Abstract
Objective
This study’s objective is to differentiate possible ADHD syndromes on the basis of symptom trajectories, prognosis, and associated clinical features in a high-risk cohort.
Method
Latent class analysis of inattentive (IA) and hyperactive–impulsive (HI) symptoms in 387 non-disabled members of a regional low birthweight/preterm birth cohort who were evaluated for ADHD at 6, 9, and 16 years. Adolescent functional outcomes and other clinical features were examined across the classes.
Results
Three latent classes were identified: unaffected (modest IA and HI symptom prevalences at six, remitting by nine), school age limited (relatively high IA and HI symptom prevalences at six and nine, declining by 16), and persistent inattentive (high IA and HI prevalences at six and nine, with high IA levels persisting to 16). The persistent inattentive class was distinctively associated with poor functioning, motor problems, other psychiatric disorders, and social difficulties as indexed by a positive screen for autism spectrum disorder at 16.
Conclusion
These findings differentiate a potential persistent inattentive syndrome relevant to ADHD evaluation and treatment.
Keywords: ADHD, ADHD subtypes, epidemiology, longitudinal study, persistence
Introduction
ADHD, as defined in the Diagnostic and Statistical Manual of Mental Disorders (5th ed.; DSM-5; American Psychiatric Association [APA], 2013), is a neurodevelopmental disorder composed of inattentive (IA) and hyperactive–impulsive (HI) symptoms. Typically first diagnosed in childhood, ADHD increases risk by adulthood for poor academic achievement (Polderman, Boomsma, Bartels, Verhulst, & Huizink, 2010; Washbrook, Propper, & Sayal, 2013), long-term occupational disability (Fredriksen et al., 2014), and a wide range of adverse physical and mental health outcomes (Balazs, Miklosi, Kereszteny, Dallos, & Gadoros, 2014; C. M. Jensen & Steinhausen, 2015; Johnson & Wolke, 2013; Ljung, Chen, Lichtenstein, & Larsson, 2014; Spencer, Faraone, Tarko, McDermott, & Biederman, 2014). In 2011, the prevalence of current ADHD among 4- to 17-year-olds in the United States was 8.8%, of whom 69% were taking medication for the disorder (Visser et al., 2014). This prevalence rate, however, hides the considerable clinical heterogeneity that exists among children and adolescents with ADHD. Given the potential long-term benefits and risks of ADHD medications (Chen et al., 2014; Hammerness et al., 2013; Molina et al., 2007; Ramos Olazagasti et al., 2013), it is important that research on potential interventions be mindful of this heterogeneity, perhaps focusing primarily on the group(s) of children at greatest risk of persistent symptoms and poor functional outcomes. Unfortunately, the widely used Diagnostic and Statistical Manual of Mental Disorders (4th ed.; DSM-IV; APA, 1994) ADHD subtypes, based on presenting profiles of IA and HI symptoms, have proved too unstable for identifying these groups (Willcutt et al., 2012). Reframing the DSM-IV ADHD subtypes (Predominantly IA, Predominantly HI, and Combined; APA, 2000) as “presentations” in DSM-5 (APA, 2013) acknowledges their instability but does not improve their predictive validity.
An approach to classification that incorporates the natural history of ADHD symptoms may better serve clinical practice and research than the present DSM approach based on current symptoms. Incorporating the natural history of ADHD symptoms has the potential for allowing the delineation of true syndromes as traditionally defined in medicine—that is, a set of symptoms that has a distinctive natural history and prognosis and is associated with a distinctive set of clinical features and risk factors (Guze, 1970; Rutter, 1983; Shaffer & Greenhill, 1979).
Prior research has found that ADHD symptoms decline in number between school age and adolescence (Faraone, Biederman, & Mick, 2006) with the decline being more rapid for HI than for IA symptoms. By adolescence, IA symptoms predominate in both clinical (Biederman, Mick, & Faraone, 2000; Hinshaw, Owens, Sami, & Fargeon, 2006; Lahey & Willcutt, 2010; Pingault et al., 2011) and population-based samples (Larsson, Dilshad, Lichtenstein, & Barker, 2011). These findings, however, use symptom counts that likely mask considerable heterogeneity in longitudinal patterns of IA and HI symptoms experienced across childhood and adolescence. The present study represents an advance over these prior studies in that it looks to differentiate longitudinal profiles of IA and HI symptoms and examines the association of these profiles with functional outcomes and other clinical features. This represents a first step toward differentiating ADHD syndromes.
The data used in this article come from a regional low birthweight/preterm (LBW/PT) birth cohort (Pinto-Martin et al., 1992) followed through mid-adolescence (Whitaker et al., 2011). It is one of very few studies to have collected longitudinal data on DSM IA and HI symptoms as well as on functional outcomes and other neurodevelopmental and psychiatric characteristics. A LBW/PT cohort is advantageous for studying ADHD symptoms (particularly IA) because their prevalence is elevated in such cohorts (Aarnoudse-Moens, Weisglas-Kuperus, van Goudoever, & Oosterlaan, 2009; Groen-Blokhuis, Middeldorp, van Beijsterveldt, & Boomsma, 2011; Hack et al., 2009; Jaekel, Wolke, & Bartmann, 2013; Johnson et al., 2010; O’Shea, Downey, & Kuban, 2013).
Method
Birth Cohort and Longitudinal Assessment
The Neonatal Brain Hemorrhage Study (NBHS) birth cohort (n = 1,105) included 90% of births <1,500 g and 85% of births <2,000 g in three New Jersey counties in 1984 to 1987. These counties were demographically representative of the nation, although somewhat more affluent (Pinto-Martin et al., 1992). The cohort was screened systematically for perinatal brain injury; a post-birth maternal interview and hospital chart abstraction provided additional prenatal, perinatal, and neonatal data (Pinto-Martin et al., 1992). Members were re-assessed at ages 2 (Pinto-Martin, Riolo, Cnaan, Holzman, & Paneth, 1995), 6 (Whitaker et al., 1996; Whitaker et al., 1997), 9 (Pinto-Martin et al., 2004), and 16 (Whitaker et al., 2011; Whitaker et al., 2006) years. Typical of longitudinal studies (Wolke et al., 2009), those lost to follow-up were of relatively lower socioeconomic status, as described in previous reports (Pinto-Martin et al., 1995; Pinto-Martin et al., 2004; Whitaker et al., 2011; Whitaker et al., 2006; Whitaker et al., 1996; Whitaker et al., 1997). This secondary analysis was approved by the New York State Psychiatric Institute Institutional Review Board.
Present Sample
The study sample (n = 387) consists of NBHS cohort members from whom DSM ADHD symptom data, using a structured diagnostic interview of parents, were obtained at all three assessment points (ages 6, 9, and 16) and who were not classified as having a major cognitive or motor disability at age 16 (IQ < 55 or untestable or unable to walk without assistance). The first supplemental table (Supplement Table 1) contrasts the study sample with two mutually exclusive comparison groups, both of which also exclude cases with major disability. The first consists of those missing one or two such ADHD assessments. The second consists of those missing all three such assessments. There were few significant differences between the groups. Members of the study sample were less likely to have had one or more social risk conditions at birth or to have engaged in heavy alcohol consumption during pregnancy, but were more likely to belong to a multiple birth.
Measures
ADHD symptoms/related impairment
ADHD symptoms were assessed using the Diagnostic Interview Schedule for Children (DISC) Parent Version (Shaffer, Fisher, Dulcan, & Schwab-Stone, 2000). The age 6 follow-up used the DISC-2.1 (Shaffer, Fisher, Piacentini, Schwab-Stone, & Wicks, 1989) based on Diagnostic and Statistical Manual of Mental Disorders (3rd ed., rev.; DSM-III-R; APA, 1987) criteria. The ages 9 and 16 follow-ups used the DISC-3.0 (Shaffer, Fisher, & Lucas, 1994) and the DISC-IV (Shaffer, Fisher, Dulcan, & Schwab-Stone, 2000), respectively, both based on DSM-IV (APA, 2000). All three versions also assessed ADHD symptom-related impairment. Here, impairment is defined as interference in at least two of three domains (family, peer, and school functioning).
Age 16 functional outcomes
Educational status was assessed with parent-report items assessing current (past year) special educational services and below-expected grade in the current academic year. Psychosocial impairment was assessed with the Columbia Impairment Scale (CIS), where a parent-report score of ≥16 defines impairment (Bird et al., 1993) and the Children’s Global Assessment Scale (CGAS) where a clinician rating of <70 defines impairment (Shaffer et al., 1983). Adaptive functioning was assessed with parent report on the Vineland Adaptive Behavior Scales (VABS) using cut points of <80 on both the Adaptive Behavior Composite Score and three subscales (Communication, Daily Living, Socialization; Sparrow, Balla, & Cicchetti, 1984). Self-reported well-being was measured with adolescent self-report on the Beck Depression Inventory using a cutoff of ≥16 (Beck, Ward, Mendelson, Mock, & Erbaugh, 1961) and with the Victim–Bully subscale of the Rigby–Slee Peer Relations Questionnaire (Rigby & Slee, 1993) using the mean score.
Other Clinical Characteristics
Neurodevelopmental features
Motor problems were assessed with the Riley Motor Problems Inventory (RMPI; Riley, 1976). Research evaluators trained on the RMPI rated the child’s performance at ages 6, 9, and 16; higher scores indicate more problems. To make the number of motor problems considered excessive the same across the three ages, a single cut point (≥9 problems) was applied at each age (Riley, 1976). General intellectual functioning was measured using standardized assessments of general cognitive ability (IQ) at 6 (Stanford–Binet [SBIV]; Thorndike, Hagen, & Sattler, 1986), 9 (Wechsler Intelligence Scale for Children [WISC-III]; Wechsler, 1991), and 16 (Wechsler Abbreviated Scale of Intelligence [WASI]; Wechsler, 1999), where a score greater than 2 SD below the mean indicated intellectual impairment (Thorndike et al., 1986; Wechsler, 1991, 1999). Social difficulties were indexed by a positive screen for autism spectrum disorder (ASD) defined as a parent report of a professional’s diagnosis of ASD, and/or a score on the Social Communication Questionnaire (SCQ; Berument, Rutter, Lord, Pickles, & Bailey, 1999) ≥9, and/or a score on the Autism Spectrum Screening Questionnaire (ASSQ; Ehlers, Gillberg, & Wing, 1999) ≥ 12 obtained at 16 as part of a two-stage study of ASD prevalence in this cohort (Pinto-Martin et al., 2011).
Other psychiatric disorders
The DISC, Version 2.1, assessed 19 DSM-III-R disorders at age 6. Three modules of the DISC-3.0 assessed ADHD and the disruptive disorders at age 9. The DISC-IV assessed 26 DSM-IV disorders at 16.
Analysis
Initial analyses sought to characterize classes of ADHD based on changes over time in the prevalence of both IA and HI symptoms. Latent Class Analysis (LCA) was conducted using all ADHD symptoms, measured at all three time points, as indicators. The temporal patterns of both IA and HI symptoms were assumed to be characterized by a single latent categorical variable with the number of classes determined by goodness of fit. The analysis was conducted using the statistical program Mplus (Muthen & Muthen, 1998–2007). LCA yielded predicted probabilities (for each case) of belonging to each class. Cases were assigned to their most likely class based on these probabilities.
Subsequent analyses examined the associations of the latent classes with DSM-defined ADHD presentations, indicators of functional outcomes at 16, and selected neurodevelopmental and psychiatric characteristics. The inferential statistics for these analyses involved omnibus tests of group differences in prevalence (χ2) or mean level (F) of the potentially associated features. When warranted, post hoc pairwise comparisons were conducted. The Type I error rate was set at 0.05 throughout.
Results
Latent Classes
Table 1 provides the fit statistics for the three- and four-class solutions. The Lo–Mendell–Rubin test (Lo, Mendell, & Rubin, 2001) associated with the four-class solution indicated a high probability that the same data could have been generated by three classes. Thus, the three-class solution was chosen as the best representation of the data. It is also worth noting that the four-class solution merely split one of the classes of the more parsimonious solution into severity variants.
Table 1.
Latent Class Analysis of ADHD Symptom Criteria at Ages 6, 9, and 16.
| Solution | Log likelihood | Bayesian information criterion | Entropy | Lo–Mendell–Rubin Likelihood Ratio Testa
|
|||
|---|---|---|---|---|---|---|---|
| 2 Times the log likelihood difference | p value | ||||||
| 3-class | −7915.5 | 16,701.0 | 0.917 | 561.9 | .01 | ||
| 4-class | −7,785.6 | 16,733.1 | 0.906 | 259.9 | .61 | ||
|
| |||||||
| Results of 3-class solution | Class 1 | Class 2 | Class 3 | ||||
|
| |||||||
| Based on estimated model | 67.2 (17.4%) | 149.8 (38.7%) | 170.0 (43.9%) | ||||
| Based on most likely class membership | 66 (17.1%) | 150 (38.8%) | 171 (44.2%) | ||||
Lo–Mendell–Rubin likelihood ratio test (LMR LRT) is a test of probability that the data were generated by a model with one fewer latent class.
Figure 1 graphs the ADHD symptom endorsement probabilities within each of the three latent classes. The largest class (44.2%) had a low prevalence of ADHD symptoms throughout most of childhood and adolescence. Although there were comparatively high prevalence rates for some symptoms in this group at age 6, at ages 9 and 16, ADHD symptoms were very rare. This class is labeled unaffected (UA). Another class, comprising 38.8% of the sample, had higher symptom prevalence rates at age 6 than the UA group, and these rates were sustained through age 9. At 16, however, symptom prevalence rates were much lower in this group. The decline was greatest for the HI symptoms. This class is labeled school age limited (SAL). Finally, a class comprising 17.1% of the sample had high probabilities of IA symptoms at all ages, whereas the prevalence of HI symptoms, which were high at ages 6 and 9, declined markedly by age 16. It is worth noting that the three impulsive symptoms declined less than the six hyperactive ones. This class is labeled persistent inattentive (PIA).
Figure 1.
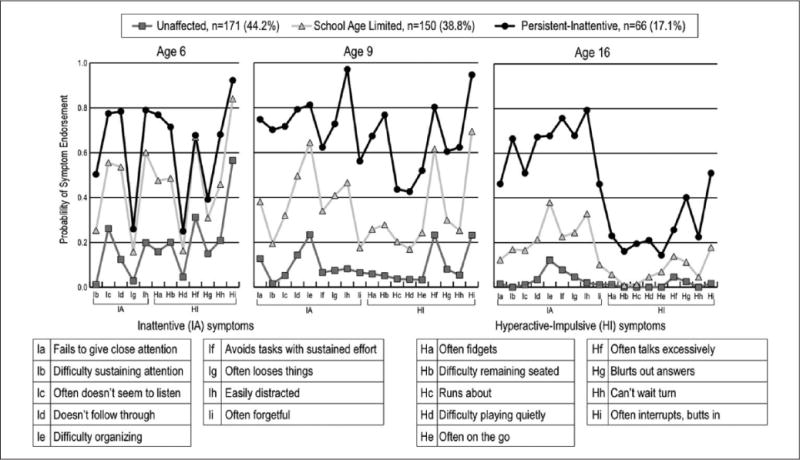
DSM-IV Inattentive and Hyperactive–Impulsive symptoms assessed at ages 6, 9, and 16.
Note. At age 6, the Diagnostic Interview Schedule for Children Version 2.1–Parent report assessed DSM-III-R symptoms of ADHD; hence at age 6, only five of the nine DSM-IV Inattentive symptoms and seven of the nine Hyperactive–Impulsive symptoms were assessed. At ages 9 and 16, Versions 3.0 and IV of the Diagnostic Interview Schedule for Children, respectively, assessed all 18 DSM-IV ADHD symptoms. DSM-IV = Diagnostic and Statistical Manual of Mental Disorders (4th ed.); DSM-III-R = Diagnostic and Statistical Manual of Mental Disorders (3rd ed., rev.).
ADHD-related impairment in the three classes over time is displayed in Table 2. The greatly elevated rates in the PIA group mirrored the consistently high symptom prevalence rates in this group. Noteworthy is that PIA impairment rates were already markedly elevated relative to the SAL group at age 6—before the large gap in symptom prevalence rates between these groups appeared; also the rates of impairment from IA symptoms, in both affected classes, were substantially higher than HI-related impairment rates. This was true even at age 9, before the prevalence rates of HI symptoms dropped.
Table 2.
ADHD Symptoms: Associated Impairment at Ages 6, 9, and 16 Years in Relation to Most Likely Latent Class.
| DISC—Derived Impairmenta | Most likely latent class
|
||||
|---|---|---|---|---|---|
| PIA % (n) | SAL % | UA % | χ2b | p value | |
| Age 6 years—Attention-deficit/hyperactivity-related | 48.5% (32/66) | 18.7% (28/150) | 1.8% (3/171) | 77.3 | <.001 |
| Age 9 years—Inattention-related | 75.8% (50/66) | 20.7% (31/150) | 1.2% (2/171) | 157.3 | <.001 |
| Age 9 years—Hyperactivity/impulsiveness-related | 48.5% (32/66) | 10.7% (16/150) | 0.0% (0/171) | 103.7 | <.001 |
| Age 16 years—Inattention-related | 72.7% (48/66) | 17.3% (26/150) | 1.2% (2/171) | 155.3 | <.001 |
| Age 16 years—Hyperactivity/impulsiveness-related | 22.7% (15/66) | 0.7 (1/150) | 0.0% (0/171) | 69.5 | <.001 |
Note. DISC = Diagnostic Interview Schedule for Children; PIA = persistent inattentive; SAL = school age limited; UA = unaffected.
Impairment is defined as having attention-deficit/hyperactivity–related difficulties in at least two domains, regardless of severity. At age 6 years, there were three possible areas of difficulty—at home, with peers, or at school; at age 9 years, there were two—home and school; at age 16 years, there were three—at home with family, with peers, or at school and/or work.
Two degrees of freedom χ2.
Adolescent Functional Outcomes by Latent Class
Table 3 shows substantial and statistically significant differences in rates of poor functional outcomes at age 16 across the three latent classes. In general, across the outcomes, the PIA class showed elevated rates relative to the SAL class, which, in turn, were significantly elevated relative to the UA class. A few exceptions are notable: The SAL class did not differ from the PIA class in the proportion below expected grade level, and the SAL class did not differ from the UA class in measures of daily living skills or depression.
Table 3.
Functional Outcomes at Age 16 Years by Three Latent Classes: Persistent Inattentive, School Age Limited and Unaffected.
| Most likely latent class % (n)
|
Total % (n) | χ2a | p value | Post hoc analysis p value
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| PIA | SAL | UA | PIA vs. SAL | SAL vs. UA | PIA vs. UA | ||||
| Educational status | |||||||||
| Any special education services? | 62.1% (41/66) | 40.7% (61/150) | 18.1% (31/171) | 34.4% (133/387) | 45.17 | <.001 | .005 | <.001 | <.001 |
| Below-expected grade? | 22.0% (13/59) | 23.7% (33/139) | 7.9% (13/164) | 16.3% (59/362) | 15.49 | <.001 | .856 | <.001 | .006 |
| Psychosocial impairment | |||||||||
| CIS ≥ 16 | 98.4% (61/62) | 82.0% (114/139) | 66.3% (106/160) | 77.8% (281/361) | 29.04 | <.001 | .001 | .002 | <.001 |
| CGAS ≤ 70 | 70.8% (46/65) | 32.6% (47/144) | 11.3% (18/160) | 30.1% (111/369) | 78.59 | <.001 | <.001 | <.001 | <.001 |
| Adaptive Functioning: Vineland Adaptive Behavior Scales | |||||||||
| Communication Standard Score < 80 | 45.3% (29/64) | 28.4% (42/148) | 14.3% (24/168) | 25.0% (95/380) | 25.27 | <.001 | .018 | .002 | <.001 |
| Daily Living Standard Score < 80 | 40.6% (26/64) | 20.1% (30/149) | 19.5% (33/169) | 23.3% (89/382) | 12.93 | .002 | .002 | 1.000 | .001 |
| Socialization Standard Score < 80 | 29.7% (19/64) | 9.3% (14/150) | 2.4% (4/169) | 9.7% (37/383) | 39.73 | <.001 | <.001 | .008 | <.001 |
| Adaptive Behavior Composite Standard Score < 80 | 46.9% (30/64) | 21.3% (32/150) | 11.2% (19/170) | 21.1% (81/384) | 35.61 | <.001 | <.001 | .015 | <.001 |
| Self-reported well-being | |||||||||
| Beck Score ≥ 16 (Depressed) | 12.1% (7/58) | 4.9% (7/144) | 3.6% (6/166) | 5.4% (20/368) | 6.129 | .046 | .121 | .778 | .025 |
| Suicidality (Beck item 9) | 17.2% (10/58) | 9.8% (14/143) | 7.2% (12/166) | 9.8% (36/367) | 4.871 | .088 | ___b | ___b | ___b |
| Victim of Bullying Scale M ± SD (n/N) | .68 ± .73 (56) | .29 ± .47 (137) | .19 ± .36 (163) | .31 ± .51 (356) | F(2, 353) = 21.43 | <.001 | <.001 | .071 | <.001 |
Note. PIA = persistent inattentive; SAL = school age limited; UA = Unaffected; CIS = Columbia Impairment Scale; CGAS = Children’s Global Assessment Scale.
Two degrees of freedom.
Post hoc unwarranted.
Other Clinical Characteristics by Latent Class
Neurodevelopmental features
At ages 6 and 9, the PIA class had the highest and the UA the lowest rate of children with excessive motor problems (Table 4), with the SAL class being intermediate. By age 16, only the PIA group retained significantly elevated rates of excessive motor problems. The PIA class screened positive for social difficulties as indexed by a positive screen for ASD at nearly twice the rate of the SAL and UA classes at age 16 and had markedly higher rates of above-threshold scores on the SCQ and ASSQ.
Table 4.
Other Clinical Characteristics by Three Latent Classes: Persistent Inattentive, School Age Limited, and Unaffected.
| Most likely latent class % (n/N)
|
Total % (n/N) | χ2a | pb value | Post hoc analysis p value
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| PIA | SAL | UA | PIA vs. SAL | SAL vs. UA | PIA vs. UA | ||||
| Neurodevelopmental features RMPI | |||||||||
| Percent scoring in the top 2% for Age 6c | |||||||||
| Age 6 years | 47.0% (31/66) | 30.0% (45/150) | 19.9% (34/171) | 28.4% (110/387) | 17.472 | <.001 | .020 | .038 | <.001 |
| Age 9 years | 65.2% (43/66) | 46.7% (70/150) | 30.0% (51/170) | 42.5% (164/386) | 25.795 | <.001 | .018 | .003 | <.001 |
| Age 16 years | 20.3% (13/64) | 8.7% (13/149) | 5.3% (9/169) | 9.2% (35/382) | 12.584 | .002 | .023 | .272 | .001 |
| IQ > Two standard deviations below M for age | |||||||||
| Stanford–Binet Age 6 years | 0.0% (0/66) | 0.7% (1/150) | 0.0% (0/171) | 0.3% (1/387) | 1.584 | .558 | ___d | ___d | ___d |
| WISC-III age 9 years | 9.4% (6/64) | 3.3% (5/150) | 0.0% (0/171) | 2.9% (11/385) | 14.948 | .001 | .090 | .021 | <.001 |
| WASI age 16 years | 9.1 6/16 (6/66) | 4.0% (6/150) | 0.0% (0/171) | 3.1% (12/387) | 13.757 | .002 | .194 | .010 | <.001 |
| Social difficulties (screening status for ASD) | |||||||||
| Screen positive for ASDe | 30.3% (20/66) | 16.0% (24/150) | 10.0% (17/170) | 15.8% (61/386) | 14.736 | .001 | .018 | .132 | <.001 |
| Age 16 years | |||||||||
| SCQ ≥ 9 | 23.7% (14/59) | 13.6% (19/140) | 7.9% (13/164) | 12.7% (46/363) | 9.957 | .007 | .095 | .134 | .003 |
| Age 16 years | |||||||||
| ASSQ ≥ 12 | 16.4% (10/61) | 3.4% (5/145) | 2.4% (4/168) | 5.1% (19/374) | 19.530 | <.001 | .002 | .038 | <.001 |
| Age 16 years | |||||||||
| Professional diagnosis of ASD (ever by age 16 years) | 6.7% (4/60) | 1.4% (2/143) | 0.6% (1/166) | 1.9% (7/369) | 9.020 | .011 | .064 | .598 | .018 |
| DSM psychiatric disorders | |||||||||
| Oppositional defiant | |||||||||
| Age 6 years | 16.7% (11/66) | 6.7% (10/150) | 1.2 (2/171) | 5.9% (23/387) | 20.688 | <.001 | .027 | .015 | <.001 |
| Age 9 years | 16.7% (11/66) | 4.7% (7/150) | 0.6% (1/171) | 4.9% (19/387) | 26.412 | <.001 | .006 | .028 | <.001 |
| Age 16 years | 27.7% (18/65) | 8.0% (12/150) | 1.8% (3/171) | 8.5% (33/386) | 40.623 | <.001 | <.001 | .014 | <.001 |
| Conduct | |||||||||
| Age 6 years | 4.6% (3/65) | 0.7% (1/150) | 0.0% (0/171) | 1.0% (4/386) | 10.110 | .008 | .084 | .467 | .020 |
| Age 9 years | 4.5% (3/66) | 2.0% (3/150) | 0.0% (0/171) | 1.6% (6/387) | 6.770 | .025 | .373 | .101 | .021 |
| Age 16 years | 10.6% (7/66) | 2.7% (4/150) | 0.6% (1/171) | 3.1% (12/387) | 16.070 | <.001 | .021 | .189 | .001 |
| Separation anxiety | |||||||||
| Age 6 years | 6.1% (4/66) | 5.3% (8/150) | 0.6% (1/171) | 3.4% (13/387) | 7.339 | .025 | 1.000 | .014 | .022 |
| Age 16 years | 7.6% (5/66) | 2.7% (4/150) | 0.0% (0/171) | 2.3% (9/387) | 12.157 | .002 | .136 | .047 | .001 |
| Social phobia | |||||||||
| Age 6 years | nef | nef | nef | nef | nef | nef | nef | nef | nef |
| Age 16 years | 15.2% (10/66) | 4.7% (7/150) | 3.5% (6/171) | 5.9% (23/387) | 12.262 | .003 | .013 | .778 | .003 |
| Major depression | |||||||||
| Age 6 years | 0.0% (0/66) | 0.7% (1/150) | 0.0% (0/171) | 0.3% (1/387) | 1.584 | .558 | ___d | ___d | ___d |
| Age 16 years | 13.6% (9/66) | 4.7% (7/150) | 1.2% (2/171) | 4.7% (18/387) | 16.689 | <.001 | .026 | .088 | <.001 |
| Obsessive compulsive | |||||||||
| Age 6 years | 12.1% (8/66) | 10.0% (15/150) | 1.2% (2/171) | 6.5% (25/387) | 14.532 | .001 | .811 | .000 | .001 |
| Age 16 years | 10.6% (7/66) | 2.7% (4/150) | 1.2% (2/171) | 3.4% (13/387) | 13.424 | .001 | .021 | .424 | .002 |
| Nocturnal enuresis | |||||||||
| Age 6 years | 19.7% (13/66) | 6.0% (9/150) | 1.2% (2/171) | 6.2% (24/387) | 28.118 | <.001 | .003 | .028 | <.001 |
| Age 16 years | 0.0% (0/66) | 2.0% (3/150) | 0.6% (1/171) | 1.0% (4/387) | 2.396 | .397 | .555 | .343 | 1.000 |
| Taking medication for attention-deficit/hyperactivity | |||||||||
| Age 6 years | 7.6% (5/66) | 0.7% (1/150) | 0.6% (1/171) | 1.8% (7/387) | 14.903 | .002 | .011 | 1.000 | .007 |
| Age 9 years | 28.8% (19/66) | 2.0% (3/150) | 0.0% (0/171) | 5.7% (22/387) | 79.809 | <.001 | <.001 | .101 | <.001 |
| Age 16 years | 31.0% (18/58) | 2.4% (3/126) | 0.7% (1/139) | 6.8% (22/323) | 65.641 | <.001 | <.001 | .349 | <.001 |
Note. Specific psychiatric disorders with prevalence ≥ 3% are shown if they differed across groups at one of the ages that they were assessed (see Supplement Table 2). Age 6 Year diagnoses are DSM-III-R; Age 9 and 16 Years diagnoses are DSM-IV. Age 16 Years Threshold diagnoses: oppositional-defiant disorder, specific phobia, and tic disorder. Age 16 Years Threshold + subthreshold diagnoses: conduct disorder, separation anxiety disorder, social phobia, major depressive disorder, obsessive-compulsive disorder, and nocturnal enuresis. Not shown are those diagnoses that did not differ at any age or were assessed at only one age: Overanxious disorder (age 6), generalized anxiety (age 16), simple phobia (age 6)/specific phobia (age 16), tic disorders.
Abbreviations: PIA = persistent inattentive; SAL = school age limited; UA = unaffected; RMPI = Riley Motors Problems Inventory; WISC-III = Wechsler Intelligence Scale for Children; WASI = Wechsler Abbreviated Scale of Intelligence; ASD = autism spectrum disorder; SCQ = Social Communication Questionnaire; ASSQ = Autism Spectrum Screening Questionnaire; DSM-III-R = Diagnostic and Statistical Manual of Mental Disorders (3rd ed., rev.); DSM-IV = Diagnostic and Statistical Manual of Mental Disorders (4th ed.).
Two degrees of freedom.
The p values are exact two-sided.
Cut point for top 2% of the age 6 distribution was extended to all three ages to equate across the ages the number of motor problems considered “excessive” (≥nine problems).
Post hoc unwarranted.
Defined as satisfying any one of three conditions: (a) a score ≥ 9 on the SCQ, (b) a score of ≥ 12 on the ASSQ, or (c) a history of professional diagnosis of ASD.
“ne” is “not evaluated.”
In view of the liberal thresholds on the two instruments (SCQ and ASSQ) used in the tripartite screen for ASD, the analyses in Table 4 involving the ASD screen were redone using more stringent cutoffs reported in the literature, namely, ≥14 for the SCQ as reported for a regional birth cohort (the United Kingdom and Ireland) of extremely preterm survivors at 11 years of age (Johnson et al., 2011) and >19 as reported for ASSQ in a sample of 6- to 17-year-olds referred to neuropsychiatric clinics in Sweden (Ehlers et al., 1999). As with the more liberal cutoffs, this version of the screen was also strongly elevated in the PIA group relative to the other two classes (see Supplemental Table 2).
DSM psychiatric disorders
Only disorders with a prevalence of greater than or equal to 3% at any age (see Supplemental Table 3) were examined in relation to the latent classes. Table 4 shows that, at age 6, the PIA class is substantially and significantly elevated relative to the other two in rates of oppositional defiant disorder, conduct disorder, and nocturnal enuresis. In the case of separation anxiety and obsessive-compulsive disorder, the PIA and SAL classes do not differ, but are both significantly elevated relative to the UA class. By age 16, the PIA class stands alone, significantly elevated relative to the other two classes for all of the disorders in the table (except nocturnal enuresis). This includes the disorders that were largely absent at age 6 (major depressive disorder) or that were not assessed at the earlier age (social anxiety disorder).
Additional Analyses
Potential confounding
As shown in Supplemental Table 4, the findings regarding adolescent functional outcomes in the PIA group remained essentially the same after controlling for cognitive and motor deficits, a positive screen for ASD, and the presence of any non-ADHD psychiatric disorder at age 16.
Co-occurrence of ADHD, ASD, and motor problems
Given current interest in the co-occurrence of these conditions (Cooper, Martin, Langley, Hamshere, & Thapar, 2014; Gustafsson et al., 2014; C. M. Jensen & Steinhausen, 2015; Mulligan et al., 2009; Papadopoulos, Rinehart, Bradshaw, & McGinley, 2013; Reiersen, Constantino, & Todd, 2008), it is notable that, at age 16, ASD and excessive motor problems were present concurrently in 10.6% of the PIA class, but only in 2.0% and 1.2% of the SAL and UA classes, respectively.
Medication
Table 4 shows that separation of the SAL and PIA classes after age 6 is not attributable to medication use by the former: only one of the 150 youth in the SAL class had received any medication for ADHD by age 6. At age 9, only 2.0% of the SAL group had received medication compared with 28.8% of the PIA class. At all ages, members of the PIA class were significantly more likely than those in the SAL and UA classes to have received medication for ADHD in the past year. An analysis presented in Supplemental Table 5 shows that much of this finding may be due to elevated ADHD-related impairment in this group.
DSM-IV ADHD subtypes
As shown in Supplemental Table 6, DSM-IV diagnoses of ADHD are far more prevalent in the PIA class than in the other two classes at all three ages. Rates of all types of ADHD symptoms decline over time. The exception is the Predominantly IA subtype, which increases to a prevalence of 45% in the PIA class at age 16. The two most common DSM-IV subtypes (Predominantly IA and Combined) are unstable between ages 9 and 16 and showed few differences in functional outcomes (Supplemental Table 7) or associated characteristics (Supplemental Table 8).
Discussion
This article used LCA to characterize heterogeneity in the course of IA and HI symptoms. The three classes that were identified—UA, SAL, and PIA—differentiate individuals in terms of the persistence of their ADHD symptoms, with the UA class largely symptom free by age 9, the SAL class largely symptom free by age 16, and the PIA class retaining high rates of symptoms (particularly, IA symptoms) at age 16.
Although the PIA class had markedly higher rates of ADHD-related impairment than the SAL class at age 6, the ADHD symptom presentations in the two classes were only modestly different at that age. The subsequent symptom remissions in the SAL class were not explained by medication use in that class and are consistent with a natural “clock setting cure” (Lambert & Bickman, 2004). This phenomenon was invoked to explain why so many children enrolled in the Multimodal Treatment of ADHD (MTA) study lost their ADHD diagnosis after 3 years regardless of intervention (P. S. Jensen et al., 2007).
Groups with persistent high IA symptoms, such as the PIA class in this study, have been identified in other longitudinal studies of clinical (Arnold et al., 2014; Biederman, Petty, Clarke, Lomedico, & Faraone, 2011) and population-based samples (Lahey & Willcutt, 2010; Larsson et al., 2011; Pingault et al., 2011). The present study, however, by examining each DSM-IV IA and HI symptom separately, was able to observe that of the nine HI symptoms, the three impulsive symptoms were more prevalent than the hyperactive ones at age 16, a finding potentially relevant to the increased risk of suicide among adults with ADHD (Ljung et al., 2014).
Functional outcomes were markedly and significantly poorer in the PIA class relative to both other classes. Outcomes in the UA class at age 16 were similar to those found in other population-based LBW/PT cohorts from the same era and similarly poorer than functional outcomes reported for the term controls in those studies (Saigal, Hoult, Streiner, Stoskopf, & Rosenbaum, 2000; Saigal, Lambert, Russ, & Hoult, 2002; Saigal, Pinelli, Hoult, Kim, & Boyle, 2003; Taylor, 2010). In general, outcomes in the SAL class were intermediate between those of the PIA and UA classes. This finding is similar to the 8-year follow-up of the MTA study, where functioning remained suboptimal even after considerable ADHD symptom improvement (Molina et al., 2009). Importantly, differences in age 16 functioning across the three classes were not explained by other associated non-ADHD clinical characteristics.
The non-ADHD clinical characteristics that were distinctive to the PIA class included high rates of non-disabling motor problems, other psychiatric disorders, and social difficulties as indicated by a positive screen for ASD. Combinations of these characteristics in children with ADHD have been noted in other samples not restricted to LBW or preterm birth (Acosta et al., 2008; Cooper et al., 2014; Gustafsson et al., 2014; Koyuncu et al., 2014; Lahey & Willcutt, 2010; Mulligan et al., 2009; Papadopoulos et al., 2013; Piek, Pitcher, & Hay, 1999; Reiersen et al., 2008).
The distinctive association between the PIA class and a positive tripartite screen at age 16 for lifetime ASD, whether more liberal or more stringent cut points for the SCQ or ASSQ are used, deserves a caveat. The vast majority of screen positives for ASD in this sample were false positives (Pinto-Martin et al., 2011). In a two-stage study of 11-year-old preterms that excluded cases with major functional disability, Johnson et al. (2011) found that false positives for ASD (using a cut point of >14 on the SCQ) were at high risk for emotional, conduct, attention/hyperactivity, and peer problems (as compared with those with true negative screens). The findings of Johnson et al. suggest that a positive screen for ASD should be interpreted conservatively as a nonspecific indicator of social difficulty rather than as specific indicator of “autism features.”
Gilger (Gilger & Kaplan, 2001) has termed the overlap of neurodevelopmental problems as “atypical brain development syndrome” as distinct from the now discredited concept of “minimal brain dysfunction” (Rutter, 1982). Gillberg (2010) has framed a similar concept from a public health/services perspective as “Syndromes Eliciting Neurodevelopmental Clinical Examinations” (ESSENCE). In the present sample, the co-occurrence of non-ADHD psychiatric disorders with ADHD latent classes appears to vary by age. For example, an elevated rate of nocturnal enuresis in the PIA class is only apparent at age 6, whereas the association of Major Depressive Disorder (MDD) with this class is not apparent until age 16, perhaps reflecting the more common adolescent onset for MDD (Costello, Copeland, & Angold, 2011). Fernell and Gillberg (2012) speculated that ADHD after PT birth may be an underdiagnosed antecedent of adult affective disorders (Nosarti et al., 2012). The present findings support this speculation and suggest the existence of a subgroup of LBW/PT infants without major cognitive or motor disability who need a higher level of support (Copeland et al., 2013). Children in the PIA class varied in their associated clinical characteristics, but as noted by Rutter (1983), “uniformity of symptomatology does not constitute a necessary criterion for the validity of a syndrome.”
Limitations of this study include the use of DSM-III-R symptom criteria at the age 6 assessment, the reliance on parent report in the assessment of ADHD symptoms, and absence of information on ASD prior to age 16. The use of a LBW/PT sample might also be seen as a limitation. A case can be made, however, for the relevance of the findings here to the general population. First, LBW/PT infants constitute a substantial subgroup of the general population (11.5% of live births in the United States in 2012 were PT [<37 weeks], 8.0% were LBW [<2,500 g]; March of Dimes, 2014). Second, the types of neonatal brain abnormalities and other perinatal risk factors previously found to be associated with ADHD (Whitaker et al., 2011; Whitaker et al., 1997) and ASD (Movsas et al., 2013) in this LBW/PT cohort can occur in utero in full-term infants (Niemann, Wakat, Krageloh-Mann, Grodd, & Michaelis, 1994; Truwit, Barkovich, Koch, & Ferriero, 1992). Third, the non-ADHD neurodevelopmental problems that aggregate with IA symptoms in this sample are consistent with those found in population-based samples not restricted to LBW/PT survivors (Gustafsson et al., 2014; Reiersen et al., 2008).
The findings of the present study suggest that future research efforts to differentiate clinically useful syndromes of ADHD might fruitfully focus on longitudinal profiles of IA and HI symptoms rather than on presenting symptoms. A next step in further exploring the potential PIA syndrome identified here is to determine whether it is distinctive in terms of familial and medical risk factors. Also important will be the identification of any child characteristics that could assist in recognition of school-aged children whose ADHD symptoms are likely to persist. A study by Biederman et al. (2011) of a clinical sample of children with ADHD followed into adolescence suggests that greater ADHD-related impairment in childhood is associated with persistence of ADHD symptoms (Biederman et al., 2011). This might also be true in our sample, given that the PIA class had a markedly higher rate of ADHD-related impairment at age 6 than the SAL class. The issue of potential early childhood predictors will be pursued in a separate report.
In conclusion, this study identified a distinctive longitudinal profile of persistent inattentive symptoms from childhood to adolescence in LBW/PT survivors without major disability. This longitudinal profile was associated with poor functional outcomes and was distinguished by excessive rates of motor problems, higher rates of other psychiatric disorder, and higher rates of social difficulties as indicated by a positive screen for lifetime ASD at age 16. These findings differentiate a potential persistent inattentive syndrome of relevance to ADHD evaluation and treatment.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge their colleagues Nigel Paneth of the College of Human Medicine, Michigan State University, and Jennifer Pinto-Martin of the University of Pennsylvania School of Nursing and Perelman School of Medicine for their contributions to the data set as principal investigators on NS20713 and NS3817, respectively, and as co-investigators on MH4583 and MH57514. They thank Kelly M. Morton and Kimberley S. Dias for their helpful comments on the article. They express their continuing gratitude to all the participants in this study.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This secondary analysis was made possible by an American Academy of Child and Adolescent Psychiatry Eli Lilly Pilot Award to Aaron Krasner, by MH81501 (Prudence Fisher and J. Blake Turner) and by a gift from Marilyn and James Simons supporting mentored training in developmental neuropsychiatry (Agnes Whitaker). The data set and relevant primary analyses were funded by National Institutes of Health R01 Grants NS20713, MH4583, NS3817, and MH57514 with cooperative funding from the March of Dimes and the John Merck Fund.
Biographies
Aaron J. Krasner, a child psychiatrist, began this study while a Whitaker Scholar in developmental neuropsychiatry at New York State Psychiatric Institute-Columbia University Medical Center.
J. Blake Turner is a sociologist and psychiatric epidemiologist with interest in the social and developmental etiologies of mental health problems.
Judith F. Feldman is a developmental cognitive neuropsychologist who has published widely on longitudinally-followed high risk samples.
Anna E. Silberman has her masters of science in Neuroscience and Education and was a Maternal and Child Health Bureau LEND scholar in Leadership Education in Neurodevelopmental and related Disabilities.
Prudence W. Fisher has her doctorate in social work and has extensive experience in the development and testing of psychiatric diagnostic measures.
Catherine C. Workman, a neonatologist, contributed to this report while a Whitaker Scholar in developmental neuropsychiatry
Jonathan E. Posner, a child psychiatrist, has both clinical and research expertise in ADHD.
Laurence L. Greenhill, a child psychiatrist, has an extensive research background in ADHD.
John M. Lorenz, a neonatologist, has a research background in outcomes after prematurity.
David Shaffer, a child psychiatrist, is an internationally recognized expert on, among other subjects, the assessment and classification of child psychiatric disorders.
Agnes H. Whitaker, a child psychiatrist and director of the Whitaker Scholar award in developmental neuropsychiatry, has published primarily on long-term outcomes in children born at low birthweight or prematurely.
Footnotes
Author Contributions
Turner, Feldman, and Whitaker had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: all authors
Acquisition, analysis, or interpretation of the data: all authors
Drafting of the manuscript: Krasner, Whitaker, Turner, Feldman
Critical revision of the manuscript for important intellectual content: all authors
Statistical analysis: Turner, Feldman, Silberman, Whitaker
Obtained funding: Krasner, Fisher, Turner, Whitaker
Administrative, technical or material support: Silberman
Study supervision: Whitaker
Authors’ Note
The sponsors had no role in the design and conduct of the study; collection, management, or interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Greenhill and Posner have research funding from Shire Pharmaceuticals; Greenhill is on the Scientific Advisory Board of Biobehavioral Diagnostics Company.
Supplemental Material
The supplemental tables are available at http://jad.sagepub.com/supplemental
References
- Aarnoudse-Moens CS, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124:717–728. doi: 10.1542/peds.2008-2816. [DOI] [PubMed] [Google Scholar]
- Acosta MT, Castellanos FX, Bolton KL, Balog JZ, Eagen P, Nee L, Muenke M. Latent class subtyping of attention-deficit/hyperactivity disorder and comorbid conditions. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47:797–807. doi: 10.1097/CHI.0b013e318173f70b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd. Washington, DC: Author; 1987. Rev. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington, DC: Author; 1994. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington, DC: Author; 2000. text rev. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Arnold LE, Ganocy SJ, Mount K, Youngstrom EA, Frazier TW, Fristad M, Marsh L. Three-year latent class trajectories of Attention-Deficit/Hyperactivity Disorder (ADHD) symptoms in a clinical sample not selected for ADHD. Journal of the American Academy of Child & Adolescent Psychiatry. 2014;53:745–760. doi: 10.1016/j.jaac.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balazs J, Miklosi M, Kereszteny A, Dallos G, Gadoros J. Attention-deficit hyperactivity disorder and suicidality in a treatment naive sample of children and adolescents. Journal of Affective Disorders. 2014:152–154. doi: 10.1016/j.jad.2013.09.026. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Berument SK, Rutter M, Lord C, Pickles A, Bailey A. Autism screening questionnaire: Diagnostic validity. British Journal of Psychiatry. 1999;175:444–451. doi: 10.1192/bjp.175.5.444. [DOI] [PubMed] [Google Scholar]
- Biederman J, Mick E, Faraone SV. Age-dependent decline of symptoms of attention deficit hyperactivity disorder: Impact of remission definition and symptom type. American Journal of Psychiatry. 2000;157:816–818. doi: 10.1176/appi.ajp.157.5.816. [DOI] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Clarke A, Lomedico A, Faraone SV. Predictors of persistent ADHD: An 11-year follow-up study. Journal of Psychiatric Research. 2011;45:150–155. doi: 10.1016/j.jpsychires.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird HR, Shaffer D, Fisher P, Gould MS, Staghezza B, Chen JY, Hoven C. The Columbia-Impairment-Scale (Cis)—Pilot findings on a measure of global impairment for children and adolescents. International Journal of Methods in Psychiatric Research. 1993;3:167–176. [Google Scholar]
- Chen Q, Sjolander A, Runeson B, D’Onofrio BM, Lichtenstein P, Larsson H. Drug treatment for attention-deficit/hyperactivity disorder and suicidal behaviour: Register based study. British Medical Journal. 2014;348 doi: 10.1136/bmj.g3769. Article g3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M, Martin J, Langley K, Hamshere M, Thapar A. Autistic traits in children with ADHD index clinical and cognitive problems. European Child & Adolescent Psychiatry. 2014;23:23–34. doi: 10.1007/s00787-013-0398-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland WE, Adair CE, Smetanin P, Stiff D, Briante C, Colman I, Angold A. Diagnostic transitions from childhood to adolescence to early adulthood. The Journal of Child Psychology and Psychiatry. 2013;54:791–799. doi: 10.1111/jcpp.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EJ, Copeland W, Angold A. Trends in psychopathology across the adolescent years: What changes when children become adolescents, and when adolescents become adults? Journal of Child Psychology and Psychiatry. 2011;52:1015–1025. doi: 10.1111/j.1469-7610.2011.02446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers S, Gillberg C, Wing L. A screening questionnaire for Asperger syndrome and other high-functioning autism spectrum disorders in school age children. Journal of Autism and Developmental Disorders. 1999;29:129–141. doi: 10.1023/a:1023040610384. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: A meta-analysis of follow-up studies. Psychological Medicine. 2006;36:159–165. doi: 10.1017/S003329170500471X. [DOI] [PubMed] [Google Scholar]
- Fernell E, Gillberg C. Preterm birth, ADHD and the ESSENCE in adult psychiatry. Acta Paediatrica. 2012;101:e568–569. doi: 10.1111/apa.12026. [DOI] [PubMed] [Google Scholar]
- Fredriksen M, Dahl A, Martinsen E, Klungsoyr O, Faraone SV, Peleikis D. Childhood and persistent ADHD symptoms associated with educational failure and long-term occupational disability in adult ADHD. ADHD Attention Deficit and Hyperactivity Disorders. 2014;6:87–99. doi: 10.1007/s12402-014-0126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilger JW, Kaplan BJ. Atypical brain development: A conceptual framework for understanding developmental learning disabilities. Developmental Neuropsychology. 2001;20:465–481. doi: 10.1207/S15326942DN2002_2. [DOI] [PubMed] [Google Scholar]
- Gillberg C. The ESSENCE in child psychiatry: Early symptomatic syndromes eliciting neurodevelopmental clinical examinations. Research in Developmental Disabilities. 2010;31:1543–1551. doi: 10.1016/j.ridd.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Groen-Blokhuis MM, Middeldorp CM, van Beijsterveldt CE, Boomsma DI. Evidence for a causal association of low birth weight and attention problems. Journal of the American Academy of Child & Adolescent Psychiatry. 2011;50:1247–1254.e2. doi: 10.1016/j.jaac.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Gustafsson P, Kerekes N, Anckarsater H, Lichtenstein P, Gillberg C, Rastam M. Motor function and perception in children with neuropsychiatric and conduct problems: Results from a population based twin study. Journal of Neurodevelopmental Disorders. 2014;6 doi: 10.1186/1866-1955-6-11. Article 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guze SB. The need for toughmindedness in psychiatric thinking. Southern Medical Journal. 1970;63:662–671. doi: 10.1097/00007611-197006000-00012. [DOI] [PubMed] [Google Scholar]
- Hack M, Taylor HG, Schluchter M, Andreias L, Drotar D, Klein N. Behavioral outcomes of extremely low birth weight children at age 8 years. Journal of Developmental & Behavioral Pediatrics. 2009;30:122–130. doi: 10.1097/DBP.0b013e31819e6a16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerness P, Joshi G, Doyle R, Georgiopoulos A, Geller D, Spencer T, Biederman J. Do stimulants reduce the risk for cigarette smoking in youth with attention-deficit hyperactivity disorder? A prospective, long-term, open-label study of extended-release methylphenidate. The Journal of Pediatrics. 2013;162:22–27.e22. doi: 10.1016/j.jpeds.2012.06.046. [DOI] [PubMed] [Google Scholar]
- Hinshaw SP, Owens EB, Sami N, Fargeon S. Prospective follow-up of girls with attention-deficit/hyperactivity disorder into adolescence: Evidence for continuing cross-domain impairment. Journal of Consulting and Clinical Psychology. 2006;74:489–499. doi: 10.1037/0022-006X.74.3.489. [DOI] [PubMed] [Google Scholar]
- Jaekel J, Wolke D, Bartmann P. Poor attention rather than hyperactivity/impulsivity predicts academic achievement in very preterm and full-term adolescents. Psychological Medicine. 2013;43:183–196. doi: 10.1017/S0033291712001031. [DOI] [PubMed] [Google Scholar]
- Jensen CM, Steinhausen HC. Comorbid mental disorders in children and adolescents with attention-deficit/hyperactivity disorder in a large nationwide study. Attention Deficit and Hyperactivity Disorders. 2015;7:27–38. doi: 10.1007/s12402-014-0142-1. [DOI] [PubMed] [Google Scholar]
- Jensen PS, Arnold LE, Swanson JM, Vitiello B, Abikoff HB, Greenhill LL, Hur K. 3-year follow-up of the NIMH MTA study. Journal of the American Academy of Child & Adolescent Psychiatry. 2007;46:989–1002. doi: 10.1097/CHI.0b013e3180686d48. [DOI] [PubMed] [Google Scholar]
- Johnson S, Hollis C, Hennessy E, Kochhar P, Wolke D, Marlow N. Screening for autism in preterm children: Diagnostic utility of the Social Communication Questionnaire. Archives of Disease in Childhood. 2011;96:73–77. doi: 10.1136/adc.2010.194795. [DOI] [PubMed] [Google Scholar]
- Johnson S, Hollis C, Kochhar P, Hennessy E, Wolke D, Marlow N. Psychiatric disorders in extremely preterm children: Longitudinal finding at age 11 years in the EPICure study. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49:453–463.e451. doi: 10.1097/00004583-201005000-00006. [DOI] [PubMed] [Google Scholar]
- Johnson S, Wolke D. Behavioural outcomes and psychopathology during adolescence. Early Human Development. 2013;89:199–207. doi: 10.1016/j.earlhumdev.2013.01.014. [DOI] [PubMed] [Google Scholar]
- Koyuncu A, Ertekin E, Yuksel C, Aslantas Ertekin B, Celebi F, Binbay Z, Tukel R. Predominantly inattentive type of ADHD is associated with social anxiety disorder. Journal of Attention Disorders. 2014;19:856–864. doi: 10.1177/1087054714533193. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Willcutt EG. Predictive validity of a continuous alternative to nominal subtypes of attention-deficit/hyperactivity disorder for DSM-V. Journal of Clinical Child & Adolescent Psychology. 2010;39:761–775. doi: 10.1080/15374416.2010.517173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert W, Bickman L. Child & adolescent psychiatry: The “clock-setting” cure: How children’s symptoms might improve after ineffective treatment. Psychiatric Services. 2004;55:381–382. doi: 10.1176/appi.ps.55.4.381. [DOI] [PubMed] [Google Scholar]
- Larsson H, Dilshad R, Lichtenstein P, Barker ED. Developmental trajectories of DSM-IV symptoms of attention-deficit/hyperactivity disorder: Genetic effects, family risk and associated psychopathology. The Journal of Child Psychology and Psychiatry. 2011;52:954–963. doi: 10.1111/j.1469-7610.2011.02379.x. [DOI] [PubMed] [Google Scholar]
- Ljung T, Chen Q, Lichtenstein P, Larsson H. NCommon etiological factors of attention-deficit/hyperactivity disorder and suicidal behavior: A population-based study in Sweden. JAMA: Psychiatry. 2014;71:958–964. doi: 10.1001/jama-psychiatry.2014.363. [DOI] [PubMed] [Google Scholar]
- Lo Y, Mendell NR, Rubin DB. Testing the number of components in a normal mixture. Biometrika. 2001;88:767–778. [Google Scholar]
- March of Dimes. Perinatal Data Snapshots: United States trends in birth outcomes. National Center for Health Statistics; 2014. Final Natality Data. Retrieved from http://www.marchofdimes.org/peristats/prematurity.aspx?reg=99. [Google Scholar]
- Molina BS, Flory K, Hinshaw SP, Greiner AR, Arnold LE, Swanson JM, Wigal T. Delinquent behavior and emerging substance use in the MTA at 36 months: Prevalence, course, and treatment effects. Journal of the American Academy of Child & Adolescent Psychiatry. 2007;46:1028–1040. doi: 10.1097/chi.0b013e3180686d96. [DOI] [PubMed] [Google Scholar]
- Molina BS, Hinshaw SP, Swanson JM, Arnold LE, Vitiello B, Jensen PS, Houck PR. The MTA at 8 years: Prospective follow-up of children treated for combined-type ADHD in a multisite study. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48:484–500. doi: 10.1097/CHI.0b013e31819c23d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movsas TZ, Pinto-Martin JA, Whitaker AH, Feldman JF, Lorenz JM, Korzeniewski SJ, Paneth N. Autism spectrum disorder is associated with ventricular enlargement in a low birth weight population. The Journal of Pediatrics. 2013;163:73–78. doi: 10.1016/j.jpeds.2012.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan A, Anney RJ, O’Regan M, Chen W, Butler L, Fitzgerald M, Gill M. Autism symptoms in attention-deficit/hyperactivity disorder: A familial trait which correlates with conduct, oppositional defiant, language and motor disorders. Journal of Autism and Developmental Disorders. 2009;39:197–209. doi: 10.1007/s10803-008-0621-3. [DOI] [PubMed] [Google Scholar]
- Muthen LK, Muthen BO. Mplus user’s guide. 5th. Los Angeles, CA: Muthen & Muthen; 1998–2007. [Google Scholar]
- Niemann G, Wakat JP, Krageloh-Mann I, Grodd W, Michaelis R. Congenital hemiparesis and periven-tricular leukomalacia: Pathogenetic aspects on magnetic resonance imaging. Developmental Medicine & Child Neurology. 1994;36:943–950. doi: 10.1111/j.1469-8749.1994.tb11790.x. [DOI] [PubMed] [Google Scholar]
- Nosarti C, Reichenberg A, Murray RM, Cnattingius S, Lambe MP, Yin L, Hultman CM. Preterm birth and psychiatric disorders in young adult life. Archives of General Psychiatry. 2012;69(6):E1–E8. doi: 10.1001/archgenpsychiatry.2011.1374. [DOI] [PubMed] [Google Scholar]
- O’Shea TM, Downey LC, Kuban KK. Extreme prematurity and attention deficit: Epidemiology and prevention. Frontiers in Human Neuroscience. 2013;7:578. doi: 10.3389/fnhum.2013.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos N, Rinehart N, Bradshaw JL, McGinley JL. Brief report: Children with ADHD without comorbid autism do not have impaired motor proficiency on the movement assessment battery for children. Journal of Autism and Developmental Disorders. 2013;43:1477–1482. doi: 10.1007/s10803-012-1687-5. [DOI] [PubMed] [Google Scholar]
- Piek JP, Pitcher TM, Hay DA. Motor coordination and kinaesthesis in boys with attention deficit–hyperactivity disorder. Developmental Medicine & Child Neurology. 1999;41:159–165. doi: 10.1111/j.1469-8749.1999.tb00575.x. [DOI] [PubMed] [Google Scholar]
- Pingault JB, Tremblay RE, Vitaro F, Carbonneau R, Genolini C, Falissard B, Cote SM. Childhood trajectories of inattention and hyperactivity and prediction of educational attainment in early adulthood: A 16-year longitudinal population-based study. American Journal of Psychiatry. 2011;168:1164–1170. doi: 10.1176/appi.ajp.2011.10121732. [DOI] [PubMed] [Google Scholar]
- Pinto-Martin JA, Levy SE, Feldman JF, Lorenz JM, Paneth N, Whitaker AH. Prevalence of autism spectrum disorder in adolescents born weighing <2000 grams. Pediatrics. 2011;128:883–891. doi: 10.1542/peds.2010-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto-Martin JA, Paneth N, Witomski T, Stein I, Schonfeld S, Rosenfeld D, Susser MW. The central New Jersey neonatal brain haemorrhage study: Design of the study and reliability of ultrasound diagnosis. Paediatric and Perinatal Epidemiology. 1992;6:273–284. doi: 10.1111/j.1365-3016.1992.tb00767.x. [DOI] [PubMed] [Google Scholar]
- Pinto-Martin JA, Riolo SA, Cnaan A, Holzman C, Paneth N. Cranial ultrasound prediction of disabling and nondisabling cerebral palsy at age two in a low birth weight population. Pediatrics. 1995;95:249–254. [PubMed] [Google Scholar]
- Pinto-Martin JA, Whitaker A, Feldman J, Cnaan A, Zhao H, Rosen-Bloch J, Paneth N. Special education services and school performance in a regional cohort of low-birthweight infants at age nine. Paediatric and Perinatal Epidemiology. 2004;18:120–129. doi: 10.1111/j.1365-3016.2003.00541.x. [DOI] [PubMed] [Google Scholar]
- Polderman TJ, Boomsma DI, Bartels M, Verhulst FC, Huizink AC. A systematic review of prospective studies on attention problems and academic achievement. Acta Psychiatrica Scandinavica. 2010;122:271–284. doi: 10.1111/j.1600-0447.2010.01568.x. [DOI] [PubMed] [Google Scholar]
- Ramos Olazagasti MA, Klein RG, Mannuzza S, Belsky ER, Hutchison JA, Lashua-Shriftman EC, Castellanos FX. Does childhood attention-deficit/hyperactivity disorder predict risk-taking and medical illnesses in adulthood? Journal of the American Academy of Child & Adolescent Psychiatry. 2013;52:153–162.e154. doi: 10.1016/j.jaac.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiersen AM, Constantino JN, Todd RD. Co-occurrence of motor problems and autistic symptoms in attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47:662–672. doi: 10.1097/CHI.0b013e31816bff88. [DOI] [PubMed] [Google Scholar]
- Rigby K, Slee PT. Dimensions of interpersonal relation among Australian children and implications for psychological well-being. The Journal of Social Psychology. 1993;133:33–42. doi: 10.1080/00224545.1993.9712116. [DOI] [PubMed] [Google Scholar]
- Riley GD. Riley motor problems inventory: Manual. Los Angeles, CA: Western Psychological Services; 1976. [Google Scholar]
- Rutter M. Syndromes attributed to “minimal brain dysfunction” in childhood. American Journal of Psychiatry. 1982;139:21–33. doi: 10.1176/ajp.139.1.21. [DOI] [PubMed] [Google Scholar]
- Rutter M. Behavioral studies: Questions and findings on the concept of a distinctive syndrome. In: Rutter M, editor. Developmental neuropsychiatry. New York, NY: Guilford; 1983. pp. 259–279. [Google Scholar]
- Saigal S, Hoult LA, Streiner DL, Stoskopf BL, Rosenbaum PL. School difficulties at adolescence in a regional cohort of children who were extremely low birth weight. Pediatrics. 2000;105:325–331. doi: 10.1542/peds.105.2.325. [DOI] [PubMed] [Google Scholar]
- Saigal S, Lambert M, Russ C, Hoult L. Self-esteem of adolescents who were born prematurely. Pediatrics. 2002;109:429–433. doi: 10.1542/peds.109.3.429. [DOI] [PubMed] [Google Scholar]
- Saigal S, Pinelli J, Hoult L, Kim MM, Boyle M. Psychopathology and social competencies of adolescents who were extremely low birth weight. Pediatrics. 2003;111:969–975. doi: 10.1542/peds.111.5.969. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP. Diagnostic Interview Schedule for Children, Version 3. New York: Division of Child and Adolescent Psychiatry, New York State Psychiatric Institute, Columbia University; 1994. (DISC-3.0) [Google Scholar]
- Shaffer D, Fisher P, Piacentini J, Schwab-Stone M, Wicks J. Diagnostic Interview Schedule for Children (DISC-2.1P): Parent version. New York: Division of Child and Adolescent Psychiatry, New York State Psychiatric Institute; 1989. [Google Scholar]
- Shaffer D, Gould MS, Brasic J, Ambrosini PJ, Fisher P, Bird H, Aluwahlia S. A Children’s Global Assessment Scale (CGAS) Archives of General Psychiatry. 1983;40:1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Greenhill L. A critical note on the predictive validity of “the hyperkinetic syndrome. Journal of Clinical Child & Adolescent Psychology. 1979;20:61–72. doi: 10.1111/j.1469-7610.1979.tb01706.x. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Schwab-Stone M, Fisher P, Cohen P, Piacentini J, Davies M, Regier D. The Diagnostic Interview Schedule for Children-Revised Version (DISC-R): I. Preparation, field testing, interrater reliability, and acceptability. Journal of the American Academy of Child & Adolescent Psychiatry. 1993;32:643–650. doi: 10.1097/00004583-199305000-00023. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Balla DA, Cicchetti DV. Vineland Adaptive Behavior Scales: Interview edition, survey form manual. Circle Pines, MN: American Guidance Service; 1984. [Google Scholar]
- Spencer TJ, Faraone SV, Tarko L, McDermott K, Biederman J. Attention-deficit/hyperactivity disorder and adverse health outcomes in adults. Journal of Nervous and Mental Disease. 2014;202:725–731. doi: 10.1097/NMD.0000000000000191. [DOI] [PubMed] [Google Scholar]
- Taylor HG. Persisting cognitive deficits in survivors of very low birthweight and their implications for adult functioning. Developmental Medicine & Child Neurology. 2010;52:1078–1079. doi: 10.1111/j.1469-8749.2010.03773.x. [DOI] [PubMed] [Google Scholar]
- Thorndike RL, Hagen EP, Sattler JM. Stanford-Binet Intelligence Scale. Chicago, IL: Riverside; 1986. [Google Scholar]
- Truwit CL, Barkovich AJ, Koch TK, Ferriero DM. Cerebral palsy: MR findings in 40 patients. American Journal of Neuroradiology. 1992;13:67–78. [PMC free article] [PubMed] [Google Scholar]
- Visser SN, Danielson ML, Bitsko RH, Holbrook JR, Kogan MD, Ghandour RM, Blumberg SJ. Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003–2011. Journal of the American Academy of Child & Adolescent Psychiatry. 2014;53:34–46.e32. doi: 10.1016/j.jaac.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washbrook E, Propper C, Sayal K. Pre-school hyperactivity/attention problems and educational outcomes in adolescence: Prospective longitudinal study. The British Journal of Psychiatry. 2013;203:265–271. doi: 10.1192/bjp.bp.112.123562. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WISC-III: Wechsler Intelligence Scale for Children. San Antonio, TX: Psychological Corporation; 1991. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Whitaker AH, Feldman JF, Lorenz JM, McNicholas F, Fisher PW, Shen S, Paneth N. Neonatal head ultrasound abnormalities in preterm infants and adolescent psychiatric disorders. Archives of General Psychiatry. 2011;68:742–752. doi: 10.1001/archgenpsychiatry.2011.62. [DOI] [PubMed] [Google Scholar]
- Whitaker AH, Feldman JF, Lorenz JM, Shen S, McNicholas F, Nieto M, Paneth N. Motor and cognitive outcomes in nondisabled low-birth-weight adolescents: Early determinants. Archives of Pediatrics & Adolescent Medicine. 2006;160:1040–1046. doi: 10.1001/archpedi.160.10.1040. [DOI] [PubMed] [Google Scholar]
- Whitaker AH, Feldman JF, Van Rossem R, Schonfeld IS, Pinto-Martin JA, Torre C, Paneth NS. Neonatal cranial ultrasound abnormalities in low birth weight infants: Relation to cognitive outcomes at six years of age. Pediatrics. 1996;98(4, Pt 1):719–729. [PubMed] [Google Scholar]
- Whitaker AH, Van Rossem R, Feldman JF, Schonfeld IS, Pinto-Martin JA, Tore C, Paneth N. Psychiatric outcomes in low-birth-weight children at age 6 years: Relation to neonatal cranial ultrasound abnormalities. Archives of General Psychiatry. 1997;54:847–856. doi: 10.1001/archpsyc.1997.01830210091012. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Nigg JT, Pennington BF, Solanto MV, Rohde LA, Tannock R, Lahey BB. Validity of DSM-IV attention deficit/hyperactivity disorder symptom dimensions and subtypes. Journal of Abnormal Psychology. 2012;121:991–1010. doi: 10.1037/a0027347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolke D, Waylen A, Samara M, Steer C, Goodman R, Ford T, Lamberts K. Selective drop-out in longitudinal studies and non-biased prediction of behaviour disorders. The British Journal of Psychiatry. 2009;195:249–256. doi: 10.1192/bjp.bp.108.053751. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


