Abstract
Objective
Plasma levels of high density lipoprotein cholesterol (HDLC) and apolipoprotein A-I (ApoA-I) are reduced in individuals with defective insulin signaling. Initial studies using liver-specific insulin receptor (InsR) knockout mice (LIRKO) identified reduced expression of Type 1 Deiodinase (Dio1) as a potentially novel link between defective hepatic insulin signaling and reduced expression of the ApoA-I gene. Our objective was to examine the regulation of ApoA-I expression by Dio1.
Approach and Results
Acute inactivation of InsR by adenoviral delivery of Cre recombinase to InsR floxed mice reduced HDLC and expression of both ApoA-I and Dio1. Overexpression of Dio1 in LIRKO restored HDLC and ApoA-I levels and increased the expression of ApoA-I. Dio1 knockout (D1KO) mice had very low expression of ApoA-I and reduced serum levels of HDLC and ApoA-I. Treatment of C57BL/6J mice with anti-sense to Dio1 reduced ApoA-I mRNA, HDLC, and serum ApoA-I. Hepatic 3,5,3′-triiodothyronine (T3) content was normal or elevated in LIRKO or D1KO mice. Knockdown of either InsR or Dio1 by siRNA in HepG2 cells decreased expression of ApoA-I as well as ApoA-I synthesis and secretion. siRNA knockdown of InsR or Dio1 decreased activity of a region of the ApoA-I promoter lacking thyroid hormone response elements (TREs) (Region B). Electrophoretic mobility shift assay demonstrated that reduced Dio1 expression decreased the binding of nuclear proteins to Region B.
Conclusions
Reductions in Dio1 expression reduce expression of ApoA-I in a T3/TRE independent manner.
Keywords: Apolipoprotein A-I, HDL Cholesterol, Type 1 Diodinase, Insulin Signaling
Introduction
High density lipoprotein (HDL) cholesterol (C) and Apolipoprotein A-I (ApoA-I) concentrations are strong predictors of risk for cardiovascular disease (CVD) 1,2. The development of a well-tolerated agent which can significantly elevate serum HDLC levels remains a therapeutic target. Statins raise HDLC only modestly and it is not clear whether that effect plays a role in the success of statins in CVD reduction. Niacin increases HDLC significantly, but two recent studies where niacin was added to statin treatment did not reduce CVD 3,4. Fibrates increase HDLC moderately, depending on baseline triglycerides, but studies of fibrates and CVD reduction have produced mixed results 5,6. There is one large ongoing trial to determine if CVD can be reduced by increasing HDLC with inhibitors of cholesteryl ester (CE) transfer protein (CETP); three previous trials with this class of drug, however, have failed 7,8,
Although niacin may increase the production of ApoA-I 9, the other HDL-raising drugs do not. Relevant to this point, data from mouse models indicate that when ApoA-I synthesis is increased by genetic approaches, atherosclerosis is reduced 10–12. Importantly, short term administration of ApoA-I has provided promising, but not definitive, evidence of beneficial effects on atherosclerotic lesions in animals 13–15 and in humans 16–18.
Low levels of HDLC are common in the people with defective insulin signaling, eg, metabolic syndrome and T2DM 19,20. Although the hypertriglyceridemia common to these disorders drives increased CETP-mediated exchange of HDL cholesteryl esters for triglycerides (TG) from very low density lipoproteins and chylomicrons, those pathways only account for about half of the inverse relationship between TG and HDLC. Mouse models of defective insulin signaling, such as insulin receptor (InsR) liver specific knockout (LIRKO) mice 21, PI3K double knockout mice 21 and mice lacking hepatic Irs 1 and Irs 2 22, all have reduced HDLC levels in the absence of CETP. The mechanism by which defective insulin signaling results in low levels of HDLC remains to be determined.
To examine the role of insulin signaling in the regulation of HDLC and ApoA-I levels, and based on our prior studies in congenital LIRKO mice 21, we created acute LIRKO mice by injecting a Cre adenovirus into InsR(fl/fl) (Lox) mice. Microarray analyses indicated that, concomitant with reductions in ApoA-I levels in serum, the mice had marked reductions in the hepatic expression of ApoA-I. In addition, and to our surprise, the largest change in gene expression was that of Type I Deiodinase (Dio1), which was reduced by 90% in the acute LIRKO mice. Here, we present results of a series of in vivo and in vitro experiments demonstrating that Dio1 can regulate ApoA-I gene expression at the level of the promoter and that this regulation is independent of previously demonstrated effects of thyroid hormone on ApoA-I gene expression. Importantly, reduced Dio1 expression resulted in reduced rates of ApoA-I synthesis and secretion from hepatocytes. Our results demonstrate a completely novel mechanism for the regulation of ApoA-I gene expression and provide new insights regarding the link between states of defective insulin signaling and reduced levels of plasma HDLC and ApoA-I.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Acute and Chronic Loss of Hepatic InsRs are Associated with Reductions in the Levels of HDLC and ApoA-I, and the Expression of ApoA-I and Dio1
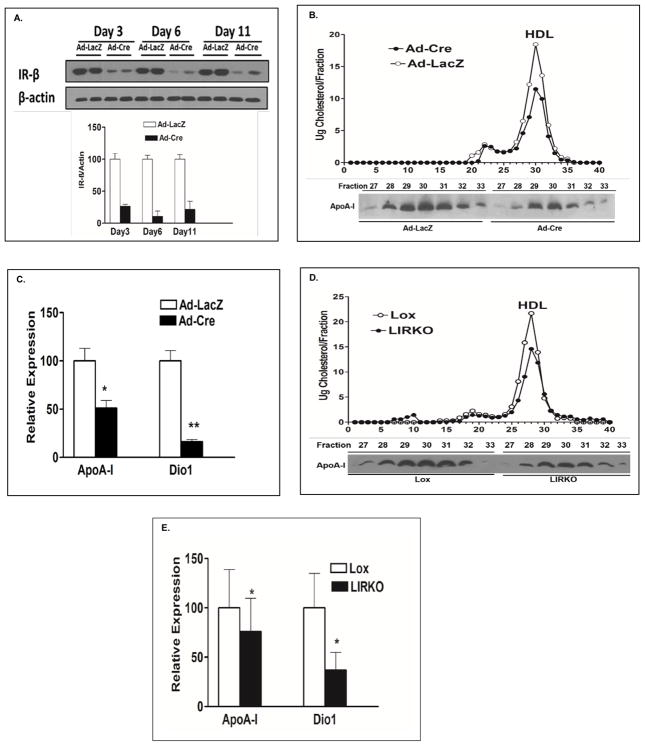
We reported previously that LIRKO mice have markedly reduced serum HDLC levels 21. In this study we produced acute liver InsR depletion by injecting 10 week old InsR (fl/fl) (Lox) mice with either an adenovirus carrying the Cre recombinase with the liver specific albumin promoter (Ad-Cre) or a control adenovirus carrying LacZ (Ad-LacZ). Levels of liver InsR levels were determined by western blotting of hepatic samples from Ad-LacZ and Ad-Cre-injected mice at day 3, day 6, and day 12. By 6 days after injection of Ad-Cre, hepatic InsR expression levels were reduced by 90% (Fig. 1A). Fast protein liquid chromatography (FPLC) analysis revealed that both HDLC and ApoA-I were significantly decreased in Lox mice infused with the Ad-Cre compared with Ad-LacZ injected mice (Fig. 1B). As noted above, microarray analyses revealed that livers from the mice injected with Ad-Cre had marked reductions in the expression of ApoA-I and Dio1 (Supplemental Fig. 1A); real time qPCR data confirmed these results (Fig. 1C). Microarray analyses of other major genes involved in HDLC metabolism, including SR-BI, ABCA1 and ABCG1, did not show significant changes in Lox mice treated with Ad-Cre (Supplemental Fig. IA). Other lipid or lipoprotein related genes that were affected by acute loss of InsR included ApoB, ApoAII, ApoCIII, ApoE, LDLR and LCAT; they were all down regulated in the microarray analysis (Supplemental Fig. IB) and, except for ApoCIII, by qPCR as well (Supplemental Fig. 1C). Based on these findings, we returned to LIRKO mice and confirmed the reduced HDLC levels we had reported previously (Fig. 1D) 21. Additionally, we found reduced ApoA-I levels in HDL fractions (Fig. 1D). We also observed that ApoA-I mRNA and Dio1 mRNA levels were significantly decreased in LIRKO mice (Fig. 1E). In our prior study, expression of constitutively active AKT reversed the low HDLC in LIRKO mice21; we repeated this and demonstrated a marked increase in Dio1 mRNA levels in LIRKO after administration of an adenovirus carrying constitutively active AKT (Supplemental Fig. II). These results are consistent with low levels of HDLC and Dio1 mRNA levels present in AKT1/2 double knockout mice (Supplemental Fig. IIIA and IIIB.).
Fig 1. Acute and chronic loss of hepatic InsRs are associated with reductions in HDLC, ApoA-I, and the expression of ApoA-I and Dio1.
A. InsR levels were determined by western blots of livers from acute LIRKO mice and their controls generated by injecting 5 X 109 viral particles of Ad-Cre or Ad-LacZ into 10 week old Lox mice (n=3 per group). Livers were harvested at day 3, day 6 and day 11 after injections. B. Serum was obtained from 10 week old Lox mice 6 days after injection with Ad-LacZ or Ad-Cre following a 6 hour fast. 200ul of pooled serum from mice (n = 6 per group) was subjected to FPLC to resolve lipoproteins and cholesterol was measured in each of the eluted fraction (upper panel). The FPLC fractions representing HDL (fractions 27–33) were used for western blotting of ApoA-I (lower panel). The data shown are from one of 3 independent experiments. C. Livers (n = 4–6 per group) were harvested 6 days after injection of Ad-LacZ or Ad-Cre into Lox mice. Real time PCR was used to verify microarray results showing significantly reduced hepatic ApoA-I and Dio1 expression levels in acute LIRKO mice. D. 200ul of serum was pooled from 16 week old LIRKO mice and Lox controls (n=5 per group) and lipoproteins were resolved by FPLC. Cholesterol and ApoA-I were determined as described in B. E. Liver ApoA-I mRNA and Dio1 mRNA were quantified by qPCR in LIRKO and Lox mice (n =10 per group). Bar graphs depict mean ± sd. * p < 0.05, ** p <0.01.
In mammals, Dio1 is highly expressed in liver and has been widely thought to be an important source of T3 in the euthyroid state. However mice lacking the Type I Deiodinase enzyme (D1), have no evidence of hepatic hypothyroidism and have normal plasma T3 levels 23,24. Indeed mice lacking all three types of deiodinase activity have a normal hepatic T3 content 25. To test if reduced expression of hepatic Dio1 results in a reduced content of T3 in liver, we measured T3 content in the livers of LIRKO mice and their control Lox mice. There was no significant difference in liver T3 content between these two groups (Supplemental Fig. IVA). Importantly, the normal hepatic T3 content in LIRKO mice suggested that any mechanistic association of defective hepatic insulin signaling, reduced Dio1 expression, and low plasma HDLC and ApoA-I levels might not involve the well described effects of T3 on ApoA-I gene expression 26,27.
HDLC, ApoA-I, and ApoA-I mRNA Levels Are Decreased in Both D1KO mice and C57BL/6J Mice Treated with Dio1 ASO
The results presented above indicated that the effects of Dio1 on ApoA-I expression were downstream of defective hepatic insulin signaling. To determine if Dio1 could regulate ApoA-I directly, we carried out studies in D1KO mice. In D1KO mice both HDLC and HDL ApoA-I levels were reduced compared to WT controls (Fig. 2A). Concomitant with these differences in blood levels, hepatic ApoA-1 mRNA levels were significantly reduced in D1KO mice (Fig. 2B). Importantly, qPCR of D1KO livers demonstrated that mRNA levels of ApoAII, ApoB, ApoCIII and ApoE, which were reduced in LIRKO mice, were not altered by direct loss of Dio1 expression (Supplemental Fig. V). Liver T3 content was actually increased in the D1KO mice compared with WT mice (Supplemental Fig. IV B), a result not unexpected based on our prior published studies in these mice 23,25. Because LIRKO mice have reduced Dio1 and ApoA-I mRNA levels in association with defective hepatic insulin signaling and insulin resistance, we measured fasting serum insulin levels in D1KO and WT mice: there were no differences between the two groups (Supplemental Fig. VI).
Fig 2. HDLC, ApoA-I, and ApoA-I mRNA levels are decreased in both D1KO mice and C57BL/6J mice treated with Dio1 ASO.
A. Serum was obtained from 16 week old D1KO mice on a normal chow diet after a 16 hour fast (WT, n = 8, D1KO, n=10). 200 ul of pooled serum from each group of mice was subjected to FPLC. Cholesterol was measured in each of the eluted fraction (upper panel). The FPLC fractions representing HDL (fractions 27–33) were used for western blotting of ApoA-I (lower panel). The data shown are from one of 3 independent experiments. B. ApoA-I mRNA was quantified by qPCR from livers of 16 week old D1KO and control mice (n=8 per group). C and D. 10 week old C57BL/6J mice were injected intraperitoneally with a dose of 12.5 mg/kg of Dio1 ASO (n=6) or Control (Ctrl) ASO (n=6) twice per week. Mice were sacrificed after 8 weeks of treatment. Liver ApoA-I mRNA was measured by qPCR.(C). 200 ul of pooled serum from each group of mice was subjected to FPLC (D). The measurement for cholesterol and ApoA-I from the fractions was described in A. Bar graphs depict mean ± sd. * p < 0.05.
To further understand the effect of Dio1 on normal mice, we knocked down Dio1 expression in C57BL/6J mice using a 16-mer cEt gap-mer antisense oligonucleotide (ASO) that was provided by Ionis Pharmaceuticals. Dio1 and control ASO were administered twice weekly at a dose of 12.5 mg/kg body weight to 10 week old C57BL/6J. After 8 weeks of treatment, liver Dio1 mRNA was decreased by 95% (Supplemental Fig. VII). The reduction in Dio1 expression was associated with a 50% decrease in ApoA-I mRNA (Fig. 2C) in the mice treated with Dio1 ASO. Serum HDLC levels and HDL ApoA-I concentrations were both significantly reduced in Dio1 ASO-treated mice compared to controls ASO (Fig. 2D). The results from both the D1KO mice and the Dio1 ASO-treated mice indicate clearly that Dio1 deficiency results in reduced ApoA-I mRNA levels independent of hepatic insulin signaling and in the presence of normal or elevated hepatic T3 content.
Overexpression of Dio1 Can Restore HDLC and ApoA-I levels, and Significantly Increase the Expression of ApoA-I in LIRKO mice
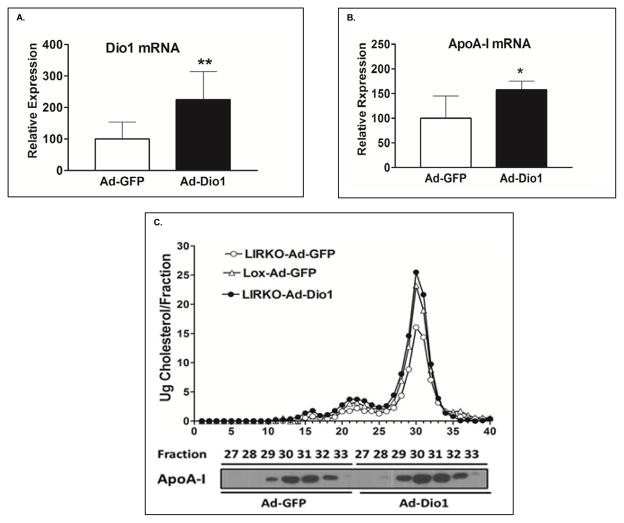
The findings indicated a direct link between Dio1 and ApoA-I expression. To extend these results, we generated an adenovirus containing the cDNA for mouse Dio1 (Ad-Dio1) and injected it into LIRKO mice. LIRKO and Lox mice received Ad-GFP as well. Twelve days after infection, LIRKO mice that had received Ad-Dio1 had an increased expression of hepatic Dio1 (Fig. 3A) as well as a significant increase in the expression of hepatic ApoA-I mRNA (Fig. 3B). FPLC profiles showed that LIRKO mice infected with Ad-Dio1 had elevated HDLC levels compared to LIRKO mice infected with Ad-GFP; LIRKO mice infected with Ad-Dio1 had the same HDLC levels as Lox mice infected with Ad-GFP (Fig. 3C, upper panel). Furthermore, immunoblotting of the HDL fractions from the FPLC profile (fractions 27–33) showed significant increases in ApoA-I in LIRKO mice infected with Ad-Dio1 (Fig. 3C, lower panel). Adenoviral-mediated overexpression of Dio1 in C57BL/6J mice had no effects on HDLC and ApoA-I mRNA levels even though Dio1 mRNA levels increased more than two fold in the C57BL/6J treated with Ad-Dio1 (Supplemental Fig. VIIIA and VIIIB). Overall these data indicate that overexpression of Dio1 corrected the abnormality in hepatic ApoA-I mRNA levels and serum HDLC and ApoA-I levels in LIRKO mice. The absence of any effect of overexpressing Dio1 in C57BL/6J mice is not surprising in view of the very high levels of D1 in normal mouse livers28.
Fig 3. Overexpression of Dio1 can restore HDLC and ApoA-I levels, and significantly increase the expression of ApoA-I in LIRKO mice.
12 week old LIRKO mice were injected intravenously with Ad-Dio1 (n=8) or Ad-GFP (n=6). After 12 days, liver Dio1mRNA (A) and ApoA-I mRNA (B) were measured by qPCR. C. 200ul of serum from mice with Ad-Dio1 or Ad-GFP was pooled and lipoproteins were resolved by FPLC, with cholesterol measured in each fraction (upper panel). ApoA-I levels were examined by western blot in FPLC fractions (27–33) (lower panel). Similar results were obtained in the two other experiments. Bar graphs depict mean ± SD. * p < 0.05. **p < 0.01
Knockdown of either InsR or Dio1 Decreases ApoA-I Expression as well as the Synthesis and Secretion of ApoA-I in HepG2 Cells
To determine the effects of reduced insulin signaling and Dio1 expression at the cellular level, we used siRNA to knock down either InsR or Dio1 in the human hepatoblastoma cell line, HepG2. Treatment of HepG2 cells with InsR siRNA resulted in a dramatic decrease in receptors by western blotting (Supplemental Fig. IXA) and a fall in InsR mRNA of more than 90% by qPCR (Supplemental Fig. IXB). These changes in InsR were associated with reductions of approximately 40% and 60% in ApoA-I mRNA and Dio1 mRNA levels, respectively (Supplemental Fig. IXB). When Dio1 expression was reduced about 90% in HepG2 cells by direct siRNA treatment, ApoA-I mRNA levels were decreased to 50% of the levels in cells treated with control siRNA (Fig. 4A). To demonstrate the effect of knockdown of either insulin signaling or Dio1 on the synthesis and secretion of ApoA-I, HepG2 cells treated with siRNAs for either InsR or Dio1 were labeled with [35S] methionine for 20 minutes. As shown in Fig. 4B, compared to control cells (lanes 1 and 2), cells treated with siRNAs for InsR (lanes 3 and 4) or Dio1 (lanes 5 and 6) had reductions of newly synthesized cellular ApoA-I of 37% and 22%. Secretion of ApoA-I into the media was concomitantly reduced (Fig. 4B, lanes 9 and 10 for InsR and lanes 11 and 12 for Dio1) by 52% and 30% compared to control cells (Fig. 4B, lanes 7 and 8). When HepG2 cells that had been treated with siRNAs for either InsR or Dio1 were labeled with [35S] methionine for 20 minutes and then chased in media without [35S] methionine for 20 minutes, essentially identical results were obtained. Thus during a 20 min chase, cells in which either InsR or Dio1 had been knocked down synthesized 41% or 26% less ApoA-I (Fig. 4C, lanes 3 and 4 for InsR and lanes 5 and 6 for Dio1), respectively, compared to control cells (Fig. 4C, lanes 1 and 2). During the 20 min chase experiment, InsR and Dio1 siRNA-treated cells (Fig. 4C, lanes 9 and 10, and 11 and 12 respectively) secreted 50% or 32% less than control cells (Fig. 4C, lanes 7 and 8). The similar reductions in synthesis and secretion in each experiment indicated that the reduction in ApoA-I secretion could be fully accounted for by decreased synthesis; there was no evidence of increased intracellular degradation of newly synthesized ApoA-I in the cells treated with either InsR or Dio1 siRNA.
Fig 4. Knockdown of either InsR or Dio1 decreases ApoA-I expression as well as the synthesis and secretion of ApoA-I in HepG2 cells.
A. Both Dio1 and ApoA-I mRNA were analyzed by qPCR after knockdown of Dio1 with siRNA for 72hr in HepG2 cells. B. The cells were labeled with [35S] methionine for 20 minutes after knockdown of InsR and Dio1 with siRNA. Then the cells and media were harvested and ApoA-I was immunoprecipitated prior to SDS PAGE and autoradiography. Each bar is representative of 3 experiments. C. The cells were labeled with [35S] methionine for 20 minutes followed by 20min chase after knockdown of InsR and Dio1 with siRNA. The method for measuring labeled ApoA-I in cells and media is described as D. Each bar represents the data from 3 experiments respectively. Statistical analyses were done using one-way ANOVA (Bonferroni post hoc test). *p < 0.05, **p < 0.01.
Dio1 regulates activity of the ApoA-I promoter through Region B, which does not contain T3 responsive elements
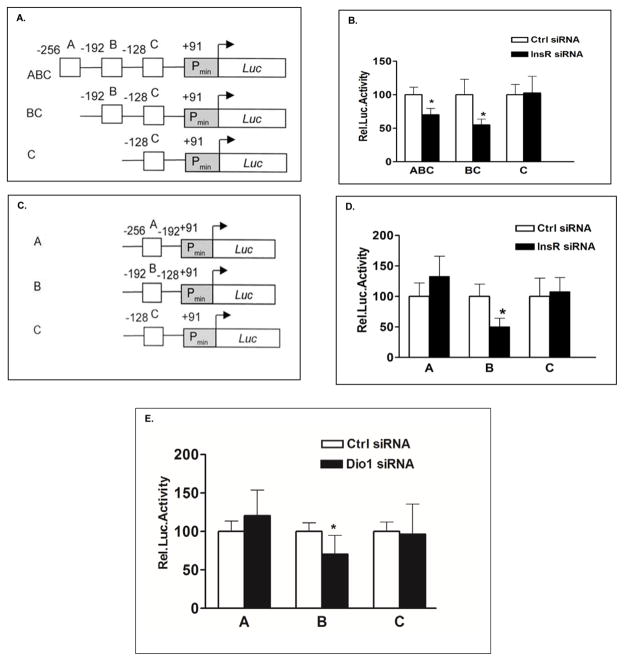
The ApoA-I promoter region from −256 to −41bp 5′ from the transcription start (+1) has been defined by some investigators as a liver-specific enhancer. It contains three transcription factor (TF) binding regions designated as Region A (−256 to −192), Region B (−192 to −128), and Region C (−128 to −41)29. We obtained pGL3-luciferase reporter plasmids including a construct containing ApoA-I gene promoter sequence from base pair −256 to +1 and two 5′ deletion constructs as gifts from Professor Bart Staels (France) (Figure 5A). HepG2 cells were transfected with scrambled control siRNA or InsR siRNA for 24 h, followed by transfection of ABC, BC, or C luciferase reporter constructs of the ApoA-I promoter for another 48h. As shown in Fig. 5B, knockdown of InsR reduced the activity of the ABC ApoA-I promoter construct by approximately 30% of the control level and the activity of the BC ApoA-I promoter construct to about 50% of control promoter activity. In contrast, knockdown of InsR had no effect on the construct containing only Region C of the promoter. These results suggested that Region B contained response elements (REs) sensitive to defective insulin signaling. We extended these findings by generating additional ApoA-I promoter constructs containing only Region A (A) or Region B (B) (Fig. 5C). Using the same experimental protocol as described above, we demonstrated that defective insulin signaling resulted in decreased ApoA-I promoter activity only through Region B (Fig. 5D). To confirm our earlier findings that defective insulin signaling reduced ApoA-I expression through reduced Dio1 expression, HepG2 cells were transfected with the three reporter constructs, A, B or C, after siRNA-mediated knockdown of Dio1. As shown in Fig. 5E, knockdown Dio1 reduced the activity of the B promoter construct by approximately 35% of the control level, and there were no effects of Dio1 knockdown on either the A or C promoter constructs.
Fig 5. Dio1 regulates activity of the ApoA-I promoter through Region B which does not contain T3 responsive elements.
A. Schematic drawing of the human ApoA-I promoter constructs as described by Staels and colleagues (24). The liver-specific enhancer sequence of the ApoAI promoter contains three transcription factor binding regions (designated region A (−256 to −192), region B (−192 to −128), and region C (−128 to −41)). Deletion constructs were made to remove only region A (BC), or regions A plus B (C). B. HepG2 cells were transfected with InsR siRNA. 24h later the cells were transfected with ABC, BC or C luciferase reporter constructs for 48hs, respectively. C. Additional deletions were made in the ApoA-I ABC construct to generate constructs containing only region A (A) or region B (B). D. HepG2 cells were transfected with InsR siRNA. 24h later the cells were transfected with A, B or C luciferase reporter constructs for 48hs, respectively. E. HepG2 cells were transfected with Dio1siRNA for 24hs, then transfected with A, B or C luciferase reporter constructs for 48hs, respectively. Values are expressed as percentage of control (set at 100%) for each condition and normalized to internal control Renilla luciferase activity. In all experiments, luciferase activity was determined using the Dual-Luciferase Reporter Assay System. Data represent the means ± sd of at least 3 independent experiments with triplicate determinations. Statistical analyses was done using one-way ANOVA (Bonferroni post hoc test). *p < 0.05.
Romney et al. had demonstrated that T3 increased ApoA-I gene expression at the level of transcription 30 and Taylor et al. identified Region A to contain a positive T3 responsive element in the rat ApoA-I promoter 31. It was, therefore, important to determine which Region(s) of the human ApoA-I promoter is/are responsive to T3 treatment. Because the actions of T3 are mediated by TRβ, HepG2 cells were co-transfected with TRβ and ApoA-I promoter constructs in the presence of T3 or vehicle. As shown in Supplemental Fig. X, T3 treatment significantly increased Region A promoter activity by approximately 35%. Neither Region B nor Region C was responsive to T3 treatment. These results provide additional evidence that Dio1 is involved in regulation of the expression of the ApoA-I promoter via effects on Region B, which does not respond to T3 treatment.
Knockdown of either InsR or Dio1 Reduces the Binding of Nuclear Proteins to Region B and Overexpression of Dio1 Restores that Binding
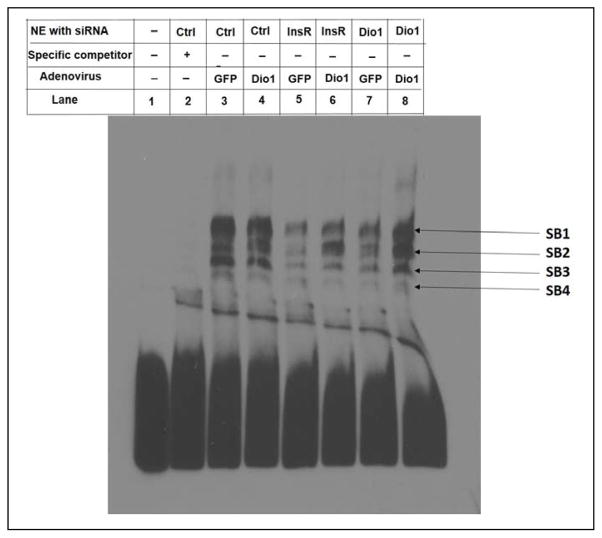
Based on the results described above, we determined if defective insulin signaling or decreased Dio1 expression induce changes in binding of transcription factors to Region B of ApoA-I promoter. In these experiments, either InsR or Dio1 was knocked down in HepG2 cells and nuclear proteins were extracted to do EMSA. The sequence of Region B was labeled as a probe. The specificity of binding of nuclear proteins to the Region B probe was confirmed by addition of excess non-labeled Region B oligonucleotide (Fig. 6, Lane 2). As shown in Lane 3, several nuclear proteins from HepG2 cells bound specifically to the DNA probe of Region B, generating specific bands (SBs) of varying mobility; these bands were not affected by overexpression of Dio1 in the cells (Lane 4). However, the intensities of SB1, SB2, SB3 and SB4 were dramatically reduced in the cells treated with either InsR siRNA or Dio1 siRNA (Lanes 5 and 7). Overexpression of Dio1 in the cells knocked down by InsR siRNA or Dio1 siRNA increased the intensities of SB1, SB2, SB3 (shown in Lane 6 or Lane 8) compared with those shown in Lane 5 or 7. Overexpression of Dio1 did not affect the intensity of SB4. EMSA analysis indicated, therefore, that the specific binding of several nuclear proteins to a Region B probe was clearly reduced when either InsR or Dio1 were knocked down compared to control cells. Over-expression of Dio1 restored binding that had been reduced by knockdown of either InsR or Dio1. These results suggest that ApoA-I is transcriptionally regulated by Dio1 expression via Region B.
Fig 6. Knockdown of either InsR or Dio1 reduces the binding of nuclear proteins to Region B and overexpression of Dio1 restores that binding.
HepG2 cells were transfected with Control siRNA (Ctrl), InsR siRNA, or Dio1 siRNA. 6 hours later, the cells were infected with Ad-Dio1 or control Ad-GFP for 48 hrs. Then nuclear extracts (NE) were prepared. The promoter sequence corresponding to Region B (−192 to −142) was used to generate the DNA probe. EMSA were conducted using a LightShift chemiluminescent EMSA kit. Lane 1: only labeled probe; Lane 2: specific competitor + NE from the cells treated with Ctrl siRNA; Lane 3, 5, 7: NE from the cells treated with Ctrl siRNA, InsR siRNA, and Dio1 siRNA respectively as well as Ad-GFP. Lane 4, 6, 8: NE from the cells treated with Ctrl siRNA, InsR siRNA, and Dio1 siRNA respectively as well as Ad-Dio1. All lanes include labeled probe. The results shown are representative of 6 experiments.
Discussion
ApoA-I, the major protein component of HDL, is expressed mainly in the liver. Interest in HDL as an anti-atherogenic lipoprotein led to extensive interrogation of the regulation of the ApoA-I gene by several groups about 20 years ago. The proximal region of the ApoA-I promoter, from −256 to −41 bp 5′ of the transcription start Region (+1), which has also been called the liver-specific enhancer, was mapped in fine detail and found to contain three transcription factor binding regions that have been designated differently by each group of investigators 32. We have used the terminology of Staels and colleagues 29, who divided the promoter into Region A (−256 to −192), Region B (−192 to −128), and Region C (−128 to −41). Several transcription factors involved in regulating ApoA-I gene transcription have been identified that bind to REs in one or more of these Regions 30,33–42. Most relevant to the present studies are the thyroid hormone receptors (TRs) 31 that increase ApoA-I promoter activity by binding to REs in Region A.
Despite the longstanding observation that HDLC and ApoA-I levels are reduced in people with insulin resistance and T2DM, studies of the role of insulin signaling on ApoA-I expression have been limited. Elshourbagy et al found that insulin increased the expression of ApoA-I in cultured primary rat hepatocytes 43. In contrast, Levy et al did not find an effect of insulin on intestinal ApoA-I expression or synthesis, suggesting tissue specific regulation 44. Haas et al demonstrated that hepatic ApoA-I mRNA levels were reduced in rats with streptozotocin-induced diabetes 45, and that insulin’s stimulatory activity occurred via binding of the TF, Sp1, to a GC-rich “insulin response core element” in the region of −411 to −404 bp of the ApoA-I gene promoter upstream from the initiation site 40. Our results complement those of Haas et al 45 in that defective insulin signaling in LIRKO mice and in HepG2 cells results in reduced ApoA-I expression, synthesis, and secretion. However, we add two new and novel findings to prior studies: First, that the effect of absent or reduced hepatic insulin signaling occurs at the level of the ApoA-I promoter at Region B of the liver-specific enhancer, between bases −128 and −192 from the transcriptional start region, and second, that the effect of absent or reduced insulin signaling is transmitted to the ApoA-I promoter via reduced expression of hepatic Dio1.
Regarding the effects of absent or reduced hepatic insulin signaling, we have extended our initial finding of low HDLC levels in LIRKO mice that was corrected by restoration of insulin signaling 21 by demonstrating a similar phenotype in an acute model of InsR knockout. Furthermore, we reproduced these findings in HepG2 cells using a specific siRNA for InsR. Thus, InsR knockdown resulted in reduced expression of ApoA-I that led to lower rates of synthesis and secretion of Apo-AI from HepG2 cells. Finally, as noted above, we demonstrated that loss of insulin signaling in HepG2 cells resulted in reduced activity of Region B of the ApoA-I promoter, and this reduced promoter activity was associated with changes in the binding of nuclear proteins to Region B of the ApoA-I promoter.
Our most important novel finding, however, was that the effects of absent or reduced hepatic insulin signaling on ApoA-I expression and promoter activity occurred via insulin signaling-mediated regulation of Dio1. Dio1 is expressed mainly in liver, kidney, thyroid, and pituitary gland, and is the source of D1, a selenoenzyme that can 5′-deiodinate thyroxine (T4) to T3. However its preferred substrate is 3,3′,5′-triiodothyronine (rT3). The D1 also has inner-ring or 5-deiodinase activity by which it can convert T4 and T3, usually as their sulfated conjugates, to their inactive derivatives, respectively rT3 or 3,3′-T2 23. The expression of Dio1 is influenced by fasting, severe illness, cytokines, nutritional status, and sex steroids, and it is markedly up regulated by T3 46. The latter effect probably occurs via two TREs that have been identified in the regions of −90 −100 and −660 −700 bps of the human Dio1 gene 47,48, although in the rodent gene, T3 stimulates Dio1 expression despite the absence of identifiable TREs in the promoter region 49. Recently, the transcription factors FOXA1 and FOXA2 were reported to regulate human Dio1 expression in the liver 50. Our present studies add hepatic insulin signaling to the list of regulators of Dio1 expression. Thus, both congenital and acute LIRKO mice have marked reductions in Dio1 expression, and the latter abnormality is reversed by delivery of constitutively active AKT. Severe insulin deficiency and metabolic derangements in rats with streptozotocin induced diabetes are associated with marked reductions in both Dio1 expression and D1 activity that can be reversed by insulin treatment 51. In contrast, LIRKO mice have minimally altered metabolism 21,52, allowing a more precise demonstration of linkage between defective hepatic insulin signaling and reductions in Dio1 expression. The molecular mechanisms underlying the link between hepatic insulin signaling and down-regulation of Dio1 expression will require additional experiments beyond the scope of the present work.
Not only is Dio1 gene expression remarkably reduced in LIRKO mice, it appears to be central to the regulation of ApoA-I gene expression by hepatic insulin signaling. When we overexpressed Dio1 in LIRKO mice, ApoA-I expression and the levels of both plasma HDLC and ApoA-I increased. Our studies with the D1KO mice and with Dio1 ASO in C57BL/6J mice confirmed the existence of a direct role for Dio1 in the regulation of ApoA-I expression, as well as the levels of plasma HDLC and ApoA-I. Critical to our model of a direct role of Dio1 in the regulation of ApoA-I expression, the D1KO mice have normal plasma insulin levels indicating normal hepatic insulin signaling. Finally, knockdown of Dio1 by siRNA in HepG2 cells produced the same pattern of nuclear protein binding in an EMSA as did knockdown of InsR. Importantly, over-expression of Dio1 normalized the pattern of nuclear protein binding in Region B in cells where expression of endogenous Dio1 or InsR had been knocked down.
It is well known that thyroid hormone status affects ApoA-I gene expression 26,27,53,54; T3 binds to a TRE present in Region A via TRs 55, resulting in increased promoter activity. Furthermore, propylthiouracil (PTU), a specific inhibitor of D1 activity, significantly decreased ApoA-I expression and ApoA-I synthesis in rat liver 53,56. There is, however, complexity in this pathway, as binding of the T3-TR complex to the other TRE, which is 3′ to the TATA box in Region C and close to the transcriptional start-region, leads to decreased promoter activity 31. In addition, in rodents, unliganded TRs can influence transcription of thyroid responsive genes in the opposite direction than the liganded TR 57. Our results add another level of complexity: the expression of Dio1 regulates ApoA-I expression at Region B, which does not have canonical TREs. Our study that T3 treatment only significantly increased Region A promoter activity in the presence of TRβ provided further evidence that Region B is not responsive to T3. Additional support for a T3-independent mechanism derives from the finding that hepatic T3 content was normal in LIRKO mice and actually increased in the D1KO mice. These findings were not unexpected based on our previous published data showing a normal content of hepatic T3 in D1KO mice 25. In addition, both D1KO 23 and C3H mice (which have very low D1 activity due to a naturally occurring mutation in Dio1) 58 appear to be euthyroid in terms of their plasma T3 levels and the expression of several “classical” T3-responsive genes in the liver. The maintenance of normal or elevated hepatic T3 content in LIRKO and D1KO mice may result from increased delivery of plasma T3 to the liver and/or reduced conversion of T3 to inactive T2 metabolites when D1 activity is reduced or absent.
Although we have demonstrated that Dio1 acts on the ApoA-I promoter via Region B, which does not contain a known TRE, and which is unresponsive to T3 in the presence of adequate TRβ, we have not as yet identified an alternative signaling pathway. Based on existing literature, we know that Region B contains response elements to which known TFs, including HNF-3beta 38,42, C/EBP59, NFY 60, or Sp161 can bind. How a transmembrane enzyme might interact and/or activate one of these (or another) TFs that would then act on the ApoA-I promoter, will require significant additional work with approaches that include RNA Seq, transciptomics, and bioinformatics analyses.
In summary, we have demonstrated that hepatic insulin signaling plays a significant role in the regulation of ApoA-I gene expression, as well as the synthesis and secretion of ApoA-I, through the regulation of Dio1. Of note, Dio1-mediated regulation of ApoA-I does not require defective hepatic insulin signaling, as shown by our studies in the D1KO mouse and with Dio1 ASO. Furthermore, loss of Dio1 expression reduces apoA-I expression without affecting expression of several other apolipoproteins that are also reduced when insulin signaling is lost. Our characterization of a novel pathway linking hepatic insulin signaling, Dio1, and ApoA-I, could have significant impact on the problem of low HDLC in people with metabolic syndrome or T2DM. Recent negative CVD clinical trials with fenofibrate, niacin, and CETP inhibitors, coupled with early but promising results from studies where blood levels of ApoA-I were increased directly, provide impetus for additional studies focused on the molecular mechanisms involved in this pathway. More importantly, identification of the links between Dio1 expression and ApoA-I promoter activity, could provide new and significant approaches to regulating plasma HDLC and ApoA-I levels.
Supplementary Material
HIGHLIGHTS.
Acute or chronic loss of insulin signaling in the LIRKO mouse resulted in significant reductions in plasma levels of HDLC, apoA-I, and apoA-I gene expression.
Dio1 expression was also significantly reduced in acute or chronic LIRKO and expression of Dio1 in LIRKO mice restored plasma levels of HDLC and apoA-I, and apoA-I gene expression.
Dio1 knockout mice and mice treated with Dio1 antisense have low plasma levels of HDLC and apoA-I, and apoA-I gene expression.
Knockdown of Dio1 in liver cells results in reduced apoA-I gene expression and apoA-I synthesis.
Dio1 regulates apoA-I promoter activity in a region that is not affected by thyroid hormone and has no thyroid response elements.
Acknowledgments
The authors wish to thank Professor Bart Staels, Member of the Institut Universitaire de France Inserm UMR1011 & UDSL, Université Lille Nord de France Institut Pasteur de Lille.
Sources of Funding: This work was supported by grants R01-HL55638 from NIH, National Heart Lung and Blood Institute and R21-HD72526-2 from Eunice Kennedy Shriver National Institute of Child Health & Human Development.
Nonstandard Abbreviations and Acronyms
- ApoA-I
Apolipoprotein A
- CE
Cholesteyll ester
- CETP
Cholesteryl ester transfer protein
- Cre
Cre recombinase
- Dio1
Type I deiodinase gene
- D1KO
Type I Deiodinase knockout mice
- EMSA
Electrophoretic mobility shifted assay
- HDLC
High density lipoprotein cholesterol
- InsR
insulin receptor
- LIRKO
Liver-specific insulin receptor knockout mice
- T3
3,5,3′-triiodothyronine
- TREs
Thyroid hormone response elements
Footnotes
Disclosures: The authors have filed a patent through Columbia University based on some of the work included in this manuscript.
References
- 1.Gofman JW, Young W, Tandy R. Ischemic heart disease, atherosclerosis, and longevity. Circulation. 1966;34:679–697. doi: 10.1161/01.cir.34.4.679. [DOI] [PubMed] [Google Scholar]
- 2.Gordon T, Kannel WB, Castelli WP, Dawber TR. Lipoproteins, cardiovascular disease, and death. The Framingham study. Arch Intern Med. 1981;141:1128–1131. [PubMed] [Google Scholar]
- 3.Investigators A-H. Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 4.Landray MJ, Haynes R, Armitage J. Niacin for reduction of cardiovascular risk. N Engl J Med. 2014;371:1943–1944. doi: 10.1056/NEJMc1411240. [DOI] [PubMed] [Google Scholar]
- 5.Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, Faas FH, Linares E, Schaefer EJ, Schectman G, Wilt TJ, Wittes J. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med. 1999;341:410–418. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- 6.Group AS, Ginsberg HN, Elam MB, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz GG, Olsson AG, Abt M, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–2099. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 9.Lamon-Fava S, Diffenderfer MR, Barrett PH, Buchsbaum A, Nyaku M, Horvath KV, Asztalos BF, Otokozawa S, Ai M, Matthan NR, Lichtenstein AH, Dolnikowski GG, Schaefer EJ. Extended-release niacin alters the metabolism of plasma apolipoprotein (Apo) A-I and ApoB-containing lipoproteins. Arterioscler Thromb Vasc Biol. 2008;28:1672–1678. doi: 10.1161/ATVBAHA.108.164541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubin EM, Krauss RM, Spangler EA, Verstuyft JG, Clift SM. Inhibition of early atherogenesis in transgenic mice by human apolipoprotein AI. Nature. 1991;353:265–267. doi: 10.1038/353265a0. [DOI] [PubMed] [Google Scholar]
- 11.Plump AS, Scott CJ, Breslow JL. Human apolipoprotein A-I gene expression increases high density lipoprotein and suppresses atherosclerosis in the apolipoprotein E-deficient mouse. Proc Natl Acad Sci U S A. 1994;91:9607–9611. doi: 10.1073/pnas.91.20.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paszty C, Maeda N, Verstuyft J, Rubin EM. Apolipoprotein AI transgene corrects apolipoprotein E deficiency-induced atherosclerosis in mice. J Clin Invest. 1994;94:899–903. doi: 10.1172/JCI117412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tangirala RK, Tsukamoto K, Chun SH, Usher D, Pure E, Rader DJ. Regression of atherosclerosis induced by liver-directed gene transfer of apolipoprotein A-I in mice. Circulation. 1999;100:1816–1822. doi: 10.1161/01.cir.100.17.1816. [DOI] [PubMed] [Google Scholar]
- 14.Borthwick F, Warnakula S, Mangat R, Uwiera RR, Russell JC, Kelly SE, Lee CY, Hryshko L, Mamo JC, Rye KA, Lopaschuk GD, Proctor SD. ApoA-1 infusion reduces arterial cholesterol and myocardial lesions in a rat model of cardiac dysfunction and insulin resistance. Atherosclerosis. 2012;222:402–408. doi: 10.1016/j.atherosclerosis.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Tardy C, Goffinet M, Boubekeur N, Ackermann R, Sy G, Bluteau A, Cholez G, Keyserling C, Lalwani N, Paolini JF, Dasseux JL, Barbaras R, Baron R. CER-001, a HDL-mimetic, stimulates the reverse lipid transport and atherosclerosis regression in high cholesterol diet-fed LDL-receptor deficient mice. Atherosclerosis. 2014;232:110–118. doi: 10.1016/j.atherosclerosis.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 16.Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, Eaton GM, Lauer MA, Sheldon WS, Grines CL, Halpern S, Crowe T, Blankenship JC, Kerensky R. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 2003;290:2292–2300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 17.Tardif JC, Gregoire J, L’Allier PL, Ibrahim R, Lesperance J, Heinonen TM, Kouz S, Berry C, Basser R, Lavoie MA, Guertin MC, Rodes-Cabau J Effect of r HDLoA-S, Efficacy I. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. JAMA. 2007;297:1675–1682. doi: 10.1001/jama.297.15.jpc70004. [DOI] [PubMed] [Google Scholar]
- 18.Waksman R, Torguson R, Kent KM, Pichard AD, Suddath WO, Satler LF, Martin BD, Perlman TJ, Maltais JA, Weissman NJ, Fitzgerald PJ, Brewer HB., Jr A first-in-man, randomized, placebo-controlled study to evaluate the safety and feasibility of autologous delipidated high-density lipoprotein plasma infusions in patients with acute coronary syndrome. J Am Coll Cardiol. 2010;55:2727–2735. doi: 10.1016/j.jacc.2009.12.067. [DOI] [PubMed] [Google Scholar]
- 19.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 20.Chahil TJ, Ginsberg HN. Diabetic dyslipidemia. Endocrinol Metab Clin North Am. 2006;35:491–510. vii–viii. doi: 10.1016/j.ecl.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Biddinger SB, Hernandez-Ono A, Rask-Madsen C, Haas JT, Aleman JO, Suzuki R, Scapa EF, Agarwal C, Carey MC, Stephanopoulos G, Cohen DE, King GL, Ginsberg HN, Kahn CR. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab. 2008;7:125–134. doi: 10.1016/j.cmet.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong XC, Copps KD, Guo S, Li Y, Kollipara R, DePinho RA, White MF. Inactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Cell Metab. 2008;8:65–76. doi: 10.1016/j.cmet.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneider MJ, Fiering SN, Thai B, Wu SY, St Germain E, Parlow AF, St Germain DL, Galton VA. Targeted disruption of the type 1 selenodeiodinase gene (Dio1) results in marked changes in thyroid hormone economy in mice. Endocrinology. 2006;147:580–589. doi: 10.1210/en.2005-0739. [DOI] [PubMed] [Google Scholar]
- 24.Galton VA, Schneider MJ, Clark AS, St Germain DL. Life without thyroxine to 3,5,3′-triiodothyronine conversion: studies in mice devoid of the 5′-deiodinases. Endocrinology. 2009;150:2957–2963. doi: 10.1210/en.2008-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galton VA, de Waard E, Parlow AF, St Germain DL, Hernandez A. Life without the iodothyronine deiodinases. Endocrinology. 2014;155:4081–4087. doi: 10.1210/en.2014-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strobl W, Chan L, Patsch W. Differential regulation of hepatic apolipoprotein A-I and A-II gene expression by thyroid hormone in rat liver. Atherosclerosis. 1992;97:161–170. doi: 10.1016/0021-9150(92)90129-5. [DOI] [PubMed] [Google Scholar]
- 27.Apostolopoulos JJ, La Scala MJ, Howlett GJ. The effect of triiodothyronine on rat apolipoprotein A-I and A-IV gene transcription. Biochem Biophys Res Commun. 1988;154:997–1002. doi: 10.1016/0006-291x(88)90238-0. [DOI] [PubMed] [Google Scholar]
- 28.Berry MJ, Grieco D, Taylor BA, Maia AL, Kieffer JD, Beamer W, Glover E, Poland A, Larsen PR. Physiological and genetic analyses of inbred mouse strains with a type I iodothyronine 5′ deiodinase deficiency. J Clin Invest. 1993;92:1517–1528. doi: 10.1172/JCI116730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vu-Dac N, Schoonjans K, Laine B, Fruchart JC, Auwerx J, Staels B. Negative regulation of the human apolipoprotein A-I promoter by fibrates can be attenuated by the interaction of the peroxisome proliferator-activated receptor with its response element. J Biol Chem. 1994;269:31012–31018. [PubMed] [Google Scholar]
- 30.Romney JS, Chan J, Carr FE, Mooradian AD, Wong NC. Identification of the thyroid hormone-responsive messenger RNA spot 11 as apolipoprotein-A1 messenger RNA and effects of the hormone on the promoter. Mol Endocrinol. 1992;6:943–950. doi: 10.1210/mend.6.6.1495493. [DOI] [PubMed] [Google Scholar]
- 31.Taylor AH, Wishart P, Lawless DE, Raymond J, Wong NC. Identification of functional positive and negative thyroid hormone-responsive elements in the rat apolipoprotein AI promoter. Biochemistry. 1996;35:8281–8288. doi: 10.1021/bi960269o. [DOI] [PubMed] [Google Scholar]
- 32.Malik S. Transcriptional regulation of the apolipoprotein AI gene. Front Biosci. 2003;8:d360–368. doi: 10.2741/1005. [DOI] [PubMed] [Google Scholar]
- 33.Ladias JA, Karathanasis SK. Regulation of the apolipoprotein AI gene by ARP-1, a novel member of the steroid receptor superfamily. Science. 1991;251:561–565. doi: 10.1126/science.1899293. [DOI] [PubMed] [Google Scholar]
- 34.Widom RL, Rhee M, Karathanasis SK. Repression by ARP-1 sensitizes apolipoprotein AI gene responsiveness to RXR alpha and retinoic acid. Mol Cell Biol. 1992;12:3380–3389. doi: 10.1128/mcb.12.8.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fuernkranz HA, Wang Y, Karathanasis SK, Mak P. Transcriptional regulation of the apoAI gene by hepatic nuclear factor 4 in yeast. Nucleic Acids Res. 1994;22:5665–5671. doi: 10.1093/nar/22.25.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan J, Nakabayashi H, Wong NC. HNF-4 increases activity of the rat Apo A1 gene. Nucleic Acids Res. 1993;21:1205–1211. doi: 10.1093/nar/21.5.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rottman JN, Widom RL, Nadal-Ginard B, Mahdavi V, Karathanasis SK. A retinoic acid-responsive element in the apolipoprotein AI gene distinguishes between two different retinoic acid response pathways. Mol Cell Biol. 1991;11:3814–3820. doi: 10.1128/mcb.11.7.3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harnish DC, Malik S, Kilbourne E, Costa R, Karathanasis SK. Control of apolipoprotein AI gene expression through synergistic interactions between hepatocyte nuclear factors 3 and 4. J Biol Chem. 1996;271:13621–13628. doi: 10.1074/jbc.271.23.13621. [DOI] [PubMed] [Google Scholar]
- 39.Berthou L, Duverger N, Emmanuel F, Langouet S, Auwerx J, Guillouzo A, Fruchart JC, Rubin E, Denefle P, Staels B, Branellec D. Opposite regulation of human versus mouse apolipoprotein A-I by fibrates in human apolipoprotein A-I transgenic mice. J Clin Invest. 1996;97:2408–2416. doi: 10.1172/JCI118687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lam JK, Matsubara S, Mihara K, Zheng XL, Mooradian AD, Wong NC. Insulin induction of apolipoprotein AI, role of Sp1. Biochemistry. 2003;42:2680–2690. doi: 10.1021/bi026984h. [DOI] [PubMed] [Google Scholar]
- 41.Claudel T, Sturm E, Duez H, Torra IP, Sirvent A, Kosykh V, Fruchart JC, Dallongeville J, Hum DW, Kuipers F, Staels B. Bile acid-activated nuclear receptor FXR suppresses apolipoprotein A-I transcription via a negative FXR response element. J Clin Invest. 2002;109:961–971. doi: 10.1172/JCI14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harnish DC, Malik S, Karathanasis SK. Activation of apolipoprotein AI gene transcription by the liver-enriched factor HNF-3. J Biol Chem. 1994;269:28220–28226. [PubMed] [Google Scholar]
- 43.Elshourbagy NA, Boguski MS, Liao WS, Jefferson LS, Gordon JI, Taylor JM. Expression of rat apolipoprotein A-IV and A-I genes: mRNA induction during development and in response to glucocorticoids and insulin. Proc Natl Acad Sci U S A. 1985;82:8242–8246. doi: 10.1073/pnas.82.23.8242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levy E, Sinnett D, Thibault L, Nguyen TD, Delvin E, Menard D. Insulin modulation of newly synthesized apolipoproteins B-100 and B-48 in human fetal intestine: gene expression and mRNA editing are not involved. FEBS Lett. 1996;393:253–258. doi: 10.1016/0014-5793(96)00896-4. [DOI] [PubMed] [Google Scholar]
- 45.Haas MJ, Pun K, Reinacher D, Wong NC, Mooradian AD. Effects of ketoacidosis on rat apolipoprotein A1 gene expression: a link with acidosis but not with ketones. J Mol Endocrinol. 2000;25:129–139. doi: 10.1677/jme.0.0250129. [DOI] [PubMed] [Google Scholar]
- 46.Koenig RJ. Regulation of type 1 iodothyronine deiodinase in health and disease. Thyroid. 2005;15:835–840. doi: 10.1089/thy.2005.15.835. [DOI] [PubMed] [Google Scholar]
- 47.Toyoda N, Zavacki AM, Maia AL, Harney JW, Larsen PR. A novel retinoid X receptor-independent thyroid hormone response element is present in the human type 1 deiodinase gene. Mol Cell Biol. 1995;15:5100–5112. doi: 10.1128/mcb.15.9.5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang CY, Kim S, Harney JW, Larsen PR. Further characterization of thyroid hormone response elements in the human type 1 iodothyronine deiodinase gene. Endocrinology. 1998;139:1156–1163. doi: 10.1210/endo.139.3.5849. [DOI] [PubMed] [Google Scholar]
- 49.Maia AL, Kieffer JD, Harney JW, Larsen PR. Effect of 3,5,3′-Triiodothyronine (T3) administration on dio1 gene expression and T3 metabolism in normal and type 1 deiodinase-deficient mice. Endocrinology. 1995;136:4842–4849. doi: 10.1210/endo.136.11.7588215. [DOI] [PubMed] [Google Scholar]
- 50.Kanamoto N, Tagami T, Ueda-Sakane Y, Sone M, Miura M, Yasoda A, Tamura N, Arai H, Nakao K. Forkhead box A1 (FOXA1) and A2 (FOXA2) oppositely regulate human type 1 iodothyronine deiodinase gene in liver. Endocrinology. 2012;153:492–500. doi: 10.1210/en.2011-1310. [DOI] [PubMed] [Google Scholar]
- 51.Tabata S, Nishikawa M, Toyoda N, Yonemoto T, Ogawa Y, Inada M. Effect of triiodothyronine administration on reduced expression of type 1 iodothyronine deiodinase messenger ribonucleic acid in streptozotocin-induced diabetic rats. Endocr J. 1999;46:367–374. doi: 10.1507/endocrj.46.367. [DOI] [PubMed] [Google Scholar]
- 52.Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000;6:87–97. [PubMed] [Google Scholar]
- 53.Apostolopoulos JJ, Howlett GJ, Fidge N. Effects of dietary cholesterol and hypothyroidism on rat apolipoprotein mRNA metabolism. J Lipid Res. 1987;28:642–648. [PubMed] [Google Scholar]
- 54.Davidson NO, Carlos RC, Drewek MJ, Parmer TG. Apolipoprotein gene expression in the rat is regulated in a tissue-specific manner by thyroid hormone. J Lipid Res. 1988;29:1511–1522. [PubMed] [Google Scholar]
- 55.Hargrove GM, Junco A, Wong NC. Hormonal regulation of apolipoprotein AI. J Mol Endocrinol. 1999;22:103–111. doi: 10.1677/jme.0.0220103. [DOI] [PubMed] [Google Scholar]
- 56.Go MF, Schonfeld G, Pfleger B, Cole TG, Sussman NL, Alpers DH. Regulation of intestinal and hepatic apoprotein synthesis after chronic fat and cholesterol feeding. J Clin Invest. 1988;81:1615–1620. doi: 10.1172/JCI113496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheng SY, Leonard JL, Davis PJ. Molecular aspects of thyroid hormone actions. Endocr Rev. 2010;31:139–170. doi: 10.1210/er.2009-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Christoffolete MA, Arrojo e Drigo R, Gazoni F, Tente SM, Goncalves V, Amorim BS, Larsen PR, Bianco AC, Zavacki AM. Mice with impaired extrathyroidal thyroxine to 3,5,3′-triiodothyronine conversion maintain normal serum 3,5,3′-triiodothyronine concentrations. Endocrinology. 2007;148:954–960. doi: 10.1210/en.2006-1042. [DOI] [PubMed] [Google Scholar]
- 59.Papazafiri P, Ogami K, Ramji DP, Nicosia A, Monaci P, Cladaras C, Zannis VI. Promoter elements and factors involved in hepatic transcription of the human ApoA-I gene positive and negative regulators bind to overlapping sites. J Biol Chem. 1991;266:5790–5797. [PubMed] [Google Scholar]
- 60.Novak EM, Bydlowski SP. NFY transcription factor binds to regulatory element AIC and transactivates the human apolipoprotein A-I promoter in HEPG2 cells. Biochem Biophys Res Commun. 1997;231:140–143. doi: 10.1006/bbrc.1997.6056. [DOI] [PubMed] [Google Scholar]
- 61.Oleaga C, Ciudad CJ, Izquierdo-Pulido M, Noe V. Cocoa flavanol metabolites activate HNF-3beta, Sp1, and NFY-mediated transcription of apolipoprotein AI in human cells. Mol Nutr Food Res. 2013;57:986–995. doi: 10.1002/mnfr.201200507. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.