Abstract
Background
Parkinson’s disease (PD) is a neurodegenerative disease characterized by motor manifestations, autonomic and neurological disorders and sensorial symptoms. Medication therapy management (MTM) consists of a service undertaken by pharmacists to optimize pharmacological therapy results. This way, the pharmacist monitors the treatment prescribed by the doctor and formulates a healthcare plan to guarantee the treatment’s effectiveness, safety and convenience, thereby improving the patient’s quality of life (QoL).
Objective
To analyze the effect of MTM upon medicine-related problems, motor symptoms, autonomic disorders and QoL of patients with Parkinson’s disease, and describe the pharmaceutical interventions.
Methods
Quasi-experimental uncontrolled before-and-after study carried out between September 2012 and March 2013 in a community pharmacy. Pharmacotherapy data were collected from medical prescriptions, patient diaries, medical charts and all the medicines (over-the-counter and prescription) brought by the patients to the appointment with the pharmacist. The medicine-related problems were classified as indication, effectiveness, safety and adherence. Adherence was measured through clinical interviews and the Morisky questionnaire. PD symptoms were assessed according to the patients’ and/or caregivers’ perceptions about the On/Off state of the motor symptoms and relief of the nonmotor symptoms. QoL was assessed using the PDQ-39 scores. The interventions were targeted to patients/caregivers and/or doctors, with pharmacological and non-pharmacological measures.
Results
Seventy patients were followed up, showing a decrease in medicine-related problems (1.67 ± 1.34 to 0.8 ± 0.9 (p < 0.001), positive impact on adherence (from 37 to 10 non-adherent patients, p < 0.001), QoL improvement related to emotional wellbeing (p = 0.012) and autonomic disorder. Most interventions were performed directly with the patients (73.8%), including non-pharmacological guidance (28.5%), pharmacological guidance (24.3%) and rescheduling (13.6%).
Conclusions
To carry out MTM with PD patients, the pharmacist’s expertise needs to transcend the technical knowledge about the PD pharmacological treatment. The study showed a positive effect with a decrease in the medicine-related problems after the interventions, especially improving adherence and patients’ QoL.
Keywords: Parkinson disease, Pharmaceutical care, Medication therapy management
Introduction
Parkinson’s disease (PD) is a neurodegenerative disease characterized by motor manifestation, autonomic disorder, sensorial symptoms and neurological disorder that compromises the patient’s quality of life (QoL) [1, 2].
Due to the progressive nature of the disease, the patient’s QoL is compromised in physical, mental/emotional, social and economic aspects. The most common and relevant factors reviewed in the literature about the worsening of PD patients’ QoL were bradykinesia, tremor, rigidity, postural instability, gait disorder, pain, fatigue, depression, and sexual and cognitive disorders [3, 4]. A Brazilian study showed that the major QoL determinants include mood disorder (mainly depression), disability, PD complications (dyskinesia and fluctuation) and educational attainment [2].
Therapy for PD is effective in the treatment of motor symptoms, but it does not prevent the disease’s progression. The worsening of motor symptoms associated with the occurrence of non-motor symptoms evolves progressively. This situation leads to dosage increase and need of new medication. Some antiparkinsonians, mainly levodopa, develop motor complications (fluctuation and dyskinesia) in the long term, making the treatment complex, increasing the demands for care and more expensive and invasive procedures [5, 6].
As a result of the treatment, PD patients can experience medicine-related problems, which are also called DRPs—drug-related problems. “A DRP exists when a patient experiences (or is likely to experience) either a disease or symptom having an actual or suspected relationship with drug therapy” [7]. Medicine-related problems include issues related to medicine effectiveness, adverse reactions and non-adherence to the treatment.
Non-adherence to the treatment is one of the most common medicine-related problems in patients who suffer from chronic diseases. It has been estimated that the PD patients’ adherence to the treatment is only 39%, compromising the benefits of the therapy [6]. Younger patients, patients with complex therapeutic regimens (several pills per day), high depression, and low QoL are less adherent to the antiparkinsonian treatment [8–10]. Clinical consequences of non-adherence to the antiparkinsonian treatment include loss of motor functions and reduction in QoL [11, 12]. The commitment of health professionals and patients together contributes to improvement of treatment adherence [5, 8, 13]. Furthermore, non-adherence increases PD-related costs because of the increase in hospital admissions, medical appointments and other healthcare services [6].
Some studies have reported that the participation of a pharmacist in a multidisciplinary healthcare team promotes clinical benefits for PD patients and is considered a valuable healthcare strategy [14, 15]. Medication therapy management (MTM) is one of the pharmacist’s duties, which consists of a service undertaken by the pharmacist in conjunction with other health professionals. MTM aims at optimizing the pharmacological therapy results, so the pharmacist monitors the results of the treatment prescribed by the doctor and elaborates a healthcare plan to guarantee the treatment’s effectiveness, safety, and convenience, and therefore improve the patients’ QoL. MTM is based on a patient-centered approach which considers the patient as an active partner in the healthcare process and takes into consideration the patient’s clinical, family, social and economic conditions [16–18].
In view of the benefits that MTM represents for PD patients, and given the complexity of the disease and treatment, this study aims to analyze the effects of MTM on the patients’ QoL, motor and non-motor symptoms, and medicine-related problems, describing the main pharmaceutical interventions performed.
Methods
Study Design
A quasi-experimental uncontrolled before-and-after study [19] was carried out from October 2012 to April 2013 in a community pharmacy (a training unit) linked to the pharmacy undergraduate course at the Federal University of Santa Catarina and to the Municipal Health Secretariat in Florianopolis, Brazil. This community pharmacy dispenses the medicines included in the Specialized Component of Pharmaceutical Assistance (SCPA) of the Brazilian Unified Health System (SUS). The SCPA aims to guarantee the integrality of the pharmacological treatment, especially for chronic diseases whose medications have a high cost or are of difficult access in the market. The SCPA supplies the following antiparkinsonian medicines: entacapone, tocapone, amantadine, pramipexole, bromocriptine, cabergoline, and selegiline. Such medicines are dispensed according to the Clinical Protocol and Therapeutic Guidelines for the PD treatment, as defined by the Ministry of Health [20, 21].
Patients
At the moment of the study, 161 patients diagnosed with Parkinson’s disease (International Classification of Disease G20) were registered in the SCPA pharmacy. The patients and/or the caregiver were contacted and invited to participate in this study during the dispensing session of antiparkinsonian medicines by pharmacist AAF during her shift. After being informed about the MTM process, the participating patients/caregivers signed an Informed Consent Form (ICF) and the first appointment was scheduled. Patients living in nursing homes or patients with discontinued treatment were excluded.
Medication Therapy Management Service
The MTM service was carried out by two pharmacists qualified for clinical pharmaceutical assessment who had knowledge about PD and its treatment. Additionally, all the parameters included in the assessment were agreed between pharmacist and patient prior to the study in order to standardize the data collection. The provision of the MTM service considered the patient’s health needs related to PD and co-morbidities.
The data record forms were designed based on the Pharmacotherapy Workup and the Dader methods [16, 22–24]. The form contained information about demographics, pharmacotherapy, lifestyle, co-morbidities, memory, and cognition, PD symptoms, and QoL.
Pharmacotherapy data (indication, dose, posology, scheduling, and reports on the access to medicines) were collected from the medical prescription, patient diary [25] and all medicines (over-the-counter and prescription medicines) brought by the patient to the appointment with the pharmacist. Such data were supplemented with information from the medical chart. Adherence was measured through clinical interviews (open questions made during the interview in the first and sixth appointments) and by the Morisky questionnaire (self-reporting by answering closed questions in the first, third, and sixth appointments) [26].
Lifestyle data were collected in the clinical interviews and from the patient’s diary. Co-morbidity data were collected from medical charts and test results brought by the patient to the appointments. Memory and cognition impairment data were collected with the mini–mental state examination test [27] which was applied in the second appointment. The PD symptoms assessment was based on the patients’ and/or caregivers’ perception about the symptoms’ progression. The patients or caregivers that reported bradykinesia, tremor and/or rigidity during the period between their doses were classified as off-state. The patients or caregivers that did not report these symptoms were classified as on-state. Non-motor symptoms (e.g. autonomic disorder, insomnia, hallucinations) were classified as relief or non-relief. These data were collected throughout the six appointments.
After analyzing the aforementioned data, the pharmacist identified potential medicine-related problems reported by the patients in the appointments. The problems were classified according to Cipolle et al. [16] as indication, effectiveness, safety and adherence. When a medicine-related problem was identified, the pharmacist designed an intervention procedure in order to solve it based on clinical, familiar, and social context data; the latter two were collected using the genogram [28, 29] and ecomap [30–32] tools.
The interventions to resolve the detected medicine-related or other health problems referring to PD were targeted to patients/caregivers and/or doctors. The interventions targeting the patients/caregivers included counseling, rescheduling, education measures, changes in the pharmacotherapy (only OTC medicines), and referral to other healthcare services (e.g., phonoaudiology, physiotherapy). The interventions were held with the support of written instructions referring to verbal counseling, educational material, personalized calendar of the pharmacological treatment and the pharmacotherapeutic diary (meal and medication reminder). The interventions targeting the doctors included suggestions of changes in the therapeutic regimen of prescription medicines (addition, discontinuation, posology, or dosage form). The doctors were informed about the recommendations by means of letters based on the best evidence available collected from searches in the databases Pubmed, Cochrane, Bireme, and Micromedex®.
In order to record the follow-up data, the SOAP (Subjective, Objective, Assessment Plan) [33] was used.
Main Outcomes Analyzed
The effects of MTM upon PD patients were assessed considering QoL improvement or maintenance as a primary outcome. QoL was measured by means of the Parkinson’s Disease Questionnaire (PDQ-39) in a legitimate Brazilian Portuguese version. This is a PD-specific questionnaire that consists of 39 questions distributed between eight multi-item domains: mobility (ten questions), activities of daily living (six questions), emotional wellbeing (six questions), social support (three questions), body discomfort (three questions), stigma (four questions); cognition (four questions); and communication (three questions) [34–36]. Primary outcomes include changes in the general and specific PDQ-39 domain and scores. Secondary outcomes include changes in the On/Off state of motor symptoms and relief of non-motor symptoms from the patient’/caregiver’ perspective, as well as solutions to medicine-related problems, especially those related to adherence.
Data Analysis
The results of the descriptive analysis were expressed as median ± standard deviation, and frequency was expressed in percentage (%). For the analysis, (1) the Student’s t test, (2) the Fisher’s Chi square test (χ 2), and (3) the one-way ANOVA test were used respectively for: (1) calculating the association between 2 averages: inter- and intra-groups; (2) calculating the association between categorical variables; and (3) comparing a categorical variable with the average of another one. SPSS-Kappa Measure of Agreement was used to analyze the concordance between the Morisky Medication Adherence Scale and the clinical interviews. Confidence intervals of 95% and values of p < 0.05 were considered significant. The data analysis was carried out using the software SPSS Statistics 15.0 for Windows.
Ethical Issues
This research was approved by the Human Ethics Committee at the Federal University of Santa Catarina with Institutional Approval No. 1963/2011. All ethical aspects were in accordance with the Declaration of Helsinki of 1964, as revised in 2013.
Results
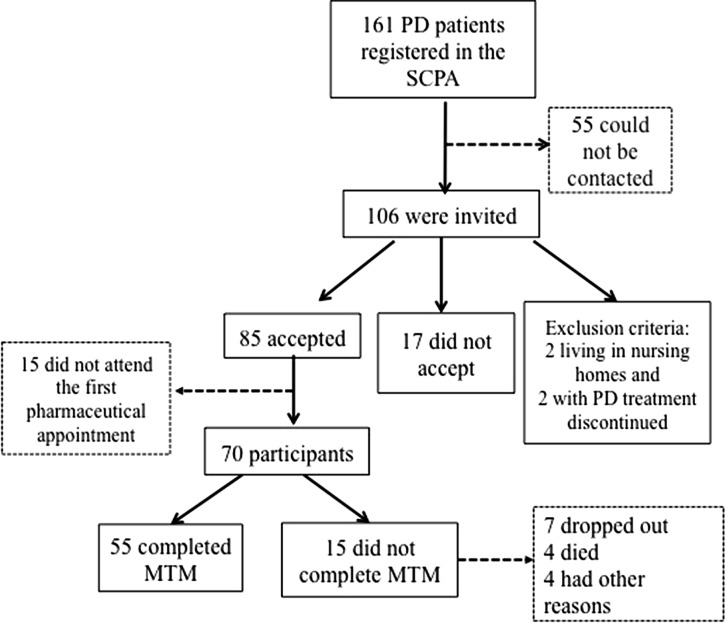
Seventy of the 161 PD patients registered in the SCPA were included in the study. The main reason why some patients declined to participate was difficulty to get to the pharmacy. Fifty-one of the 70 participating patients completed the expected 6 months of MTM. Figure 1 shows the sampling flowchart. The socio-demographical baseline data of the studied population is shown in Table 1.
Fig. 1.
Sampling flowchart
Table 1.
Socio-demographical baseline data of PD patients in the study (N = 70)
| Sex | Male: 64.3% |
| Female: 35.7% | |
| Average age ± SD | 69.4 ± 11 |
| Marital status | Married: 65.7% |
| Single: 5.7% | |
| Widowed: 18.6% | |
| Separated: 10% | |
| Type of medical assistance | Public: 22.9% |
| Private: 62.9% | |
| Mixeda: 14.2% | |
| Self-declared skin color | White: 85.7% |
| Black: 5.7% | |
| Multiracial: 8.6% | |
| Productive sapacity | Retired: 82.9% |
| Active: 12.9% | |
| Home worker: 4.3% | |
| Education | Illiterate: 5.7% |
| 1–3 years: 7.1% | |
| 4–8 years: 21.1% | |
| More than 8 years: 55.7% | |
| Assisted by a caregiver | 37.1% |
SD standard deviation
aMixed = public + private medical assistance
In the first appointment, 35 patients reported rigidity and 35 reported tremors as predominant PD motor symptoms. The most frequent autonomic disorders were constipation (43%), speech disorder (36%), dysphagia (28%), and urinary dysfunction (28%). The most frequent neurological co-morbidities were depression (42.3%), sleep disorder (30.8%), and dementia associated with PD (28%).
Medicine-Related Problems
Types and frequency of the medicine-related problems are shown in Table 2.
Table 2.
Classification, type, and frequency of medicine-related problems
| Classification | Type | Frequency (%) |
|---|---|---|
| Indication | Unnecessary medication | 2 (1.7) |
| Necessity of additional medication | 16 (13.8) | |
| Effectiveness | Ineffective medication | 3 (2.6) |
| Underdosage | 16 (13.8) | |
| Safety | Adverse reaction | 19 (16.4) |
| High dosage | 17 (14.6) | |
| Adherence | Non-adherence | 43 (37.1) |
| Total | 116 (100.0) |
Based on Cipolle et al. [16]
One hundred sixteen medicine-related problems were identified (mean 1.7 ± 1.3 per patient). Of the patients, 87.1% presented at least one medicine-related problem in the first appointment. Nine (7.8%) patients did not present medicine-related problems over the 6 months of MTM.
The most frequent medicine-related problem types were Adherence (non-adherence) (37.1%) followed by Safety (31.0%), Ineffectiveness (16.4%), and Indication (15.5%). Of the Ineffectiveness type, 84.2% were related to underdose, and in 88.9% of the Indication type an additional medicine was needed.
Interventions
A total of 404 pharmaceutical interventions were performed (mean 5.8 ± 3.1 interventions/patient). A number of 279 interventions (69.1%) were accepted and in 213 of them (76.3%) the health problem (medicine-related or other health problems) was resolved (Table 3). 103 (25.5%) of the 404 interventions were aimed to resolve the medicine-related problems. These interventions were more effective to resolve the problems related to non-adherence (n = 43; 46.5%) and adverse reactions (n = 13; 68%). For some of the identified problems, interventions were not carried out by the pharmacist because the patient had had a follow-up visit prior to the appointment with the pharmacist and his/her doctor had changed the treatment.
Table 3.
Pharmaceutical interventions performed, accepted, and solved by conduct (n = 404)
| Conduct | N (%) | N accepted (%) | N solved (%) |
|---|---|---|---|
| Non-pharmacological treatment guidance (nutrition, exercises, etc.) | 115 (28.5) | 74 (64.3) | 53 (71.6) |
| Pharmacological treatment guidance (information about medication) | 98 (24.4) | 72 (73.5) | 60 (83.3) |
| Rescheduling | 55 (13.6) | 49 (89.1) | 33 (67.3) |
| Guidance on PD | 49 (12.1) | 36 (73.5) | 30 (83.3) |
| Referral to specialistsa | 17 (4.2) | 8 (47.1) | 4 (50.0) |
| Education for habit changing | 13 (3.2) | 10 (76.9) | 7 (70.0) |
| Addition of a new medicationb | 12 (3.0) | 6 (50.0) | 4 (66.7) |
| Guidance on the access to the medication | 11 (2.7) | 4 (36.4) | 3 (75.0) |
| Medication discontinuationb | 10 (2.5) | 6 (60.0) | 6 (100.0) |
| Guidance on another pathology | 7 (1.7) | 5 (71.4) | 4 (80.0) |
| Change of medicationb | 6 (1.5) | 2 (33.3) | 2 (100.0) |
| Guidance on the medication use | 5 (1.2) | 4 (80.0) | 4 (100.0) |
| Change of dosageb | 3 (0.7) | 3 (100.0) | 3 (100.0) |
| Change of dosage formb | 3 (0.7) | 0 (0.0) | – |
| Total | 404 | 279 | 213 |
aPhysical therapists, speech therapists, physical education professionals. These health professionals were voluntary workers at the Parkinson Association of Santa Catarina—APASC
bIf the medicine was OTC, the pharmacist added a new medication. In the case of prescription medicines, the pharmacist sent a letter to the doctor suggesting the changes
Furthermore, out of the 297 interventions (73.5%) aimed to resolve PD-related problems, 200 (67.3%) were accepted and in 152 of these (76%) the problem was resolved. The most frequent PD-related interventions included guidance on the disease (24.6%), pharmacological (23.5%), and non-pharmacological (20.1%) treatments. Most of the interventions (N = 92) were carried out in order to resolve health problems related to autonomic disorders, especially concerning non-pharmacological measures, rescheduling, and pharmaceutical indication.
The main interventions to resolve sleeping problems included non-pharmacological and rescheduling measures. Most of these interventions were targeted to the patients (87.5%); 71.4% of them were accepted and all of them resolved the sleeping problems.
The number of interventions decreased over time. 177 interventions (43.8%) were carried out in the first appointment and 27 (15%) in the sixth appointment. Among the factors associated with the number of interventions were age (inversely proportional variable), QoL (significant positive association only in the stigma domain; p = 0.055), and diagnosis time (significant positive association p = 0.022) for patients diagnosed with PD in a period of up to 2 years and between 5 and 10 years.
The MTM sessions showed that issues such as self-knowledge, empowerment facing the disease and treatment, stigma, participation in support groups, caregiver/family strengthening, encouragement to non-pharmacological treatments and healthier life were not stimulated by any health professional involved in the patient’s care. However, the patients that were part of the APASC support group (15.7% of the patients) were already being helped with these issues.
MTM Outcomes
The outcomes before and after the MTM service are described in Table 4. It consists of an intention-to-treat (ITT) analysis.
Table 4.
Outcomes reached after 6 months of MTM (N = 70)
| Outcome | Initial value | After 6 months | p | |
|---|---|---|---|---|
| QoL | PDQ 39 | Reduction in all domains | NS | |
| PDQ39_3 (emotional wellbeing) (mean ± SD) | 39.12 ± 26.54 | 36.89 ± 27.56 | 0.012 | |
| Motor symptomsa | On | 60% | 58.6% | NS |
| Off | 40% | 22.9% | ||
| Not assessedb | 0 | 18.6% | ||
| Non-motor symptoms (patients) | Constipation | 27 | 9 | <0.001 |
| Dysphagia | 8 | 3 | 0.001 | |
| Gastric dysfunction | 7 | 0 | 0.008 | |
| Speech disorder | 14 | 11 | <0.001 | |
| Hypotension | 6 | 0 | 0.014 | |
| Urinary incontinence | 2 | 1 | 0.029 | |
| Sleep disorder | 6 | 3 | <0.001 | |
| Anxiety | 3 | 1 | 0.043 | |
| DRP | Problem resolved (mean ± SD) | 1.7 ± 1.3 | 0.8 ± 0.9 | <0.001 |
| Adherence | Clinical interview | 37 non-adherent | 10 non-adherent | 0.001 |
| Morisky–Green–Levine | 42 non-adherent | 20 non-adherent | NS | |
NS non-significant, PDQ-39_3 third domain of PDQ-39, SD standard deviation, DRP drug-related problem
aThe patient’s perception about the On/Off state after taking the medicines
bNot assessed: when neither the patient or the caregiver was able to attend the appointment and someone else went to the pharmacy to get the medicines for them
Quality of Life
After the MTM service, all the PDQ-39 scores improved; however, a statistically significant improvement (p = 0.012) was perceived only in the item related to emotional wellbeing (PDQ3). In spite of the short period of analysis, these data represent a positive clinical impact of MTM upon the PD patients’ QoL. The improvement of the PDQ-39 scores before and after MTM was not correlated with adherence, cognition, age, or sex.
Motor and Non-Motor Symptoms
The On/Off state of motor symptoms from the perspective of the patients/caregivers did not vary from the first to the sixth appointments.
The results for the non-motor symptoms showed that in general all the symptoms were to some extent relieved after the MTM service. Best results were obtained for the control of constipation, dysphagia, gastric dysfunction, and hypotension.
Medicine-Related Problems Resolution
After the interventions, the number of medicine-related problems decreased significantly (p < 0.001) from 1.7 ± 1.3 in the first appointment to 0.8 ± 0.9 in the sixth appointment. The adherence evaluation in the clinical interview verified a decline from 37 to 10 non-adherent patients (p < 0.001). When evaluated by the Morisky questionnaire, adherence had a significant increase only between the first and the third appointments, from 28 to 34 adherent patients (p = 0.005). The difference between the non-adherent patients assessed in the first and the sixth appointments was not statistically significant.
It was not possible to carry out a post-MTM assessment with 12 of the patients (15.7%) as they did not complete the 6 months of follow-up, in addition to four other patients who were not able to attend the appointment. It is worth noting that there were differences in the results of the adherent patients at the end of the MTM process (appointment 6) between the Morisky and Clinical Interviews methods (kappa value = 0.304; α 95%, p = 0.017); despite a considerable concordance (but not high) found between the methods in the first appointment (kappa value = 0.333, α 95%; p = 0.007).
Discussion
Parkinson’s disease is a complex condition due to the progressive nature of its motor and non-motor symptoms, in addition to the fact that PD medicines quickly lose their effectiveness and cause long-term side effects. These characteristics create the necessity of a comprehensive approach towards a constant monitoring of the pharmacological therapy and the PD effects on the patients’ QoL [37]. These characteristics consider QoL as a primary outcome in PD [3]. Furthermore, because of the particularities of the disease, individualized interventions are required.
As previously indicated, most of the interventions were performed in a period of up to 2 years and between 5 and 10 years after the diagnosis, as these are the two critical periods in the PD patient’s life. Up to 2 years, the patient experiences a period of adaptation and acceptance of the diagnosis. In the period from 5 to 10 years, the PD patient faces complications related to the treatment and the disease’s progression [38].
Based on MTM, the analysis detected health problems related and not related to medicines. Our results demonstrate that even though most of the identified health problems were not medicine-related, they could be resolved by pharmaceutical interventions. For instance, autonomic disorders were one of the most frequent problems not related to medicines and can result from the disease’s progression and/or antiparkinsonian medication side effects. Their negative effects on the PD patients’ QoL were also mentioned in other studies [39–41]. The symptoms can be relieved by means of nonpharmacological measures or by OTC medicines. Therefore, this type of interventions became important in the pharmaceutical practice.
Although there are few studies about medicine-related problems in PD patients, the results of the present study were similar to those of other published papers. Our results show that the medicine-related problems were more frequently related to non-adherence, adverse reactions, necessity of additional medication and underdose. In another study carried out in community pharmacies, most DRPs concentrated on the need of additional medicines (26.3%) and adverse reactions (12.4%) [14]. In another study at a nursing home, most DRPs concentrated on unnecessary medications (28%) and underdose (14%) [15]. According to Schröder et al. [14], underdose may be associated with the prevention of adverse reactions to dopaminergic medications. Moreover, the medicine-related problems associated with the necessity of additional medication may stem from the fact that the clinical practice focuses on motor rather than non-motor symptoms, which remain underestimated and untreated.
Similarly to other studies, our results show that non-adherence is the main medicine-related problem. This finding highlights the importance of health professionals following up the patient’s medication use in order to investigate if the symptoms are a consequence of non-adherence or of the disease’s progression. In this way, unnecessary dosage change or addition of new medicines can be avoided, reducing the risk of adverse events [8, 14, 42, 43].
One of the key factors to improve adherence is the patients’/caregivers’ knowledge about the disease and awareness of the consequences of discontinuing the medicine. This is the reason why it is crucial to consider the patient as an active partner in the healthcare process. Good communication between the health professional and the patient is fundamental for effective clinical practice and for adherence improvement. Some causes of non-adherence include the patient’s search for other treatment options, especially for the cure (mysticism, beliefs), and discontinuation of the treatment in case-positive effects are not experienced.
Moreover, our results corroborate those reported by Navarro-Peternella and Marcon [44] ,which demonstrated the necessity of individualized professional interventions based on actions that represent the real needs of the PD patient and/or caregiver. Guidance and information about the disease, its progression, and ways of facing it are fundamental for patient, caregiver, and family. The healthcare services for PD patients must aim to minimize the limitations resulting from the disease’s progression and contribute to the improvement or maintenance of their QoL [3].
Regarding the outcomes of the MTM service related to QoL, and despite the short period of analysis, this study produced a positive clinical impact. It is worth remarking that one of the health professional’s biggest challenges is to prepare his/her therapeutic plan in a way that the patient’s QoL reaches a level that can be considered good [44, 45].
The improvement of the PDQ-39 score regarding emotional wellbeing after the MTM process confirms the previously reported humanistic positive impacts of MTM on the patient’s life [18, 46]. During this process, the patient is seen as an individual with rights, knowledge, and experience and is treated as a partner in the planning of his/her care, being responsible for taking the final decision in the therapeutic conduct [16]. This horizontal relationship between pharmacist and patient generates a transparent environment of trust and credibility necessary for establishing a therapeutic alliance. In addition the perception of the patient as the central figure of the pharmaceutical care within their family and social context, singularity, complexity, and subjectivity contributed to a more comprehensive care. This way of generating healthcare may have contributed to the improvement of the PD patient’s wellbeing.
MTM was not able to improve or stabilize the patients’ motor symptoms. A contributing factor is the absence of medicines that actually delay the disease’s progression, which undoubtedly leads to the worsening of the motor symptoms. Nevertheless, MTM has a considerable impact on the relief of non-motor symptoms, thus contributing to the PD patients’ QoL.
Variations in the results of adherence assessed by different methods were also reported by Perseguer-Torregrosa et al. [47]. This difference reflects the way that each method considers the patient’s role during the therapy. While the Morisky questionnaire evaluates adherence as the simple act of taking the medication (compliance), the clinical interview method sees the patient as an active partner in the adherence process and considers the patient’s will to collaborate with the treatment (concordance) [48, 49]. In this sense, once the most practical method to evaluate the treatment adherence, especially for elderly patients, is by interviewing the patients or their caregivers [50], the adherence improvement assessed in our study can be considered a significant result of the MTM service for PD patients.
Limitations
This study was conducted at a public community pharmacy which is the only outpatient setting that dispenses antiparkinsonian medicines in the city, except levodopa which is dispensed at primary healthcare pharmacies. As we used the convenience sampling method including only the patients that we could contact instead of a random sample, a selection bias may have occurred. However, the characteristics of the population under analysis are similar to those reported in other studies [51–55].
The sample loss can be considered a limiting factor of the statistical analysis. Nevertheless, the results can be considered relevant from a clinical point of view, once there was a decrease from 42 to 20 in the number of non-adherent patients. Since 15 patients did not complete the MTM process, the number of interventions per patient over the 6 months may have been underestimated.
Another limitation of the study was the unavailability of a neurologist. In an MTM service, this partnership is very important to achieve the desired outcomes, since many interventions need the doctor’s collaboration.
The quantitative data on humanistic aspects were out of the scope of the study. Therefore, further studies are needed for statistical analyses. For the reason that some interventions to improve adherence were related to changes in the medication regimen, it was not possible to analyze whether the outcomes were driven by medication changes or compliance.
Conclusion
In order to carry out an MTM service with PD patients, the pharmacist’s expertise needs to transcend their knowledge about the pharmacological treatment. Non-pharmacological measures are fundamental to relieve non-motor symptoms (autonomic and neurological disorders), which are not assessed by the PDQ-39 but have an important impact on the PD patients’ QoL. The changes in the individual, family, and social dynamics caused by the disease must be also understood by the pharmacist so that he/she can perform a service based on a comprehensive care perspective.
The interventions resulting from the MTM service contributed to improve or to maintain the PD patients’ QoL, especially their emotional wellbeing. MTM had a positive effect in the decrease of medicine-related problems, especially regarding improvements in the treatment adherence. These results represent clinical and humanistic outcomes of the MTM service.
Acknowledgments
The authors would like to thank Dr. Bernd Storb for the statistical analysis, and the reviewers of this journal for their comments which contributed to improve this manuscript. No funding or sponsorship was received for this study or publication of this article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript and take responsibility for the integrity of the work as a whole, and gave final approval to the version to be published. The concept and design of the study were developed by Foppa and Chemello. Foppa was primarily in charge of the data collection; and Chemello, Foppa and Vargas-Peláez were primarily responsible for the data analysis. The manuscript was primarily written by Foppa with assistance from Chemello and Farias, and was revised by Chemello, Vargas-Pelaez, and Farias. This paper is part of Foppa’s master’s thesis with the title “Qualification of clinical pharmaceutical service based on the Medication Therapy Management for patients with Parkinson’s disease” advised by Farias and co-advised by Chemello.
Disclosures
Aline Aparecida Foppa, Clarice Chemello, Claudia Marcela Vargas-Peláez and Mareni Rocha Farias have nothing to disclose.
Compliance with Ethics Guidelines
This research was approved by the Human Ethics Committee of the Federal University of Santa Catarina with Institutional Approval No. 1963/2011. All ethical aspects are in accordance with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all the participants in the study.
Financing
This project was partially financed by CNPq (National Center for Science and Technology Development) and by CAPES (Coordination for the Improvement of Higher Education Personnel).
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/66D4F060318369C4.
References
- 1.Prediger RD, Bortolanza M, Issy CCA, Dos Santos BL, Bel ED, Vozari RR. Dopaminergic neurons in Parkinson’s disease. In: Kostrzewa R, editor. Handobook of neurotoxicity. New York: Springer; 2013. pp. 01–36. [Google Scholar]
- 2.Carod-Artal FJ, Vargas AP, Martinez-Martin P. Determinants of quality of life in Brazilian patients with Parkinson’s disease. Mov Disord. 2007;22(10):1408–1415. doi: 10.1002/mds.21408. [DOI] [PubMed] [Google Scholar]
- 3.Marinus J, Ramaker C, Van Hilten JJ, Stiggelbout AM. Health related quality of life in Parkinson’s disease: a systematic review of disease specific instruments. J Neurol Neurosurg Psychiatry. 2002;72(2):241–248. doi: 10.1136/jnnp.72.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camargos ACR, Cópio FCQ, Sousa TRR, Goulart F. O impacto da doença de Parkinson na qualidade de vida: uma revisão de literatura. Revista Brasileira de Fisioterapia. 2004;8(3):267–272. [Google Scholar]
- 5.Kulkarni AS, Balkrishnan R, Anderson RT, Edin HM, Kirsch J, Stacy MA. Medication adherence and associated outcomes in medicare health maintenance organization-enrolled older adults with Parkinson’s disease. Mov Disord. 2008;23(3):359–365. doi: 10.1002/mds.21831. [DOI] [PubMed] [Google Scholar]
- 6.Davis KL, Edin HM, Allen JK. Prevalence and cost of medication nonadherence in Parkinson’s disease: evidence from administrative claims data. Mov Disord. 2010;25(4):474–480. doi: 10.1002/mds.22999. [DOI] [PubMed] [Google Scholar]
- 7.Strand LM, Morley PC, Cipolle RJ, Ramsey R, Lamsam GD. Drug-related problems: their structure and function. DICP. 1990;24(11):1093–1097. doi: 10.1177/106002809002401114. [DOI] [PubMed] [Google Scholar]
- 8.Grosset KA, Bone I, Grosset DG. Suboptimal medication adherence in Parkinson’s disease. Mov Disord. 2005;20(11):1502–1507. doi: 10.1002/mds.20602. [DOI] [PubMed] [Google Scholar]
- 9.Grosset D, European PD Therapy Compliance Study Group Therapy adherence issues in Parkinson’s disease. J Neurol Sci. 2010;289:115–118. doi: 10.1016/j.jns.2009.08.053. [DOI] [PubMed] [Google Scholar]
- 10.Grosset KA, Grosset DG. Effect of educational intervention on medication timing in Parkinson’s disease: a randomized controlled trial. BMC Neurol. 2007;7:01–06. doi: 10.1186/1471-2377-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pasina L, Brucato AL, Falcone C, Cucchi E, Bresciani A, Sottocorno M, Taddei GC, Casati M, Franchi C, Djade CD, Nobili A. Medication non-adherence among elderly patients newly discharged and receiving polypharmacy. Drugs Aging. 2014;31(4):283–289. doi: 10.1007/s40266-014-0163-7. [DOI] [PubMed] [Google Scholar]
- 12.Brooks DJ. Optimizing levodopa therapy for Parkinson’s disease with levodopa/carbidopa/entacapone: implications from a clinical and patient perspective. Neuropsychiatr Dis Treat. 2008;4(1):39–47. doi: 10.2147/NDT.S1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daley DJ, Deane KH, Gray RJ, Worth PF, Clark AB, Sabanathan K, Pfeil M, Myint PK. The use of carer assisted adherence therapy for people with Parkinson’s disease and their carers (CAAT-PARK): study protocol for a randomized controlled trial. Trials. 2011;12:01–12. doi: 10.1186/1745-6215-12-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schröder S, Martus P, Odin P, Schaefer M. Drug-related problems in Parkinson’s disease: the role of community pharmacists in primary care. Int J Clin Pharm. 2011;33(4):674–682. doi: 10.1007/s11096-011-9526-x. [DOI] [PubMed] [Google Scholar]
- 15.Poon LH, Lee AJ, Chiao TB, Kang GA, Heath S, Glass GA. Pharmacist’s role in a Parkinson’s disease and movement disorders clinic. Am J Health Syst Pharm. 2012;69(6):518–520. doi: 10.2146/ajhp110127. [DOI] [PubMed] [Google Scholar]
- 16.Cipolle RJ, Strand LM, Morley PC. Pharmaceutical care practice: the patient centered approach to medication management. 3. New York: McGraw-Hill; 2012. [Google Scholar]
- 17.Perlroth D, Marrufo G, Montesinos A, et al. Medication therapy management in chronically ill populations: final report. Blackwell, S.A. 2013. http://innovation.cms.gov/files/reports/mtm_final_report.pdf. Accessed July 24, 2015.
- 18.Oliveira DR. Atenção Farmacêutica: da filosofia ao gerenciamento da terapia medicamentosa. 1. São Paulo: RCN Editora; 2011. [Google Scholar]
- 19.Grimshaw J, Campbell M, Ecclesa M, Steena N. Experimental and quasi-experimental designs for evaluating guideline implementation strategies. Fam Pract. 2000;17(sppl1):S11–S16. doi: 10.1093/fampra/17.suppl_1.S11. [DOI] [PubMed] [Google Scholar]
- 20.Brasil. Portaria n0 204, de 29 de janeiro de 2007. Regulamenta o financiamento e a transferência dos recursos federais para as ações e os serviços de saúde, na forma de blocos de financiamento, com o respectivo monitoramento e controle. Diário Oficial [da] República Federativa do Brasil, Poder Executivo, Brasília, DF, Jan 2007.
- 21.Brasil. Portaria no 2.981, de 26 de novembro de 2009. Aprova o Componente Especializado da Assistência Farmacêutica. Diário Oficial [da] República Federativa do Brasil, Brasília, DF, Nov 2009.
- 22.Mc-Whinney IR. A evolução do método clínico. In: Stewart M, Brown JB, Weston WW, Mc-Whinney IA, Mc William CL, Freeman TR, editors. Medicina centrada na pessoa: transformando o método clínico. 2. Porto Alegre: Artmed; 2010. pp. 35–48. [Google Scholar]
- 23.Weed LL. Medical records, patient care, and medical education. Ir J Med Sci. 1964;39(6):271–282. doi: 10.1007/BF02945791. [DOI] [PubMed] [Google Scholar]
- 24.Machuca M, Fernández-Llimós F, Faus MJ. Dáder Method: Manual de Acompanhamento Farmacoterapêutico. GIAF-UGR: Granada; 2003. [Google Scholar]
- 25.Papapetropoulos SS. Patient diaries as a clinical endpoint in Parkinson’s disease clinical trials. CNS Neurosci Ther. 2012;18(5):380–387. doi: 10.1111/j.1755-5949.2011.00253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 28.Asen KE, Tomson P. Atención Familiar: Guia práctica para los profisionales de la salud. 1. Barcelona: Paidós; 1997. [Google Scholar]
- 29.Nascimento LC, Rocha SMM, Hayes VE. Contribuição do Genograma e Ecomapa para o Estudo de Famílias em Enfermagem Pediátrica. Revista Texto and Contexto Enfermagem. 2005;14(2):280–286. doi: 10.1590/S0104-07072005000200017. [DOI] [Google Scholar]
- 30.Cecagno S, Souza MD, Jardim VMR. Compreendendo o contexto familiar no processo saúde-doença. Acta Scientiarum Health Sci. 2004;26(1):107–112. [Google Scholar]
- 31.More CLO. As redes pessoais significativas como instrumento de intervenção interferences psicológica no contexto comunitário. Paidéia. 2005;15(31):287–297. doi: 10.1590/S0103-863X2005000200016. [DOI] [Google Scholar]
- 32.Sluzki CE. A rede social na pratica sistêmica: alternativas terapêuticas. 2. São Paulo: Casa do Psicólogo; 2003. [Google Scholar]
- 33.Zierler-Brown S, Brown TR, Chen D, Blackburn RW. Clinical documentation for patient care: models, concepts, and liability considerations for pharmacists. Am J Health Syst Pharm. 2007;64(17):1851–1858. doi: 10.2146/ajhp060682. [DOI] [PubMed] [Google Scholar]
- 34.Lana RC, Goulart FRP, Maia T, Prudente C, Cardoso, F. Estudo da confiabilidade do Questionário de Qualidade de Vida na Doença de Parkinson-39 (PDQ-39). In: Anais do VIII Encontro de Extensão da UFMG. Belo Horizonte: UFMG; 2005. p. 01–06.
- 35.Souza RG, Borges V, Silva SMCA, Ferraz HB. Quality of life scale in Parkinson’s disease PDQ-39—(Brazilian Portuguese version) to assess patients with and without levodopa motor fluctuation. Arq Neuropsiquiatr. 2007;65(3):787–791. doi: 10.1590/S0004-282X2007000500010. [DOI] [PubMed] [Google Scholar]
- 36.Martinez-Martin P, Jeukens-Visser M, Lyons KE, et al. Health-related quality-of-life scales in Parkinson’s disease: critique and recommendations. Mov Disord. 2011;26(13):2371–2380. doi: 10.1002/mds.23834. [DOI] [PubMed] [Google Scholar]
- 37.Schrag A, Jahanshahi M, Quinn N. What contributes to quality of life in patients with Parkinson’s disease? J Neurol Neurosurg Psychiatry. 2000;69(3):308–312. doi: 10.1136/jnnp.69.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goodman LS, Gilman AG, Brunton LL. As Bases Farmacológicas da Terapêutica. 11. Rio de Janeiro: McGraw Hill; 2006. [Google Scholar]
- 39.Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23(6):837–844. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- 40.Hely MA, Morris JG, Reid WG, Trafficante R. Sydney multicenter study of Parkinson’s disease: non-l-dopa-responsive problems dominate at 15 years. Mov Disord. 2005;20(2):190–199. doi: 10.1002/mds.20324. [DOI] [PubMed] [Google Scholar]
- 41.Barone P, Antonini A, Colosimo C, et al. The priamo study: a multicenter assessment of non-motor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord. 2009;24(11):1641–1649. doi: 10.1002/mds.22643. [DOI] [PubMed] [Google Scholar]
- 42.Grosset KA, Bone I, Grosset DG. Medicine-taking behavior: implications of suboptimal compliance in Parkinson’s disease. Mov Disord. 2005;20(11):1397–1404. doi: 10.1002/mds.20525. [DOI] [PubMed] [Google Scholar]
- 43.Chemello C, de Souza F, de Souza EP, Farias MR. Pharmaceutical care as a strategy to improve the safety and effectiveness of patients pharmacotherapy at a Pharmacy School: a practical proposal. Braz J Pharm Sci. 2014;50(1):185–193. doi: 10.1590/S1984-82502011000100019. [DOI] [Google Scholar]
- 44.Navarro-Peternella FM, Marcon SS. Qualidade de vida quality of life de indivíduos com Parkinson e sua relação com tempo de evolução e gravidade da doença. Revista Latino americana de Enfermagem. 2012;20(2):384–391. doi: 10.1590/S0104-11692012000200023. [DOI] [PubMed] [Google Scholar]
- 45.Ai AL, Carretta H, Beitsch LM, Watson L, Munn J, Mehriary S. Medication therapy management programs: promises and pitfalls. J Manag Care Spec Pharm. 2014;20(12):1162–1182. doi: 10.18553/jmcp.2014.20.12.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Viswanathan M, Kahwati LC, Golin CE, Blalock SJ, Coker-Schwimmer E, Posey R, Lohr KN. Medication therapy management interventions in outpatient settings: a systematic review and meta-analysis. JAMA Intern Med. 2015;175(1):76–87. doi: 10.1001/jamainternmed.2014.5841. [DOI] [PubMed] [Google Scholar]
- 47.Perseguer-Torregrosa Z, Orozco-Beltrán D, Gil-Guillen VF, et al. Magnitude of pharmacological nonadherence in hypertensive patients taking antihypertensive medication from a community pharmacy in Spain. J Manag Care Spec Pharm. 2014;20(12):1217–1225. doi: 10.18553/jmcp.2014.20.12.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gusmão JL, Junior Mion D. Adesão ao tratamento—conceitos. Revista Brasileira de Hipertensão. 2006;13(1):23–25. [Google Scholar]
- 49.World Health Organization. Adherence to long-term therapies: evidence for action. Geneva: World Health Organization; 2003. http://www.who.int/chp/knowledge/publications/adherence_report/en/ Accessed July 26, 2015.
- 50.MacLaughlin EJ, Raehl CL, Treadway AK, Sterling TL, Zoller DP, Bond CA. Assessing medication adherence in the elderly: which tools to use in clinical practice? Drugs Aging. 2005;22(3):231–255. doi: 10.2165/00002512-200522030-00005. [DOI] [PubMed] [Google Scholar]
- 51.De Lau LML, Breteler MMB. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5(6):525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 52.Twelves D, Perkins KSM, Counsell C. Systematic review of incidence studies of Parkinson’s disease. Mov Disord. 2003;18(1):19–31. doi: 10.1002/mds.10305. [DOI] [PubMed] [Google Scholar]
- 53.Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, Nelson LM. Incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. Am J Epidemiol. 2003;157(11):1015–1022. doi: 10.1093/aje/kwg068. [DOI] [PubMed] [Google Scholar]
- 54.Elbaz A, Bower JH, Maraganore DM, McDonnell SK, Peterson BJ, Ahlskog JE, et al. Risk tables for parkinsonism and Parkinson’s disease. J Clin Epidemiol. 2002;55(1):25–31. doi: 10.1016/S0895-4356(01)00425-5. [DOI] [PubMed] [Google Scholar]
- 55.Wirdefeldt K, Adami HO, Cole P, Trichopoulos D, Mandel J. Epidemiology and etiology of Parkinson’s disease: a review of the evidence. Eur J Epidemiol. 2011;8(1):1–58. doi: 10.1007/s10654-011-9581-6. [DOI] [PubMed] [Google Scholar]