Abstract
Background & Aims
A proportion of patients with Barrett's esophagus (BE) are diagnosed with esophageal adenocarcinoma (EAC) within 1 year of an endoscopic examination that produced negative findings. These cases of missed cancers have not been well studied, despite current surveillance strategies for BE. We performed a systematic review and meta-analysis to determine the magnitude of missed EAC in cohorts of patients with BE.
Methods
We searched MEDLINE, EMBASE, and Web of Science from their inception to May 31, 2015 to identify cohort studies of adults with BE (baseline nondysplastic BE ± BE with low-grade dysplasia) and at least a 3-year follow-up period, providing data on missed and incident EACs (diagnosed within 1 year and diagnosed more than 1 year after the initial endoscopy in which BE was diagnosed, respectively). The main outcome measure was pooled proportion of missed and incident EACs (of all EACs detected after initial endoscopy) among BE cohorts, using a random effects model.
Results
In a metaanalysis of 24 studies reporting on 820 missed and incident EACs, 25.3% were classified as missed (95% confidence interval: 16.4%–36.8%) and 74.7% as incident EACs (95% CI: 63.2%–83.6%), although there was substantial heterogeneity among studies (I2 = 74%). When the analysis was restricted to nondysplastic BE cohorts (15 studies), 23.9% of EACs were classified as missed (95% confidence interval: 15.3%–35.4%; I2 = 0%). In a meta-analysis of 10 studies with follow-up periods of ≥5 years (a total of 239 EACs), 22.0% were classified as missed (95% confidence interval: 8.7%–45.5%), with substantial heterogeneity (I2 = 68%).
Conclusions
Among adults with nondysplastic BE (or BE with low-grade dysplasia) at their index endoscopy and at least a 3-year follow-up period, 25% of EACs are diagnosed within 1 year after the index endoscopy. Additional resources should be allocated to detect missed EAC.
Keywords: Esophageal Cancer, Endoscopy, Prevalence, Surveillance
During the last 3 decades, the incidence of esophageal adenocarcinoma (EAC) has been rising. Barrett's esophagus (BE) is the primary precursor lesion for EAC, and therefore, efforts to reduce EAC are directed toward identifying patients at high risk for BE, performing endoscopic surveillance of patients with nondysplastic BE (NDBE) every 3–5 years, and endoscopic treatment of patients with BE with high-grade dysplasia (HGD). However, the impact of these strategies on EAC incidence is uncertain. Estimates of progression to EAC in NDBE appear to be lower than previously estimated.1–3 Recent data on the impact of surveillance on EAC outcomes are also conflicting, with some studies finding that outcomes of EAC diagnosed on surveillance, when performed adequately, are better than those when EAC is diagnosed outside of surveillance programs,4,5 while others do not report such benefit.6 This has led investigators to question the appropriation of substantial health care resources for endoscopic surveillance in patients with NDBE, which might not be cost effective.7
Conversely, most patients with EAC are diagnosed at the initial endoscopy; in other words, the prevalence of EAC is far greater than the incidence.8,9 Furthermore, as most cancer screening tools have a miss rate, one can speculate that there might be cancers that are missed at the index endoscopy during which an initial BE diagnosis is made. Indeed, in recent large population-based studies with long-term follow-up, 58%–66% of EACs detected were diagnosed within 1 year of index endoscopy. 2,3 Using data from the Olmsted County population-based cohort of BE patients, we observed that 2% of patients might have missed HGD or EAC and 10% have missed dysplasia when endoscopy is repeated in 1–2 years.9 As a result, one has to question whether the missed EAC group is an important BE population to target for detection of dysplasia via methods, such as a 1-year follow-up endoscopy, which currently is not recommended by any of the 3 major gastroenterologic societies.10–12
To better understand the magnitude of missed EAC in patients with BE, we performed a systematic review with meta-analysis of all cohort studies in adults with BE (baseline NDBE ± BE with low-grade dysplasia [LGD]) with at least 3-year follow-up, to accurately estimate the proportion of EAC missed at index endoscopy (ie, diagnosed within 1 year of index endoscopy, during which BE diagnosis was made) as compared with those patients with EAC found during surveillance (ie, incident EAC, diagnosed >1 year after BE diagnosis).
Methods
Definitions
EAC reported by studies were divided into 2 categories: missed and incident. EAC diagnosed within 1 year of negative index endoscopy (in which BE was diagnosed) was defined as “missed” EAC because, given the usual time course of progression from NDBE to EAC, it is highly likely that these had been missed at index endoscopy, rather than being a true incident cancer.13 “Incident” EAC patients were those diagnosed more than 1 year after negative index endoscopy. In studies that did not report a self-defined time frame for missed EAC, but provided sufficient information regarding the timing of EAC detection, a cutoff of 12 months was retrospectively applied to determine the numbers of missed and incident EAC. Likewise, we defined missed and incident HGD-EAC as diagnosis of HGD or EAC within 1 year or after 1 year of index negative endoscopy, respectively. Only studies that sufficiently reported timings of detection for both HGD and EAC were eligible for the HGD-EAC analysis. In cases where EAC arose in a background of HGD or progressed from HGD, one case of EAC was recorded based on the timing of its detection.
Data regarding the numbers of prevalent EACs detected during index endoscopy were also ascertained. However, these were ultimately excluded due to the paucity of information provided by included studies, and inability to determine if these EAC were truly detected during screening in asymptomatic patients vs investigative workup in patients with tumor-related symptoms (ie, dysphagia/odynophagia or weight loss).
Data Sources and Searches
We conducted a systematic search of the electronic databases MEDLINE (1966 through May 31, 2015), EMBASE (1988 through May 31, 2015), and Web of Science (1993 through May 31, 2015) with the help of an experienced medical librarian after receiving input from study investigators (details in the Supplementary Material). Two investigators (KV and RK) independently reviewed the titles and abstracts of the studies identified by the primary search and excluded studies that did not address the research question of interest based on pre-specified inclusion and exclusion criteria. The full text of the remaining articles was reviewed for additional relevant information. The bibliographies of selected articles as well as pertinent systematic or narrative reviews on the topic were reviewed for additional articles. Any discrepancy regarding article selection was resolved by discussion with the senior investigators (DAK and PGI).
Study Selection
Articles were initially screened for nonsurgical or nonprocedural BE cohorts that provided absolute numbers or incident rates of EAC or HGD-EAC detection. Those meeting screening criteria were then reviewed in full text and included in this meta-analysis if they met the following specific criteria: the cohort of patients had endoscopic- and/or biopsy-proven BE; included subjects with NDBE ± subjects with BE-LGD; had a reported mean/median follow-up of at least 3 years from the time of BE diagnosis; specified the number of patients with missed EAC or HGD-EAC (or provided data on the timing of detection for all EAC or HGD-EAC, after negative index endoscopy, to identify missed and incident lesions); and specified the number of patients found to have incident EAC or HGD-EAC.
Studies were excluded if mean/median follow-up was <3 years; there were insufficient data to determine the numbers of missed and incident EAC or HGD-EAC; or BE cohorts included patients with baseline BE-HGD and outcomes for subjects with baseline NDBE or BE-LGD could not be determined separately. Surgical studies and procedural series (eg, radiofrequency ablation or photodynamic therapy) were also excluded. In cases where multiple articles were published from the same institution or using the same cohort, the most recent publication with sufficient information was included.
Data Extraction and Quality Assessment
Data regarding study- and patient-related characteristics were abstracted independently by 2 investigators (KV and RK) using a standardized abstraction form (details in the Supplementary Material).
A formal quality assessment was performed to ascertain the risk of bias. A scale modified from the Newcastle-Ottawa scale for cohort studies was used,14 and consisted of 9 questions, each scored up to 1 point (Supplementary Table 1). A total score of >6, 5–6, and <5 was suggestive of a high-, medium-, and low-quality study, respectively.
Data Synthesis and Analysis
The primary outcome of interest was the proportion of missed and incident EAC among all EAC detected after negative index endoscopy in cohorts of BE patients with NDBE ± BE-LGD with at least 3-year follow-up. Secondary outcomes included proportion of missed EAC in patients with baseline NDBE, proportion of missed EAC in BE cohorts with at least 5-year follow-up, and proportion of missed HGD-EAC in BE cohorts with at least 3-year and 5-year follow-up.
To assess stability of findings and identify sources of heterogeneity, subgroup analyses based on study quality, design (population-based vs other), setting (multicenter vs single center), region (Europe vs United States), publication date, and follow-up duration after negative index endoscopy were also performed to estimate the proportion of missed vs incident EAC among all EAC detected after negative index endoscopy. All analyses were repeated for the combined end point of HGD-EAC.
Statistical Analysis
We adhered to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines using a predefined protocol.15 Proportions were calculated for missed and incident as follows:
To account for differences in study size, proportions were pooled and weighted, using random effects meta-analysis. We assessed heterogeneity using the I2 statistic. Values of <30%, 30%–60%, 61%–75%, and >75% were classified as low, moderate, substantial, and considerable heterogeneity, respectively.16 Between-study sources of heterogeneity were assessed using predefined subgroup analyses, and a P value for differences between subgroups of <.10 was considered statistically significant. Publication bias was assessed quantitatively using Egger's regression test (publication bias considered present if P ≤ .10),17 and qualitatively, by visual inspection of funnel plots.18 All analyses were performed by using Comprehensive Meta-Analysis software (version 2, Biostat, Englewood, NJ).
Results
Search Results
A total of 2482 studies were identified using our search strategy, of which 27 studies fulfilled our inclusion criteria and were included in the meta-analysis (Figure 1).2,3,9,19–42 Twenty-four of these studies cumulatively reported 320 missed EAC and 500 incident EAC, and 20 studies reported the combined end points of HGD-EAC with 638 missed HGD-EAC and 863 incident HGD-EAC (Table 1). Twelve studies were excluded from meta-analysis due to overlapping cohorts.43–54 Four studies were excluded because numbers of missed EAC could not be ascertained,55 baseline histology of the cohort could not be ascertained,56 or the study cohort consisted of only patients requiring esophageal surgery,57 or having achalasia.58
Figure 1.
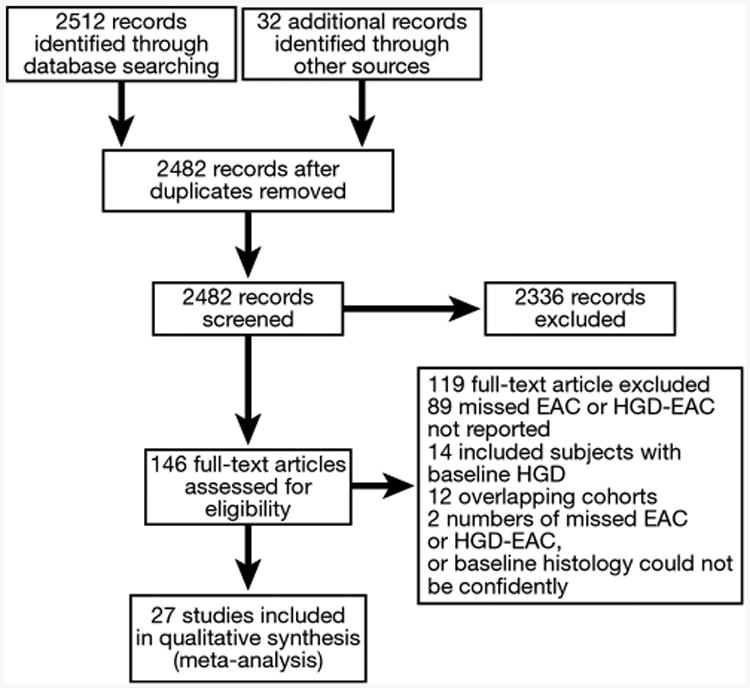
Flow diagram summarizing study identification and selection.
Table 1. Numbers of Missed and Incident EAC and HGD-EAC, Categorized by Duration of Follow-Up and Baseline Histology of Cohort.
| EAC | HGD-EAC | |||
|---|---|---|---|---|
|
|
|
|||
| Study, first author, year | Missed | Incident | Missed | Incident |
| Follow-up ≥5 y | ||||
| NDBE and BE-LGD | ||||
| Hameeteman, 198940,a | 0 | 4 | NR | NR |
| Spechler, 2001,36 199268 | 0 | 4 | NR | NR |
| Conio, 200329 | 0 | 5 | 0 | 5 |
| Parrilla, 200328 | 0 | 2 | NR | NR |
| Martinek, 200823 | 0 | 2 | 0 | 2 |
| Bhat, 20112 | NR | 62 | 189 | 98 |
| den Hoed, 201119 | NR | NR | 2 | 13 |
| Hvid-Jensen, 20113 | 131 | 66 | 203 | 148 |
| NDBE only | ||||
| Teodori, 199841 | 0 | 4 | 0 | 5 |
| Eckardt, 200130 | 0 | 2 | 0 | 2 |
| Gladman, 200625 | 0 | 4 | 1 | 5 |
| Vieth, 200624 | 5 | 10 | 5 | 10 |
| Follow-up ≥3 y | ||||
| NDBE and BE-LGD | ||||
| Spechler, 198435 | 0 | 2 | NR | NR |
| Miros, 199134,a | 0 | 1 | 0 | 1 |
| Williamson, 199138,a | 1 | 4 | 1 | 4 |
| Katz, 199833 | 0 | 3 | 0 | 6 |
| Reid, 200037,a | 0 | 9 | NR | NR |
| Dulai, 200527 | 2 | 4 | 9 | 15 |
| Murphy, 200526 | 0 | 3 | NR | NR |
| Rossi, 200922,a | 2 | 4 | 2 | 7 |
| de Jonge, 201021 | 168 | 337 | 212 | 454 |
| Vogt, 201020 | 0 | 0 | 2 | 1 |
| Kastelein, 201139 | NR | 12 | 3 | 38 |
| Rugge, 201242 | 3 | 7 | 6 | 22 |
| Visrodia, 20159 | 1 | 7 | 3 | 23 |
| NDBE only | ||||
| Bani-Hani, 200032 | 7 | 12 | NR | NR |
| Macdonald, 200031 | 0 | 4b | 0 | 4b |
NR, not reported.
Outcomes for patients with baseline BE with HGD were excluded from all tables and analyses.
One squamous cell carcinoma excluded.
Characteristics and Quality of Included Studies
The characteristics and the quality of the included studies are shown in Supplementary Tables 2 and 3, respectively. Of 27 studies, the majority were performed in Europe (n = 20) and the United States (n = 6). Mean/median follow-up was ≥5 years in 12 studies, including ≥8 years in 4 studies. Four studies were population-based (median follow-up, 5.0 years),2,3,9,21 4 were multicenter studies (median follow-up, 4.7 years),27,29,39,42 and the remaining were single-center studies (median follow-up, 4.8 years).19,20,22–26,28,30–38,40,41 Most studies reported or permitted a missed vs incident cutoff of exactly 12 months to be applied, with the exception of 2 studies reporting cutoffs of 9 and 6 months,32,39 and 1 study for which only a cutoff of 14 months could be applied.24 In these 3 studies, EAC (and HGD-EAC) detected before and after their respective cutoffs were classified as missed and incident, respectively. Ten studies followed cohorts composed of >200 BE subjects. Six study cohorts included subjects with baseline NDBE only; 14 included subjects with baseline NDBE and BE-LGD only (4 of which provided sufficient information separately for patients with NDBE); 5 studies including patients with baseline NDBE, BE-LGD, and BE-HGD were also included because they provided sufficient information separately for patients with NDBE and BE-LGD, and NDBE alone (Supplementary Table 4), thereby allowing patients with baseline BE-HGD to be excluded. Nineteen studies reported a surveillance protocol, of which 15 were in accordance with current guidelines. Twenty studies described a biopsy protocol, including 7 reporting 4-quadrant biopsies every 1–2 cm. Attrition rate was <20% in 21 studies, and not reported in the remainder. Overall, 3 of the 27 studies were considered high quality, 19 were considered medium quality, and 5 were considered low quality. Of the 3 high-quality studies, 2 were multicenter studies, 1 was a single-center study, and none were population-based studies.
Missed Esophageal Adenocarcinoma and High-Grade Dysplasia–Esophageal Adenocarcinoma Among All Barrett's Esophagus Cohorts
On meta-analysis of 24 studies reporting numbers of missed and incident EAC, the pooled proportion of missed EAC was 25.3% (95% confidence interval [CI]: 16.4%–36.8%) vs incident EAC of 74.7% (95% CI: 63.2%-83.6%), that is, 25.3% of all EAC diagnosed in BE cohorts with at least 3-year follow-up were detected within 1 year of negative index endoscopy (Table 2). Substantial heterogeneity was noted (I2 = 74%). After analysis was restricted to 10 studies with at least 5-year follow-up, the pooled proportion of missed EAC was 22.0% (95% CI: 8.7%–45.5%) vs incident EAC of 78.0% (95% CI: 54.5%–91.3%), with substantial heterogeneity (I2 = 68%).
Table 2. Proportion of Missed and Incident EAC and HGD-EAC Among Included Studies, Categorized by Duration of Follow-Up and Baseline Histology of Cohort.
| Missed | Incident | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| No. of studies | Total cases | n | Pooled weighted proportion, % | 95% CI | n | Pooled weighted proportion,% | 95% CI | Heterogeneity I2,% | |
| Follow-up ≥3 y | |||||||||
| NDBE ± BE-LGD | |||||||||
| EAC | 24 | 820 | 320 | 25.3 | 16.4–36.8 | 500 | 74.7 | 63.2–83.6 | 74 |
| HGD-EAC | 20 | 1501 | 638 | 26.6 | 17.9–37.6 | 863 | 73.4 | 62.4–82.1 | 89 |
| NDBE only | |||||||||
| EAC | 15 | 74 | 12 | 23.9 | 15.3–35.4 | 62 | 76.1 | 64.6–84.7 | 0 |
| HGD/EAC | 11 | 55 | 7 | 19.0 | 10.5–31.8 | 48 | 81.0 | 68.2–89.5 | 0 |
| Follow-up ≥5 y | |||||||||
| NDBE ± BE-LGD | |||||||||
| EAC | 10 | 239 | 136 | 22.0 | 8.7–45.5 | 103 | 78.0 | 54.5–91.3 | 68 |
| HGD-EAC | 9 | 688 | 400 | 41.1 | 28.4–55.2 | 288 | 58.9 | 44.8–71.6 | 75 |
| NDBE only | |||||||||
| EAC | 8 | 33 | 5 | 23.0 | 11.8–40.1 | 28 | 77.0 | 59.9–88.2 | 0 |
| HGD-EAC | 6 | 33 | 6 | 23.5 | 12.1–40.8 | 27 | 76.5 | 59.2–87.9 | 0 |
On meta-analysis of 20 studies reporting numbers of missed and incident HGD-EAC, the pooled proportion of missed HGD-EAC was 26.6% (95% CI: 17.9%–37.6%) vs incident HGD-EAC of 73.4% (95% CI: 62.4%–82.1%). Considerable heterogeneity was observed (I2 = 89%). When analysis was restricted to 9 studies with at least 5-year follow-up, the pooled proportion of missed HGD-EAC was 41.1% (95% CI: 28.4%–55.2%) vs incident EAC of 58.9% (95% CI: 44.8%–71.6%), with substantial heterogeneity (I2 = 75%).
Missed Esophageal Adenocarcinoma and High-Grade Dysplasia–Esophageal Adenocarcinoma Among Nondysplastic Barrett's Esophagus Cohorts
On restricting analysis to 15 studies reporting outcomes for patients with baseline NDBE alone, the pooled proportion of missed EAC was 23.9% (95% CI: 15.3%–35.4%), and of missed HGD-EAC was 19.0% (95% CI: 10.5%–31.8%), that is, 23.9% of all EAC (and 19.0% of all HGD-EAC) diagnosed in patients with baseline NDBE with at least 3-year follow-up, were detected within 1 year of initial negative endoscopy (Table 2). Minimal heterogeneity (I2 = 0%) was observed in both analyses. On restriction to cohorts with at least 5-year follow-up, the pooled proportion of missed EAC (n = 8 studies) and missed HGD-EAC (n = 6 studies) remained similar at 23.0% (95% CI: 11.8%–40.1%) and 23.5% (95% CI: 12.1%–40.8%), respectively, with minimal heterogeneity (I2 = 0%).
Subgroup Analysis and Publication Bias
The overall rates of missed EAC and of missed HGD-EAC were stable on multiple a priori subgroup analyses, based on quality of included studies (high vs medium vs low), study design (population-based vs other), study setting (multicenter vs single-center), study region (Europe vs United States), year of publication (before 2000, 2000–2009, after 2010), and duration of follow-up (3–5 years, 5–8 years, and >8 years), except for a higher proportion of missed HGD-EAC in medium-quality studies as compared with high-quality studies, and in population-based studies as compared with single-center or multi-center studies (Table 3).
Table 3. Sensitivity Analysis to Examine Sources of Heterogeneity.
| Missed | Incident | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Subgroup analysis | No. of studies | Total cases | n | Pooled weighted proportion | 95% CI | n | Pooled weighted proportion | 95% CI | Heterogeneity I2,% | Heterogeneity between groups, P valuea |
| Study quality | ||||||||||
| EAC | .63 | |||||||||
| High | 2 | 7 | 0 | 11.7 | 1.6–51.8 | 7 | 88.3 | 48.2–98.4 | 0 | |
| Medium | 17 | 777 | 313 | 27.7 | 16.8–42.2 | 464 | 72.3 | 57.8–83.2 | 81 | |
| Low | 5 | 36 | 7 | 27.1 | 14.5–45.0 | 29 | 72.9 | 55.0–85.5 | 0 | |
| HGD-EAC | <.01 | |||||||||
| High | 3 | 48 | 3 | 8.2 | 3.1–19.9 | 45 | 91.8 | 80.1–96.9 | 0 | |
| Medium | 17 | 1453 | 635 | 30.2 | 20.4–42.3 | 818 | 69.8 | 57.7–79.6 | 90 | |
| Low | 0 | — | — | — | — | — | — | — | — | |
| Study design | ||||||||||
| EAC | .26 | |||||||||
| Population-based | 3 | 710 | 300 | 40.8 | 16.9–70.1 | 410 | 59.2 | 29.9–83.1 | 97 | |
| Other | 21 | 110 | 20 | 24.7 | 17.3–33.9 | 90 | 75.3 | 66.1–82.7 | 0 | |
| HGD-EAC | .04 | |||||||||
| Population-based | 4 | 1330 | 607 | 42.5 | 24.3–62.9 | 723 | 57.5 | 37.1–75.7 | 4 | |
| Other | 16 | 171 | 31 | 21.7 | 15.5–29.5 | 140 | 78.3 | 70.5–84.5 | 98 | |
| Study setting | ||||||||||
| EAC | .34 | |||||||||
| Multicenter | 6 | 731 | 305 | 34.6 | 17.4–57.2 | 426 | 65.4 | 42.8–82.6 | 92 | |
| Single-center | 18 | 89 | 15 | 24.0 | 16.0–34.3 | 74 | 76.0 | 65.7–84.0 | 0 | |
| HGD-EAC | .28 | |||||||||
| Multicenter | 8 | 1428 | 625 | 31.3 | 19.1–46.9 | 803 | 68.7 | 53.1–80.9 | 95 | |
| Single-center | 12 | 73 | 13 | 22.0 | 13.5–33.6 | 60 | 78.0 | 66.4–86.5 | 0 | |
| Study regionb | ||||||||||
| EAC | .21 | |||||||||
| Europe | 16 | 782 | 316 | 28.9 | 17.7–43.3 | 466 | 71.1 | 56.7–82.3 | 81 | |
| United States | 7 | 37 | 4 | 16.8 | 7.6–33.2 | 33 | 83.2 | 66.8–92.4 | 0 | |
| HGD-EAC | .47 | |||||||||
| Europe | 15 | 1439 | 625 | 28.5 | 18.4–41.4 | 814 | 71.5 | 58.6–81.6 | 91 | |
| United States | 4 | 61 | 13 | 20.7 | 8.8–41.5 | 48 | 79.3 | 58.5–91.2 | 44 | |
| Publication date | ||||||||||
| EAC | .27 | |||||||||
| Before 2000 | 6 | 19 | 1 | 14.1 | 4.6–35.9 | 18 | 85.9 | 64.1–95.4 | 0 | |
| 2000–2009 | 13 | 81 | 16 | 26.2 | 17.5–37.4 | 65 | 73.8 | 62.6–82.5 | 0 | |
| After 2010 | 5 | 720 | 303 | 38.6 | 17.9–64.4 | 417 | 61.4 | 35.6–82.1 | 95 | |
| HGD-EAC | .36 | |||||||||
| Before 2000 | 4 | 17 | 1 | 12.2 | 3.0–38.2 | 16 | 87.8 | 61.8–97.0 | 0 | |
| 2000–2009 | 8 | 67 | 17 | 28.7 | 19.0–40.9 | 50 | 71.3 | 59.1–81.0 | 0 | |
| After 2010 | 8 | 1417 | 620 | 31.4 | 18.8–47.5 | 797 | 68.6 | 52.6–81.2 | 95 | |
| Duration of follow-up, y | ||||||||||
| EAC | .32 | |||||||||
| 3–5 | 14 | 581 | 184 | 32.5 | 28.8–36.4 | 397 | 67.5 | 63.6–71.2 | 0 | |
| 5–8 | 7 | 229 | 136 | 26.8 | 9.9–55.0 | 93 | 73.2 | 45.0–90.1 | 70 | |
| ≥8 | 3 | 10 | 0 | 11.8 | 2.4–42.5 | 10 | 88.2 | 57.5–97.6 | 0 | |
| HGD-EAC | <.01 | |||||||||
| 3–5 | 11 | 813 | 238 | 22.5 | 14.8–32.7 | 575 | 77.5 | 67.2–85.2 | 52 | |
| 5–8 | 6 | 666 | 398 | 52.3 | 40.0–64.2 | 268 | 47.7 | 35.8–60.0 | 71 | |
| ≥8 | 3 | 22 | 2 | 12.8 | 4.2–33.0 | 20 | 87.2 | 67.0–95.8 | 0 | |
P ≤ .10, explains source of heterogeneity between groups and appear in bold.
One Australian study was excluded.34
On time-trend analysis (based on year of publication of study), the proportions of missed EAC and HGD-EAC were comparable across decades. When analysis was stratified based on duration of cohort follow-up after negative index endoscopy, the proportion of missed EAC predictably decreased in comparison with the proportion of detected incident EAC (which increased with longer duration of follow-up), although this difference was not statistically significant. The subgroup and sensitivity analyses were repeated for studies with mean/median follow-up ≥5 years, with similar results (Supplementary Table 5).
On analysis of funnel plot for the primary outcomes of proportion of missed EAC, there was evidence of asymmetry (Supplementary Figure 1), and Egger's regression test demonstrated publication bias (P = .06). Using the trim-and-fill method, which conservatively imputes hypothetical negative unpublished studies to mirror the positive studies that cause funnel plot asymmetry,59 we estimated the pooled proportion of missed EAC (after imputing missed EAC rate for 9 hypothetical studies) to be 33.2% (95% CI: 23.6%–44.4%).
Discussion
Surveillance in patients with BE is time- and resource-intensive, and has not yielded a consistent population-level reduction in the incidence of EAC. Several factors may be contributing to this, and we specifically evaluated the problem of missed EAC in patients with BE. Through a systematic review and meta-analysis of 24 cohort studies in adults with BE (baseline NDBE ± BE-LGD) followed for at least 3 years after negative index endoscopy (in which BE was diagnosed), we made several key observations. First, about 25% of EACs diagnosed in these patients are classified as missed, that is, diagnosed within 1 year of negative endoscopy. On restricting to studies with at least 5-year follow-up, 22% of EACs were still classified as missed. Second, when considering only patients with baseline NDBE, for whom current guidelines recommend repeating endoscopy in 3–5 years,10–12 23.9% and 23% of EACs were classified as missed in studies with at least 3- or 5-year follow-up, respectively. These findings were stable on multiple a priori defined subgroup and sensitivity analyses, based on study quality, design, region, and setting. These findings have important implications for the surveillance in patients with BE. The index endoscopy and early repeat endoscopy, within 1 year of BE diagnosis, may be vital exams for patients with BE, given the magnitude of missed EAC. The potential increased yield of dysplasia and EAC as well as the cost-effectiveness of this approach of initial intensive surveillance (at diagnosis and 1 year after initial diagnosis) should be compared with the current recommendations of repeating endoscopy every 3–5 years in those with NDBE.
Methods to increase detection of dysplasia and EAC, similar to those utilized in colorectal cancer screening,60–63 such as use of advanced imaging techniques,64–66 greater time examining BE segments,67 and greater number of targeted biopsies, may potentially identify occult neoplastic lesions and decrease the burden of missed EAC. Although such an approach might seemingly increase the already expensive costs of surveillance in BE, the high yield of an early exam detecting HGD or early EAC (which could be treated nonsurgically) could offset such a cost, and continued efforts to identify patients with BE at highest risk of progression to EAC using clinical characteristics and biomarkers to personalize surveillance exams would decrease the long-term costs of repeated exams performed over decades.
The strengths of this study include a robust search of multiple databases with the aid of an experienced medical librarian; strict inclusion and exclusion criteria using clinically useful time cutoffs (at least 3-year and 5-year follow-up intervals); exclusion of patients with prevalent EAC (ie, EAC diagnosed at index endoscopy), given the difficulty in differentiating symptomatic from screen-detected cancers (based on published literature); careful avoidance of redundant studies; application of strict definitions; multiple a priori subgroup/sensitivity analyses; and a detailed quality assessment in an effort to identify risk of bias in the literature.
Our study has several potential limitations. First, most studies did not follow patients until death or development of EAC. As a result, we cannot be sure of the true incidence of EAC in comparison with missed EAC. However, by using studies with up to a 15-year, and an overall mean 5.6-year, follow-up, we still found that the proportion of missed HGD and EAC was substantial to that found on surveillance. Second, we were unable to accurately identify the magnitude of prevalent EAC in patients with BE, that is, what proportion of (asymptomatic) patients with BE are identified during their index (screening) endoscopy. This was because the published literature on prevalent EAC did not distinguish those identified based on clinical suspicion (symptomatic patients) from those that were screen-detected. Moreover, classification as missed EAC operates under the assumption that patients had no baseline symptoms (ie, dysphagia and weight loss) or endoscopic evidence (ie, grossly visible lesion) of EAC despite initially negative biopsies, a scenario in which good medicine would dictate urgent repeat endoscopy as opposed to enrollment in a BE surveillance protocol. Using data from the Olmsted County BE database, we observed that missed EACs indeed occur in patients with no grossly visible lesion during index endoscopy.9 Future strategies to lower the missed rate can focus on improvement in both endoscopic and histologic detection. Third, there was substantial unexplained heterogeneity observed in our analysis, despite multiple preplanned subgroup and sensitivity analyses. Such statistical heterogeneity is not uncommon in studies of prevalence/proportion; conceptually, these studies were similar based on our strict inclusion and exclusion criteria. As a result, if incorrect, this assumption could overestimate the missed rate. Fourth, we assumed that HGD and EAC detected within 1 year of negative index endoscopy were missed lesions (ie, present at time of index exam, but just not identified) as opposed to true rapid neoplastic progression. Unfortunately, data on EAC stage were not reported, which would have provided perspective on prognosis of EACs found within 1 year, especially compared with those detected during long-term surveillance. Furthermore, there were limited data on the roles of inter-observer variability (both endoscopically and histologically) or the inclusion of “presence of goblet cells,” as a definition of BE, thereby preventing their analysis, as potential confounders. We also recognize that in 9 studies, requiring a follow-up endoscopy was not specified in the inclusion criteria, potentially resulting in an underestimation of the miss rate for HGD/EAC. Finally, there was considerable evidence of publication bias; on statistical imputation of hypothetical unpublished studies, the rate of missed EAC was 33.2%, higher than the observed 25.3%. However, in the presence of substantial heterogeneity, the implications of funnel plot asymmetry should be interpreted with caution.
In conclusion, based on a systematic review and meta-analysis of 24 cohort studies in adults with BE (baseline NDBE or BE-LGD) followed for at least 3 years after negative index endoscopy (in which BE was diagnosed), we observed a high magnitude of missed EAC, that is, EAC diagnosed within 1 year of negative index endoscopy. These data persist over a wide variety of study types and chronological time periods of BE cohort studies. Additional studies need to be performed to determine if enhanced endoscopic detection using advanced imaging techniques, longer inspection time, an increased number of biopsies, and more assiduous application of the early repeat endoscopy (ie, within 1 year of diagnosis) would enable BE surveillance to reach its real potential in decreasing the burden of EAC; cost-effectiveness analyses of such an approach are warranted to understand the implications of these findings.
Supplementary Material
Supplementary Figure 1. Funnel plot for the primary outcome of proportion missed EAC.
Supplementary Table 1. Quality Scoring Scheme
Supplementary Table 2. Characteristics of Included Studies, Categorized by Duration of Follow-Up and Baseline Histology of Cohort
Supplementary Table 3. Quality of the Studies, Categorized by Baseline Histology of Cohort
Supplementary Table 4. Additional Extraction of Outcomes for Subjects With NDBE Only From Studies With Cohorts Composed of Subjects With Baseline NDBE, BE-LGD, and/or BE-HGD
Supplementary Table 5. Sensitivity Analysis to Examine Sources of Heterogeneity for Studies With at Least 5 Years Follow-Up
Search Strategy
The search strategy included a combination of the following keywords: “Barrett esophagus/oesopohagus,” “Barrett metaplasia,” “Barrett mucosa,” “Barrett epithelium,” “columnar lined epithelium,” “specialized intestinal metaplasia,” “adenocarcinoma,” “dysplasia,” “malignant progression.” The search was restricted to studies in humans published in English in peer-reviewed journals.
Data Abstraction
The following study characteristics were abstracted from each study: author, year of publication, country of the population studied, study institution(s), cohort size, surveillance and biopsy protocols, mean/median and minimum follow-up period, and attrition rate (percent lost to follow-up). Patient-related characteristics included baseline histology of cohort subjects (ie, numbers of patients with baseline NDBE, BE-LGD, or BE-HGD), in addition to sex, age, BE segment length, and percentage of cohort with long-segment BE. The numbers of missed and incident EAC or HGD-EAC were recorded. Esophageal squamous cell carcinomas, gastric carcinomas, and histologically unspecified carcinomas were excluded where possible. Discrepancies were resolved by consensus and review with a lead investigator.
Acknowledgments
The authors thank Patricia Erwin, Medical Librarian at Mayo Clinic Library, for her expertise and help in conducting the literature search for this systematic review and meta-analysis.
Abbreviations used in this paper
- BE
Barrett's esophagus
- EAC
esophageal adenocarcinoma
- HGD
high-grade dysplasia
- LGD
low-grade dysplasia
- NDBE
nondysplastic Barrett's esophagus
Footnotes
Supplementary Material: Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at http://dx.doi.org/10.1053/j.gastro.2015.11.040.
Author names in bold designate shared co-first authorship.
Conflicts of interest: These authors disclose the following: David A. Ahlquist: Exact Sciences. Kenneth K. Wang: Medical Advisory Board and Mauna Kea Technologies. Prasad G. Iyer: Intromedic and Exact Sciences. The remaining authors disclose no conflicts.
References
- 1.Desai TK, Krishnan K, Samala N, et al. The incidence of oesophageal adenocarcinoma in non-dysplastic Barrett's oesophagus: a meta-analysis. Gut. 2012;61:970–976. doi: 10.1136/gutjnl-2011-300730. [DOI] [PubMed] [Google Scholar]
- 2.Bhat S, Coleman HG, Yousef F, et al. Risk of malignant progression in Barrett's esophagus patients: results from a large population-based study. J Natl Cancer Inst. 2011;103:1049–1057. doi: 10.1093/jnci/djr203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hvid-Jensen F, Pedersen L, Drewes AM, et al. Incidence of adenocarcinoma among patients with Barrett's esophagus. N Engl J Med. 2011;365:1375–1383. doi: 10.1056/NEJMoa1103042. [DOI] [PubMed] [Google Scholar]
- 4.Verbeek RE, Leenders M, Ten Kate FJW, et al. Surveillance of Barrett's esophagus and mortality from esophageal adenocarcinoma: a population-based cohort study. Am J Gastroenterol. 2014;109:1215–1222. doi: 10.1038/ajg.2014.156. [DOI] [PubMed] [Google Scholar]
- 5.El-Serag HB, Naik AD, Duan Z, et al. Surveillance endoscopy is associated with improved outcomes of oesophageal adenocarcinoma detected in patients with Barrett's oesophagus. Gut. 2015 Aug 26; doi: 10.1136/gutjnl-2014-308865. http://dx.doi.org/10.1136/gutjnl-2014-308865. Epub ahead of print. [DOI] [PubMed]
- 6.Corley DA, Mehtani K, Quesenberry C, et al. Impact of endoscopic surveillance on mortality from Barrett's esophagus-associated esophageal adenocarcinomas. Gastroenterology. 2013;145:312–319.e1. doi: 10.1053/j.gastro.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inadomi JM, Sampliner R, Lagergren J, et al. Screening and surveillance for Barrett esophagus in high-risk groups: a cost-utility analysis. Ann Intern Med. 2003;138:176–186. doi: 10.7326/0003-4819-138-3-200302040-00009. [DOI] [PubMed] [Google Scholar]
- 8.Dulai GS, Guha S, Kahn KL, et al. Preoperative prevalence of Barrett's esophagus in esophageal adenocarcinoma: a systematic review. Gastroenterology. 2002;122:26–33. doi: 10.1053/gast.2002.30297. [DOI] [PubMed] [Google Scholar]
- 9.Visrodia K, Iyer PG, Schleck CD, et al. Yield of repeat endoscopy in Barrett's esophagus with no dysplasia and low-grade dysplasia: a population-based study. Digest Dis Sci. 2016;61:158–167. doi: 10.1007/s10620-015-3697-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spechler SJ, Sharma P, Souza RF, et al. American Gastroenterological Association technical review on the management of Barrett's esophagus. Gastroenterology. 2011;140:e18–e52. doi: 10.1053/j.gastro.2011.01.031. quiz e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ASBE Standard of Practice Committee. Evans JA, Early DS, et al. Standards of Practice Committee of the American Society for Gastrointestinal Endoscopy. The role of endoscopy in Barrett's esophagus and other premalignant conditions of the esophagus. Gastrointest Endosc. 2012;76:1087–1094. doi: 10.1016/j.gie.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Shaheen NJ, Falk GW, Iyer PG, et al. ACG clinical guideline: diagnosis and management of Barrett's esophagus. Am J Gastroenterol. 2015 Nov 3; doi: 10.1038/ajg.2015.322. http://dx.doi.org/10.1038/ajg.2015.322. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 13.Schnell TG, Sontag SJ, Chejfec G, et al. Long-term nonsurgical management of Barrett's esophagus with high-grade dysplasia. Gastroenterology. 2001;120:1607–1619. doi: 10.1053/gast.2001.25065. [DOI] [PubMed] [Google Scholar]
- 14.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. W64. [DOI] [PubMed] [Google Scholar]
- 16.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence—inconsistency. J Clin Epidemiol. 2011;64:1294–1302. doi: 10.1016/j.jclinepi.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 17.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Easterbrook PJ, Berlin JA, Gopalan R, et al. Publication bias in clinical research. Lancet. 1991;337:867–872. doi: 10.1016/0140-6736(91)90201-y. [DOI] [PubMed] [Google Scholar]
- 19.Den Hoed CM, Van Blankenstein M, Dees J, et al. The minimal incubation period from the onset of Barrett's oesophagus to symptomatic adenocarcinoma. Br J Cancer. 2011;105:200–205. doi: 10.1038/bjc.2011.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vogt N, Schonegg R, Gschossmann JM, et al. Benefit of baseline cytometry for surveillance of patients with Barrett's esophagus. Surg Endosc. 2010;24:1144–1150. doi: 10.1007/s00464-009-0741-7. [DOI] [PubMed] [Google Scholar]
- 21.de Jonge PJ, van Blankenstein M, Looman CW, et al. Risk of malignant progression in patients with Barrett's oesophagus: a Dutch nationwide cohort study. Gut. 2010;59:1030–1036. doi: 10.1136/gut.2009.176701. [DOI] [PubMed] [Google Scholar]
- 22.Rossi E, Grisanti S, Villanacci V, et al. HER-2 over-expression/amplification in Barrett's oesophagus predicts early transition from dysplasia to adenocarcinoma: a clinico-pathologic study. J Cell Mol Med. 2009;13:3826–3833. doi: 10.1111/j.1582-4934.2008.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinek J, Benes M, Brandtl P, et al. Low incidence of adenocarcinoma and high-grade intraepithelial neoplasia in patients with Barrett's esophagus: a prospective cohort study. Endoscopy. 2008;40:711–716. doi: 10.1055/s-2008-1077502. [DOI] [PubMed] [Google Scholar]
- 24.Vieth M, Schubert B, Lang-Schwarz K, et al. Frequency of Barrett's neoplasia after initial negative endoscopy with biopsy: a long-term histopathological follow-up study. Endoscopy. 2006;38:1201–1205. doi: 10.1055/s-2006-944993. [DOI] [PubMed] [Google Scholar]
- 25.Gladman L, Chapman W, Iqbal TH, et al. Barrett's oesophagus: an audit of surveillance over a 17-year period. Eur J Gastroenterol Hepatol. 2006;18:271–276. doi: 10.1097/00042737-200603000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Murphy SJ, Dickey W, Hughes D, et al. Surveillance for Barrett's oesophagus: results from a programme in Northern Ireland. Eur J Gastroenterol Hepatol. 2005;17:1029–1035. doi: 10.1097/00042737-200510000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Dulai GS, Shekelle PG, Jensen DM, et al. Dysplasia and risk of further neoplastic progression in a regional Veterans Administration Barrett's cohort. Am J Gastroenterol. 2005;100:775–783. doi: 10.1111/j.1572-0241.2005.41300.x. [DOI] [PubMed] [Google Scholar]
- 28.Parrilla P, Martinez de Haro LF, Ortiz A, et al. Long-term results of a randomized prospective study comparing medical and surgical treatment of Barrett's esophagus. Ann Surg. 2003;237:291–298. doi: 10.1097/01.SLA.0000055269.77838.8E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conio M, Blanchi S, Lapertosa G, et al. Long-term endoscopic surveillance of patients with Barrett's esophagus. Incidence of dysplasia and adenocarcinoma: a prospective study. Am J Gastroenter. 2003;98:1931–1939. doi: 10.1111/j.1572-0241.2003.07666.x. [DOI] [PubMed] [Google Scholar]
- 30.Eckardt VF, Kanzler G, Bernhard G. Life expectancy and cancer risk in patients with Barrett's esophagus: a prospective controlled investigation. Am J Med. 2001;111:33–37. doi: 10.1016/s0002-9343(01)00745-8. [DOI] [PubMed] [Google Scholar]
- 31.Macdonald CE, Wicks AC, Playford RJ. Final results from 10 year cohort of patients undergoing surveillance for Barrett's oesophagus: observational study. BMJ. 2000;321:1252–1255. doi: 10.1136/bmj.321.7271.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bani-Hani K, Sue-Ling H, Johnston D, et al. Barrett's oesophagus: results from a 13-year surveillance programme. Eur J Gastroenterol Hepatol. 2000;12:649–654. [PubMed] [Google Scholar]
- 33.Katz D, Rothstein R, Schned A, et al. The development of dysplasia and adenocarcinoma during endoscopic surveillance of Barrett's esophagus. Am J Gastroenterol. 1998;93:536–541. doi: 10.1111/j.1572-0241.1998.161_b.x. [DOI] [PubMed] [Google Scholar]
- 34.Miros M, Kerlin P, Walker N. Only patients with dysplasia progress to adenocarcinoma in Barrett's oesophagus. Gut. 1991;32:1441–1446. doi: 10.1136/gut.32.12.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spechler SJ, Robbins AH, Rubins HB, et al. Adenocarcinoma and Barrett's esophagus. An overrated risk? Gastroenterology. 1984;87:927–933. [PubMed] [Google Scholar]
- 36.Spechler SJ, Lee E, Ahnen D, et al. Long-term outcome of medical and surgical therapies for gastroesophageal reflux disease: follow-up of a randomized controlled trial. JAMA. 2001;285:2331–2338. doi: 10.1001/jama.285.18.2331. [DOI] [PubMed] [Google Scholar]
- 37.Reid BJ, Levine DS, Longton G, et al. Predictors of progression to cancer in Barrett's esophagus: baseline histology and flow cytometry identify low- and high-risk patient subsets. Am J Gastroenterol. 2000;95:1669–1676. doi: 10.1111/j.1572-0241.2000.02196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williamson WA, Ellis FH, Jr, Gibb SP, et al. Barrett's esophagus. Prevalence and incidence of adenocarcinoma. Arch Intern Med. 1991;151:2212–2216. doi: 10.1001/archinte.151.11.2212. [DOI] [PubMed] [Google Scholar]
- 39.Kastelein F, Spaander MC, Biermann K, et al. Nonsteroidal anti-inflammatory drugs and statins have chemopreventative effects in patients with Barrett's esophagus. Gastroenterology. 2011;141:2000–2008. doi: 10.1053/j.gastro.2011.08.036. quiz e13–e14. [DOI] [PubMed] [Google Scholar]
- 40.Hameeteman W, Tytgat GN, Houthoff HJ, et al. Barrett's esophagus: development of dysplasia and adenocarcinoma. Gastroenterology. 1989;96:1249–1256. doi: 10.1016/s0016-5085(89)80011-3. [DOI] [PubMed] [Google Scholar]
- 41.Teodori L, Gohde W, Persiani M, et al. DNA/protein flow cytometry as a predictive marker of malignancy in dysplasia-free Barrett's esophagus: thirteen-year follow-up study on a cohort of patients. Cytometry. 1998;34:257–263. doi: 10.1002/(sici)1097-0320(19981215)34:6<257::aid-cyto3>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 42.Rugge M, Zaninotto G, Parente P, et al. Barrett's esophagus and adenocarcinoma risk: the experience of the North-Eastern Italian Registry (EBRA) Ann Surg. 2012;256:788–795. doi: 10.1097/SLA.0b013e3182737a7e. [DOI] [PubMed] [Google Scholar]
- 43.Kastelein F, Spaander MC, Steyerberg EW, et al. Proton pump inhibitors reduce the risk of neoplastic progression in patients with Barrett's esophagus. Clin Gastroenterol Hepatol. 2013;11:382–388. doi: 10.1016/j.cgh.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 44.Sikkema M, Looman CW, Steyerberg EW, et al. Predictors for neoplastic progression in patients with Barrett's Esophagus: a prospective cohort study. Am J Gastroenterol. 2011;106:1231–1238. doi: 10.1038/ajg.2011.153. [DOI] [PubMed] [Google Scholar]
- 45.Ajumobi A, Bahjri K, Jackson C, et al. Surveillance in Barrett's esophagus: an audit of practice. Digest Dis Sci. 2010;55:1615–1621. doi: 10.1007/s10620-009-0917-y. [DOI] [PubMed] [Google Scholar]
- 46.Lim CH, Treanor D, Dixon MF, et al. Low-grade dysplasia in Barrett's esophagus has a high risk of progression. Endoscopy. 2007;39:581–587. doi: 10.1055/s-2007-966592. [DOI] [PubMed] [Google Scholar]
- 47.Cook MB, Wild CP, Everett SM, et al. Risk of mortality and cancer incidence in Barrett's esophagus. Cancer Epidemiol Biomarkers Prev. 2007;16:2090–2096. doi: 10.1158/1055-9965.EPI-07-0432. [DOI] [PubMed] [Google Scholar]
- 48.Falk GW, Thota PN, Richter JE, et al. Barrett's esophagus in women: demographic features and progression to high-grade dysplasia and cancer. Clin Gastroenterol Hepatol. 2005;3:1089–1094. doi: 10.1016/s1542-3565(05)00606-3. [DOI] [PubMed] [Google Scholar]
- 49.Bani-Hani KE, Bani-Hani BK, Martin IG. Characteristics of patients with columnar-lined Barrett's esophagus and risk factors for progression to esophageal adenocarcinoma. World J Gastroenterol. 2005;11:6807–6814. doi: 10.3748/wjg.v11.i43.6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hage M, Siersema PD, van Dekken H, et al. Oesophageal cancer incidence and mortality in patients with long-segment Barrett's oesophagus after a mean follow-up of 12.7 years. Scand J Gastroenterol. 2004;39:1175–1179. doi: 10.1080/00365520410003524. [DOI] [PubMed] [Google Scholar]
- 51.O'Connor JB, Falk GW, Richter JE. The incidence of adenocarcinoma and dysplasia in Barrett's esophagus: report on the Cleveland Clinic Barrett's Esophagus Registry. Am J Gastroenterol. 1999;94:2037–2042. doi: 10.1111/j.1572-0241.1999.01275.x. [DOI] [PubMed] [Google Scholar]
- 52.Ferraris R, Bonelli L, Conio M, et al. Incidence of Barrett's adenocarcinoma in an Italian population: an endoscopic surveillance programme. Gruppo Operativo per lo Studio delle Precancerosi Esofagee (GOSPE) Eur J Gastroenterol Hepatol. 1997;9:881–885. doi: 10.1097/00042737-199709000-00011. [DOI] [PubMed] [Google Scholar]
- 53.vanderBurgh A, Dees J, Hop WCJ, et al. Oesophageal cancer is an uncommon cause of death in patients with Barrett's oesophagus. Gut. 1996;39:5–8. doi: 10.1136/gut.39.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van der Veen AH, Dees J, Blankensteijn JD, et al. Adenocarcinoma in Barrett's oesophagus: an overrated risk. Gut. 1989;30:14–18. doi: 10.1136/gut.30.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wright TA, Gray MR, Morris AI, et al. Cost effectiveness of detecting Barrett's cancer. Gut. 1996;39:574–579. doi: 10.1136/gut.39.4.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Manabe N, Haruma K, Imamura H, et al. Does short-segment columnar-lined esophagus elongate during a mean follow-up period of 5.7 years? Digest Endosc. 2011;23:166–172. doi: 10.1111/j.1443-1661.2010.01073.x. [DOI] [PubMed] [Google Scholar]
- 57.Peters JH, Clark GWB, Ireland AP, et al. Outcome of adenocarcinoma arising in Barrett's esophagus in endoscopically surveyed and nonsurveyed patients. J Thoracic Cardiovasc Surg. 1994;108:813–822. [PubMed] [Google Scholar]
- 58.Leeuwenburgh I, Scholten P, Calje TJ, et al. Barrett's esophagus and esophageal adenocarcinoma are common after treatment for achalasia. Digest Dis Sci. 2013;58:244–252. doi: 10.1007/s10620-012-2157-9. [DOI] [PubMed] [Google Scholar]
- 59.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 60.Barclay RL, Vicari JJ, Doughty AS, et al. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med. 2006;355:2533–2541. doi: 10.1056/NEJMoa055498. [DOI] [PubMed] [Google Scholar]
- 61.Dik VK, Moons LM, Siersema PD. Endoscopic innovations to increase the adenoma detection rate during colonoscopy. World J Gastroenterol. 2014;20:2200–2211. doi: 10.3748/wjg.v20.i9.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gralnek IM, Siersema PD, Halpern Z, et al. Standard forward-viewing colonoscopy versus full-spectrum endoscopy: an international, multicentre, randomised, tandem colonoscopy trial. The Lancet Oncology. 2014;15:353–360. doi: 10.1016/S1470-2045(14)70020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chung SJ, Kim D, Song JH, et al. Comparison of detection and miss rates of narrow band imaging, flexible spectral imaging chromoendoscopy and white light at screening colonoscopy: a randomised controlled back-to-back study. Gut. 2014;63:785–791. doi: 10.1136/gutjnl-2013-304578. [DOI] [PubMed] [Google Scholar]
- 64.Boerwinkel DF, Holz JA, Kara MA, et al. Effects of autofluorescence imaging on detection and treatment of early neoplasia in patients with Barrett's esophagus. Clin Gastroenterol Hepatol. 2014;12:774–781. doi: 10.1016/j.cgh.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 65.Wang KK, Carr-Locke DL, Singh SK, et al. Use of probe-based confocal laser endomicroscopy (pCLE) in gastrointestinal applications. A consensus report based on clinical evidence. United European Gastroenterol J. 2015;3:230–254. doi: 10.1177/2050640614566066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Curvers WL, Alvarez Herrero L, Wallace MB, et al. Endoscopic tri-modal imaging is more effective than standard endoscopy in identifying early-stage neoplasia in Barrett's esophagus. Gastroenterology. 2010;139:1106–1114. doi: 10.1053/j.gastro.2010.06.045. [DOI] [PubMed] [Google Scholar]
- 67.Gupta N, Gaddam S, Wani SB, et al. Longer inspection time is associated with increased detection of high-grade dysplasia and esophageal adenocarcinoma in Barrett's esophagus. Gastrointesti Endosc. 2012;76:531–538. doi: 10.1016/j.gie.2012.04.470. [DOI] [PubMed] [Google Scholar]
- 68.Spechler SJ. Comparison of medical and surgical therapy for complicated gastroesophageal reflux disease in veterans. The Department of Veterans Affairs Gastroesophageal Reflux Disease Study Group. N Engl J Med. 1992;326:786–792. doi: 10.1056/NEJM199203193261202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Funnel plot for the primary outcome of proportion missed EAC.
Supplementary Table 1. Quality Scoring Scheme
Supplementary Table 2. Characteristics of Included Studies, Categorized by Duration of Follow-Up and Baseline Histology of Cohort
Supplementary Table 3. Quality of the Studies, Categorized by Baseline Histology of Cohort
Supplementary Table 4. Additional Extraction of Outcomes for Subjects With NDBE Only From Studies With Cohorts Composed of Subjects With Baseline NDBE, BE-LGD, and/or BE-HGD
Supplementary Table 5. Sensitivity Analysis to Examine Sources of Heterogeneity for Studies With at Least 5 Years Follow-Up


