Abstract
Objective
Plasma high-density-lipoproteins (HDL) have several putative anti-atherogenic effects, including preservation of endothelial functions. This is thought to be mediated, in part, by the ability of HDL to promote cholesterol efflux from endothelial cells (ECs). The ATP binding cassette transporters A1 and G1 (ABCA1 and ABCG1) interact with HDL to promote cholesterol efflux from ECs. To determine the impact of endothelial cholesterol efflux pathways on atherogenesis, we prepared mice with endothelial-specific knockout of Abca1 and/or Abcg1.
Approach and Results
Generation of mice with EC-ABCA1 and ABCG1 deficiency required crossbreeding Abca1fl/flAbcg1fl/flLdlr−/− mice with the Tie2Cre strain, followed by irradiation and transplantation of Abca1fl/flAbcg1fl/fl bone marrow to abrogate the effects of macrophage ABCA1 and ABCG1 deficiency induced by Tie2Cre. After 20–22 weeks of Western type diet (WTD), both single EC-Abca1 and Abcg1 deficiency increased atherosclerosis in the aortic root and whole aorta. Combined EC-Abca1/g1 deficiency caused a significant further increase in lesion area at both sites. EC-Abca1/g1 deficiency dramatically enhanced macrophage lipid accumulation in the branches of the aorta that are exposed to disturbed blood flow, decreased aortic eNOS activity, and increased monocyte infiltration into the atherosclerotic plaque. Abca1/g1 deficiency enhanced LPS-induced inflammatory gene expression in mouse aortic ECs, which was recapitulated by ABCG1 deficiency in human aortic ECs.
Conclusions
These studies provide direct evidence that endothelial cholesterol efflux pathways mediated by ABCA1 and ABCG1 are non-redundant and athero-protective, reflecting preservation of eNOS activity and suppression of endothelial inflammation, especially in regions of disturbed arterial blood flow.
Keywords: endothelium, HDL, ABC transporters, atherosclerosis, angiogenesis
Introduction
Plasma high-density-lipoprotein (HDL) levels correlate inversely with the incidence of cardiovascular disease in humans.1 While the causative nature of this association in humans has been called into question by Mendelian randomization studies,2 abundant evidence in animal models and limited evidence in humans indicate that increased production or infusion of HDL is anti-atherogenic.3–8 A plethora of anti-atherogenic mechanisms has been invoked to explain these beneficial effects of HDL;9–11 however, relatively few of these have been adequately tested in vivo.
Endothelial dysfunction brought about by hypercholesterolemia and disturbed arterial blood flow is a key early event in atherogenesis.12–14 The ability of HDL to preserve endothelial function is thought to be central to its overall anti-atherogenic role.11, 15–18 In humans, HDL levels correlate with endothelium-dependent vasodilation in atherosclerotic coronary arteries,19 and, compellingly, infusion of cholesterol poor reconstituted HDL improves forearm blood-flow in subjects with heterozygous ABCA1 deficiency and reduced HDL levels.20 Studies in cultured endothelial cells (ECs) and mice have shown that HDL preserves endothelial function by maintaining the activity of endothelial NO synthase (eNOS).11, 15–18 HDL also preserves endothelial function by suppressing the expression of vascular cell adhesion molecule-1 (VCAM-1) and intracellular adhesion molecule-1 (ICAM-1) in ECs.21–23 While some of these effects are thought to be mediated by the stimulation of cholesterol efflux pathways,18, 24 the role of endothelial cholesterol efflux pathways in atherosclerosis has not been directly tested, and a variety of alternative protective effects of HDL on endothelial function have been proposed.25, 26
Various cholesterol efflux pathways have been shown to operate in cultured ECs including those mediated by Scavenger Receptor BI (SR-BI), passive diffusion and the ATP-Binding Cassette Transporters A1 and G1 (ABCA1 and ABCG1).17, 18 ABCA1 and ABCG1 mediate cholesterol efflux to apolipoprotein A1 (apoA1) and HDL, respectively.18, 24, 27 Studies in human aortic ECs (HAECs) have shown high expression of ABCG1 but not ABCA1,18 and our studies in cultured mouse ECs have suggested a predominant role of ABCG1 in preservation of endothelial function.18, 24 An athero-protective effect of vascular ABCG1 was suggested by transplantation of Abcg1+/+ bone marrow into mice with whole body Abcg1 deficiency on the Ldlr−/− background28 and ABCG1 was shown to have a major role in suppressing pro-atherogenic VCAM-1 expression in ECs.29 However, these studies did not directly test the role of endothelial ABCG1 in endothelial inflammation and atherosclerosis in vivo. Moreover, endothelial Abca1 as well as Abcg1 expression is upregulated by laminar blood flow in the thoracic aorta in mice,30 and endothelial Abca1 expression is upregulated in the aortic arch by treatment with a pro-atherogenic stimulus.31
Thus, there could be roles for both ABC transporters in preservation of endothelial functions in vivo. Relevant functions suggested by previous studies include preservation of arterial eNOS activity and suppression of endothelial inflammation.18, 24, 29 In addition, elegant studies in the zebrafish model have demonstrated roles for ABCA1 and ABCG1 in the suppression of angiogenesis.32 We have developed Abca1fl/fl and Abcg1fl/fl mice,33 allowing us to address the role of cell-specific pathways mediating cholesterol efflux in atherosclerosis. The goals of this study were to assess the impact of deleting endothelial ABCA1 and/or ABCG1 on atherogenesis and to assess the relevance of potential anti-atherogenic mechanisms.18, 24, 29, 32
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
ABCA1 and ABCG1 expression in VecadherinCreAbca1fl/flAbcg1fl/fl and Tie2CreAbca1fl/flAbcg1fl/fl mice
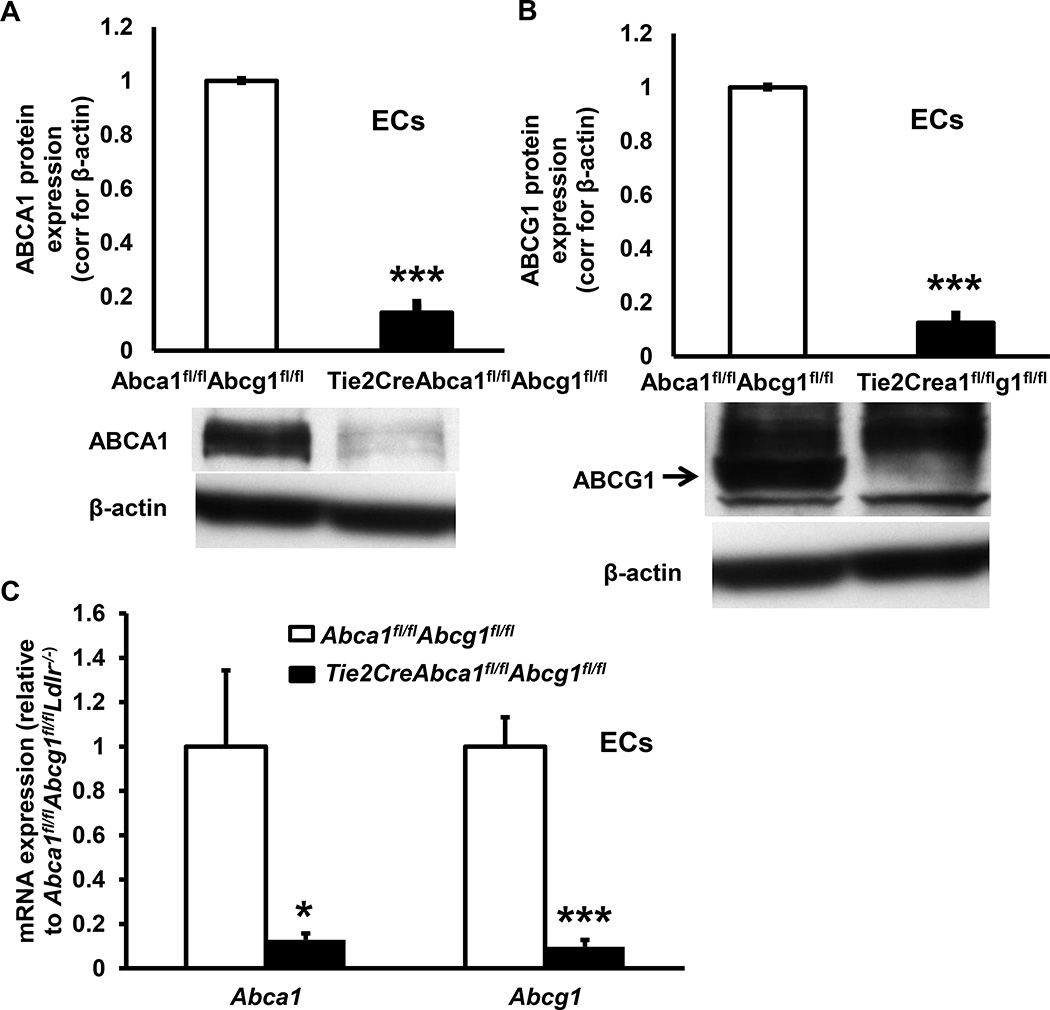
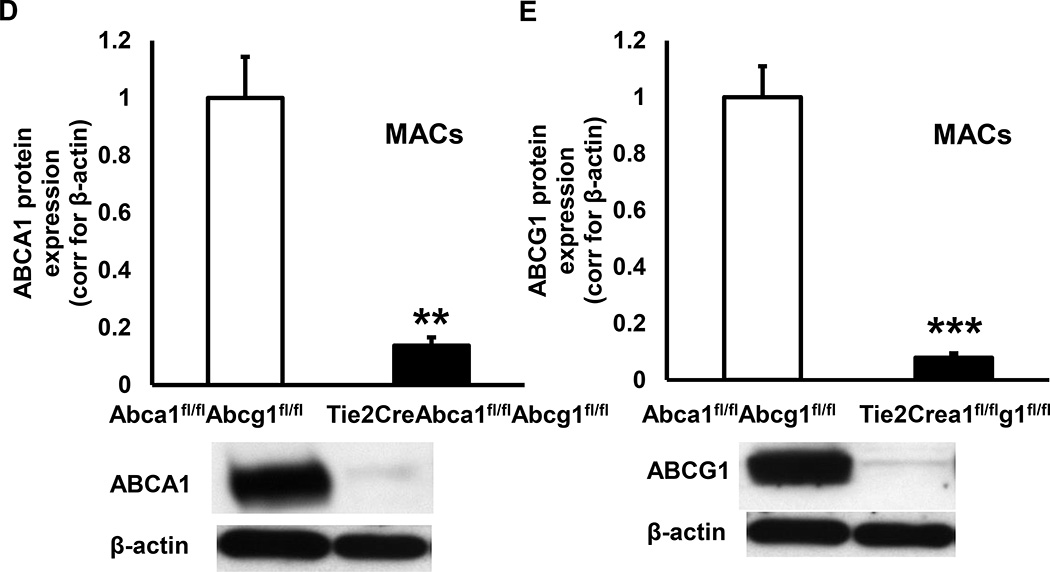
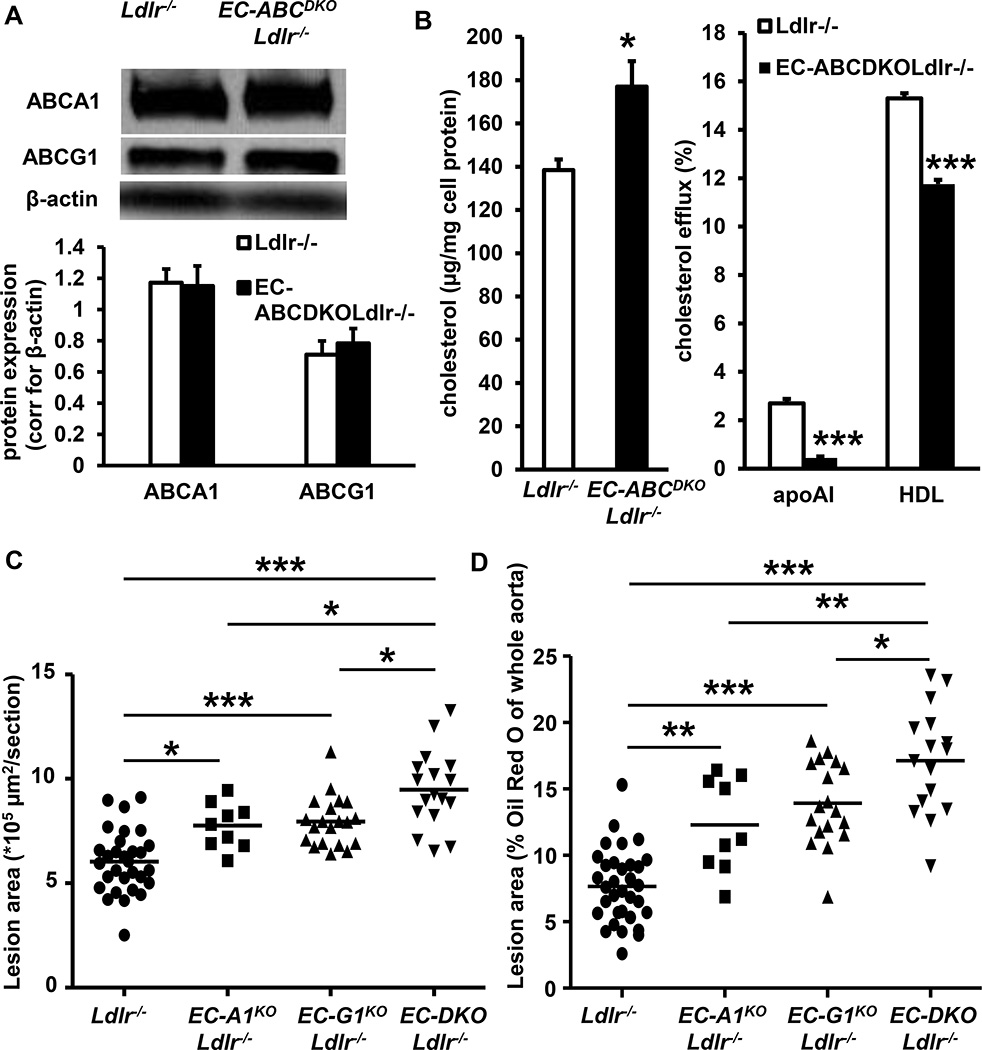
We attempted to develop an endothelial-specific Abca1/g1 deficient mouse model, by crossbreeding Abca1fl/flAbcg1fl/fl mice with mice expressing the endothelial-specific VecadherinCre promoter (also referred to as Cdh5Cre)34 to generate VecadherinCreAbca1fl/flAbcg1fl/fl and Abca1fl/flAbcg1fl/fl mice. However, VecadherinCreAbca1fl/flAbcg1fl/fl mice did not show reduced ABCA1 and ABCG1 protein expression in ECs compared to their littermate Abca1fl/flAbcg1fl/fl controls (Supplemental Figure I). We therefore crossbred Abca1fl/flAbcg1fl/fl mice with mice expressing the endothelial-specific Tie2Cre promoter.35 We found that ABCA1 and ABCG1 protein levels were reduced by >90% in Tie2CreAbca1fl/flAbcg1fl/fl mouse lung ECs as compared to littermate Abca1fl/flAbcg1fl/fl controls (Figure 1A–B and Supplemental Figure II). In mouse aortic ECs, Abca1 and Abcg1 mRNA expression were reduced by >90% in ECs isolated from Tie2CreAbca1fl/flAbcg1fl/fl mice compared to littermate Abca1fl/flAbcg1fl/fl controls (Figure 1C). In line with several other models,35–37 ABCA1 and ABCG1 protein expression were reduced by >90% in Tie2CreAbca1fl/flAbcg1fl/fl macrophages compared to Abca1fl/flAbcg1fl/fl controls (Figure 1D–E and Supplemental Figure III), necessitating transplantation of floxed control bone marrow (BM) to allow us to focus on endothelial functions. In order to study the role of EC Abca1/g1 in atherosclerosis, we bred Ldlr−/− mice with Abca1fl/flAbcg1fl/fl, Tie2CreAbca1fl/fl, Tie2CreAbcg1fl/fl, and Tie2CreAbca1fl/flAbcg1fl/fl mice, and transplanted those mice with Abca1fl/flAbcg1fl/fl BM to generate Ldlr−/−, EC-ABCA1KOLdlr−/−, EC-ABCG1KOLdlr−/−, and EC-ABCDKOLdlr−/− mice, respectively. We tested the BM reconstitution by measuring ABCA1 and ABCG1 protein expression in macrophages and measuring Ldlr in DNA from peripheral blood. At 8 weeks after BM transplantation, macrophage ABCA1 and ABCG1 protein expression was similar in EC-ABCDKOLdlr−/− mice and their Ldlr−/− controls (Figure 2A), as were blood Ldlr levels (results not shown), indicating successful BM reconstitution.
Figure 1. Tie2CreAbca1fl/flAbcg1fl/fl mice show >90% decreased ABCA1 and ABCG1 protein expression in endothelial cells (ECs) and macrophages (MACs) compared to Abca1fl/flAbcg1fl/fl controls.
A and B. Lung ECs were isolated using ICAM-2 positive beads. After two purifications, cells were incubated with the liver X receptor (LXR) ligand T0901317 (3 µM, 24 h), and ABCA1 (A), and ABCG1 (B) protein expression was assessed using Western blot, quantified, and corrected for β-actin. C. Aortic ECs were isolated and Abca1 and Abcg1 mRNA expression determined and corrected for the housekeeping gene m36B4. D and E. Thioglycollate-elicited MACs were isolated, incubated with T0901317 (3 µM, 24 h), and ABCA1 (D), and ABCG1 (E) protein expression was assessed as indicated in A–B. n=5 per genotype. **P<0.01, ***P<0.001, by t-test.
Figure 2. Deficiency of ABCA1 and ABCG1 in ECs increases cholesterol accumulation and accelerates atherosclerosis.
A. Tie2CreAbca1fl/flAbcg1fl/flLdlr−/− and Abca1fl/flAbcg1fl/flLdlr−/− mice were transplanted with Abca1fl/flAbcg1fl/fl BM. These mice are referred to as EC-ABCDKOLdlr−/− and Ldlr−/− mice, respectively. Eight weeks after BM transplantation, thioglycollate-elicited macrophages were isolated, and ABCA1 and ABCG1 protein expression was assessed by Western blot, quantified, and corrected for β-actin. n=6 per genotype. B. EC-ABCDKOLdlr−/− and Ldlr−/− mice were fed Western-type diet for 12 weeks, lung ECs were isolated using ICAM-2 coated beads and cholesterol accumulation was assessed using an enzymatic assay. ECs were also loaded with [3H]cholesterol and cholesterol-phospholipid liposomes, and cholesterol efflux to apoAI (25 µg/mL) and HDL (50 µg/mL) was assessed, and corrected for cholesterol efflux to serum albumin. n=4–5 per group. *P<0.05 and ***P<0.001, by t-test. C and D. Ldlr−/−, EC-ABCA1KOLdlr−/−, EC-ABCG1KOLdlr−/−, and EC-ABCDKOLdlr−/− mice were fed Western-type diet (WTD) for 20–22 weeks, and atherosclerosis was assessed after H&E staining at the level of the aortic root (C) or after Oil Red O staining in the whole aorta (D). Each datapoint represents a single mouse. n=9–32. *P<0.05, **P<0.01, and ***P<0.001, by one-way ANOVA.
EC-Abca1 and Abcg1 deficiency increases cholesterol accumulation and accelerates atherosclerosis
We first evaluated whether EC Abca1 and Abcg1 deficiency affected cholesterol accumulation. We fed EC-ABCDKOLdlr−/− and Ldlr−/− mice the Western type diet (WTD) for 12 weeks and isolated ECs. Abca1/g1 deficiency increased EC cholesterol accumulation by ~25% (Figure 2B), which reflected an increase in free cholesterol accumulation, as free cholesterol and total cholesterol levels were similar (results not shown). Abca1/g1 deficiency decreased cholesterol efflux to apoA1 and HDL in ECs (Figure 2B), suggesting a role for both ABCA1 and ABCG1 in mediating cholesterol efflux from ECs.
We next examined the role of EC cholesterol efflux pathways in atherogenesis. We used male mice for these studies, since we previously observed that vascular Abcg1 deficiency decreased eNOS activity in male, but not in female mice,28 possibly reflecting an estrogen effect.38 Ldlr−/−, EC-ABCA1KOLdlr−/−, EC-ABCG1KOLdlr−/−, and EC-ABCDKOLdlr−/− mice were fed the WTD. Total plasma cholesterol levels were similar in all groups of mice as were HDL-cholesterol levels (Table). Unlike models with hematopoietic or macrophage Abca1/g1 deficiency,33 EC-Abca1/g1 deficiency did not affect blood leukocyte levels (Supplemental Figure IV). After 20–22 weeks of WTD feeding, we sacrificed the mice and assessed atherosclerotic lesion area at the level of the aortic root. Both EC-Abca1 and EC-Abcg1 deficiency significantly increased atherosclerotic lesion area compared to the Ldlr−/− controls; combined EC-Abca1/g1 deficiency caused a significant further increase in lesion area (Figure 2C). We then assessed atherosclerotic lesion area in the whole aorta by performing en face analysis using Oil Red O staining. Both single and combined EC-Abca1 and EC-Abcg1 deficiency increased atherosclerotic lesion area compared to the Ldlr−/− controls (Figure 2D and Supplemental Figure V). EC-Abca1 and EC-Abcg1 deficiency increased atherosclerosis in the whole aorta to a larger extent than in the aortic root; the effects being most dramatic in combined EC-Abca1/g1 deficiency compared to Ldlr−/− controls (P<0.001; Figure 2D). These findings provide direct evidence that endothelial cholesterol efflux pathways mediated by both ABCA1 and ABCG1 suppress atherosclerosis in mice, and that the effects are non-redundant with approximately equal additive contributions in the double knockout.
Table.
Plasma cholesterol and HDL-cholesterol levels in Ldlr−/−, EC-ABCA1KOLdlr−/−, EC-ABCG1KOLdlr−/−, and EC-ABCDKOLdlr−/− mice fed WTD for 20 weeks.
| Total plasma cholesterol (mg/dL) | Plasma HDL-cholesterol (mg/dL) | |
|---|---|---|
| Ldlr−/− | 1516 ± 83 | 50.98 ± 2.62 |
| EC-ABCA1KOLdlr−/− | 1556 ± 183 | 50.63 ± 2.15 |
| EC-ABCG1KOLdlr−/− | 1605 ± 81 | 55.18 ± 4.63 |
| EC-ABCDKOLdlr−/− | 1481 ± 101 | 53.47 ± 4.02 |
n=9–20 mice per group.
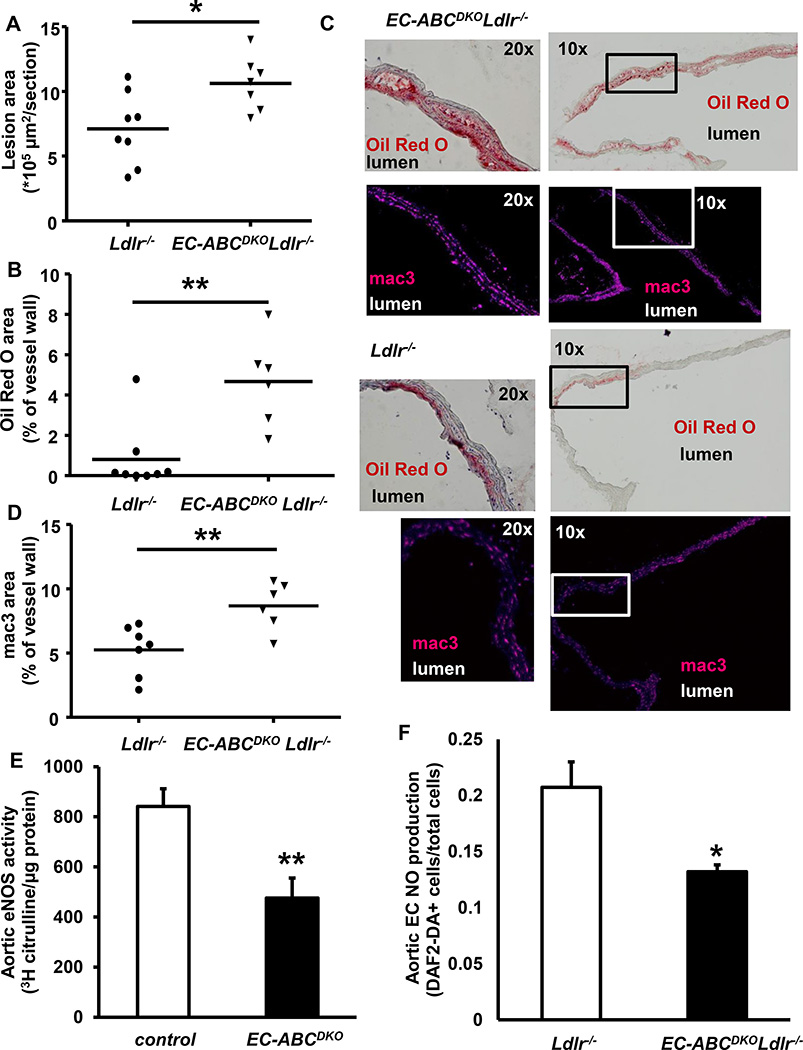
EC-Abca1 and Abcg1 deficiency accelerate atherosclerosis in aortic branch areas
Since the effects on atherosclerosis were most pronounced in the setting of combined EC-Abca1/g1 deficiency, we used EC-ABCDKOLdlr−/− mice and Ldlr−/− controls for our subsequent studies. We performed a short term atherosclerosis study by feeding EC-ABCDKOLdlr−/− and Ldlr−/− mice the WTD for 12 weeks. Similar to our findings in the 20–22 week study (Figure 2C), EC-Abca1/g1 deficiency increased atherosclerotic lesion area in the aortic root (Figure 3A). Interestingly, EC-Abca1/g1 deficiency markedly increased lipid accumulation in the branches of the aorta (4.7-fold; P<0.001; Figure 3B and C). We observed this lipid accumulation specifically in EC-ABCDKOLdlr−/− mice; only one of the Ldlr−/− control mice showed detectable lipid accumulation in the branch areas (Figure 3B and C). We stained the sections of the aorta for the macrophage marker mac3, and found that EC-Abca1/g1 deficiency also increased mac3 staining in the vessel wall of the aortas (Figure 3C and D). The areas showing lipid accumulation corresponded to those positive for mac3 staining (Figure 3C), suggesting macrophage foam cell formation. These data indicate that EC-Abca1/g1 deficiency dramatically increases the onset of atherosclerosis in the branches of the aorta that are exposed to disturbed blood flow.
Figure 3. Deficiency of ABCA1 and ABCG1 in ECs accelerates the initiation of atherosclerosis in the aortic root and branches of the aorta and decreases eNOS activity in the aorta after 12 weeks of WTD and in aortic ECs.
EC-ABCDKOLdlr−/− and Ldlr−/− mice were fed the WTD for 12 weeks, and atherosclerosis was assessed after H&E staining at the level of the aortic root. Each datapoint represents a single mouse. n=7–8 (A). Frozen sections of the aortic arch and branch areas were prepared and stained with Oil Red O (B,C) and mac3 (B,D). B. Quantification of Oil Red O positive areas corrected for the total vessel wall area of the aortic section. C. Oil Red O positive areas of representative sections (branch area) and corresponding mac3+ areas are indicated for both genotypes at two magnifications. A square indicates the magnified area in the 10× enlarged pictures. D. Mac3+ area, quantified similar to C. n=6–8. *P<0.05, **P<0.01, by Mann-Whitney. E. EC-ABCDKO and control mice were fed the WTD for 12 weeks. Aortas were collected, and the eNOS activity assay, measuring the conversion of [3H]arginine into [3H]citrulline in aortic lysates, was performed. n=6–8. **P<0.01, by t-test. F. Aortic ECs were isolated from Ldlr−/− and EC-ABCDKOLdlr−/− mice and NO production was determined using the DAF assay. n=6. *P<0.05, by t-test.
EC-Abca1 and Abcg1 deficiency decrease eNOS activity and increase LPS-induced expression of inflammatory genes
Our previous studies have shown that on cholesterol-rich diets, combined deficiency of Abca1/g1 or Abcg1 deficiency alone led to decreased endothelium-dependent vasorelaxation in femoral arteries, which we attributed to endothelial Abca1/g1 or Abcg1 deficiency and decreased eNOS activity; however, a contribution of other vascular cells could not be excluded.18, 28 We tested this hypothesis in our EC-Abca1/g1 deficient model. EC-ABCDKO and control mice were fed the WTD for 12 weeks. Aortas were isolated and aortic eNOS activity was measured in an [3H]arginine to [3H]citrulline conversion assay. EC-Abca1/g1 deficiency decreased the conversion of [3H]arginine into [3H]citrulline by ~40–50% (P<0.01; Figure 3E), reflecting decreased eNOS activity. We then assessed eNOS mediated NO production in aortic ECs isolated from EC-ABCDKOLdlr−/− and Ldlr−/− mice using the NO-specific fluorescent dye 4,5-diaminofluorescein diacetate (DAF-2 DA). Abca1/g1 deficiency decreased the DAF2-DA+ cells by ~40% (*P<0.05; Figure 3F), reflecting reduced NO production. These results indicate that our previous findings regarding decreased endothelium-dependent vasorelaxation in Abca1−/−Abcg1−/− mice on WTD18 were most likely the consequence of decreased eNOS activity in Abca1/g1 deficient ECs. Deficiency of eNOS has been reported to moderately increase blood pressure.39 However, we did not find any effect of EC-Abca1/g1 deficiency on blood pressure at several time-points after the start of the WTD feeding (Supplemental Figure VI), likely reflecting our finding that eNOS activity was decreased by ~40–50% (Figure 3E–F), whereas a moderate increase in blood pressure depends on complete eNOS deficiency.39
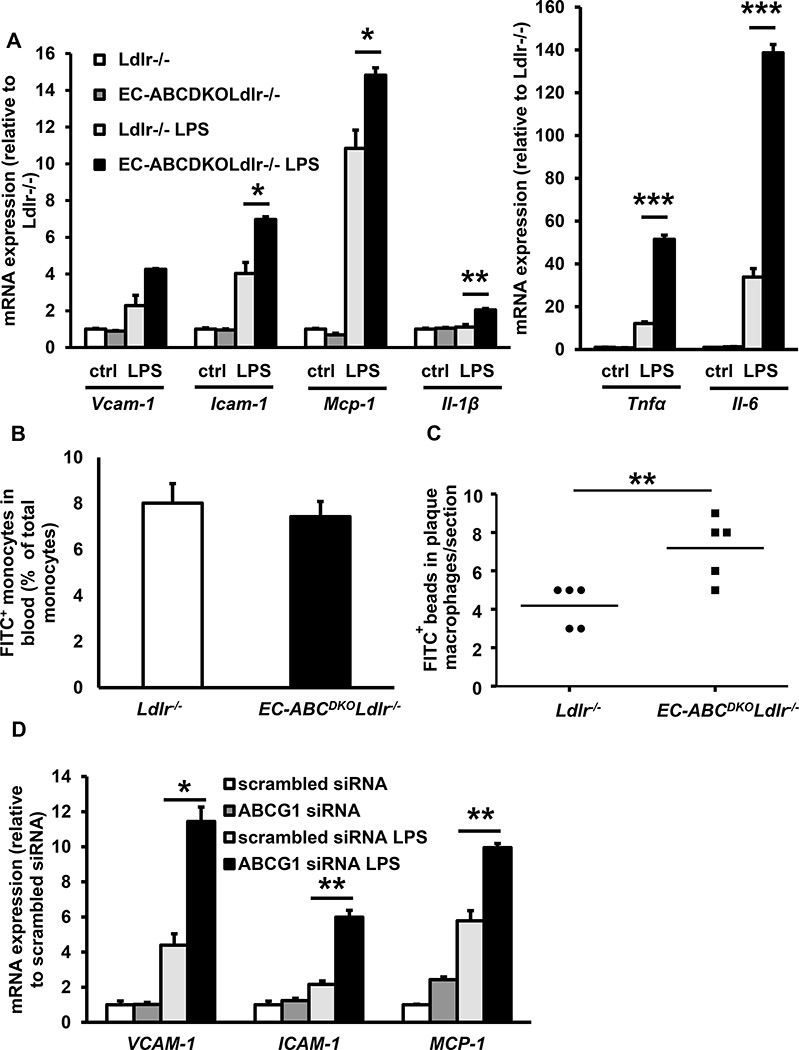
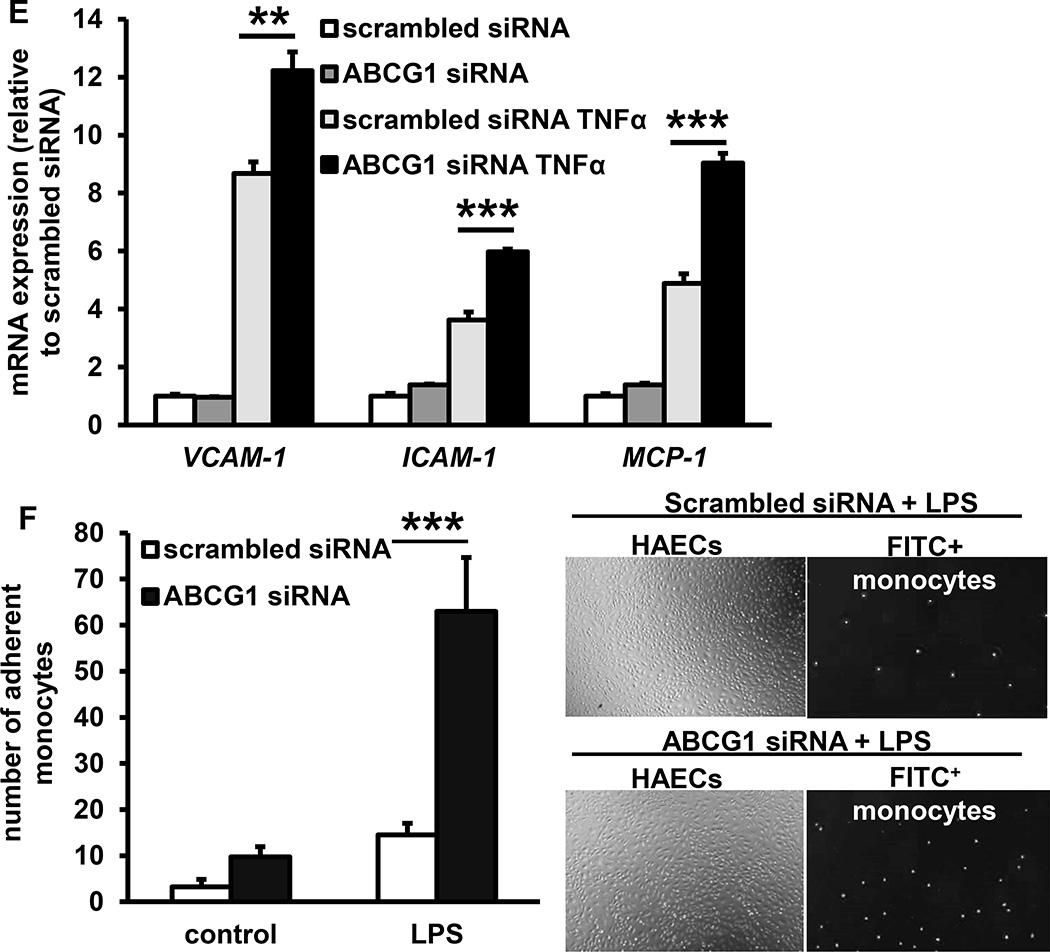
Hypercholesterolemia, or treatment with a low dose of lipopolysaccharide (LPS) in Ldlr−/− mice increases endothelial NF-κB activation in regions of the aorta exposed to disturbed blood flow,13 predisposing these sites to atherosclerosis. To investigate a role for Abca1/g1 in endothelial inflammation, we isolated aortic ECs from the EC-ABCDKOLdlr−/− mice and Ldlr−/− controls, and incubated them with LPS. EC-Abca1/g1 deficiency enhanced the LPS induced mRNA expression of Vcam-1, Icam-1, Monocyte chemoattractant protein-1 (Mcp-1), Interleukin-1β (Il-1β), Tumor necrosis factor α (Tnf-α), and Il-6 (Figure 4A). To examine whether these changes would contribute to monocyte infiltration in atherosclerotic plaques, EC-ABCDKOLdlr−/− and Ldlr−/− mice were fed WTD for 12 weeks, and injected with FITC+ beads that accumulate in monocytes.40 The percentage of FITC+ beads incorporated in blood monocytes was similar between mice from both genotypes (Figure 4B), but EC-ABCDKOLdlr−/− mice showed an ~50% increase in FITC+ bead accumulation in macrophage positive areas of the atherosclerotic plaques compared to their Ldlr−/− controls, reflecting enhanced monocyte infiltration (Figure 4C and Supplemental Figure VII). We then asked whether cholesterol efflux pathways also regulate inflammatory gene expression in HAECs. As shown previously, in HAECs ABCG1 is the main transporter mediating cholesterol efflux to HDL, whereas ABCA1 expression is almost undetectable.18 We efficiently knocked down ABCG1 in HAECs using siRNA,18, 24 and incubated HAECs with LPS or the pro-atherogenic cytokine TNFα. ABCG1 knockdown enhanced both LPS- and TNFα-induced expression of VCAM-1, ICAM-1, and MCP-1 in HAECs (Figure 4D–E). We then assessed whether these increases resulted in enhanced monocyte adhesion, and co-incubated ABCG1 and scrambled siRNA treated HAECs with FITC+ monocytes during the LPS exposure. ABCG1 knockdown in HAECs markedly enhanced the LPS induced monocyte adhesion to the EC monolayer (Figure 4F).
Figure 4. Deficiency of ABCA1 and ABCG1 in ECs increases inflammatory gene expression, monocyte adhesion and monocyte infiltration in atherosclerotic plaques.
A and B. Deficiency of ABCA1 and ABCG1 increases inflammatory gene expression in mouse ECs (A) and monocyte infiltration in atherosclerotic plaques (B). A. Mouse aortic ECs were isolated from EC-ABCDKOLdlr−/− and Ldlr−/− mice and subsequently incubated with or without LPS (4 h, 10 ng/ml). mRNA expression of the indicated genes was assessed. n=4. B and C. Ldlr−/− and EC-ABCDKOLdlr−/− mice were fed WTD for 12 weeks and injected with FITC+ beads to label monocytes. At 48 h after injection, FITC+ blood monocytes were assessed using flow cytometry (B). Mice were then sacrificed, hearts isolated, frozen sections were made of the aortic root and stained for mac3. FITC+ monocytes were assessed by counting cells positive for FITC+ beads in the mac3+ area of the atherosclerotic plaque. Each datapoint represents an individual mouse (C). n=5. *P<0.05, **P<0.01, ***P<0.001, by t-test. D, E, and F. Deficiency of ABCG1 in human aortic ECs enhances inflammatory gene expression and monocyte adhesion. Human aortic ECs were transfected with scrambled or ABCG1 siRNA, and incubated with or without LPS (4 h, 10 ng/ml) (D) or TNFα (4 h, 0.01 ng/ml) (E). mRNA expression of the indicated genes was assessed. F. Human aortic ECs were treated as described in D, and during the last 30 min of the 4 h LPS treatment, incubated with FITC+ THP-1 monocytes. Non-adherent THP-1 monocytes were washed off, and monocyte adhesion was quantified. n=4–6. *P<0.05, **P<0.01, ***P<0.001, by one-way ANOVA.
Together, these findings show that endothelial Abca1/g1 deficiency decreased eNOS activity in aortas of WTD-fed mice and NO production in aortic ECs. NO has been shown to be anti-atherogenic by decreasing cytokine-induced NF-κB activation in ECs, resulting in decreased EC vascular adhesion molecules and monocyte adhesion.41 Endothelial Abca1/g1 deficiency also increased LPS-induced inflammatory gene expression in aortic ECs and monocyte infiltration into atherosclerotic plaques. The findings on inflammatory gene expression are recapitulated in HAECs, and resulted in enhanced monocyte adhesion, indicating that the findings in mice also may be relevant to humans. These observations likely explain the increased atherosclerosis in EC-Abca1/g1 deficiency.
EC-Abca1 and Abcg1 deficiency enhance new vessel sprouting in aortic rings
Recent studies have suggested that cholesterol efflux pathways regulate angiogenesis in zebrafish, and that deficiency of Abca1 and Abcg1 causes excessive angiogenesis, impairing proper development and growth.32 Whether cholesterol efflux pathways play a role in modulating angiogenesis in mammals is unclear. ECs play a key role in angiogenesis,42 and angiogenesis worsens advanced atherosclerosis in mice.43 We evaluated the role of endothelial Abca1/g1 expression in new vessel sprouting in aortic rings ex vivo. Aortic rings were embedded in matrigel and incubated with endothelial growth factors. After 5 days, we observed increased new vessel sprouting in EC-Abcg1 and EC-Abca1/g1 deficient aortic rings, but not in EC-Abca1 deficient aortic rings, compared to controls (Figure 5A and B), suggestive of increased angiogenesis. We then examined whether increased vessel sprouting in EC-Abca1/g1 deficient aortas could have played a role in the increased atherosclerosis in EC-ABCDKOLdlr−/− mice. Using Von Willebrand factor staining, we observed only a few new blood vessels in the intima or adventitia of advanced atherosclerotic lesions in EC-ABCDKOLdlr−/− mice and their controls, with no difference between the genotypes (Supplemental Figure VIII). We performed further in vivo studies on the role of cholesterol efflux pathways in angiogenesis in mice, using the retinal angiogenesis assay that can be used as a model for angiogenesis during development,44 but also these studies showed no effect of EC-Abca1 and Abcg1 on angiogenesis (Figure 5C–D and Supplemental Figure IX).
Figure 5. Deficiency of ABCA1 and ABCG1 in ECs increases new vessel sprouting in aortic rings, but does not affect retinal angiogenesis.
A–B. Rings from the thoracic aorta of Abca1fl/flAbcg1fl/fl control Ldlr−/−, EC-ABCA1KOLdlr−/−, EC-ABCG1KOLdlr−/−, and EC-ABCDKOLdlr−/− mice were isolated, embedded in matrigel, and incubated with endothelial growth cell factors for 5 days. New vessel sprouting was quantified using Image J. A. Representative pictures of new vessel sprouting from aortic rings. B. Quantification of vessel sprouting. n=6. C–D. Control and EC-ABCDKO mice were sacrificed at postnatal day 5. Whole mount retinas were stained for isolectin B4 to visualize ECs. C. Maximal vascular length was calculated as maximal length from the center to the tip of the vessels and corrected for bodyweight (BW). D. Vascular density was quantified using Image J and corrected for BW. *P<0.05, **P<0.01, ***P<0.001, by one-way ANOVA.
Together, these results demonstrated that EC-Abca1/g1 deficiency enhanced angiogenesis in aortic rings ex vivo, but not during retina vascular developments in vivo. Our data demonstrated that unlike in zebrafish, EC cholesterol efflux pathways may not be essential for proper angiogenesis during development in mice; however, this issue deserves further exploration. The observations on new vessel formation in atherosclerotic lesions suggest that this mechanism does not play a role in the increased atherosclerosis in EC-ABCDKOLdlr−/− mice. Previous studies have suggested that 36–60 weeks of cholesterol-rich diet feeding is required to observe angiogenesis in lesions of Apoe−/− mice,43 presumably explaining why we only observed a few new vessels in the Ldlr−/− model fed the WTD for 20–22 weeks.
Discussion
This study shows that cholesterol efflux pathways mediated by both ABCA1 and ABCG1 in ECs are anti-atherogenic, and have a key role in preserving eNOS activity and suppressing endothelial inflammation. Although we previously showed that cholesterol efflux pathways are involved in maintaining eNOS activity,18 we here demonstrated that this is specifically due to their action in ECs, leading to suppression of atherosclerosis. Our studies provide strong support for the idea that the beneficial effects of infusion of HDL on endothelial function20, 23 are mediated at least in part by promotion of cholesterol efflux from, and restoration of eNOS activity in ECs.
Interestingly, in ECs, not only combined Abca1/g1 deficiency accelerates atherosclerosis, but also single Abca1 or Abcg1 deficiency (Figure 2). This is strikingly different to macrophages, in which single deficiencies of Abca1 or Abcg1 do not increase atherosclerosis.27, 45–47 Likely this is because deletion of Abca1 leads to upregulation of Abcg1 expression and vice versa,27 so that only the combined deficiency of macrophage Abca1/g1 accelerates atherosclerosis.33 Why such compensation occurs in macrophages and presumably not ECs is unclear but could perhaps be related to the ability of macrophages to store cholesterol and increase endogenous liver X receptor (LXR) ligands.48 One study addressed the role of endothelial ABCA1 in atherosclerosis using an endothelium-specific ABCA1 overexpression mouse model.49 Surprisingly, endothelial ABCA1 overexpression increased HDL-cholesterol levels by ~40%, potentially due to a 2.6-fold increase in EC cholesterol efflux to apolipoprotein A1 compared to controls.49 In contrast, we found no effect of endothelial Abca1 deficiency on HDL levels (Table). The increased HDL levels most likely explain why endothelial ABCA1 overexpression reduced atherosclerosis in this model,49 and hamper interpretation as to whether EC-ABCA1 affected atherosclerosis locally in the vessel wall.
Several studies have shown that SR-BI plays a role in the HDL-preserving effects on eNOS activity and endothelial function, possibly by mediating the efflux of oxysterols and cholesterol from ECs to HDL.11, 15–17 Srb1−/− mice show reduced endothelium-dependent vasorelaxation,16 and Srb1 deficiency accelerates atherosclerosis in Apoe−/− mice.50 Endothelial Srb1 deficiency could have contributed to this effect; however Srb1 deficiency also increased pro-atherogenic VLDL-sized cholesterol-enriched particles and led to abnormally enlarged HDL.50 The significance of the endothelial-preserving effects of SR-BI for atherogenesis thus remains to be directly tested in an endothelium-specific Srb1-deficient mouse model.
We found that EC-Abca1/g1 deficiency especially increased the onset of atherosclerosis in the branches of the aorta (Figure 3) that are exposed to disturbed blood flow. Abca1 is upregulated in aortic arch ECs following a pro-atherogenic stimulus,31 suggesting a particular anti-atherogenic role for the Abca1 transporter in this region of the aorta. Interestingly, a pro-atherogenic stimulus involving either LPS or pro-atherogenic diet feeding increased NF-κB activation particularly at sites of the aorta exposed to disturbed blood flow, suggesting a link between EC cholesterol accumulation and NF-κB activation in these regions. We explored this link in Abca1/g1 deficient aortic ECs. EC-Abca1/g1 deficiency enhanced LPS-induced expression of NF-κB target genes (Figure 4), possibly due to enhanced surface expression of Toll-like receptor 4 in lipid rafts, similar to our previous observations in macrophages.51 Interestingly, EC-Abca1/g1 deficiency also enhanced monocyte infiltration in atherosclerotic plaques in vivo (Figure 4), and particularly enhanced macrophage foam cell formation at sites of disturbed blood flow (Figure 3D). Together, these findings provide evidence for the idea13 that endothelial cholesterol accumulation contributes to the initiation of atherogenesis in regions of the aorta exposed to disturbed blood flow.
In sum, our studies demonstrate an athero-protective role of endothelial cholesterol efflux pathways mediated by ABCA1 and ABCG1 that help to maintain eNOS activity and suppress endothelial inflammation. We cannot exclude a possible contribution of other actions of these transporters to our results, such as effects of ABCG1 on intracellular cholesterol homeostasis.52, 53 These studies add to the understanding of the athero-protective effects of HDL and LXR activators. LXR activators could be anti-atherogenic in part by increasing Abca1/g1 expression in ECs. Infusions of cholesterol poor reconstituted HDL that are currently in clinical trials for athero-thrombosis,54 are likely to exert beneficial effects by stimulating endothelial cholesterol efflux mediated by ABCA1/G1 and other pathways.
Supplementary Material
Highlights.
Deficiency of Abca1 and Abcg1 in ECs decreases eNOS activity, and enhances EC inflammation, monocyte adhesion, and monocyte infiltration into atherosclerotic plaques.
Both single and combined deficiency of Abca1 and Abcg1 in ECs accelerate atherosclerosis in mice.
Our data provide evidence for a link between EC cholesterol accumulation, inflammation, and atherosclerosis in vivo.
Acknowledgments
None.
Sources of Funding
This project was supported by the Leducq Foundation and the National Institutes of Health grants HL107653 and HL87123 (to A.R. Tall), and HL112626 (to J. Kitajewski). Marit Westerterp was supported by VIDI grant 91715350 from the Netherlands Organization of Sciences and a Rosalind Franklin Fellowship from the University Medical Center Groningen. Kyoichiro Tsuchiya was supported by a postdoctoral fellowship from the Japan Society for the Promotion of Science. Flow cytometry experiments were performed in the CCTI Flow Cytometry Core, supported in part by the Office of the Director, NIH under awards S10RR027050. The content is solely the responsibility of the authors and does not necessarily represent the views of the NIH.
Abbreviation List
- ABCA1/G1
ATP-Binding Cassette Transporter A1/G1
- ApoA1/E
Apolipoprotein A1/E
- ECs
Endothelial cells
- EC-ABCA1KO
Endothelial cell Abca1 deficient
- EC-ABCG1KO
Endothelial cell Abcg1 deficient
- EC-ABCDKO
Endothelial cell Abca1/g1 deficient
- eNOS
Endothelial NO synthase
- HAECs
Human aortic endothelial cells
- HDL
High-density-lipoprotein(s)
- ICAM-1/2
Intracellular adhesion molecule-1/2
- Interleukin-1/6
IL-1/6
- LXR
Liver X Receptor
- MACs
Macrophages
- MCP-1
Monocyte chemoattractant protein-1
- SRB-I
Scavenger Receptor B-I
- TLR4
Toll-like receptor 4
- TNF-α
Tumor necrosis factor-α
- VCAM-1
Vascular cell adhesion molecule-1
- WTD
Western type diet
Footnotes
Disclosures
A.R. Tall is a consultant to Amgen, Arisaph, and CSL. The other authors report no conflicts.
References
- 1.Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The framingham study. Am J Med. 1977;62:707–714. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- 2.Voight BF, Peloso GM, Orho-Melander M, et al. Plasma hdl cholesterol and risk of myocardial infarction: A mendelian randomisation study. Lancet. 2012;380:572–580. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fond AM, Lee CS, Schulman IG, Kiss RS, Ravichandran KS. Apoptotic cells trigger a membrane-initiated pathway to increase abca1. J Clin Invest. 2015;125:2748–2758. doi: 10.1172/JCI80300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy AJ, Funt S, Gorman DJ, Tall AR, Wang N. Pegylation of hdl decreases plasma clearance and enhances anti-atherogenic activity. Circ Res. 2013;113:e1–e9. doi: 10.1161/CIRCRESAHA.113.301112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plump AS, Scott CJ, Breslow JL. Human apolipoprotein a-i gene expression increases high density lipoprotein and suppresses atherosclerosis in the apolipoprotein e-deficient mouse. Proc Natl Acad Sci USA. 1994;91:9607–9611. doi: 10.1073/pnas.91.20.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicholls SJ, Tuzcu EM, Sipahi I, Schoenhagen P, Crowe T, Kapadia S, Nissen SE. Relationship between atheroma regression and change in lumen size after infusion of apolipoprotein a-i milano. J Am Coll Cardiol. 2006;47:992–997. doi: 10.1016/j.jacc.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 7.Dansky HM, Charlton SA, Barlow CB, Tamminen M, Smith JD, Frank JS, Breslow JL. Apo a-i inhibits foam cell formation in apo e-deficient mice after monocyte adherence to endothelium. J Clin Invest. 1999;104:31–39. doi: 10.1172/JCI6577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tardif JC, Gregoire J, L'Allier PL, Ibrahim R, Lesperance J, Heinonen TM, Kouz S, Berry C, Basser R, Lavoie MA, Guertin MC, Rodes-Cabau J Effect of r HDLoA-S, Efficacy I. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: A randomized controlled trial. JAMA. 2007;297:1675–1682. doi: 10.1001/jama.297.15.jpc70004. [DOI] [PubMed] [Google Scholar]
- 9.Assmann G, Gotto AM., Jr Hdl cholesterol and protective factors in atherosclerosis. Circulation. 2004;109:III8–III14. doi: 10.1161/01.CIR.0000131512.50667.46. [DOI] [PubMed] [Google Scholar]
- 10.Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of hdl. Circ Res. 2004;95:764–772. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 11.Mineo C, Deguchi H, Griffin JH, Shaul PW. Endothelial and antithrombotic actions of hdl. Circ Res. 2006;98:1352–1364. doi: 10.1161/01.RES.0000225982.01988.93. [DOI] [PubMed] [Google Scholar]
- 12.d'Uscio LV, Smith LA, Katusic ZS. Hypercholesterolemia impairs endothelium-dependent relaxations in common carotid arteries of apolipoprotein e-deficient mice. Stroke. 2001;32:2658–2664. doi: 10.1161/hs1101.097393. [DOI] [PubMed] [Google Scholar]
- 13.Hajra L, Evans AI, Chen M, Hyduk SJ, Collins T, Cybulsky MI. The nf-kappa b signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc Natl Acad Sci USA. 2000;97:9052–9057. doi: 10.1073/pnas.97.16.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohara Y, Peterson TE, Harrison DG. Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest. 1993;91:2546–2551. doi: 10.1172/JCI116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uittenbogaard A, Shaul PW, Yuhanna IS, Blair A, Smart EJ. High density lipoprotein prevents oxidized low density lipoprotein-induced inhibition of endothelial nitric-oxide synthase localization and activation in caveolae. J Biol Chem. 2000;275:11278–11283. doi: 10.1074/jbc.275.15.11278. [DOI] [PubMed] [Google Scholar]
- 16.Yuhanna IS, Zhu Y, Cox BE, Hahner LD, Osborne-Lawrence S, Lu P, Marcel YL, Anderson RG, Mendelsohn ME, Hobbs HH, Shaul PW. High-density lipoprotein binding to scavenger receptor-bi activates endothelial nitric oxide synthase. Nat Med. 2001;7:853–857. doi: 10.1038/89986. [DOI] [PubMed] [Google Scholar]
- 17.Assanasen C, Mineo C, Seetharam D, Yuhanna IS, Marcel YL, Connelly MA, Williams DL, de la Llera-Moya M, Shaul PW, Silver DL. Cholesterol binding, efflux, and a pdz-interacting domain of scavenger receptor-bi mediate hdl-initiated signaling. J Clin Invest. 2005;115:969–977. doi: 10.1172/JCI200523858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terasaka N, Yu S, Yvan-Charvet L, Wang N, Mzhavia N, Langlois R, Pagler T, Li R, Welch CL, Goldberg IJ, Tall AR. Abcg1 and hdl protect against endothelial dysfunction in mice fed a high-cholesterol diet. J Clin Invest. 2008;118:3701–3713. doi: 10.1172/JCI35470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeiher AM, Schachlinger V, Hohnloser SH, Saurbier B, Just H. Coronary atherosclerotic wall thickening and vascular reactivity in humans. Elevated high-density lipoprotein levels ameliorate abnormal vasoconstriction in early atherosclerosis. Circulation. 1994;89:2525–2532. doi: 10.1161/01.cir.89.6.2525. [DOI] [PubMed] [Google Scholar]
- 20.Bisoendial RJ, Hovingh GK, Levels JH, Lerch PG, Andresen I, Hayden MR, Kastelein JJ, Stroes ES. Restoration of endothelial function by increasing high-density lipoprotein in subjects with isolated low high-density lipoprotein. Circulation. 2003;107:2944–2948. doi: 10.1161/01.CIR.0000070934.69310.1A. [DOI] [PubMed] [Google Scholar]
- 21.Cockerill GW, Rye KA, Gamble JR, Vadas MA, Barter PJ. High-density lipoproteins inhibit cytokine-induced expression of endothelial cell adhesion molecules. Arterioscler Thromb Vasc Biol. 1995;15:1987–1994. doi: 10.1161/01.atv.15.11.1987. [DOI] [PubMed] [Google Scholar]
- 22.Nicholls SJ, Dusting GJ, Cutri B, Bao S, Drummond GR, Rye KA, Barter PJ. Reconstituted high-density lipoproteins inhibit the acute pro-oxidant and proinflammatory vascular changes induced by a periarterial collar in normocholesterolemic rabbits. Circulation. 2005;111:1543–1550. doi: 10.1161/01.CIR.0000159351.95399.50. [DOI] [PubMed] [Google Scholar]
- 23.Wu BJ, Chen K, Shrestha S, Ong KL, Barter PJ, Rye KA. High-density lipoproteins inhibit vascular endothelial inflammation by increasing 3beta-hydroxysteroid-delta24 reductase expression and inducing heme oxygenase-1. Circ Res. 2013;112:278–288. doi: 10.1161/CIRCRESAHA.111.300104. [DOI] [PubMed] [Google Scholar]
- 24.Terasaka N, Westerterp M, Koetsveld J, Fernandez-Hernando C, Yvan-Charvet L, Wang N, Sessa WC, Tall AR. Atp-binding cassette transporter g1 and high-density lipoprotein promote endothelial no synthesis through a decrease in the interaction of caveolin-1 and endothelial no synthase. Arterioscler Thromb Vasc Biol. 2010;30:2219–2225. doi: 10.1161/ATVBAHA.110.213215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Besler C, Heinrich K, Rohrer L, et al. Mechanisms underlying adverse effects of hdl on enos-activating pathways in patients with coronary artery disease. J Clin Invest. 2011;121:2693–2708. doi: 10.1172/JCI42946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riwanto M, Rohrer L, Roschitzki B, Besler C, Mocharla P, Mueller M, Perisa D, Heinrich K, Altwegg L, von Eckardstein A, Luscher TF, Landmesser U. Altered activation of endothelial anti- and proapoptotic pathways by high-density lipoprotein from patients with coronary artery disease: Role of high-density lipoprotein-proteome remodeling. Circulation. 2013;127:891–904. doi: 10.1161/CIRCULATIONAHA.112.108753. [DOI] [PubMed] [Google Scholar]
- 27.Yvan-Charvet L, Ranalletta M, Wang N, Han S, Terasaka N, Li R, Welch C, Tall AR. Combined deficiency of abca1 and abcg1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J Clin Invest. 2007;117:3900–3908. doi: 10.1172/JCI33372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Westerterp M, Koetsveld J, Yu S, Han S, Li R, Goldberg IJ, Welch CL, Tall AR. Increased atherosclerosis in mice with vascular atp-binding cassette transporter g1 deficiency. Arterioscler Thromb Vasc Biol. 2010;30:2103–2105. doi: 10.1161/ATVBAHA.110.212985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whetzel AM, Sturek JM, Nagelin MH, Bolick DT, Gebre AK, Parks JS, Bruce AC, Skaflen MD, Hedrick CC. Abcg1 deficiency in mice promotes endothelial activation and monocyte-endothelial interactions. Arterioscler Thromb Vasc Biol. 2010;30:809–817. doi: 10.1161/ATVBAHA.109.199166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu M, Fu Y, Hou Y, Wang N, Guan Y, Tang C, Shyy JY, Zhu Y. Laminar shear stress regulates liver x receptor in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:527–533. doi: 10.1161/ATVBAHA.107.143487. [DOI] [PubMed] [Google Scholar]
- 31.Erbilgin A, Siemers N, Kayne P, Yang WP, Berliner J, Lusis AJ. Gene expression analyses of mouse aortic endothelium in response to atherogenic stimuli. Arterioscler Thromb Vasc Biol. 2013;33:2509–2517. doi: 10.1161/ATVBAHA.113.301989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fang L, Choi SH, Baek JS, Liu C, Almazan F, Ulrich F, Wiesner P, Taleb A, Deer E, Pattison J, Torres-Vazquez J, Li AC, Miller YI. Control of angiogenesis by aibp-mediated cholesterol efflux. Nature. 2013;498:118–122. doi: 10.1038/nature12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Westerterp M, Murphy AJ, Wang M, et al. Deficiency of atp-binding cassette transporters a1 and g1 in macrophages increases inflammation and accelerates atherosclerosis in mice. Circ Res. 2013;112:1456–1465. doi: 10.1161/CIRCRESAHA.113.301086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alva JA, Zovein AC, Monvoisin A, Murphy T, Salazar A, Harvey NL, Carmeliet P, Iruela-Arispe ML. Ve-cadherin-cre-recombinase transgenic mouse: A tool for lineage analysis and gene deletion in endothelial cells. Dev Dyn. 2006;235:759–767. doi: 10.1002/dvdy.20643. [DOI] [PubMed] [Google Scholar]
- 35.Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-cre transgenic mice: A new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 36.Kanda T, Brown JD, Orasanu G, Vogel S, Gonzalez FJ, Sartoretto J, Michel T, Plutzky J. Ppargamma in the endothelium regulates metabolic responses to high-fat diet in mice. J Clin Invest. 2009;119:110–124. doi: 10.1172/JCI36233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rask-Madsen C, Li Q, Freund B, et al. Loss of insulin signaling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein e null mice. Cell Metab. 2010;11:379–389. doi: 10.1016/j.cmet.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chambliss KL, Yuhanna IS, Mineo C, Liu P, German Z, Sherman TS, Mendelsohn ME, Anderson RG, Shaul PW. Estrogen receptor alpha and endothelial nitric oxide synthase are organized into a functional signaling module in caveolae. Circ Res. 2000;87:E44–E52. doi: 10.1161/01.res.87.11.e44. [DOI] [PubMed] [Google Scholar]
- 39.Kuhlencordt PJ, Gyurko R, Han F, Scherrer-Crosbie M, Aretz TH, Hajjar R, Picard MH, Huang PL. Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease in apolipoprotein e/endothelial nitric oxide synthase double-knockout mice. Circulation. 2001;104:448–454. doi: 10.1161/hc2901.091399. [DOI] [PubMed] [Google Scholar]
- 40.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ ccr2, ccr5, and cx3cr1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Caterina R, Libby P, Peng HB, Thannickal VJ, Rajavashisth TB, Gimbrone MA, Jr, Shin WS, Liao JK. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest. 1995;96:60–68. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Millauer B, Wizigmann-Voos S, Schnurch H, Martinez R, Moller NP, Risau W, Ullrich A. High affinity vegf binding and developmental expression suggest flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993;72:835–846. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- 43.Moulton KS, Heller E, Konerding MA, Flynn E, Palinski W, Folkman J. Angiogenesis inhibitors endostatin or tnp-470 reduce intimal neovascularization and plaque growth in apolipoprotein e-deficient mice. Circulation. 1999;99:1726–1732. doi: 10.1161/01.cir.99.13.1726. [DOI] [PubMed] [Google Scholar]
- 44.Gariano RF, Gardner TW. Retinal angiogenesis in development and disease. Nature. 2005;438:960–966. doi: 10.1038/nature04482. [DOI] [PubMed] [Google Scholar]
- 45.Bi X, Zhu X, Gao C, Shewale S, Cao Q, Liu M, Boudyguina E, Gebre AK, Wilson MD, Brown AL, Parks JS. Myeloid cell-specific atp-binding cassette transporter a1 deletion has minimal impact on atherogenesis in atherogenic diet-fed low-density lipoprotein receptor knockout mice. Arterioscler Thromb Vasc Biol. 2014;34:1888–1899. doi: 10.1161/ATVBAHA.114.303791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brunham LR, Singaraja RR, Duong M, Timmins JM, Fievet C, Bissada N, Kang MH, Samra A, Fruchart JC, McManus B, Staels B, Parks JS, Hayden MR. Tissue-specific roles of abca1 influence susceptibility to atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:548–554. doi: 10.1161/ATVBAHA.108.182303. [DOI] [PubMed] [Google Scholar]
- 47.Out R, Hoekstra M, Habets K, Meurs I, de Waard V, Hildebrand RB, Wang Y, Chimini G, Kuiper J, Van Berkel TJ, Van Eck M. Combined deletion of macrophage abca1 and abcg1 leads to massive lipid accumulation in tissue macrophages and distinct atherosclerosis at relatively low plasma cholesterol levels. Arterioscler Thromb Vasc Biol. 2008;28:258–264. doi: 10.1161/ATVBAHA.107.156935. [DOI] [PubMed] [Google Scholar]
- 48.Spann NJ, Garmire LX, McDonald JG, et al. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell. 2012;151:138–152. doi: 10.1016/j.cell.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vaisman BL, Demosky SJ, Stonik JA, Ghias M, Knapper CL, Sampson ML, Dai C, Levine SJ, Remaley AT. Endothelial expression of human abca1 in mice increases plasma hdl cholesterol and reduces diet-induced atherosclerosis. J Lipid Res. 2012;53:158–167. doi: 10.1194/jlr.M018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trigatti B, Rayburn H, Vinals M, Braun A, Miettinen H, Penman M, Hertz M, Schrenzel M, Amigo L, Rigotti A, Krieger M. Influence of the high density lipoprotein receptor sr-bi on reproductive and cardiovascular pathophysiology. Proc Natl Acad Sci USA. 1999;96:9322–9327. doi: 10.1073/pnas.96.16.9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, Ishibashi M, Li R, Wang N, Tall AR. Increased inflammatory gene expression in abc transporter-deficient macrophages: Free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 2008;118:1837–1847. doi: 10.1161/CIRCULATIONAHA.108.793869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang N, Ranalletta M, Matsuura F, Peng F, Tall AR. Lxr-induced redistribution of abcg1 to plasma membrane in macrophages enhances cholesterol mass efflux to hdl. Arterioscler Thromb Vasc Biol. 2006;26:1310–1316. doi: 10.1161/01.ATV.0000218998.75963.02. [DOI] [PubMed] [Google Scholar]
- 53.Tarling EJ, Edwards PA. Atp binding cassette transporter g1 (abcg1) is an intracellular sterol transporter. Proc Natl Acad Sci USA. 2011;108:19719–19724. doi: 10.1073/pnas.1113021108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gille A, Easton R, D'Andrea D, Wright SD, Shear C. Csl112 enhances biomarkers of reverse cholesterol transport after single and multiple infusions in healthy subjects. Arterioscler Thromb Vasc Biol. 2014;34:2106–2114. doi: 10.1161/ATVBAHA.114.303720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.