Abstract
Background
Activation of corticotropin releasing factor type 1 receptors (CRF-R1) in the ventral tegmental area (VTA) represents a critical mechanism for social defeat to escalate cocaine self-administration in adult rats.
Objective
We determined the acute effect of a CRF-R1 antagonist (CP376395) microinfusion into the VTA prior to each episode of social defeat in adolescent rats and determined whether this drug treatment could prevent later escalation of cocaine taking in early adulthood.
Methods
Rats were implanted with bilateral cannulae aimed at the VTA five days before the first social defeat. Bilateral microinfusion of CP376395 (500ng/side) or vehicle occurred 20 min before each episode of social defeat on postnatal days (P) 35, 38, 41, and 44. Behavior was quantified on P35 and P44. On P57, rats were implanted with intra-jugular catheters, and subsequent cocaine self-administration was analyzed.
Results
CP376395-treated adolescent rats walked less and were attacked more slowly, but were socially investigated more than vehicle-treated adolescents. Vehicle-treated rats showed increased social and decreased non-social exploration from P35 to P44, while CP376395-treated rats did not. Socially defeated, vehicle-treated adolescents took more cocaine during a 24-hour unlimited access binge during adulthood. The latency to supine posture on P44 was inversely correlated with later cocaine self-administration during fixed and progressive ratio schedules of reinforcement and during the binge.
Conclusions
CP376395 treatment in adolescence blocked escalation of cocaine taking in adulthood. Episodes of social defeat stress engender neuroadaptation in CRF-R1s in the VTA that alter coping with social stress and that persist into adulthood.
Keywords: Adolescence, CRF, stress, social behavior, VTA
Introduction
Brief episodes of social stress can escalate drug self-administration in humans and rats (Boyson et al. 2014; Covington and Miczek 2005; Sinha 2009). A large percentage of adolescent humans (10–30%) are victims of social stress (Nansel et al. 2001; Newman et al. 2005), which is associated with increased illicit substance use (Hoffmann et al. 2000; Tharp-Taylor et al. 2009). Our recent study modeled this connection between stress and drug self-administration by applying social defeat stress to adolescent male rats from postnatal day (P) 35–44 and then measuring several features cocaine self-administration in early adulthood (P65-85). After brief episodes of social defeat during mid-adolescence, rats acquired intravenous cocaine self-administration faster, attained a greater number of cocaine infusions under a progressive ratio schedule of reinforcement (PR), and escalated cocaine intake during a 24-h continuous access binge later on in adulthood (Burke and Miczek 2015). Past studies also implicate the mesocorticolimbic dopamine system in adolescent social defeat cross-sensitization to psychostimulants (Burke et al. 2013; Burke and Miczek 2014; Burke et al. 2010; Burke et al. 2011). These effects were only observed when the rats were allowed social experiences during adolescence. The neural mechanisms underlying the cross-sensitization between adolescent stress and adult drug seeking behavior remain largely elusive, but may involve neuropeptides such as corticotropin releasing factor (CRF) (Brielmaier et al. 2012; Nader et al. 2012).
CRF not only plays a neuroendocrine regulatory role in the hypothalamic-pituitary- adrenal (HPA) stress response, but also is involved in stress reactivity through action in extrahypothalamic brain sites (Heinrichs et al. 1992) and in drug reward (Koob and Zorrilla 2010). Our recent work implicates the CRF system in the VTA in neuroadaptations that are induced during episodes of adult social defeat to subsequent cocaine self-administration, cocaine-stimulated motor activity, and cocaine-stimulated dopamine release in the nucleus accumbens shell (Boyson et al. 2014; Boyson et al. 2011). The CRF-R1 antagonist CP395376 is a highly selective antagonist for CRF-R1 based on previous in vivo studies (Chen et al. 2008; Schulz et al. 1996). Microinjections of CP395376 prior to each social defeat dose-dependently prevented the subsequent escalation of cocaine intake during a 24 hour binge in singly housed adult rats (Boyson et al. 2014).
The extrahypothalamic CRF system has been investigated mostly in the extended amygdala during adolescent development (Eghbal-Ahmadi et al. 1998; Viau et al. 2005; Weathington and Cooke 2012), but not in the adolescent VTA. Stressors such as exposure to fox odor or placement on an elevated platform, during adolescence decreases CRF-R1 gene expression in subregions of the hippocampus and amygdala, and antagonism of CRF-R1 following adolescent stress attenuates behavioral indices of long-term anxiety states (Veenit et al. 2014) and also depressive-like behavior (Bourke et al. 2014). Systemic administration of CRF-R1 antagonists during adolescence prevents stress sensitization to conditioned place preference for psychostimulants (Brielmaier et al. 2012; Nader et al. 2012). The current study aims to determine whether microinjection of the CRF-R1 antagonist CP359376 into the VTA prior to each episode of adolescent social defeat will block putative neuroadaptations from occurring that promote escalated cocaine self-administration in early adulthood. Furthermore, we investigated the acute effect of CP359376 on social defeat behavior and searched for adolescent behavioral predictors of adult cocaine taking.
Experimental Methods
Long-Evans rats from Charles River Laboratories (Wilmington, MA) were housed in a vivarium that was maintained at 21 ± 1°C with 35–40% humidity on a reverse light/dark cycle (lights on 2000 to 0800 h). Non-littermate male rats (N=64, 5 separate 1-day shipments over 7 months) arrived between P20 and P22 and were housed in pairs in standard polycarbonate cages (45 × 24 × 20 cm). Food (LabDiet 5001 rodent chow) and tap water were available ad libitum. Reliably aggressive adult male rats (500–700 g; N=20) were housed with females in large stainless steel cages (71 × 46 × 46 cm) in a separate room, but within the same vivarium (Miczek 1979). Facilities and procedures were approved by the Tufts Institutional Animal Care and Use Committee in adherence with the guidelines established by the National Institutes of Health (National Research Council 2011).
Intracranial surgery
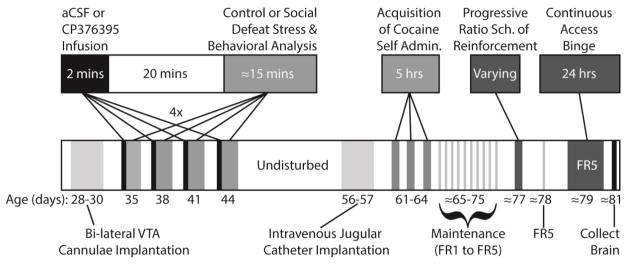
Between P28–P30 rats were anesthetized with ketamine (100 mg/kg) and xylazine (6 mg/kg) and surgically implanted with bilateral stainless steel cannulae (23 ga, 9 mm length, Plastics One) aimed 1 mm above the VTA (anterior-posterior: −5.2 mm from bregma; medial-lateral: ±1.8 mm from bregma; dorsal-ventral: −7.1 mm from skull) at a 10° angle. Protection and patency of the cannulae were maintained by inserting obdurators between microinjections. Rats were allowed to recover until P35 before the first microinjection.
Microinjections and social defeat
All rats were microinjected with CP376395 (500 ng/side) or artificial cerebrospinal fluid (aCSF) into the VTA as previously reported for adult rats (Boyson et al. 2014; Boyson et al. 2011). After infusion, injectors were left in place for one additional minute to prevent backflow and allow for diffusion; then the obdurators were replaced and the rats were returned to their home cages (Figure 1). Twenty minutes after the start of the microinjection, rats were placed into a resident’s cage. Before the first microinjections, all resident males had multiple encounters with non-experimental adolescent intruders and the 6 most consistently aggressive residents were selected based on short latency to attack, frequent aggressive postures, and consistent attack bites. Adolescents were defeated 4 times over the course of 10 days, following the intermittent episodic social defeat design of previous experiments (Boyson et al. 2014; Burke and Miczek 2015; Covington and Miczek 2001; Miczek et al. 2011). The interaction was terminated 5 mins after the first attack bite or earlier if the intruder displayed a submissive supine posture for >8 seconds or if 13 attack bites occurred. The intruder was then placed in a protective mesh cage (20 × 30 × 20 cm), which was placed inside the resident’s home cage for 10 min. Intruders were exposed to a different resident during each episode of defeat. Control rats remained undisturbed during the defeats, and cage-mates always had the same treatment, either both defeated or both control.
Figure 1.
Summary of experimental procedures and timeline.
Behavioral Analysis
Rat behavior was measured using The Observer XT v9.0.436 (Noldus Information Technology, The Netherlands). A trained observer (intra-observer reliability r>0.93) measured previously described social defeat behaviors (Buwalda et al. 2013; Koolhaas et al. 1980; Miczek 1979; Plyusnina et al. 2011). Since the duration of the fights (latency to submission) varied, all Observer-coded data were expressed as a percent of total time or number per minute. Resident behaviors quantified were: attack bite, leg attack/lunging, dragging intruder, frontal threat, lateral sideways threat, allogrooming, and sniffing. The term “attack” refers to attack bites, leg attacks, and dragging. Intruder behaviors quantified were: Non-social exploration (rearing and sniffing), walking, supine posture, freezing posture, stretch attend posture, walking slowly, tail rattle, upright defensive posture, follow the resident, approach resident, crawl under the resident, and social investigation (sniffing resident). Freezing and stretch attend were combined and referred to as freezing. Social investigation, following, and crawling under were defined as social exploration. Immobility was calculated by subtracting percent of time engaged in behaviors requiring mobility (e.g., following) from the percent of time not walking. A time lag of +2 sec from the attack bite was used to determine the probability of intruder behaviors in response to an attack bite.
Cocaine Self-administration
Rats were anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (6 mg/kg). An indwelling catheter (Silastic silicone tubing, Dow Corning, ID 0.63 mm, OD 1.17 mm) was implanted into the right jugular vein as previously described (Burke and Miczek 2015; Covington and Miczek 2001) on P57–59. Following 5–6 days of recovery, rats were transported to a custom-built chamber (30 × 30.5 × 24.5 cm) within a sound- and light-attenuating enclosure.
All self-administration procedures and equipment were closely similar to those used in previous experiments (Boyson et al. 2014; Burke and Miczek 2015; Figure 1). Med PC IV (Med Associates Inc., St. Albans, VT) controlled experimental events and recorded operant responding.
During acquisition, rats were allowed to self-administer cocaine (0.75 mg/kg per infusion) according to a fixed ratio (FR) 1 schedule with the active and inactive levers present once per day. The session was terminated after 5 hours or 30 infusions. On the 2nd and 3rd day of acquisition the experimenter simulated a response using the computer, which resulted in a single priming infusion of cocaine to increase motor activity. Rats were considered to have acquired the task when they obtained 15 infusions for two out of the three sessions. If rats did not achieve this requirement within 3 sessions (15 hours of access to cocaine), the experimenter shaped the rat’s behavior. No behavioral shaping was used during measurement of dependent variables. After acquisition, the FR was gradually increased to 5 and after 2 stable days of FR5 responding the motivation to self-administer cocaine was studied according to a progressive ratio schedule of reinforcement (PR).
Rats were given access to cocaine on a PR that required an increasing number of responses to be reinforced by an infusion of cocaine (0.3 mg/kg/infusion). A lower unit dose of cocaine was used for this phase (0.3 mg/kg/infusion) based on previous studies suggesting that reliably augmented self-administration following adult social defeat is observed at this lower dose (Covington and Miczek 2001, 2005). The PR session was followed by 1 or more FR5 maintenance sessions until responding stabilized again. The following day rats were given access to cocaine (0.3 mg/kg/infusion) for 24 hours on an FR5 schedule.
Histology
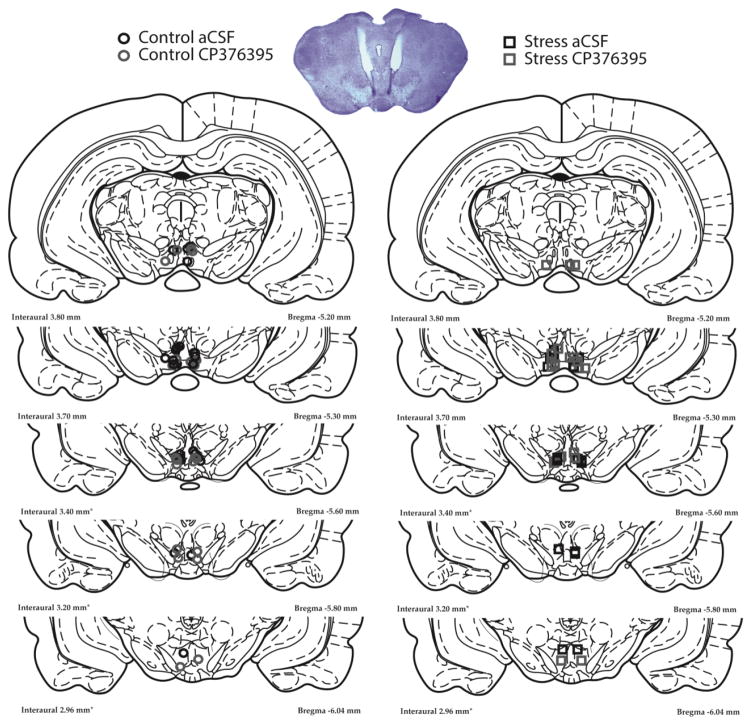
After the 24-h binge, rats were deeply anesthetized and perfused with saline and 4% PFA, and their brains were fixed in PFA, then sucrose. 60 μm sections were mounted on slides and stained with cresyl violet to identify exact injection sites using light microscopy and a rat brain atlas (Paxinos and Watson 2007; Figure 2).
Figure 2.
Representative photo of cresyl violet stained midbrain illustrating bilateral cannula placement 1 mm above the VTA and injector tracks entering the VTA. Each figure corresponds to coronal sections of the rat brain −5.2 to −6.04 mm from bregma. Shown are symbols representing injection sites for control aCSF (black circle, N=13), control CP376395 (grey circle, N=12), stress aCSF (black square, N=9), and stress CP376395 (grey square, N=11) treatment groups.
Drugs
CP376395 (Tocris Bioscience) was dissolved in aCSF. The chosen dose had proven to be most effective in similar studies in adult rats (Boyson et al. 2014; Boyson et al. 2011). Cocaine hydrochloride was obtained from the Research Technology Branch of the National Institute on Drug Abuse (Rockville, MD) and dissolved in sterile 0.9% saline.
Statistical Analysis
Behaviors during social defeat encounters were analyzed with a repeated measures two-way ANOVA with the factors Day (first or fourth defeat) x Drug (aCSF or CP376395). Two-way ANOVA (Drug x Treatment [Control or Social Defeat] was used to analyze the cocaine self- administration data and body weights. The percent increase in body weight was calculated by examining the difference between the average weight from P51-56 (pre-surgery) and the average weight on P31-35 (pre-stress) for each individual. A Kaplan Meier survival analysis followed by Mantel-Cox Log Rank Tests was used to analyze the percent of rats reaching the criteria for acquisition of cocaine self-administration. Separate linear regression analyses identified significant correlations between adolescent behavior and adult cocaine taking. Separate Grubbs’ tests were applied to cocaine self-administration data (except acquisition) and behavioral data during social defeat to identify outliers (Grubbs 1969). The outcome removed subjects from the Binge (N=1), PR (N=1), Maintenance (N=0), and from several behavioral data sets (N=1–3). Survival analysis and log ranks were performed using IBM SPSS Statistics v21 (IBM Corp.). Sigma Plot v11 (Systat Software Inc.) was used for the remainder of statistical tests. The alpha level was always 0.05 for statistical significance.
Results
Histology
Intra-VTA placements were evenly distributed across the anterior-posterior dimension of the VTA (Figure 2). There were too few subjects with placements outside of the VTA (N=6) to allow statistical comparison, and there were no misses in one treatment group.
Social Defeat Behavior
Two-way repeated measures ANOVAs yielded several significant effects of day of adolescent defeat stress and drug infusion during the first (P35) and fourth (P44) social defeat (Table 1). Both vehicle and CP376395 infused rats were quicker to freeze after repeated defeats as evidenced by the main effect of day for latency to freeze. The CP376395 infused rats exhibited a longer latency to submission. The drug x day interaction for social exploration and following behavior as well as non-social exploration behavior was significant, whereby aCSF infused rats increased social exploration, but decreased non-social exploration from P35 to P44. These effects were absent in CP376395 infused rats. CP376395 infused rats walked less and spent more time immobile compared to aCSF infused rats as indicated by main effects of drug. There was a significant main effect of day for the probability of displaying the supine posture when attacked, suggesting that rats were more likely to adopt the supine posture when attacked on P44 compared to P35.
Table 1.
Mean (± SEM) social defeat behaviors of intruders infused with aCSF or CP376395 prior to first defeat (P35) and fourth defeat (P44).
| aCSF P35 | aCSF P44 | CP376395 P35 | CP376395 P44 | Statistics | |
|---|---|---|---|---|---|
| Intruder Behaviors | |||||
| Latency to Freeze (s) | 65.0 ± 12.5 | 14.6 ± 6.4 | 44.3 ± 9.4 | 22.4 ± 8.3 | Day: F(1,17)=21.9, p<0.001 |
| Latency to Supine (s) | 52.9 ± 13.5 | 49.7 ± 10.3 | 63.2 ± 10.5 | 62.5 ± 16.3 | Not Significant |
| Latency to Submission (s) | 321.8 ± 25.7 | 271.4 ± 37.1 | 326.2 ± 19.2 | 378.8 ± 23.4 | Drug: F(1,18)=4.57, p=0.045 |
| Freezing (% of time) | 8.12 ± 2.08 | 10.15 ± 2.78 | 8.80 ± 1.98 | 10.84 ± 2.19 | Not Significant |
| Supine (% of time) | 2.34 ± 1.51 | 4.08 ± 1.79 | 3.05 ± 1.33 | 2.51 ± 0.73 | Not Significant |
| Upright Defensive (%of time) | 0.47 ± 0.32 | 0.87 ± 0.36 | 0.05 ± 0.04 | 0.79 ± 0.32 | Not Significant |
| Approach (#/min) | 0.60 ± 0.21 | 0.98 ± 0.28 | 0.82 ± 0.17 | 0.81 ± 0.17 | Not Significant |
| Social Exploration & Following (% of time) | 4.57 ± 1.33† | 10.30 ± 2.70 | 6.32 ± 1.82 | 6.23 ± 1.21 | Drug x Day: F(1,17)=6.22, p=0.023 |
| Non-social Exploration (% of time) | 15.5 ± 2.3† | 8.6 ± 1.2 | 10.4 ± 1.7 | 12.4 ± 2.0 | Drug x Day: F(1,17)=8.50, p=0.009 |
| Walking (% of time) | 21.8 ± 4.0 | 20.1 ± 3.9 | 13.7 ± 2.6 | 13.2 ± 1.7 | Drug: F(1,17)=4.35, p=0.049 |
| Immobility (% of time) | 67.5 ± 5.0 | 58.6 ± 5.1 | 73.7 ± 3.3 | 75.8 ± 2.9 | Drug: F(1,17)=6.13, p=0.022 |
| Resident Behaviors
| |||||
| Latency to Attack (s) | 25.1 ± 5.2 | 22.4 ± 3.1 | 26.1 ± 6.1 | 43.3 ± 6.0 | Drug: F(1,17)=4.61, p=0.043 |
| Latency to Sniff (s) | 1.95 ± 0.33 | 2.44 ± 0.44 | 2.64 ± 0.37† | 2.01 ± 0.32 | Drug x Day: F(1,18)=5.94, p=0.025 |
| Attacks (#/min) | 2.73 ± 0.45† | 5.34 ± 1.06 | 2.91 ± 0.83 | 2.65 ± 0.51§ | Drug: F(1,16)=5.81, p=0.028; Drug x Day: F(1,16)=6.56, p=0.020 |
| Sniff Intruder (% of time) | 10.5 ± 2.1 | 10.3 ± 2.5 | 13.7 ± 1.8 | 14.2 ± 1.2 | Drug: F(1,17)=5.67, p=0.025 |
| Probability of Adolescent Behavior after Attack Bite
| |||||
| Freezing | 0.11 ± 0.04 | 0.23 ± 0.06 | 0.15 ± 0.04 | 0.22 ± 0.05 | Not Significant |
| Supine | 0.06 ± 0.03 | 0.17 ± 0.05 | 0.14 ± 0.04 | 0.23 ± 0.05 | Day: F(1,19)=8.70, p=0.008 |
different from P44 within group (p<0.05)
different from aCSF on P44 (p<0.05)
The main effect of drug on latency to attack was significant, suggesting that the residents were quicker to attack aCSF infused rats than to attack CP376395 infused rats. There were also significant main effects of drug on the number of attacks per minute and a drug by day interaction. Residents attacked P44 intruders at a higher rate than P35 intruder only if the intruder was infused with aCSF prior to the social interaction. The residents also attacked aCSF infused intruders at a higher rate than CP376395 intruders on P44. However, the residents spent more time sniffing the CP376395 intruders as evidenced by a main effect of drug. There was a significant drug by day interaction on the latency to sniff the intruder, revealing that resident were slower to sniff CP376395 intruders on P35 compared to P44.
Body Weight Gain
The percent increase in body weight gain was 57.7 ± 0.6 for aCSF Control, 58.6 ± 0.8 for CP376395 Control, 57.1 ± 0.6 for aCSF Stress, and 59.4 ± 1.0 for CP376395 Stress. The main effect of drug on body weight gain reached significance (F(1,45)=3.98, p≤0.05) indicated that rats infused with the CRF-R1 antagonist gained slightly more body weight.
Cocaine Self Administration
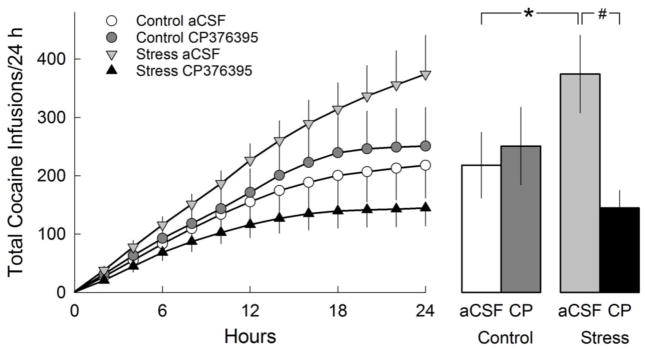
Rats infused with intra-VTA aCSF prior to each adolescent social defeat acquired the i.v. cocaine self-administration task in early adulthood significantly faster than rats infused with CP376395 prior to defeat (x2=4.2, p<0.05; Table 2). A significant main effect of drug on stable responding for i.v. cocaine at FR5, referred to as “maintenance” in Table 2 (F(1,36)=7.30, p≤0.01), indicating that adult rats infused with aCSF prior to adolescent defeat responded at a higher rate for i.v. cocaine than rats infused with CP376395. For PR (Table 2), a significant effect of drug (F(1,34)=8.27, p<0.01) suggested that CP376395 infusion prior to adolescent defeat attenuated the number cocaine infusions achieved during the PR test compared to aCSF infused rats. There was a significant drug x stress treatment interaction for total cocaine infusions obtained during the 24 hour binge (F(1,34)=5.51, p<0.05; Figure 3, right panel). Post hoc comparisons indicated that within rats infused with aCSF during adolescence, the socially defeated adolescent rats consumed more cocaine than non-defeated controls during the 24 hour binge in adulthood (p≤0.05). Furthermore, among the socially defeated rats, infusion of CP376395 prior to each adolescent defeat blocked the escalated cocaine consumption during the 24 hour binge observed in aCSF infused rats (p<0.01).
Table 2.
Adult cocaine self-administration following social defeat stress during adolescence
| Percent Reaching Acquisition Criterion | Maintenance (Responses/min) | Progressive Ratio (Infusions) | |
|---|---|---|---|
| Control aCSF | 38.5 % | 0.89 ± 0.11 | 11.55 ± 0.55 |
| Control CP376395 | 33.3 % | 0.78 ± 0.12 | 9.78 ± 0.78 |
| Stress aCSF | 66.7 % | 1.12 ± 0.17 | 10.56 ± 0.44 |
| Stress CP376395 | 25.0 %† | 0.57 ± 0.08 | 8.20 ± 0.96 |
different from aCSF within stress (p<0.05)
Figure 3.
Number of cocaine infusions (mean± SEM) accumulated in 2 hour bins (left) and during the entire 24-h binge (right) by non-stressed controls infused with aCSF (N=11), non-stressed controls infused with CP376395 (N=9), stressed rats infused with aCSF (N=9), and stressed rats infused with CP376395 (N=10). Abbreviations: artificial cerebrospinal fluid (aCSF); CP376395 (CP). *Significantly different from control aCSF group. #Significantly different from stressed CP376395 group.
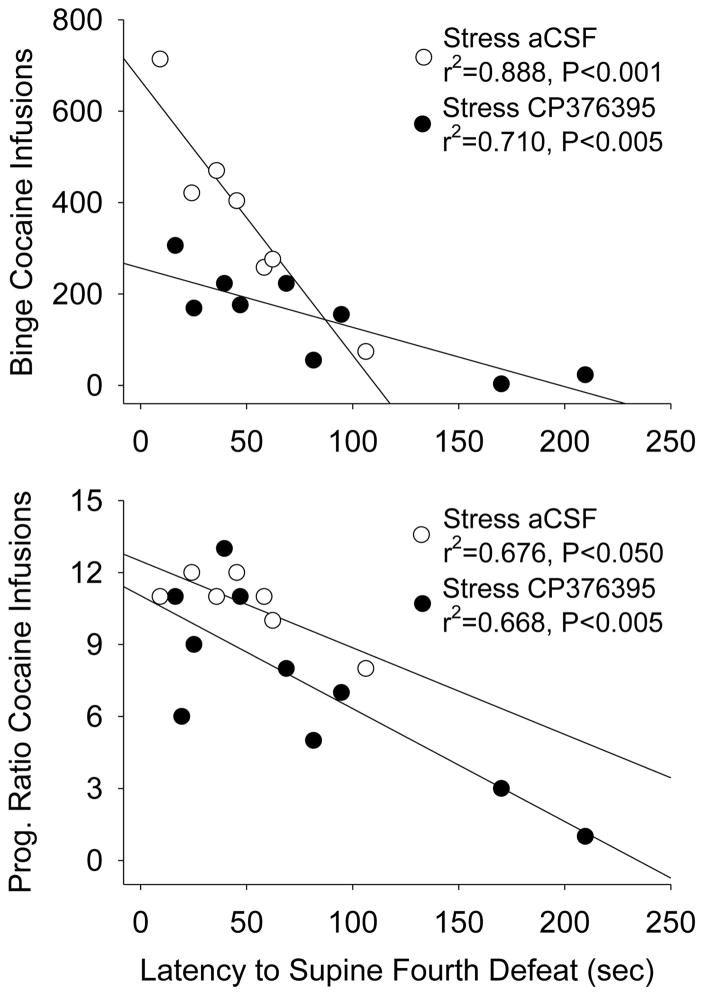
The latency to exhibit the supine posture on the last day of adolescent social defeat (P44) was negatively correlated with total cocaine infusions during the 24 hour binge for aCSF defeated group (p≤0.001, r2=0.89) and the CP376795 defeated group (p<0.004, r2=0.71) as assessed in early adulthood (Figure 4, top). The latency to display the supine posture was also negatively correlated with cocaine infusions obtained under PR for aCSF defeated group (p=0.023, r2=0.68) and the CP376795 defeated group (p=0.004, r2=0.67; Figure 4 bottom). When aCSF and CP376395 defeated groups were combined, latency to display the supine posture was still negatively correlated with cocaine infusions during the binge (p=0.002, r2=0.52), PR (p<0.001, r2=0.66), and also with responses per minute during maintenance (p=0.018, r2=0.32).
Figure 4.
Top, Latency to adopt the supine posture during the fourth defeat on P44 was negatively correlated with the number of cocaine infusions obtained durting the 24 hour binge for rats infused with aCSF prior to social defeat stress and those infused with CP376395. Bottom, Latency to supine on P44 also was inversely correlated with cocaine infusions obtained during progressive ratio schedule of reinforcement.
Discussion
The present experiment confirmed that social defeat stress during adolescence escalated adult cocaine self-administration during the 24-h binge (Burke and Miczek 2015). This effect was prevented by CRF-R1 antagonism in the VTA prior to each adolescent social defeat episode, suggesting that VTA CRF-R1 receptors are essential for adolescent social defeat to initiate a neuroadaptive process that escalates later drug self-administration in adulthood. Furthermore, the latency to assume the supine posture after repeated social defeat episodes was highly predictive of adult cocaine taking both after vehicle and CP376395 infusion into the VTA prior to the social defeat, indicating that this salient element of submissive behavior during adolescence is somehow related to adult drug taking.
Adaptive changes in both social and non-social behaviors over the course of repeated social defeats characterized vehicle infused control rats, while these effects were absent in rats infused with CP376395. Notably, rats infused with CP376395 prior to each social defeat walked less and were more immobile during interactions with the aggressive adult. These results suggest that CRF-R1s in the VTA are important for locomotion behavior under high stress conditions, and for adaptive behaviors over repeated exposures to social stress. Meanwhile, the residents were less aggressive toward these CP376395-injected rats on P44, but more likely to socially investigate them, suggesting that the lack of adaptive changes in CP376395-infused rats impacted resident social behavior during the 4th social defeat (P44).
Both CP154526 and CP376395 CRF-R1 antagonists infused into the VTA prior to social defeat in adulthood prevent escalation of cocaine self-administration (Boyson et al. 2014; Boyson et al. 2011). Systemic and intracerebroventricular infusion of both these CRF-R1 antagonists causes transient reductions in alcohol drinking (Hwa et al. 2016), and CP376395 reduces stress-induced escalation of alcohol drinking (Hwa et al. 2015). CP154526 has high affinity (Ki<10) for CRF-R1 (Schulz et al. 1996) and is a prototypic CRF-R1 antagonist. CP376395 is more selective for CRF-R1 (IC50=5) and more water-soluble than CP154526 (Chen et al. 2008). The high dose of CP376395 (500 ng/side) was chosen based on previous studies (Blacktop et al. 2011; Holly et al. 2015) and because it most consistently blocked neural and behavioral cross-sensitization in our previous study of adult rats (Boyson et al. 2014).
The investigation of behavior during social defeat 20 minutes after intra-VTA CRF-R1 antagonist infusion was previously unexplored. There was evidence for habituation in the non-social exploration behavior in aCSF treated rats from P35 to P44 confirming our previous study (Burke and Miczek 2015). In addition, the aCSF-treated rats attended to the resident more on P44 compared to P35. However, the CP376395 treated rats did not adapt to repeated social defeat encounter as the aCSF-treated rats did, suggesting a potential role of VTA CRF-R1 in behavioral adaptation during adolescent stress. Confrontation with an aggressive adult opponent activates the HPA stress response in adolescent rats (Watt et al. 2009). The HPA axis is initiated by hypothalamic CRF release, which eventually causes the synthesis of corticosterone in the adrenals and systemic corticosterone exerts negative feedback on further CRF release (Herman et al. 2003). Our finding that intra-VTA CP376395 reduces motor activity agrees with the attenuating effect of systemic administration of the CRF-R1 antagonist antalarmin on i.c.v. CRF-induced motor activity (Zorrilla et al. 2002). Low motor activity of adolescent rats injected with CP376395 could explain the reduced rates of attacks and the increased latency to attack toward these less active intruders, since reduced intruder locomotion is thought to reduce resident aggressive behavior (Buwalda et al. 2012; Paul et al. 2011). As the residents spent less time attacking the CP376395-infused intruders they spent more time sniffing them, suggesting residents were no less interested in the drug-treated intruders, just less aggressive toward them. The behavior of the intruder rat has been shown to influence resident aggression (Blanchard and Blanchard 1977; Miczek 1979; Burke and Miczek 2015). For example, lower levels of motor activity during the social defeat test are thought to reduce further aggression when the intruder is an adolescent (van den Berg et al., 1999) and when it is an adult (Buwalda et al., 2012; Nocjar et al., 2012; Paul et al., 2011). It is not possible to determine whether the increase in aggression from P35 to P44 is a result of the conditioned behavior of the intruder or the increased probability of attacking a larger and older adolescent intruder undergoing sexual maturation (Burke and Miczek 2014).
The latency to display the supine posture after repeated social defeats predicted greater risk for escalated cocaine taking about 5 weeks later in early adulthood. The probability of the intruder exhibiting the supine posture increased over the course of repeated defeats, indicating increased submissive behavior during repeated aggressive encounters. In agreement with our previous study (Burke and Miczek 2015), rats were quicker to freeze from P35 to P44 when confronted with the resident. Attack-induced freezing during adolescent social defeat predicted adult cocaine taking in our previous experiment (Burke and Miczek 2015); yet, this correlation was absent in the present study. Discrepancies between the current and past results may be the result of additional handling during the microinjection procedure, the change in duration of supine posture required for fight termination (4 s in the previous study to 8 s in the current study), or the exclusion of the single housing factor. Overall, adaptive behaviors during adolescence that prevent further attacks such as decreased latency to supine in this study and increased attack-induced freezing in our previous study (Burke and Miczek 2014) have interesting relevance to the escalation of cocaine taking in adulthood.
Rats infused with aCSF prior to social defeat self-administered more cocaine during the 24 hour unlimited access binge as previously observed (Burke and Miczek 2015). Pretreatment with bilateral CP376395 into the VTA prior to each adolescent social defeat blocked escalation of cocaine self-administration during the binge in adulthood. This result is similar to the prevention of escalated cocaine taking during the 24-h binge by CRF-R1 antagonism in the VTA of adult rats prior to social defeat (Boyson et al. 2014; Boyson et al. 2011). The major methodological differences between the current study and Boyson et al. (2014) are that the intruders were adolescents and that they were housed in pairs. This agreement between studies with adolescent and adult rats is concordant with the observations that CRF-R1 mRNA in the rat mesocorticolimbic system peaks in the brain prior to adolescence between P12 and P21 (Avishai-Eliner et al. 1996; Insel et al. 1988). In the VTA, CRF-R1 and CRF-R2 expression remains constant during the stages of adolescence into adulthood (Lukkes et al. 2016). Thus, the role of CRF-R1 in the VTA in escalation of cocaine self-administration following social defeat stress is not age-dependent.
One potential neural mechanism for social defeat episodes during adolescence to escalate cocaine self-administration in adulthood involves the mesocorticolimbic dopamine system. Both cocaine and intermittent social defeat stress during adulthood increase extracellular dopamine in the NAc shell in rats with a history of intermittent social defeat (Miczek et al. 2011), which is attenuated by intra-VTA CP376395 prior to each defeat (Boyson et al. 2014). While adolescent social defeat does not increase extracellular dopamine in the NAc core stimulated by amphetamine in adulthood (Burke et al. 2013), it remains to be determined whether social defeat during adolescence increases subsequent extracellular NAc shell dopamine in response to cocaine. The current results for the 24-h binge are consistent with other findings suggesting that CRF receptors in the VTA are involved in escalated cocaine self-administration (Specio et al. 2008), stress-induced reinstatement (Blacktop et al. 2011; Chen et al. 2014; Wang et al. 2005), and the induction of neuroadaptive changes that take place during social stress to escalate later cocaine self-administration (Boyson et al. 2014; Boyson et al. 2011). Other studies suggest that CRF-R1 antagonism prevents adolescent non-social stress from increasing preference for contexts paired with experimenter administered nicotine and cocaine (Brielmaier et al. 2012; Nader et al. 2012). The current results are the first evidence of a role of CRF-R1 for adolescent social stress to induce neuroadaptive changes that escalate voluntary drug self-administration. These novel results also implicate the adolescent VTA CRF system in the propensity for escalated cocaine taking after adolescent adverse experience.
Not only salient rewarding events (Schultz 2002) but also stressful experiences, such as social defeat of adult rats, increase tonic and phasic dopamine signaling in the mesocorticoaccumbal system (Anstrom et al. 2009; Holly et al. 2015; Tidey and Miczek 1996), which presumably sensitizes the dopamine system to subsequent psychostimulant challenges. Stressful events also result in the release of CRF into the VTA (Wang et al. 2005), and VTA dopamine neurons increase their firing rate through activation of CRF-R1 receptors (Wanat et al. 2008). Thus, CRF-R1 antagonism prior to social defeat may prevent this increase in dopamine firing rate of VTA neurons, which in turn reduces cross-sensitization to cocaine, and which attenuates cocaine self-administration during the 24-h binge in the CP376395 stressed group. Recent data investigating adult rat dopamine release during social defeat did not detect attenuated extracellular dopamine by the CRF-R1 antagonist (Holly et al. 2015). Because the dopamine system is undergoing substantial maturation during the mid-adolescent period (P34-46) when adolescent social defeat occurs (Burke and Miczek 2014), it is necessary to investigate tonic and rapid phasic firing of the mesocorticolimbic dopamine system during social defeat stress and CRF receptor manipulation during adolescence to completely rule out this hypothesis. Future studies need to investigate multiple doses of the CRF-R1 antagonist and determine the role of CRF type 2 receptors that are implicated in adult social defeat studies (Boyson et al. 2014; Holly et al. 2015) and other aspects of the CRF system (e.g., binding protein) for a role in adolescent social defeat behavioral adaptations over repeated stressful episodes.
We found that CRF-R1 within the VTA plays an important role in the link between adolescent social stress to adult escalated cocaine taking during unlimited access. Presumably by blocking CRF-R1 in the VTA we are preventing social stress-induced neuroadaptations that would normally increase the risk for cocaine abuse. The CRF-R1s in the VTA are essential for normal acclimation to repeated social defeats during adolescence and also for motor behavior under stressful conditions. The impact of CRF-R1 on intruder behavior may impede aggressiveness of the resident, but does not reduce social exploration. Further studies are required to determine whether other drugs that alter intruder behavior similarly to the pharmacological manipulation in this study can also reduce cocaine taking in adulthood. Finally, individuals that were quicker to submission after repeated aggressive confrontations during adolescence were more likely to uncontrollably self-administer cocaine when given unlimited access in adulthood, suggesting that greater severity of social stress equates to greater risk for drug abuse. Although limited to this animal model, these findings implicate a potential neural mechanism explaining how victimization during adolescence increases risk for substance abuse and addiction in humans.
Acknowledgments
The authors acknowledge support from the National Institutes of Health R01DA031734 (KAM), F32DA032226 (ARB), Tufts University Office of the Vice Provost for Research, and Tufts University Graduate School of Arts and Sciences, and thank Tufts University undergraduate assistants Shreya Bhatia and Rebecca Nardulli.
Footnotes
Disclosure: The authors declare no conflicts of interest.
References
- Anstrom KK, Miczek KA, Budygin EA. Increased phasic dopamine signaling in the mesolimbic pathway during social defeat in rats. Neuroscience. 2009;161:3–12. doi: 10.1016/j.neuroscience.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avishai-Eliner S, Yi SJ, Baram TZ. Developmental profile of messenger RNA for the corticotropin-releasing hormone receptor in the rat limbic system. Brain Res Dev Brain Res. 1996;91:159–163. doi: 10.1016/0165-3806(95)00158-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacktop JM, Seubert C, Baker DA, Ferda N, Lee G, Graf EN, Mantsch JR. Augmented cocaine seeking in response to stress or CRF delivered into the ventral tegmental area following long-access self-administration is mediated by CRF receptor type 1 but not CRF receptor type 2. J Neurosci. 2011;31:11396–11403. doi: 10.1523/JNEUROSCI.1393-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Aggressive behavior in the rat. Behav Biol. 1977;21:197–224. doi: 10.1016/s0091-6773(77)90308-x. [DOI] [PubMed] [Google Scholar]
- Bourke CH, Glasper ER, Neigh GN. SSRI or CRF antagonism partially ameliorate depressive-like behavior after adolescent social defeat. Behav Brain Res. 2014;270:295–299. doi: 10.1016/j.bbr.2014.05.035. [DOI] [PubMed] [Google Scholar]
- Boyson CO, Holly EN, Shimamoto A, Albrechet-Souza L, Weiner LA, DeBold JF, Miczek KA. Social stress and CRF-dopamine interactions in the VTA: role in long-term escalation of cocaine self-administration. J Neurosci. 2014;34:6659–6667. doi: 10.1523/JNEUROSCI.3942-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyson CO, Miguel TT, Quadros IM, DeBold JF, Miczek KA. Prevention of social stress-escalated cocaine self-administration by CRF-R1 antagonist in the rat VTA. Psychopharmacology. 2011;218:257–269. doi: 10.1007/s00213-011-2266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brielmaier J, McDonald CG, Smith RF. Effects of acute stress on acquisition of nicotine conditioned place preference in adolescent rats: a role for corticotropin-releasing factor 1 receptors. Psychopharmacology. 2012;219:73–82. doi: 10.1007/s00213-011-2378-1. [DOI] [PubMed] [Google Scholar]
- Burke AR, Forster GL, Novick AM, Roberts CL, Watt MJ. Effects of adolescent social defeat on adult amphetamine-induced locomotion and corticoaccumbal dopamine release in male rats. Neuropharmacology. 2013;67:359–369. doi: 10.1016/j.neuropharm.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke AR, Miczek KA. Stress in adolescence and drugs of abuse in rodent models: role of dopamine, CRF, and HPA axis. Psychopharmacology. 2014;231:1557–1580. doi: 10.1007/s00213-013-3369-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke AR, Miczek KA. Escalation of cocaine self-administration in adulthood after social defeat of adolescent rats: Role of social experience and adaptive coping behavior. Psychopharmacology. 2015;232:3067–3079. doi: 10.1007/s00213-015-3947-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buwalda B, Scholte J, de Boer SF, Coppens CM, Koolhaas JM. The acute glucocorticoid stress response does not differentiate between rewarding and aversive social stimuli in rats. Horm Behav. 2012;61:218–226. doi: 10.1016/j.yhbeh.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Buwalda B, Stubbendorff C, Zickert N, Koolhaas JM. Adolescent social stress does not necessarily lead to a compromised adaptive capacity during adulthood: A study on the consequences of social stress in rats. Neuroscience. 2013;26:258–270. doi: 10.1016/j.neuroscience.2012.12.050. [DOI] [PubMed] [Google Scholar]
- Chen NA, Jupp B, Sztainberg Y, Lebow M, Brown RM, Kim JH, Chen A, Lawrence AJ. Knockdown of CRF1 receptors in the ventral tegmental area attenuates cue- and acute food deprivation stress-induced cocaine seeking in mice. J Neurosci. 2014;34:11560–11570. doi: 10.1523/JNEUROSCI.4763-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YL, Obach RS, Braselton J, Corman ML, Forman J, Freeman J, Gallaschun RJ, Mansbach R, Schmidt AW, Sprouse JS, Tingley FD, III, Winston E, Schulz DW. 2-aryloxy-4-alkylaminopyridines: discovery of novel corticotropin-releasing factor 1 antagonists. J Med Chem. 2008;51:1385–1392. doi: 10.1021/jm070579c. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Miczek KA. Repeated social-defeat stress, cocaine or morphine. Effects on behavioral sensitization and intravenous cocaine self-administration “binges”. Psychopharmacology. 2001;158:388–398. doi: 10.1007/s002130100858. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Miczek KA. Intense cocaine self-administration after episodic social defeat stress, but not after aggressive behavior: dissociation from corticosterone activation. Psychopharmacology. 2005;183:331–340. doi: 10.1007/s00213-005-0190-5. [DOI] [PubMed] [Google Scholar]
- Eghbal-Ahmadi M, Hatalski CG, Lovenberg TW, Avishai-Eliner S, Chalmers DT, Baram TZ. The developmental profile of the corticotropin releasing factor receptor (CRF2) in rat brain predicts distinct age-specific functions. Brain Res Dev Brain Res. 1998;107:81–90. doi: 10.1016/s0165-3806(98)00002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubbs FE. Procedures for detecting outlying observations in samples. Technometrics. 1969;11:1–21. [Google Scholar]
- Heinrichs SC, Pich EM, Miczek KA, Britton KT, Koob GF. Corticotropin-releasing factor antagonist reduces emotionality in socially defeated rats via direct neurotropic action. Brain Res. 1992;581:190–197. doi: 10.1016/0006-8993(92)90708-h. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Hoffmann JP, Cerbone FG, Su SS. A growth curve analysis of stress and adolescent drug use. Subst Use Misuse. 2000;35:687–716. doi: 10.3109/10826080009148417. [DOI] [PubMed] [Google Scholar]
- Holly EN, DeBold JF, Miczek KA. Increased mesocorticolimbic dopamine during acute and repeated social defeat stress: modulation by corticotropin releasing factor receptors in the ventral tegmental area. Psychopharmacology. 2015;232:4469–4479. doi: 10.1007/s00213-015-4082-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, Holly EN, DeBold JF, Miczek KA. Social stress-escalated intermittent alcohol drinking: modulation by CRF-R1 in the ventral tegmental area and accumbal dopamine in mice. Psychopharmacology. 2015;233:681–90. doi: 10.1007/s00213-015-4144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, Shimamoto A, Kayyali T, Norman KJ, Valentino RJ, DeBold JF, Miczek KA. Dissociation of μ-opioid receptor and CRF-R1 antagonist effects on escalated ethanol consumption and mPFC serotonin in C57BL/6J mice. Addict Biol. 2016;21:111–124. doi: 10.1111/adb.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Battaglia G, Fairbanks DW, De Souza EB. The ontogeny of brain receptors for corticotropin-releasing factor and the development of their functional association with adenylate cyclase. J Neurosci. 1988;8:4151–4158. doi: 10.1523/JNEUROSCI.08-11-04151.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Zorrilla EP. Neurobiological mechanisms of addiction: focus on corticotropin-releasing factor. Curr Opin Investig Drugs. 2010;11:63–71. [PMC free article] [PubMed] [Google Scholar]
- Koolhaas JM, Schuurman T, Wiepkema PR. The organization of intraspecific agonistic behaviour in the rat. Prog Neurobiol. 1980;15:247–268. doi: 10.1016/0301-0082(80)90024-6. [DOI] [PubMed] [Google Scholar]
- Lukkes JL, Norman KJ, Meda S, Andersen SL. Sex differences in the ontogeny of CRF receptors during adolescent development in the dorsal raphe nucleus and ventral tegmental area. Synapse. 2016;70:125–132. doi: 10.1002/syn.21882. [DOI] [PubMed] [Google Scholar]
- Miczek KA. A new test for aggression in rats without aversive stimulation: differential effects of d-amphetamine and cocaine. Psychopharmacology. 1979;60:253–259. doi: 10.1007/BF00426664. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Nikulina EM, Shimamoto A, Covington HE., 3rd Escalated or suppressed cocaine reward, tegmental BDNF, and accumbal dopamine caused by episodic versus continuous social stress in rats. J Neurosci. 2011;31:9848–9857. doi: 10.1523/JNEUROSCI.0637-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader J, Chauvet C, Rawas RE, Favot L, Jaber M, Thiriet N, Solinas M. Loss of environmental enrichment increases vulnerability to cocaine addiction. Neuropsychopharmacology. 2012;37:1579–1587. doi: 10.1038/npp.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nansel TR, Overpeck M, Pilla RS, Ruan WJ, Simons-Morton B, Scheidt P. Bullying behaviors among US youth: prevalence and association with psychosocial adjustment. JAMA. 2001;285:2094–2100. doi: 10.1001/jama.285.16.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. 8. National Academies Press; Washington, D.C: 2011. [Google Scholar]
- Newman ML, Holden GW, Delville Y. Isolation and the stress of being bullied. J Adolesc. 2005;28:343–357. doi: 10.1016/j.adolescence.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Nocjar C, Zhang J, Feng P, Panksepp J. The social defeat animal model of depression shows diminished levels of orexin in mesocortical regions of the dopamine system, and of dynorphin and orexin in the hypothalamus. Neuroscience. 2012;218:138–153. doi: 10.1016/j.neuroscience.2012.05.033. [DOI] [PubMed] [Google Scholar]
- Paul ED, Hale MW, Lukkes JL, Valentine MJ, Sarchet DM, Lowry CA. Repeated social defeat increases reactive emotional coping behavior and alters functional responses in serotonergic neurons in the rat dorsal raphe nucleus. Physiol Behav. 2011;104:272–282. doi: 10.1016/j.physbeh.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6. Academic Press/Elsevier; Amsterdam: 2007. [Google Scholar]
- Plyusnina IZ, Solov’eva MY, Oskina IN. Effect of domestication on aggression in gray Norway rats. Behav Genet. 2011;41:583–592. doi: 10.1007/s10519-010-9429-y. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Schulz DW, Mansbach RS, Sprouse J, Braselton JP, Collins J, Corman M, Dunaiskis A, Faraci S, Schmidt AW, Seeger T, Seymour P, Tingley FD, 3rd, Winston EN, Chen YL, Heym J. CP-154,526: a potent and selective nonpeptide antagonist of corticotropin releasing factor receptors. Proc Natl Acad Sci U S A. 1996;93:10477–10482. doi: 10.1073/pnas.93.19.10477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. Stress and addiction: a dynamic interplay of genes, environment, and drug intake. Biol Psychiatry. 2009;66:100–101. doi: 10.1016/j.biopsych.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specio SE, Wee S, O’Dell LE, Boutrel B, Zorrilla EP, Koob GF. CRF1 receptor antagonists attenuate escalated cocaine self-administration in rats. Psychopharmacology. 2008;196:473–482. doi: 10.1007/s00213-007-0983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharp-Taylor S, Haviland A, D’Amico EJ. Victimization from mental and physical bullying and substance use in early adolescence. Addict Behav. 2009;34:561–567. doi: 10.1016/j.addbeh.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. Brain Res. 1996;721:140–149. doi: 10.1016/0006-8993(96)00159-x. [DOI] [PubMed] [Google Scholar]
- Veenit V, Riccio O, Sandi C. CRHR1 links peripuberty stress with deficits in social and stress-coping behaviors. J Psychiatr Res. 2014;53:1–7. doi: 10.1016/j.jpsychires.2014.02.015. [DOI] [PubMed] [Google Scholar]
- Viau V, Bingham B, Davis J, Lee P, Wong M. Gender and puberty interact on the stress-induced activation of parvocellular neurosecretory neurons and corticotropin-releasing hormone messenger ribonucleic acid expression in the rat. Endocrinology. 2005;146:137–146. doi: 10.1210/en.2004-0846. [DOI] [PubMed] [Google Scholar]
- Wanat MJ, Hopf FW, Stuber GD, Phillips PE, Bonci A. Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. J Physiol. 2008;586:2157–1270. doi: 10.1113/jphysiol.2007.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt MJ, Burke AR, Renner KJ, Forster GL. Adolescent male rats exposed to social defeat exhibit altered anxiety behavior and limbic monoamines as adults. Behav Neurosci. 2009;123:564–576. doi: 10.1037/a0015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathington JM, Cooke BM. Corticotropin-releasing factor receptor binding in the amygdala changes across puberty in a sex-specific manner. Endocrinology. 2012;153:5701–5705. doi: 10.1210/en.2012-1815. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Nozulak J, Koob GF, Markou A. Effects of antalarmin, a CRF type 1 receptor antagonist, on anxiety-like behavior and motor activation in the rat. Brain Res. 2002;952:188–199. doi: 10.1016/s0006-8993(02)03189-x. [DOI] [PubMed] [Google Scholar]