family. In the Asia-Pacific Cohort Studies Collection, BMI was measured at enrollment; however, the study had an acknowledged limitation of non-standardization of data collection. Most studies adjusted for the following confounders: age (12 of 13), sex (8 of 13), race (4 of 13), diabetes mellitus (9 of 13), smoking (11 of 13), and stage (5 of 13).
Body Mass Index and All-cause Mortality in Patients With Pancreatic Cancer
Of 13 studies, 9 studies observed higher mortality in patients with higher premorbid BMI, and this was statistically significant in 5 studies. On meta-analysis, when comparing patients in the highest category of BMI with the lowest category, we observed that patients with high BMI had 33% higher mortality (aHR, 1.33; 95% CI, 1.19–1.49), with moderate heterogeneity (I2 = 35%) (Figure 2A).
Figure 2.
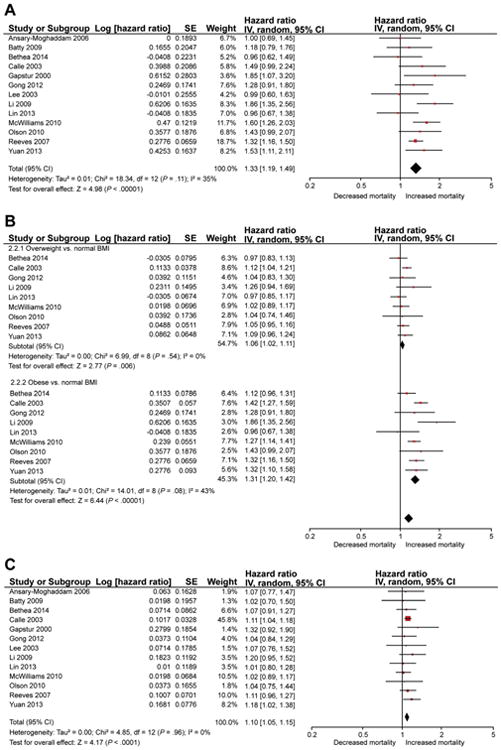
(A) Summary of maximally adjusted HR for association between premorbid BMI and pancreatic cancer-related mortality, comparing patients in the highest category of BMI with the lowest category. The size of the box corresponds to the weight of the given study. (B) Summary of maximally adjusted HR for association between premorbid BMI and pancreatic cancer-related mortality, comparing overweight (BMI 25.0–29.9 kg/m2) and obese (BMI ≥30 kg/m2) patients with normal BMI patients. The size of the box corresponds to the weight of the given study. (C) Summary of maximally adjusted HR for evaluation of dose-response relationship between premorbid BMI and pancreatic cancer–related mortality. The estimate represents mortality increase per 1 kg/m2 increase in BMI. SE, standard error.
Dose-response Relationship
On comparing 9 studies that allowed estimation of a summary estimate for obese and overweight patients, we observed an incremental and significant increase in mortality in both overweight patients (aHR, 1.06; 95% CI, 1.02–1.11; I2 = 0%) and obese patients with pancreatic cancer (aHR, 1.31; 95% CI, 1.20–1.42; I2 = 43%), as compared with normal BMI pancreatic cancer patients (Figure 2B); 4 studies were excluded from this analysis because they provided data only as tertiles, quartiles, or nonconventional BMI categories (without conventional World Health Organization–defined categories for overweight and obesity). There was significant difference between summary estimates for overweight and obese patients, suggesting a dose-response relationship (P value for trend, < .001).
On inputing mortality risk per unit BMI, we again observed a dose-response relationship, with each unit increase in BMI above normal associated with a 10% increase in mortality (aHR, 1.10, 95% CI, 1.05–1.15) with minimal heterogeneity (Figure 2C).
Subgroup Analysis
To assess robustness of the association between BMI and mortality in patients with pancreatic cancer, we performed several pre-planned subgroup analyses. For these analyses, we used World Health Organization–defined categories for overweight and obesity for studies where such data were available; when these data were not available, available BMI categories were transformed into 3 categories, and lowest BMI category was used as reference, middle category was regarded as overweight, and highest category was regarded as obese. We observed that incremental and significant increase in mortality in both overweight and obese patients with pancreatic cancer was seen only in Western studies (11 studies; overweight vs normal BMI: aHR, 1.07; 95% CI, 1.02–1.12; obese vs normal BMI: aHR, 1.32; 95% CI, 1.22–1.42) but not in studies performed in the Asia-Pacific region (2 studies; overweight vs normal BMI: aHR, 0.98; 95% CI, 0.87–1.11; obese vs normal BMI: aHR, 0.98; 95% CI, 0.76–1.27) (Table 3). The association between obesity and mortality was stronger in individual studies as compared with pooled cohorts, although 2 out of 3 pooled cohorts were performed in Asia-Pacific regions. Both population-based and hospital-based subgroups reported increase in mortality to a similar degree. There was no differences in summary estimates that were based on subgroups defined by method of exposure ascertainment and time period in which the study was conducted (ie, the association between obesity and mortality was stable over time).
Table 3. Subgroup Analysis.
| Subgroups | Categories | No. of studies | Overweight | Obese |
|---|---|---|---|---|
| Location | Asia-Pacific | 2 | 0.98 (0.87–1.11), I2 = 0 | 0.98 (0.76–1.27), I2 = 0 |
| Western | 11 | 1.07 (1.02–1.12), I2 = 0 | 1.32 (1.22–1.42), I2 = 30 | |
| Study type | Pooled cohort | 3 | 0.98 (0.89–1.08), I2 = 0 | 1.08 (0.95–1.23), I2 = 3 |
| Individual site | 10 | 1.08 (1.03–1.13), I2 = 0 | 1.35 (1.27–1.43), I2 = 5 | |
| Study setting | Population-based | 10 | 1.06 (1.01–1.11), I2 = 0 | 1.26 (1.15–1.37), I2 = 60 |
| Hospital-based | 3 | 1.06 (0.94–1.18), I2 = 0 | 1.45 (1.14–1.84), I2 = 60 | |
| Adjusted for diabetes | Yes | 9 | 1.03 (0.95–1.10), I2 = 0 | 1.27 (1.11–1.46), I2 = 37 |
| No | 4 | 1.08 (1.02–1.14), I2 = 2 | 1.30 (1.18–1.44), I2 = 50 | |
| Whether primary tumor underwent surgical resection | Yes | 2 | — | 2.29 (1.13–4.67), I2 = 39 |
| No | 2 | — | 1.53 (1.17–2.01), I2 = 0 (P value for difference, .30) | |
| Adjusted for stage and/or treatment of pancreatic cancer and/or performance status | Yes | 5 | 1.07 (0.98–1.16), I2 = 0 | 1.35 (1.21–1.51), I2 = 21 |
| No | 8 | 1.06 (1.01–1.12), I2 = 0 | 1.23 (1.09–1.38), I2 = 49 (P value for difference between groups, .26) | |
| Ascertainment of exposure | Self-reported | 3 | 1.05 (0.93–1.18), I2 = 0 | 1.26 (1.00–1.58), I2 = 41 |
| Measured | 10 | 1.06 (1.02–1.11), I2 = 0 | 1.30 (1.18–1.42), I2 = 41 (P value for difference between groups, .83) | |
| Time period of recruitment of patients | Before 1980s | 3 | 1.04 (0.85–1.26), I2 = 0 | 1.26 (0.90–1.75), I2 = 32 |
| 1980–2000 | 5 | 1.07 (1.02–1.13), I2 = 0 | 1.28 (1.13–1.45), I2 = 42 | |
| 2001–2010 | 3 | 1.06 (0.94–1.18), I2 = 0 | 1.45 (1.14–1.84), I2 = 60 | |
| Across 1980–2010 | 2 | 1.04 (0.93–1.16), I2 = 21 | 1.21 (1.03–1.42), I2 = 43 (P value for difference between groups = .66) |
To evaluate for potential confounding by presence of diabetes and differences in treatment and/or performance status between obese and normal BMI patients, we performed stratified analysis of studies that did and did not adjust for these variables in their analysis. Higher mortality in overweight and obese patients persisted after adjusting for diabetes, suggesting a diabetes independent effect (Table 3). Similarly, no difference was observed in the association between obesity and mortality outcome in studies that did or did not adjust for pancreatic cancer stage, treatment, or performance status. Because obesity increases risk of surgical mortality in patients with pancreatic cancer, we analyzed the association between obesity and mortality in patients who underwent resection and those who did not. Obese patients (vs normal BMI) had increased mortality in both patients who underwent surgical resection (2 studies; aHR, 2.29; 95% CI, 1.13–4.67) and those who did not undergo resection of primary tumor (2 studies; aHR, 1.53; 95% CI, 1.17–2.01), with comparable summary estimates (P value for difference between groups, .30).12,24
Sensitivity Analysis and Publication Bias
The results were stable on restricting analysis to studies performed in patients with established pancreatic cancer12,21,23–25 (overweight vs normal BMI: aHR, 1.07; 95% CI, 0.98–1.16; I2 = 0; obese vs normal BMI: aHR, 1.35; 95% CI, 1.21–1.51; I2 = 21%).
To assess whether any one study had a dominant effect on the meta-analytic HR, we excluded each study at a time and analyzed its effect on the main summary estimate. On this analysis, the overall summary estimate for the comparison between obesity and mortality in pancreatic cancer patients ranged from 1.26 to 1.32, with no single study significantly affecting summary estimate. On the basis of the visual inspection of the funnel plot (Supplementary Figure 1) as well as on quantitative measurement by using the Egger regression test, there was no evidence of publication bias (P = .67).
Discussion
Although obesity has been associated with increased risk of developing pancreatic cancer, in this systematic review we made several key observations on the association between premorbid obesity and pancreatic cancer–related mortality. First, we observed that obesity (present before diagnosis of pancreatic cancer) is associated with increased pancreatic cancer–related mortality both in patients with established pancreatic cancer and in cancer-free individuals at baseline in a dose-dependent and consistent manner. Both overweight (BMI 25.0–29.9 kg/m2) and obese patients (BMI ≥30 kg/m2) had 6% and 31% higher mortality, respectively, as compared with patients with normal BMI, without significant heterogeneity. Second, this association between obesity and increased mortality is observed only in the Western regions but not in patients with pancreatic cancer diagnosed in the Asia-Pacific regions. Third, the association is independent of diabetes and pancreatic cancer stage and treatment and is observed regardless of whether patients underwent surgical resection. Because of the very poor prognosis of patients with pancreatic cancer even with aggressive intervention, this observation mandates further research into the role of obesity-associated proinflammatory state in the progression of pancreatic cancer and merits evaluation of obesity as an effect modifier in treatment trials of pancreatic cancer.
Several observational and preclinical interventional studies have demonstrated an association between obesity and pancreatic cancer. The underlying mechanism of how obesity drives pancreatic tumorigenesis is related to complex interplay between obesity/high-fat diet, associated altered metabolism including insulin resistance, chronic inflammation, and oncogenic KRAS signaling. Metabolically active visceral fat exerts systemic proinflammatory effects that may promote, independently or synergistically, pancreatic carcinogenesis.39–42 Recent evidence suggests that obesity is associated with intrapancreatic fatty infiltration, which is associated with pancreatic intraepithelial neoplasia, a precursor of pancreatic cancer, thus suggesting a paracrine effect of these mediators. Obesity-induced insulin resistance and peripheral hyperinsulinemia have also been associated with increased risk of developing pancreatic cancer. Insulin-like growth factor-1 and insulin-like growth factor-1 receptor are highly expressed in pancreatic cancer cell lines, and insulin-like growth factor-1 mediated signalling promotes proliferation, invasion, and expression of angiogenic factors and decreases apoptosis in these cell lines. Hence, obesity may induce a more aggressive phenotype of pancreatic cancer.
We observed that obesity, as measured by BMI, was associated with increased mortality only in the Western population but not in the Asia-Pacific regions. This may be related to underlying differences in the pathophysiology and progression of pancreatic cancer in these different populations or may be a reflection of the failure of overall obesity to capture underlying metabolic syndrome. It is well-known that Asians, in particular South Asians, may have higher rates of metabolic syndrome even with apparently normal BMI; hence, Asians may be experiencing similar effects of adiposity-associated chronic inflammation and insulin resistance at seemingly normal levels of BMI. In their pooled analysis of the Asia-Pacific Cohort Studies Collaboration, Ansary-Moghaddam et al26 did not observe any significant association between premorbid BMI and pancreatic cancer mortality but observed that each 2-cm increase in waist circumference was associated with 8% increase in mortality (aHR, 1.08; 95% CI, 1.02–1.14). Additional studies evaluating ethnicity as an effect modifier of obesity in pancreatic cancer mortality are warranted.
Obesity also creates unique challenges in delivering appropriate therapy that may lead to worse survival outcomes for obese patients. It can limit the extent of a cancer operation and increase surgical morbidity and mortality. Obesity also complicates the planning or delivery of radiation therapy and is associated with inadequate dosing of chemotherapeutic agents. Despite guidelines recommending dosing that is based on actual body weight, practice pattern studies demonstrate that up to 40% of obese patients receive limited chemotherapy doses that are not based on actual body weight because of concerns about toxicity or overdosing.43 Hence, one of the factors linking obesity and worse survival could be inadequate chemotherapy dosing in obese patients.
Strengths and Limitations
The strengths of this systematic review include (1) comprehensive and systematic literature search with well-defined inclusion criteria, carefully excluding redundant studies and studies in which BMI was assessed at time of pancreatic cancer diagnosis; (2) rigorous evaluation of study quality by using a validated tool for prognostic studies; (3) subgroup and sensitivity analyses to evaluate the stability of findings, regardless of presence or absence of heterogeneity; and (4) rigorous assessment of a dose-response relationship by using 2 approaches, adding biological credibility to findings.
There are several limitations in our study. First, the meta-analysis included only observational studies with inherent biases and suboptimal control of confounders. Second, not all studies were restricted to patients with established pancreatic cancer; several studies were large cohort studies that enrolled cancer-free individuals at baseline and followed them for development of several outcomes including pancreatic cancer–related mortality; however, our results were stable on restricting analysis to the former group of studies. Third, all studies did not consistently account for relevant covariates, including pancreatic cancer stage and treatment, and despite adjusting for several covariates, it is not possible to eliminate the potential of residual confounding, especially with regard to factors that go into determining treatment choice for patients with medically complicated obesity. However, subgroup analyses that are based on adjustment for diabetes and pancreatic cancer stage and treatment did not suggest significant impact of these factors. Fourth, we did not specifically analyze all-cause and cancer-related mortality separately because of paucity of such data in the included studies. However, competing risk for mortality is unlikely to play a significant part in pancreatic cancer because of high mortality associated with this disease. The included studies had inherent limitations, including variability in method and timing of exposure ascertainment and outcome assessment.
In conclusion, we observed that premorbid obesity adversely influences pancreatic cancer–related mortality in a dose-dependent manner, independent of presence or absence of diabetes or type of treatment. This increased risk is observed primarily in the Western population but not in Asia-Pacific regions. Premorbid obesity and its underlying metabolic alterations and associated chronic inflammation may be a potential therapeutic target in improving mortality in this highly lethal cancer. In current treatment schemes, premorbid obesity should be considered as a marker of poor prognosis, with a focus on more aggressive intervention, and may be used as a stratification variable in interventional studies.
Supplementary Material
Supplementary Figure 1. Funnel plot, showing no evidence of publication bias. SE, standard error.
Supplementary Table 1. Study-level Quality Assessment by Using the Quality In Prognosis Studies Tool
Abbreviations used in this paper
- aHR
adjusted hazard ratio
- BMI
body mass index
- CI
confidence interval
- HR
hazard ratio
Footnotes
Supplementary Material: Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at http://dx.doi.org/10.1016/j.cgh.2015.09.036.
Conflicts of interest: The authors disclose no conflicts.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Aune D, Greenwood DC, Chan DS, et al. Body mass index, abdominal fatness and pancreatic cancer risk: a systematic review and non-linear dose-response meta-analysis of prospective studies. Ann Oncol. 2012;23:843–852. doi: 10.1093/annonc/mdr398. [DOI] [PubMed] [Google Scholar]
- 3.Bao Y, Giovannucci EL, Kraft P, et al. A prospective study of plasma adiponectin and pancreatic cancer risk in five US cohorts. J Natl Cancer Inst. 2013;105:95–103. doi: 10.1093/jnci/djs474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolpin BM, Bao Y, Qian ZR, et al. Hyperglycemia, insulin resistance, impaired pancreatic beta-cell function, and risk of pancreatic cancer. J Natl Cancer Inst. 2013;105:1027–1035. doi: 10.1093/jnci/djt123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grote VA, Rohrmann S, Nieters A, et al. Diabetes mellitus, glycated haemoglobin and C-peptide levels in relation to pancreatic cancer risk: a study within the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Diabetologia. 2011;54:3037–3046. doi: 10.1007/s00125-011-2316-0. [DOI] [PubMed] [Google Scholar]
- 6.Li N, Grivennikov SI, Karin M. The unholy trinity: inflammation, cytokines, and STAT3 shape the cancer microenvironment. Cancer Cell. 2011;19:429–431. doi: 10.1016/j.ccr.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gukovsky I, Li N, Todoric J, et al. Inflammation, autophagy, and obesity: common features in the pathogenesis of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1199–1209. doi: 10.1053/j.gastro.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parkin E, O'Reilly DA, Sherlock DJ, et al. Excess adiposity and survival in patients with colorectal cancer: a systematic review. Obes Rev. 2014;15:434–451. doi: 10.1111/obr.12140. [DOI] [PubMed] [Google Scholar]
- 9.Chan DS, Vieira AR, Aune D, et al. Body mass index and survival in women with breast cancer: systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25:1901–1914. doi: 10.1093/annonc/mdu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X, Zhou G, Sun B, et al. Impact of obesity upon prostate cancer-associated mortality: a meta-analysis of 17 cohort studies. Oncol Lett. 2015;9:1307–1312. doi: 10.3892/ol.2014.2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castillo JJ, Reagan JL, Ingham RR, et al. Obesity but not overweight increases the incidence and mortality of leukemia in adults: a meta-analysis of prospective cohort studies. Leuk Res. 2012;36:868–875. doi: 10.1016/j.leukres.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 12.Li D, Morris JS, Liu J, et al. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA. 2009;301:2553–2562. doi: 10.1001/jama.2009.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin Y, Fu R, Grant E, et al. Association of body mass index and risk of death from pancreatic cancer in Asians: findings from the Asia Cohort Consortium. Eur J Cancer Prev. 2013;22:244–250. doi: 10.1097/CEJ.0b013e3283592cef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269, W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 15.Hayden JA, van der Windt DA, Cartwright JL, et al. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158:280–286. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 16.Chene G, Thompson SG. Methods for summarizing the risk associations of quantitative variables in epidemiologic studies in a consistent form. Am J Epidemiol. 1996;144:610–621. doi: 10.1093/oxfordjournals.aje.a008971. [DOI] [PubMed] [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 21.McWilliams RR, Matsumoto ME, Burch PA, et al. Obesity adversely affects survival in pancreatic cancer patients. Cancer. 2010;116:5054–5062. doi: 10.1002/cncr.25465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reeves GK, Pirie K, Beral V, et al. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335:1134. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan C, Bao Y, Wu C, et al. Prediagnostic body mass index and pancreatic cancer survival. J Clin Oncol. 2013;31:4229–4234. doi: 10.1200/JCO.2013.51.7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olson SH, Chou JF, Ludwig E, et al. Allergies, obesity, other risk factors and survival from pancreatic cancer. Int J Cancer. 2010;127:2412–2419. doi: 10.1002/ijc.25240. [DOI] [PubMed] [Google Scholar]
- 25.Gong Z, Holly EA, Bracci PM. Obesity and survival in population-based patients with pancreatic cancer in the San Francisco Bay Area. Cancer Causes Control. 2012;23:1929–1937. doi: 10.1007/s10552-012-0070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ansary-Moghaddam A, Huxley R, Barzi F, et al. The effect of modifiable risk factors on pancreatic cancer mortality in populations of the Asia-Pacific region Cancer. Epidemiol Bio-markers Prev. 2006;15:2435–2440. doi: 10.1158/1055-9965.EPI-06-0368. [DOI] [PubMed] [Google Scholar]
- 27.Bethea TN, Kitahara CM, Sonderman J, et al. A pooled analysis of body mass index and pancreatic cancer mortality in african americans. Cancer Epidemiol Biomarkers Prev. 2014;23:2119–2125. doi: 10.1158/1055-9965.EPI-14-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 29.Batty GD, Kivimaki M, Morrison D, et al. Risk factors for pancreatic cancer mortality: extended follow-up of the original Whitehall Study. Cancer Epidemiol Biomarkers Prev. 2009;18:673–675. doi: 10.1158/1055-9965.EPI-08-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gapstur SM, Gann PH, Lowe W, et al. Abnormal glucose metabolism and pancreatic cancer mortality. JAMA. 2000;283:2552–2558. doi: 10.1001/jama.283.19.2552. [DOI] [PubMed] [Google Scholar]
- 31.Lee IM, Sesso HD, Oguma Y, et al. Physical activity, body weight, and pancreatic cancer mortality. Br J Cancer. 2003;88:679–683. doi: 10.1038/sj.bjc.6600782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arnold LD, Patel AV, Yan Y, et al. Are racial disparities in pancreatic cancer explained by smoking and overweight/obesity? Cancer Epidemiol Biomarkers Prev. 2009;18:2397–2405. doi: 10.1158/1055-9965.EPI-09-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura K, Nagata C, Wada K, et al. Cigarette smoking and other lifestyle factors in relation to the risk of pancreatic cancer death: a prospective cohort study in Japan. Jpn J Clin Oncol. 2011;41:225–231. doi: 10.1093/jjco/hyq185. [DOI] [PubMed] [Google Scholar]
- 34.Stevens RJ, Roddam AW, Spencer EA, et al. Factors associated with incident and fatal pancreatic cancer in a cohort of middle-aged women. Int J Cancer. 2009;124:2400–2405. doi: 10.1002/ijc.24196. [DOI] [PubMed] [Google Scholar]
- 35.Coughlin SS, Calle EE, Patel AV, et al. Predictors of pancreatic cancer mortality among a large cohort of United States adults. Cancer Causes Control. 2000;11:915–923. doi: 10.1023/a:1026580131793. [DOI] [PubMed] [Google Scholar]
- 36.Lin Y, Ueda J, Yagyu K, et al. A prospective cohort study of shift work and the risk of death from pancreatic cancer in Japanese men. Cancer Causes Control. 2013;24:1357–1361. doi: 10.1007/s10552-013-0214-0. [DOI] [PubMed] [Google Scholar]
- 37.Balentine CJ, Enriquez J, Fisher W, et al. Intra-abdominal fat predicts survival in pancreatic cancer. J Gastrointest Surg. 2010;14:1832–1837. doi: 10.1007/s11605-010-1297-5. [DOI] [PubMed] [Google Scholar]
- 38.Wang D, Zheng W, Wang SM, et al. Estimation of cancer incidence and mortality attributable to overweight, obesity, and physical inactivity in China. Nutr Cancer. 2012;64:48–56. doi: 10.1080/01635581.2012.630166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev. 2009;18:2569–2578. doi: 10.1158/1055-9965.EPI-09-0372. [DOI] [PubMed] [Google Scholar]
- 40.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bullo M, Garcia-Lorda P, Megias I, et al. Systemic inflammation, adipose tissue tumor necrosis factor, and leptin expression. Obes Res. 2003;11:525–531. doi: 10.1038/oby.2003.74. [DOI] [PubMed] [Google Scholar]
- 43.Griggs JJ, Mangu PB, Anderson H, et al. Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2012;30:1553–1561. doi: 10.1200/JCO.2011.39.9436. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Funnel plot, showing no evidence of publication bias. SE, standard error.
Supplementary Table 1. Study-level Quality Assessment by Using the Quality In Prognosis Studies Tool


