Abstract
Here we analyze gray matter indices before and after completing a challenging adaptive cognitive training program based on the n-back task. The considered gray matter indices were cortical thickness (CT) and cortical surface area (CSA). Twenty-eight young women (age range 17–22 years) completed 24 training sessions over the course of 3 months (12 weeks, 24 sessions), showing expected performance improvements. CT and CSA values for the training group were compared with those of a matched control group. Statistical analyses were computed using a ROI framework defined by brain areas distinguished by their genetic underpinning. The interaction between group and time was analyzed. Middle temporal, ventral frontal, inferior parietal cortices, and pars opercularis were the regions where the training group showed conservation of gray matter with respect to the control group. These regions support working memory, resistance to interference, and inhibition. Furthermore, an interaction with baseline intelligence differences showed that the expected decreasing trend at the biological level for individuals showing relatively low intelligence levels at baseline was attenuated by the completed training.
Keywords: Cognitive training, Brain plasticity, Surface-based morphometry, Cortical thickness, Cortical surface area
Introduction
There has been a recent constructive discussion regarding the robustness of reported findings related to structural changes in the brain after training (Thomas and Baker 2013a, b; Draganski and Kherif 2013; Erickson 2013; Fields 2013). Limitations of experimental design, statistical methods, and methodological artifacts have been noted. The overwhelming majority of published studies have applied voxel-based approaches, which involves looking for changes in regional gray matter volumes in response to training (Bueti et al. 2012; Draganski et al. 2004, 2006; Kwok et al. 2011; Landi et al. 2011; Lustig et al. 2009; Takeuchi et al. 2010; Thomas et al. 2009; Woollett and Maguire 2011). However, it is acknowledged that these volumes combine two relatively independent cortical measures, namely, cortical thickness (CT) and cortical surface area (CSA) (Burgaleta et al. 2014; Colom et al. 2013a; Panizzon et al. 2009; Román et al. 2014; Vuoksimaa et al. 2015). Surface-Based Morphometry (SBM) provides measures of CSA and CT across the human neocortex. The number of radial columns perpendicular to the pial surface defines cortical surface area and it is thought to represent functional units. The horizontal layers in the cortical columns define cortical thickness and it is thought to organize cortical connectivity (Chance et al. 2008; la Fougère et al. 2011; Lyttelton et al. 2009; Rakic 1988; Thompson et al. 2007).
To our knowledge, there are only three published studies that have applied SBM to studying gray matter changes in the neocortex after training. Haier et al. (2009) compared a group that played Tetris (a well-known commercial complex visuospatial game), focusing their analyses on cortical thickness. The training group played an online version of the game for 3 months (1.5 h per week on average). The findings revealed changes in temporal and frontal regions in the training group. Engvig et al. (2010) trained a group of individuals during 8 weeks on a memory task (MoL, visualization mnemonic technique) for improving serial verbal recollection memory, and similarly, they focused on changes in cortical thickness. Finally, Colom et al. (2012) compared a group that played with Professor Layton and The Pandora Box (by Nintendo)—a cognitively complex commercial videogame—with a control group. The training was completed across 4 weeks (4 h per week). This study failed to find statistically significant changes in cortical thickness or cortical surface area after completing the game.
In the present study, a group that completed a demanding adaptive working memory program based on an n-back task is compared with a matched control group (Colom et al. 2013b reported the behavioral results for these groups). The training program has been employed extensively in previous research. The recent meta-analysis by Au et al. (2014) concluded that cognitive training programs based on the n-back task has a positive impact on fluid intelligence (d = 0.24: 3.6 IQ points). Here we consider 12 regions of interest (ROIs) defined by brain areas distinguished by their genetic substrate (Chen et al. 2012, 2013); genetic influences over CT or CSA were maximally similar within ROIs and maximally different between ROIs. The regions defined for CT and CSA are shown in Fig. 1. To our knowledge this is the first study focused on the analysis of the structural changes, isolating CT and CSA, after n-back training.
Fig. 1.
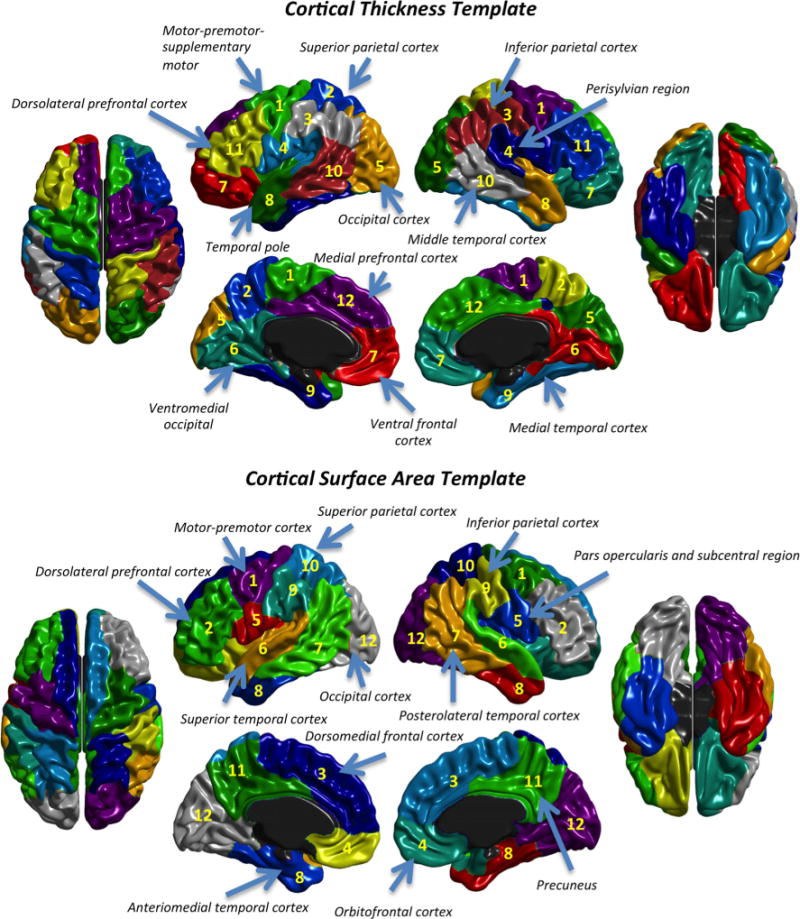
Genetic template for cortical thickness (top) and cortical surface area (bottom)
The cerebral cortex is a highly organized and complex structure that is divided into anatomically distinct and functionally specialized regions (Chen et al. 2013). However, there is no definitive division because available neuroimaging software is based on different brain parcellations (e.g., FreeSurfer, BrainSuite, IBASPM, ANIMAL, etc.). Chen et al.’s (2012, 2013) studies applied a data-driven approach for generating a genetically based parcellation for CSA and CT. As a result, they found an anterior-posterior division for CSA, along with a dorsal–ventral division for CT. The genetic patterning of both gray matter indices corresponds to functional specializations and their genetic contributions show very small correlations. Further, principles underlying genetically defined regions are different for CSA and CT: the regions/clusters for CSA show large genetic proximity within the same brain lobe, whereas regions for CT show remarkable genetic relatedness regarding maturational timing (primary vs. association cortex). Therefore, CT and CSA show differential neuro developmental mechanisms. Our study focuses on the analysis of which brain regions, under remarkable genetic control, are sensitive to the completed challenging cognitive training. Also, published reports have massively analyzed volumetric changes (as noted above) and it is known that volume combines CSA and CT, which, as described above, are genetically unrelated. Therefore, the separate analysis of these two gray matter indices would be highly informative.
The main prediction regarding potential gray matter responsiveness after training nominates anterior frontal, parietal, and middle temporal regions. We expect increases or preservation/conservation in these regions since (a) performance in the n-back task is related to intelligence and working memory (Colom et al. 2013b), and parietal and anterior frontal regions are relevant for intelligence (Jung and Haier 2007) and for working memory (Burgess et al. 2011; Colom et al. 2013a); (b) cortical thickness preservation in anterior frontal and temporal areas has been found in previous training research focusing on visuospatial skills (Haier et al. 2009); and (c) they are known to be involved in information integration and evaluation cognitive processes, which are relevant for working memory (Buschman et al. 2011, Hampson et al. 2006; Rottschy et al. 2012; Zou et al. 2013). Furthermore, we expect cortical thinning for the control group, since CT shows a spontaneous decrease with age (Wierenga et al. 2014; Zhou et al. 2013). For CSA, changes during brain development have been less studied and results are less clear (see Burgaleta et al. 2014; Wierenga et al. 2014; Zhou et al. 2015). Thus, we are unable to provide a specific hypothesis for this latter index. Note, however, that there are studies suggesting that developmental changes in CT and CSA are modulated by baseline differences in intelligence (Burgaleta et al. 2014; Schnack et al. 2015; Shaw et al. 2006), and, therefore, the interaction between brain changes and intelligence differences will be analyzed.
Method
Participants
One hundred and sixty-nine university undergraduates completed a cognitive battery comprising 12 tasks, which assess fluid-abstract intelligence (Gf), crystallized-verbal intelligence (Gc), working memory capacity (WMC), and attention control (ATT) (see Supplementary 1 for further details).1 Afterwards, 56 young women (mean age 18.29 years, SD = 1.09; age range 17–22) were selected to represent a wide range of general intelligence scores, and divided into two groups (training and control), matched by these scores. All participants were right-handed, as evaluated by the Edinburgh Test (Oldfield 1971). They were paid for their participation: 200€ for the training group and 100 € for the control group. Participants also completed a set of questions regarding medical or psychiatric disorders, as well as substance intake. The recruitment process followed the Helsinki guidelines (World Medical Association 2008) and the local ethics committee approved the study.
Basic design
Prior to recruitment, the participants completed the first psychological assessment. One hundred and sixty-nine students completed assessment sessions tapping fluid-abstract intelligence (Gf), crystallized-verbal intelligence (Gc), working memory capacity (WMC), and attention control (ATT). (Specific details about the tests/tasks administered can be found in the first supplementary document). Next, we recruited 56 of those participants and they were MRI-scanned. Afterwards, the training group completed the training program based on the n-back task over the course of 3 months (see Cognitive training section below for further details). Finally, participants were scanned again and they also completed the second psychological assessment. The interval between MRI scans was, on average, 116 days (SD = 9 days; range 88–133).
Cognitive training (n-back)
The training group (N = 28) completed the cognitive program based on the dual n-back task (Jaeggi et al. 2008). However, we began with eight sessions using the single n-back task, both in visual and auditory versions. The completed program lasted for 12 weeks and 24 sessions (approximately 30 min per session). The first four sessions were devoted to the visual modality, and the subsequent four sessions to the auditory modality. Afterwards, participants completed 16 dual sessions (visual + auditory). (See Supplementary 2 for details regarding these tasks.) All sessions were completed within individual cabins under strict supervision in the laboratory. Data were checked every week and participants received systematic feedback about their performance. The control group did not complete any training and simply came in for pre- and post-testing at the same interval as the experimental group.
MRI acquisition
Images were acquired in a General Electric Signa 3T MR Scanner (General Electric Healthcare, Farfield, CT) using a whole-body radiofrequency (RF) coil for signal excitation and quadrature 8-channel coil for reception. For the structural images analyzed here, a high-resolution 3D T1-weighted Gradient Echo-SPGR was applied, with parameters: TE = 4.1 ms, TR = 9.1 ms, TI = 450 ms, flip angle = 10°, 170 sagittal slices, acquisition matrix = 256 mm × 256 mm, isotropic voxel size = 1 mm3.
Image preprocessing
First, scan pairs for the same participants were carefully inspected. A warping distortion between the pre-test and post-test scans was observed, namely, a stretch expansion in the temporal lobe area and the opposite in the parietal area. This distortion was due to (a) the use of high field strength in the scanner and (b) the offset of iso-center in the longitudinal scans. Note that we corrected all the images to have equivalent 3D distortion correction in order to reduce the differences in residual distortion due to differences in participant positioning. These corrections were required as we expect subtle changes and any distortion might engulf the signal of interest. Therefore, recommended corrections were applied using a tool called “Grinder.” This tool corrects for geometric distortions due to uneven field strength within the scanner. The applied image pre-processing steps for this correction involved Grinder + N3 + bias correction from SPM5 unified segmentation, which is similar to the protocol applied in Alzheimer’s Disease Neuroimaging Initiative and has been validated using phantoms (Jack et al. 2008). These steps were successful with the exception of two participants from each group (Nfinal = 52).
Surface-Based Morphometry
The corrected MRIs were processed through the CIVET pipeline (version 2.0) (Ad-Dab’bagh et al. 2006; Kim et al. 2005). CIVET implements a surface-based technique for estimating cortical thickness (CT) and cortical surface area (CSA). Specific stages for the analyses are as follows: (1) linear registration (12-parameter) to MNI-Talairach (ICBM152) space; (2) images corrected for radio-frequency non-uniformities and a brain mask computed; (3) tissue classification into white matter (WM), gray matter (GM), and cerebrospinal fluid (CSF); (4) generation of high-resolution hemispheric surfaces with 163,842 vertices each; (5) registration of surfaces to a high-resolution template; (6) cortical thickness is computed by evaluating the distance, in mm, between the original WM and GM surfaces transformed back to the native space of the original MR images, then interpolated onto the surface template; (7) vertex-based areas computed directly on the resampled surfaces and measure local variations of area/volume contraction and expansion relative to the vertex distribution on the surface template; (8) smoothing using a 30-mm kernel for CT and 40-mm for CSA.
Statistical analysis
Statistical analyses were computed using SurfStat (http://www.math.mcgill.ca/keith/surfstat/), which was created for MATLAB 7 (The Math-Works, Inc.). Descriptive maps (means and standard deviations) for CT and CSA were computed at the vertex level. As noted above, we relied on the genetic templates provided by Chen et al. (2012, 2013) for checking potential changes in 12 brain regions for CSA (Chen et al. 2012) and 12 regions for CT (Chen et al. 2013).
The original genetic templates (Chen et al. 2012, 2013) were built with the “pial templates” of FreeSurfer 4.5. Each vertex on the FreeSurfer template was associated with one ROI using numerical labels (from 1 to 12). FreeSurfer templates and MNI ICBM 152 template have different coordinates, and, therefore, the first step was devoted to the translation of their respective spaces. As a result, each vertex in the MNI space was associated to one ROI following the same distribution as in the original templates. The following steps were focused on the computation of mean CT and sum of CSA within ROIs. For achieving this goal, a mask was generated for each ROI including only the vertices associated with same numerical label (same ROI). Afterwards, the mean of the CT values (or the sum of the CSA values) within vertices of each ROI were computed for each participant. These steps were done separately for each cerebral hemisphere. Therefore, our final division was based on 24 ROIs (12 per hemisphere).
With the obtained values, we computed standardized changes using the following formula: (after training – before training)/SD (before training) (Jaeggi et al. 2011). These standardized changes were submitted to analyses of covariance (ANCOVA)2 where group was the independent variable, standardized change for each ROI was the dependent variable, and the covariate was the mean for CT or sum of CSA before training for the corresponding ROI. A p level (one-tailed) was considered. Significance level (α = 0.05) was modified according to the number of comparisons and the correlations between ROIs to correct for multiple comparisons using the partial Bonferroni correction, since multiple measurements were computed (24 ROIs per index and per subject).
Finally, we checked for effects of intelligence differences measured at baseline in the regions where a statistically significant group effect was found in ANCOVA analyses. The sample was divided according to fluid intelligence scores (Gf) obtained in the first psychological assessment (baseline). The mean for the Gf factor (resulting from RAPM, DAT-AR, and PMA-R scores before training) was 102. Therefore, participants with scores ≤102 were assigned to the low intelligence group (mean score = 91.17, SD = 9.944), whereas participants with scores ≥102 were assigned to the high intelligence group (mean score = 110.79, SD = 5.912). The sample sizes were as follows: (1) high intelligence-training group (N = 14); (2) high intelligence-control group (N = 15); (3) low intelligence-training group (N = 12); (4) low intelligence-control group (N = 11). Repeated ANOVAs measuring 2 × 2 × 2 (Time × Group × Intelligence group) were computed to study the effect of intelligence. Group (Training and Control) and Intelligence group (Low and High) were between-factor, while pre-test and post-test gray matter indices were within-factor. Results for the triple interaction are reported here. Post-hoc analyses showed significant pre-test vs. post-test changes for all groups: (1) high intelligence-training group, (2) high intelligence-control group, (3) low intelligence-training group and (4) low intelligence-control group.
Results
Behavioral results
This is a summary of results reported by Colom et al. (2013b). First, the training group showed a systematic improvement in n-back performance over time. For the visual condition the improvement was 41 %, for the auditory condition it was 39 %, and for the dual condition it was 53 %. Second, this performance systematically correlated across sessions with Gf, Gc, and WMC, but not with ATT. Finally, changes at the construct level between the pre-test and the post-test sessions were not significant. Nevertheless, post hoc analyses suggested that specific tests and tasks tapping visuospatial processing across cognitive domains were sensitive to training. Further details can be found in the Colom et al. (2013b) report.
Descriptive gray matter maps
Supplementary 3 (Figs. 1, 2, 3, and 4) show the distribution (means and standard deviations) in both groups for cortical thickness (CT) and cortical surface area (CSA) at each vertex before and after training. The maps were largely similar for both groups at the two time points for CT; the region showing the highest mean thickness (>5 mm) was the insula. Maps for CSA were also highly alike; mean surface area was highest for parietal, frontal and temporal regions.
Fig. 2.
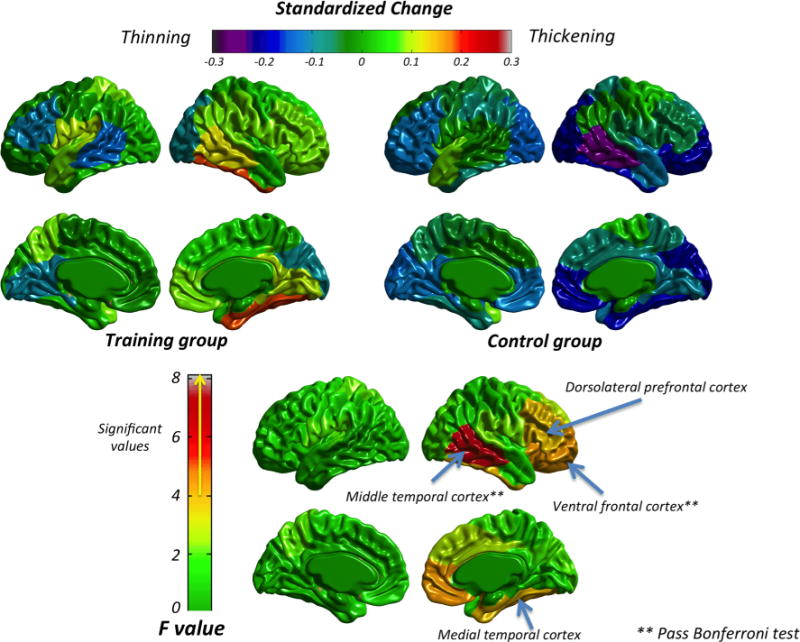
Standardized Change for the training and control groups (top panel) and ANCOVA results (bottom panel) for cortical thickness
Fig. 3.
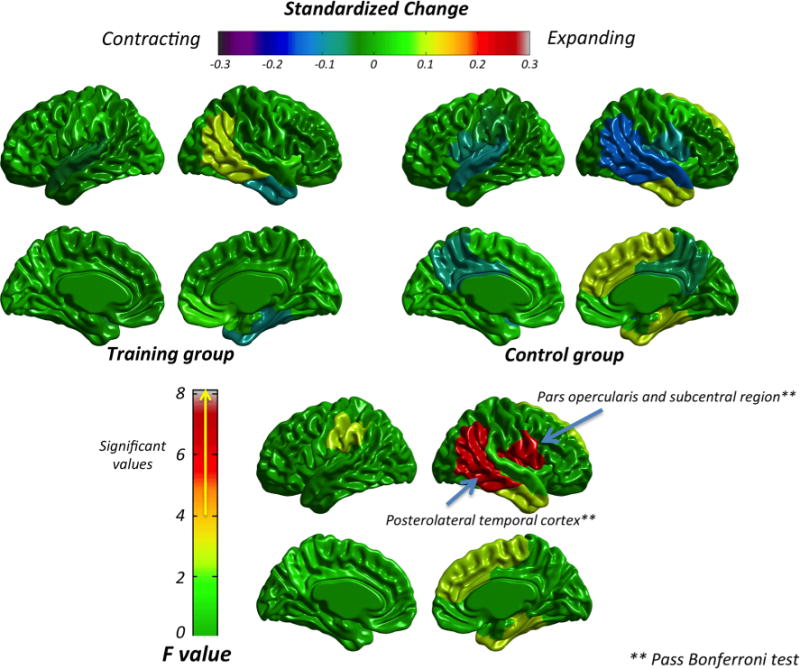
Standardized Change for the training and control group (top panel) and ANCOVA results (bottom panel) in cortical surface area
Fig. 4.
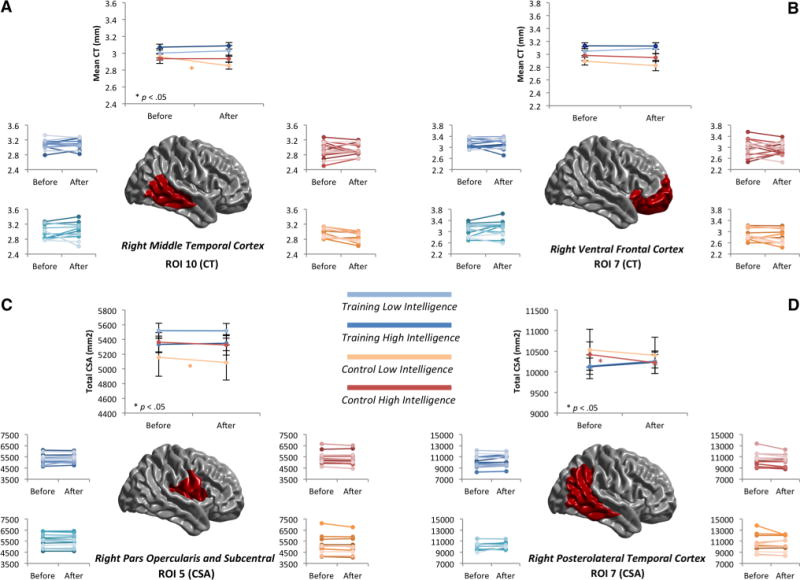
a Interaction analysis (Group × Intelligence × ROI): ROI 10–Cortical Thickness. Top panel shows the mean change in each group. Bottom panel shows the changes for each participant. Colors show different groups: dark blue (high intelligence-training group), light blue (low intelligence-training group), red (high intelligence-control group) and orange (low intelligence-control group). b Interaction analysis (Group × Intelligence × ROI): ROI 7–Cortical Thickness. Top panel shows the mean changes in each group. Bottom panel shows the changes for each participant. Colors show different groups: dark blue (high intelligence-training group), light blue (low intelligence-training group), red (high intelligence control group) and orange (low intelligence-control group). c Interaction analysis (Group × Intelligence × ROI): ROI 5–Cortical Surface Area. Top panel shows the mean changes in each group. Bottom panel shows the changes for each participant. Colors show different groups: dark blue (high intelligence training group), blue (low intelligence training group), red (high intelligence control group) and orange (low intelligence control group). d Interaction analysis (Group × Intelligence × ROI): ROI 7–Cortical Surface Area. Top panel shows the mean changes in each group. Bottom panel shows the changes for each participant. Colors show different groups: dark blue (high intelligence training group), blue (low intelligence training group), red (high intelligence control group) and orange (low intelligence control group)
Regions of interest (ROIs)
As noted above, we selected regions defined by two templates based on the genetic underlying organization (Chen et al. 2012, 2013). Mean CT and sum of CSA values were computed at each region for the training and the control groups before and after training. Afterwards, we computed the standardized change (Jaeggi et al. 2011) for each ROI.
ROIs for cortical thickness
Figure 2 (top panel) shows the standardized change for cortical thickness in the training (left) and control (right) groups. The control group was characterized by a generalized thinning, whereas the training group hardly showed any change (minor thinning in the left middle temporal, frontal and occipital areas, along with minor thickening in the right temporal region were rare exceptions). The descriptive statistics are shown in Supplementary Table 1.
The bottom panel of Fig. 2 shows the ANCOVA results (F value); the specific F value and the associated effect are shown in Supplementary Table 1. Values greater than 4 were statistically significant. Regions within the right hemisphere showing significant results included the ventral frontal cortex, medial and middle temporal cortex, and dorsolateral prefrontal cortex. However, only the ventral frontal and middle temporal cortex survived the Bonferroni correction (p < 0.02, for r = 0.75, number of comparisons = 24). All significant regions had a moderate effect size ( ; Cohen, 1992). Standardized changes in significant regions indicated thinning in the control group (−0.05 to −0.25) and thickening in the training group (0.08–0.19).
ROIs for cortical surface area
Figure 3 (top panel) shows the standardized change for cortical surface area in the training (left) and control (right) groups (Supplementary Table 2 shows the descriptive results for each group and each ROI). Changes were generally absent for both groups. However, a small contracting effect was found for the control group in the right superior and posterolateral temporal cortex, along with a small expanding effect in the right dorsomedial frontal and anteromedial temporal cortex. For the training group, only a small expanding effect was found in the posterolateral temporal cortex.
ANCOVA results (see bottom panel of Fig. 3 and Supplementary Table 2) showed significant values in the right posterolateral temporal cortex and right pars opercularis cortex. Both regions passed Bonferroni correction (p < 0.04, for r = 0.98, number of comparisons = 24) and effect sizes were moderate ( ; Cohen 1992). The control group showed a contracting effect of −0.15 (right posterolateral temporal), whereas the training group showed an expanding effect of 0.10 in the same region. For the right pars opercularis, the changes revealed a contracting effect in the control group (−0.09) and a small expanding effect (0.01) in the training group.
Group × Intelligence × ROI
The control and training groups were divided according to their baseline levels in fluid intelligence (Gf), as described above, and the main findings (Fig. 4) were as follows:
Right middle temporal cortex (ROI 10–CT)
Figure 4a depicts the changes for cortical thickness in ROI 10. The top panel shows the mean for each group. The high intelligence training group is represented in dark blue and the low intelligence training group is represented in light blue. The high-intelligence control group is represented in red and the low intelligence-control group is represented in orange. The bottom panel shows the changes for each participant (same color code).
Only the low intelligence-control group showed remarkable changes indicating a thinning process (p < 0.05). Cortical thickness was preserved/conserved in the high intelligence groups, as well as in the low intelligence-training group.
Right ventral frontal cortex (ROI 7–CT)
Figure 4b shows changes for cortical thickness in ROI 7.
Results showed thinning in the two control groups and preservation/conservation in the two training groups. Nevertheless, the thinning process was only marginally significant (p < 0.10) for low intelligence participants in the control group.
Right pars opercularis and subcentral region (ROI 5–CSA)
Figure 4c depicts results for CSA in ROI 5. Cortical surface preservation/conservation was found in the two training groups and in the high intelligence-control group, while a significant contracting effect (p < 0.05) was found for the low intelligence-control group.
Right posterolateral temporal cortex (ROI 7–CSA)
Figure 4d displays results for CSA in ROI 7. An expanding effect was found for the training groups, whereas a contracting effect was found for the control groups. However, this latter effect was significant in the high intelligence-control group only.
Discussion
In the current study we have analyzed gray matter changes after completing a challenging adaptive cognitive training requiring working memory related skills. The measures were obtained from cortical thickness (CT) and cortical surface area (CSA) indices computed after applying Surface-Based Morphometry (SBM). The analyses were calculated for a set of brain regions distinguished by their genetic underpinning (Chen et al. 2012, 2013). The interaction between group (training vs. control) and time (before vs. after training) was analyzed, as recommended (Thomas and Baker 2013a, b). We hypothesized that anterior frontal, parietal and middle temporal regions would show changes after cognitive training (Burgess et al. 2011; Buschman et al. 2011; Colom et al. 2007, Hampson et al. 2006; Rottschy et al. 2012; Zou et al. 2013). The main findings are discussed below in successive sections: cortical thickness; cortical surface area; and interactions between baseline intelligence differences, group, and biological indices.
Changes in cortical thickness (CT)
Regarding CT, thinning in the control group was observed (Fig. 2). These results are consistent with the findings reported in brain maturation studies (Shaw et al. 2006; Zhou et al. 2015). For instance, Zhou et al. (2015) scanned children (6–10 years old), adolescents (10–20 years old) and adults (20–32 years old) at different time points. They found that participants scanned during adolescence showed a generalized decrease (92 %) or no changes (8 %) in their CT for the whole brain. This result was obtained for all age groups, although the thinning process was more pronounced during adolescence. This thinning process is usually attributed to synaptic pruning (Huttenlocher and Dabholkar 1997), ongoing myelination, and dendritic arborization (Paus 2010; Chklovskii et al. 2004; Sur and Rubenstein 2005; Thompson et al. 2007).
In general, the training group did not show any CT changes, although small variations were observed at some ROIs: (a) minor thickening in the right middle and medial temporal and left pars opercularis; (b) minor thinning in left middle temporal, dorsolateral prefrontal frontal and ventromedial cortex, and right occipital cortex. This preservation/thickening for the training group might be a response to training, which is consistent with previous research (Engvig et al. 2010; Haier et al. 2009).
The comparison between the training and control groups was significant in ventral frontal, dorsolateral prefrontal, middle, and medial temporal ROIs (Fig. 2), although only differences in ventral frontal and middle temporal survived the Bonferroni correction. Parietal regions did not show any appreciable difference between groups. Differences between groups were moderate regarding the observed effect size (Cohen 1992). Also, regions where the training group showed a preservation/thickening with respect to the control group have been associated with working memory processes; the meta-analysis by Duncan and Owen (2000) revealed that regions in the frontal lobe relevant for working memory are the dorsolateral prefrontal cortex, inferior ventral frontal cortex, and dorsal anterior cingulate cortex. Specifically, the right ventral frontal cortex has been related to the inhibition of responses required to cancel an intended movement (Aron et al. 2004), while it has been suggested that the right dorsolateral prefrontal cortex connects working memory with complex reasoning (Prabhakaran et al. 2011). There are studies suggesting that the right temporal cortex acts as an interface between the dorsal and ventral streams for visual processing, allowing the exploration of both object-related and space-related information (Karnath 2001). Moreover, as is the case with the right inferior ventral cortex, the middle temporal lobe is relevant for interference resolution (Kirwan and Stark 2007; Yassa et al. 2011, Yassa and Stark 2011), which was particularly taxed during n-back training.
Changes in cortical surface area (CSA)
With respect to cortical surface area, changes in both groups were subtler than for cortical thickness (Fig. 3). These findings are consistent with studies showing less significant and less steep changes across development for CSA (Østby et al. 2009; Raznahan et al. 2011) than those for CT. Indeed, the control group showed mixed results with small expanding trends in right dorsomedial frontal and anteromedial temporal cortex, along with small contracting trends in right temporal (superior and middle), right pars opercularis, left precuneus, and superior temporal cortex. Some studies suggest that changes in cortical surface area have a cubic relationship with age (Wierenga et al. 2014); there seems to be a contracting effect for CSA in 18–20 year olds. However, Zhou et al. (2015) reported generalized expanding (71 %) between the period ranging from 10 to 20 years of age, although they did not divide the brain into different regions for studying CSA development.
Note that changes in cortical surface area might not show the same trajectory across regions. In this respect, Burgaleta et al. (2014) found a contraction of CSA associated with age in right occipital and temporal regions, in contrast to an expansion of CSA in bilateral dorsomedial prefrontal cortex, left ventromedial frontal, and left motor cortex. Our results for the control group are consistent to some degree with Burgaleta et al. (2014), since we found a contraction of CSA in the right temporal area, and an expansion of CSA in right dorsomedial frontal cortex. Changes in the training group for CSA were absent, except for a small surface expansion in right middle temporal cortex.
The development in CSA may be related to changes in gyrification (Wierenga et al. 2014). Nevertheless, studies about these developmental changes in gyrification are disparate, since there are studies showing increased folding with the age (Blanton et al. 2001), while others consider that sulcal architecture takes place mostly at birth, because neurogenesis and neural migration are almost complete after gestation (Hill et al. 2010). There might be indirect processes accounting for changes in cortical surface area (Burgaleta et al. 2014), such as the mechanistic pressures exerted by the size and complexity of the dendritic arbors (Hill et al. 2010), the size of intracortical elements, or the volume of white matter adjacent to a given gyrus or sulcus (Feczko et al. 2009).
Differences in CSA changes between groups were found in right pars opercularis and right posterolateral temporal cortex with a moderate effect size. The posterolateral temporal region can be considered as belonging to the middle temporal and to the inferior parietal regions (Fig. 4d). As noted above, the middle temporal lobe supports working memory processes (e.g. Hampson, et al. 2006; Zou et al. 2013), and interference resolution (Kirwan and Stark 2007; Yassa et al. 2011, Yassa and Stark 2011). Moreover, inferior parietal cortex is considered one of the most important regions supporting intelligence and high-level processing (Jung and Haier 2007; Vendetti and Bunge 2014). Finally, the pars opercularis is associated with inhibition process in go-no go tasks (Forstmann et al. 2008).
Changes in cortical thickness and cortical surface area: summary of findings
Note that the reported developmental changes must be considered as tentative, since the time gap between scans is rather short (4 months). Regions where the training group showed preservation with respect to the control group included right ventral frontal cortex (CT), right pars opercularis (CSA), right middle temporal cortex (CT and CSA), and one small region in the right inferior parietal region (Fig. 5).
Fig. 5.
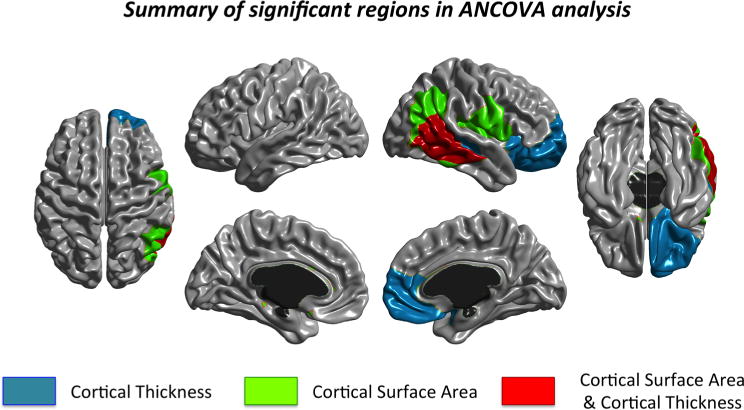
Summary of significant regions passing Bonferroni in the ANCOVA analyses
Interestingly, most of the statistically significant regions were located in lateral regions. Parcellations for CT and CSA were similar in the lateral view, but differed in the medial view. Lateral parcellations are more related to structural and functional regions (Chen et al. 2013). Also, note that statistically significant regions were primarily located in temporal and frontal regions, which are regions that, despite their lack of spatial contiguity, have similar genetic influences (Rimol et al. 2010). In fact, the cross-regional genetic patterning for cortical thickness may report to main fiber tract structures (e.g., thalamocortical or intracortical connections) (Chen et al. 2013; see also Zilles and Amunts 2012).
The highlighted regions (see Fig. 5) are known to support working memory, interference resolution, inhibition, and reasoning. These abilities and skills are thought to contribute to n-back performance (Jaeggi et al. 2010). Changes in the right middle temporal lobe were observed for both CT and CSA (Fig. 5). This suggests that the training regime might have evoked changes in the temporal lobe (Hsu et al. 2013; Zhang et al. 2014). Note that the appreciated behavioral changes are congruent with this suggestion. Thus, for instance, the training group showed significant improvements in the Simon task after training. Specifically, the training group produced similar reaction times in compatible and incompatible trials (Colom et al. 2013b).
Of note is that all the significant standardized changes reported here were located in the right hemisphere. It is known that young people are more lateralized than older people (HAROLD model; Cabeza 2002). In this regard, the right hemisphere might be more involved in spatial than in verbal working memory tasks (Reuter-Lorenz et al. 2000). The behavioral results for this sample, extensively discussed elsewhere (Colom et al. 2013b), revealed transfer effects in measures with a substantial spatial component across cognitive domains. This latter finding is consistent with the neuroimaging results reported here.
Interaction with intelligence baseline levels
As noted in the introduction section, there are reports showing that age changes in cortical indices (CT and CSA) interact with baseline intelligence differences (Shaw et al. 2006; Schnack et al. 2015). In this regard, Schnack et al. (2015) reported greater CT changes (the direction of these changes were dependent upon age group; see below) in people with higher intelligence scores. Contrary to this finding, Burgaleta et al. (2014) reported CT preservation over time in youngsters showing increased intelligence scores, and CT reduction over time in those showing decreased intelligence scores.
Schnack et al. (2015) showed that changes in CT for people with high intelligence have different stages: (a) pronounced cortical thinning for the left hemisphere in childhood (until 10 years old); (b) a weakened association between intelligence and thinning until adulthood (10–21 years old); (c) a thickening process (after 21 years old). However, changes in the right hemisphere are not associated with intelligence until approximately 47 years of age. These results are consistent with findings reported by Shaw et al. (2006) in childhood, but contrary to the results found by Karama et al. (2014), who showed a positive association between CT and intelligence in childhood (11 years of age) and in adulthood (70 years of age). This latter result is in tension with the thinning process attributed to people with higher intelligence scores in the Shaw et al. (2006) and Schnack et al. (2015) reports.
The findings observed in the present study are highly consistent with Burgaleta et al. (2014) and Karama et al. (2014), since the thinning found in the control group was circumscribed to participants with low intelligence at baseline (Fig. 4a, b). The training group revealed remarkable gray matter preservation, irrespective of the participants’ baseline intelligence level. We suggest that the completed training helps to compensate for low baseline intelligence levels (Draganski et al. 2004; Haier et al. 2009; Lerch et al. 2011; Fu and Zuo 2011). Practicing a challenging cognitive task might interact with spontaneous dendritic and spine elimination processes (Hensch 2004; Knudsen, 2004). Relatedly, it has been shown that dendritic and spine rearrangement and elimination occurring during childhood and adolescence may be related to social and educational interactions (Petanjek et al. 2008).
Lastly, the interaction between intelligence and cortical surface area is much less clear. Schnack et al. (2015) found that individuals with higher intelligence scores showed less pronounced surface expansion in both hemispheres during childhood and adolescence. A more pronounced surface contraction was found in adulthood. The results observed here for the control group are consistent with Schnack et al. (2015) to some extent. Participants with low intelligence scores at baseline showed CSA contracting effects (Fig. 4c, d), while people with high intelligence scores showed CSA preservation in pars opercularis and a high contracting process in the temporal lobe. These results serve as further examples of the complex nature of CSA development (Wierenga et al. 2014). Note that CSA results for the training group followed the same pattern as that observed for CT: preservation was appreciated regardless of baseline intelligence. Burgaleta et al. (2014) found that changes in CSA were not related to changes in intelligence. Note, however, that these researchers studied spontaneous changes, while here we have analyzed changes as a function of short-term cognitive training.
It warrants speculation that high intelligence individuals may tend to seek out challenging experiences/situations. For example, Jaeggi et al. (2014) found that people with a high need for cognition are most likely to sign up (and complete) the training intervention. These kinds of behaviors may help to prevent spontaneous declines in crucial brain indices, which, in turn, might delay associated cognitive declines that can interfere with everyday capability and independence (Kirkwood et al. 2008; see Deary et al. 2010 for a review addressing the relationship between intelligence differences and health outcomes). Nevertheless, further studies are required in this regard.
Conclusion
In conclusion, here we have reported changes in brain indices, quantified by cortical thickness (CT) and cortical surface area (CSA), after a challenging adaptive cognitive training based on the n-back task. The reported changes were primarily focused in temporal and frontal regions known to support cognitive processes required for successful performance in the completed training regime. The observed results seem consistent with the transfer effects at the task level reported by Colom et al. (2013b), namely, a positive effect of the completed training over performance in visuospatial tests and tasks across cognitive domains. Furthermore, the effect of the completed training was more beneficial for low intelligence individuals; the reported gray matter preservation was equivalent for high intelligence individuals in both the training and control groups. We suggest that people with high intelligence seek out challenging experiences/situations, which prevents this decline in the analyzed brain indices.
Supplementary Material
Acknowledgments
This research was supported by Grant PSI2010-20364 (Ministerio de Ciencia e Innovación, Spain). FJR is supported by BES-2011-043527 (Ministerio de Ciencia e Innovación, Spain). KM is supported by AP2008-00433 (Ministerio de Educación, Spain). MB was funded by the Spanish Ministerio de Economía y Competitividad (MINECO-FPDI-2013-17528). Also, WSK was supported by Grants R01 AG022381, AG018386A, and AG018384 (U.S. National Institute on Aging).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00429-015-1168-7) contains supplementary material, which is available to authorized users.
Colom et al. (2013b) reported the behavioral results for this same sample.
Analyses were also computed with repeated ANOVA 2 × 2 (Time × Group) and the results found were similar to those obtained after ANCOVA analyses.
References
- Ad-Dab’bagh Y, Lyttelton O, Muehlboeck JS, Lepage C, Einarson D, Mok K, Ivanov O, Vincent RD, Lerch J, Fombonne E, Evans AC. In: Corbetta M, editor. The CIVET image processing environment: A fully automated comprehensive pipeline for anatomical neuroimaging research; Proceedings of the 12th Annual Meeting of the Organization for Human Brain Mapping NeuroImage; Florence, Italy. 2006. [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in cognitive sciences. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Au J, Sheehan E, Tsai N, Duncan GJ, Buschkuehl M, Jaeggi SM. Improving fluid intelligence with training on working memory: a meta-analysis. Psychonomic bulletin and review. 2014:1–12. doi: 10.3758/s13423-014-0699-x. [DOI] [PubMed] [Google Scholar]
- Blanton RE, Levitt JG, Thompson PM, Narr KL, Capetillo-Cunliffe L, Nobel A, Toga AW. Mapping cortical asymmetry and complexity patterns in normal children. Psychiatry Res Neuroimag. 2001;107:29–43. doi: 10.1016/s0925-4927(01)00091-9. [DOI] [PubMed] [Google Scholar]
- Bueti D, Lasaponara S, Cercignani M, Macaluso E. Learning about time: plastic changes and interindividual brain differences. Neuron. 2012;75:725–737. doi: 10.1016/j.neuron.2012.07.019. [DOI] [PubMed] [Google Scholar]
- Burgaleta M, Johnson W, Waber DP, Colom R, Karama S. Cognitive ability changes and dynamics of cortical thickness development in healthy children and adolescents. NeuroImage. 2014;84:810–819. doi: 10.1016/j.neuroimage.2013.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess GC, Gray JR, Conway AR, Braver TS. Neural mechanisms of interference control underlie the relationship between fluid intelligence and working memory span. J Exp Psychol Gen. 2011;140:674–692. doi: 10.1037/a0024695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschman TJ, Siegel M, Roy JE, Miller EK. Neural substrates of cognitive capacity limitations. Proc Natl Acad Sci. 2011;108:11252–11255. doi: 10.1073/pnas.1104666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Chance SA, Casanova MF, Switala AE, Crow TJ. Auditory cortex asymmetry, altered minicolumn spacing and absence of ageing effects in schizophrenia. Brain. 2008;131:3178–3192. doi: 10.1093/brain/awn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Gutierrez ED, Thompson W, Panizzon MS, Jernigan TL, Eyler LT, Dale AM. Hierarchical genetic organization of human cortical surface area. Science. 2012;335:1634–1636. doi: 10.1126/science.1215330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Fiecas M, Gutiérrez ED, Panizzon MS, Eyler LT, Vuoksimaa E, Kremen WS. Genetic topography of brain morphology. Proc Natl Acad Sci. 2013;110:17089–17094. doi: 10.1073/pnas.1308091110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chklovskii DB, Mel BW, Svoboda K. Cortical rewiring and information storage. Nature. 2004;431:782–788. doi: 10.1038/nature03012. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Colom R, Jung RE, Haier RJ. General intelligence and memory span: evidence for a common neuroanatomic framework. Cognit Neuropsychol. 2007;24:867–878. doi: 10.1080/02643290701781557. [DOI] [PubMed] [Google Scholar]
- Colom R, Quiroga M, Solana AB, Burgaleta M, Román FJ, Privado J, Karama S. Structural changes after videogame practice related to a brain network associated with intelligence. Intelligence. 2012;40:479–489. [Google Scholar]
- Colom R, Burgaleta M, Román FJ, Karama S, Álvarez-Linera J, Abad FJ, Haier RJ. Neuroanatomic overlap between intelligence and cognitive factors: morphometry methods provide support for the key role of the frontal lobes. Neuroimage. 2013a;72:143–152. doi: 10.1016/j.neuroimage.2013.01.032. [DOI] [PubMed] [Google Scholar]
- Colom R, Román FJ, Abad FJ, Shih PC, Privado J, Froufe M, Jaeggi SM. Adaptive n-back training does not improve fluid intelligence at the construct level: Gains on individual tests suggest that training may enhance visuospatial processing. Intelligence. 2013b;41:712–727. [Google Scholar]
- Deary IJ, Weiss A, Batty GD. Intelligence and personality as predictors of illness and death how researchers in differential psychology and chronic disease epidemiology are collaborating to understand and address health inequalities. Psychol Sci Publ Interest. 2010;11:53–79. doi: 10.1177/1529100610387081. [DOI] [PubMed] [Google Scholar]
- Draganski B, Kherif F. In vivo assessment of use-dependent brain plasticity—beyond the “one trick pony” imaging strategy. NeuroImage. 2013;73:255–259. doi: 10.1016/j.neuroimage.2012.08.058. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Kempermann G, Kuhn HG, Winkler J, Büchel C, May A. Temporal and spatial dynamics of brain structure changes during extensive learning. J Neurosci. 2006;26(23):6314–6317. doi: 10.1523/JNEUROSCI.4628-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Engvig A, Fjell AM, Westlye LT, Moberget T, Sundseth Ø, Larsen VA, Walhovd KB. Effects of memory training on cortical thickness in the elderly. Neuroimage. 2010;52:1667–1676. doi: 10.1016/j.neuroimage.2010.05.041. [DOI] [PubMed] [Google Scholar]
- Erickson KI. Evidence for structural plasticity in humans: comment on Thomas and Baker (2012) NeuroImage. 2013;73:237–238. doi: 10.1016/j.neuroimage.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Feczko E, Augustinack JC, Fischl B, Dickerson BC. An MRI-based method for measuring volume, thickness and surface area of entorhinal, perirhinal, and posterior parahippocampal cortex. Neurobiol Aging. 2009;30:420–431. doi: 10.1016/j.neurobiolaging.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD. Changes in brain structure during learning: fact or artifact? Reply to Thomas and Baker. NeuroImage. 2013;73:260–264. doi: 10.1016/j.neuroimage.2012.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstmann B, van den Wildenberg W, Ridderinkhof K. Neural mechanisms, temporal dynamics, and individual differences in interference control. J Cogn Neurosci. 2008;20:1854–1865. doi: 10.1162/jocn.2008.20122. [DOI] [PubMed] [Google Scholar]
- Fu M, Zuo Y. Experience-dependent structural plasticity in the cortex. Trends Neurosci. 2011;34:177–187. doi: 10.1016/j.tins.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haier RJ, Karama S, Leyba L, Jung RE. MRI assessment of cortical thickness and functional activity changes in adolescent girls following three months of practice on a visual-spatial task. BMC Res Notes. 2009;2:174. doi: 10.1186/1756-0500-2-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. J Neurosci. 2006;26:13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK. Critical period regulation. Ann Rev Neurosci. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- Hill J, Inder T, Neil J, Dierker D, Harwell J, Van Essen D. Similar patterns of cortical expansion during human development and evolution. Proc Natl Acad Sci. 2010;107:13135–13140. doi: 10.1073/pnas.1001229107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu NS, Buschkuehl M, Jonides J, Jaeggi SM. Potential Mechanisms Underlying Working Memory Training and Transfer; Poster presented at the Psychonomic Society Annual Meeting; Toronto, Ontario. 2013. [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Jack CR, Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, Weiner MW. The Alzheimer’s disease neuroimaging initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27:685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Jonides J, Perrig WJ. Improving fluid intelligence with training on working memory. Proc Natl Acad Sci. 2008;105:6829–6833. doi: 10.1073/pnas.0801268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Perrig WJ, Meier B. The concurrent validity of the N-back task as a working memory measure. Memory. 2010;18:394–412. doi: 10.1080/09658211003702171. [DOI] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Jonides J, Shah P. Short-and long-term benefits of cognitive training. Proc Natl Acad Sci. 2011;108:10081–10086. doi: 10.1073/pnas.1103228108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Shah P, Jonides J. The role of individual differences in cognitive training and transfer. Memory Cognition. 2014;42:464–480. doi: 10.3758/s13421-013-0364-z. [DOI] [PubMed] [Google Scholar]
- Jung RE, Haier RJ. The Parieto-frontal integration theory (P-FIT) of intelligence: converging neuroimaging evidence. Behav Brain Sci. 2007;30:135–154. doi: 10.1017/S0140525X07001185. [DOI] [PubMed] [Google Scholar]
- Karama S, Bastin ME, Murray C, Royle NA, Penke L, Maniega SM, Deary IJ. Childhood cognitive ability accounts for associations between cognitive ability and brain cortical thickness in old age. Molecular psychiatry. 2014;19:555–559. doi: 10.1038/mp.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnath HO. New insights into the functions of the superior temporal cortex. Nat Rev Neurosci. 2001;2:568–576. doi: 10.1038/35086057. [DOI] [PubMed] [Google Scholar]
- Kim JS, Singh V, Lee JK, Lerch J, Ad-Dab’bagh Y, MacDonald D, Evans AC. Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. Neuroimage. 2005;27:210–221. doi: 10.1016/j.neuroimage.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Kirkwood T, Bond J, May C, McKeith I, Teh M. Mental capital through life: Future challenges. The Government Office for Science; London: 2008. Foresight mental capital and wellbeing project. [Google Scholar]
- Kirwan CB, Stark CE. Overcoming interference: An fMRI investigation of pattern separation in the medial temporal lobe. Learn Memory. 2007;14:625–633. doi: 10.1101/lm.663507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen E. Sensitive periods in the development of the brain and behavior. Cognit Neurosci J. 2004;16:1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- Kwok V, Niu Z, Kay P, Zhou K, Mo L, Jin Z, Tan LH. Learning new color names produces rapid increase in gray matter in the intact adult human cortex. Proc Natl Acad Sci. 2011;108:6686–6688. doi: 10.1073/pnas.1103217108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- la Fougère C, Grant S, Kostikov A, Schirrmacher R, Gravel P, Schipper HM, Thiel A. Where in vivo imaging meets cytoarchitectonics: The relationship between cortical thickness and neuronal density measured with high-resolution [18F] flumazenil-PET. Neuroimage. 2011;56:951–960. doi: 10.1016/j.neuroimage.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Landi SM, Baguear F, Della-Maggiore V. One week of motor adaptation induces structural changes in primary motor cortex that predict long-term memory 1 year later. J Neurosci. 2011;31:11808–11813. doi: 10.1523/JNEUROSCI.2253-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch JP, Yiu AP, Martinez-Canabal A, Pekar T, Bohbot VD, Frankland PW, Sled JG. Maze training in mice induces MRI-detectable brain shape changes specific to the type of learning. Neuroimage. 2011;54:2086–2095. doi: 10.1016/j.neuroimage.2010.09.086. [DOI] [PubMed] [Google Scholar]
- Lustig C, Shah P, Seidler R, Reuter-Lorenz PA. Aging, training, and the brain: a review and future directions. Neuropsychol Rev. 2009;19:504–522. doi: 10.1007/s11065-009-9119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyttelton OC, Karama S, Ad-Dab’bagh Y, Zatorre RJ, Carbonell F, Worsley K, Evans AC. Positional and surface area asymmetry of the human cerebral cortex. Neuroimage. 2009;46:895–903. doi: 10.1016/j.neuroimage.2009.03.063. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Østby Y, Tamnes CK, Fjell AM, Westlye LT, Due-Tønnessen P, Walhovd KB. Heterogeneity in subcortical brain development: a structural magnetic resonance imaging study of brain maturation from 8 to 30 years. J Neurosci. 2009;29:11772–11782. doi: 10.1523/JNEUROSCI.1242-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, Kremen WS. Distinct genetic influences on cortical surface area and cortical thickness. Cerebral Cortex. 2009:bhp026. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Growth of white matter in the adolescent brain: myelin or axon? Brain Cogn. 2010;72:26–35. doi: 10.1016/j.bandc.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Petanjek Z, Judaš M, Kostović I, Uylings HB. Lifespan alterations of basal dendritic trees of pyramidal neurons in the human prefrontal cortex: a layer-specific pattern. Cereb Cortex. 2008;18:915–929. doi: 10.1093/cercor/bhm124. [DOI] [PubMed] [Google Scholar]
- Prabhakaran V, Rypma B, Narayanan NS, Meier TB, Austin BP, Nair VA, Gabrieli JD. Capacity-speed relationships in prefrontal cortex. PLoS One. 2011;6:e27504. doi: 10.1371/journal.pone.0027504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;24:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Shaw P, Lalonde F, Stockman M, Wallace GL, Greenstein D, Giedd JN. How does your cortex grow? J Neurosci. 2011;31:7174–7177. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, Koeppe RA. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J Cogn Neurosci. 2000;12:174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- Rimol LM, Panizzon MS, Fennema-Notestine C, Eyler LT, Fischl B, Franz CE, Dale AM. Cortical thickness is influenced by regionally specific genetic factors. Biol Psychiatry. 2010;67:493–499. doi: 10.1016/j.biopsych.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Román FJ, Abad FJ, Escorial S, Burgaleta M, Martínez K, Álvarez-Linera J, Colom R. Reversed hierarchy in the brain for general and specific cognitive abilities: A morphometric analysis. Hum Brain Mapp. 2014;35:3805–3818. doi: 10.1002/hbm.22438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottschy C, Langner R, Dogan I, Reetz K, Laird AR, Schulz JB, Eickhoff SB. Modelling neural correlates of working memory: a coordinate-based meta-analysis. Neuroimage. 2012;60:830–846. doi: 10.1016/j.neuroimage.2011.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnack HG, van Haren NEM, Brouwer RM, Evans A, Durston S, Hulshoff Pol HH. Changes in thickness and surface area of the human cortex and their relationship with intelligence. Cereb Cortex. 2015;25:1608–1617. doi: 10.1093/cercor/bht357. [DOI] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Giedd J. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Sur M, Rubenstein JL. Patterning and plasticity of the cerebral cortex. Science. 2005;310:805–810. doi: 10.1126/science.1112070. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Sekiguchi A, Taki Y, Yokoyama S, Yomogida Y, Komuro N, Kawashima R. Training of working memory impacts structural connectivity. J Neurosci. 2010;30:3297–3303. doi: 10.1523/JNEUROSCI.4611-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Baker CI. Teaching an adult brain new tricks: a critical review of evidence for training-dependent structural plasticity in humans. Neuroimage. 2013a;73:225–236. doi: 10.1016/j.neuroimage.2012.03.069. [DOI] [PubMed] [Google Scholar]
- Thomas C, Baker CI. On evidence, biases and confounding factors: Response to commentaries. NeuroImage. 2013b;73:265–267. doi: 10.1016/j.neuroimage.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AG, Marrett S, Saad ZS, Ruff DA, Martin A, Bandettini PA. Functional but not structural changes associated with learning: an exploration of longitudinal voxel-based morphometry (VBM) Neuroimage. 2009;48:117–125. doi: 10.1016/j.neuroimage.2009.05.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Dutton RA, Chiang MC, Leow AD, Sowell ER, Toga AW. Tracking Alzheimer’s disease. Ann N Y Acad Sci. 2007;1097:183–214. doi: 10.1196/annals.1379.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendetti MS, Bunge SA. Evolutionary and developmental changes in the lateral frontoparietal network: a little goes a long way for higher-level cognition. Neuron. 2014;84:906–917. doi: 10.1016/j.neuron.2014.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuoksimaa E, Panizzon MS, Chen CH, Fiecas M, Eyler LT, Fennema-Notestine C, Kremen WS. The genetic association between neocortical volume and general cognitive ability is driven by global surface area rather than thickness. Cereb Cortex. 2015;25:2127–2137. doi: 10.1093/cercor/bhu018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga LM, Langen M, Oranje B, Durston S. Unique developmental trajectories of cortical thickness and surface area. NeuroImage. 2014;87:120–126. doi: 10.1016/j.neuroimage.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Woollett K, Maguire EA. Acquiring “the Knowledge” of London’s layout drives structural brain changes. Curr Biol. 2011;21:2109–2114. doi: 10.1016/j.cub.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Medical Association. Declaration of Helsinki—ethical principles for medical research involving human subjects. 59th WMA General Assembly; Seoul, Korea: 2008. [Google Scholar]
- Yassa MA, Stark CE. Pattern separation in the hippocampus. Trends Neurosci. 2011;34:515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Mattfeld AT, Stark SM, Stark CE. Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. Proc Natl Acad Sci. 2011;108:8873–8878. doi: 10.1073/pnas.1101567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Buschkuehl M, Bernat E, Jaeggi SM. EEG power changes as a function of working memory training; Poster presented at the 26th Annual Convention of the Association for Psychological Science; San Francisco. 2014. [Google Scholar]
- Zhou D, Lebel C, Evans A, Beaulieu C. Cortical thickness asymmetry from childhood to older adulthood. NeuroImage. 2013;83:66–74. doi: 10.1016/j.neuroimage.2013.06.073. [DOI] [PubMed] [Google Scholar]
- Zhou D, Lebel C, Treit S, Evans A, Beaulieu C. Accelerated longitudinal cortical thinning in adolescence. NeuroImage. 2015;104:138–145. doi: 10.1016/j.neuroimage.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Zilles K, Amunts K. Segregation and wiring in the brain. Science. 2012;335:1582–1584. doi: 10.1126/science.1221366. [DOI] [PubMed] [Google Scholar]
- Zou Q, Ross TJ, Gu H, Geng X, Zuo XN, Hong LE, Yang Y. Intrinsic resting-state activity predicts working memory brain activation and behavioral performance. Hum Brain Mapp. 2013;34:3204–3215. doi: 10.1002/hbm.22136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


