Abstract
The SLC (solute carrier)-type transporters (∼400 in number) in mammalian cells consist of 52 distinct gene families, grouped solely based on the amino acid sequence (primary structure) of the transporter proteins and not on their transport function. Among them are the transporters for amino acids. Fourteen of them, capable of transporting glutamine across the plasma membrane, are found in four families: SLC1, SLC6, SLC7, and SLC38. However, it is generally thought that the members of the SLC38 family are the principal transporters for glutamine. Some of the glutamine transporters are obligatory exchangers whereas some function as active transporters in one direction. While most glutamine transporters mediate the influx of the amino acid into cells, some actually mediate the efflux of the amino acid out of the cells. Glutamine transporters play important roles in a variety of tissues, including the liver, brain, kidney, and placenta, as clearly evident from the biological and biochemical phenotypes resulting from the deletion of specific glutamine transporters in mice. Owing to the obligatory role of glutamine in growth and proliferation of tumor cells, there is increasing attention on glutamine transporters in cancer biology as potential drug targets for cancer treatment. Selective blockers of certain glutamine transporters might be effective in preventing the entry of glutamine and other important amino acids into tumor cells, thus essentially starving these cells to death. This could represent the beginning of a new era in the discovery of novel anticancer drugs with a previously unexplored mode of action.
1. Introduction
Glutamine is one of the twenty different amino acids involved in protein synthesis in mammalian cells, but the biological functions of this particular amino acid go far beyond its role in protein synthesis. It is the most abundant amino acid in human blood, its concentrations ranging from 0.4 mM to 0.7 mM [1, 2]. The average concentration of the next most abundant amino acid, namely alanine, is far less (0.35 mM) than that of glutamine. The carbon skeleton of glutamine can be incorporated into glucose as well as fatty acids while the nitrogen is used in the synthesis of purines and pyrimidines [3]. As such, glutamine occupies a central place in the metabolism of all major macromolecules in mammalian cells. In addition, glutamine is also the precursor for the synthesis of various biologically important molecules including glutathione (an antioxidant), glutamate (an excitatory neurotransmitter), and γ-aminobutyrate (GABA, an inhibitory neurotransmitter). It also serves as a carrier of ammonia from tissues such as the skeletal muscle and the brain to the liver where ammonia is extracted from glutamine for subsequent conversion to urea. Skeletal muscle represents the principal site as the reservoir of glutamine in the body; amino acid metabolism leads to the generation of glutamine in this tissue as a mechanism of detoxification of ammonia that is produced in the metabolism [4, 5]. Under physiologic conditions, a major portion of glutamine in the plasma is derived from the skeletal muscle. Another important function of glutamine is to replenish anaplerotic carbon in the citric acid cycle in the form of α-ketoglutarate (glutamine → glutamate → α-ketoglutarate). Two additional metabolic pathways also depend on glutamine, namely glutamate/GABA-glutamine cycle that occurs in the brain between glutamatergic/GABAergic neurons and astrocytes, and glutaminolysis that occurs in cancer cells where glutamine enters the citric acid cycle in the form of α-ketoglutarate and gets converted to malate, which then becomes pyruvate through the action of malic enzyme and then lactate through the action of lactate dehydrogenase. Glutamine also plays an important role in acid-base balance; it is used by the tubular epithelial cells in the kidney as a source of NH3, which then is used to carry H+ (NH4+) into urine, a process that occurs during metabolic acidosis as a means to eliminate excess acid [3].
From the plethora of biological functions that glutamine is known to play in mammalian cells, the metabolic versatility of this amino acid is quite obvious. Nonetheless, glutamine is a non-essential amino acid, meaning that it does not have to be provided in the diet; it can be synthesized endogenously from the citric acid cycle intermediate α-ketoglutarate by a two-step process involving glutamate dehydrogenase, which converts α-ketoglutarate into glutamate using NADPH, and glutamine synthetase, which converts glutamate into glutamine using NH3. But still, most cells do depend on extracellular glutamine to some extent because of the inadequacy of the endogenous biosynthetic pathway to meet the normal demands of the cells for this important amino acid. This is particularly true for cells with high proliferative rates (e.g., immune cells, intestinal epithelial cells) and for cancer cells. Rapid proliferation means increased demand for DNA/RNA synthesis, a process that is dependent on glutamine as the source of nitrogen for the generation of purine and pyrimidines. Certain cancer cell types are actually “addicted” to glutamine as evident from their failure to grow and proliferate in the absence of extracellular supply of this amino acid [6, 7].
As glutamine is hydrophilic and water-soluble, extracellular glutamine cannot simply diffuse into cells across the plasma membrane; the transmembrane transfer of glutamine into cells needs mediation by transporters. To date, fourteen transporters have been identified at the molecular level in the mammalian cell plasma membrane that accept glutamine as a substrate (Table 1). It is important to note that none of these transporters is exclusively selective for glutamine and that not all of these transporters function in the influx of glutamine into cells. Even though all of these transporters have multiple substrate selectivity, some accept only neutral amino acids whereas others accept neutral as well as cationic amino acids, and yet others neutral, cationic, and anionic amino acids. Similarly, while most of these transporters mediate the entry of extracellular glutamine into cells, some facilitate the release of intracellular glutamine into the extracellular medium under normal physiologic conditions. According to the Human Genome Organization nomenclature, these transporters belong to four distinct gene families: SLC1, SLC6, SLC7, and SLC38. The distribution of the fourteen transporters in these four gene families is as follows: one transporter in SLC1, two transporters in SLC6, five transporters in SLC7, and six transporters in SLC38 (Table 1).
Table 1. Plasma membrane transporters for glutamine in mammalian cells.
| Gene name | Protein name | Amino acid substrates | Direction of glutamine flux |
|---|---|---|---|
| SLC1A5 | ASCT2 | Neutral | Influx/Efflux |
| SLC6A14 | ATB0,+ | Neutral & Cationic | Influx |
| SLC6A19 | B0AT1 | Neutral | Influx |
| SLC7A5 | LAT1 | Neutral | Influx/Efflux |
| SLC7A6 | y+LAT2 | Neutral & Cationic | Influx |
| SLC7A7 | y+LAT1 | Neutral & Cationic | Influx |
| SLC7A8 | LAT2 | Neutral | Efflux |
| SLC7A9 | b0,+AT | Neutral & Cationic | Efflux |
| SLC38A1 | SNAT1 (SA2) | Neutral | Influx |
| SLC38A2 | SNAT2 (SA1) | Neutral | Influx |
| SLC38A3 | SNAT3 (SN1) | Neutral | Influx/Efflux |
| SLC38A5 | SNAT5 (SN2) | Neutral | Influx/Efflux |
| SLC38A7 | SNAT7 | Neutral & Cationic & Anionic | Influx |
| SLC38A8 | SNAT8 | Neutral, Cationic & Anionic | Influx |
2. SLC1A5
The SLC1 gene family consists of transporters for anionic amino acids (aspartate and glutamate) or neutral amino acids [8]. SLC1A4 (also known as ASCT1) and SLC1A5 (also known as ASCT2) are the transporters for neutral amino acids, the former being selective for alanine, serine, and cysteine and the latter for alanine, serine, cysteine, threonine, and glutamine, all of which are neutral amino acids. The term “ASCT” stands for “Alanine-Serine-Cysteine Transporter.” Both transporters are Na+-coupled and function as obligatory exchangers. SLC1A5 is the only transporter in this gene family that accepts glutamine as a substrate. Since it is an obligatory exchanger, it is capable of mediating either the influx or efflux of glutamine depending on the concentration gradients for the five amino acid substrates across the plasma membrane in a given cell. The transport process is electroneutral, involving the influx of Na+/amino acid coupled to the efflux of Na+/amino acid (Fig. 1), thus contributing to the homeostasis of neutral amino acids inside the cells. It is expressed in the intestine, kidney, lung, testis, skeletal muscle, and adipose tissue.
Fig. 1.
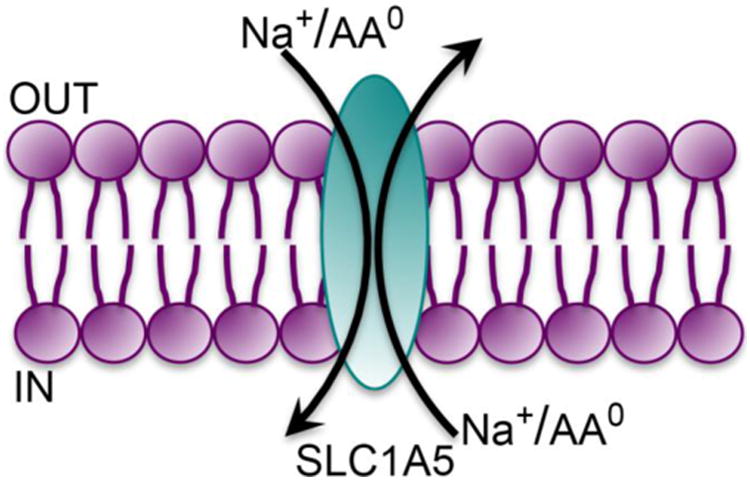
Transport mode of SLC1A5.
AA0, neutral amino acids (net charge of zero on the molecule).
In recent years, SLC1A5 is receiving increasing attention for its potential role in cancer because its expression is up-regulated in many cancer types, including triple-negative breast cancer [9, 10], colon cancer [11], lung cancer [12], melanoma [13], neuroblastoma [14], glioblastoma [15], and prostate cancer [16]. In glioblastoma and neuroblastoma, the expression of the transporter appears to be under the control of the Myc oncogenes, c-Myc in glioblastoma [15] and n-Myc in neuroblastoma [14]; activation of these oncogenes induces SLC1A5 expression. The transporter is also under the control of the retinoblastoma protein Rb; the expression of SLC1A5 is down-regulated by this tumor suppressor protein [17]. It is interesting that this transporter is widely up-regulated in cancers considering its narrow substrate selectivity and its mode of transport as an obligatory exchanger. Even though glutamine is a substrate, none of the essential amino acids is a substrate for the transporter. How could this transporter support the growth and proliferation of tumor cells? The answer to this question seems to rest upon the potential coupling of this transporter to two other amino acid transporters (SLC7A5 and SLC7A11) at the functional level. SLC1A5 mediates the Na+-coupled influx of glutamine (in exchange for the efflux of the other four amino acid substrates), and SLC7A5 then mediates the efflux of glutamine from the cells in exchange for the influx of leucine, an essential amino acid and also a potent activator of mTORC1. This co-operation between SLC1A5 and SLC7A5 in tumor cells might underlie the promotion of tumor growth by these transporters [18]. Similarly, the Na+-coupled influx of glutamine via SLC1A5 could also fuel the transport function of SLC7A11 to potentiate the antioxidant machinery in tumor cells [9]. SLC7A11 is a cystine-glutamate exchanger, which functions always in the import of cystine into cells under physiologic conditions, and the imported cystine is then used in the synthesis of the antioxidant molecule glutathione. For this transporter to work optimally, there must be a source of intracellular glutamate, and the SLC1A5-mediated accumulation of glutamine inside the cells could serve as the source of this glutamate [7]. This mode of functional coupling between SLC1A5 and SLC7A11 has been shown to be important for the survival and proliferation of triple-negative breast cancer cells [9]. In fact, the expression of SLC7A11 is also increased in several cancers [19]; as SLC7A11 also works as an exchanger for extracellular glutamate and intracellular glutamate, the transporter can be exploited for PET (positron emission tomography) imaging of tumors using 18F-labelled glutamate derivatives [20]. Recent studies have shown a similar phenomenon involving the co-operation between SLC1A5 and SLC7A5 with the resultant mTORC1 activation in the development of pro-inflammatory CD4+ Th1 and Th17 responses [21]. In this case, the T-cell receptor controls the expression of SLC1A5 in CD4+ cells.
As SLC1A5 appears to play a critical role in the promotion of at least certain types of cancers and also in the development of pro-inflammatory immune responses, this transporter represents a potential drug target in cancer therapy and in the treatment of immunological diseases. Blockade of the transporter function with small molecules has been shown to yield promising results in decreasing the growth of SLC1A5-positive cancer cells in vitro and in vivo, but the currently available blockers are not necessarily selective for this particular amino acid transporter and also exhibit low affinity for the transporter. Identification of selective high-affinity blockers of SLC1A5 would likely have a significant impact in the treatment of SLC1A5-positive tumors. Information on the crystal structure of the transporter would be very useful towards achieving this goal, but neither SLC1A5 nor any other members of the SLC1 gene family has been crystallized. However, the structure of a homolog of the SLC1 gene family members, the aspartate transporter GltPh from Pyrococcus horikoshii, is available [22, 23], which could be used as a template for homology modeling for the design of selective blockers of SLC1A5 [24].
A knockout mouse line for Slc1a5 has been generated [21]; the mutant mice show no obvious abnormalities in growth and survival. The immune system function is normal in Slc1a5-/- mice under normal conditions, but the development of pro-inflammatory T cell responses is impaired under experimental conditions of immunity and autoimmunity. The knockout mouse line has not yet been used in cancer biology to determine if the deletion of the transporter blocks the growth of tumors in spontaneous models of cancer.
3. SLC6A14 and SLC6A19
The SLC6 gene family is known as the Na+/Cl- -coupled neurotransmitter transporter family because of the inclusion of transporters for a variety of neurotransmitters (e.g., GABA, serotonin, dopamine, norepinephrine, and glycine) in this family [25, 26]. However, the SLC6 gene family does contain transporters for amino acids that do not function as neurotransmitters; among these transporters are the glutamine transporters SLC6A14 and SLC6A19.
3.1. SLC6A14
SLC6A14 is unique among the amino acid transporters in a number of ways. It is the only transporter with a broad substrate selectivity and obligatorily coupled to a Na+ gradient as well as a Cl- gradient [27, 28]. It recognizes 18 of the 20 amino acids as substrates, with glutamate and aspartate being the only two amino acids excluded by the transporter. As the transporter accepts all of the neutral and cationic amino acids, it is also called ATB0,+ with “AT” referring to amino acid transporter, “B” referring to broad selective, and “0,+” referring to neutral and cationic amino acids. SLC6A14 is highly concentrative and electrogenic, its transport function thus coupled to three different driving forces, namely a Na+ gradient, a Cl- gradient, and a membrane potential. Irrespective of whether it transports neutral or cationic amino acid, the Na+:Cl-:amino acid stoichiometry is 2:1:1 (Fig. 2A).
Fig. 2. Transport modes of SLC6A14 (A) and SLC6A19 (B).
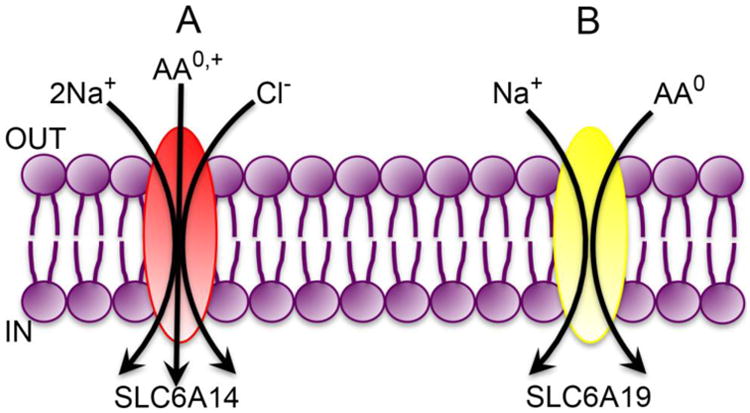
AA0,+, neutral (net charge of zero on the molecule) and cationic (net positive charge on the molecule) amino acids; AA0, neutral amino acids (net charge of zero on the molecule).
SLC6A14 is expressed in the lung, trachea, salivary gland, intestinal tract, and pituitary. Its expression in the lung and ileum is of interest because of the recent findings that the transporter is a modifier of cystic fibrosis phenotype, including meconium ileus and the severity of lung disease [29, 30]. Certain specific single nucleotide polymorphisms (SNPs) in the gene coding for the transporter have been found to have a strong link to these phenotypes in children with cystic fibrosis, but the molecular mechanisms underlying the connection remain unknown. In fact, it is not even known what these specific SNPs do to the expression of the transporter. The transporter is also linked to obesity [31-33]; again, nothing is known on the molecular basis of the connection. Similarly, the transporter is also associated with inflammatory bowel disease; the expression of the transporter is induced several-fold in the colon of patients with ulcerative colitis [34, 35]. In all of these cases, the ability of the transporter to transport most of the amino acids in a highly concentrative manner is likely to have a role. For example, as SLC6A14 is a transporter for Na+ and Cl- coupled to the absorption of amino acids, its function might have a significant impact on water absorption as well in the lung and ileum; this could link the function of the transporter to the incidence of meconium ileus and the progression of lung disease in cystic fibrosis patients. The expression of the transporter in the pituitary suggests that it might function as a sensor of circulating levels of amino acids in the brain and hence could be an important determinant of satiety control by the pituitary, thus explaining the link between the transporter and obesity. As SLC6A14 is a Na+/Cl- -coupled concentrative transporter for arginine, its up-regulation in ulcerative colitis is likely to be related to the role of arginine as a donor of nitric oxide, a critical participant in the progression of inflammation.
SLC6A14 exhibits several functional features that are highly suitable to support tumor growth; this includes the robust energy coupling for concentrative uptake of amino acid substrates into cells and the ability to transport not only all essential amino acids but also most of non-essential amino acids such as glutamine, arginine, serine, and glycine, which have been shown to be important for various metabolic pathways critical for tumor growth. SLC6A14 does indeed get up-regulated in certain cancer types: colon cancer [36], cervical cancer [37], estrogen receptor-positive breast cancer [38], and pancreatic cancer [39, 40]. The tumor-promoting role of SLC6A14 has been documented unequivocally in estrogen receptor-positive breast cancer using α-methyltryptophan, a small molecule blocker of the transporter [38, 41].
Slc6a14-knockout mice have been generated [42]; these mice do not have any noticeable physical or metabolic phenotype, including body habitus and plasma levels of amino acids. But, when crossed with MMTV-PyMT mice, a model for spontaneous breast cancer, the development and progression of breast cancer were markedly suppressed on Slc6a14-null background, thus demonstrating the tumor-promoting role of the transporter in mammary gland [42]. Similar studies are yet to be done for other cancer types that are associated with the up-regulation of the transporter.
Based on the convincing evidence for SLC6A14 as a tumor promoter at least in certain specific cancers and also on the known up-regulation of the transporter in ulcerative colitis, it is tempting to speculate that SLC6A14 is a viable drug target for treatment of cancer and possibly also for treatment of inflammatory bowel disease. Pharmacological blockade of the transporter using small molecules or monoclonal antibodies is likely to be therapeutically effective in these cases. Details of the membrane topology and the structural motifs of the substrate-binding site for the transporter would greatly facilitate the design and development of such blockers for clinical use. The crystal structures of a prokaryotic homolog LeuT [43] and a drosophila homolog drDAT [44] have been elucidated; homology modeling of SLC6A14 based on the structural features of LeuT and drDAT has potential to aid in the design of high-affinity and selective ligands for the transporter for potential use in cancer therapy.
3.2. SLC6A19
In the early days of the amino acid transport field, the focus was entirely on the small intestine and kidney because of the role of these organs in the absorption of protein-digestion products (small intestine) and in the reabsorption of amino acids filtered at the glomerulus (kidney). Prior to the discovery of various amino acid transporters at the molecular level, amino acid absorption in these two tissues was shown to occur via group-specific transport processes in terms of amino acid substrates, each selective for a given group of amino acids: one for neutral amino acids, one for cationic amino acids, one for anionic amino acids, and one for imino acids. The transport process that is selective for neutral amino acids is defective in a genetic disorder known as Hartnup disease, which is characterized by increased urinary excretion of neutral amino acids. The excretion of cationic amino acids and anionic amino acids is normal in these patients, demonstrating the selectivity of the transport process for neutral amino acids that is defective in Hartnup disease. Based on the broad selectivity of the transport process for all neutral amino acids, the transporter responsible for the process is referred to as B0 (“B” for broad; uppercase letter denoting Na+-dependent active transport, and the superscript “0” indicating the net charge on the neutral amino acid substrates). Subsequently the transporter that is mutated in Hartnup disease was identified as SLC6A19, also known as B0AT1 (i.e., B0 Amino acid Transporter 1) [45, 46]. It transports all neutral amino acids including glutamine. Interestingly, even though SLC6A19 belongs to the SLC6 gene family in which most of the previously known members are coupled to Na+ as well as Cl-, SLC6A19 is coupled only to Na+. The transport process is however electrogenic with a Na+:amino acid stoichiometry of 1:1 (Fig. 2B). The transporter is expressed primarily in the lumen-facing apical membrane of the absorptive cells of the small intestine and kidney. Recent studies have shown that the stability and trafficking to the plasma membrane of SLC6A19 depend on two specific proteins, collectrin in the small intestine and ACE2 (angiotensin converting enzyme 2) in the kidney [47, 48]. It is not readily apparent as to why the same transporter protein (SLC6A19) interacts with two different chaperones in different tissues. Nonetheless, deletion of collectrin in mice affects the stability and plasma membrane localization of Slc6a19 in the kidney but not in the small intestine; in contrast, deletion of Ace2 in mice affects the stability and plasma membrane localization of Slc6a19 in the small intestine but not in the kidney. However, neither collectrin nor Ace2 plays any direct role in the transport process mediated by SLC6A19.
Slc6a19-knockout mouse has been generated [49]; the mice show no Na+-dependent uptake of neutral amino acids in the apical membranes of the intestinal and renal epithelial cells, indicating that this transporter represents the major route for the energy-coupled active absorption of neutral amino acids in these two tissues. Analysis of amino acids in the urine shows a general neutral aminoaciduria similar to that seen in patients with Hartnup disease. With the loss of active absorption of neutral amino acids in the intestine and the increased excretion of neutral amino acids in the urine due to their defective reasbsorption in the kidney, the knockout mice exhibit decreased body weight; furthermore, the intestinal and renal epithelial cells suffer from amino acid deficiency as evident from decreased mTORC1 signaling.
4. SLC7A5, SLC7A6, SLC7A7, SLC7A8, and SLC7A9
Most members of the amino acid transporters in the SLC7 gene family are unique because they function as heterodimers, consisting of a “transporter proper” subunit and a chaperone subunit that interacts with the transporter during biosynthesis and brings it to the plasma membrane [50]. There are two chaperones related to the amino acid transporters in the SLC7 gene family; they themselves are classified as the members of a separate solute carrier gene family, namely SLC3. Among the five glutamine transporters in the SLC7 gene family, four (SLC7A5, SLC7A6, SLC7A7, and SLC7A8) specifically interact with SLC3A2 (also known as 4F2hc; heavy chain of the cell-surface antigen 4F2) whereas one interacts specifically with SLC3A1 (also known as rBAT; related to b0,+ amino acid transporter). Each of these heterodimeric transporters functions as an obligatory exchanger, meaning that the transport process is associated with the influx of some amino acid substrates into the cells obligatorily coupled to the efflux of some other amino acid substrates out of the cells. Again, the chaperones participate only in the trafficking of the transporter proteins to the plasma membrane but have little effect on the functions of the transporters themselves.
4.1. SLC7A5 and SLC7A8
SLC7A5 and SLC7A8 are known as LAT1 and LAT2 respectively; “LAT” refers to “system L amino acid transporter” because the transport process mediated by these two transporters represent the amino acid transport system originally known as “system L” owing to its preference for leucine. Both transporters do interact with all neutral amino acids but prefer large amino acids such as leucine, isoleucine, valine, tyrosine, phenylalanine, tryptophan, glutamine, and methionine. SLC7A5 exhibits relatively higher affinity for its substrates than SLC7A8. Both are expressed in a wide variety of tissues and cells. SLC7A5 is the primary transporter for neutral amino acids in the endothelial cells lining the blood-brain barrier [51, 52]. It is also expressed at high levels in placental syncytiotrophoblast where it plays a role in the transfer of amino acids from the mother to the developing fetus [53]. An important aspect of this transporter is its substrate selectivity that includes all essential amino acids, with the exception of arginine (a conditionally essential amino acid) and lysine. Relative to SLC7A5, SLC7A8 has a narrower tissue expression pattern. It is highly expressed in the absorptive cells of the intestine and kidney where it is present in the basolateral membrane, thus participating in the efflux of amino acids from the cells into the circulation. Both SLC7A5 and SLC7A8 are Na+-independent and obligatory exchangers (Fig. 3A).
Fig. 3.
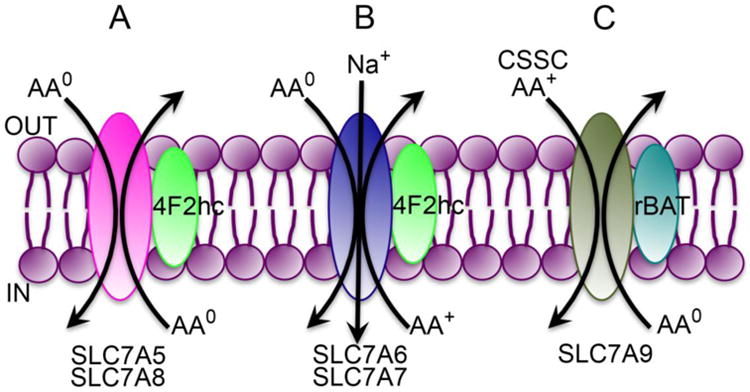
Transport modes of the glutamine transporters in SLC7 gene family.
AA0, neutral amino acids (net charge of zero on the molecule); AA+, cationic amino acids (net positive charge on the molecule); CSSC, cystine (oxidized form of cysteine)
SLC7A5 is receiving increasing attention in recent years for its potential role in cancer and its relevance to mTORC1 signaling [54, 55]. Its expression is increased in many cancers, particularly in melanoma, lung cancer, and colon cancer [55]. As this transporter is an obligatory exchanger, it is unlikely that the mechanism of tumor promotion by this transporter is the general supply of amino acids. Even though it can transport almost all essential amino acids, the obligatory exchange mechanism means that influx of an amino acid substrate into the tumor cells is coupled to the efflux of some other amino acid substrate out of the tumor cells. What might be happening in tumor cells is a functional coupling between SLC7A5 and some other transporter that can bring a specific amino acid substrate of SLC7A5 into the cells by a concentrative mechanism; the consequent increase in the intracellular concentration of this amino acid then results in its efflux via SLC7A5 coupled to the influx of leucine into the cells via the obligatory exchange mode [7, 56]. Currently available evidence identifies the coupled transporter as SLC1A5, which brings in glutamine into tumor cells actively by a Na+-dependent mechanism, and then SLC7A5 mediates the efflux of glutamine coupled to the influx of leucine into the tumor cells. The relevance of leucine to tumor promotion is that it is a potent activator of mTORC1 signaling [57, 58]. In those tumor cells where the functional coupling has been demonstrated between SLC7A5 and SLC1A5, both transporters are up-regulated, thus making the interaction between the two transporters as an underlying mechanism for activation of mTORC1 and resultant tumor promotion. The induction of SLC1A5 and SLC7A5 in cancer cells might involve the oncogene c-Myc [15] and the hypoxia-inducible factor 2α [59], respectively. In theory, a similar functional coupling can also occur between SLC6A14 and SLC7A5 in certain cancers [7, 56], but this possibility has not yet been investigated.
Recent studies have investigated the consequences of Slc7a5 deletion in mice [60]. While the heterozygous knockout mice have no overt phenotype, homozygous knockout mice are embryonically lethal. The lethality resulting from the complete absence of the transporter is understandable based on the fact that it is the primary transporter in the blood-brain barrier for the delivery of most of the essential amino acids to the brain. This however undermines the potential of this transporter as a therapeutic target for cancer treatment because high-affinity blockers of the transporter as anticancer drugs are likely to interfere with amino acid delivery to the brain. Conditional deletion of the transporter in skeletal muscle has provided unequivocal evidence for the role of the transporter in mTORC1 signaling pathway [60].
SLC7A8 is very similar to SLC7A5 in substrate selectivity and function. It plays a critical role in the release of absorbed amino acids from the intestinal and renal epithelial cells into blood. Accordingly, homozygous deletion of Slc7a8 in mice leads to aminoaciduria even though the growth and development remain unaffected [61]. Despite the similarity between SLC7A5 and SLC7A8 in function, there is very little known on whether SLC7A8 plays any role in cancer. Data from Oncomine do suggest significant up-regulation of SLC7A8 in many cancers, but none of these has been validated independently.
4.2. SLC7A6 and SLC7A7
SLC7A6 (y+LAT2) and SLC7A7 (y+LAT1) exhibit an interesting transport function; both mediate the influx into cells of neutral amino acids in a Na+-coupled manner, but the process is obligatorily coupled to the efflux of cationic amino acids (Fig. 3B). “y+” refers to the ability of the transporters to mediate the transfer of cationic amino acids in a Na+-independent manner whereas “LAT” refers to the ability of the transporters to mediate the transfer of amino acid substrates of system L (large neutral amino acids) but in a Na+-coupled manner. Both transporters have similar substrate selectivity, which is true for cationic amino acids as well as neutral amino acids. These transporters are expressed in the basolateral membrane of absorptive epithelial cells of the intestine and kidney where they play an essential role in the release of cationic amino acids into blood following their absorption from the lumen across the apical membrane. The presence of an inside-negative membrane potential across the plasma membrane in these cells poses a problem for the release of cationic amino acids from the cells, which is overcome by the obligatory exchange mode of transport by SLC7A6 and SLC7A7 that links the Na+-coupled active influx of neutral amino acids into the cells and the efflux of cationic amino acids out of the cells. This mode of transport renders the entire process electroneutral (transfer of cationic amino acid in one direction coupled to the co-transfer of Na+ and neutral amino acid in the opposite direction). Without such a mechanism, it would be difficult to release positively charged cationic amino acids from the cells in the presence of the inside-negative membrane potential.
Inactivating mutations in SLC7A7 (y+LAT1) cause a genetic disease of amino acid transport, known as lysinuric protein intolerance [62]. Untreated children with the disorder suffer from growth failure, hepatosplenomegaly, mental retardation, osteoporosis, and altered immune function. As the transporter is critical for the efflux of cationic amino acids from the renal and intestinal epithelial cells into circulation, defects in the transporter lead to decreased absorption of cationic amino acids (lysine and arginine) in both organs, thus causing lysinuria and lysine malabsorption. Patients with the disease do not tolerate proteins in the diet as the presence of proteins in the diet makes them sick; this feature coupled with urinary excretion of lysine highlight the two important clinical symptoms of lysinuric protein intolerance. Knockout mice with homozygous deletion of Slc7a7 recapitulate the clinical phenotype of the patients [63]. The knockout mice exhibit significant neonatal lethality, and those that survive display all the symptoms and metabolic abnormalities seen in patients with lysinuric protein intolerance.
4.3. SLC7A9
SLC7A9 mediates a transport process that is identified as b0,+ at the functional level. The transport is Na+-independent, and the process involves an obligatory exchange of cationic amino acids with neutral amino acids. It is expressed predominantly in the absorptive tissues such as the intestine, kidney, and placenta, and is located on the apical membrane of the absorptive epithelial cells in these tissues. Under physiologic conditions, the transporter facilitates the influx of cationic amino acids in the cells coupled to the efflux of neutral amino acids from the cells (Fig. 3C). Interestingly, the disulfide amino acid cystine is a substrate for the transporter. In fact, SLC7A9 provides the principal mechanism for the absorption of cystine and cationic amino acids (lysine, arginine) in the kidney and intestine [50]. Inactivating mutations in SLC7A9 are the cause of the amino acid transport disease cystinuria [64]. Patients with the disease however do not exhibit any evidence of protein malnutrition, but excrete abnormally high levels of cystine and cationic amino acids in urine. The major clinical symptom in cystinuria patients is the kidney damage caused by cystine stones. Normal plasma contains cysteine mostly in the form of cystine (the oxidized form of cysteine). Cystine has poor solubility in water (300 mg/L), but its plasma levels under normal conditions (10 mg/L) are well below this solubility limit. But when the blood is filtered at the glomerulus, if the filtered cystine is not reabsorbed by the tubular epithelial cells due to mutations in SLC7A9, this amino acid accumulates in the tubular lumen because of the normal absorption of water; this has potential to increase the tubular concentrations of cystine by ∼100-fold, thus leading to the precipitation and crystallization of cystine. This is the molecular pathogenesis of cystine stones in the kidney in patients with cystinuria. Along with cystine, the levels of cationic amino acids are also increased in the urine.
Slc7a9-knockout mouse develop cystinuria and also cystine urolithiasis [65]. Calculi develop during the first month of life itself and continue to grow throughout the lifespan of the animals. As a result of these stones in the kidneys, the mice show evidence of tubular necrosis and chronic nephritis.
5. SLC38A1, SLC38A2, SLC38A3, SLC38A5, SLC38A7, and SLC38A8
The SLC38 gene family consists of amino acid transporters that mediate transport processes that are functionally identified as amino acid transport systems A and N; system A (“A” stands for alanine-preferring) refers to a Na+-dependent transport process selective for neutral amino acids including alanine while system N (“N” stands for amino acids with nitrogen in the side chain) refers to a Na+-dependent transport process selective for glutamine, asparagine, and histidine, which all contain nitrogen atom in the side chain. All transporters belonging to this gene family are called SNATs (sodium-coupled neutral amino acid transporters) [66, 67]. One of the key distinguishing features between system A and system N transporters is the ability of the former to transport the non-metabolizable amino acid (methylamino)isobutyric acid (MeAIB); in addition, the transport process mediated by system A transporters is electrogenic involving the co-transport of Na+ and amino acid with a 1:1 stoichiometry (Fig. 4A) whereas the transport process mediated by system N transporters is electroneutral involving the movement of Na+ and amino acid (stoichiometry, 1:1) in one direction coupled to the movement of H+ in the opposite direction (Fig. 4B). As such, the function of system N transporters is associated with H+ flux with a significant impact on intracellular pH. It is important to note that the activity of both groups of transporters is increased in the presence of an alkaline pH in the extracellular medium, but H+ is a transportable co-substrate only for system N transporters; even though pH influences the activity of system A transporters, transmembrane transfer of H+ does not occur as a part of the transport process. SLC38A1 and SLC38A2 belong to the group of system A transporters; SLC38A3 and SLC38A5 belong to the group of system N transporters; detailed functional studies are not available for SLC38A7 and SLC38A8 for definitive classification into either system A group or system N group. Another important distinction between system A and system N transporters is that the latter is capable of changing the direction of amino acid flux either into the cell or out of the cell, depending on the metabolic phenotype of a given cell; in contrast, system A transporters are capable of transporting their amino acid substrates only into the cells.
Fig. 4.
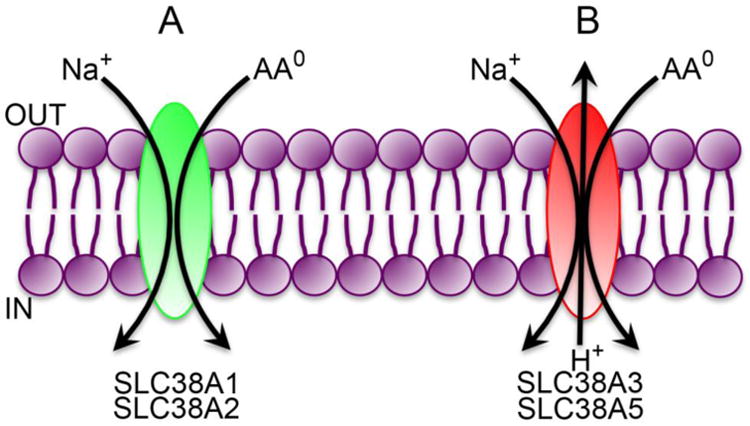
Transport modes of the glutamine transporters in SLC38 gene family.
AA0, neutral amino acids (net charge of zero on the molecule).
5.1. SLC38A1 and SLC38A2
SLC38A1 and SLC38A2 are Na+-coupled transporters for neutral amino acids including glutamine; aromatic amino acids, though neutral, are excluded as substrates by the transporters [68, 69]. Both transporters are expressed ubiquitously in mammalian tissues. Owing to the relatively narrow substrate selectivity, these transporters represent one of the major routes of glutamine entry into cells under physiologic conditions. In the central nervous system, SLC38A1 and SLC38A2 are expressed almost exclusively in neurons where they function in glutamate/GABA-glutamine cycle that takes place between neurons and astrocytes [70]. Astrocytes take up the neurotransmitter glutamate from the synapse and extrasynaptic regions and convert it into glutamine, which is then released into the extracellular medium via system N transporters (see below); the released glutamine is taken up by the neurons via SLC38A1 and SLC38A2 and re-used as the source of glutamate, which is then released into the synaptic cleft during neuronal activation. This glutamate/GABA-glutamine cycle plays a critical role in glutamatergic neurotransmission. A similar metabolic cycle also occurs in GABAergic neurotransmission because glutamine also serves as the precursor for the synthesis of GABA with glutamate as an intermediate [70]. SLC38A2 also plays an important role in ammonia metabolism and urea synthesis in the liver where the metabolically distinct perivenous hepatocytes and periportal hepatocytes both take up glutamine via the transporter [71]. Recent studies have shown that glutamine entry into the α cells of the pancreas via SLC38A2 is an important regulator of glucagon secretion [72]. SLC38A2 is also critically involved in the transfer of amino acids from the mother to the developing fetus across the placenta; the placental expression of SLC38A2 is an important determinant of placental and fetal growth [73].
There is some evidence that SLC38A2 might be involved in promoting tumor growth and in chemotherapy. The gene encoding this transporter is a transcriptional target for the tumor suppressor p53; the expression of SLC38A2 is repressed by active p53 [74]. SLC6A14 is also a target gene for p53, and the expression of this transporter is also suppressed by active p53. As most cancers are associated with mutations in p53, it is likely that the expression of SLC38A2 and SLC6A14 is increased in p53-mutant cancers, thus providing glutamine to the tumor cells. SLC38A2 is also regulated by proteasomal degradation; the chemotherapeutic agent paclitaxel induces endoplasmic reticulum stress, which induces the activity of the ubiquitin ligase RNF5 with subsequent ubiquitination and degradation of SLC38A2 [75]. The same mechanism also leads to degradation of SLC1A5. The resultant decrease in glutamine entry into tumor cells might represent at least a part of the mechanism underlying the therapeutic efficacy of this drug in cancer treatment.
5.2. SLC38A3 and SLC38A5
SLC38A3 and SLC38A5 are referred to as SN1 (system N1) and SN2 (system N2) transporters. These are Na+-coupled, to some extent Li+-tolerant (i.e., the transport process is at least partly active even when Na+ is replaced with Li+), and selective for glutamine, asparagine, and histidine. SLC38A5 recognizes alanine and serine as additional substrates. SLC38A3 is expressed abundantly in the liver, brain, retina, and pancreas [76, 77] whereas SLC38A5 is expressed primarily in the intestinal tract, kidney, retina, lung, and cervix [78, 79]. Because of the involvement of H+ as a transportable co-substrate for both transporters, the transport process is associated with changes in intracellular pH. When the transporters function in the influx of Na+/amino acid into cells, H+ is effluxed out of the cells, thus leading to intracellular alkalization. However, these transporters are capable of working in both directions; in certain cell types, these transporters mediate the release of glutamine (and other substrates) from the cells, a process in which the efflux of Na+/amino acid out of the cells is coupled to the influx of H+ into the cells [67]. In this mode, the transport process leads to intracellular acidification. In the brain, both transporters are expressed in astrocytes where they function in the release of glutamine from the cells as a part of the glutamate/GABA-glutamine cycle. The transport process occurs in this particular direction in these cells because of the robust activity of glutamine synthetase that generates glutamine from glutamate or GABA, which increases the intracellular concentrations of glutamine, consequently changing the direction of the transport process of the two transporters. In the liver, the transporters function differentially in periportal hepatocytes versus perivenous hepatocytes. In the periportal hepatocytes, which express both SLC38A3 and SLC38A5, the transporters mediate the influx of Na+/glutamine into cells from the portal blood, thus transferring NH3 in the form of glutamine from the intestinal tract for subsequent conversion into urea in the liver. On the other hand, in the perivenous hepatocytes, which express mostly SLC38A3, the transporter functions to release Na+/glutamine from the cells into the venous circulation. Renal tubular epithelial cells express predominantly SLC38A5; the function of this transporter in this tissue is in acid-base balance. During metabolic acidosis, the expression of SLC38A5 in the kidney is induced, resulting in active entry of Na+/glutamine into cells from the blood; once inside the cells, glutamine is hydrolyzed by glutaminase to release ammonia, which then serves to carry H+ into the urine in the form of NH4+. Retina is another tissue in which both SLC38A3 and SLC38A5 are expressed abundantly [80, 81], but their precise physiological function in this tissue remains unexplored.
The ability of SLC38A3 and SLC38A5 to transport glutamine into cells in a Na+-coupled manner and the associated intracellular alkalization in the process are uniquely suitable for tumor cells not only because of the provision of glutamine for the tumor cell-specific metabolic pathways but also because of the intracellular alkalization. Tumor cells are at increased risk for intracellular acidification due to their robust glycolytic activity resulting in lactic acid production, and any biological process leading intracellular alkalization would be beneficial to these cells. As such, it may be advantageous for tumor cells to up-regulate SLC38A3 and/or SLC38A5. Notwithstanding this attractive logic, few studies have examined the expression of SLC38A3 and SLC38A5 in tumor tissues. It is of importance to note that SLC38A5 is a transcriptional target for the oncogene c-Myc [15], making it likely that some cancer subtypes might actually up-regulate this transporter as a part of their tumor-promoting gene expression program.
5.3. SLC38A7 and SLC38A8
SLC38A7 and SLC38A8 are Na+-coupled glutamine transporters that are expressed in the central nervous system, almost exclusively in the neurons [82, 83]. As such, these two transporters may also play a role in glutamate/GABA-glutamine cycle between the neurons and astrocytes. Even though SLC38A7 has been classified as a system N transporter [82] and SLC38A8 as a system A transporter [83], additional studies might be necessary to confirm and corroborate this classification. SLC38A7 is Na+-dependent but does not tolerate Li+. It recognizes not only glutamine, asparagine and histidine, but also the anionic amino acids glutamate and aspartate and the cationic amino acid arginine as substrates. It does not transport the system A-selective substrate MeAIB. Thus, the transport characteristics of SLC38A7 do not allow classification of the transporter as a system N transporter unequivocally. Li+-tolerance may not be a reliable distinguishing feature between system A and system N transporters [67], but the involvement of H+ as a transportable co-substrate is. However, there is no information as to whether or not SLC38A7 is influenced by transmembrane H+ gradient and whether or not the transport process involves transmembrane movement of H+. SLC38A8 is also Na+-dependent but does not tolerate Li+ [83]. It recognizes glutamine, asparagine, histidine, alanine, aspartate, and arginine as substrates. Its transport process is electrogenic, associated with substrate-induced currents. Curiously, even though the transporter interacts with the system A-selective substrate MeAIB, it induces outward currents in the presence of this amino acid derivative; but it does transport this derivative into cells, making it difficult to explain the differences in the currents between MeAIB and other transportable substrates. Further, we have no information on the impact of transmembrane H+ gradient on the transport process. Both transporters, when expressed heterologously in X. laevis oocytes, localize to the plasma membrane as evident from the appearance of the transporter-specific amino acid uptake activity [82, 83], but whether this is true in situ in the neurons, which express these transporters, has not yet established.
Among the known members of the SLC38 gene family, the consequences of gene deletion are known thus far only for Slc38a3 [84]. The knockout mice show growth retardation, hypoglycemia, and impaired mTORC1 signaling; the mice die within 20 days of birth. The levels of glutamate and GABA in the brain are significantly reduced, possibly highlighting the role of the transporter in glutamate/GABA-glutamine cycle in the brain; as a consequence, the mice are ataxic. Urinary excretion of NH4+ is also reduced in these mice as expected from the essential role of the transporter in the kidney as the supplier of glutamine in the maintenance of acid-base balance.
6. Conclusions
Mammalian cells express several transporters in the plasma membrane that can mediate the transmembrane transfer of glutamine, but none of these transporters is selective exclusively for this amino acid. Nonetheless, the members of the SLC38 gene family are generally considered as the major glutamine transporters in mammalian cells. Different cell types express different sets of glutamine transporters depending on their metabolic phenotype. Accordingly, some transporters mediate the influx of glutamine into cells whereas others do the opposite, mediating the efflux of glutamine out of the cells. In fact, the directionality of glutamine movement associated with a given transporter might vary in different cell types. Many of these transporters are becoming increasingly relevant to tumor growth; this is not surprising given the fact that glutamine plays an obligatory role in tumor cell-specific metabolic pathways. Some of these transporters might actually have potential as drug targets in the design of novel anticancer agents to deprive the tumor cells of this important tumor-promoting amino acid.
Highlights.
Glutamine is an amino acid with a plethora of functions in physiology and cancer
Tumor cells are addicted to glutamine
There are multiple glutamine transporters in the plasma membrane of mammalian cells
Tumor cells induce selective transporters to meet their high demands for glutamine
Some of the glutamine transporters have potential as drug targets in cancer therapy
Acknowledgments
This work was supported in part by the National Institutes of Health grant CA190710 and the Welch Endowed Chair in Biochemistry, Grant No. BI-0028, at Texas Tech University Health Sciences Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rennie MJ, Edwards RHT, Krywawych S, Davies CTM, Halliday D, Waterlow JC, Millward DJ. Effect of exercise on protein turnover in man. Clin Sci (London) 1981;61:627–639. doi: 10.1042/cs0610627. [DOI] [PubMed] [Google Scholar]
- 2.Planche T, Dzeing A, Emmerson AC, Onanga M, Kremsner PG, Engel K, Kombila M, Ngou-Milama E, Krishna S. Plasma glutamine and glutamate concentrations in Gabonese children with Plasmodium falciparum infection. Q J Med. 2002;95:89–97. doi: 10.1093/qjmed/95.2.89. [DOI] [PubMed] [Google Scholar]
- 3.Newsholme P, Procopio J, Lima MM, Pithon-Curi TC, Curi R. Glutamine and glutamate-their central role in cell metabolism and function. Cell Biochem Funct. 2003;21:1–9. doi: 10.1002/cbf.1003. [DOI] [PubMed] [Google Scholar]
- 4.Kuhn K, Schuhmann K, Stehle P, Darmaun D, Furst P. Determination of glutamine in muscle protein facilitates accurate assessment of proteolysis and de novo synthesis-derived endogenous glutamine production. Am J Clin Nutr. 1999;70:484–489. doi: 10.1093/ajcn/70.4.484. [DOI] [PubMed] [Google Scholar]
- 5.Chang TW, Goldberg AL. The metabolic fates of amino acids and the formation of glutamine in skeletal muscle. J Biol Chem. 1978;252:3685–3695. [PubMed] [Google Scholar]
- 6.Wise DR, Thompson CB. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci. 2010;35:427–433. doi: 10.1016/j.tibs.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhutia YD, Babu E, Ramachandran S, Ganapathy V. Amino acid transporters in cancer and their relevance to “glutamine addiction”: novel targets for the design of a new class of anticancer drugs. Cancer Res. 2015;75:1782–1788. doi: 10.1158/0008-5472.CAN-14-3745. [DOI] [PubMed] [Google Scholar]
- 8.Kanai Y, Clemencon B, Simonin A, Leuenberger M, Lochner M, Weisstanner M, Hediger MA. The SLC1 high-affinity glutamate and neutral amino acid transporter family. Mol Aspects Med. 2013;34:108–120. doi: 10.1016/j.mam.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Timmerman LA, Holton T, Yuneva M, Louie RJ, Pedro M, Daemen A, Hu M, Chan DA, Ethier SP, van't Veer LJ, Polyak K, McCormick F, Gray JW. Glutamine sensitivity analysis identifies the xCT antiporter as a common triple-negative breast tumor therapeutic target. Cancer Cell. 2013;24:450–465. doi: 10.1016/j.ccr.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Geldermalsen M, Wang Q, Nagarajah R, Marshall AD, Thoeng A, Gao D, Ritchie W, Feng Y, Bailey CG, Deng N, Harvey K, Beith JM, Selinger CI, O'Toole SA, Rasko JE, Holst J. ASCT2/SLC1A5 controls glutamine uptake and tumour growth in triple-negative basal-like breast cancer. Oncogene. 2015 doi: 10.1038/onc.2015.381. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang F, Zhao Y, Zhao J, Wu S, Jiang Y, Ma H, Zhang T. Upregulated SLC1A5 promotes cell growth and survival in colorectal cancer. Int J Clin Exp Pathol. 2014;7:6006–6014. [PMC free article] [PubMed] [Google Scholar]
- 12.Hassanein M, Qian J, Hoeksema MD, Wang J, Jacobovitz M, Ji X, Harris FT, Harris BK, Boyd KL, Chen H, Eisenberg R, Massion PP. Targeting SLC1A5-mediated glutamine dependence in non-small cell lung cancer. Int J Cancer. 2015;137:1587–1597. doi: 10.1002/ijc.29535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Q, Beaumont KA, Otte NJ, Font J, Bailey CG, van Geldermalsen M, Sharp DM, Tiffen JC, Ryan RM, Jormakka M, Haass NK, Rasko JE, Holst J. Targeting glutamine transport to suppress melanoma cell growth. Int J Cancer. 2014;135:1060–1071. doi: 10.1002/ijc.28749. [DOI] [PubMed] [Google Scholar]
- 14.Ren P, Yue M, Xiao D, Xiu R, Gan L, Liu H, Qing G. ATF4 and N-Myc coordinate glutamine metabolism in MYCN-amplified neuroblastoma cells through ASCT2 activation. J Pathol. 2015;235:90–100. doi: 10.1002/path.4429. [DOI] [PubMed] [Google Scholar]
- 15.Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, Nissim I, Daikin E, Yudkoff M, McMahon SB, Thompson CB. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci U S A. 2008;105:18782–18787. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Q, Hardie RA, Hoy AJ, van Geldermalsen M, Gao D, Fazli L, Sadowski MC, Balaban S, Schreuder M, Nagarajah R, Wong JJ, Metierre C, Pinello N, Otte NJ, Lehman ML, Gleave M, Nelson CC, Bailey CG, Ritchie W, Rasko JE, Holst J. Targeting ASCT2-mediated glutamine uptake blocks prostate cancer growth and tumour development. J Pathol. 2015;236:278–289. doi: 10.1002/path.4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reynolds MR, Lane AN, Robertson B, Kemp S, Liu Y, Hill BG, Dean DC, Clem BF. Control of glutamine metabolism by the tumor suppressor Rb. Oncogene. 2014;33:556–566. doi: 10.1038/onc.2012.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, Wilson C, Myer VE, MacKeigan JP, Porter JA, Wang YK, Cantley LC, Finan PM, Murphy LO. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lo M, Wang YZ, Gout PW. The x-c-cystine/glutamate antiporter: a potential target for therapy of cancer and other diseases. J Cell Physiol. 2008;215:593–602. doi: 10.1002/jcp.21366. [DOI] [PubMed] [Google Scholar]
- 20.Koglin N, Mueller A, Berndt M, Schmitt-Willich H, Toschi L, Stephens AW, Gekeler V, Friebe M, Dinkelborg LM. Specific PET imaging of x-c transporter activity using a 18F-labeled glutamate derivative reveals a dominant pathway in tumor metabolism. Clin Cancer Res. 2011;17:6000–6011. doi: 10.1158/1078-0432.CCR-11-0687. [DOI] [PubMed] [Google Scholar]
- 21.Nakaya M, Xiao Y, Zhou X, Chang JH, Chang M, Cheng X, Blonska M, Lin X, Sun SC. Inflammatory T cell responses rely on amino acid transporter ASCT2 facilitation of glutamine uptake and mTORC1 kinase activation. Immunity. 2014;40:692–705. doi: 10.1016/j.immuni.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yernool D, Boudker O, Jin Y, Gouaux E. Structure of a glutamate transporter homologue from Pyrococcus horikoshii. Nature. 2004;431:811–818. doi: 10.1038/nature03018. [DOI] [PubMed] [Google Scholar]
- 23.Boudker O, Ryan RM, Yernool D, Shimamot K, Gouaux E. Coupling substrate and ion binding to extracellular gate of a sodium-dependent aspartate transporter. Nature. 2007;445:387–393. doi: 10.1038/nature05455. [DOI] [PubMed] [Google Scholar]
- 24.Colas C, Grewer C, Otte NJ, Gameiro A, Albers T, Singh K, Shere H, Bonomi M, Holst J, Schlessinger A. Ligand discovery for the alanine-serine-cysteine transporter (ASCT2, SLC1A5) from homology modeling and virtual screening. PLoS Comput Biol. 2015 doi: 10.1371/journal.pcbi.1004477. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rudnick G, Kramer R, Blakely RD, Murphy DL, Verrey F. The SLC6 transporters: perspectives on structure, functions, regulation, and models for transporter dysfunction. Pflugers Arch. 2014;466:25–42. doi: 10.1007/s00424-013-1410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Broer S, Gether U. The solute carrier 6 family of transporters. Br J Pharmacol. 2012;167:256–278. doi: 10.1111/j.1476-5381.2012.01975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ganapathy ME, Ganapathy V. Amino acid transporter ATB0,+ as a delivery system for drugs and prodrugs. Curr Drug Targets Endocr Metabol Disord. 2005;5:357–364. doi: 10.2174/156800805774912953. [DOI] [PubMed] [Google Scholar]
- 28.Bhutia YD, Babu E, Prasad PD, Ganapathy V. The amino acid transporter SLC6A14 in cancer and its potential use in chemotherapy. Asian J Pharm Sci. 2014;9:293–303. [Google Scholar]
- 29.Sun L, Rommens JM, Corvol H, Li W, Li X, Chiang TA, lin F, Dorfman R, Busson PF, Parekh RV, Zelenika D, Blackman SM, Corey M, Doshi VK, Henderson L, Naughton KM, O'Neal WK, Pace RG, Stonebraker JR, Wood SD, Wright FA, Zielenski J, Clement A, Drumm ML, Boelle PY, Cutting GR, Knowles MR, Durie PR, Strug LJ. Multiple apical membrane constituents are associated with susceptibility to meconium ileus in individuals with cystic fibrosis. Nat Genet. 2012;44:562–569. doi: 10.1038/ng.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corvol H, Blackman SM, Boelle PY, Gallins PJ, Pace RG, Stonebraker JR, Accurso FJ, Clement A, Collaco JM, Dang H, Dang AT, Franca A, Gong J, Guillot L, Keenan K, Li W, Lin F, Patrone MV, Raraigh KS, Sun L, Zhou YH, O'Neal WK, Sontag MK, Levy H, Durie PR, Rommens JM, Drumm ML, Wright FA, Strug LJ, Cutting GR, Knowles MR. Genome-wide association meta-analysis identifies five modifier loci of lung disease severity in cystic fibrosis. Nat Commun. 2015;6:8382. doi: 10.1038/ncomms9382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suviolahti E, Oksanen LJ, Ohman M, Cantor RM, Ridderstrale M, Tuomi T, Kaprio J, Rissanen A, Mustajoki P, Jousilahti P, Vartiainen E, Silander K, Kilpikari R, Salomaa V, Groop L, Kontula K, Peltonen L, Pajukanta P. The SLC6A14 gene shows evidence of association with obesity. J Clin Invest. 2003;112:1762–1772. doi: 10.1172/JCI17491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Durand E, Boutin P, Meyre D, Charles MA, Clement K, Dina C, Froguel P. Polymorphisms in the amino acid transporter solute carrier family 6 (neurotransmitter transporter) member 14 gene contribute to polygenic obesity in French Caucasians. Diabetes. 2004;53:2483–2486. doi: 10.2337/diabetes.53.9.2483. [DOI] [PubMed] [Google Scholar]
- 33.Miranda RC, Vetter SB, Genro JP, Campagnolo PD, Mattevi VS, Vitolo MR, Almeida S. SLC6A14 and 5-HTR2C polymorphisms are associated with food intake and nutritional status in children. Clin Biochem. 2015 doi: 10.1016/j.clinbiochem.2015.07.003. in press. [DOI] [PubMed] [Google Scholar]
- 34.Flach CF, Eriksson A, Jennische E, Lange S, Gunnerek C, Lonnroth I. Detection of elafin as a candidate biomarker for ulcerative colitis by whole-genome microarray screening. Inflamm Bowel Dis. 2006;12:837–842. doi: 10.1097/01.mib.0000232469.23574.11. [DOI] [PubMed] [Google Scholar]
- 35.Yanai H, Ben-Shachar S, Baram L, Elad H, Gitstein G, Brazowski E, Tulchinsky H, Pasmanik-Chor M, Dotan I. Gene expression alterations in ulcerative colitis patients after restorative proctocolectomy extend to the small bowel proximal to the pouch. Gut. 2015;64:756–764. doi: 10.1136/gutjnl-2014-307387. [DOI] [PubMed] [Google Scholar]
- 36.Gupta N, Miyauchi S, Martindale RG, Herdman AV, Podolsky R, Miyake K, Mager S, Prasad PD, Ganapathy ME, Ganapathy V. Upregulation of the amino acid transporter ATB0,+ (SLC6A14) in colorectal cancer and metastasis in humans. Biochim Biophys Acta. 2005;1741:215–223. doi: 10.1016/j.bbadis.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 37.Gupta N, Prasad PD, Ghamande S, Moore-Martin P, Herdman AV, Martindale RG, Podolsky R, Mager S, Ganapathy ME, Ganapathy V. Up-regulation of the amino acid transporter ATB0,+ (SLC6A14) in carcinoma of the cervix. Gynecol Oncol. 2006;100:8–13. doi: 10.1016/j.ygyno.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 38.Karunakaran S, Umapathy NS, Thangaraju M, Hatanaka T, Itagaki S, Munn DH, Prasad PD, Ganapathy V. Interaction of tryptophan derivatives with SLC6A14 (ATB0,+) reveals the potential of the transporter as a drug target for cancer chemotherapy. Biochem J. 2008;414:343–355. doi: 10.1042/BJ20080622. [DOI] [PubMed] [Google Scholar]
- 39.Gutierrez ML, Corchete L, Teodosio C, Sarasquete ME, del Mar Abad M, Iglesias M, Esteban C, Sayagues JM, Orfao A, Munoz-Bellvis L. Identification and characterization of the gene expression profiles for protein coding and non-coding RNAs of pancreatic ductal adenocarcinoma. Oncotarget. 2015;6:19070–19086. doi: 10.18632/oncotarget.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Penheiter AR, Erdogan S, Murphy SJ, Hart SN, Lima JF, Rohakhtar FR, O'Brien DR, Bamlet WR, Wuertz RE, Smyrk TC, Couch FJ, Vasmatzis G, Bender CE, Carlson SK. Transcriptomic and immunohistochemical profiling of SLC6A14 in pancreatic ductal adenocarcinoma. Biomed Res Int. 2015;2015 doi: 10.1155/2015/593572. article ID 593572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karunakaran S, Ramachandran S, Coothankandaswamy V, Elangovan S, Babu E, Periyasamy-Thandavan S, Gurav A, Gnanaprakasam JP, Singh N, Schoenlein PV, Prasad PD, Thangaraju M, Ganapathy V. SLC6A14 (ATB0,+) protein, a highly concentrative and broad specific amino acid transporter, is a novel and effective drug target for treatment of estrogen receptor-positive breast cancer. J Biol Chem. 2011;286:31830–31838. doi: 10.1074/jbc.M111.229518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Babu E, Bhutia YD, Ramachandran S, Gnanaparakasam JP, Prasad PD, Thangaraju M, Ganapathy V. Deletion of the amino acid transporter Slc6a14 suppresses tumour growth in spontaneous mouse models of breast cancer. Biochem J. 2015;469:17–23. doi: 10.1042/BJ20150437. [DOI] [PubMed] [Google Scholar]
- 43.Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl- -dependent neurotransmitter transporters. Nature. 2005;437:215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- 44.Penmatsa A, Wang KH, Gouaux E. X-ray structure of dopamine transporter elucidates antidepressant mechanism. Nature. 2013;503:85–90. doi: 10.1038/nature12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kleta R, Romeo E, Ristic Z, Ohura T, Stuart C, Arcos-Buros M, Dave MH, Wagner CA, Camargo SR, Inoue S, Matsuura N, Helip-Wooley A, Bockenhauer D, Warth R, Bernardini I, Visser G, Eggermann T, Lee P, Chairoungdua A, Jutabha P, Babu E, Nilwarangkoon S, Anzai N, Kanai Y, Verrey F, Gahl WA, Koizumi A. Mutations in SLC6A19, encoding B0AT1, cause Hartnup disorder. Nat Genet. 2004;36:999–1002. doi: 10.1038/ng1405. [DOI] [PubMed] [Google Scholar]
- 46.Seow HF, Broer S, Broer A, Bailey CG, Potter SJ, Cavanaugh JA, Rasko JE. Hartnup disorder is caused by mutations in the gene encoding the neutral amino acid transporter SLC6A19. Nat Genet. 2004;36:1003–1007. doi: 10.1038/ng1406. [DOI] [PubMed] [Google Scholar]
- 47.Singer D, Camargo SM. Collectrin and ACE2 in renal and intestinal amino acid transport. Channels (Austin) 2011;5:410–423. doi: 10.4161/chan.5.5.16470. [DOI] [PubMed] [Google Scholar]
- 48.Fairweather SJ, Broer A, O'Mara ML, Broer S. Intestinal peptidases form complexes with the neutral amino acid transporter B0AT1. Biochem J. 2012;446:135–148. doi: 10.1042/BJ20120307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Broer A, Juelich T, Vanslambrouck JM, Tietze N, Solomon PS, Holtz J, Bailey CG, Rasko JE, Broer S. Impaired nutrient signaling and body weight control in a Na+/neutral amino acid cotransporter (Slc6a19)-deficient mouse. J Biol Chem. 2011;286:26638–26651. doi: 10.1074/jbc.M111.241323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fotiadis D, Kanai Y, Palacin M. The SLC3 and SLC7 families of amino acid transporters. Mol Aspects Med. 2013;34:139–158. doi: 10.1016/j.mam.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 51.Boado RJ, Li JY, Nagaya M, Zhang C, Pardridge WM. Selective expression of the large neutral amino acid transporter at the blood-brain barrier. Proc Natl Acad Sci U S A. 1999;96:12079–12084. doi: 10.1073/pnas.96.21.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matsuo H, Tsukada S, Nakata T, Chairoungdua A, Kim DK, Cha SH, Inatomi J, Yorifuji H, Fukuda J, Endou H, Kanai Y. Expression of a system L neutral amino acid transporter at the blood-brain barrier. Neuroreport. 2000;11:3507–3511. doi: 10.1097/00001756-200011090-00021. [DOI] [PubMed] [Google Scholar]
- 53.Ganapathy ME, Leibach FH, Mahesh VB, Howard JC, Devoe LD, Ganapathy V. Characterization of tryptophan transport in human placental brush-border membrane vesicles. Biochem J. 1986;238:201–208. doi: 10.1042/bj2380201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pochini L, Scalise M, Calluccio M, Indiveri C. Membrane transporters for the special amino acid glutamine: structure/function relationships and relevance to human health. Front Chem. 2014;2:61. doi: 10.3389/fchem.2014.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Q, Holst J. L-Type amino acid transporter and cancer: targeting the mTORC1 pathway to inhibit neoplasia. Am J Cancer Res. 2015;5:1281–1294. [PMC free article] [PubMed] [Google Scholar]
- 56.Ganapathy V, Thangaraju M, Prasad PD. Nutrient transporters in cancer: relevance to Warburg hypothesis and beyond. Pharmacol Ther. 2009;121:29–40. doi: 10.1016/j.pharmthera.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 57.Jewell JL, Russell RC, Guan KL. Amino acid signaling upstream of mTOR. Nat Rev Mol Cell Biol. 2013;14:133–139. doi: 10.1038/nrm3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taylor PM. Role of amino acid transporters in amino acid sensing. Am J Clin Nutr. 2014;99(suppl):223S–230S. doi: 10.3945/ajcn.113.070086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Elorza A, Soro-Arnaiz I, Melendez-Rodriguez F, Rodriguez-Vaello V, Marsboom G, de Carcer G, Acosta-Iborra B, Albacete-Albacete L, Ordonez A, Serrano-Oviedo L, Gimenez-Bachs JM, Vara-Vega A, Salinas A, Sanchez-Prieto R, Martin del Rio R, Sanchez-Madrid F, Malumbres M, Landazuri MO, Aragones J. HIF2α acts as an mTORC1 activator through the amino acid carrier SLC7A5. Mol Cell. 2012;48:681–691. doi: 10.1016/j.molcel.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 60.Poncet N, Mitchell FE, Ibrahim AF, McGuire VA, English G, Arthur JS, Shi YB, Taylor PM. The catalytic subunit of the system L1 amino acid transporter (slc7a5) facilitates nutrient signaling in mouse skeletal muscle. PLoS One. 2014;9:e89547. doi: 10.1371/journal.pone.0089547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Braun D, Wirth EK, Wohlgemuth F, Reix N, Klein MO, Gruters A, Kohrle J, Schweizer U. Aminoaciduria, but normal thyroid hormone levels and signaling, in mice lacking the amino acid and thyroid hormone transporter Slc7a8. Biochem J. 2011;439:249–255. doi: 10.1042/BJ20110759. [DOI] [PubMed] [Google Scholar]
- 62.Sperandeo MP, Andria G, Sebastio G. Lysinuric protein intolerance: update and extended mutation analysis of the SLC7A7 gene. Hum Mutat. 2008;29:14–21. doi: 10.1002/humu.20589. [DOI] [PubMed] [Google Scholar]
- 63.Sperandeo MP, Annunziata P, Bozzato A, Piccolo P, Maiuri L, D'Armiento M, Ballabio A, Corso G, Andria G, Borsani G, Sebastio G. Slc7a7 disruption causes fetal growth retardation by downregulating Igf1 in the mouse model of lysinuric protein intolerance. Am J Physiol Cell Physiol. 2007;293:C191–C198. doi: 10.1152/ajpcell.00583.2006. [DOI] [PubMed] [Google Scholar]
- 64.Chillaron J, Font-Llitjos M, Fort J, Zorzano A, Goldfarb DS, Nunes V, Palacin M. Pathophysiology and treatment of cystinuria. Nat Rev Nephrol. 2010;6:424–434. doi: 10.1038/nrneph.2010.69. [DOI] [PubMed] [Google Scholar]
- 65.Feliubadalo L, Arbones ML, Manas S, Chillaron J, Visa J, Rodes M, Rousaud F, Zorzano A, Palacin M, Nunes V. Slc7a9-deficient mice develop cystinuria non-I and cystine urolithiasis. Hum Mol Genet. 2003;12:2097–2108. doi: 10.1093/hmg/ddg228. [DOI] [PubMed] [Google Scholar]
- 66.Mackenzie B, Erickson JD. Sodium-coupled neutral amino acid (System N/A) transporters of the SLC38 gene family. Pflugers Arch. 2004;447:784–795. doi: 10.1007/s00424-003-1117-9. [DOI] [PubMed] [Google Scholar]
- 67.Broer S. The SLC38 family of sodium-amino acid co-transporters. Pflugers Arch. 2014;466:155–172. doi: 10.1007/s00424-013-1393-y. [DOI] [PubMed] [Google Scholar]
- 68.Wang H, Huang W, Sugawara M, Devoe LD, Leibach FH, Prasad PD, Ganapathy V. Cloning and functional expression of ATA1, a subtype of amino acid transporter A, from human placenta. Biochem Biophys Res Commun. 2000;273:1175–1179. doi: 10.1006/bbrc.2000.3061. [DOI] [PubMed] [Google Scholar]
- 69.Sugawara M, Nakanishi T, Fei YJ, Huang W, Ganapathy ME, Leibach FH, Ganapathy V. Cloning of an amino acid transporter with functional characteristics and tissue expression pattern identical to that of system A. J Biol Chem. 2000;275:16473–16477. doi: 10.1074/jbc.C000205200. [DOI] [PubMed] [Google Scholar]
- 70.Bak LK, Schousboe A, Waagepetersen HS. The glutamate-GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J Neurochem. 2006;98:641–653. doi: 10.1111/j.1471-4159.2006.03913.x. [DOI] [PubMed] [Google Scholar]
- 71.Brosnan ME, Brosnan JT. Hepatic glutamate metabolism: a tale of 2 hepatocytes. Am J Clin Nutr. 2009;90:857S–861S. doi: 10.3945/ajcn.2009.27462Z. [DOI] [PubMed] [Google Scholar]
- 72.Jenstad M, Chaudhry FA. The amino acid transporters of the glutamate-GABA-glutamine cycle and their impact on insulin and glucagon secretion. Front Endocrinol (Lausanne) 2013;4:199. doi: 10.3389/fendo.2013.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Desforges M, Sibley CP. Placental nutrient supply and fetal growth. Int J Dev Biol. 2010;54:377–390. doi: 10.1387/ijdb.082765md. [DOI] [PubMed] [Google Scholar]
- 74.Riley T, Sontag E, Chen P, Levine A. Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Biol. 2008;9:402–412. doi: 10.1038/nrm2395. [DOI] [PubMed] [Google Scholar]
- 75.Jeon YJ, Khelifa S, Ratnikov B, Scott DA, Feng Y, Parisi F, Ruller C, Lau E, Kim H, Brill LM, Jiang T, Rimm DL, Cardiff RD, Mills GB, Smith JW, Osterman AL, Kluger Y, Ronai ZA. Regulation of glutamine carrier proteins by RNF5 determines breast cancer response to ER stress-inducing chemotherapies. Cancer Cell. 2015;27:354–369. doi: 10.1016/j.ccell.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chaudhry FA, Reimer RJ, Krizaj D, Barber D, Storm-Mathisen J, Copenhagen DR, Edwards RH. Molecular analysis of system N suggests novel physiological role in nitrogen metabolism and synaptic transmission. Cell. 1999;99:769–780. doi: 10.1016/s0092-8674(00)81674-8. [DOI] [PubMed] [Google Scholar]
- 77.Fei YJ, Sugawara M, Nakanishi T, Huang W, Wang H, Prasad PD, Leibach FH, Ganapathy V. Primary structure, genomic organization, and functional and eletrogenic characteristics of human system N1, a Na+- and H+-coupled glutamine transporter. J Biol Chem. 2000;275:23707–23717. doi: 10.1074/jbc.M002282200. [DOI] [PubMed] [Google Scholar]
- 78.Nakanishi T, Sugawara M, Huang W, Martindale RG, Leibach FH, Ganapathy ME, Prasad PD, Ganapathy V. Structure, function, and tissue distribution pattern of human SN2, a subtype of the amino acid transport system N. Biochem Biophys Res Commun. 2001;281:1343–1348. doi: 10.1006/bbrc.2001.4504. [DOI] [PubMed] [Google Scholar]
- 79.Nakanishi T, Kekuda R, Fei YJ, Hatanaka T, Sugawara M, Martindale RG, Leibach FH, Prasad PD, Ganapathy V. Cloning and functional characterization of a new subtype of the amino acid transport system N. Am J Physiol Cell Physiol. 2001;281:C1757–C1768. doi: 10.1152/ajpcell.2001.281.6.C1757. [DOI] [PubMed] [Google Scholar]
- 80.Umapathy NS, Li W, Mysona BA, Smith SB, Ganapathy V. Expression and function of glutamine transporters SN1 (SNAT3) and SN2 (SNAT5) in retinal Muller cells. Invest Ophthalmol Vis Sci. 2005;46:3980–3987. doi: 10.1167/iovs.05-0488. [DOI] [PubMed] [Google Scholar]
- 81.Umapathy NS, Dun Y, Martin PM, Duplantier JN, Roon P, Prasad PD, Smith SB, Ganapathy V. Expression and function of system N transporters (SN1/SN2 or SNAT3/SNAT5) in retinal ganglion cells. Invest Ophthalmol Vis Sci. 2008;49:5151–5160. doi: 10.1167/iovs.08-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hagglund MGA, Sreedharan S, Nilsson VCO, Shaik JHA, Almkvist IM, Backlin S, Wrange O, Fredriksson R. Identification of SLC38A7 (SNAT7) protein as a glutamine transporter expressed in neurons. J Biol Chem. 2011;286:20500–20511. doi: 10.1074/jbc.M110.162404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hagglund MGA, Hellsten SV, Bagchi S, Philippot G, Lofqvist E, Nilsson VCO, Almkvist I, Karlsson E, Sreedharan S, Tafreshiha A, Fredriksson R. Transport L-glutamine, L-alanine, L-arginine and L-histidine by the neuron-specific Slc38a8 (SNAT8) in CNS. J Mol Biol. 2015;427:1495–1512. doi: 10.1016/j.jmb.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 84.Chan K, Busque SM, Sailer M, Stoeger C, Broer S, Daniel H, Rubio-Aliaga I, Wagner CA. Loss of function mutation of the Slc38a3 glutamine transporter reveals its critical role for amino acid metabolism in the liver, brain, and kidney. Pflugers Arch. 2015 doi: 10.1007/s00424-015-1742-0. in press. [DOI] [PubMed] [Google Scholar]


