Leukemic transformation in patients with polycythemia vera (PV) is a known, but uncommon event. Acute myeloid leukemia (AML) developing in the setting of pre-existing myeloproliferative neoplasm (MPN) such as PV generally carries a poor prognosis.1,2 Traditionally, risk factors predictive for leukemic transformation in patients with PV included older age, longer disease duration, leukocytosis, exposure to chlorambucil, phosphorus32 and pipobroman.3 In two large series, the incidence of secondary AML in patients with PV was 3-10%.4,5 Median survival of these patients was 5 months. 4,5 Molecular events including the precise mechanistic roles of mutations such as JAK2, MPL, CALR, TET2 and ASXL1 in the leukemic transformations of different MPN's are still unclear. In one study, comprehensive sequencing was performed in paired samples of chronic MPN patients who later developed leukemic transformation and discovered the presence of spliceosome mutations, specifically SRSF2 as a significant predictor of adverse outcome.6 Two other studies have shown that acquisition of two or more non JAK2 mutations such as TET2 and DNMT3A was associated with adverse outcomes, high risk of leukemic transformation 7 and poor response to therapy with pegylated interferon α-2a in patients with MPN8. It is believed that sub-clones of different mutations may exist at the time of initial diagnosis of MPN7,9 and clonal evolution over time may predispose to leukemic transformation. On the contrary, PV/MPN developing after the diagnosis of AML is very rarely reported. In several reports,10-12 it has been suggested that the JAK2 clone was present at the time of initial diagnosis of AML, expanded over time, and led to the development of PV, 3-5 years following achievement of remission of AML despite intensive chemotherapy given for the treatment of AML.
Herein, we present a 59 year-old Caucasian man who initially presented to our hospital in December 2011 with fever, fatigue and progressive shortness of breath. The patient was diagnosed with PV in January 2011 at an outside hospital and received therapeutic phlebotomies 3 times since the initial diagnosis. Of note, the patient also had a history of hepatitis C which was unsuccessfully treated with the combination of interferon and ribavirin approximately 10 years prior to presentation. His physical examination at the time of initial presentation was unremarkable except for fever of 39.5°C. A complete blood count (CBC) demonstrated anemia (hemoglobin 8.6 g/dL), hematocrit 25.1%, thrombocytopenia (platelets 44×109/L), with a white blood cell count of 50.7 ×109/L. A peripheral blood smear showed 34% blasts with monocytoid features. Granulocytes showed marked dysplasia with hypogranularity. Serum erythropoietin level was 368 mIU/mL (normal 5.2-25.3 mIU/mL). Bone marrow biopsy confirmed the diagnosis of AML. The aspirate smear showed 22% blasts, monocytoid appearing, with dispersed chromatin and moderate amount of agranular cytoplasm (Figure-1A). Special stains on bone marrow aspirate showed 58% blasts positive for myeloperoxidase (MPO) and a loose network of reticulin with many intersections (MF-1) (Figure-1B), trichrome stain was negative. Flow cytometric immunophenotyping on the bone marrow aspirate showed aberrant cell population in the area of the blast and monocyte gate. The neoplastic cells were positive for CD13, CD14 (subset), CD33, CD38, CD49d, CD64, CD117 (subset) and MPO (partial/subset). The neoplastic cells were negative for CD2, cytoplasmic CD3, CD5, CD7, CD9, CD10, CD19, CD34, CD41, CD56 and TdT. Cytogenetic analysis revealed diploid karyotype. FISH studies and multiplex leukemia translocation assay was negative for common fusion translocations in AML. PCR based DNA sequencing analysis was negative for FLT3, IDH1, IDH2, KIT, KRAS, NPM1, and NRAS. A mutation was detected in codon 617 of JAK2 leading to a change in the coding amino acid from Val to Phe. (V617F). The percentage of PCR product with the mutation V617F was 41.05. The patient was started on induction chemotherapy with idarubicin/cytarabine (IA) for AML, idarubicin 12 mg/m2/day daily for 3 days and cytarabine 1.5 g/m2/day continuous infusion for 4 days. On day 28, a BM examination demonstrated a minimal residual disease (MRD) negative complete remission (CR) with normalization of blood counts but also revealed morphological features of PV (Figure 1C-D), consistent with the reemergence of previously diagnosed PV. The patient received a total of 7 cycles of IA chemotherapy until July 2012 (7 months after diagnosis of AML) and maintained MRD negative CR for AML. The patient was found unfit for stem cell transplantation due to chronic hepatitis C infection with advanced liver disease. Due to neutropenic complications from cytotoxic chemotherapy, the patient's regimen was switched to single agent decitabine 20 mg/m2 for 5 days as consolidation cycles for a total of 4 cycles until January 2013. In the meantime, we noticed that the patient's hemoglobin and hematocrit began to rise in July 2012 (6 months after initial remission of AML), together with rise in JAK2 allele burden and morphologic changes suggestive of recurrence of PV with the AML remaining in CR. For the management of the PV, the patient was started on aspirin, hydroxyurea and intermittent phlebotomies in order to achieve a target goal of hematocrit ≤4513. Temporal profile of JAK2 allele burden over time is presented in Figure-1E. Allele burden decreased to 15.15 at the time of initial remission and subsequent increases are documented in Figure-1E. The patient remains under active surveillance for AML (which remains in CR1) and remains on active therapy for PV at present. The patient has maintained MRD negative CR for AML over the past 3 years and 9 months and continues to receive aspirin, hydroxyurea, and phlebotomies for PV therapy. Successive BM assessments over time showed minimal morphologic evidence of PV. Interestingly, 2 years after achieving leukemia remission in March 2014, the patient was treated for chronic hepatitis C with pegylated interferon alfa 2a, ribavirin and sofosbuvir achieving sustained virological response regarded as cure of hepatitis C. Since acquisition of non-JAK2 mutations is associated with inadequate response to therapy with pegylated interferon alfa 2a, we explored whether the patient has non-JAK2 mutations in the BM. A next generation sequencing (NGS)-based analysis for the detection of somatic mutations in the coding sequence of a total of 28 genes was performed on the DNA extracted from the most recent BM sample obtained in July 2015. We found point mutations in exon 14 of JAK2 (V617F) and exon 23 of DNMT3A (R288H). In addition, we detected point mutations in exon 3 (Q916) and exon 9 (V1371F) and frame shift mutations in exon 7 and exon 11 of TET2 gene. We have summarized the mutational profile of the patient over time in Table-1 and the details of these mutations in Table-2. Unfortunately we were unable to access the pre-AML BM samples with the initial diagnosis of PV for this analysis. Ideally, paired NGS based analysis of BM samples prior to AML and the current BM would have revealed the temporal profile of these mutations. In the absence of such data, we have shown the morphologic and molecular evolution of PV over time. Clinical presentation of this patient is highly unusual and it has raised certain questions about the molecular ontogeny of transformed AML in PV.14 The duration of PV in this patient prior to developing AML was 11 months, he did not receive any prior chemotherapy for PV, and had a diploid karyotype. The resurgence of the JAK2 mutational allele burden, and the changes in the BM suggestive of PV reappeared shortly after bringing the AML under remission. Furthermore, the patient maintained MRD negative CR for almost 4 years and is currently under active surveillance for PV and AML. It is interesting to note that this patient was treated for HCV with interferon alfa-2a and this treatment might have had an impact on PV as pegylated interferon alfa 2a has been shown to improve responses in patients with PV15 and a decrease in HCV viral load might have diminished the possible chronic antigenic stimulation by HCV on BM stem cells. Although, non- JAK2V617F mutations have been described in patients with myeloproliferative neoplasms16, simultaneous presence of JAK2V617F and DNMT3A, TET2 mutations in PV is rarely reported in patients with PV. In one study, DNMT3A mutations (R882H and R488fs) were detected in 2/30 patients with de novo PV and none had aggressive disease course.17 Previous reports have suggested that presence of TP53 mutations or ≥1 non-JAK2 mutations in MPN (MF) predict for adverse prognosis or poor response to ruxolitinib therapy.7,18 The overall clinical picture of this patient suggests that this may not be the case in PV, when ≥1 non-JAK2 mutations are detected simultaneously in the absence of TP53 mutations, possibly indicating that the patient might have had a genetically canalized trait.19 Moreover, co-existence, or the order20, in which the non-JAK2 mutations are acquired in PV, in the absence of TP53 mutations, may have a different prognostic impact. Experimental approaches using a knock out mouse model for PV or siRNA for JAK2V617F, DNMT3A and TET2 mutations and the mechanisms of leukemic transformation or clonal evolution in PV are lacking, and further studies in this regard would be warranted to better understand the pathogenesis of transformations and may help predict the risk of leukemic transformation in PV.
Figure 1. (A-E)Evolution of polycythemia vera (PV) to Acute myeloid leukemia (AML) and resurgence of PV is shown in the bone marrow aspirate and biopsy (BM) slides and temporal profile of JAK2 allele burden over time is shown A-B).
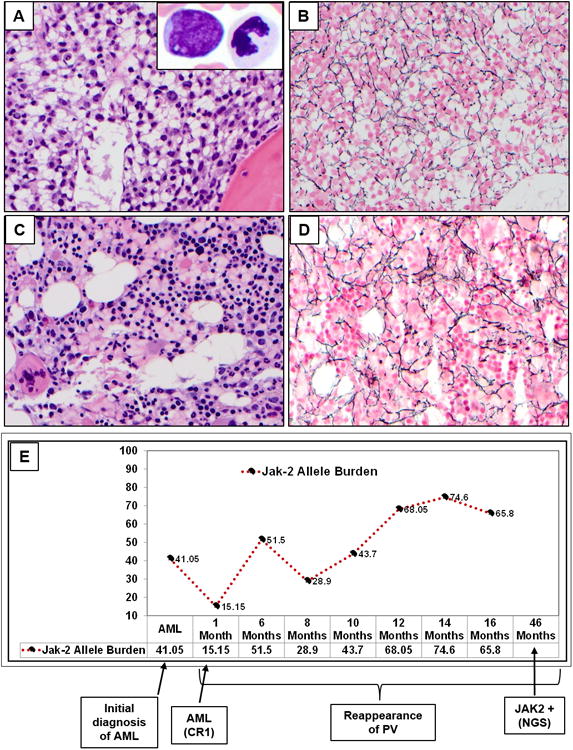
BM core biopsy (H&E, 50×) at the time of diagnosis of AML. Hypercellular marrow with diffuse interstitial infiltrate of immature mononucleated immature cells, morphologically consistent with blasts. They have oval to irregular nuclei, dispersed chromatin and moderate amount of eosinophilic cytoplasm. Dilated sinusoids were present. Background trilineage hematopoiesis was significantly decreased. The insert (100×) shows bone marrow smear with a blast and adjacent dysplastic neutrophil. On smears, blasts have oval to irregular nuclei, open chromatin and moderate amount of agranular cytoplasm. No Auer rods were identified. Neutrophils show dysplastic features with abnormal lobation and hypograluation; Reticulin stain showed loose network of reticulin fibers with intersections, MF-1 (myelofibrosis). C-D) AML in remission with re-emergence of PV: BM showed a hypercellular marrow with panmyelosis, a characteristic feature for polycythemia vera. Large megakaryocytes with hyperlobated nuclei are present. No residual acute myeloid leukemia was identified; Reticulin stain showed similar findings as previous biopsy, consistent with MF-1. This shows the morphologic evolution from PV/AML- Back to PV E) JAK2 allele burden over time from the time of initial diagnosis of AML to the most recent report.
Table 1. Summary of mutations over disease course.
| Name of Mutation | Initial BM (Only PV) | PV transformation to AML | PV alone (AML in Remission) |
|---|---|---|---|
| JAK2-V617F | Not Done | Positive | Positive |
| FLT3 | Not Done | Negative | Negative |
| IDH1, IDH2 | Not Done | Negative | Negative |
| KIT | Not Done | Negative | Negative |
| KRAS, NRAS | Not Done | Negative | Negative |
| NPM1 | Not Done | Negative | Negative |
| DNMT3A | Not Done | Not Done | Positive |
| TET2 | Not Done | Not Done | Positive |
Miscellaneous other mutations were tested in the bone marrow in remission for AML with presence of PV and were negative - ABL1, ASXL1, BRAF, EGFR, EZH2, FLT3, GATA1, GATA2, HRAS, IDH1, IDH2, IKZF2, KIT, KRAS, MDM2, MLL, MPL, MYD88, NOTCH1, NPM1, NRAS, PTPN11, RUNX1, TP53 and WT1 were not detected.
Table 2. Summary of JAK2, DNMT3 and TET2 gene mutations with sequence reads.
| Gene | HGVS | Locus | Type | Mutation Frequency | Total Reads | Mutant Reads |
|---|---|---|---|---|---|---|
| DNMT3A | NM_022552.4(DNMT3A):c.2645G>A p.R882H | Exon 23 | SNV | 44.99131 | 3453 | 1554 |
| JAK2 | NM_004972.3(JAK2):c.1849G>T p.V617F | Exon 14 | SNV | 58.78312 | 7132 | 4193 |
| TET2 | NM_001127208.2(TET2):c.2746C>T p.Q916* | Exon 3 | SNV | 12.47476 | 4456 | 559 |
| TET2 | NM_001127208.2(TET2):c.4111G>T p.V1371F | Exon 9 | SNV | 13.51902 | 1471 | 199 |
| TET2 | NM_001127208.2(TET2):c.3854_3855insTA p.S1286fs*78 | Exon 7 | Indel | 18.27192 | 3935 | 721 |
| TET2 | NM_001127208.2(TET2):c.4989_4993dupCCAAG p.D1665fs*32 | Exon 11 | Indel | 3.924171 | 5275 | 207 |
Acknowledgments
The University of Texas MD Anderson Cancer Center is supported in part by the National Institutes of Health through a Cancer Center Support Grant (P30 CA16672).
Disclosures – NP, ZE, SV consultant to Incyte, H. A. T. is a consultant for Janssen Pharmaceuticals, Inc., Gilead Sciences, Merck & Co., Inc., Vertex Pharmaceuticals, Novartis, Genentech, Astellas, Pfizer, and Theravance, Inc., and has received research grants from Gilead Sciences, Merck & Co., Inc., and Vertex Pharmaceuticals.
Footnotes
Contributions –: PJ, WW, SL, and NP gathered data and organized the figures.
PJ, WW, SL, KP and NP evaluated the pathologic findings and prepared images.
NP, SV, ZE, HAT managed the patient.
All authors critically reviewed and approved the manuscript.
References
- 1.Passamonti F, Rumi E, Pietra D, et al. A prospective study of 338 patients with polycythemia vera: the impact of JAK2 (V617F) allele burden and leukocytosis on fibrotic or leukemic disease transformation and vascular complications. Leukemia. 2010;24(9):1574–1579. doi: 10.1038/leu.2010.148. [DOI] [PubMed] [Google Scholar]
- 2.Gangat N, Strand J, Li CY, Wu W, Pardanani A, Tefferi A. Leucocytosis in polycythaemia vera predicts both inferior survival and leukaemic transformation. Br J Haematol. 2007;138(3):354–358. doi: 10.1111/j.1365-2141.2007.06674.x. [DOI] [PubMed] [Google Scholar]
- 3.Tefferi A, Rumi E, Finazzi G, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia. 2013;27(9):1874–1881. doi: 10.1038/leu.2013.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tefferi A, Guglielmelli P, Larson DR, et al. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood. 2014;124(16):2507–2513. doi: 10.1182/blood-2014-05-579136. quiz 2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marchioli R, Finazzi G, Landolfi R, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 2005;23(10):2224–2232. doi: 10.1200/JCO.2005.07.062. [DOI] [PubMed] [Google Scholar]
- 6.Zhang SJ, Rampal R, Manshouri T, et al. Genetic analysis of patients with leukemic transformation of myeloproliferative neoplasms shows recurrent SRSF2 mutations that are associated with adverse outcome. Blood. 2012;119(19):4480–4485. doi: 10.1182/blood-2011-11-390252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lundberg P, Karow A, Nienhold R, et al. Clonal evolution and clinical correlates of somatic mutations in myeloproliferative neoplasms. Blood. 2014;123(14):2220–2228. doi: 10.1182/blood-2013-11-537167. [DOI] [PubMed] [Google Scholar]
- 8.Quintas-Cardama A, Abdel-Wahab O, Manshouri T, et al. Molecular analysis of patients with polycythemia vera or essential thrombocythemia receiving pegylated interferon alpha-2a. Blood. 2013;122(6):893–901. doi: 10.1182/blood-2012-07-442012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rao N, Butcher CM, Lewis ID, et al. Clonal and lineage analysis of somatic DNMT3A and JAK2 mutations in a chronic phase polycythemia vera patient. Br J Haematol. 2012;156(2):268–270. doi: 10.1111/j.1365-2141.2011.08837.x. [DOI] [PubMed] [Google Scholar]
- 10.Portell CA, Sekeres MA, Rogers HJ, Tiu RV. De novo polycythaemia vera arising 5 years following acute myeloid leukemia remission: suggestion of a chemotherapy resistant JAK2 clone. Br J Haematol. 2012;157(2):266–267. doi: 10.1111/j.1365-2141.2011.08972.x. [DOI] [PubMed] [Google Scholar]
- 11.Belotti A, Doni E, Elli E, Rossi V, Pioltelli P, Pogliani EM. Development of polycythemia vera after chemotherapy-induced remission of acute myeloid leukemia: a case report. Acta Haematol. 2011;126(1):52–53. doi: 10.1159/000324468. [DOI] [PubMed] [Google Scholar]
- 12.Youk HJ, Cho CH, Lee JH, Choi CW, Lim CS, Yoon SY. A rare case of polycythemia vera following acute undifferentiated leukemia remission. Ann Lab Med. 2014;34(6):469–470. doi: 10.3343/alm.2014.34.6.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368(1):22–33. doi: 10.1056/NEJMoa1208500. [DOI] [PubMed] [Google Scholar]
- 14.Hazani A, Tatarsky I, Barzilai D. Prolonged remission of leukemia associated with polycythemia vera. Cancer. 1977;40(3):1297–1299. doi: 10.1002/1097-0142(197709)40:3<1297::aid-cncr2820400344>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 15.Quintas-Cardama A, Kantarjian H, Manshouri T, et al. Pegylated interferon alfa-2a yields high rates of hematologic and molecular response in patients with advanced essential thrombocythemia and polycythemia vera. J Clin Oncol. 2009;27(32):5418–5424. doi: 10.1200/JCO.2009.23.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nangalia J, Massie CE, Baxter EJ, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013;369(25):2391–2405. doi: 10.1056/NEJMoa1312542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stegelmann F, Bullinger L, Schlenk RF, et al. DNMT3A mutations in myeloproliferative neoplasms. Leukemia. 2011;25(7):1217–1219. doi: 10.1038/leu.2011.77. [DOI] [PubMed] [Google Scholar]
- 18.Patel KP, Newberry KJ, Luthra R, et al. Correlation of mutation profile and response in patients with myelofibrosis treated with ruxolitinib. Blood. 2015;126(6):790–797. doi: 10.1182/blood-2015-03-633404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hornstein E, Shomron N. Canalization of development by microRNAs. Nat Genet. 2006;38(1):S20–24. doi: 10.1038/ng1803. [DOI] [PubMed] [Google Scholar]
- 20.Ortmann CA, Kent DG, Nangalia J, et al. Effect of mutation order on myeloproliferative neoplasms. N Engl J Med. 2015;372(7):601–612. doi: 10.1056/NEJMoa1412098. [DOI] [PMC free article] [PubMed] [Google Scholar]


