Abstract
Objectives
To evaluate the utility of preoperative multiparametric magnetic resonance imaging (MP-MRI) in predicting biochemical recurrence (BCR) following radical prostatectomy (RP).
Materials/Methods
From March 2007 to January 2015, 421 consecutive patients with prostate cancer (PCa) underwent preoperative MP-MRI and RP. BCR-free survival rates were estimated using the Kaplan-Meier method. Cox proportional hazards models were used to identify clinical and imaging variables predictive of BCR. Logistic regression was performed to generate a nomogram to predict three-year BCR probability.
Results
Of the total cohort, 370 patients met inclusion criteria with 39 (10.5%) patients experiencing BCR. On multivariate analysis, preoperative prostate-specific antigen (PSA) (p = 0.01), biopsy Gleason score (p = 0.0008), MP-MRI suspicion score (p = 0.03), and extracapsular extension on MP-MRI (p = 0.03) were significantly associated with time to BCR. A nomogram integrating these factors to predict BCR at three years after RP demonstrated a c-index of 0.84, outperforming the predictive value of Gleason score and PSA alone (c-index 0.74, p = 0.02).
Conclusion
The addition of MP-MRI to standard clinical factors significantly improves prediction of BCR in a post-prostatectomy PCa cohort. This could serve as a valuable tool to support clinical decision-making in patients with moderate and high-risk cancers.
Introduction
Prostate cancer (PCa) is the leading noncutaneous cancer in men in the United States, responsible for nearly 30,000 deaths and 230,000 new cases per year [1]. Radical prostatectomy (RP) is an established treatment for localized disease [2]. However, rates of biochemical recurrence (BCR) after RP are reportedly as high as 27% with two-thirds of BCR occurring within two years after RP [3,4]. Furthermore, BCR has been associated with progression to distant metastases and cancer-specific mortality [5]. Several studies have attempted to identify predictors of BCR after RP but with limited accuracy [6]. Thus, a more reliable method to predict BCR would be clinically useful in treatment decision-making.
Tools such as the Kattan nomogram and Han tables have enabled clinicians to use clinical parameters such as pretreatment prostate-specific antigen (PSA), clinical stage, and biopsy Gleason score to predict the probability of BCR [7,8]. Attempts to increase the accuracy of these models with additional clinical parameters have produced limited added benefit [9]. Magnetic resonance imaging (MRI) has been recognized as an excellent modality to stage and localize PCa due to its exceptional soft-tissue contrast and high spatial resolution [10]. Several studies have explored the utility of prostate MRI in predicting BCR but with varied results [11,12].
Advances in multiparametric MRI (MP-MRI), consisting of T2-weighted (T2W), diffusion-weighted (DW), and dynamic contrast enhanced (DCE) imaging have improved detection and localization of clinically significant PCa [13,14]. Although MP-MRI has been extensively studied for its diagnostic capabilities, its significance in predicting postoperative outcomes is less well understood [15,16]. A predictive model for the likelihood of recurrence after treatment, combining standard clinical factors with imaging results, could be a valuable tool for patients and clinicians. Therefore, we evaluated the performance of preoperative MP-MRI characteristics in predicting BCR following RP.
Materials and Methods
Patient selection, assessment, treatment, and follow-up
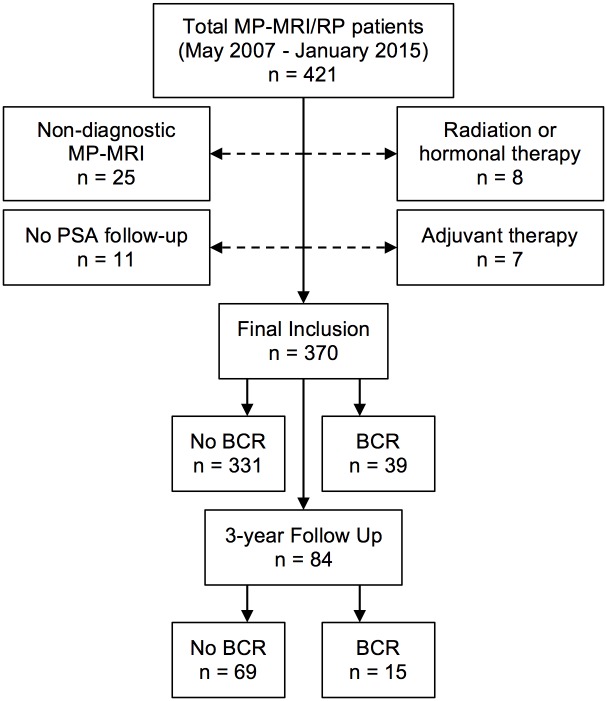
Patients were enrolled under an institutional review board (IRB) approved (ClinicalTrials.gov: NCT00102544), prospective trial with all data collection and follow-up performed in accordance with the United States Health Insurance Portability and Accountability Act. Patients provided written informed consent with approval of the consent procedure by the IRB. From May 2007 to January 2015, 421 consecutive patients underwent MP-MRI followed by robotic-assisted RP at a single institution (Fig 1). Patients were excluded if they had no recorded PSA values postoperatively, had non-diagnostic MRI (e.g. hemorrhage, hip prosthesis, movement artifacts), received prior hormone or radiation therapy, or had adjuvant treatments before documented BCR.
Fig 1. Flowchart of patient selection.
MP-MRI = multiparametric magnetic resonance imaging; PSA = prostate-specific antigen; RP = radical prostatectomy.
All patients underwent total serum PSA screening, digital rectal exam (staging per American Joint Committee on Cancer, 7th Edition), standard 12-core systematic transrectal ultrasound (TRUS) guided biopsy, as well as MP-MRI of the prostate. A subset of patients also underwent a targeted MRI/TRUS fusion guided biopsy. MP-MRI data including lesion number, total prostate volume, MP-MRI suspicion score, and MRI-based suspicion for extracapsular extension (mECE) and seminal vesicle invasion (mSVI) together with biopsy Gleason score (highest from either standard or targeted MRI/TRUS fusion guided biopsy) were obtained.
All RP procedures were performed by a single urologist (PAP) and all pathology was reviewed by a single genitourinary pathologist (MJM) with pathologic grade, stage, margin, and lymph node status noted. Follow-up protocol involved monitoring serum PSA levels at one, three, and six months after RP, with annual PSA levels subsequently. BCR was defined following the guidelines of the American Urological Association Localized Prostate Cancer Update Panel report [17] as a serum PSA ≥ 0.2 ng/ml with a confirmatory value of ≥ 0.2 ng/ml, a single PSA ≥ 0.4 ng/ml, or by receipt of salvage therapy specifically due to an increasing postoperative PSA.
Imaging protocol
Diagnostic MP-MRI was performed on a 3.0 Tesla MRI scanner (Achieva, Philips Healthcare, Best, Netherlands) with a 16-channel cardiac surface coil (SENSE, Philips Healthcare, Cleveland OH) positioned over the pelvis and an endorectal coil (BPX-30, Medrad, Pittsburgh, PA) as previously described [13]. The MRI protocol included T2W imaging, DW imaging with apparent diffusion coefficient mapping (ADC), and axial three-dimensional fast field echo DCE MRI sequences. These images underwent blinded centralized radiological evaluation by two radiologists (BT, PLC) with 8 and 16 years of prostate MRI experience, respectively. PCa suspicion scores (low, moderate, or high) were assigned to each lesion using previously described criteria, which have been associated with both the occurrence of PCa and tumor grade [18, 19]. The now standardized PI-RADS (Prostate Imaging Reporting and Data System) criteria were not routinely used during the time frame of this study [20]. However, a low, moderate, and high MP-MRI suspicion score is analogous to 1–2, 3–4, and 5 score, respectively using the PI-RADS system [21].
Outcome measures and statistical analysis
Statistical analysis was performed using JMP® 11.0 (SAS Institute Inc., Cary, NC) with a threshold for significance of p <0.05. Cox proportional hazards regression models were constructed for all clinical and imaging variables. Variables meeting a threshold of p <0.15 were included in the multivariate analysis. BCR-free survival (BCRFS) was estimated using the Kaplan-Meier method. Patients without recurrence were censored by their last follow-up time with survival curves compared using the log-rank test. Collinearity was assessed in the multivariate analysis by examining the effects of adding a combined term for potentially collinear covariates.
Utilizing factors found to be associated with BCR on multivariate regression, a nomogram was generated using the R statistical software package (http://www.r-project.org). A subset of 84 men from the total cohort who had complete three years of follow-up was examined to generate the nomogram with three-year BCR chosen as the outcome of interest. Stepwise analysis of the impact of each individual predictor on the c-index of the nomogram was examined and PSA was excluded as it had minimal improvement on the overall predictive ability of the nomogram. A calibration curve to assess the performance of the nomogram was generated using bootstrap analysis with n = 40 to assess predicted against actual probability of BCR from the nomogram.
Results
Of the 421 patients who underwent RP, 370 patients met the inclusion criteria for analysis (Fig 1). In this population, 39 patients (10.5%) experienced BCR with a median time to recurrence of 14.0 months (IQR 3.7–27.5). The median follow-up time for patients with no biochemical recurrence was 22.3 months (IQR 11.9–33.9). Within the 370 patients, 206 (55.7%) underwent fusion biopsy, and 84 had complete 3-year follow up with 15 (17.9%) experiencing BCR. Preoperative patient characteristics for both cohorts are displayed in Table 1. Median patient age and preoperative PSA for the total cohort were 60 years (IQR 55–65) and 5.8 ng/ml (IQR 4.1–9.6), respectively. Post-prostatectomy variables for both cohorts including pathologic Gleason score, pathologic stage, lymph node invasion, and positive surgical margins are displayed in Table 2.
Table 1. Preoperative clinical and multiparametric magnetic resonance imaging characteristics of patients undergoing radical prostatectomy.
| Clinical Characteristics | ||
| Median (IQR) | Total Cohort | Nomogram Cohort |
| Age, yr | 60 (55–65) | 58 (55–63) |
| BMI | 28 (26–31) | 28 (25–31) |
| Preoperative PSA, ng/ml | 5.5 (4.0–8.7) | 5.2 (3.7–9.9) |
| No (%) | ||
| Race | ||
| White | 240 (72.5) | 54 (64.3) |
| Black | 66 (19.9) | 19 (22.6) |
| Other | 25 (7.6) | 11 (13.1) |
| Clinical stage | ||
| T1c | 301 (90.9) | 71 (84.5) |
| ≥ T2a | 30 (9.1) | 13 (15.5) |
| Biopsy Gleason score | ||
| 6 | 90 (27.2) | 29 (34.5) |
| 7 | 184 (55.6) | 46 (54.8) |
| ≥ 8 | 57 (17.2) | 9 (10.7) |
| MP-MRI Characteristics | ||
| Median (IQR) | Total Cohort | Nomogram Cohort |
| MRI prostate volume, cc | 37 (30–47) | 39 (28–49) |
| MRI lesions, no | 2 (1–3) | 2 (1–3) |
| No (%) | ||
| MP—MRI suspicion score | ||
| Low | 45 (13.6) | 17 (20.2) |
| Moderate | 208 (62.8) | 37 (44.1) |
| High | 78 (23.6) | 30 (35.7) |
| mECE | ||
| Absent | 232 (70.0) | 56 (66.7) |
| Present | 99 (30.0) | 28 (33.3) |
| mSVI | ||
| Absent | 322 (97.3) | 83 (98.8) |
| Present | 9 (2.7) | 1 (1.2) |
BMI = body mass index, IQR = interquartile range, MRI = magnetic resonance imaging, MP-MRI = multiparametric magnetic resonance imaging, mECE = extracapsular extension on magnetic resonance imaging, mSVI = seminal vesicle invasion on magnetic resonance imaging, PSA = prostate-specific antigen.
Table 2. Postoperative pathologic patient characteristics of patients undergoing radical prostatectomy.
| Postoperative Characteristics | ||
|---|---|---|
| No. (%) | Total Cohort | Nomogram Cohort |
| Pathologic Gleason score | ||
| 6 | 38 (10.3) | 11 (13.1) |
| 7 | 235 (63.5) | 48 (57.1) |
| ≥ 8 | 97 (26.2) | 25 (29.8) |
| Pathologic stage | ||
| pT2a—pT2b | 52 (14.0) | 10 (11.9) |
| pT2c | 243 (65.7) | 53 (63.1) |
| pT3a | 58 (15.7) | 16 (19.0) |
| pT3b—pT4 | 17 (4.6) | 5 (6.0) |
| Lymph node invasion | ||
| Absent | 352 (95.1) | 77 (91.7) |
| Present | 18 (4.9) | 7 (8.3) |
| Margins (all) | ||
| Not involved | 322 (87.0) | 70 (83.3) |
| Involved | 48 (13.0) | 14 (16.7) |
| Margins (≤ pT2c) | ||
| Not Involved | 269 (91.2) | 55 (87.3) |
| Involved | 26 (8.8) | 8 (12.7) |
Cox proportional hazards regression analyses for BCR after RP are shown in Table 3. On univariate analysis, preoperative PSA, clinical stage, biopsy Gleason score, MP-MRI suspicion score, and mECE demonstrated a significant association with time to BCR (all p <0.01). Upon multivariate analysis, preoperative PSA, biopsy Gleason score, MP-MRI suspicion score, and mECE remained significantly associated with BCR (all p <0.05). Presence of mECE (HR = 2.10, p = 0.04) and higher suspicion lesions on MP-MRI (HR = 1.97, p = 0.02) preoperatively were both associated with an increased risk of BCR after RP.
Table 3. Univariate and multivariate Cox proportional hazards regression model of preoperative clinical and imaging variables predicting biochemical recurrence.
| Covariate | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age, years | 0.98 (0.94–1.02) | 0.3 | - | - |
| BMI | 0.99 (0.92–1.07) | 0.8 | - | - |
| Preoperative PSA, ng/ml | 1.05 (1.02–1.07) | <0.0001 | 1.04 (1.01–1.07) | 0.01 |
| Race | ||||
| White | 1 | - | - | - |
| Black | 0.89 (0.35–1.92) | 0.8 | - | - |
| Other | 1.35 (0.46–3.23) | 0.6 | - | - |
| Clinical stage | 2.87 (1.37–5.61) | 0.007 | 1.38 (0.63–2.90) | 0.4 |
| Biopsy Gleason score | 3.76 (2.27–6.40) | <0.0001 | 2.35 (1.37–4.14) | 0.002 |
| MRI prostate volume, cc | 0.98 (0.96–1.00) | 0.06 | 0.98 (0.96–1.00) | 0.08 |
| MRI lesions, no | 0.80 (0.59–1.07) | 0.1 | 0.93 (0.68–1.26) | 0.7 |
| MP—MRI suspicion score | 3.49 (2.02–6.39) | <0.0001 | 1.97 (1.09–3.73) | 0.02 |
| mECE | 4.34 (2.27–8.72) | <0.0001 | 2.10 (1.05–4.40) | 0.04 |
| mSVI | 1.85 (0.30–6.05) | 0.4 | - | - |
BMI = body mass index; CI = confidence interval; HR = hazard ratio; PSA = prostate-specific antigen; MP-MRI = multiparametric magnetic resonance imaging; MRI = magnetic resonance imaging. Statistical significance denoted by bold face.
In order to address the influence of margin status on preoperative predictors of BCR, a subgroup Cox regression analysis was performed for patients with negative surgical margins. Of the 370 patients, 322 (87.0%) had negative surgical margins and 22 (6.8%) men in this cohort experienced BCR. On multivariate analysis, preoperative PSA, biopsy Gleason score, and mECE remained predictive of BCR (all p <0.05, Table 4), similar to as noted in the total cohort.
Table 4. Univariate and multivariate Cox proportional hazards regression model of clinical and imaging variables predicting biochemical recurrence: patients with negative surgical margins.
| Covariate | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age, years | 0.99 (0.94–1.05) | 0.7 | - | - |
| BMI | 1.03 (0.93–1.12) | 0.5 | - | - |
| Preoperative PSA, ng/ml | 1.07 (1.04–1.09) | <0.0001 | 1.05 (1.02–1.08) | 0.003 |
| Race | ||||
| White | 1 | - | - | - |
| Black | 0.62 (0.14–1.83) | 0.4 | - | - |
| Other | 0.98 (0.16–3.43) | 1 | - | - |
| Clinical stage | 3.54 (1.35–8.42) | 0.01 | 1.89 (0.69–4.75) | 0.2 |
| Biopsy Gleason score | 6.71 (3.26–15.28) | <0.0001 | 4.89 (2.24–11.61) | <0.0001 |
| MRI prostate volume, cc | 0.99 (0.96–1.01) | 0.3 | - | - |
| MRI lesions, no | 0.79 (0.52–1.17) | 0.3 | - | - |
| MP—MRI suspicion score | 3.81 (1.83–8.70) | 0.0002 | 1.84 (0.84–4.41) | 0.1 |
| mECE | 5.45 (2.30–14.29) | 0.0001 | 3.37 (1.35–9.32) | 0.009 |
| mSVI | 1.60 (0.09–7.65) | 0.7 | - | - |
BMI = body mass index; CI = confidence interval; HR = hazard ratio; PSA = prostate-specific antigen; MP-MRI = multiparametric magnetic resonance imaging; MRI = magnetic resonance imaging. Statistical significance denoted by bold face.
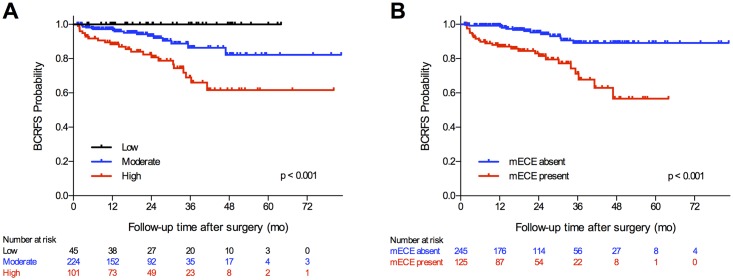
To further assess the impact of imaging on prediction of BCR, Kaplan-Meier analyses were performed examining BCRFS stratified by MP-MRI suspicion score and mECE (Fig 2). BCRFS rates differed between suspicion levels and between presence of mECE (all p <0.001). The BCRFS rate for those with low MP-MRI suspicion scores remained at 100%. However, patients with high MP-MRI suspicion scores had significantly lower BCRFS than those with moderate MP-MRI suspicion scores 3 years after RP (66% vs. 86%, respectively) and 5 years after RP (62% vs. 82%, respectively). The BCRFS rate at 3 years after RP for patients with mECE was 68% compared to 89% for patients without mECE. This BCRFS difference amplified at 5 years after RP as BCRFS decreased to 57% for patients with mECE while remaining the same for patients without mECE (89%).
Fig 2. Kaplan-Meier analysis of biochemical recurrence-free survival for patients after radical prostatectomy.
(A) By multiparametric magnetic resonance imaging suspicion score. (B) By extracapsular extension on magnetic resonance imaging. BCRFS = biochemical recurrence-free survival; mECE = extracapsular extension on magnetic resonance imaging; SVI = seminal vesicle invasion on magnetic resonance imaging.
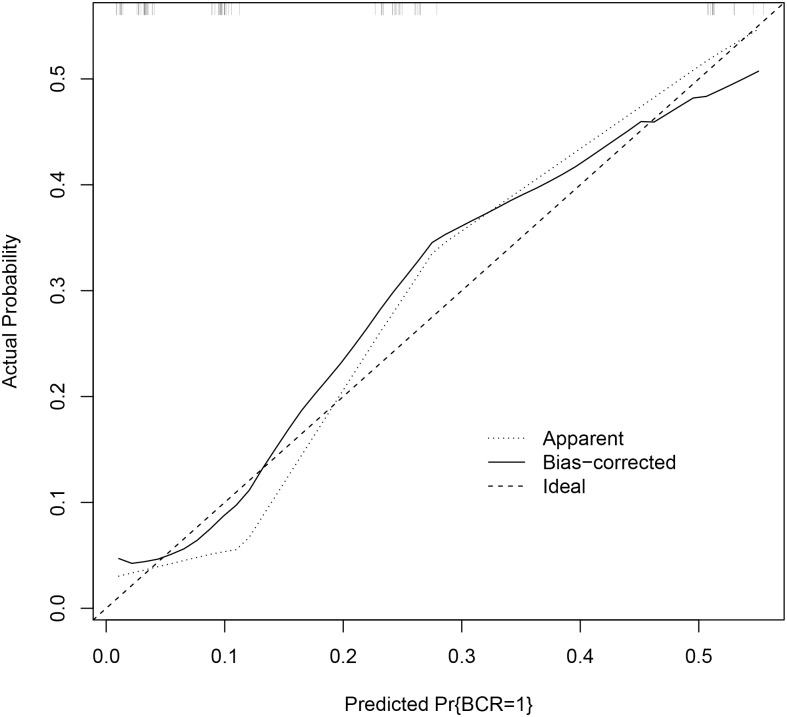
These findings provided sufficient evidence to support the generation of a nomogram with incorporation of pre-operative MP-MRI features for the prediction of BCR (Fig 3). The specific outcome of three-year BCR was chosen as the outcome of interest for this nomogram resulting in 84 patients being included in the analysis (Fig 1). Although PSA demonstrated a statistically significant association with BCR, the amplitude of this association was low and PSA did not exert a clinically significant impact on the prediction of BCR. Consequently, PSA was removed from this final model. The c-index of the final nomogram was 0.84. A predictive model created not including the imaging-derived features of mECE and MP-MRI suspicion score yielded a c-index of 0.74 (p = 0.02). A calibration plot demonstrated a mean absolute error of 2.9% for prediction of BCR (Fig 4). The nomogram did not consistently over-predict or under-predict throughout the entire probability range. However, it tended to under-predict in intermediate probabilities while over predicting the rate of three-year BCR at higher and lower extremes.
Fig 3. Nomogram to predict biochemical recurrence at 36 months after radical prostatectomy incorporating both clinical and multiparametric magnetic resonance imaging parameters.
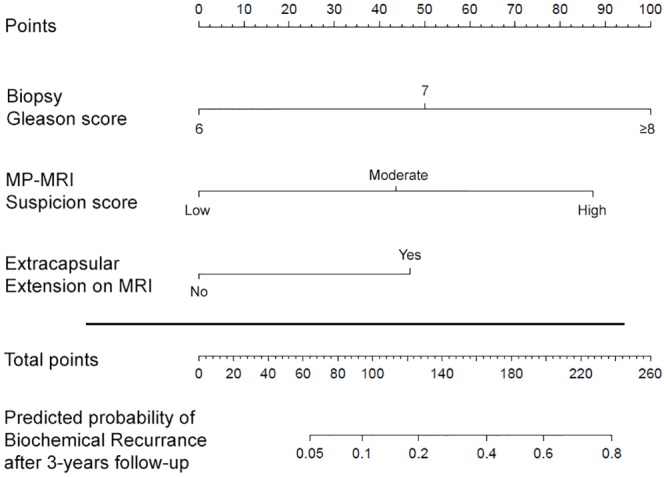
MP-MRI = multiparametric magnetic resonance imaging; MRI = magnetic resonance imaging.
Fig 4. Calibration curve demonstrating performance of predicted probability of 3-year biochemical recurrence versus actual probability noted within the study.
BCR = biochemical recurrence.
Discussion
Biochemical recurrence of prostate cancer after radical prostatectomy can be a harbinger of more advanced disease and has been associated with increased rates of metastasis and prostate cancer specific mortality [22]. While RP is curative in most patients, rates of BCR following RP have been reported as high as 27% in cohorts with a median follow up of ten years [3, 23]. Thus, a preoperative predictive model for BCR could serve as an invaluable clinical tool for risk stratification and patient counseling.
MP-MRI allows for imaging-based identification and characterization of PCa, improving diagnostic accuracy [13, 14]. Yet, few studies have determined the value of MP-MRI as a non-invasive means for the prediction of BCR. Our results show that MP-MRI suspicion score and mECE, together with previously validated clinical parameters such as preoperative PSA and biopsy Gleason score, have significant added benefit in prediction of BCR after RP [7, 8].
MP-MRI suspicion score is a clinically useful method to detect and characterize PCa, especially in patients with Gleason ≥ seven, which comprised 90% of this cohort [24]. Patients who harbor highly suspicious lesions as well as those with mECE are nearly twice as likely to experience BCR, as compared to patients with only moderately suspicious lesions or those without mECE. No patients in this study with a low MP-MRI suspicion score experienced BCR, suggesting that low MP-MRI suspicion in the setting of RP corresponds to long-term BCRFS. In addition, mECE remains a significant predictor of BCR even when accounting for surgical margins, which was a confounder in previous studies [25].
Preoperative predictive models for prostate cancer recurrence have been a previously explored area of study, producing widely recognized tools such as the Kattan nomogram and the Han tables [7,8]. Imaging factors such as lesion suspicion or mECE have been associated with BCR [26–28]. However, few studies have explored the incorporation of preoperative MP-MRI variables into predictive models. Poulakis et al. showed that an artificial neural model combining 1 T pelvic-coil MRI findings with preoperative clinical variables was superior to both the Kattan nomogram and Han tables in predicting recurrence [11]. A study by Nishida et al. showed that inclusion of MRI staging significantly increased the predictive value for BCR (AUC = 0.79) in comparison to the Han tables alone (AUC = 0.67, p = 0.047) [15]. The MP-MRI nomogram we have developed is unique in its ability to incorporate clinical parameters with both MP-MRI as a biomarker (MRI suspicion score) and anatomic staging (mECE) to reliably predict BCRFS after RP in the preoperative setting.
Predictive models for BCR can serve as a crucial tool for managing patient expectations on the possibility of receiving adjuvant therapy, especially when RP is viewed as a curative measure in most patients. Also, many secondary treatments can have negative implications for quality of life [29,30]. Of the 39 patients with BCR in this cohort, a majority of these patients (85%) went on to receive salvage therapy, including external beam radiation therapy as well as androgen deprivation therapy. A nomogram developed from our predictive model was reliable (c-index = 0.84) in predicting these cases of BCR. With further validation, this MP-MRI based nomogram may be useful in risk stratification of patients before RP as well as managing patient expectations on the possibility of adjuvant interventions.
Many of our patients (55.7%) went on to receive a targeted MRI-TRUS fusion biopsy, which could be a potential confounder in this study. In order to control for this, additional analysis was performed incorporating a covariate in the multivariate analysis of fusion biopsy. This variable was not associated with BCR on univariate or multivariate analysis. Furthermore, addition of this covariate did not change the outcome of the multivariate analysis. Since MRI-TRUS fusion guided biopsies typically detect more clinically significant disease and less clinically insignificant disease, the biopsy results could also assist in the accuracy of the nomogram [31].
A limitation of this study is that it is a single institution retrospective study. Also, our MP-MRI images were performed on a single MRI unit, mostly by a single MR technologist, and read by genitourinary radiologists experienced with prostate imaging. This would all tend to improve the quality of the MRI and hence its value. The sensitivity and specificity of mECE for pathologic ECE was 58.5% and 73.3% respectively. Although significant associations were demonstrated with MRI findings consistent with ECE and biochemical recurrence, the imaging modality continues to have much room for improvement in the actual prediction of pathologic ECE. Our median follow-up time of 22.3 months is relatively short, though prediction of early BCR is clinically meaningful as nearly two-thirds of recurrences occur within two years of RP [4]. Lastly, our nomogram was generated from 84 patients with 15 (17.9%) experiencing BCR, which may limit the power and robustness of this clinical tool. As we continue with our work, we hope to increase the sample size and thus applicability of the MP-MRI based nomogram.
Additional future work will concentrate on validating the nomogram in a multicenter or prospective cohort, which could support its future clinical use. Furthermore, while the NIH suspicion score system has been validated, the incorporation of the PI-RADS system may enhance the utility of this study and is expected to perform in a similar manner.
In conclusion, the addition of MP-MRI parameters to standard clinical factors better predicts BCR in a post-prostatectomy PCa cohort. Presence of mECE and higher suspicion lesions on preoperative MP-MRI were associated with nearly twice the risk of BCR after RP. When validated, the MP-MRI based nomogram could serve as a helpful tool to support clinical decision-making.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, the Center for Cancer Research, and the Center for Interventional Oncology.
Data Availability
All relevant data are within the paper.
Funding Statement
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, the Center for Cancer Research, and the Center for Interventional Oncology. NIH and Philips Healthcare/Invivo Corp. have a cooperative research and development agreement. NIH and Philips share intellectual property in the field. This research was also made possible through the National Institutes of Health Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from Pfizer Inc., The Doris Duke Charitable Foundation, The Alexandria Real Estate Equities, Inc. and Mr. and Mrs. Joel S. Marcus, and the Howard Hughes Medical Institute, as well as other private donors. For a complete list, please visit the Foundation website at: http://fnih.org/what-we-do/current-education-and-training-programs/mrsp. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin [Internet]. 2015. January 5 [cited 2015 Jan 9];65(1):5–29. Available: http://www.ncbi.nlm.nih.gov/pubmed/25559415 10.3322/caac.21254 [DOI] [PubMed] [Google Scholar]
- 2.Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, et al. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol [Internet]. 2014. January [cited 2014 Dec 31];65(1):124–37. Available: http://www.sciencedirect.com/science/article/pii/S0302283813010403 10.1016/j.eururo.2013.09.046 [DOI] [PubMed] [Google Scholar]
- 3.Diaz M, Peabody JO, Kapoor V, Sammon J, Rogers CG, Stricker H, et al. Oncologic outcomes at 10 years following robotic radical prostatectomy. Eur Urol. Switzerland; 2015. June;67(6):1168–76. [DOI] [PubMed] [Google Scholar]
- 4.Walz J, Chun FK-H, Klein EA, Reuther A, Saad F, Graefen M, et al. Nomogram predicting the probability of early recurrence after radical prostatectomy for prostate cancer. J Urol [Internet]. 2009. February [cited 2015 May 1];181(2):601–7; discussion 607–8. Available: http://www.ncbi.nlm.nih.gov/pubmed/19084864 10.1016/j.juro.2008.10.033 [DOI] [PubMed] [Google Scholar]
- 5.Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA [Internet]. American Medical Association; 2005. July 27 [cited 2015 Apr 6];294(4):433–9. Available: http://jama.jamanetwork.com/article.aspx?articleid=201291#REF-JOC50067-11 [DOI] [PubMed] [Google Scholar]
- 6.Shariat SF, Karakiewicz PI, Roehrborn CG, Kattan MW. An updated catalog of prostate cancer predictive tools. Cancer [Internet]. 2008. December 1 [cited 2015 Apr 29];113(11):3075–99. Available: http://www.ncbi.nlm.nih.gov/pubmed/18823041 10.1002/cncr.23908 [DOI] [PubMed] [Google Scholar]
- 7.Kattan MW, Eastham JA, Stapleton AM, Wheeler TM, Scardino PT. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst [Internet]. 1998. May 20 [cited 2015 Apr 30];90(10):766–71. Available: http://www.ncbi.nlm.nih.gov/pubmed/9605647 [DOI] [PubMed] [Google Scholar]
- 8.Han M, Partin AW, Zahurak M, Piantadosi S, Epstein JI, Walsh PC. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J Urol [Internet]. 2003. March [cited 2015 Apr 30];169(2):517–23. Available: http://www.ncbi.nlm.nih.gov/pubmed/12544300 [DOI] [PubMed] [Google Scholar]
- 9.D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Fondurulia J, Chen MH, et al. Clinical utility of the percentage of positive prostate biopsies in defining biochemical outcome after radical prostatectomy for patients with clinically localized prostate cancer. J Clin Oncol [Internet]. 2000. March [cited 2015 May 28];18(6):1164–72. Available: http://www.ncbi.nlm.nih.gov/pubmed/10715284 [DOI] [PubMed] [Google Scholar]
- 10.Heenan SD. Magnetic resonance imaging in prostate cancer. Prostate Cancer Prostatic Dis [Internet]. 2004. January [cited 2015 May 3];7(4):282–8. Available: http://www.ncbi.nlm.nih.gov/pubmed/15592440 [DOI] [PubMed] [Google Scholar]
- 11.Poulakis V, Witzsch U, de Vries R, Emmerlich V, Meves M, Altmannsberger H-M, et al. Preoperative neural network using combined magnetic resonance imaging variables, prostate-specific antigen, and gleason score for predicting prostate cancer biochemical recurrence after radical prostatectomy. Urology [Internet]. 2004. December [cited 2015 May 1];64(6):1165–70. Available: http://www.ncbi.nlm.nih.gov/pubmed/15596191 [DOI] [PubMed] [Google Scholar]
- 12.Hattori S, Kosaka T, Mizuno R, Kanao K, Miyajima A, Yasumizu Y, et al. Prognostic value of preoperative multiparametric magnetic resonance imaging (MRI) for predicting biochemical recurrence after radical prostatectomy. BJU Int [Internet]. 2014. May [cited 2015 Apr 30];113(5):741–7. Available: http://www.ncbi.nlm.nih.gov/pubmed/23937660 10.1111/bju.12329 [DOI] [PubMed] [Google Scholar]
- 13.Turkbey B, Pinto PA, Mani H, Bernardo M, Pang Y, McKinney YL, et al. Prostate cancer: value of multiparametric MR imaging at 3 T for detection—histopathologic correlation. Radiology [Internet]. 2010. April [cited 2015 Mar 17];255(1):89–99. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2843833&tool=pmcentrez&rendertype=abstract 10.1148/radiol.09090475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frye TP, Pinto PA, George AK. Optimizing Patient Population for MP-MRI and Fusion Biopsy for Prostate Cancer Detection. Curr Urol Rep [Internet]. 2015. July [cited 2015 Jun 16];16(7):521 Available: http://www.ncbi.nlm.nih.gov/pubmed/26063625 [DOI] [PubMed] [Google Scholar]
- 15.Nishida K, Yuen S, Kamoi K, Yamada K, Akazawa K, Ito H, et al. Incremental value of T2-weighted and diffusion-weighted MRI for prediction of biochemical recurrence after radical prostatectomy in clinically localized prostate cancer. Acta Radiol [Internet]. 2011. March 1 [cited 2015 Apr 30];52(1):120–6. Available: http://www.ncbi.nlm.nih.gov/pubmed/21498337 10.1258/ar.2010.100293 [DOI] [PubMed] [Google Scholar]
- 16.Panebianco V, Barchetti F, Sciarra A, Musio D, Forte V, Gentile V, et al. Prostate cancer recurrence after radical prostatectomy: the role of 3-T diffusion imaging in multi-parametric magnetic resonance imaging. Eur Radiol [Internet]. 2013. February 2 [cited 2015 Apr 20];23(6):1745–52. Available: http://www.ncbi.nlm.nih.gov/pubmed/23377546 10.1007/s00330-013-2768-3 [DOI] [PubMed] [Google Scholar]
- 17.Cookson MS, Aus G, Burnett AL, Canby-Hagino ED, D’Amico AV, Dmochowski RR, et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: the American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the re. J Urol [Internet]. 2007. March [cited 2015 Mar 29];177(2):540–5. Available: http://www.ncbi.nlm.nih.gov/pubmed/17222629 [DOI] [PubMed] [Google Scholar]
- 18.Yerram NK, Volkin D, Turkbey B, Nix J, Hoang AN, Vourganti S, et al. Low suspicion lesions on multiparametric magnetic resonance imaging predict for the absence of high-risk prostate cancer. BJU Int [Internet]. 2012. December [cited 2015 Apr 24];110(11 Pt B):E783–8. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3808160&tool=pmcentrez&rendertype=abstract 10.1111/j.1464-410X.2012.11646.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinto PA, Chung PH, Rastinehad AR, Baccala AA, Kruecker J, Benjamin CJ, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol [Internet]. 2011. October [cited 2015 Jan 21];186(4):1281–5. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3193933&tool=pmcentrez&rendertype=abstract 10.1016/j.juro.2011.05.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G, et al. ESUR prostate MR guidelines 2012. Eur Radiol [Internet]. 2012. April [cited 2014 Jul 9];22(4):746–57. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3297750&tool=pmcentrez&rendertype=abstract 10.1007/s00330-011-2377-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siddiqui MM, Truong H, Rais-Bahrami S, Stamatakis L, Logan J, Walton-Diaz A, et al. Clinical Implications of a Multiparametric Magnetic Resonance Imaging Based Nomogram Applied to Prostate Cancer Active Surveillance. J Urol [Internet]. 2015. January 26 [cited 2015 Apr 23];193(6):1943–9. Available: http://www.ncbi.nlm.nih.gov/pubmed/25633923 10.1016/j.juro.2015.01.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Partin AW. Time to prostate specific antigen recurrence after radical prostatectomy and risk of prostate cancer specific mortality. J Urol [Internet]. 2006. October [cited 2015 May 1];176(4 Pt 1):1404–8. Available: http://www.ncbi.nlm.nih.gov/pubmed/16952644 [DOI] [PubMed] [Google Scholar]
- 23.Ung JO, Richie JP, Chen M-H, Renshaw AA, D’Amico AV. Evolution of the presentation and pathologic and biochemical outcomes after radical prostatectomy for patients with clinically localized prostate cancer diagnosed during the PSA era. Urology [Internet]. 2002. September [cited 2015 Apr 28];60(3):458–63. Available: http://www.sciencedirect.com/science/article/pii/S0090429502018149 [DOI] [PubMed] [Google Scholar]
- 24.Rais-Bahrami S, Siddiqui MM, Turkbey B, Stamatakis L, Logan J, Hoang AN, et al. Utility of multiparametric magnetic resonance imaging suspicion levels for detecting prostate cancer. J Urol [Internet]. 2013. November [cited 2015 Mar 12];190(5):1721–7. Available: http://www.sciencedirect.com/science/article/pii/S0022534713044170 10.1016/j.juro.2013.05.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Schnall M, Tomaszewski JE, et al. A Multivariate Analysis of Clinical and Pathological Factors that Predict for Prostate Specific Antigen Failure after Radical Prostatectomy for Prostate Cancer. J Urol [Internet]. 1995. July [cited 2015 Apr 30];154(1):131–8. Available: http://www.sciencedirect.com/science/article/pii/S0022534701672483 [PubMed] [Google Scholar]
- 26.Fuchsjäger MH, Shukla-Dave A, Hricak H, Wang L, Touijer K, Donohue JF, et al. Magnetic resonance imaging in the prediction of biochemical recurrence of prostate cancer after radical prostatectomy. BJU Int [Internet]. 2009. August [cited 2015 Apr 30];104(3):315–20. Available: http://www.ncbi.nlm.nih.gov/pubmed/19220263 10.1111/j.1464-410X.2009.08406.x [DOI] [PubMed] [Google Scholar]
- 27.Park JJ, Kim CK, Park SY, Park BK, Lee HM, Cho SW. Prostate cancer: role of pretreatment multiparametric 3-T MRI in predicting biochemical recurrence after radical prostatectomy. AJR Am J Roentgenol [Internet]. 2014. May [cited 2015 Apr 23];202(5):W459–65. Available: http://www.ncbi.nlm.nih.gov/pubmed/24758681 10.2214/AJR.13.11381 [DOI] [PubMed] [Google Scholar]
- 28.D’Amico AV, Whittington R, Malkowicz B, Schnall M, Schultz D, Cote K, et al. Endorectal magnetic resonance imaging as a predictor of biochemical outcome after radical prostatectomy in men with clinically localized prostate cancer. J Urol [Internet]. 2000. September [cited 2015 Apr 26];164(3 Pt 1):759–63. Available: http://www.ncbi.nlm.nih.gov/pubmed/10953141 [DOI] [PubMed] [Google Scholar]
- 29.Thompson IM, Valicenti RK, Albertsen P, Davis BJ, Goldenberg SL, Hahn C, et al. Adjuvant and salvage radiotherapy after prostatectomy: AUA/ASTRO Guideline. J Urol [Internet]. 2013. August [cited 2015 Apr 6];190(2):441–9. Available: http://www.ncbi.nlm.nih.gov/pubmed/23707439 10.1016/j.juro.2013.05.032 [DOI] [PubMed] [Google Scholar]
- 30.Swindle PW, Kattan MW, Scardino PT. Markers and meaning of primary treatment failure. Urol Clin North Am [Internet]. 2003. May [cited 2015 Apr 29];30(2):377–401. Available: http://www.ncbi.nlm.nih.gov/pubmed/12735513 [DOI] [PubMed] [Google Scholar]
- 31.Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, Shakir N, et al. Comparison of MR/Ultrasound Fusion–Guided Biopsy With Ultrasound-Guided Biopsy for the Diagnosis of Prostate Cancer. JAMA [Internet]. 2015. January 27 [cited 2015 Jan 28];313(4):390 Available: http://www.ncbi.nlm.nih.gov/pubmed/25626035 10.1001/jama.2014.17942 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.