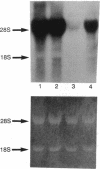
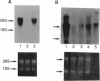
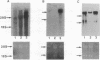
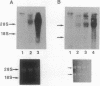
Abstract
We previously reported that mouse NIH 3T3 cells transformed by transfection of activated human c-Ha-ras become apparently normal upon treatment with the antibiotic azatyrosine. The revertant cells maintain their normal phenotype during prolonged culture in the absence of azatyrosine, although activated p21ras is still expressed. The normal phenotype induced by azatyrosine could be due to activation of expression of some cellular gene(s) in the cells that results in suppression of ras function. To identify the genes with increased expression in the revertant cells, we adopted differential screening of recombinants from a phage cDNA library made from mRNA of the revertant cells, hybridized with 32P-labeled cDNAs made from mRNAs of the ras-transformed NIH 3T3 cells and the revertant cells. Two clones thus isolated were found to be almost identical to the ras recision gene (rrg), which was identified as a tumor-suppressor gene by Contente et al. [Contente, S., Kenyon, K., Rimoldi, D. & Friedman, R. M. (1990) Science 249, 796-798]. Other genes identified were the collagen type III and rhoB genes. Approximately half the clones were found to contain a sequence corresponding to that of the murine retrovirus-like intracisternal A particle. We speculate that azatyrosine activates several cellular genes in the ras-transformed cells and that some of these genes, including rrg, act cooperatively to counteract ras function, resulting in reversion of the ras-transformed cells to the normal phenotype.
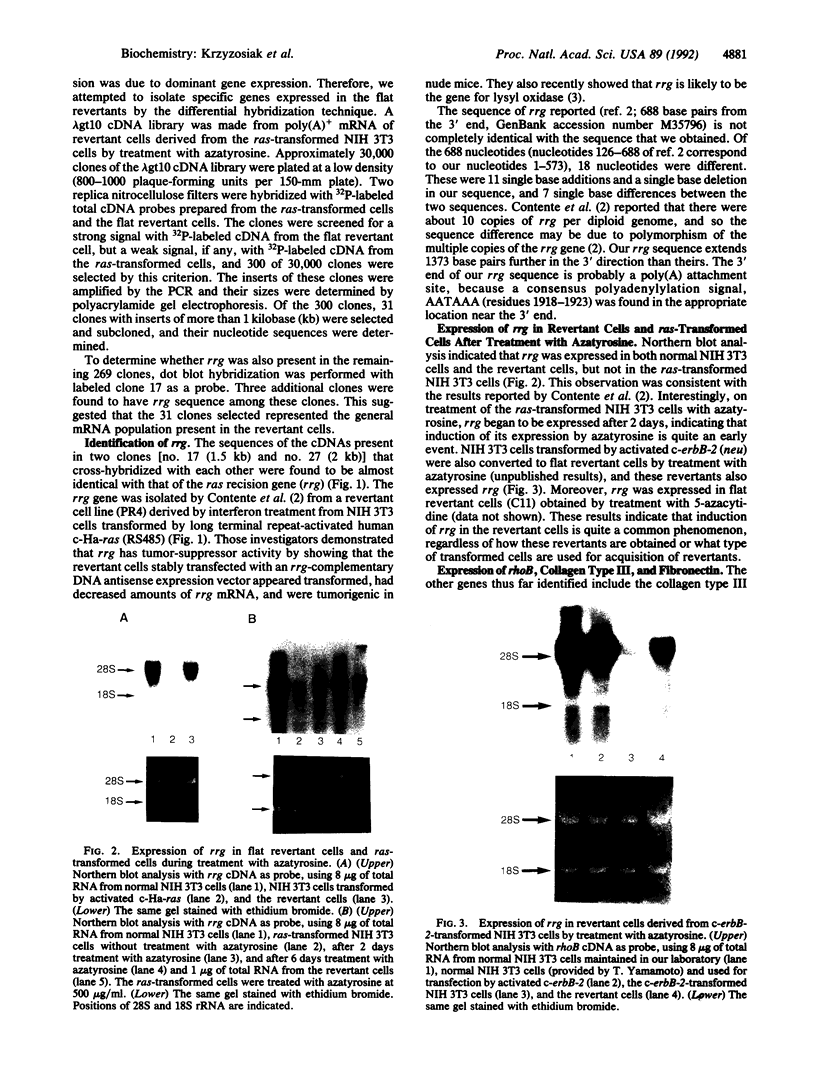
Full text
PDF

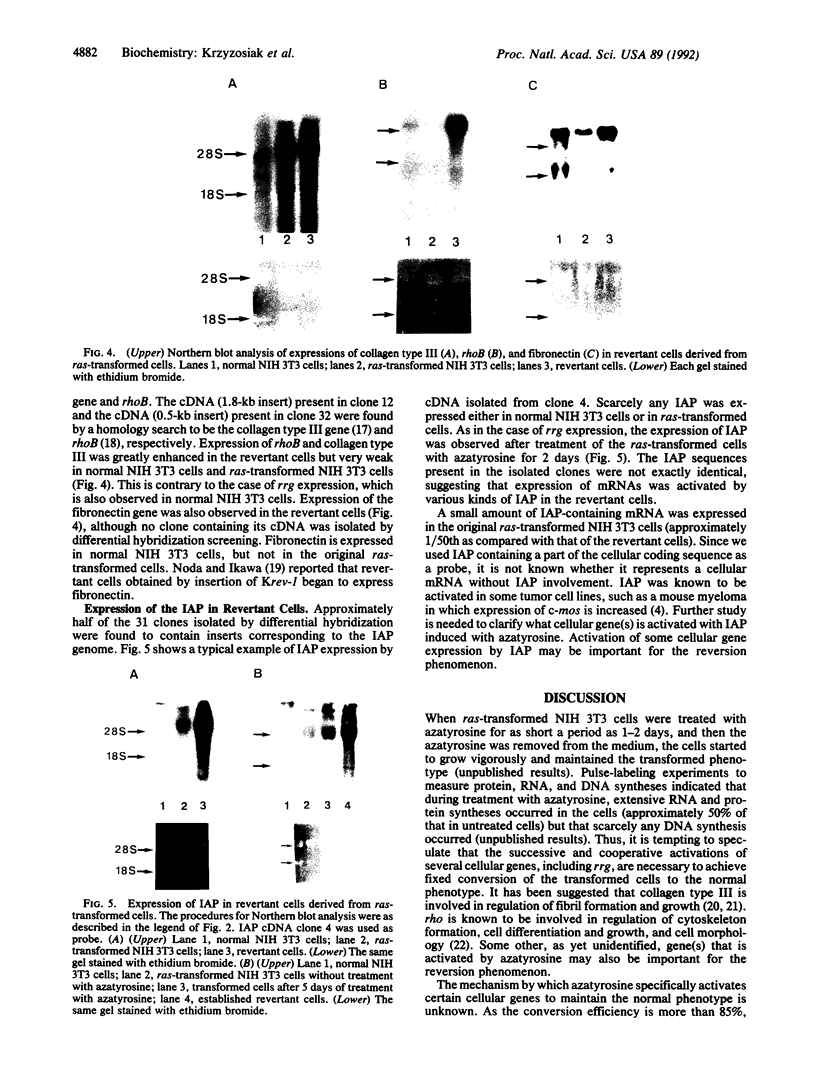

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brattain M. G., Fine W. D., Khaled F. M., Thompson J., Brattain D. E. Heterogeneity of malignant cells from a human colonic carcinoma. Cancer Res. 1981 May;41(5):1751–1756. [PubMed] [Google Scholar]
- Canaani E., Dreazen O., Klar A., Rechavi G., Ram D., Cohen J. B., Givol D. Activation of the c-mos oncogene in a mouse plasmacytoma by insertion of an endogenous intracisternal A-particle genome. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7118–7122. doi: 10.1073/pnas.80.23.7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Contente S., Kenyon K., Rimoldi D., Friedman R. M. Expression of gene rrg is associated with reversion of NIH 3T3 transformed by LTR-c-H-ras. Science. 1990 Aug 17;249(4970):796–798. doi: 10.1126/science.1697103. [DOI] [PubMed] [Google Scholar]
- Drebin J. A., Link V. C., Stern D. F., Weinberg R. A., Greene M. I. Down-modulation of an oncogene protein product and reversion of the transformed phenotype by monoclonal antibodies. Cell. 1985 Jul;41(3):697–706. doi: 10.1016/s0092-8674(85)80050-7. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hatamochi A., Ono M., Ueki H., Namba M. Regulation of collagen gene expression by transformed human fibroblasts: decreased type I and type III collagen RNA transcription. J Invest Dermatol. 1991 Apr;96(4):473–477. doi: 10.1111/1523-1747.ep12470171. [DOI] [PubMed] [Google Scholar]
- Hawley R. G., Shulman M. J., Hozumi N. Transposition of two different intracisternal A particle elements into an immunoglobulin kappa-chain gene. Mol Cell Biol. 1984 Dec;4(12):2565–2572. doi: 10.1128/mcb.4.12.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz M., Luria S., Rechavi G., Givol D. Mechanism of activation of the mouse c-mos oncogene by the LTR of an intracisternal A-particle gene. EMBO J. 1984 Dec 1;3(12):2937–2941. doi: 10.1002/j.1460-2075.1984.tb02235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Shomura T., Tsuruoka T., Ogawa Y., Watanabe H. L-beta-(5-hydroxy-2-pyridyl)-alanine and L-beta-(3-hydroxyureido)-alanine from Streptomyces. Chem Pharm Bull (Tokyo) 1975 Nov;23(11):2669–2677. doi: 10.1248/cpb.23.2669. [DOI] [PubMed] [Google Scholar]
- Kenyon K., Contente S., Trackman P. C., Tang J., Kagan H. M., Friedman R. M. Lysyl oxidase and rrg messenger RNA. Science. 1991 Aug 16;253(5021):802–802. doi: 10.1126/science.1678898. [DOI] [PubMed] [Google Scholar]
- Kuff E. L., Feenstra A., Lueders K., Smith L., Hawley R., Hozumi N., Shulman M. Intracisternal A-particle genes as movable elements in the mouse genome. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1992–1996. doi: 10.1073/pnas.80.7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizusawa S., Nishimura S., Seela F. Improvement of the dideoxy chain termination method of DNA sequencing by use of deoxy-7-deazaguanosine triphosphate in place of dGTP. Nucleic Acids Res. 1986 Feb 11;14(3):1319–1324. doi: 10.1093/nar/14.3.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Selinger Z., Scolnick E. M., Bassin R. H. Flat revertants isolated from Kirsten sarcoma virus-transformed cells are resistant to the action of specific oncogenes. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5602–5606. doi: 10.1073/pnas.80.18.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens R. J., Baralle F. E. Exon structure of the collagen-binding domain of human fibronectin. FEBS Lett. 1986 Aug 18;204(2):318–322. doi: 10.1016/0014-5793(86)80836-5. [DOI] [PubMed] [Google Scholar]
- Paterson H. F., Self A. J., Garrett M. D., Just I., Aktories K., Hall A. Microinjection of recombinant p21rho induces rapid changes in cell morphology. J Cell Biol. 1990 Sep;111(3):1001–1007. doi: 10.1083/jcb.111.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya T., Fushimi M., Hori H., Hirohashi S., Nishimura S., Sugimura T. Molecular cloning and the total nucleotide sequence of the human c-Ha-ras-1 gene activated in a melanoma from a Japanese patient. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4771–4775. doi: 10.1073/pnas.81.15.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba K., Kamata N., Toyoshima K., Yamamoto T. A v-erbB-related protooncogene, c-erbB-2, is distinct from the c-erbB-1/epidermal growth factor-receptor gene and is amplified in a human salivary gland adenocarcinoma. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6497–6501. doi: 10.1073/pnas.82.19.6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo-Okada N., Makabe O., Nagahara H., Nishimura S. Permanent conversion of mouse and human cells transformed by activated ras or raf genes to apparently normal cells by treatment with the antibiotic azatyrosine. Mol Carcinog. 1989;2(3):159–167. doi: 10.1002/mc.2940020309. [DOI] [PubMed] [Google Scholar]
- Yamada H., Sakamoto H., Taira M., Nishimura S., Shimosato Y., Terada M., Sugimura T. Amplifications of both c-Ki-ras with a point mutation and c-myc in a primary pancreatic cancer and its metastatic tumors in lymph nodes. Jpn J Cancer Res. 1986 Apr;77(4):370–375. [PubMed] [Google Scholar]
- Yamada H., Yoshida T., Sakamoto H., Terada M., Sugimura T. Establishment of a human pancreatic adenocarcinoma cell line (PSN-1) with amplifications of both c-myc and activated c-Ki-ras by a point mutation. Biochem Biophys Res Commun. 1986 Oct 15;140(1):167–173. doi: 10.1016/0006-291x(86)91072-7. [DOI] [PubMed] [Google Scholar]
- Yeramian P., Chardin P., Madaule P., Tavitian A. Nucleotide sequence of human rho cDNA clone 12. Nucleic Acids Res. 1987 Feb 25;15(4):1869–1869. doi: 10.1093/nar/15.4.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ymer S., Tucker W. Q., Sanderson C. J., Hapel A. J., Campbell H. D., Young I. G. Constitutive synthesis of interleukin-3 by leukaemia cell line WEHI-3B is due to retroviral insertion near the gene. Nature. 1985 Sep 19;317(6034):255–258. doi: 10.1038/317255a0. [DOI] [PubMed] [Google Scholar]