Abstract
Objective
The epidemiology of post-gastric bypass surgery hypoglycemia (PGBH) is incompletely understood. This study aimed to evaluate the risk of PGBH among nondiabetic patients and associated factors.
Methods
A cohort study of nondiabetic patients who underwent Roux-en-Y gastric bypass (RYGB) was conducted. PGBH was defined by any postoperative record of glucose < 60 mg/dL, diagnosis of hypoglycemia, or any medication use for treatment of PGBH. Kaplan-Meier analysis was used to describe PGBH occurrence, log-rank tests, and Cox regression to examine associated factors.
Results
Of the 1,206 eligible patients, 86% were female with mean age of 43.7 years, mean preoperative BMI of 48.7 kg/m2, and a mean follow-up of 4.8 years. The cumulative incidence of hypoglycemia at 1 and 5 years post-RYGB was 2.7% and 13.3%, respectively. Incidence of PGBH was identified in 158 patients and was associated with lower preoperative BMI (P = 0.048), lower preoperative HbA1c (P = 0.012), and higher 6-month percent of excess body weight loss (%EWL) (P = 0.001). A lower preoperative HbA1c (HR = 1.73, P = 0.0034) and higher 6-month %EWL (HR = 1.96, P = 0.0074) remained independently correlated with increased risk for PGBH in multi-regression analysis.
Conclusions
The 5-year incidence of PGBH among nondiabetic individuals was 13.3% and was associated with a lower preoperative HbA1c and greater weight loss at 6 months following surgery.
Introduction
Bariatric surgery, especially the Roux-en-Y gastric bypass (RYGB), is a highly effective treatment for obesity and its comorbidities, such as diabetes (1–4). With growing evidence supporting the benefits of RYGB and the increased number of these procedures being performed, we are also becoming better aware of its metabolic and nutritional complications.
Unlike hypoglycemia caused by insulinoma or sulfonylurea ingestion, post-gastric bypass hypoglycemia (PGBH) occurs exclusively postprandially starting approximately 6 months or later following surgery (5,6). While thought to be rare, severe PGBH can be life threatening with reports of associated seizure, syncope, and motor vehicle accidents (7,8). The mechanisms underlying PGBH are incompletely understood but may include inappropriate secretion of insulin and gut hormones, increased beta cell response to oral stimuli, increased glucose effectiveness, dysfunction of counter-regulatory hormones such as glucagon, and rapid post-weight loss improvement in insulin sensitivity (7–13). In addition, a recent study suggested reduced rates of insulin clearance as a contributing factor in PGBH (14). Although most cases are successfully treated with low-carbohydrate diet, some cases require medical therapies such as acarbose and diazoxide and sometimes even surgical therapies such as partial or total pancreatectomy, reduction of gastric pouch size, or reversal of gastric bypass (8,15–17).
Reports on the prevalence of PGBH have been limited and contrasting thus far. For example, two large studies based on hospitalization record or self-report of PGBH reported a prevalence less than 1% (18,19). In contrast, smaller studies using hypoglycemic symptoms questionnaire, continuous glucose monitor, or mixed meal tolerance have found that between 10% and 75% of the subjects were affected by PGBH (20–22).
In order to better evaluate the cumulative incidence of PGBH and associated risk factors, we conducted a cohort study of nondiabetic patients who underwent bariatric surgery at Geisinger Medical Center.
The Geisinger bariatric program collects a wide range of pre- and postsurgical data, providing an excellent resource for exploring the incidence of PGBH and its risk factors in greater detail. Our goals in this study were to estimate the incidence of PGBH among nondiabetic patients and to determine factors associated with PGBH.
Methods
This is a single center, retrospective study based on the electronic medical record (EMR) data available within the Geisinger Health Systems (Danville, PA, USA). A clinical registry for patients with obesity and bariatric surgery patients at Geisinger was established in 2004 with regularly scheduled data transfers from its EMR to the registry for longitudinal data collection. The details of the clinical registry and its interface with the EMR have been previously described (23). This study was approved by the Institute Review Boards of the Geisinger Medical Center and was reviewed by the Institute Review Boards of the Johns Hopkins University School of Medicine. All study participants provided written informed consent.
The registry was queried to identify all patients with body mass index (BMI) ≥ 35 kg/m2 who underwent RYGB at Geisinger Medical Center between 2004 and December 2014. From the N = 3097 individuals identified, we excluded patients on diabetes medications (biguanides, sulfonylureas, thiazolidinedione, and/or insulin) or with hemoglobin A1c≥ 6.5%, fasting glucose > 125 mg/dL, or diagnosis of diabetes preoperatively (n = 1,868 excluded). An additional 23 patients were excluded due to a preoperative diagnosis of hypoglycemia. The final analytical sample included 1,206 individuals.
Preoperative data available included demographics, BMI, clinical diagnoses such as diabetes or hypoglycemia, medications, and laboratory data such as hemoglobin A1c, serum glucose, and fasting insulin. Among those with concurrent fasting glucose and fasting insulin data (N = 502), we calculated insulin resistance using the following homeostasis model assessment insulin resistance (HOMA-IR) formula: fasting serum insulin (μU/mL) × fasting plasma glucose (mmol/L)/22.5 (24). During the immediate postoperative period, the percent of excess body weight loss (%EWL) and percent of initial body weight loss (%IBWL) at 6 months after surgery were calculated using methods described elsewhere (25).
We defined PGBH using EMR data, as: 1) measured glucose less than 60 mg/dL, 2) any postoperative outpatient or inpatient encounter with a diagnosis of hypoglycemia as defined by ICD9 code 251.0 (hypoglycemic coma), 251.1 (other specified hypoglycemia), or 251.2 (hypoglycemia, unspecified), or 3) prescription order or active medication use for treatment of PGBH occurring at least 1 month after surgery (acarbose, glucagon, diazoxide, octreotide). Severe hypoglycemia was defined as: any glucose <40 mg/dL, any visit to emergency room or hospitalization due to hypoglycemia, or gastric bypass reversal due to hypoglycemia.
Kaplan-Meier analysis was used to estimate the cumulative incidence of PGBH. Kaplan-Meier analysis and log-rank tests were used to determine if the risk of hypoglycemia was associated with age, sex, preoperative BMI, preoperative hemoglobin A1c, HOMA-IR, initial 6-month %EWL, and/or 6-month %IBWL after RYGB surgery. For this analysis, continuous variables were categorized according to known clinical cutoff values (i.e., preoperative BMI), or according to distribution tertiles if clinical cutoffs are unknown (HOMA-IR, %EWL, %IBWL), or a combination of clinical cutoffs and distribution tertiles (i.e., hemoglobin A1c). Cox regression was used to determine if preoperative characteristics were independently associated with PGBH after adjusting for age, sex, and preoperative BMI. Due to the small number of severe hypoglycemia cases, we only present an analysis for the outcome of any hypoglycemia. SAS version 9.4 was used for statistical analysis and P values < 0.05 were considered significant.
Results
Among the entire study cohort (N = 3,097), we limited our analysis to the 1,206 (39%) individuals without pre-existing diabetes and without a preoperative diagnosis of hypoglycemia. The mean age was 43.7 years and 86% were female (Table 1). Mean follow-up for the entire study cohort was 4.8 years (range: <0.1–11.2 years).
TABLE 1.
Baseline characteristics of 1,206 nondiabetic patients who underwent gastric bypass at Geisinger Medical Center
| Mean (SD) [Range] |
Overall population, N = 1,206 | Incident hypoglycemia, N = 158 | No hypoglycemia, N = 1,048 | P value |
|---|---|---|---|---|
| Age | 43.7 (10.5) [18, 72] |
44.0 (10.1) [18, 68] |
43.6 (10.6) [19, 72] |
0.665a |
| Sex | ||||
| Female % (n) | 86% (n = 1,032) | 91% (n = 139) | 85% (n = 893) | 0.076b |
| Male % (n) | 14% (n = 174) | 9% (n = 15) | 15% (n = 159) | |
| Preoperative BMI | 48.7 (8.2) [35.0, 94.3] |
47.1 (7.3) [35.0, 75.0] |
49.0 (8.3) [35.0, 94.3] |
0.0094a |
| Preoperative HbA1c | 5.6 (0.4) [4.1, 6.4] |
5.5 (0.3) [4.4, 6.4] |
5.6 (0.4) [4.1, 6.4] |
0.0010a |
| Follow-up time (years) | 4.8 (2.8) [<0.1, 11.2] |
6.1 (2.3) [0.9, 11.2] |
4.6 (2.8) [<0.1, 11.1] |
<0.0001a |
| 6-month postsurgical EWL (%)* | 68.5 (18.3) [22.0, 147.9] |
72.4 (19.1) [29.3, 147.9] |
67.9 (18.1) [22.0, 142.3] |
0.0040a |
| 6-month postsurgical IBWL (%)* | 29.7 (5.5) [7.2, 51.1] |
30.1 (5.8) [13.8, 51.1] |
29.6 (5.5) [7.2, 47.4] |
0.301a |
| Pre-op HOMA-IR* | 4.3 (2.8) [0.5, 31.7] |
4.3 (2.7) [0.9, 15.9] |
4.3 (2.8) [0.5, 31.7] |
0.948a |
HOMA-IR was available for 502 patients (58 with hypoglycemia and 444 without hypoglycemia). 6-month %EWL and 6-month %IBWL were available for 1,186 patients (158 with hypoglycemia and 1,028 without hypoglycemia).
Two-sample t-test.
Chi-square test.
BMI: body mass index; HOMA-IR: homeostasis model assessment insulin resistance; EWL: excess weight loss; IBWL: initial body weight loss.
Of the 1,206 patients, 158 patients (13.1%) met at least one of the following criteria for hypoglycemia after surgery: diagnosis of hypoglycemia (N = 92), postoperative glucose less than 60 mg/dL (N = 77), new use of medications associated with PGBH such as acarbose and diazoxide (N = 23 total including 9 with octreotide, 8 with acarbose, 5 with glucagon, and 1 with a combination of diazoxide, acarbose, and glucagon). We did not find any patient who underwent revision of bariatric surgery due to hypoglycemia. Most patients (n = 127, 80%) qualified by meeting only a single criterion (Table 2). Of the 158 patients identified with hypoglycemia, 8 (5%) met at least one of the following two criteria for severe hypoglycemia: glucose <40 (N = 7) or emergency room or hospitalization due to hypoglycemia (Table 2).
TABLE 2.
Criteria used to identify 158 patients with post-gastric bypass hypoglycemia (PGBH)
| Number of PGBH cases | Number of severe cases | |
|---|---|---|
| Patients identified by one criterion (N = 127) | ||
| Diagnosis only | ||
| ICD9 251.2 | 55 | 0 |
| ICD9 251.1 | 6 | 0 |
| ICD9 251.1 and 251.2 | 4 | 0 |
| ICD9 251.0 and 251.2 | 1 | 0 |
| Glucose<60 only | 54 | 4 |
| Medication only | ||
| Octreotide | 4 | 0 |
| Glucagon | 3 | 0 |
| Patients identified by two criteria (N = 28) | ||
| Diagnosis and glucose<60 | ||
| ICD9 251.2 and glucose<60 | 14 | 2 |
| ICD9 251.1 and 251.2 and glucose<60 | 1 | 0 |
| Diagnosis and medication | ||
| ICD9 251.2 and acarbose | 6 | 0 |
| ICD9 251.1 and acarbose | 1 | 0 |
| ICD9 251.1 and 251.2 and glucagon | 1 | 0 |
| Glucose<60 and medication | ||
| Glucose<60 and octreotide | 5 | 1 |
| Patients identified by three criteria (N = 3) | ||
| Diagnosis and glucose<60 and medication | ||
| ICD9 251.2 and glucose<60 and glucagon | 1 | 0 |
| ICD9 251.2 and glucose<60 and acarbose | 1 | 0 |
| ICD9 251.1 and 251.2 and glucose<60 and glucagon and acarbose and diazoxide | 1 | 1 |
| TOTAL | 158 | 8 |
The lost to follow-up rate was calculated by the number of patients who underwent bariatric surgery within the period of interest and have missing follow-up data divided by the number of patients who underwent bariatric surgery and eligible for further follow-up (i.e., patients who underwent RYGB at least 1 month ago and without evidence of PGBH by the end of the period of interest). We found an increasing rate of lost to follow-up: 1.1% at Year 1, 11.4% at Year 2, 20.2% at Year 3, 24.5% at Year 4 and 27.9% at Year 5. At their last follow-up (Year 5), the 158 cases in the hypoglycemia group had a mean BMI of 31.5 kg/m2. This is similar to the 1,052 patients without evidence of hypoglycemia (mean = 33.9 kg/m2 at their last follow-up).
The Kaplan-Meier estimate of the cumulative incidence for any hypoglycemia occurrence at 1, 3, and 5 years post-gastric bypass surgery was 2.7%, 10.0%, and 13.3%, respectively. The estimates for severe hypoglycemia were relatively low (0.1% at 1-year, 0.3% at 3-years, and 0.7% at 5-years). The Kaplan-Meier curves demonstrating the interval until the occurrence of postoperative hypoglycemia (any and severe) are shown in Figure 1.
Figure 1.
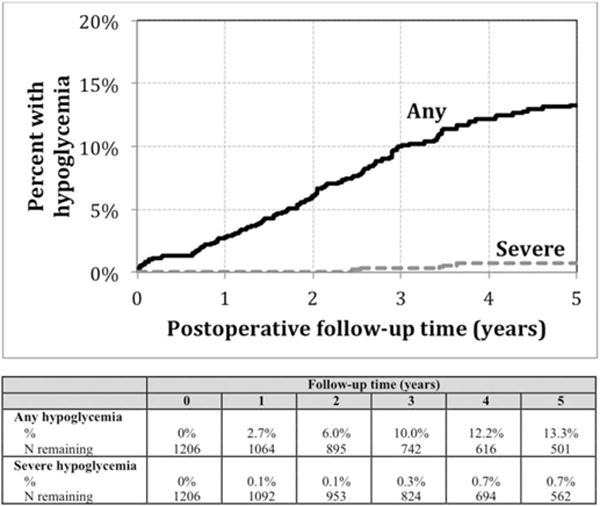
Kaplan-Meier analysis of incidence of post-gastric bypass hypoglycemia from 0–5 years after surgery.
Log-rank analysis indicated that the time until hypoglycemia occurrence was not associated with age (P = 0.816), sex (P = 0.223), HOMA-IR (P = 0.926), or %IBWL (P = 0.369) (Table 3). In contrast, in this univariate analyses, lower preoperative hemoglobin A1c (P = 0.012, Figure 2A), lower preoperative BMI (P = 0.048; Figure 2B), and higher 6-month %EWL (P = 0.001; Figure 2C) were associated with increased risk of any hypoglycemia. In multivariate analyses, lower preoperative hemoglobin A1c and higher 6-month %EWL were again significantly associated with increased risk of any hypoglycemia: after adjusting for age, sex, and preoperative BMI, there was an increased risk for PGBH associated with lower preoperative hemoglobin A1c (hazard ratio 1.73, CI:1.20, 2.50, P = 0.0034, when A1c of <5.5% compared to A1c between 5.7% and 6.4%) and largest 6-month %EWL (hazard ratio 1.96, CI: 1.20, 3.20, P = 0.0074, when EWL of ≥75% compared to <60%). On the other hand, lower preoperative BMI was no longer associated with increased risk of any hypoglycemia after adjusting for age, sex, preoperative hemoglobin A1c, and 6-month %EWL (Table 4).
TABLE 3.
Kaplan-Meier estimates of hypoglycemia at 1, 3, and 5-years by baseline characteristics
| N | % any hypoglycemia
|
Log-rank P value | |||
|---|---|---|---|---|---|
| 1 year (%) | 3 year (%) | 5 year (%) | |||
| Age | |||||
| 18–39 | 445 | 2.4 | 8.6 | 12.9 | 0.816 |
| 40–54 | 560 | 3.3 | 11.0 | 13.1 | |
| 55+ | 201 | 1.5 | 10.2 | 14.8 | |
| Sex | |||||
| Male | 174 | 3.0 | 9.4 | 10.5 | 0.223 |
| Female | 1,032 | 2.6 | 10.1 | 13.7 | |
| Pre-op BMI | |||||
| 35–39 | 98 | 5.2 | 16.8 | 20.6 | 0.048 |
| 40–49 | 674 | 3.1 | 10.2 | 13.4 | |
| 50–59 | 319 | 1.9 | 9.2 | 12.7 | |
| 60+ | 115 | 0.0 | 5.4 | 8.0 | |
| Pre-op HbA1c | |||||
| <5.5 | 415 | 4.1 | 13.5 | 15.9 | 0.012 |
| 5.5–5.6 | 260 | 2.0 | 9.9 | 14.6 | |
| 5.7–6.4 | 531 | 2.0 | 7.4 | 10.7 | |
| Pre-op HOMA-IR* | |||||
| <3.0 | 176 | 4.8 | 10.5 | 14.2 | 0.926 |
| 3.0–4.6 | 171 | 3.1 | 10.2 | 12.2 | |
| 4.7+ | 155 | 1.4 | 12.0 | 13.5 | |
| 6-month EWL* | |||||
| <60% | 399 | 1.8 | 6.7 | 9.9 | 0.0010 |
| 60–74% | 403 | 1.9 | 10.1 | 12.2 | |
| 75%+ | 384 | 4.6 | 13.6 | 18.5 | |
| 6-month IBWL* | |||||
| <27% | 386 | 2.1 | 7.7 | 12.4 | 0.369 |
| 27%–31% | 419 | 3.2 | 10.9 | 13.6 | |
| 32%+ | 381 | 2.8 | 11.6 | 14.2 | |
HOMA-IR was available for 502 patients (58 with hypoglycemia and 444 without hypoglycemia). 6-month EWL was available for 1,186 patients (158 with hypoglycemia and 1,028 without hypoglycemia).
BMI: body mass index; HOMA-IR: homeostasis model assessment insulin resistance; EWL: excess weight loss; IBWL: initial body weight loss.
Figure 2.
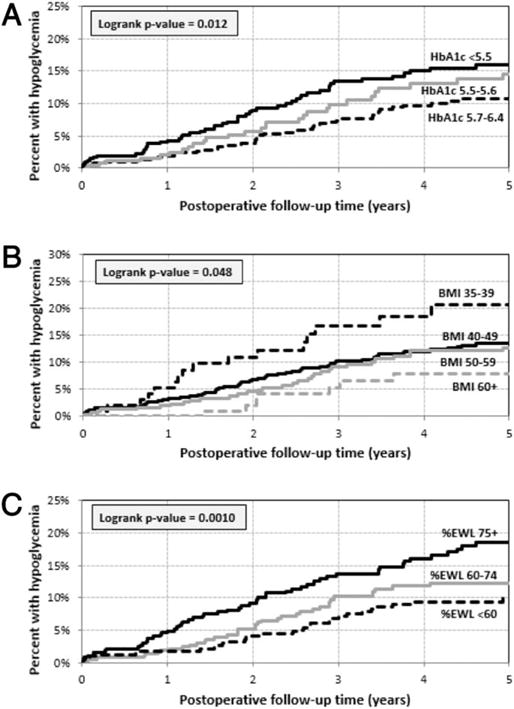
Percent with post-gastric bypass hypoglycemia by (A) hemoglobin A1c categories, (B) BMI categories, and (C) 6-month %EWL categories.
TABLE 4.
Cox regression results for presence of any hypoglycemia using preoperative characteristics and postoperative 6-month weight loss
| HR | 95% CI | P value | |
|---|---|---|---|
| HbA1c | |||
| <5.5 | 1.73 | [1.20, 2.50] | 0.0034 |
| 5.5–5.6 | 1.37 | [0.89, 2.11] | 0.148 |
| 5.7–6.4 | Reference | ||
| 6-month %EWL | |||
| <60% | Reference | ||
| 60–74% | 1.38 | [0.87, 2.19] | 0.170 |
| 75%+ | 1.96 | [1.20, 3.20] | 0.0074 |
| Age | |||
| 18–39 | Reference | ||
| 40–54 | 1.20 | [0.84, 1.72] | 0.320 |
| 55+ | 1.24 | [0.75, 2.04] | 0.400 |
| Sex | |||
| Male | Reference | ||
| Female | 1.23 | [0.74, 2.05] | 0.430 |
| BMI | |||
| 35–39 | 1.39 | [0.57, 3.39] | 0.468 |
| 40–49 | 1.03 | [0.49, 2.17] | 0.935 |
| 50–59 | 1.10 | [0.54, 2.25] | 0.799 |
| 60+ | Reference |
EWL: excess weight loss; BMI: body mass index.
Discussion
In a study of 1,206 individuals without diabetes undergoing RYGB surgery, we found incidence of PGBH of 2.7% and 13.3% at 1 and 5 years, respectively. We found fewer cases when we limited our hypoglycemia cases to severe hypoglycemia as defined by glucose lower than 40 mg/dL or requiring emergency room visit or hospitalization; the incidence of severe PGBH was 0.1% and 0.7% at 1 and 5 years after surgery, respectively. Low preoperative hemoglobin A1c (<5.5%) and increased 6-month weight loss (≥ 75% EWL) were significantly and independently associated with increased risk of any hypoglycemia after gastric bypass surgery.
Marsk et al. (n = 5,040 of which 1,013 had pre-existing diabetes) used inpatient hospitalization ICD codes without further details such as laboratory data or outpatient records to define PGBH. After a median follow-up period of 2.7 years, the study’s estimated incidence of PGBH was 0.2%, which is similar to our data on the incidence of severe PGBH (18). Sarwar et al. (n = 145,500) used self-reported data to define PGBH and estimated a 0.02% incidence of PGBH among patients not on diabetes medications or insulin prior to surgery (n = 145,500) (19). These findings match the low incidence of severe hypoglycemia in our study, thus suggesting that severe hypoglycemia associated with neuroglycemic symptoms and requirement for assistance is rare after bariatric surgery.
In contrast, nonsevere hypoglycemia may be more common as suggested by more recent studies and the results of our study. In our previous survey study (n = 450 of which 36% had preexisting diabetes), we found that up to a third of patients who underwent bariatric surgery reported symptoms of hypoglycemia (20). A study by Abrahamsson et al. (N = 30) observed that post-gastric bypass patients (mean preoperative hemoglobin A1c 5.4%) spent more time in hypoglycemia as defined by glucose less than 60 mg/dL (2.9% compared to none in nondiabetic overweight controls) as captured by continuous glucose monitoring during three days of normal activities (26). In addition, Kefurt and colleagues observed hypoglycemic episodes in 30 out of 40 asymptomatic post-RYGB patients who underwent continuous glucose monitoring during 5 days of normal activities (21). Furthermore, we observed in our study cohort that the number of PGBH cases increase from 13.1% to 16.9% at 5 years if we use a broader definition of PGBH used in previous studies by including any inpatient or emergency department visit due to syncope or confusion or any record of postoperative outpatient or inpatient visit due to dumping syndrome (18). These studies together with our findings suggest that PGBH is likely under-recognized and underestimated.
While the clinical implication of severe hypoglycemia after bariatric surgery is clear (e.g., syncope, seizure, death), it is less clear for nonsevere hypoglycemia. However, emerging evidence suggests an adverse impact of nonsevere and asymptomatic hypoglycemia; a recent study demonstrated a link between asymptomatic hypoglycemia and increased susceptibility to cardiac arrhythmia among patients with diabetes (27). Another study showed that insulin-induced hypoglycemia (glucose < 40 mg/dL) in healthy adults correlated with increased proinflammatory cytokines, markers of lipid peroxidation, reactive oxygen species, and leukocytosis (28). Given that the long-term effect of bariatric surgery on all-cause and cardiovascular mortality appears favorable, nonsevere hypoglycemia is less likely to be contributing to mortality outcomes after bariatric surgery (29). However, further studies on the clinical impact of nonsevere hypoglycemia may be warranted on other outcomes of bariatric surgery such as long-term diabetes complications, quality of life, and weight maintenance.
To our knowledge, this is the first study that demonstrated a strong, independent correlation between lower preoperative hemoglobin A1c and greater weight loss at 6 months after surgery with increased risk for PGBH among those without diabetes. In contrast, a lower preoperative BMI was associated with increased risk for PGBH in our initial univariate analysis but was no longer statistically significant (and showed an attenuated effect size) after adjustment for age, sex, preoperative hemoglobin A1c, and 6-month %EWL, suggesting that the effect of lower preoperative BMI on PGBH was confounded by these other clinical factors. A lower preoperative hemoglobin A1c may be a surrogate marker of normal beta cell function compared to those with a higher preoperative hemoglobin A1c in the prediabetic range. This finding agrees with the previous study that reported an increased risk for PGBH in the absence of pre-existing diabetes, suggesting a normal beta cell function at baseline as a potential risk factor for PGBH (20). Also, a more rapid loss of weight after surgery may increase the risk of PGBH by unmasking the preoperative beta cell hyperfunction or hypertrophy developed with prior obesity. However, beta cell hypertrophy as the underlying mechanism behind PGBH is controversial (8,11). Interestingly, there was no association between preoperative insulin resistance as measured by HOMA-IR and the risk of PGBH among a subset of patients who had preoperative HOMA-IR measured (42%) possibly due to insufficient sample size. Further studies are needed to define the characteristics of patients at an increased risk of PGBH.
The strength of this study lies in the use of the large, comprehensive, longitudinal database of the Geisinger Healthcare System, which provides extensive clinical data pre-and post-gastric bypass surgery as well as sufficient long-term follow-up needed to identify PGBH and its risk factors. The limitations include the use of EHR-based algorithm to identify PGBH cases, which limits our ability to identify PGBH cases to care received at Geisinger Medical Center. It is also plausible that we may have under-or overestimated the number or seriousness of PGBH cases given the lack of individual chart reviews, unknown timing of the blood glucose measurement in relationship to food, and lack of correlation with any symptoms of hypoglycemia, or dynamic glucose measurements such as continuous glucose monitor or meal tolerance tests. However, we evaluated various clinical factors such as ICD-9 codes associated with PGBH in the context of visits to the emergency room, outpatient clinic or hospitalization as well as clinical data including laboratory measurements of serum glucose and new medication data following the surgery over a median follow-up period of 4.5 years, which provides a unique, detailed view into PGBH in a large cohort. Our follow-up data were limited by an increasing number of individuals lost to follow-up, which in turn could lead to selection bias. Our HOMA-IR data were limited to 502 (42%) individuals who had fasting glucose, and fasting insulin measurements.
In conclusion, post-bariatric surgery hypoglycemia may occur more frequently than previously thought, affecting up to one in eight non-diabetic patients at 5 years after surgery. A lower preoperative hemoglobin A1c and faster weight loss after surgery conferred an increased risk for post-bariatric surgery hypoglycemia, suggesting the contribution of normal presurgical beta cell function and potential improvement in insulin sensitivity following more rapid postsurgical weight loss in development of PGBH. Further studies are needed to confirm these findings in a prospective study that utilizes frequent, dynamic blood glucose measurements such as continuous glucose monitoring before and after bariatric surgery with a longer follow-up period. In addition, a better tool to predict an individual’s risk of developing post-bariatric surgery hypoglycemia would be clinically helpful. Ultimately, a better understanding of the epidemiology and mechanism of post-bariatric surgery hypoglycemia and its risk factors will enhance the ability of clinicians to better screen and counsel patients prior to bariatric surgery to minimize their risk of developing post-bariatric surgery hypoglycemia.
Acknowledgments
We are grateful for the extraordinary cooperation of the patients enrolled in the Geisinger bariatric surgery program without which these studies would not have been possible.
Funding agencies: This study was partially supported by Grant Number KL2 (5KL2TR001077-02, CJL) as part of the Institute for Clinical and Translational Research Grant from the NIH/NCAT and K24AI120834 (TTB).
Footnotes
Disclosure: The authors declared no conflict of interest.
Author contributions: CJL, TTB, and JMC conceived and carried out the study. GCW, ML, and PB conceived the study and analyzed data. All authors were involved in writing the article and had final approval of the submitted and published versions.
References
- 1.Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248–256.e5. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 2.Sjostrom L. Bariatric surgery and reduction in morbidity and mortality: experiences from the SOS study. Int J Obes (Lond) 2008;32:S93–S97. doi: 10.1038/ijo.2008.244. [DOI] [PubMed] [Google Scholar]
- 3.Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes–3-year outcomes. N Engl J Med. 2014;370:2002–2013. doi: 10.1056/NEJMoa1401329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Courcoulas AP, Belle SH, Neiberg RH, et al. Three-year outcomes of bariatric surgery vs lifestyle intervention for type 2 diabetes mellitus treatment: a randomized clinical trial. JAMA Surg. 2015;150:931–940. doi: 10.1001/jamasurg.2015.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tack J, Arts J, Caenepeel P, De Wulf D, Bisschops R. Pathophysiology, diagnosis and management of postoperative dumping syndrome. Nat Rev Gastroenterol Hepatol. 2009;6:583–590. doi: 10.1038/nrgastro.2009.148. [DOI] [PubMed] [Google Scholar]
- 6.Won JG, Tseng HS, Yang AH, et al. Clinical features and morphological characterization of 10 patients with noninsulinoma pancreatogenous hypoglycaemia syndrome (NIPHS) Clin Endocrinol (Oxf) 2006;65:566–578. doi: 10.1111/j.1365-2265.2006.02629.x. [DOI] [PubMed] [Google Scholar]
- 7.Service GJ, Thompson GB, Service FJ, Andrews JC, Collazo-Clavell ML, Lloyd RV. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med. 2005;353:249–254. doi: 10.1056/NEJMoa043690. [DOI] [PubMed] [Google Scholar]
- 8.Patti ME, McMahon G, Mun EC, et al. Severe hypoglycaemia post-gastric bypass requiring partial pancreatectomy: evidence for inappropriate insulin secretion and pancreatic islet hyperplasia. Diabetologia. 2005;48:2236–2240. doi: 10.1007/s00125-005-1933-x. [DOI] [PubMed] [Google Scholar]
- 9.Goldfine AB, Mun EC, Devine E, et al. Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. J Clin Endocrinol Metab. 2007;92:4678–4685. doi: 10.1210/jc.2007-0918. [DOI] [PubMed] [Google Scholar]
- 10.Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis. 2007;3:597–601. doi: 10.1016/j.soard.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meier JJ, Butler AE, Galasso R, Butler PC. Hyperinsulinemic hypoglycemia after gastric bypass surgery is not accompanied by islet hyperplasia or increased beta-cell turnover. Diabetes Care. 2006;29:1554–1559. doi: 10.2337/dc06-0392. [DOI] [PubMed] [Google Scholar]
- 12.Cummings DE. Endocrine mechanisms mediating remission of diabetes after gastric bypass surgery. Int J Obes (Lond) 2009;33:S33–S40. doi: 10.1038/ijo.2009.15. [DOI] [PubMed] [Google Scholar]
- 13.Patti ME, Li P, Goldfine AB. Insulin response to oral stimuli and glucose effectiveness increased in neuroglycopenia following gastric bypass. Obesity (Silver Spring) 2015;23:798–807. doi: 10.1002/oby.21043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salehi M, Gastaldelli A, D’Alessio DA. Altered islet function and insulin clearance cause hyperinsulinemia in gastric bypass patients with symptoms of postprandial hypoglycemia. J Clin Endocrinol Metab. 2014;99:2008–2017. doi: 10.1210/jc.2013-2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kellogg TA, Bantle JP, Leslie DB, et al. Post-Gastric bypass hyperinsulinemic hypoglycemia syndrome: characterization and response to a modified diet. Surg Obes Relat Dis. 2008;4:492–499. doi: 10.1016/j.soard.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Clancy TE, Moore FD, Jr, Zinner MJ. Post-gastric bypass hyperinsulinism with nesidioblastosis: subtotal or total pancreatectomy may be needed to prevent recurrent hypoglycemia. J Gastrointest Surg. 2006;10:1116–1119. doi: 10.1016/j.gassur.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez-Esparrach G, Lautz DB, Thompson CC. Peroral endoscopic anastomotic reduction improves intractable dumping syndrome in Roux-en-Y gastric bypass patients. Surg Obes Relat Dis. 2010;6:36–40. doi: 10.1016/j.soard.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Marsk R, Jonas E, Rasmussen F, Naslund E. Nationwide cohort study of post-gastric bypass hypoglycaemia including 5,040 patients undergoing surgery for obesity in 1986–2006 in sweden. Diabetologia. 2010;53:2307–2311. doi: 10.1007/s00125-010-1798-5. [DOI] [PubMed] [Google Scholar]
- 19.Sarwar H, Chapman WH, 3rd, Pender JR, et al. Hypoglycemia after Roux-en-Y gastric bypass: the BOLD experience. Obes Surg. 2014;24:1120–1124. doi: 10.1007/s11695-014-1260-8. [DOI] [PubMed] [Google Scholar]
- 20.Lee CJ, Clark JM, Schweitzer M, et al. Prevalence of and risk factors for hypoglycemic symptoms after gastric bypass and sleeve gastrectomy. Obesity (Silver Spring) 2015;23:1079–1084. doi: 10.1002/oby.21042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kefurt R, Langer FB, Schindler K, Shakeri-Leidenmuhler S, Ludvik B, Prager G. Hypoglycemia after Roux-en-Y gastric bypass: detection rates of continuous glucose monitoring (CGM) versus mixed meal test. Surg Obes Relat Dis. 2015;11:564–569. doi: 10.1016/j.soard.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Pigeyre M, Vaurs C, Raverdy V, Hanaire H, Ritz P, Pattou F. Increased risk of OGTT-induced hypoglycemia after gastric bypass in severely obese patients with normal glucose tolerance. Surg Obes Relat Dis. 2014 doi: 10.1016/j.soard.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Wood GC, Chu X, Manney C, et al. An electronic health record-enabled obesity database. BMC Med Inform Decis Mak. 2012;12:45. doi: 10.1186/1472-6947-12-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 25.Still CD, Wood GC, Chu X, et al. Clinical factors associated with weight loss outcomes after Roux-en-Y gastric bypass surgery. Obesity (Silver Spring) 2014;22:888–894. doi: 10.1002/oby.20529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abrahamsson N, Eden Engstrom B, Sundbom M, Karlsson FA. Hypoglycemia in everyday life after gastric bypass and duodenal switch. Eur J Endocrinol. 2015;173:91–100. doi: 10.1530/EJE-14-0821. [DOI] [PubMed] [Google Scholar]
- 27.Chow E, Bernjak A, Williams S, et al. Risk of cardiac arrhythmias during hypoglycemia in patients with type 2 diabetes and cardiovascular risk. Diabetes. 2014;63:1738–1747. doi: 10.2337/db13-0468. [DOI] [PubMed] [Google Scholar]
- 28.Razavi Nematollahi L, Kitabchi AE, Stentz FB, et al. Proinflammatory cytokines in response to insulin-induced hypoglycemic stress in healthy subjects. Metabolism. 2009;58:443–448. doi: 10.1016/j.metabol.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 29.Kwok CS, Pradhan A, Khan MA, et al. Bariatric surgery and its impact on cardiovascular disease and mortality: a systematic review and meta-analysis. Int J Cardiol. 2014;173:20–28. doi: 10.1016/j.ijcard.2014.02.026. [DOI] [PubMed] [Google Scholar]


