Abstract
Objective
Calcific Aortic Valve Disease (CAVD) is the most prevalent type of heart valve disease, affecting ~2% of the US population. CAVD is characterized by the presence of calcific nodules resulting in aortic valve (AoV) stenosis; however, the underlying mechanisms driving disease remain unknown. Studies of human diseased AoV provide initial evidence that BMP signaling, essential for normal bone formation, is activated during CAVD. Mice deficient in Klotho, an FGF23 transmembrane co-receptor, exhibit premature aging and develop AoV calcific nodules as occurs in human CAVD. The role of BMP signaling in the development of CAVD was examined in porcine aortic valve interstitial cells (VICs) and Klotho−/− mice.
Approach & Results
We show that activation of BMP signaling, as indicated by pSmad1/5/8 expression, precedes and later localizes with AoV calcification in Klotho−/− mice. In addition, cellular and ECM changes resembling features of normal bone formation are accompanied by increased osteochondrogenic gene induction in calcified Klotho−/− AoV. Likewise, osteogenic media (OM) treatment of porcine VICs results in BMP pathway activation, increased osteochondrogenic gene induction and formation of calcific nodules in vitro. We demonstrate that genetic inactivation of the Bmpr1a receptor in Klotho−/− aortic VICs, as well as BMP pathway inhibition of OM-treated aortic VICs in vitro, results in the inhibition of AoV calcification.
Discussion
BMP signaling and osteochondrogenic gene induction are active in calcified Klotho−/− AoV in vivo and calcified porcine aortic VICs in vitro. Importantly, BMP signaling is required for the development of AoV calcification in vitro and in vivo.
Keywords: Aortic Valve Calcification, BMP, Klotho
INTRODUCTION
Calcific Aortic Valve Disease (CAVD) is a progressive disease, initially presenting with aortic valve (AoV) sclerosis, often leading to AoV stenosis and insufficiency later in life. The risk for developing CAVD increases with age, and CAVD is becoming more prevalent with increased average lifespan1. AoV stenosis affects 2.8% of individuals over the age of 75, and AoV regurgitation presents in ~2% of the elderly2. To date, the only clinical therapy available to patients is AoV replacement surgery, which has significant limitations of coagulation and degeneration, and is often contraindicated in the elderly2–4. Thus, understanding the molecular mechanisms driving CAVD pathogenesis is crucial to the development of new therapeutic approaches.
Because of its association with aging, CAVD was believed to be a passive, degenerative disease. However, recent studies have demonstrated that CAVD is an active, cellular-driven process5. A hallmark in the progression of CAVD is AoV stenosis caused by extensive calcific deposits6. In adult healthy valves, resident valve interstitial cells (VICs) remain quiescent with fibroblast-like characteristics. Interestingly, in human CAVD, markers of endochondral bone formation – including Runx2, Alkaline Phosphatase, Osteopontin and Osteocalcin – have been observed in VICs associated with areas of calcification7, 8, supporting activation of an osteoblast-like cell phenotype in CAVD. Bone Morphogenetic Protein (BMP) signaling is essential during heart valve development and in bone formation9, 10. Notably, studies of human CAVD revealed increased BMP2 and BMP4 ligand expression in calcified AoV11, as well as increased pSmad1/5/8, indicative of canonical BMP signaling, on the fibrosa side of AoV where most calcification occurs8, 12. Altogether these data demonstrate that osteochondrogenic markers and BMP signaling are active in human CAVD. However, the in vivo requirements of BMP signaling for osteochondrogenic gene induction and AoV calcification remain unknown.
The Klotho−/− mouse model, which exhibits premature aging associated with hyperphosphatemia, low vitamin D and increased FGF23, has been used to study age-related diseases, including chronic kidney disease and vascular calcification13. Similar to human CAVD, Klotho−/− mice exhibit AoV calcification at the hinge region of the fibrosa side14. This is in contrast to hyperlipidemic mouse models of AoV disease, in which calcification occurs throughout the aorta and valve leaflet surface, accompanied by lipid accumulation and inflammation15, 16. Instead, calcification occurs independent of inflammation in Klotho−/− mice, making these mice useful for examination of molecular mechanisms of AoV calcification that are distinct from hyperlipidemia.
The goal of this study is to examine the requirements for BMP signaling during AoV calcification. Klotho−/− AoV exhibit features of endochondral bone formation and localized pSmad1/5/8 activation in the hinge region preceding calcific nodule formation. The ability of a small molecule inhibitor of BMP signaling to inhibit porcine aortic VIC calcification was tested in culture. Genetic inactivation of BMP receptor Bmpr1a in aortic VICs of Klotho−/− mice was used to demonstrate the in vivo requirement for BMP signaling in the development of AoV calcification.
MATERIALS AND METHODS
Materials and Methods are available in the online-only Data Supplement.
RESULTS
Calcification of AoV in Klotho−/− mice exhibits features of endochondral bone formation
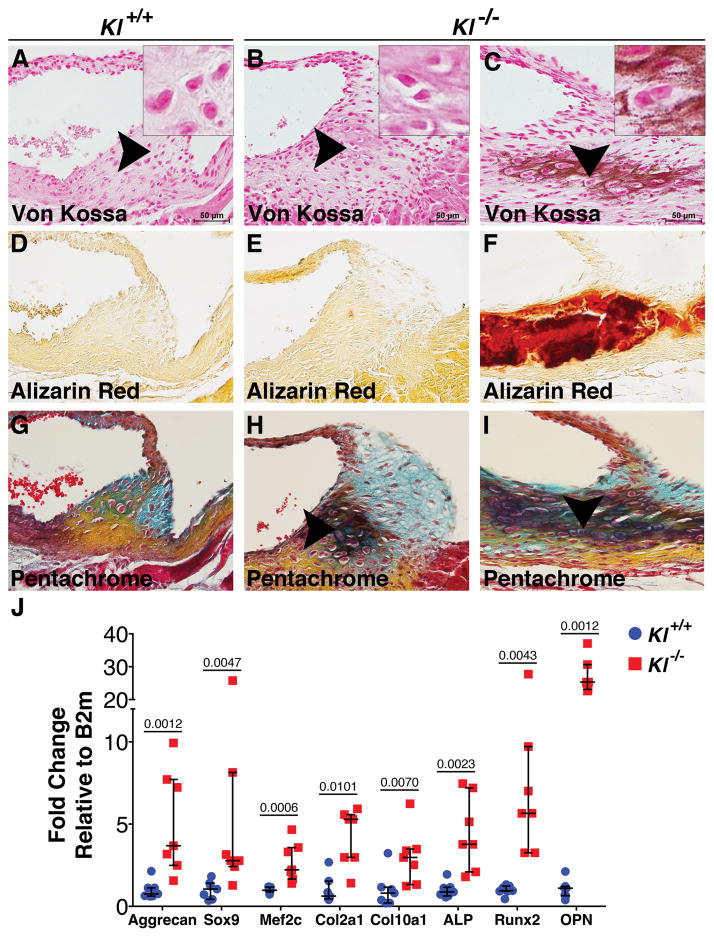
There is increasing evidence that the progression of human CAVD includes features of endochondral bone formation, including cartilage and bone-lineage gene induction8, 11. As demonstrated by Movat’s pentachrome staining, which labels extracellular matrix (ECM) constituents of the Klotho−/− AoV, areas of cell condensation can be observed prior to calcification (Figure 1B,E,H). These condensed areas are associated with increased proteoglycans and the presence of chondrocyte-like cells as indicated by morphology (Figure 1H). Likewise, proteoglycan-rich areas are localized to calcific nodules surrounded by chondrocyte-like cells (Figure 1C,F,I). Thus, VICs localized to calcific nodules undergo cellular changes that resemble endochondral bone formation.
Figure 1. Calcification of Klotho−/− AoV has features of endochondral bone formation.
Von Kossa staining detects the presence of calcific deposits in 6-week old Klotho−/− AoV (brown, C) compared to controls (A). Chondrocyte-like cells are present in Klotho−/− AoV hinge regions (B,C arrowheads and insets). As a secondary method of detection for calcification, Alizarin Red staining was performed (red, D–F). Movat’s Pentachrome staining shows changes in the ECM in Klotho−/− AoV (H,I arrowheads). Yellow stains collagen, blue stains glycosaminoglycan, deep green denotes intense overlapping yellow and blue staining. At least 3 hearts were analyzed for each phenotype at 6 weeks of age (A–F). Osteochondrogenic gene expression was determined in Klotho−/− AoV compared to control AoV at 6 weeks (n=7/group). Gene expression was normalized to Beta-2-microglobulin (B2m) control expression. Values were normalized to wild type control averages and are represented as fold changes (J). Error bars are shown as interquartile means and scatter plot. Statistical significance was determined by Mann-Whitney U-test (p< 0.05 are indicated).
Analysis of gene expression of 6-week-old Klotho−/− AoV demonstrates a significant increase in mRNA levels of cartilage markers Sox9, Aggrecan, Col2a1, Col10a1 and Mef2c (Figure 1J), which are critical factors in chondrogenesis that precedes normal bone formation. In addition, osteogenic genes involved in bone mineralization, including Alkaline Phosphatase (ALP), Runx2 and Osteopontin (OPN), are also significantly increased in these AoV, compared to control Klotho+/+ AoV (Figure 1J). Together, these findings demonstrate that AoV calcification in the Klotho−/− mice is associated with increased cartilage and bone marker expression, supporting an endochondral bone-like process of calcification.
Calcific nodules are populated by endothelial- and neural crest-derived cells in Klotho−/− mice
Adult semilunar VICs include cells derived from endocardial cushion endothelial cells and neural crest of the developing embryo17. However, the contribution of each cell type to the areas of calcification and disease pathogenesis during adult CAVD remains unclear. The developmental origins of cells populating mature AoV leaflets were assessed using Tie2Cre labeling of endothelial-derived cells in comparison to Wnt1Cre lineage cells derived from embryonic neural crest. Tie2Cre and Wnt1Cre mice were crossed with ROSA26mTmG mice in order to identify Cre-recombined cells found in the AoV. These Cre-positive cells are marked by membrane-targeted green fluorescent protein (GFP), compared to expression of membrane-targeted red fluorescence protein (RFP) in cells that do not express Cre18. Endothelial-derived Tie2Cre-positive cells, indicated by GFP, are distributed throughout all three leaflets in wild type AoV (Supplementary Figure I). Neural crest-derived Wnt1Cre GFP-positive cells are also present in all leaflets of wild type AoV, but are predominantly located in the hinge region (Supplementary Figure I). Thus, Tie2Cre and Wnt1Cre GFP-positive cells populate the AoV hinge region.
The embryonic origins of calcified VICs were examined in calcified Klotho−/− AoV. Both Tie2Cre and Wnt1Cre-derived cells are present in the calcified region of the AoV hinge in Klotho−/− mice (Supplementary Figure II). Approximately 60% of the Wnt1Cre-expressing cells in the AoV hinge are localized to calcified regions in Klotho−/−;Wnt1Cre AoV. In contrast, Tie2Cre-positive cells have a more uniform distribution in the hinge and only 20% are localized in the calcified regions. Interestingly, Tie2Cre and Wnt1Cre lineages each contribute ~25% of the total number of cells that populate the calcified areas of Klotho−/− AoV. Together these data demonstrate that both endothelial and neural crest derivatives contribute to calcified nodules in Klotho−/− AoV.
pSmad1/5/8 activation precedes and later localizes with calcific nodules in Klotho−/− AoV
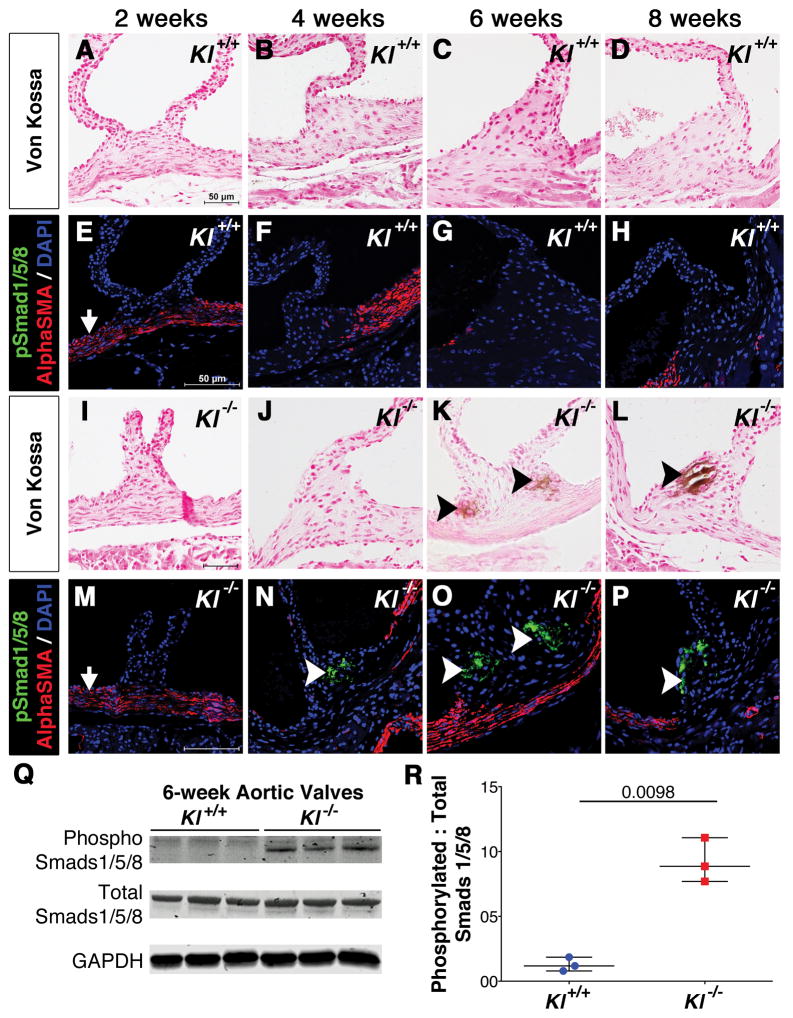
The expression of pSmad1/5/8 was examined during the progression of AoV calcification in Klotho−/− mice as an indicator of BMP signaling status. pSmad1/5/8 is initially detected in the AoV hinge prior to onset of disease at 4 weeks of age (Figure 2J,N). Later, pSmad1/5/8 localizes with calcific nodules at 6 (Figure 2K,O) and 8 (Figure 2L,P) weeks in Klotho−/− AoV, compared to age-matched wild type controls where there is no positive antibody staining or calcification (Figure 2A–H). pSmad1/5/8 expression is restricted to the AoV hinge region interstitial cells, and is not detected in smooth muscle cells of the aorta that express Alpha Smooth Muscle Actin (αSMA) (Figure 2E–H and M–P). Likewise, Western blot analysis of isolated AoV at 6 weeks demonstrates increased pSmad1/5/8 protein expression in Klotho−/− mice when compared to wild type controls (Figure 2Q–R). Interestingly, there is no significant difference in the prevalence of AoV calcification between sexes. Thus BMP signaling, as indicated by pSmad1/5/8, is activated prior to and during development of AoV calcification in Klotho−/− mice.
Figure 2. pSmad1/5/8 activation precedes and later localizes with calcific nodules in Klotho−/− AoV.
AoV calcification in Klotho−/− mice (I–L) compared to WT littermate controls (A–D) was examined at 2, 4, 6 and 8 weeks of age. Evidence of calcification is first observed at 6 weeks and persists through 8 weeks of age, as detected by von Kossa staining (K–L, arrowheads). pSmad1/5/8 (green) activation precedes calcification at 4 weeks (N, white arrowheads), and then localizes with calcific nodules at 6–8 weeks (O–P) in Klotho−/− AoV. Smooth muscle cells of the aorta are detected by AlphaSMA staining (red) in Klotho−/− and control AoV (E–H, M–P). At least 3 hearts were analyzed for each phenotype and time-point. Western blot analysis of phosphoSmad1/5/8, total Smad1/5/8 and GAPDH protein levels in Klotho−/− AoV compared to WT littermate control (Q). Quantification of phosphoSmad1/5/8 levels normalized to total Smads and GAPDH (R); n=3 was used for each Klotho−/− and control group in which each n is equal to 10–12 AoV. Error bars are shown as mean ±SEM and scatter plot. Statistical significance was determined by unpaired t-test with Welch’s correction (p< 0.05).
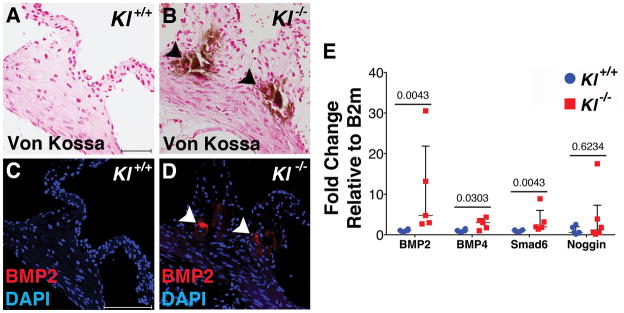
Expression of BMP ligands and signaling intermediates was examined in Klotho−/− AoV. BMP2 protein is localized to calcific nodules of Klotho−/− AoV at 6 weeks (Figure 3C,D), but BMP2 expression was not detected in controls (Figure 3A,B). In addition, BMP2 and BMP4 ligand gene expression is increased in Klotho−/− AoV compared to controls at 6 weeks (Figure 3E). Gene expression of the BMP pathway downstream target Smad6 also is significantly increased 6 weeks (Figure 3E). Thus, active BMP pathway components are localized to areas of AoV calcification in Klotho−/− mice in vivo.
Figure 3. BMP pathway components are active in calcified Klotho−/− AoV.
BMP2 ligand is expressed in calcified AoV of Klotho−/− mice (B,D) but not control AoV (A,C) at 6 weeks of age, as determined by immunofluorescence (red). BMP signaling network gene expression was evaluated by qRT-PCR of RNA isolated from Klotho−/− AoV compared to WT controls at 6 weeks of age (E). There is a significant increase in BMP2 and BMP4 ligand mRNA levels, as well as Smad6 (n=5/group). Gene expression was normalized to B2m control expression. Values were normalized to wild type control averages and are represented as fold changes. Error bars are shown as interquartile means and scatter plot. Statistical significance was determined by Mann-Whitney U-test (p< 0.05).
BMP pathway inhibition prevents calcific nodule formation and osteochondrogenic gene induction in aortic VICs cultured in vitro
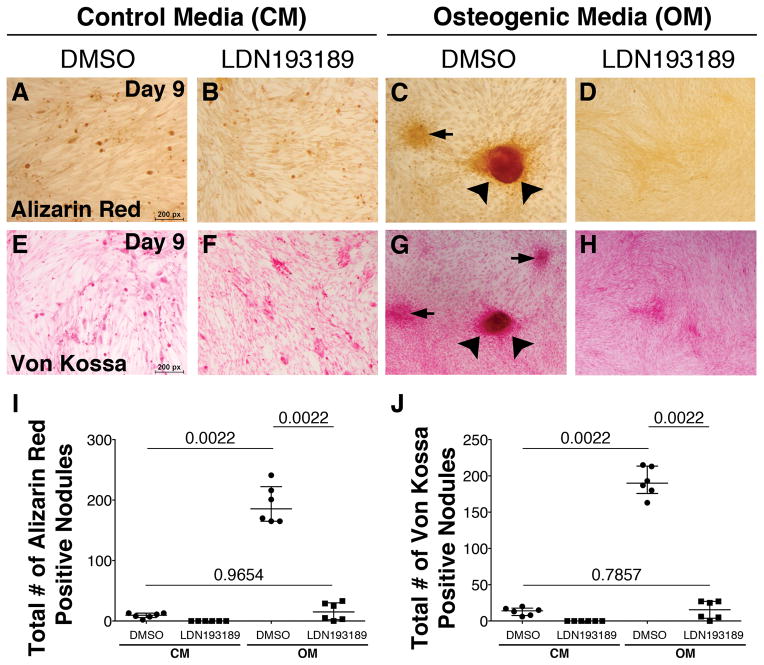
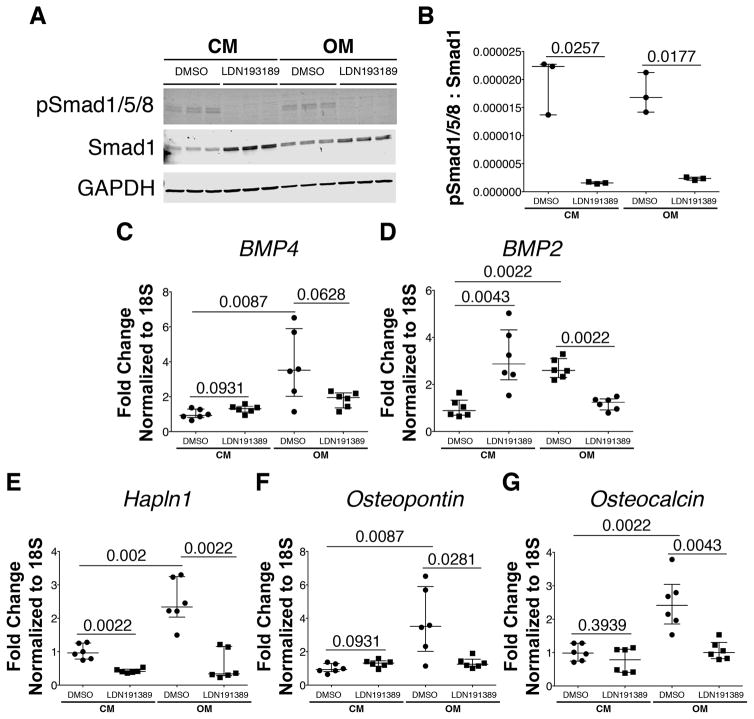
As previously shown19, porcine VICs treated with Osteogenic Media (OM) form calcific nodules, as indicated by positive Alizarin Red (Figure 4C) and von Kossa (Figure 4G) staining after 9 days in culture. In contrast, VICs maintained in control media do not form calcific nodules (Figure 4A,E). The necessity for BMP signaling in OM-induced calcific nodule formation was determined using small molecule inhibitor of BMP signaling, LDN-19318920, 21. LDN-193189 treatment inhibits OM-induced formation of calcific nodules (Figure 4D,H) when compared to cells treated only with OM (Figure 4C,G), as demonstrated by quantification of the number of nodules/well (Figure 4I–J). In addition, LDN-193189 treatment significantly inhibits phosphorylation of Smads1/5/8 as illustrated by Western blot analysis and quantification (Figure 5A,B). Moreover, OM-induced BMP2, but not BMP4, ligand expression is suppressed by LDN-193189 treatment (Figure 5C,D). Furthermore, addition of LDN-193189 to OM-treated cells results in decreased expression of OM-induced chondrogenic gene Hapln1 (Figure 5E), as well as osteogenic genes Osteopontin (Figure 5F) and Osteocalcin (Figure 5G). However, BMP2 treatment alone in the absence of OM is not sufficient to promote calcification of aortic VICs in vitro (Supplementary Fig III). Notably, treatment of porcine VICs for 9 days does not result in apoptosis (Supplementary Figure IV). Together, these data demonstrate that BMP signaling is required, but is not sufficient, for the formation of calcific nodules and osteochondrogenic gene induction in cultured aortic VICs.
Figure 4. LDN-193189 treatment prevents formation of calcific nodules in porcine aortic VICs in vitro.
Porcine aortic VICs were cultured in Osteogenic Media (OM) or Control Media (CM) for 9 days, ± BMP inhibitor LDN-193189. OM-induced calcific nodules can be observed by Alizarin Red (red, C) and von Kossa (brown, G) staining, and formation of these nodules is inhibited by LDN-193189 (D,H). The total number of calcific nodules for each treatment group, as detected by Alizarin Red (I) and von Kossa (J) staining, was quantified. Each dot is representative of the total number of calcific nodules quantified per well for each treatment group. Error bars are shown as interquartile means and scatter plot. Statistical significance was determined by Mann-Whitney U-test (p< 0.05) for comparisons indicated by lines.
Figure 5. LDN-193189 treatment inhibits OM-induced osteochondrogenic gene induction in porcine aortic VICs in vitro.
Western blot analysis shows pSmad1/5/8 relative to total Smad 1 and GAPDH control proteins in porcine aortic VICs cultured in Osteogenic Media (OM) or Control Media (CM), ± BMP inhibitor LDN-193189 (A,B). Gene expression profiles of BMP ligands BMP4 (C) and BMP2 (D), cartilage marker Hapln1 (E) and bone markers Osteopontin (F) and Osteocalcin (G), evaluated by qRT-PCR of RNA isolated from porcine aortic VICs cultured in OM and CM for 9 days, ± BMP LDN-193189. Displayed values represent 2 independent experiments performed in triplicate (C–G). Values are representative of 5 different independent experiments. Gene expression was normalized to 18S control expression. Values were normalized to control DMSO averages and are represented as fold changes. Error bars are shown as interquartile means in scatter plots. Statistical significance was determined by Mann-Whitney U-test (p< 0.05).
Genetic inactivation of Bmpr1a does not affect AoV development or maturation in vivo
The requirement for BMP signaling in normal aortic VICs was examined in vivo using PostnCre;Bmpr1aflox/flox mice. PostnCre22 is active in aortic VICs, but not in endothelial cells, and is expressed robustly throughout the AoV23. The BMP Type IA receptor ALK3 (Bmpr1a) is required for BMP-mediated phosphorylation of Smad1/5/8 in many tissues, including heart valve progenitors24. Tie2Cre and Wnt1Cre are active in a subset of aortic VICs, and deletion of Bmpr1a in either of these lineages causes embryonic lethality25, 26. Therefore, the requirements for Bmpr1a in PostnCre lineage cells, present in embryonic and adult valves after endocardial cushion formation, were examined. Although BMP signaling plays essential roles during endocardial cushion EndMT24, 26, PostnCre;Bmpr1aflox/flox mice are viable and the hearts are grossly normal at postnatal day (P)1, similar to Bmpr1aflox/flox control mice (Supplementary Figure V). In addition, Pentachrome staining demonstrates normal heart morphology, in addition to normal heart valve stratification and ECM composition of PostnCre;Bmpr1aflox/flox aortic and mitral valves (Supplementary Figure V), when compared to control P1 hearts. PostnCre expression is observed in other regions outside of the AoV VICs, including fibroblasts located at the atrioventricular junction22. However, since PostnCre is activated after endocardial cushion formation23, these data demonstrate that PostnCre-driven deletion of Bmpr1a does not affect late stage AoV development or neonatal maturation.
PostnCre;Bmpr1aflox/flox mice are viable and fertile as adults, with no observed embryonic or postnatal lethality. AoV analyzed at 8 weeks of age exhibit normal heart morphology, as well as normal AoV and ECM composition, as indicated by Pentachrome staining when compared to control Bmpr1aflox/flox mice (Supplementary Figure VI). Although the valves are apparently normal, PostnCre;Bmpr1aflox/flox mice exhibit a significant decrease in body weight compared to control mice (Supplementary Figure VI). In spite of this difference, PostnCre-driven deletion of Bmpr1a does not affect overall heart and valve structure and homeostasis in adult mice.
Genetic inactivation of Bmpr1a prevents development of AoV calcification in Klotho−/− mice with CAVD
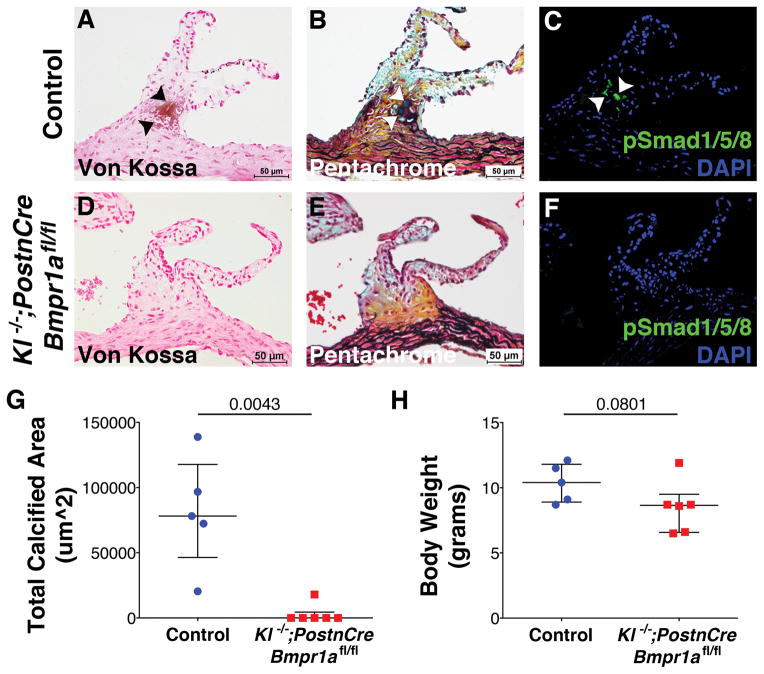
The requirement for BMP signaling, through Bmpr1a, in the development of CAVD in Klotho−/− mice was examined. In contrast to Tie2Cre or Wnt1Cre-lineages, PostnCre derived cells are located throughout the Klotho−/− AoVs (Supplementary Figure VII) and adult mice are viable for analysis. Thus 7–9 week-old Klotho−/−;PostnCre;Bmpr1aflox/flox mice were used to determine if BMP signaling is required in the development of AoV calcification. Klotho−/− mice with or without PostnCre-mediated Bmpr1a deletion are smaller by weight than normal adult mice27 (Figure 6H). However, inactivation of Bmpr1a in Klotho−/− AoV results in a significant decrease of AoV calcification, as evident by analysis of von Kossa staining, when compared to control mice that exhibit AoV calcification (Figure 6A,D). BMP pathway inactivation through loss of Bmpr1a also preserves normal ECM organization and cellular morphology (Figure 6B,E). Importantly, no pSmad1/5/8 immunoreactivity is observed in Klotho−/−;PostnCre; Bmpr1aflox/flox AoV (Figure 6F). Total areas of calcification were measured and quantified for Klotho−/−; PostnCre;Bmpr1aflox/flox and control Klotho−/− AoV von Kossa stained images. Quantitative data show a significant decrease in the total calcified area of AoV in Klotho−/−;PostnCre;Bmpr1aflox/flox mice when compared to Klotho−/− mice (Figure 6G). Together these data demonstrate the necessity of BMP-pSmad1/5/8 signaling for the development of AoV calcification in Klotho−/− mice.
Figure 6. Genetic inactivation of the BMP receptor Bmpr1a prevents AoV calcification in Klotho−/− mice.
Von Kossa staining was used for the detection of AoV calcification in Klotho−/−;PostnCre;Bmpr1aflox/flox compared to control mice at 7–9 weeks of age (A,D, black arrowheads). Control mice include both Klotho−/−;PostnCre;Bmpr1a+/+ and Klotho−/−;Bmpr1a+/+, since there is no difference in the amount of AoV calcification seen in both phenotypes. Pentachrome staining illustrates ECM and cellular composition (B,E). IHC for pSmad1/5/8 (green) was used for detection of active BMP signaling (C,F). Total calcified area of the AoV was quantified from von Kossa stained histological sections of Klotho−/−;PostnCre;Bmpr1aflox/flox (n=6) mice compared to control mice (n=5) (G). A significant decrease in the total calcified area in Klotho−/−;PostnCre;Bmpr1aflox/flox AoV is observed when compared to calcified areas in control AoV (C, p= 0.0043). Total body weight is not significantly different between groups (H). Error bars are shown as interquartile means in scatter plots. Statistical significance was determined by Mann-Whitney U-test (p< 0.05).
DISCUSSION
Here, we demonstrate that expression of pSmad1/5/8, indicative of active BMP signaling, precedes and later localizes with AoV calcification in Klotho−/− mice with CAVD. Likewise, BMP ligands BMP2 and BMP4 are significantly increased in Klotho−/− AoV. Consistent with previous studies, BMP signaling is required for osteogenic gene induction and calcification in cultured porcine VICs, similar to human VICs28–30. pSmad1/5/8 activation and increased BMP2 and BMP4 expression have been reported in human CAVD8, 11, 12. However, in vivo requirements for BMP signaling in AoV calcification have not been demonstrated previously. Here, we show that BMP pathway inhibition through genetic inactivation of Bmpr1a in Klotho−/− aortic VICs prevents AoV calcification in vivo. Thus BMP signaling is required for CAVD.
BMP signaling has previously been implicated in valve development and disease. In developing valves, Bmpr1a-mediated BMP signaling is required for proper formation of the atrioventricular junction and endocardial cushions24, 26. Moreover, genetic deletion of the related receptor ALK2 (Acvr1) in the cushion mesenchyme leads to AoV defects, including Bicuspid Aortic Valve (BAV)31. In this study, we show that PostnCre-driven deletion of Bmpr1a after endocardial cushion formation does not affect valve development or adult homeostasis, but does inhibit AoV calcification in Klotho−/− mice. Interestingly, increased BMP signaling due to haploinsufficiency of the BMP inhibitor Smad6 is sufficient to promote aortic calcification32. Thus BMP signaling has multiple critical roles in valve development and disease.
There is increasing evidence that AoV calcification during CAVD occurs via an endochondral bone formation-like process11. VICs derived from both endothelial and neural crest embryonic lineages are calcified, suggesting that the potential to calcify is associated with location within the valve, rather than a specific embryonic origin or cell lineage. Interestingly, BMP signaling via pSmad1/5/8 plays essential roles during endochondral bone formation by regulating expression of critical cartilage and bone factors33, 34. In accordance, osteochondrogenic gene induction, accompanied by cellular and ECM changes that resemble features of normal endochondral bone formation, occurs in calcified Klotho−/− AoV, where BMP signaling is active. Loss of BMP signaling through genetic deletion of Bmpr1a in vivo or inhibition of BMP receptor kinase activity by LDN-193189 in culture leads to loss of AoV osteochondrogenic characteristics and prevents formation of calcific nodules. Together these data demonstrate that BMP signaling is required for induction of endochondral bone formation-like processes during AoV calcification.
There are a number of risk factors associated with CAVD, including valve developmental defects, notably BAV, inflammation due to increased lipid deposition, and hyperphosphatemia as a result of chronic kidney disease (CKD). Consequently, the underlying pathogenesis of CAVD may vary depending on the accompanying risk factors6, 35–37. Previous studies have shown that AoV calcification in Klotho−/− mice occurs independent of inflammation14, or a myofibroblast intermediate19. Furthermore, Klotho−/− mice develop hyperphosphatemia secondary to CKD and prior to the onset of AoV calcification along with other age-related phenotypes13, 27. The Klotho protein is a cofactor for fibroblast growth factor FGF23 and Klotho-deficiency leads to abnormal mineral metabolism characterized by increased phosphate, calcium and vitamin D serum levels38. Klotho-deficiency also leads to increased Wnt signaling39, 40, which together with BMP signaling, promotes endochondral bone formation and contributes to vascular calcification41. BMP signaling is not sufficient to induce AoV calcification, but the contributions of these additional factors in conjunction with increased BMP signaling to AoV pathogenesis remains to be elucidated.
CAVD is a progressive disease, and currently there are no pharmacologic-based therapies that can prevent or inhibit CAVD. The current standard of treatment is AoV replacement, which is associated with significant complications that increase with age42. This study shows that BMP pathway inhibition prevents calcific nodule formation, a hallmark of CAVD pathology, demonstrating that BMP signaling is required for the development of CAVD. Importantly, in vitro treatment of porcine aortic VICs with BMP inhibitor LDN-193189 leads to inhibition of OM-induced calcific nodule formation. LDN-193189 is a small molecule inhibitor that specifically targets the BMP receptor Bmpr1a and has been shown to reduce soft tissue calcification21, as well as vascular calcification and atherosclerosis43, 44. However, LDN-193189 has not yet been tested in the context of CAVD. Our data demonstrates that targeting BMP signaling might serve as a new therapeutic approach in the prevention of AoV calcification during CAVD.
Supplementary Material
HIGHLIGHTS.
Increased BMP signaling, osteochondrogenic gene induction, cellular morphology and ECM changes resembling endochondral bone formation are observed in calcified Klotho−/− AoV, supporting an endochondral bone-like process of calcification via BMP signaling.
Treatment with BMP receptor inhibitor LDN-193189 inhibits OM-induced osteochondrogenic gene induction and formation of calcific nodules in vitro.
Genetic inactivation of the Bmpr1a receptor in Klotho−/− aortic VICs, results in the inhibition of AoV calcification. Thus BMP signaling is required for AoV calcification in vivo.
Acknowledgments
We thank Alexia Hulin and Christina Alfieri for their scientific discussions and technical support.
SOURCES OF FUNDING
Funding for this project was provided by the American Heart Association (13PRE16230006), the University of Cincinnati Research Council Graduate Student Fellowship and the National Institutes of Health (NIH) T32HL125204 (MVGS), and NIH R01 HL114682 (KEY).
NONSTANDARD ABBREVIATIONS AND ACRONYMS
- CAVD
Calcific Aortic Valve Disease
- AoV
Aortic Valve
- VIC
Valve Interstitial Cell
- BMP
Bone Morphogenetic Protein
- αSMA
Alpha Smooth Muscle Actin
Footnotes
DISCLOSURES
None.
References
- 1.Carabello BA, Paulus WJ. Aortic stenosis. Lancet. 2009;373:956–966. doi: 10.1016/S0140-6736(09)60211-7. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, et al. American Heart Association Statistics C, Stroke Statistics S. Heart disease and stroke statistics--2015 update. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 3.Zilla P, Brink J, Human P, Bezuidenhout D. Prosthetic heart valves: Catering for the few. Biomaterials. 2008;29:385–406. doi: 10.1016/j.biomaterials.2007.09.033. [DOI] [PubMed] [Google Scholar]
- 4.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, 3rd, Guyton RA, O’Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM, 3rd, Thomas JD American College of Cardiology/American Heart Association Task Force on Practice G. 2014 aha/acc guideline for the management of patients with valvular heart disease. J Am Coll Cardiol. 2014;63:e57–185. doi: 10.1016/j.jacc.2014.02.536. [DOI] [PubMed] [Google Scholar]
- 5.Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Demer LL, Heistad DD, Simmons CA, Masters KS, Mathieu P, O’Brien KD, Schoen FJ, Towler DA, Yoganathan AP, Otto CM. Calcific aortic valve disease: Not simply a degenerative process: A review and agenda for research from the national heart and lung and blood institute aortic stenosis working group. Executive summary: Calcific aortic valve disease-2011 update. Circulation. 2011;124:1783–1791. doi: 10.1161/CIRCULATIONAHA.110.006767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O’Brien KD. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90:844–853. doi: 10.1161/01.cir.90.2.844. [DOI] [PubMed] [Google Scholar]
- 7.Rajamannan NM, Subramaniam M, Rickard DJ, Stock SR, Donovan J, Springett M, Orszulak T, Fullerton DA, Tajik AJ, Bonow RO, Spelsberg TC. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation. 2003;107:2181–2184. doi: 10.1161/01.CIR.0000070591.21548.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wirrig EE, Hinton RB, Yutzey KE. Differential expression of cartilage and bone-related proteins in pediatric and adult diseased aortic valves. J Mol Cell Cardiol. 2011;50:561–569. doi: 10.1016/j.yjmcc.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Combs MD, Yutzey KE. Heart valve development: Regulatory networks in development and disease. Circ Res. 2009;105:408–421. doi: 10.1161/CIRCRESAHA.109.201566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: Molecular clones and activities. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 11.Mohler ER, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522–1528. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- 12.Ankeny RF, Thourani VH, Weiss D, Vega JD, Taylor WR, Nerem RM, Jo H. Preferential activation of smad1/5/8 on the fibrosa endothelium in calcificed human aortic valves - association with low bmp antagonists and smad6. PLoS One. 2011;6:e20969. doi: 10.1371/journal.pone.0020969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuro-o M. Klotho and aging. Biochim Biophys Acta. 2009;1790:1049–1058. doi: 10.1016/j.bbagen.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheek JD, Wirrig EE, Alfieri CM, James JF, Yutzey KE. Differential activation of valvulogenic, chondrogenic, and osteogenic pathways in mouse models of myxomatous and calcific aortic valve disease. J Mol Cell Cardiol. 2012;52:689–700. doi: 10.1016/j.yjmcc.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller JD, Weiss RM, Heistad DD. Calcific aortic valve stenosis: Methods, models, and mechanisms. Circ Res. 2011;108:1392–1412. doi: 10.1161/CIRCRESAHA.110.234138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sider KL, Blaser MC, Simmons CA. Animal models of calcific aortic valve disease. Int J Inflam. 2011;2011:364310. doi: 10.4061/2011/364310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armstrong EJ, Bischoff J. Heart valve development: Endothelial cell signaling and differentiation. Circ Res. 2004;95:459–470. doi: 10.1161/01.RES.0000141146.95728.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo LQ. A global double-fluorescent cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 19.Wirrig EE, Gomez MV, Hinton RB, Yutzey KE. Cox2 inhibition reduces aortic valve calcification in vivo. Arterioscler Thromb Vasc Biol. 2015;35:938–947. doi: 10.1161/ATVBAHA.114.305159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cuny GD, Yu PB, Laha JK, Xing X, Liu JF, Lai CS, Deng DY, Sachidanandan C, Bloch KD, Peterson RT. Structure-activity relationship study of bone morphogenetic protein (bmp) signaling inhibitors. Bioorg Med Chem Lett. 2008;18:4388–4392. doi: 10.1016/j.bmcl.2008.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu PB, Deng DY, Lai CS, et al. Bmp type i receptor inhibition reduces heterotopic ossification. Nat Med. 2008;14:1363–1369. doi: 10.1038/nm.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snider P, Standley KN, Wang J, Azhar M, Doetschman T, Conway SJ. Origin of cardiac fibroblasts and the role of periostin. Circ Res. 2009;105:934–947. doi: 10.1161/CIRCRESAHA.109.201400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang M, Alfieri CM, Hulin A, Conway SJ, Yutzey KE. Loss of beta-catenin promotes chondrogenic differentiation of aortic valve interstitial cells. Arterioscler Thromb Vasc Biol. 2014;34:2601–2608. doi: 10.1161/ATVBAHA.114.304579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lockhart MM, Boukens BJ, Phelps AL, Brown CL, Toomer KA, Burns TA, Mukherjee RD, Norris RA, Trusk TC, van den Hoff MJ, Wessels A. Alk3 mediated bmp signaling controls the contribution of epicardially derived cells to the tissues of the atrioventricular junction. Dev Biol. 2014;396:8–18. doi: 10.1016/j.ydbio.2014.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaartinen V, Dudas M, Nagy A, Sridurongrit S, Lu MM, Epstein JA. Cardiac outflow tract defects in mice lacking alk2 in neural crest cells. Development. 2004;131:3481–3490. doi: 10.1242/dev.01214. [DOI] [PubMed] [Google Scholar]
- 26.Song L, Fassler R, Mishina Y, Jiao K, Baldwin HS. Essential functions of alk3 during av cushion morphogenesis in mouse embryonic hearts. Dev Biol. 2007;301:276–286. doi: 10.1016/j.ydbio.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 28.Balachandran K, Sucosky P, Jo H, Yoganathan AP. Elevated cyclic stretch induces aortic valve calcification in a bone morphogenic protein-dependent manner. Am J Pathol. 2010;177:49–57. doi: 10.2353/ajpath.2010.090631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poggio P, Sainger R, Branchetti E, Grau JB, Lai EK, Gorman RC, Sacks MS, Parolari A, Bavaria JE, Ferrari G. Noggin attenuates the osteogenic activation of human valve interstitial cells in aortic valve sclerosis. Cardiovasc Res. 2013;98:402–410. doi: 10.1093/cvr/cvt055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouchareb R, Mahmut A, Nsaibia MJ, et al. Autotaxin derived from lipoprotein(a) and valve interstitial cells promotes inflammation and mineralization of the aortic valve. Circulation. 2015;132:677–690. doi: 10.1161/CIRCULATIONAHA.115.016757. [DOI] [PubMed] [Google Scholar]
- 31.Thomas PS, Sridurongrit S, Ruiz-Lozano P, Kaartinen V. Deficient signaling via alk2 (acvr1) leads to bicuspid aortic valve development. PLoS One. 2012;7:e35539. doi: 10.1371/journal.pone.0035539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galvin KM, Donovan MJ, Lynch CA, Meyer RI, Paul RJ, Lorenz JN, Fairchild-Huntress V, Dixon KL, Dunmore JH, Gimbrone MAJ, Falb D, Huszar D. A role for smad6 in development and homeostasis of the cardiovascular system. Nat Genet. 2000;24:171–174. doi: 10.1038/72835. [DOI] [PubMed] [Google Scholar]
- 33.Nishimura R, Hata K, Matsubara T, Wakabayashi M, Yoneda T. Regulation of bone and cartilage development by network between bmp signalling and transcription factors. J Biochem. 2012;151:247–254. doi: 10.1093/jb/mvs004. [DOI] [PubMed] [Google Scholar]
- 34.Retting KN, Song B, Yoon BS, Lyons KM. Bmp canonical smad signaling through smad1 and smad5 is required for endochondral bone formation. Development. 2009;136:1093–1104. doi: 10.1242/dev.029926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adeney KL, Siscovick DS, Ix JH, Seliger SL, Shlipak MG, Jenny NS, Kestenbaum BR. Association of serum phosphate with vascular and valvular calcification in moderate ckd. J Am Soc Nephrol. 2009;20:381–387. doi: 10.1681/ASN.2008040349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linefsky JP, O’Brien KD, Katz R, de Boer IH, Barasch E, Jenny NS, Siscovick DS, Kestenbaum B. Association of serum phosphate levels with aortic valve sclerosis and annular calcification: The cardiovascular health study. J Am Coll Cardiol. 2011;58:291–297. doi: 10.1016/j.jacc.2010.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts WC. The congenitally bicuspid aortic valve. A study of 85 autopsy cases. Am J Cardiol. 1970;26:72–83. doi: 10.1016/0002-9149(70)90761-7. [DOI] [PubMed] [Google Scholar]
- 38.Razzaque MS. The fgf23-klotho axis: Endocrine regulation of phosphate homeostasis. Nat Rev Endocrinol. 2009;5:611–619. doi: 10.1038/nrendo.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou L, Li Y, Zhou D, Tan RJ, Liu Y. Loss of klotho contributes to kidney injury by derepression of wnt/beta-catenin signaling. J Am Soc Nephrol. 2013;24:771–785. doi: 10.1681/ASN.2012080865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, Malide D, Rovira II, Schimel D, Kuo CJ, Gutkind JS, Hwang PM, Finkel T. Augmented wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803–806. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- 41.Shao JS, Cai J, Towler DA. Molecular mechanisms of vascular calcification: Lessons learned from the aorta. Arterioscler Thromb Vasc Biol. 2006;26:1423–1430. doi: 10.1161/01.ATV.0000220441.42041.20. [DOI] [PubMed] [Google Scholar]
- 42.Bonow RO, Carabello BA, Kanu C, et al. Acc/aha 2006 guidelines for the management of patients with valvular heart disease. Circulation. 2006;114:e84–231. doi: 10.1161/CIRCULATIONAHA.106.176857. [DOI] [PubMed] [Google Scholar]
- 43.Derwall M, Malhotra R, Lai CS, Beppu Y, Aikawa E, Seehra JS, Zapol WM, Bloch KD, Yu PB. Inhibition of bone morphogenetic protein signaling reduces vascular calcification and atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:613–622. doi: 10.1161/ATVBAHA.111.242594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malhotra R, Burke MF, Martyn T, et al. Inhibition of bone morphogenetic protein signal transduction prevents the medial vascular calcification associated with matrix gla protein deficiency. PLoS One. 2015;10:e0117098. doi: 10.1371/journal.pone.0117098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.