Abstract
Introduction
Management of Lennox–Gastaut syndrome (LGS) in adulthood can be particularly challenging. Published reports describing the use of rufinamide specifically in adult patients with LGS are scarce. A post hoc subgroup analysis of data from a phase III trial was conducted to investigate the efficacy and safety/tolerability of rufinamide in adults with LGS.
Methods
A randomized, double-blind, placebo-controlled trial was conducted in patients with LGS, aged 4 years and above. During an 84-day, double-blind treatment period, patients received either adjunctive rufinamide therapy or placebo. Efficacy and safety/tolerability were assessed in a post hoc subgroup analysis of adult patients (≥18 years). Efficacy was assessed as change from baseline in 28-day seizure frequency, 50% responder rate, and seizure freedom rate; each calculated for total seizures and drop attacks. Safety/tolerability assessments included the evaluation of adverse events (AEs).
Results
Thirty-one adults aged 18–37 years with LGS received treatment with either rufinamide (n = 21) or placebo (n = 10). Three patients in the rufinamide group did not complete the trial. The median change from baseline in seizure frequency was −31.5% for rufinamide versus +22.1% for placebo (P = 0.008) for all seizures and −54.9% versus +21.7% (P = 0.002) for drop attacks. Responder rates were 33.3% for rufinamide versus 0% for placebo (P = 0.066) for all seizures and 57.1% versus 10.0% (P = 0.020) for drop attacks. No patient achieved freedom from all seizures but two rufinamide-treated patients (9.5%) became free of drop attacks. Overall, 71.4% of patients treated with rufinamide and 60.0% of patients treated with placebo experienced AEs; most commonly, somnolence (33.3% vs. 20.0%) and vomiting (19.0% vs. 0%). Most AEs were of mild or moderate intensity.
Conclusion
Rufinamide demonstrated favorable efficacy and was generally well tolerated when used as adjunctive treatment for adults with LGS.
Funding
Eisai.
Electronic supplementary material
The online version of this article (doi:10.1007/s40120-016-0041-9) contains supplementary material, which is available to authorized users.
Keywords: Adult, Antiepileptic drug, Epilepsy, Lennox–Gastaut syndrome, Rufinamide
Introduction
Lennox–Gastaut syndrome (LGS) is a severe, chronic, epileptic encephalopathy that is associated with considerable morbidity and mortality [1, 2]. It is characterized by a triad of symptoms: multiple seizure types, abnormal electroencephalogram (EEG) features with slow spike-wave discharges, and cognitive impairment [1]. To date, only a few antiepileptic drugs (AEDs) have demonstrated efficacy against the multiple seizure types associated with LGS [3].
Rufinamide is a triazole derivative, structurally unrelated to other AEDs [4], which is approved for adjunctive treatment of seizures associated with LGS in patients aged ≥4 years [5–7]. The efficacy and safety/tolerability of rufinamide in this setting were established in a phase III, international, multicenter, randomized, double-blind, placebo-controlled trial, in which 138 LGS patients, aged 4–37 years, were randomized to receive adjunctive therapy with either rufinamide or placebo [3].
Although LGS typically begins during childhood, it frequently persists through adolescence and into adulthood, and may also, rarely, have late onset during adolescence or adulthood [2]. Diagnosis of LGS is complicated by the fact that the seizure types and other features by which it is defined and characterized evolve and change over time, and, in adulthood, the way in which it presents may not be consistent with the typical features associated with early-onset LGS [2].
The objective of this study was to investigate further the efficacy and safety/tolerability of rufinamide in adults with LGS.
Methods
Study Design
A post hoc subgroup analysis was conducted of adult data (aged 18 years and above) from a phase III, randomized, double-blind, placebo-controlled trial, conducted between March 1998 and September 2000 [3]. The trial comprised a 28-day baseline period, followed by an 84-day treatment period (14-day titration plus 70-day maintenance). Rufinamide (Inovelon®, Eisai Ltd; Banzel®, Eisai Inc.) was administered as adjunctive therapy to one to three concomitant AEDs, and initiated and titrated according to approved recommendations [6, 7]. The dose administered at the end of the titration period was used for the entire maintenance period and study visits were conducted on Days 0, 7, 14, 28, 56, and 84 after randomization.
Study Population
The overall trial population included patients aged ≥4 years with a history of multiple seizure types, including atypical absence seizures and drop attacks (tonic–atonic or astatic seizures). Patients were required to have ≥90 seizures in the month prior to the baseline period, an EEG within 6 months of study entry demonstrating a pattern of slow spike-and-wave complexes (<2.5 Hz), and a computed tomography or magnetic resonance imaging scan confirming the absence of a progressive lesion. They were also required to be on a fixed-dose regimen of one to three concomitant AEDs during the baseline period and to provide written informed consent. Patients were excluded if they had a correctable seizure etiology (such as active infection), history of generalized tonic–clonic status epilepticus within 30 days before baseline, or history of any non-neurological medical condition; and if they were pregnant or failed to use adequate contraception.
Study Assessments
Efficacy was assessed as change from baseline in 28-day seizure frequency (i.e., number of seizures per 28 days), responder rate (response defined as ≥50% seizure frequency reduction from baseline), and seizure freedom rate; each calculated for total seizures and drop attacks (tonic–atonic seizures). Safety/tolerability assessments included the evaluation of adverse events (AEs), physical/neurological examinations, vital signs, laboratory parameters, and electrocardiogram (ECG) recordings [3]. AEs were defined as any undesirable effects experienced by the patient, irrespective of relation to the study drug. AEs were considered serious if they were fatal or life-threatening, permanently disabling, or required inpatient or prolonged hospitalization.
Statistical Methodology
A post hoc subgroup analysis of the adult data was conducted. Efficacy analysis was performed for the intention-to-treat population, defined as all randomized patients who received the double-blind study drug. All 84 days of double-blind treatment (i.e., titration period plus maintenance period) were included in the intention-to-treat analysis. Median change from baseline in 28-day frequency was compared between groups using the Wilcoxon Rank Sum Test (unadjusted). Responder and seizure freedom rates were analyzed using frequency analysis. Responder rates were compared between groups using Fisher’s Exact Test. All statistical tests were performed using SAS version 9 (SAS Institute Inc., Cary, NC, USA). The safety population comprised all patients who received at least one dose of study drug.
Ethics
All procedures were conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for inclusion in the study.
Results
Patient Baseline Characteristics and Disposition
The trial included a total of 31 adult patients, aged 18–37 years, randomized to treatment with rufinamide (n = 21) or placebo (n = 10). Overall, 18/21 (85.7%) patients in the rufinamide group and 10/10 (100%) patients in the placebo group completed the trial. Reasons for discontinuation in the rufinamide group were AEs (anorexia, somnolence, and vomiting; n = 1), lack of efficacy (n = 1), and withdrawal of consent (n = 1). The mean age of the adult patients was 25.2 and 29.3 years in the rufinamide and placebo groups, respectively (Table 1). The most frequently used concomitant AEDs at baseline were lamotrigine, valproate, and phenytoin.
Table 1.
Baseline characteristics of adult patients with LGS (n = 31)
| Characteristics | Rufinamide (n = 21) | Placebo (n = 10) |
|---|---|---|
| Sex, n (%) | ||
| Male | 15 (71.4) | 5 (50.0) |
| Female | 6 (28.6) | 5 (50.0) |
| Ethnicity, n (%) | ||
| Caucasian | 20 (95.2) | 9 (90.0) |
| Black | 0 (0.0) | 1 (10.0) |
| Asian | 1 (4.8) | 0 (0.0) |
| Age, years | ||
| Mean (SD) | 25.2 (4.7) | 29.3 (7.1) |
| Median (range) | 25.0 (18–35) | 31.5 (18–37) |
| Time since LGS diagnosis, years | ||
| Mean (SD) | 18.5 (8.9) | 25.5 (8.1) |
| Median (range) | 21 (0–33) | 28.5 (8–34) |
| Number of concomitant AEDs, n (%) | ||
| 2 | 10 (47.6) | 4 (40.0) |
| 3 | 11 (52.4) | 6 (60.0) |
| Most frequently used concomitant AEDs (≥5% patients), n (%) | ||
| Lamotrigine | 10 (47.6) | 4 (40.0) |
| Valproate | 9 (42.9) | 9 (90.0) |
| Phenytoin | 5 (23.8) | 4 (40.0) |
| Topiramate | 6 (28.6) | 1 (10.0) |
| Carbamazepine | 5 (23.8) | 2 (20.0) |
| Clonazepam | 4 (19.0) | 3 (30.0) |
| Phenobarbital | 3 (14.3) | 0 (0.0) |
| Clobazam | 2 (9.5) | 0 (0.0) |
| Gabapentin | 2 (9.5) | 0 (0.0) |
| Vigabatrin | 1 (4.8) | 1 (10.0) |
| Oxcarbazepine | 0 (0.0) | 2 (20.0) |
AED antiepileptic drug, LGS Lennox–Gastaut syndrome, SD standard deviation
Rufinamide Treatment
The mean (standard deviation [SD]) maximum dose of rufinamide administered to the adult patients during the trial was 2476.2 (594.9) mg/day (median, 2400 mg/day; range, 1600–3200 mg/day). The mean (SD) final rufinamide dose administered was 2171.4 (886.1) mg/day (median, 2400 mg/day; range, 200–3200 mg/day).
Efficacy
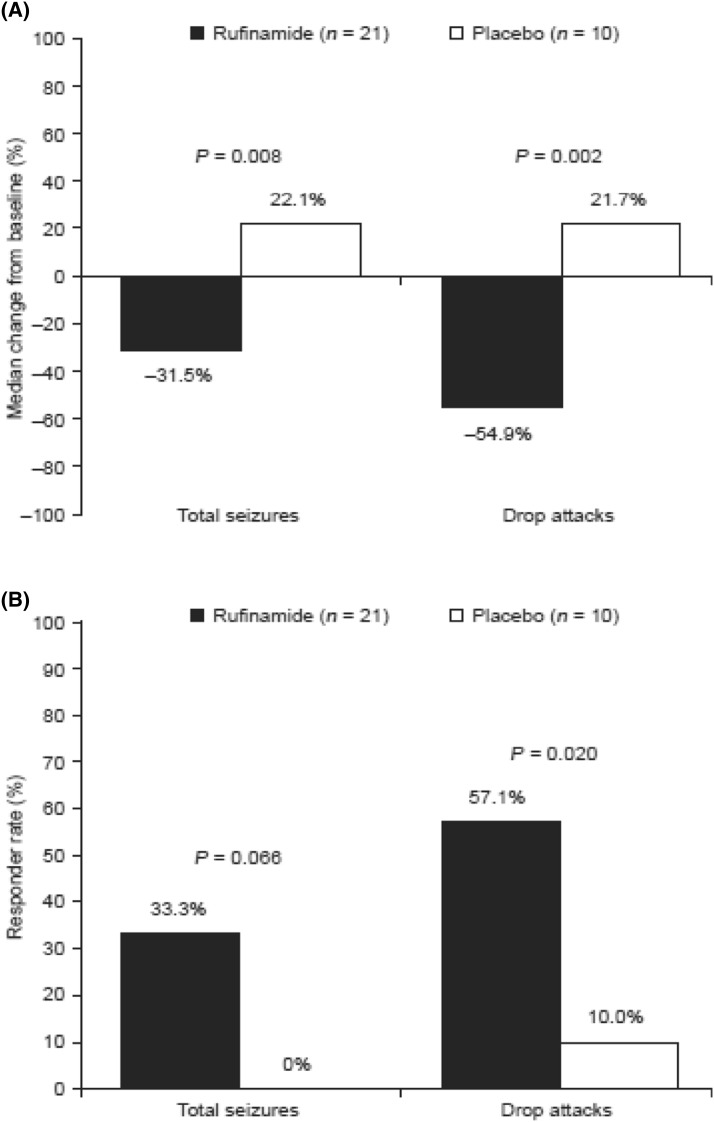
The median change from baseline in 28-day frequency of total seizures was −31.5% (mean, −26.9%; SD, 52.5%; range, −92.3 to 136.5%) with rufinamide versus +22.1% (mean, 67.5%; SD, 173.1%; range, −36.6 to 550.6%) with placebo (P = 0.008; Fig. 1a). The median change from baseline in 28-day frequency of drop attacks was −54.9% (mean, −19.0%; SD, 111.7%; range, −55.9 to 406.7%) with rufinamide versus +21.7% (mean, 136.2%; SD, 255.2%; range, −55.9 to 709.6%) with placebo (P = 0.002; Fig. 1a).
Fig. 1.
a Median percentage changes from baseline in 28-day frequency and b responder rates for total seizures and drop attacks in adult patients with Lennox–Gastaut syndrome (n = 31). Response was defined as ≥50% seizure frequency reduction from baseline
Responder rates for total seizures were 33.3% with rufinamide versus 0% with placebo (P = 0.066; Fig. 1b). Responder rates for drop attacks were 57.1% with rufinamide versus 10.0% with placebo (P = 0.020; Fig. 1b). No patient achieved seizure freedom (i.e., freedom from all seizures), but two patients treated with rufinamide (9.5%) became free of drop attacks during the trial.
Safety/Tolerability
Overall, 15/21 (71.4%) patients treated with rufinamide and six of 10 (60.0%) patients treated with placebo experienced AEs (Table 2). The most frequently reported AEs were somnolence and vomiting (Table 2). The majority of AEs were of mild or moderate intensity. Three rufinamide patients experienced severe AEs (somnolence, somnolence and hostility, and constipation). No patient experienced a serious AE. One patient experienced status epilepticus while receiving rufinamide 1400 mg/day. This patient was later withdrawn from the study due to other AEs (anorexia, somnolence, and vomiting). Rufinamide treatment was not associated with clinically significant changes in vital signs, physical examinations, ECG recordings, or laboratory tests [3].
Table 2.
Summary of AEs reported by adult patients with Lennox–Gastaut syndrome (n = 31)
| Rufinamide (n = 21) | Placebo (n = 10) | |
|---|---|---|
| Patients with any AE, n (%) | 15 (71.4) | 6 (60.0) |
| Patients with any serious AE, n (%) | 0 (0.0) | 0 (0.0) |
| Patients with AEs leading to discontinuation, n (%) | 1 (4.8) | 0 (0.0) |
| AEs reported by >10% patients in either group, n (%) | ||
| Somnolence | 7 (33.3) | 2 (20.0) |
| Vomiting | 4 (19.0) | 0 (0.0) |
| Ecchymosis | 3 (14.3) | 1 (10.0) |
| Fatigue | 3 (14.3) | 0 (0.0) |
| Ataxia | 3 (14.3) | 0 (0.0) |
| Decreased appetite | 3 (14.3) | 0 (0.0) |
| Headache | 2 (9.5) | 2 (20.0) |
| Pyrexia | 0 (0.0) | 2 (20.0) |
AE adverse event
Discussion
In this post hoc subgroup analysis, rufinamide demonstrated favorable efficacy when used as adjunctive treatment for adults with LGS. Rufinamide treatment significantly reduced the frequency of total seizures compared with placebo. Rufinamide was particularly efficacious in reducing the frequency of drop attacks, resulting in a median reduction from baseline in 28-day frequency of 55% and a responder rate of 57%, with two patients becoming free of drop attacks during the trial. These findings therefore support recent guidelines suggesting that rufinamide might be preferable to other AEDs as a second-line treatment for LGS when drop attacks are frequent [8]. The findings are also in line with a study demonstrating the long-term effectiveness of rufinamide for the treatment of pharmacoresistant myoclonic-atonic seizures in children with Doose syndrome [9].
Rufinamide treatment was generally well tolerated; the most frequently reported AEs (somnolence and vomiting) were the same as those most frequently reported for the overall population in the original trial [3]. There were no serious AEs and only one patient discontinued due to AEs associated with rufinamide treatment. It should be noted that published reports suggest that, in clinical practice, where treatment is individualized, a ‘lower and slower’ dosing strategy tends to be adopted, which does not appear to compromise rufinamide’s efficacy, but may provide improvements in tolerability [5].
An acknowledged limitation of this analysis is that it was conducted in a relatively small subgroup of adult patients with LGS. Other clinical trials and clinical practice studies have demonstrated the efficacy and safety/tolerability of adjunctive rufinamide treatment for LGS in patient populations that have included a limited number of adult patients as well as pediatric patients [10–18]. However, published reports describing the use of rufinamide specifically in adult patients with LGS are scarce. In a single-center study conducted in France, clinically significant weight loss (≥7% decrease from baseline) was reported in seven of 15 consecutive adult patients treated with adjunctive rufinamide, five of whom had LGS [19]. The authors concluded that a lower starting dose and slower titration rate might help minimize the possibility of weight loss, although it was acknowledged that this requires confirmation [19]. Weight loss is known to be a common AE with rufinamide treatment [6]. In the current analysis, decreased weight was reported as an AE for one rufinamide-treated patient. In addition, AEs of decreased appetite and anorexia were reported for three and two rufinamide-treated patients, respectively. In a single-center study conducted in Germany, the mean QT interval of 19 consecutive adult patients treated with adjunctive rufinamide, nine of whom had LGS, shortened significantly with rufinamide treatment [20], consistent with rufinamide’s known safety profile [6]. However, during a mean follow-up of 3.6 years, no symptomatic cardiac arrhythmias occurred and no associated AEs were reported [20]. In the present analysis, no AEs associated with QT interval or other ECG parameters were reported. Prescribing guidelines recommend that clinical judgment be used when assessing whether to prescribe rufinamide to patients at risk from further shortening of their QTc interval [6].
Although beyond the scope of the present analysis, given the limited number of adult patients, in future studies it will be important to establish the extent to which the clinical and EEG features of LGS in adulthood differ from those in childhood. A recent retrospective analysis of the long-term prognosis of 68 patients with LGS found that the characteristic EEG features of LGS (diffuse slow spike-wave and generalized paroxysmal fast activity) ceased in half of the patients over a mean follow-up duration of approximately 19 years [21]. Such findings might therefore support a need to broaden or adapt the diagnostic criteria for LGS in adulthood, to ensure that patients receive the most appropriate treatment.
Conclusion
This analysis demonstrated that rufinamide was efficacious and generally well tolerated when used as an adjunctive treatment in adult patients with LGS. Further studies are needed to confirm its utility in this setting.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This phase III trial and post hoc analysis were funded by Eisai. The article processing charges for this publication were funded by Eisai. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship of this manuscript, take responsibility for the integrity of this work as a whole, and have given final approval to the version to be published. Editorial assistance in the preparation of this manuscript was provided by John Scopes of mXm Medical Communications and was funded by Eisai Ltd, Hatfield, UK.
Disclosures
Rob McMurray is a current employee of Eisai Europe Ltd. Pasquale Striano has been an invited speaker for, and participated in advisory boards organized by, Shire, Eisai Ltd, and Ultragenyx.
Compliance with Ethics Guidelines
All procedures were conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for inclusion in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
References
- 1.Arzimanoglou A, Resnick T. All children who experience epileptic falls do not necessarily have Lennox–Gastaut syndrome… but many do. Epileptic Disord. 2011;13(Suppl 1):S3–S13. doi: 10.1684/epd.2011.0422. [DOI] [PubMed] [Google Scholar]
- 2.Kerr M, Kluger G, Philip S. Evolution and management of Lennox–Gastaut syndrome through adolescence and into adulthood: are seizures always the primary issue? Epileptic Disord. 2011;13(Suppl 1):S15–S26. doi: 10.1684/epd.2011.0409. [DOI] [PubMed] [Google Scholar]
- 3.Glauser T, Kluger G, Sachdeo R, Krauss G, Perdomo C, Arroyo S. Rufinamide for generalized seizures associated with Lennox–Gastaut syndrome. Neurology. 2008;70:1950–1958. doi: 10.1212/01.wnl.0000303813.95800.0d. [DOI] [PubMed] [Google Scholar]
- 4.Jain KK. An assessment of rufinamide as an anti-epileptic in comparison with other drugs in clinical development. Expert Opin Investig Drugs. 2000;9:829–840. doi: 10.1517/13543784.9.4.829. [DOI] [PubMed] [Google Scholar]
- 5.Resnick T, Arzimanoglou A, Brown LW, et al. Rufinamide from clinical trials to clinical practice in the United States and Europe. Epileptic Disord. 2011;13(Suppl 1):S27–S43. doi: 10.1684/epd.2011.0421. [DOI] [PubMed] [Google Scholar]
- 6.Inovelon® (rufinamide) Summary of Product Characteristics. Eisai Ltd. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000660/WC500032937.pdf. Accessed 15 Jan 2016.
- 7.Banzel® (rufinamide) Prescribing Information. Eisai Inc. http://www.banzel.com/pdfs/BanzelPI.pdf (2013). Accessed 15 Jan 2016.
- 8.Coppola G, Besag F, Cusmai R, et al. Current role of rufinamide in the treatment of childhood epilepsy: literature review and treatment guidelines. Eur J Paediatr Neurol. 2014;18:685–690. doi: 10.1016/j.ejpn.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 9.von Stülpnagel C, Coppola G, Striano P, Müller A, Staudt M, Kluger G. First long-term experience with the orphan drug rufinamide in children with myoclonic-astatic epilepsy (Doose syndrome) Eur J Paediatr Neurol. 2012;16:459–463. doi: 10.1016/j.ejpn.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Kluger G, Kurlemann G, Haberlandt E, et al. Effectiveness and tolerability of rufinamide in children and adults with refractory epilepsy: first European experience. Epilepsy Behav. 2009;14:491–495. doi: 10.1016/j.yebeh.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 11.Kluger G, Haberlandt E, Kurlemann G, et al. First European long-term experience with the orphan drug rufinamide in childhood-onset refractory epilepsy. Epilepsy Behav. 2010;17:546–548. doi: 10.1016/j.yebeh.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Coppola G, Grosso S, Franzoni E, et al. Rufinamide in children and adults with Lennox–Gastaut syndrome: first Italian multicenter experience. Seizure. 2010;19:587–591. doi: 10.1016/j.seizure.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Vendrame M, Loddenkemper T, Gooty VD, et al. Experience with rufinamide in a pediatric population: a single center’s experience. Pediatr Neurol. 2010;43:155–158. doi: 10.1016/j.pediatrneurol.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Kim SH, Eun SH, Kang HC, et al. Rufinamide as an adjuvant treatment in children with Lennox–Gastaut syndrome. Seizure. 2012;21:288–291. doi: 10.1016/j.seizure.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Kim JY, Lee CG, Yu HJ, Nam SH, Lee J, Lee M. The efficacy and tolerability of rufinamide in intractable pediatric epilepsy. J Epilepsy Res. 2012;2:33–37. doi: 10.14581/jer.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee EH, Yum MS, Ko TS. Effectiveness and tolerability of rufinamide in children and young adults with Lennox–Gastaut syndrome: a single center study in Korea. Clin Neurol Neurosurg. 2013;115:926–929. doi: 10.1016/j.clineuro.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 17.Ohtsuka Y, Yoshinaga H, Shirasaka Y, Takayama R, Takano H, Iyoda K. Rufinamide as an adjunctive therapy for Lennox–Gastaut syndrome: a randomized double-blind placebo-controlled trial in Japan. Epilepsy Res. 2014;108:1627–1636. doi: 10.1016/j.eplepsyres.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Thome-Souza S, Kadish NE, Ramgopal S, et al. Safety and retention rate of rufinamide in 300 patients: a single pediatric epilepsy center experience. Epilepsia. 2014;55:1235–1244. doi: 10.1111/epi.12689. [DOI] [PubMed] [Google Scholar]
- 19.Mourand I, Crespel A, Gelisse P. Dramatic weight loss with rufinamide. Epilepsia. 2013;54:e5–e8. doi: 10.1111/j.1528-1167.2012.03579.x. [DOI] [PubMed] [Google Scholar]
- 20.Schimpf R, Veltmann C, Papavassiliu T, et al. Drug-induced QT-interval shortening following antiepileptic treatment with oral rufinamide. Heart Rhythm. 2012;9:776–781. doi: 10.1016/j.hrthm.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim HJ, Kim HD, Lee JS, Heo K, Kim DS, Kang HC. Long-term prognosis of patients with Lennox–Gastaut syndrome in recent decades. Epilepsy Res. 2015;110:10–19. doi: 10.1016/j.eplepsyres.2014.11.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.