Abstract
Intellectual disability is genetically heterogeneous, and it is likely that many of the responsible genes have not yet been identified. We describe three siblings with isolated, severe developmental encephalopathy. After extensive uninformative genetic and metabolic testing, whole exome sequencing identified a homozygous novel variant in glutamic pyruvate transaminase 2 (GPT2) or alanine transaminase 2 (ALT2), c.459 C>G p.Ser153Arg that segregated with developmental encephalopathy in the family. This variant was predicted to be damaging by all in silico prediction algorithms. GPT2 is the gene encoding ALT2 which is responsible for the reversible transamination of alanine and 2-oxoglutarate to form pyruvate and glutamate. GPT2 is expressed in brain and is in the pathway to generate glutamate, an excitatory neurotransmitter. Functional assays of recombinant wild-type and mutant ALT2 proteins demonstrated the p.Ser153Arg mutation resulted in a severe loss of enzymatic function. We suggest that recessively inherited loss of function GPT2 mutations are a novel cause of intellectual disability.
Introduction
Intellectual disability (ID) affects 3 % of the general population (Daily et al 2000) and is defined as an intelligence quotient (IQ) less than 70. The etiology of ID is heterogeneous, and includes genetic, teratogenic, and developmental causes (Kaufman et al 2010). The underlying etiology of ID cannot be determined in 75–80 % of mild ID and in 20–50 % of severe ID (Utine et al 2014). The cause of ID is thought to be genetic in 25–50 % of cases, although this percentage increases with ID severity (Kaufman et al 2010). Approximately 15 % of ID is attributable to cytogenetically visible abnormalities (Topper et al 2011), and copy number variants (CNVs) are estimated to account for ~10 % of ID (Kaufman et al 2010). Identification of critical pathogenic CNVs regions has led to the detection of mutations in single genes responsible for syndromic and non-syndromic ID (Ellison et al 2013). Genetic studies have identified more than 450 single candidate genes for Mendelian forms of ID (Kaufman et al 2010). Homozygosity mapping in consanguineous families with multiple affected offspring was used in one study of 136 families to identify homozygous mutations in 23 genes associated with ID and/or other neurologic disorders, as well as possibly disease-causing variants in 50 novel candidate genes (Najmabadi et al 2011).
Exome sequencing provides the most comprehensive approach to genetic evaluation of ID because it provides the ability to interrogate known genes for ID as well as novel genes not previously implicated in ID. We describe three similarly affected siblings with isolated severe ID who were found to have a homozygous novel variant in glutamic pyruvate transaminase 2 (GPT2) and demonstrate that this amino acid substitution is a loss of function mutation. GPT2 is a pyridoxal dependent enzyme expressed in brain and muscle and catalyzes the reversible transamination between alanine and 2-oxoglutarate to form pyruvate and glutamate. GPT2 has not been previously associated with any human disease. This family study demonstrates the strength of exome sequencing as a powerful tool for genetic diagnosis of neurodevelopmental disorders.
Case presentation
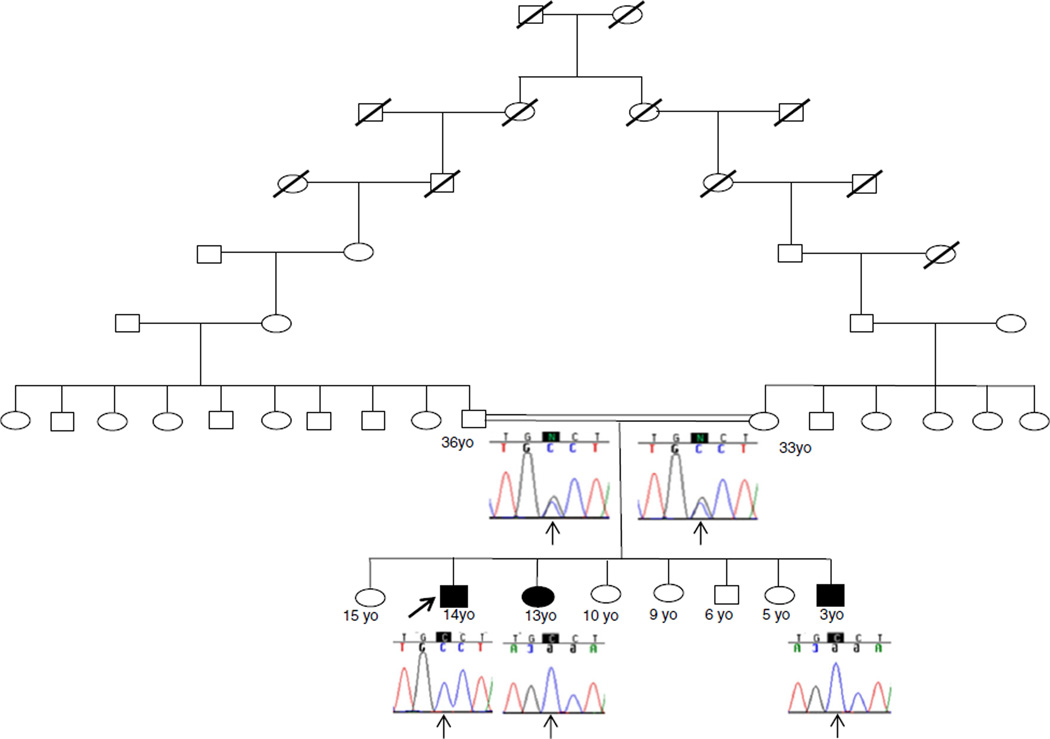
We describe a distantly consanguineous Mizrahi Jewish family (parents share great, great, great, great grandparents) with three similarly affected siblings, two males and one female, with static encephalopathy associated with severe ID (Fig. 1).
Fig. 1.
Family pedigree. Shaded symbols indicate affected individuals. Electropherograms show the GPT2 c.459C>G genotype
The proband and oldest affected sibling is a 15 year old boy, born full term by vaginal delivery with birth weight of 3.6 kg (50 %) and no prenatal complications (Table 1). He smiled at 4 months of age, rolled over at 6 months of age, reached for objects at 8 months of age, sat without support at 18 months of age and walked at 3 years of age. He never talked and does not use signs. During his first year of life, neurological examination revealed pronounced motor delay and extreme hypotonia. At 2 years old, his weight was 10.4 kg (5 %), height was 82 cm (10 %), and head circumference was 44 cm (<3 %). On physical examination he was found to have mildly dysmorphic features with bifrontal narrowing and microcephaly, hypotelorism and low set ears and an abnormal neurological exam with hypotonia with brisk reflexes, ankle clonus and bilateral extensor plantar responses, and wide based ataxic gait and ataxic movements of axial muscles and limbs. He had inverted feet bilaterally and sialorrhea. A series of genetic and metabolic tests were performed, all of which were normal including karyotype, subtelomeric FISH, FISH for Angelman syndrome/Prader-Willi, fragile X, quantitative plasma amino acids, urine organic acids, urine purines and pyrimidines, urine oligosaccharides, carbohydrate deficient transferrin, quantitative cerebrospinal fluid (CSF) amino acids, CSF neurotransmitter metabolites (3-O-methyldopa, 5-Hydroxyindolacetic acid, and homovanillic acid, neopterin and tetrahydrobiopterin). He began having generalized tonic-clonic seizures at 10 years of age and was treated with divalproex sodium and levetiracetam. Electroencephalogram (EEG) was abnormal showing mild-to-moderate diffuse slowing (a non-specific indicator of diffuse cerebral dysfunction), with frequent epileptiform discharges in the left frontal region. Computed tomography (CT) of the brain was normal. His current seizure frequency is one every 6 months. At the age of 15 he is able to sit without support and crawl, but does not walk or talk.
Table 1.
Clinical summary. A) Major clinical signs and symptoms. B) Biochemical data showing normal quantitative amino acids in cerebrospinal fluid (CSF) and plasma amino acids except for low plasma alanine
| Proband | Affected sister | Affected brother | |||
|---|---|---|---|---|---|
| A) | |||||
| Developmental delay | Yes | Yes | Yes | ||
| Speech | Non-verbal | Non-verbal | Non-verbal | ||
| Seizures | Generalized tonic-clonic seizures | Absence seizures | No seizures | ||
| Dysmorphic features | Microcephalic with bitemporal narrowing, hypotelorism, and low set ears |
Dolichocephalic, highly arched palate, posteriorly rotated ears, and epicanthal folds |
Microcephalic with bitemporal narrowing |
||
| Muscle tone | Hypotonia and brisk reflexes | Hypotonia | Hypertonia and fisting | ||
| B) | Normal Value | Proband | Sister | Brother | |
| Umol/L | |||||
| CSF | Alanine | 12.5–47.3 | 15 | 13.8 | 15.2 |
| Glutamine | 230.7–637.4 | 395.2 | 370.9 | 583.2 | |
| Glutamate | 0.0–15.0 | 0.9 | 1.7 | 1.9 | |
| Pyruvate | 0.04–0.13 | 0.08 | 0.06 | 0.06 | |
| Lactic Acid | 0.6–2.2 | N/A | 1 | 1.2 | |
| Plasma | Alanine | 240–600 | 143 | 184 | 196 |
| Glutamine | 410–700 | 488 | 498 | 485 | |
| Glutamate | 10 –129 | 22 | 49 | 38 | |
| Urine | Pyruvate | 0–30 | N/A | 12 | 18 |
| Lactic Acid | 0–50 | N/A | 31 | 43 |
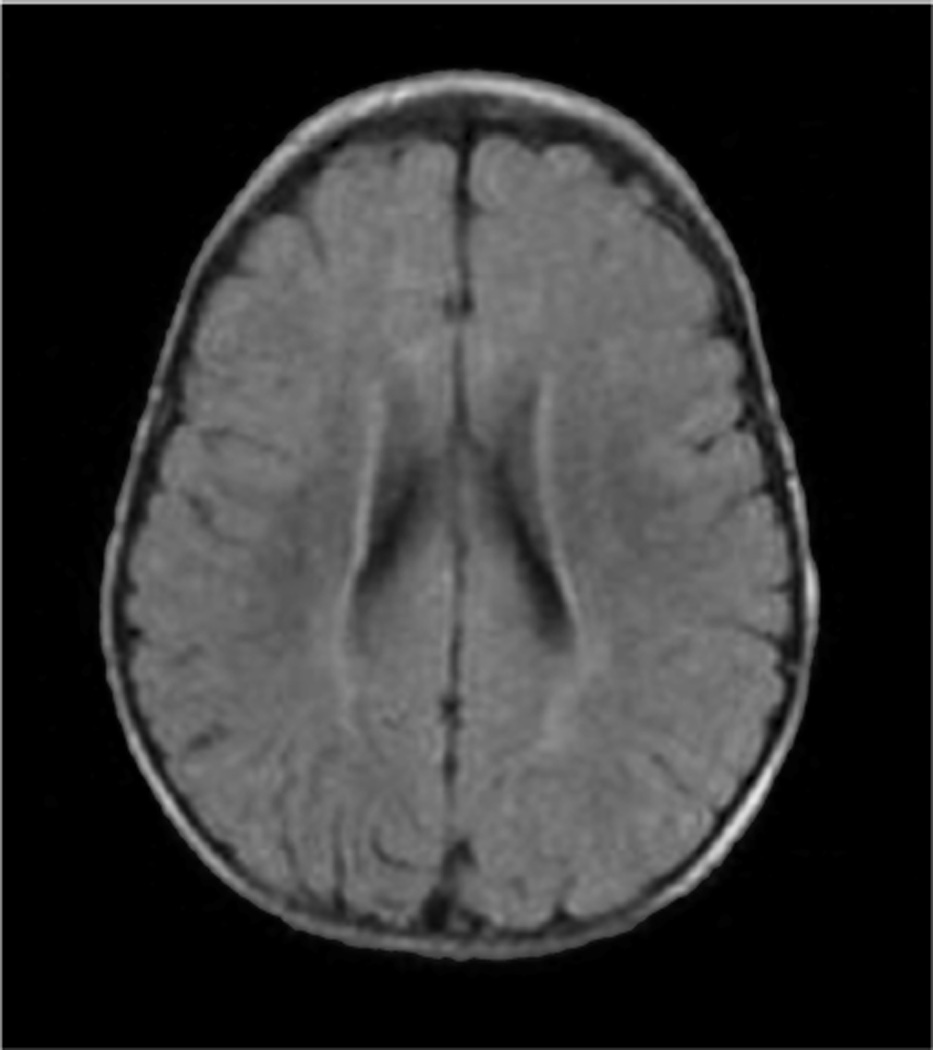
His affected sister is currently a 14 years old girl, born full term by vaginal delivery with no prenatal complications. Her birth weight was 3.2 kg (25 %) and height was 52 cm (50 %). At 3 1/2 months of age her weight was 3.8 kg (<3 %), and she was admitted for failure to thrive, requiring nasogastric tube feeding. Hepatic function studies, thyroid function studies, and abdominal ultrasound were normal. Sweat test was negative. A G-tube placement was required at the age of 6 months. On physical examination she appeared dolichocephalic and hypotonic, had a highly arched palate, posteriorly rotated ears and epicanthal folds. Developmentally she smiled at 2 months of age, sat with support at 12 months of age and has never walked or talked. An extensive genetic and metabolic evaluation was performed and included normal results for karyotype, subtelomeric FISH, MECP2 sequencing, Smith-Lemli-Opitz syndrome, fragile X, mitochondrial point mutations and deletions, congenital disorders of glycosylation, very long chain fatty acids, lactate, pyruvate, ammonia, acylcarnitine profile, muscle biopsy, quantitative plasma and CSF amino acids, CSF neurotransmitter metabolites (3-O-methyldopa, 5-hydroxyindolacetic acid, and homovanillic acid, neopterin and tetrahydrobiopterin). Magnetic resonance image (MRI) of the brain at 2 years of age showed subtle incomplete and abnormal myelination involving the subcortical white matter (Fig. 2). Muscle biopsy at 3 years of age showed some atrophic fibers with mild excess of variability in fiber size. Electron microscopy showed normal mitochondria. Oxidative phosphorylation showed normal enzyme activity. There was no accumulation of storage materials. At the age of 14 she had her first absence seizure and was treated with levetiracetam. Neurological examination revealed no spasticity or contractures. EEG was abnormal showing diffuse generalized background slowing with bursts of high voltage delta waves lasting two to four seconds.
Fig. 2.
Affected sister’s brain MRI showing subtle incomplete and abnormal myelination involving the subcortical white matter
The youngest affected sibling is a 4 year old boy who was born full term by vaginal delivery with a birth weight of 3.5 kg (50 %), length of 50 cm (50 %) and head circumference of 36 cm (50 %). The prenatal and neonatal courses were uncomplicated. At 2.5 months of age he developed bronchiolitis and aspiration pneumonia. On physical examination he was microcephalic with bitemporal narrowing, hypertonia, and fisting. By 6 months he was failing to thrive and developmentally delayed. On physical examination, his weight was 6 kg (<3 %), height was 63.5 cm (<3 %) and head circumference was 41 cm (<3 %). Developmentally, he was always delayed. He sat without support and rolled over at 2.5 years of age, and crawled at 3 years of age. He has never walked or talked, and he has never had a seizure. Currently, his weight is 8.7 kg (<3 %), height 84 cm (<3 %), and head circumference is 47 cm (<3 %).
All three siblings had normal CSF quantitative amino acids (including alanine, glutamine and glutamate). Plasma quantitative levels of amino acids were normal, except alanine (Table 1B).
There are four additional sisters and one brother of normal intelligence. The family is from Syria and Lebanon.
Materials and methods
Genomic DNA from whole blood from the three affected siblings was used with the SureSelect XT2 All Exon V4 kit (Agilent Technologies, Santa Clara, CA). The exome libraries were sequenced using 100 bp paired-end reads on the Illumina HiSeq 2000 (Illumina, San Diego, CA). The sequence data was aligned to the reference human genome (hg19) using BWA 0.5.9 (Li and Durbin 2009). Local realignment around insertion-deletion sites was performed using the Genome Analysis Toolkit v1.6 (DePristo et al 2011). Variant calls were generated simultaneously on all sequenced family members using SAMtools v0.1.18 (Li et al 2009). All coding exons and surrounding intron/exon boundaries were analyzed. Automated filtering removed common sequence changes (defined as >10 % frequency present in 1000Genomes database). The targeted coding exons and splice junctions of the known protein-coding RefSeq genes were assessed for the average depth of coverage and data quality threshold values. Whole exome sequence data for all sequenced family members was analyzed using GeneDx’s XomeAnalyzer (a variant annotation, filtering, and viewing interface for WES data), which includes nucleotide and amino acid annotations, population frequencies (NHLBI Exome Variant Server and 1000 Genomes databases), in silico prediction tools, amino acid conservation scores, and mutation references. Variants were filtered based on inheritance patterns, gene lists of interest, phenotype and population frequencies, as appropriate. Resources including the Human Gene Mutation Database (HGMD), 1000 Genomes database, NHLBI Exome Variant Server, OMIM, PubMed, and ClinVar were used to evaluate genes and detected sequence changes of interest. Additional searches were performed using specific gene lists related to ID. Identified sequence changes of interest were confirmed in all members of the trio by conventional di-deoxy DNA sequence analysis using an ABI3730 (Life Technologies, Carlsbad, CA) and standard protocols with a new DNA preparation.
Recombinant wild-type and mutant GPT2 cDNA constructs and functional expression analysis in bacteria and 293HEK cells
Construction of expression vector of wild-type (WT) and p.Ser153Arg (S153R) mutant GPT2
Cloning and bacterial expression vector of the WT of human GPT2 cDNA plasmid have been reported (Yang et al 2002). The plasmid served as a template for PCR amplification of the mutant GPT2 protein-coding region with mutagenesis forward primer p3285 5’-cctacagtgctaggcagggtgtcaactgcatccg-3’ and reverse primer p3286 5’-gcagttgacaccctgcctagcactgtaggaccccag-3’ (mutation of C to G on the sense strand is in bold) with high-fidelity Phusion polymerase (New England Biolabs, Ipswich, MA). The resultant PCR product was digested with DpnI and transformed into bacterial competent cells for plasmid preparation. The mutagenesis will disrupt a NheI site in the wild type GPT2 cDNA, and hence, the resultant successful plasmid subclones were screened by using NheI digestion for making bacterial expression vector pET28a-S153R mutant ALT2. To construct mammalian human GPT2 expression vectors, WT and mutant human GPT2 cDNAs were subcloned into pENTR1A (Life Technologies, Grand Island, NY), respectively, followed by Gateway recombination (Life Technology) to make pSMPUW-CMV-wt and -S153R mutant GPT2 vectors. Both wild type and mutant GPT2 inserts in the bacterial and mammalian expression vectors were sequence verified without PCR-introduced mutation.
Expression and enzymatic activity assay of recombinant wild type and mutant ALT2 proteins
For bacterial expression, pET28a-wtALT2, pET28a-S153R mutant ALT2 and control (empty) plasmid constructs were used to transform E. coli (Turner, Novagen) competent cells, respectively. The resultant bacterial colonies were grown in 50 mL LB medium containing 30 mg/mL kanamycin. Once the OD600 reached approximately 0.7, isopropyl-β-D-thiogalactopyranoside (IPTG) was added (final concentration of 1 mM) to induce expression of recombinant proteins for 3 hours. Subsequently, 20 mL of cell culture pellet was suspended in 0.5 mL of 1× PBS buffer and subjected to three rounds of 10-second sonication intervals on ice (setting 3, Fisher 550 Sonic Dismemrator). After centrifugation at 10,000×g for 15 minutes at 4 °C, supernatant was collected for standard SDS-PAGE analysis and enzyme activity assays. For mammalian cell expression, 293 HEK cells were grown in 6-well plate in DMEM medium with 10 % fetal bovine serum. Cells were transfected with recombinant pSMPUW plasmid of the wild type, mutant ALT2 or the EGFP control using LipoD293 transfection reagent (SignaGen, Gaithersburg, MD). At 48 hours post-transfection, cells were washed with 1× PBS and collected in 300 µl of 50 mM Tris buffer (pH 7.4) and lysed by briefly sonication (5 second two times) on ice. The supernatant of cell lysates were assayed for ALT activity (described below) and protein expression. The ALT2 protein expression was examined by Western analysis. Briefly, 15 µg of bacterial lysates or 50 µg of 293HEK cell lysates were separated by electrophoresis on 7.5 % polyacrylamide gels. Following electrophoresis, proteins were transferred onto PVDF membranes and bound proteins were probed with primary rabbit polyclonal anti-ALT2 antibody (Liu et al 2008) (Yang et al 2009) or GAPDH (Millipore, Billerica MA), and developed with an alkaline phosphatase-conjugated second antibody.
ALT activity assay
ALT activity assays were performed using the DiscretPak ALT substrate Reagent (Catachem, Bridgeport, Connecticut) per manufacturer’s instruction with a few modifications. Briefly, 5 µL of cell lysates were incubated with a 200 µL mixture of reagent A and B containing L-alanine, NADH, LDH and 2-oxoglutarate at 25 °C. Absorbances at 340 nm were recorded every 30 seconds for 7 minutes. The rate of decrease in absorbance of the reaction mixture was used to calculate ALT enzyme activity in the cell lysate sample. We defined one unit of ALT activity as the amount of enzyme that catalyzes the formation of 1 µmol/L of NAD+ per minute under conditions of the assay at 25 °C. Final ALT activities were normalized by protein concentration in the lysate, as measured by Coomassie brilliant blue G250 (BioRad), using bovine serum albumin as standard. Similar levels of ALT2 expression were confirmed by Western blotting. The results were the average of three independent experiments in which three to five colonies in each group were tested in each experiment.
Results
Whole exome sequencing
Exome sequencing of all three affected siblings produced an average of ~21 GB of sequence per sample. Mean coverage of captured regions was ~190× per sample, with >95%covered with at least 10× coverage, an average of >93%of base call quality of Q30 or greater, and an overall average mean quality score of >Q35. Filtering of common SNPs (>10 % frequency present in 1000Genomes database) resulted in ~4000 variants per sample. Thirty four genes (53 unique sequence changes) of interest were identified (Table 2). Evaluation of these 34 genes eliminated 32 genes lacking clinical overlap with the patients’ phenotype, leaving two genes, each with one variant of interest (Table 3). COX10 had only a single sequence change detected despite 100 % coverage of the coding region and no evidence of a deletion/duplication of COX10 and was therefore eliminated because COX10 deficiency is an autosomal recessive disorder. The remaining gene, GPT2, had a homozygous novel c.459 C>G, p.Ser153Arg missense variant detected in all three affected siblings. The GPT2 variant was confirmed by Sanger sequencing. Both parents and the five unaffected siblings were all heterozygous for the c.459 C>G, p.Ser153Arg missense variant. The GPT2 p.Ser153Arg variant represents a nonconservative amino acid substitution of a polar, neutral serine residue replaced by a positively charged arginine residue. The serine 153 amino acid residue is highly conserved throughout evolution and is predicted to be damaging/deleterious by PolyPhen, SIFT, and MutationTaster. The NHLBI Exome Variant Server did not detect the p.Ser153Arg variant in approximately 6000 individuals of European and African American ancestry, in the Database of Single Nucleotide Polymorphisms (dbSNP), or in a local database of over 6000 exomes.
Table 2.
Number of genes with novel variants identified from whole exome sequencing after filtering of results and then manual review of the genes. Number of variants listed in parentheses
| Filtering results |
Manual review |
Resulting genes of interest |
|
|---|---|---|---|
| Homozygous | 9 (10) | 1 (1) | 1 (1) |
| Compound Heterozygous | 13 (30) | 0 (0) | 0 (0) |
| Heterozygous | 2 (2) | 1 (1) | 0 (0) |
| X-linked genes | 10 (11) | 0 (0) | 0 (0) |
| Total genes | 34 (53) | 2 (2) | 1 (1) |
Table 3.
Variants of potential interest then filtered based on mode of inheritance
| Gene | Novel/known disease gene |
Variant | Genotype | SIFT | PolyPhen | 1000 Genome frequency |
ESP frequency |
|---|---|---|---|---|---|---|---|
| COX10 | Known | c.1193 G>A, R398H | Heterozygous | Damaging | Damaging | N/A | 0.02 % |
| GPT2 | Novel | c.459 C>G, S153R | Homozygous | Damaging | Damaging | N/A | N/A |
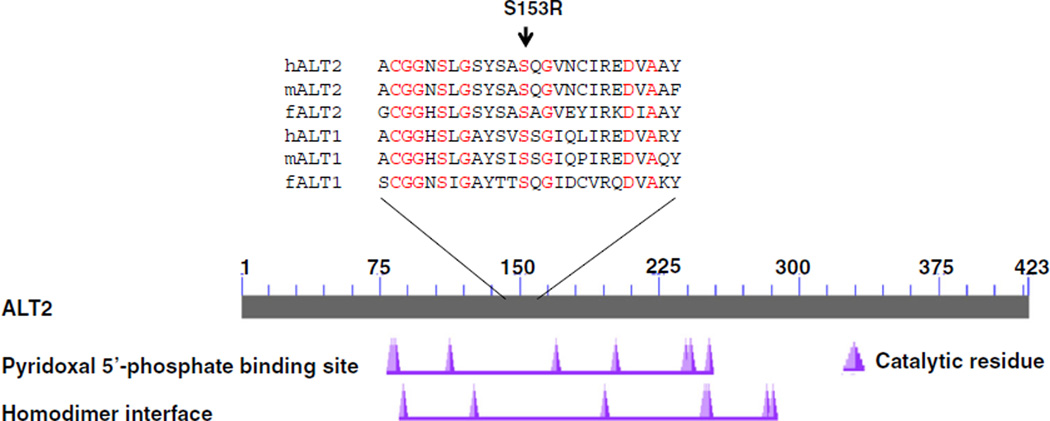
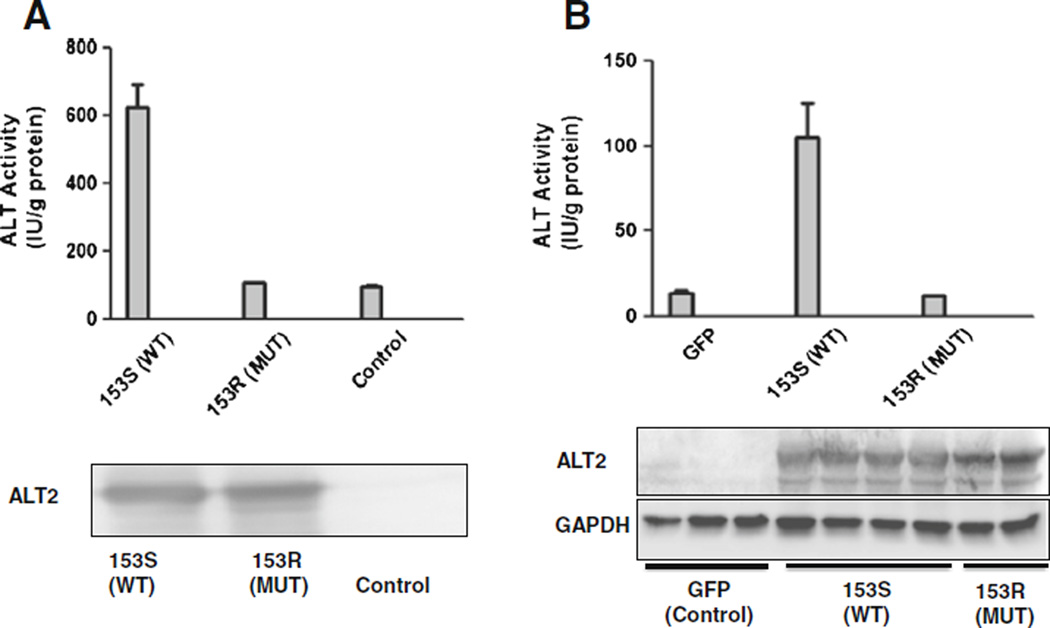
Serine at the 153 amino acid position of human ALT2 falls in the overlapped regions of the function domain of pyridocal 5’-phosphate binding site and homodimer is conserved among known ALT1 and ALT2 of vertebrate species including human, mouse and zebrafish (Fig. 3), suggesting a functional significance of this amino acid. To further examine the functional consequences of S153R, we conducted in vitro functional studies by expressing the recombinant wild type and mutant S153R in bacteria and measuring ALT activity. As shown in Fig. 4a, the basal ALT activities were about 100 IU/gram protein. Overexpression of the S153R mutant ALT2 did not change the activity, but that of wild type ALT significantly increased the activity by fivefold to 615. Western analysis showed a similar level of the mutant and wild type ALT2 expression, indicating that the mutant S153R has no enzymatic activity. The mutation may result in destablization in mammalian cells. To further investigate whether the S153R mutation may change the protein stability, we overexpressed wild type and mutant ALT2, alone with GFP control, in the mammalian kidney cell line HEK293 and measured ALT activity. As shown in Fig. 4b, the basal activity of the cells transfected with GFP control vector was about 14 IU/gram protein. ALT activity levels were increased to ~104 when the cells were expressing wild type ALT2. In comparison, lysates from cells transfected with the mutant ALT2 had almost the same level ALT activity as the control. Western analysis revealed approximately the same level of ALT2 expression in the cells compared to the house-keeping protein GAPDH. These studies demonstrate that the S153R variant does not affect its protein expression in mammalian cells but is enzymatically inactive.
Fig. 3.
Location and conservation of S153R in ALT2 proteins. Conserved functional domains of human ALT2 are revealed by Blast (www.ncbi.nlm.nih.gov) against GenBank protein database. The mutation is indicated by the arrow. hALT2: human ALT2 (GenBank accession#: NP_597700); mALT2: murine ALT2 (NP_776291); fALT2: zebrafish ALT2 (NP_001092227); hALT1: human ALT1 (NP_005300); mALT1: murine ALT1 (NP_877957) and fALT1: zebrafishALT1 (NP_001136246
Fig. 4.
Expression and functional analyses of recombinant wild type and mutant ALT2 proteins a) Upper panel: ALT activities were measured in lysates of bacteria transformed with vector expressing none (control), the 153S wild-type (WT) or 153R mutant (MUT) protein; lower panel: representative Western blot of bacteria lysates (15 µg protein/lane) blotted with anti-ALT2 antibody. (b) Upper panel: ALT activities were measured in lysates of 293HEK cells transfected with vector expressing (GFP), the 153S wild-type (WT) or 153R mutant (MUT) protein; lower panel: representative Western blot of 293HEK lysates (50 µg protein/lane) blotted with anti-ALT2 antibody. Data are expressed as mean+SD, n=3
Discussion
Using WES we identified a novel homozygous variant, c.459 C>G p.Ser153Arg in GPT2 in all three affected siblings with severe developmental encephalopathy. This variant was heterozygous in both parents and all five unaffected siblings. Functional studies demonstrate that this is a severe loss of function allele. We suggest that this is likely the cause of the three siblings’ developmental encephalopathy in this family, representing a novel genetic cause of ID.
Glutamic pyruvate transaminase 2 (GPT2), also known as alanine aminotransferase 2 (ALT2), encodes one of the two pyridoxal dependent enzymes that catalyzes a reversible transamination reaction between L-alanine and 2-oxoglutarate to produce glutamate and pyruvate, required for amino acid metabolism and neurotransmitter production. ALT2 is expressed in the brain and muscle, while ALT1 is expressed in liver, muscle, adipose tissue, and kidney. As such, ALT1 accounts for most of the ALT (also known as serum GPT or SGPT) activity in the clinically standard liver function tests. Within the brain ALT2 is expressed in gray matter more than white matter and strongly expressed in the granular cell layer of the cerebellum (Lindblom et al 2007). ALT2 may be the mitochondrial glutamate-pyruvate transaminase, with a unique role forming alanine with branch chain amino acid metabolism in muscle (Snell and Duff 1985). ALT2 is important for generating glutamate, an important excitatory neurotransmitter (Yang et al 2002).
The glutamate-glutamine/lactate-alanine shuttle pathway is important in glutamatergic neurons and astrocytes, to produce glutamate, an excitatory neurotransmitter. Disruption of the production of this major neurotransmitter would be predicted to have catastrophic and generalized effects on brain function but would not be predicted to affect other parts of the body since the expression of the ALT1 isoform should compensate in tissues other than the brain which expresses only ALT2 (Yang et al 2002). In brain as well the phenotype may be ameliorated by availability of glutamate derived from other reactions, such as glutaminase, mutation of which is lethal in an animal model (Masson et al 2006). Because this is a missense substitution and because ALT2 uses pyridoxine as an enzymatic cofactor, we tried to supplement the patients with 200 mg of pyridoxine daily, but with only minimal improvement in responsiveness and muscle tone.
In summary, we conclude that the novel p.Ser153Arg variant in GPT2 disturbs neurotransmitter release at the synapse leading to generalized central nervous system dysfunction and ID. A major limitation of this study is that there is only a single family identified with the novel variant in GPT2. Functional in vitro analysis however confirms that this is a loss of function mutation. We encourage other groups to include this as a candidate gene for ID to replicate our findings and enable description of the full phenotypic spectrum of ALT2 deficiency.
Acknowledgments
We thank the patients and their family for their contribution. Research reported in this publication was supported by the National Institutes of Health under award number: T32GM082771, Maryland Clinical Nutrition Research Unit (DK072488), and The Baltimore Diabetes Research and Training Center (P60-DK-079637). Authors acknowledge Alan Shuldiner for helpful discussions and critical review of the manuscript.
Conflicts of Interest Eden Haverfield is an employee of GeneDx, Inc. Wendy K. Chung is a consultant to BioReference Laboratories.
Footnotes
Human and animal rights and informed consent This article does not contain any studies with human or animal subjects performed by the any of the authors.
Contributor Information
Katrina Celis, Departments of Pediatrics and Medicine, Columbia University Medical Center, 1150 St. Nicholas Avenue, Room 620, New York, NY 10032, USA.
Scott Shuldiner, University of Maryland, College Park, MD, USA.
Eden V. Haverfield, GeneDx, Inc, Gaithersburg, MD, USA
Joshua Cappell, Departments of Pediatrics and Medicine, Columbia University Medical Center, 1150 St. Nicholas Avenue, Room 620, New York, NY 10032, USA; Department of Neurology, Columbia University Medical Center, New York, NY, USA.
Rongze Yang, University of Maryland, College Park, MD, USA.
Da-Wei Gong, University of Maryland, College Park, MD, USA.
Wendy K. Chung, Email: wkc15@columbia.edu, Departments of Pediatrics and Medicine, Columbia University Medical Center, 1150 St. Nicholas Avenue, Room 620, New York, NY 10032, USA.
References
- Daily DK, et al. Identification and evaluation of mental retardation. Am Fam Physician. 2000;61(4):1059–1067. [PubMed] [Google Scholar]
- DePristo M, Banks E, Poplin R, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43:491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison JW, et al. Genetic Basis of Intellectual Disability. Annu Rev Med. 2013;64:441–450. doi: 10.1146/annurev-med-042711-140053. [DOI] [PubMed] [Google Scholar]
- Kaufman L, et al. The genetic basis of non-syndromic intellectual disability: a review. J Neuro Dev Disord. 2010;2:182–209. doi: 10.1007/s11689-010-9055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, et al. The Sequence alignment/map (SAM) format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblom P, Rafter I, Glinghammar B. Isoforms of alanine aminotransferases in human tissues and serum—differential tissue expression using novel antibodies. Arch Biochem Biophys. 2007;466:66–77. doi: 10.1016/j.abb.2007.07.023. [DOI] [PubMed] [Google Scholar]
- Liu L, Zhong S, Yang R, et al. Expression, purification, and initial characterization of human alanine aminotransferase (GPT2) isoenzyme 1 and 2 in high-five insect cells. Protein Expr Purif. 2008;60:225–231. doi: 10.1016/j.pep.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson J, Darmon M, Hen R, Rayport S. Mice lacking brain/kidney phosphate-activated glutaminase have impaired glutamatergic synaptic transmission, altered breathing, disorganized goal-directed behavior and die shortly after birth. J Neurosci. 2006;26(17):4660–4671. doi: 10.1523/JNEUROSCI.4241-05.2006. 4241-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najmabadi H, Hu H, Garshasbi M, et al. Deep sequencing reveals 50 novel genes for recessive cognitive disorders. Nature. 2011;478:57–63. doi: 10.1038/nature10423. [DOI] [PubMed] [Google Scholar]
- Snell K, Duff DA. Branched-chain amino acid metabolism and alanine formation in rat muscles in vitro. Mitochondrial-cytosolic interrelationships. Biochem J. 1985;225(3):737–743. doi: 10.1042/bj2250737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topper S, et al. Exome sequencing and the genetics of intellectual disability. Clin Genet. 2011;2011(80):117–126. doi: 10.1111/j.1399-0004.2011.01720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utine GE, Haliloğlu G, Volkan-Salancı B, et al. Etiological yield of SNP microarrays in idiopathic intellectual disability. Eur J Pediatr Neurol. 2014;18(3):327–337. doi: 10.1016/j.ejpn.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Yang RZ, et al. cDNA cloning, genomic structure, chromosomal mapping, and functional expression of a novel human alanine aminotransferase. Genomics. 2002;79:445–450. doi: 10.1006/geno.2002.6722. [DOI] [PubMed] [Google Scholar]
- Yang RZ, Park S, Reagan WJ, et al. Alanine aminotransferase isoenzymes: molecular cloning and quantitative analysis of tissue expression in rats and serum elevation in liver toxicity. Hepatology. 2009;49:598–607. doi: 10.1002/hep.22657. [DOI] [PMC free article] [PubMed] [Google Scholar]