Abstract
Objective
To estimate the influence of parental rheumatoid arthritis on child morbidity.
Design
Nationwide cohort study.
Setting
Individual linkage to nationwide Danish Registries.
Participants
All singletons born in Denmark 1977–2008 (N=1 917 723) were followed for an average of 16 years.
Main outcome measures
Adjusted hazard ratios for child morbidity; i.e. 11 main diagnostic groups and specific autoimmune diseases within the International Classification of Diseases 8th and 10th version.
Results
Compared to unexposed children, children exposed to maternal rheumatoid arthritis (“clinical” and “preclinical”) (N=13 566) had up to 26% higher morbidity in 8 of 11 main diagnostic groups. Similar tendencies were found in children exposed to paternal rheumatoid arthritis (“clinical” and “preclinical”) (N=6330), with statistically significantly higher morbidity in 6 of 11 diagnostic groups. Hazard ratios (HR) were highest for autoimmune diseases with up to three times increased risk of juvenile idiopathic arthritis (HR, 95%CI: 3.30, 2.71-4.03 and 2.97, 2.20-4.01) and increased risk of up to 40% of diabetes mellitus type 1 (HR, 95%CI: 1.37, 1.12-1.66 and 1.44 ,1.09-1.90) and up to 30% increased HR of asthma (HR, 95%CI: 1.28, 1.20-1.36 and 1.15, 1.04-1.26). Conclusions were roughly similar for children exposed to maternal clinical RA and for children only followed up to 16 years of age.
Conclusions
Children of parents with rheumatoid arthritis had consistent excess morbidity. If the associations reflect biological mechanisms, genetic factors seem to play an important role. These findings call for attention given to children of parents with rheumatoid arthritis.
Keywords: parental rheumatoid arthritis, maternal exposure, paternal exposure, child health, epidemiology
Introduction
Rheumatoid Arthritis (RA) is a chronic systemic autoimmune disease [1] with a lifetime incidence of about 1% in the population but women are more often affected than men [2].
Previous studies have linked maternal RA with preterm birth [3-8] and low birth weight [5,6,8-11]. These associations could be caused by fetal programming induced by the disease, prenatal exposure to RA treatment or be related to genetic factors.
Fetal programming postulates that intrauterine exposures affect not only perinatal conditions but also susceptibility to diseases later in life [12-15]. Long-term health in children of mothers with RA is not well known [16-19]. Autoimmune diseases have been found to aggregate in families [16-18] e.g. RA and juvenile idiopathic arthritis (JIA) was found more common among children of parents with autoimmune disease [17-19], and one study suggests increased risk of type 1 Diabetes Mellitus (DM) in children of parents with RA [16]. These findings indicate importance of genetic sharing between diseases [17-19].
In a cohort of 1 917 723 children born in Denmark, we found that maternal clinical RA (RA diagnosed before childbirth) was associated with substantially increased risk of preterm birth and slightly reduced birth weight [8], which point to a possible higher susceptibility to diseases later in life. Maternal “preclinical” RA (RA diagnosed after childbirth) revealed similar increased risks, indicating that treatment for RA need not be the causal factor, whereas genetic factors or “preclinical” disease activity could play a role [8]. In this study, we follow the same cohort of almost two million children to assess long-term morbidity with regard to the most common diagnostic groups in the International Classification of Diseases System (ICD) [20] and the risk of specific autoimmune related diseases such as JIA, DM and asthma. We extended the exposure definition to children exposed either to “clinical” or “preclinical” RA at time of birth. We hypothesized that if children of mothers with RA (“clinical” or “preclinical”) were programmed in fetal life, we would find an increased risk of a broad range of diseases in these children; while no alteration of disease-risk would be expected in children born by women without RA who had a male partner with RA. On the other hand, if genetic factors were the underlying causal factor, children exposed either to maternal or paternal RA would have an increased risk of disease; particularly of other autoimmune diseases. Also, the influence of exposure to “maternal RA at time of birth” versus “maternal RA diagnosed several years after childbirth” would be roughly similar.
Methods
Data sources
Since 1960, all residents in Denmark have had a personal identification number, registered in the Civil Registration System [21]. The personal identification number allows valid linkage between various national registries [21].
A nationwide cohort was established by linkage of Danish national registries including the Civil Registration System, which contains information on date of birth, immigration and emigration status, deaths and actual residence [21], the Danish National Hospital Registry, which holds nationwide data on all admissions to any Danish Hospital since 1977 and all outpatient visits since 1995 [22], and the Medical Birth Registry, which includes information on all births since 1973 [23]. Finally, registries at Statistics Denmark provided data on demographic factors.
Study Population
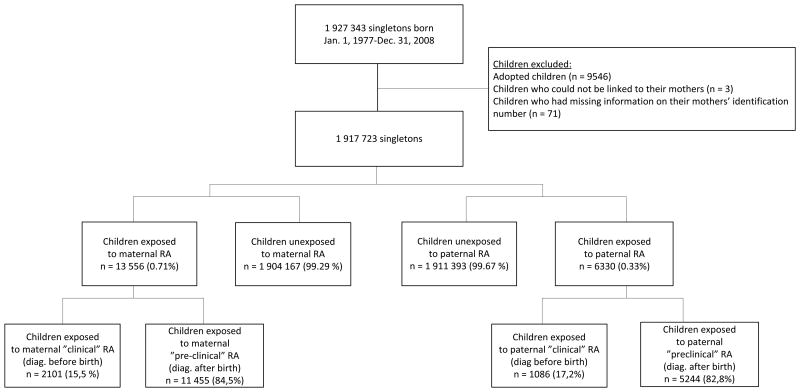
We identified all singletons born alive in Denmark between January 1, 1977, and December 31, 2008 (N = 1 927 343) from the Medical Birth Registry (Figure 1).
Figure 1. Identification of study population.
The study was approved by The Danish Data Protection Agency (Jr. no. 2010-41-5535). Research Ethic Approval was not required according to Danish law.
Parental rheumatoid arthritis
Information on maternal and paternal RA was obtained in the years from 1977 until 2008 from The Danish National Hospital Registry. We used diagnoses from the International Classification of Diseases, 8th Revision (ICD-8) from 1977 through 1993 (ICD 8: 712.19, 712.39, 712.59) and the ICD, 10th Revision (ICD-10) from 1994 onwards (ICD 10: M05 and M06 (except M06.1 Stills Disease)).
Children were classified as “exposed” to maternal or paternal RA if their mother or father were diagnosed with a main discharge diagnosis or a secondary discharge diagnosis of RA. The first hospitalization or first contact with the outpatient clinic associated with a diagnosis of RA was used as the date of “clinically” diagnosed RA. Timing of first diagnosis was used to identify fetal exposure to “clinical RA” (i.e. RA diagnosed prior to birth) or “preclinical RA” (i.e. RA diagnosed after birth).
Long-term child morbidity
Since little is known about disease occurrence in offspring of parents with RA, we included 11 common diagnostic groups and three specific autoimmune diseases according to the ICD system.
Information on disease incidence rates in the offspring was obtained from The Danish National Hospital Registry by using the first hospitalization or first contact with an outpatient clinic with the diagnostic code of interest; from January 1, 1977 until December 31, 2010. The diagnostic groups and the specific diagnoses (ICD-8 and the ICD-10 codes) are listed in the online supplementary methods and results section.
Covariates
Covariates included information at the time of birth on: gestational age (weeks), birth weight (gram), sex of the child, parity, year of birth, parental age (years), RA in the other parent, maternal education (low/middle/high), maternal and paternal income (quartiles) and maternal smoking (smoker/non-smoker) (smoking available from 1997).
Statistical Analysis
Cox proportional hazards models were used to calculate hazard ratios (HRs) and associated 95% confidence intervals for risk of disease among children exposed to parental RA (maternal or paternal RA, respectively). Age of the children (in days) was used as the underlying time scale. Follow up started at birth and ended at the age of the event, age at death, age at emigration or at age at the end of follow-up (December 31, 2010), whichever came first. The reference group was children born in the same time period and not exposed to maternal or paternal RA, respectively.
The analyses were conducted separately for each diagnostic group and for each autoimmune disease. The crude hazard models included exposure variables with age of the child as underlying time scale and year of birth. Adjusted models additionally included maternal age at birth, maternal education, and paternal or maternal RA, respectively.
A number of sensitivity analyses addressed other potential confounding factors or mediators and effectmodification by using the variables mentioned in the covariates section (See online supplementary methods and results section).
To explore a potential higher risk of morbidity in children exposed to “clinical” RA during pregnancy and at time of conception, analyses were stratified on timing of maternal RA diagnosis according to childbirth (“clinical” versus “preclinical RA”). Analyses of exposure to “preclinical RA” were further stratified by time from childbirth until a diagnosis of RA (< 5 years after childbirth versus ≥5 years after childbirth).
Tests for interaction on the multiplicative scale between sex and parental exposure to RA were conducted by inclusion of interaction terms in the model. A 5% significance level was used.
To address risk of diseases in childhood only all analyses were additionally conducted with follow-up of offspring terminated at the age of 16 years. Finally, the risk of infections and parasitic diseases were assessed among children younger than 6 years, and among children between the age of 6 and 16 years, respectively.
“Complete case analyses” were conducted including children with complete information in the fully adjusted models (94%). All statistical analyses were performed using SAS statistical software (version 9.2).
Results
From 1977 to 2008 a complete national cohort of 1 917 723 newborn singletons was identified and followed until 2010 with an average follow-up of 16 years (range 0 –34 years). The number of children exposed to maternal RA was 13 556, while 6330 children were exposed to paternal RA (Figure 1). Compared with unexposed children, children exposed to parental RA had mothers with shorter education at time of birth. Maternal smoking at the time of birth was more frequent among the exposed children, whereas parity and parental age at birth was approximately similar for exposed and unexposed children (Table 1). Children exposed to “clinical RA” at time of birth had older mothers with higher educational level and fewer mothers were smokers, compared to the unexposed children [8].
Table 1. Characteristics of the cohort at time of birth.
| Unexposed to maternal RA | Maternal RA | Unexposed to paternal RA | Paternal RA | |
|---|---|---|---|---|
| (n = 1 904 167) | (n = 13 556) | (n=1 911 393) | (n = 6 330 ) | |
| Maternal age, mean ± SD years | 28.35 (4.9) | 28.46 (5.1) | 28.35 (4.9) | 28.41 (5.1) |
| Maternal education, no. (%)a | ||||
| Low | 691 042 (36.3) | 5 852 (43.2) | 694 132 (36.3) | 2 762 (43.6) |
| Middle | 587 882 (30.9) | 3 970 (29.3) | 589 998 (30.9) | 1 854 (29.3) |
| High | 578 780 (30.4) | 3 388 (25.0) | 580 601 (30.4) | 1 567 (24.8) |
| Parity, mean ± SD | 1.75 (0.9) | 1.77 (0.9) | 1.75 (0.9) | 1.78 (0.87) |
| Maternal smoking, no (%)a,b | ||||
| Smoker | 128 600 (17.2) | 597 (20.5) | 128 933 (17.2) | 264 (21.0) |
| Non-smoker | 591 922 (79.1) | 2 172 (74.5) | 593 154 (79.1) | 940 (74.7) |
| Paternal age, mean ± SD years | 31.14 (5.8) | 31.10 (6.0) | 31.14 (5.8) | 32.08 (6.4) |
| Other parent RA, no. (%) | ||||
| Yes | 6 273 (0.3) | 57 (0.4) | 13 499 (0.7) | 57 (0.9) |
| No | 1 897 894 (99.7) | 13 499 (99.6) | 1 897 894 (99.3) | 6 273 (99.1) |
| Birth year, no. (%) | ||||
| 1977-1980 | 230 120 (12.1) | 3066 (22.6) | 231 704 (12.1) | 1482 (23.4) |
| 1981-1984 | 199 873 (10.5) | 2198 (16.2) | 201 056 (10.5) | 1015 (16.0) |
| 1985-1988 | 215 627 (11.3) | 1952 (14.4) | 216 657 (11.3) | 922 (14.6) |
| 1989-1992 | 247 294 (13.0) | 1884 (13.9) | 248 249 (13.0) | 929 (14.7) |
| 1993-1996 | 262 817 (13.8) | 1542 (11.4) | 263 635 (13.8) | 724 (11.4) |
| 1997-2000 | 255 132 (13.4) | 1195 (8.8) | 255 789 (13.4) | 538 (8.5) |
| 2001-2004 | 246 348 (12.9) | 930 (6.9) | 246 878 (12.9) | 400 (6.3) |
| 2005-2008 | 249 956 (13.0) | 789 (5.8) | 247 425 (12.9) | 320 (5.1) |
| Gender, no. (%) | ||||
| Boy | 978 038 (51.4) | 6 920 (51.1) | 981 668 (51.4) | 3 290 (52.0) |
| Girl | 926 129 (48.6) | 6 636 (48.9) | 929 725 (48.6) | 3 040 (48.0) |
Abbreviations: RA, rheumatoid arthritis; SD, standard deviation
Columns does not sum to 100 % because of missing values
1997-2008
Long-term morbidity
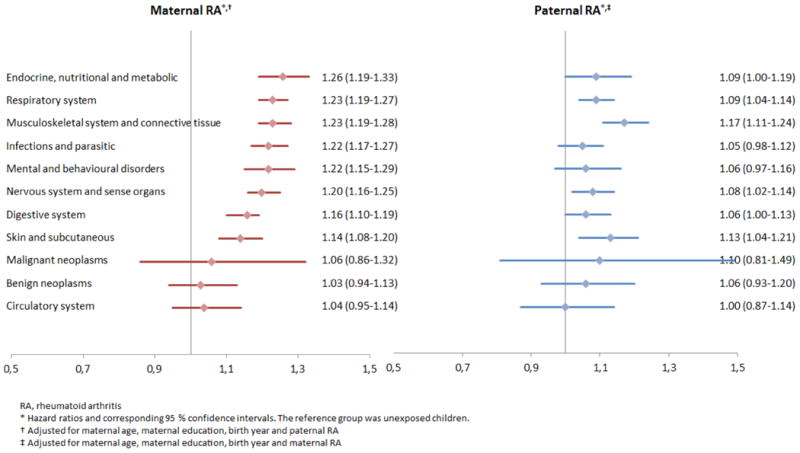
When comparing unexposed children with children exposed to parental RA the point estimates indicate an excess morbidity for all diagnostic groups examined, except for circulatory system diseases among children exposed to paternal RA (Figure 2). In 8 diagnostic groups out of 11 investigated, the hazard ratios (HR's) were statistically significantly increased among children exposed to maternal RA, including “endocrine, nutritional and metabolic” diseases, “respiratory system”, “musculoskeletal system and connective tissue”, “infectious and parasitic” diseases, “mental and behavioral disorders”, “nervous system and sense organs”, “digestive system” and “skin and subcutaneous” diseases (Figure 2). Similar tendencies were found for children exposed to paternal RA, but the HR's of “infectious and parasitic” diseases and “mental and behavioral disorders” were not statistically significantly increased (Figure 2).
Figure 2. Hazard ratios for overall morbidity in children exposed to parental RA.
Increased risks of autoimmune diseases were found in children exposed to maternal RA or paternal RA; for JIA the HR was 3.30 and 2.96, respectively. For DM type 1 the HR was 1.37 and 1.44, and for asthma the HR was 1.28 and 1.14 (Table 2).
Table 2. Hazard ratios (HR) for autoimmune diseases in children exposed to parental RA.
| Maternal RA* | Paternal RA† | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| N | Personyears | Cases | HR (95 % CI) | N | Personyears | Cases | HR | ||
| Juvenile Idiopathic Arthritis | |||||||||
| RA | 13 210 | 272 819 | 100 | 3.30 (2.71-4.03) | 6183 | 130 258 | 43 | 2.97 (2.20-4.01) | |
| No RA | 1 857 704 | 30 824 532 | 4134 | 1 [Reference] | 1 864 731 | 30 967 093 | 4191 | 1 [Reference] | |
| Diabetes type 1 | |||||||||
| RA | 13 210 | 272 867 | 101 | 1.37 (1.12-1.66) | 6183 | 130 331 | 51 | 1.44 (1.09-1.90) | |
| No RA | 1 857 704 | 30 798 413 | 8028 | 1 [Reference] | 1 864 731 | 30 940 949 | 8078 | 1 [Reference] | |
| Asthma | |||||||||
| RA | 13 210 | 26.3 262 | 940 | 1.28 (1.20-1.36) | 6183 | 126 153 | 397 | 1.15 (1.04-1.26) | |
| No RA | 1 857 703 | 29 792 452 | 110 591 | 1 [Reference] | 1 864 730 | 29 929 561 | 111 134 | 1 [Reference] | |
RA, rheumatoid arthritis; CI, confidence interval
adjusted for maternal age, maternal education, birth year and paternal RA
adjusted for maternal age, maternal education, birth year and maternal RA
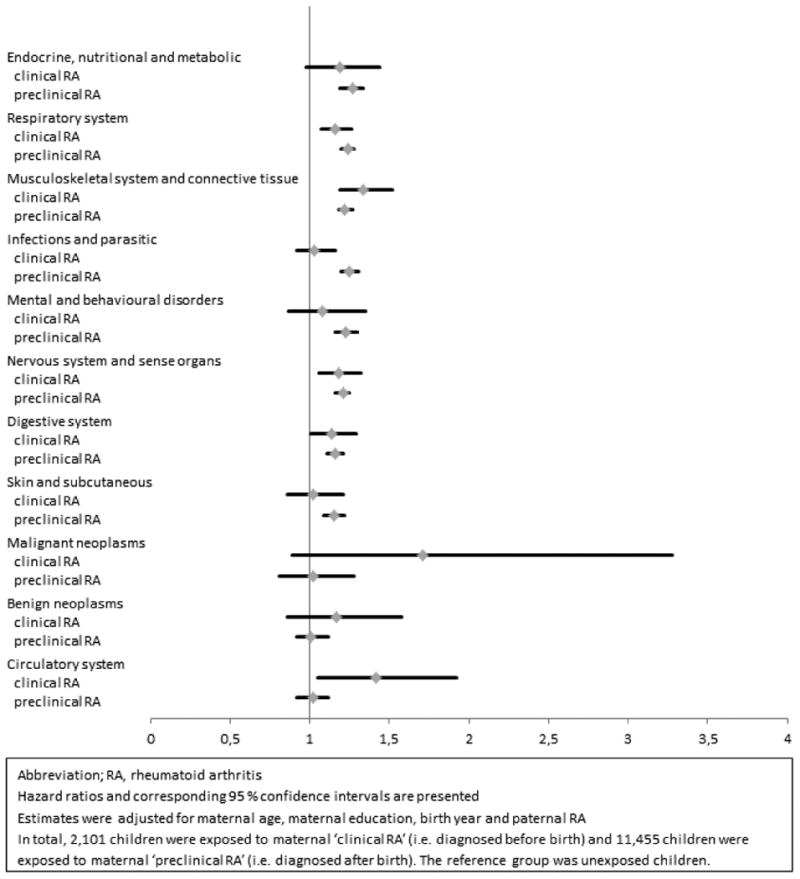
For the majority of morbidities examined the point estimates were increased both among children exposed to maternal “clinical” RA and “preclinical RA” at the time of birth. However, an increased risk of diseases of the “circulatory system” was found only among children exposed to maternal “clinical” RA (HR, 1.42) but not among children of mothers with “preclinical RA” (HR, 1.02) (Figure 3). Among children exposed to maternal “preclinical” RA whom were “diagnosed 5 or more years after childbirth” (N = 9245), the HR's were approximately similar to the HR's among children exposed to maternal “preclinical” RA “diagnosed less than 5 years after childbirth” (See online supplementary methods and results section Table S1).
Figure 3. Hazard ratios for overall morbidity according to timing of maternal RA diagnosis.
For some diseases sex related differences were found; e.g. among girls higher HR's of diseases of the “musculoskeletal system and connective tissues” was found, than among boys. HR's of JIA was also higher among girls exposed to maternal RA (See online supplementary methods and results section Table S2).
The sensitivity analyses showed results similar to the main analyses (See online supplementary methods and results section).
Childhood morbidity
When comparing children not older than 16 years at the end of follow up, the point estimates in children exposed to parental RA also indicated an excess morbidity regarding all diagnostic groups examined except for benign neoplasms among children exposed to paternal RA. Despite lower statistical power, the HR's were statistically significantly increased in 10 of 11 main diagnostic groups among children exposed to maternal RA and in 5 of 11 main diagnostic groups among children exposed to paternal RA (See online supplementary methods and results section Figure S1).
Also HR's of JIA, asthma and DM type 1 were increased in children exposed to maternal RA or paternal RA. The point estimates were approximately similar to the point estimates based on all included children (age 0-34 years) (See online supplementary methods and results section Table S5). HR's for infectious and parasitic diseases did not change after restricting analyses to; 1) children below the age of 6 years or 2) children from 6 to 16 years of age (data not shown).
Discussion
For all diagnostic groups examined, the point estimates indicated excess long-term morbidity in children exposed to parental RA. For children exposed to maternal RA, a statistically significant increase in morbidity was found in 8 of 11 diagnostic groups, while 6 were statistically significant among children exposed to paternal RA. The risk of autoimmune diseases was increased 30% for DM type 1 and up to three times for JIA. With few exceptions similar associations between exposure to parental RA and risk of morbidities in childhood (below 16 years of age) were found.
The circumstance that increased child morbidity apparently was found in both children of mothers with RA and fathers with RA (i.e. mothers without RA) point to a genetic etiology.
Our findings are supported by familial association studies, in which an aggregation of autoimmune diseases has been consistently demonstrated [16-18]. One study reported a two-fold increased risk of DM in children of a parent with RA [16]. Another study documented a threefold increased risk of RA in children of a parent with RA or other autoimmune diseases [17] and finally, an increased frequency of other autoimmune diseases was detected among relatives of children with JIA [18,19].
It is well known, that RA is associated with increased co-morbidity [24-27] and it is possible that genetic factors have pleiotropic effects and predispose both to RA and other diseases in the parent as well as in the child [28]. Also, the particularly increased risk of specific autoimmune diseases in children of parents with RA indicates that genetic factors play a role.
Among boys and girls the relative morbidity risks were similar for most diagnostic groups. However, a higher risk of diseases of the musculoskeletal system and connective tissues was found among girls exposed to parental RA, than among boys; including JIA among girls exposed to maternal RA. This finding is consistent with the well-known female predominance of these diseases. Other sex related differences in morbidity among children exposed to parental RA has not been documented before and should be verified in independent data sources.
Risk of infectious and parasitic diseases as well as mental and behavioral disorders was increased only in children exposed to maternal RA. Furthermore, stronger associations were found in children exposed to maternal RA compared to children exposed to paternal RA. Thus, it is possible that fetal programming also play a role in the etiology. Fetal adaption to the rheumatic intrauterine environment may induce epigenetic changes that enhance disease susceptibility in the offspring [29-31], but treatment for RA [32-34] seems not a plausible programming explanation. Estimates were not higher among children who had mothers with “clinical” RA (i.e. treated with specific RA treatments) than for those children who had mothers with “preclinical RA” (i.e. untreated for RA). One exception was the risk of circulatory system diseases, which was increased exclusively among children of mothers with RA diagnosed before birth; pointing to a potential detrimental programming effect of RA or RA treatment, which needs further investigation.
More children were exposed to maternal “preclinical RA” than maternal “clinical RA”, which may explain why more diagnostic groups were statistically significant in this group. On the other hand, it is possible that also untreated, latent inflammatory disease could induce fetal programming. “Preclinical RA” has recently been recognized as a period with similar elevations of disease-related biomarkers, including autoimmune antibodies, prior to the development of RA [35]. Another contributing factor might be self-medication with e.g. non-steroidal anti-inflammatory (NSAID) drugs and acetaminophen [36]. However, HR's for “very early preclinical RA during pregnancy” were similar, which again point to genetic factors as the most likely explanation for our findings. Moreover an increased morbidity was found in children born by mothers without RA but exposed to paternal RA (hereof 82.8% “preclinical” RA at time of conception). Thus, the impact of RA treatment or antibodies during pregnancy seems not to have a major impact on the fetus.
This is the first study to investigate long-term health conditions in children of parents with RA. We investigated risks of a broad range of diseases in the long-term among offspring of parents with RA; and among offspring not older than 16 years. Exposure to both maternal and paternal RA was examined, as well as maternal “clinical RA” versus maternal “preclinical RA” [35]. Assessment of long-term health in children exposed to parental RA requires access to a large study population. This study includes more than 19 000 children exposed to parental RA with a mean follow-up of 16 years. We were able to adjust for a large number of potential confounders including educational level, parental income, and maternal smoking (See online supplementary methods and results section). Moreover, approximately similar HR's were found in children of mothers whom less often were smokers (maternal “clinical” RA), indicating that maternal smoking does not play a major role for our results.
Most of the diseases assessed in our study require a hospital admission and thus referral from primary care. Therefore a potential different health behavior by parents with RA is not assumed to have a major impact on the results.
Parental RA was identified in the Danish National Hospital Registry by the first hospitalization with RA or the first visit at an outpatient clinic. A Danish study found that 59% of RA cases registered in The Danish National Hospital Registry between 1977 and 2001 could be confirmed as RA cases at that time [37]. The proportion however, increased when registered as inpatient (up to 80 %); and when having more than one diagnosis of RA (up to 91%) [37]. In our study, estimates remained similar after restricting to those of a parent diagnosed with RA while hospitalized; while estimates slightly strengthened after restricting to children of a parent diagnosed with RA more than once (See online supplementary method and results section). The latter was expected as over diagnosing of RA will most likely underestimate the effects we estimate.
The coverage of inpatients in the Danish National Hospital Registry is assumed high, since almost all hospitals in Denmark are public hospitals with services free of charge and since this register forms the basis for government payment to public hospitals [22,38]. Outpatient data was included from 1995 and the upcoming of a private healthcare sector in Denmark in the 2000s also necessitated notification from private hospitals and clinics to The Danish National Hospital Registry. Thus, some RA patients were not captured in this study; i.e. those whom were diagnosed and treated as outpatients before 1995 or at private practitioners exclusively. However, associations were quite similar before versus after the year of 1995 (See online supplementary method and results section), indicating no impact on results of some children falsely classified as unexposed; which is not surprising since the unexposed group comprises almost 2 million unexposed children giving limited room for false negatives. Contact to general practitioner was not included. However, in Denmark patients with RA are treated in hospital or by practicing specialists. Moreover, patients treated by specialists will often have contact to the hospital, especially pregnant women will be followed in hospital were RA will be recorded as a secondary diagnosis.
Finally, we cannot exclude potential unknown confounding and studies within other populations should be carried out before causality can be established. In spite of this, the results indicate an overall increased susceptibility toward diseases among these children and clinicians should be aware of this. (See online supplementary discussion section for further considerations).
In conclusion, children of parents with RA had consistent excess long-term morbidity and childhood morbidity. Assuming these associations reflect biological mechanisms this study indicates an important role of genetic factors. These are novel findings relevant for rheumatologists and family doctors.
Supplementary Material
Acknowledgments
Funding: This study was supported by the National Institutes of Health (grant number 5R21AR059931-02), The Danish Council for Independent Research and The Augustinus Foundation. None of the funding sources had a role in the design or conduct of the study; the management, analysis, or interpretation of the data; nor in preparation, review, and approval of the manuscript or decision to submit the manuscript for publication.
Footnotes
Contributors: Study concept or design: ALR, JO and LSM
Acquisition, analysis, or interpretation of data: ALR, CSW, JO, DJ, MH, BO and LSM
Drafting of the manuscript: ALR, JO and LSM
Critical revision of the manuscript for important intellectual content: ALR, CSW, JO, DJ, MH, BO and LSM
Final approval of the version to be published: ALR, CSW, JO, DJ, MH, BO and LSM
Ethical approval: Research Ethical Approval was not required according to Danish law.
Data sharing: Raw data are available from Statistics Denmark (www.dst.dk)
No competing interests: “All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organization for the submitted work; no financial relationships with any organization's that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.”
References
- 1.Symmons DP, Barrett EM, Bankhead CR, Scott DG, Silman AJ. The incidence of rheumatoid arthritis in the United Kingdom: results from the Norfolk Arthritis Register. British journal of rheumatology. 1994 Aug;33(8):735–739. doi: 10.1093/rheumatology/33.8.735. [DOI] [PubMed] [Google Scholar]
- 2.Borchers AT, Naguwa SM, Keen CL, Gershwin ME. The implications of autoimmunity and pregnancy. Journal of Autoimmunity. 2010 May;34(3):J287–99. doi: 10.1016/j.jaut.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 3.Langen ES, Chakravarty EF, Liaquat M, El-Sayed YY, Druzin ML. High rate of preterm birth in pregnancies complicated by rheumatoid arthritis. American Journal of Perinatology. 2014 Jan;31(1):9–14. doi: 10.1055/s-0033-1333666. [DOI] [PubMed] [Google Scholar]
- 4.Wallenius M, Salvesen KA, Daltveit AK, Skomsvoll JF. Rheumatoid arthritis and outcomes in first and subsequent births based on data from a national birth registry. Acta Obstetricia et Gynecologica Scandinavica. 2013 Dec 21; doi: 10.1111/aogs.12324. [DOI] [PubMed] [Google Scholar]
- 5.Wallenius M, Skomsvoll JF, Irgens LM, Salvesen KA, Nordvag BY, Koldingsnes W, et al. Pregnancy and delivery in women with chronic inflammatory arthritides with a specific focus on first birth. Arthritis and Rheumatism. 2011 Jun;63(6):1534–1542. doi: 10.1002/art.30210. [DOI] [PubMed] [Google Scholar]
- 6.Norgaard M, Larsson H, Pedersen L, Granath F, Askling J, Kieler H, et al. Rheumatoid arthritis and birth outcomes: a Danish and Swedish nationwide prevalence study. Journal of internal medicine. 2010 Oct;268(4):329–337. doi: 10.1111/j.1365-2796.2010.02239.x. [DOI] [PubMed] [Google Scholar]
- 7.Reed SD, Vollan TA, Svec MA. Pregnancy outcomes in women with rheumatoid arthritis in Washington State. Maternal and child health journal. 2006 Jul;10(4):361–366. doi: 10.1007/s10995-006-0073-3. [DOI] [PubMed] [Google Scholar]
- 8.Rom AL, Wu CS, Olsen J, Kjaergaard H, Jawaheer D, Hetland ML, et al. Fetal growth and preterm birth in children exposed to maternal or paternal rheumatoid arthritis: a nationwide cohort study. Arthritis & rheumatology (Hoboken, NJ) 2014 Dec;66(12):3265–3273. doi: 10.1002/art.38874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin HC, Chen SF, Lin HC, Chen YH. Increased risk of adverse pregnancy outcomes in women with rheumatoid arthritis: a nationwide population-based study. Annals of the Rheumatic Diseases. 2010 Apr;69(4):715–717. doi: 10.1136/ard.2008.105262. [DOI] [PubMed] [Google Scholar]
- 10.de Man YA, Dolhain RJ, van de Geijn FE, Willemsen SP, Hazes JM. Disease activity of rheumatoid arthritis during pregnancy: results from a nationwide prospective study. Arthritis and Rheumatism. 2008 Sep 15;59(9):1241–1248. doi: 10.1002/art.24003. [DOI] [PubMed] [Google Scholar]
- 11.de Man YA, Hazes JM, van der Heide H, Willemsen SP, de Groot CJ, Steegers EA, et al. Association of higher rheumatoid arthritis disease activity during pregnancy with lower birth weight: results of a national prospective study. Arthritis and Rheumatism. 2009 Nov;60(11):3196–3206. doi: 10.1002/art.24914. [DOI] [PubMed] [Google Scholar]
- 12.Barker DJ. Human growth and cardiovascular disease. Nestle Nutrition workshop series. Paediatric programme. 2008;61:21–38. doi: 10.1159/000113163. [DOI] [PubMed] [Google Scholar]
- 13.Barker DJ. The origins of the developmental origins theory. Journal of internal medicine. 2007 May;261(5):412–417. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- 14.Calkins K, Devaskar SU. Fetal origins of adult disease. Current problems in pediatric and adolescent health care. 2011 Jul;41(6):158–176. doi: 10.1016/j.cppeds.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuh D, Ben-Shlomo Y, Lynch J, Hallqvist J, Power C. Life course epidemiology. Journal of epidemiology and community health. 2003 Oct;57(10):778–783. doi: 10.1136/jech.57.10.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hemminki K, Li X, Sundquist J, Sundquist K. Familial association between type 1 diabetes and other autoimmune and related diseases. Diabetologia. 2009 Sep;52(9):1820–1828. doi: 10.1007/s00125-009-1427-3. [DOI] [PubMed] [Google Scholar]
- 17.Hemminki K, Li X, Sundquist J, Sundquist K. Familial associations of rheumatoid arthritis with autoimmune diseases and related conditions. Arthritis and Rheumatism. 2009 Mar;60(3):661–668. doi: 10.1002/art.24328. [DOI] [PubMed] [Google Scholar]
- 18.Prahalad S, O'brien E, Fraser AM, Kerber RA, Mineau GP, Pratt D, et al. Familial aggregation of juvenile idiopathic arthritis. Arthritis and Rheumatism. 2004 Dec;50(12):4022–4027. doi: 10.1002/art.20677. [DOI] [PubMed] [Google Scholar]
- 19.Prahalad S, Shear ES, Thompson SD, Giannini EH, Glass DN. Increased prevalence of familial autoimmunity in simplex and multiplex families with juvenile rheumatoid arthritis. Arthritis and Rheumatism. 2002 Jul;46(7):1851–1856. doi: 10.1002/art.10370. [DOI] [PubMed] [Google Scholar]
- 20. [December/15, 2014];International Classification of Diseases version10. Available at: http://apps.who.int/classifications/icd10/browse/2010/en.
- 21.Pedersen CB, Gotzsche H, Moller JO, Mortensen PB. The Danish Civil Registration System. A cohort of eight million persons. Danish medical bulletin. 2006 Nov;53(4):441–449. [PubMed] [Google Scholar]
- 22.Andersen TF, Madsen M, Jorgensen J, Mellemkjoer L, Olsen JH. The Danish National Hospital Register. A valuable source of data for modern health sciences. Danish medical bulletin. 1999 Jun;46(3):263–268. [PubMed] [Google Scholar]
- 23.Knudsen LB, Olsen J. The Danish Medical Birth Registry. Danish medical bulletin. 1998 Jun;45(3):320–323. [PubMed] [Google Scholar]
- 24.Gron KL, Ornbjerg LM, Hetland ML, Aslam F, Khan NA, Jacobs JW, et al. The association of fatigue, comorbidity burden, disease activity, disability and gross domestic product in patients with rheumatoid arthritis. Results from 34 countries participating in the Quest-RA program. Clinical and experimental rheumatology. 2014 Oct 20; [PubMed] [Google Scholar]
- 25.Wright K, Crowson CS, Gabriel SE. Cardiovascular comorbidity in rheumatic diseases: a focus on heart failure. Heart failure clinics. 2014 Apr;10(2):339–352. doi: 10.1016/j.hfc.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogdie A, Yu Y, Haynes K, Love TJ, Maliha S, Jiang Y, et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population-based cohort study. Annals of the Rheumatic Diseases. 2014 Oct 28; doi: 10.1136/annrheumdis-2014-205675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dougados M, Soubrier M, Antunez A, Balint P, Balsa A, Buch MH, et al. Prevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring: results of an international, cross-sectional study (COMORA) Annals of the Rheumatic Diseases. 2014 Jan;73(1):62–68. doi: 10.1136/annrheumdis-2013-204223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith GC, Pell JP, Walsh D. Pregnancy complications and maternal risk of ischaemic heart disease: a retrospective cohort study of 129,290 births. Lancet. 2001 Jun 23;357(9273):2002–2006. doi: 10.1016/S0140-6736(00)05112-6. [DOI] [PubMed] [Google Scholar]
- 29.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. The New England journal of medicine. 2008 Jul 3;359(1):61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gluckman PD, Hanson MA, Pinal C. The developmental origins of adult disease. Maternal & child nutrition. 2005 Jul;1(3):130–141. doi: 10.1111/j.1740-8709.2005.00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanson MA, Gluckman PD. Developmental origins of health and disease: new insights. Basic & clinical pharmacology & toxicology. 2008 Feb;102(2):90–93. doi: 10.1111/j.1742-7843.2007.00186.x. [DOI] [PubMed] [Google Scholar]
- 32.Krause ML, Amin S, Makol A. Use of DMARDs and biologics during pregnancy and lactation in rheumatoid arthritis: what the rheumatologist needs to know. Therapeutic advances in musculoskeletal disease. 2014 Oct;6(5):169–184. doi: 10.1177/1759720X14551568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ostensen M, Forger F. Management of RA medications in pregnant patients. Nature reviews. Rheumatology. 2009 Jul;5(7):382–390. doi: 10.1038/nrrheum.2009.103. [DOI] [PubMed] [Google Scholar]
- 34.Ostensen M, Forger F. How safe are anti-rheumatic drugs during pregnancy? Current opinion in pharmacology. 2013 Jun;13(3):470–475. doi: 10.1016/j.coph.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Deane KD. Preclinical rheumatoid arthritis (autoantibodies): an updated review. Current rheumatology reports. 2014 May;16(5) doi: 10.1007/s11926-014-0419-6. 419-014-0419-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liew Z, Ritz B, Rebordosa C, Lee PC, Olsen J. Acetaminophen use during pregnancy, behavioral problems, and hyperkinetic disorders. JAMA pediatrics. 2014 Apr;168(4):313–320. doi: 10.1001/jamapediatrics.2013.4914. [DOI] [PubMed] [Google Scholar]
- 37.Pedersen M, Klarlund M, Jacobsen S, Svendsen AJ, Frisch M. Validity of rheumatoid arthritis diagnoses in the Danish National Patient Registry. European journal of epidemiology. 2004;19(12):1097–1103. doi: 10.1007/s10654-004-1025-0. [DOI] [PubMed] [Google Scholar]
- 38.Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scandinavian Journal of Public Health. 2011 Jul;39(7 Suppl):30–33. doi: 10.1177/1403494811401482. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.