Abstract
Objective
Fat mass and obesity-associated (FTO) gene polymorphisms have been reported to be associated with differences in body mass index (BMI), obesity, and type 2 diabetes. However, previous studies have been predominantly conducted in younger individuals across a spectrum of body weight while little information is available in the older population. We examined the association of FTO gene polymorphisms with cardiometabolic risks among adults who were both obese (BMI≥30 kg/m2) and older (age ≥65 years).
Methods
One-hundred-sixty-five frail, obese older adults were genotyped for FTO (rs9939609 and rs8050136) single nucleotide polymorphisms and studied for associations with body weight and body composition, components and prevalence of the metabolic syndrome, insulin response to an oral glucose tolerance test (OGTT), and levels of adipocytokines (e.g. leptin) and vitamin D.
Results
Carriers of the A allele (CA/AA) of the FTO SNP rs8050136 had lower body weight, BMI, body fat, and trunk fat than those without the A allele (CC genotype) (all p<0.05). Moreover, genotype CA/AA was associated with lower levels of triglyceride and higher levels of HDL-cholesterol levels and had a trend for lower waist circumference, resulting in lower prevalence of the metabolic syndrome than genotype CC. The insulin area under the curve during the OGTT was lower in genotype CA/AA. Despite the lower insulin levels, the glucose area under the curve was unchanged, resulting in higher insulin sensitivity index. Leptin levels were also lower and adiponectin and 25-hydroxyvitamin levels tended to be higher in genotype CA/AA than genotype CC. No differences were observed for rs9939609.
Conclusions
Unlike results from studies in younger individuals, the risk A allele may confer a favorable cardiometabolic risk profile in obese older adults, suggesting selective survival of obese adults into old age. If confirmed in a larger sample of surviving obese older adults, these findings may have implications regarding clinical approach to obesity in this population.
Keywords: FTO, obesity, aging, cardiometabolic risks, insulin sensitivity
INTRODUCTION
The prevalence of obesity is increasing worldwide in all age groups especially in the older population ≥65 years old [1]. If nothing is done to halt the epidemic, more than 1 billion adults are projected to be obese by 2030 [2]. This will prove to be a huge burden for the health care system, not because of obesity itself but because of the associated complications such as coronary heart disease, stroke, type 2 diabetes mellitus (T2D), cancers, and physical disability. In addition, obesity in older adults increases the risk of institutionalization and mortality [3]. The surge in obesity can be attributed to the changes in lifestyle, for instance, wide availability of high calorie food and decreased physical activity. However, genetic factors are also thought to play a role. In 2007, a genome wide association study for type T2D identified relationship between several single nucleotide polymorphisms (SNPs) in the first intron of the fat mass and obesity related (FTO) gene and T2D [4-6]. However, further analysis showed that the association between FTO SNPs and T2D disappeared after adjustment for body mass index (BMI), suggesting that this relationship was mainly related to the influence of these SNPs on BMI. This association has been replicated in other studies with from different racial/ethnic backgrounds [5-8]. Most notable among the SNPs are rs9939609 and rs8050136, both located in intron 1. The rs9939609 SNP involves a base change from T to A while rs8050136 is a base change from C to A. In both SNPs, the A allele is considered to be the risk allele. Most studies involving the FTO gene have been done in children and adolescents. In children and adolescents, rs8050136 highly contributes to early onset obesity [9]. Studies on rs9939609 in children and adolescent proposed that the risk allele A is associated with increased BMI and intake of high energy foods [10]. In adults, rs8050136 is associated with increased BMI, lean mass, and body fat [11], and persistent central obesity and metabolic syndrome in a 12 year longitudinal study [12].
Among the few studies investigating the association between FTO SNPs and obesity and cardiometabolic risks in the elderly population, only a weak association has been found between the presence of risk A allele for rs9939609 and body composition [13]. In another study among patients 70 years of age or older, no association was found with parameters of body composition and body weight for rs8050136 [14]. However, no information is available on the effect of FTO SNPs for older patients with obesity, the population at the greatest risk for cardiometabolic complications [15]. The objective of this study was to investigate the association between FTO SNPs in intron 1 (rs9939609 and rs8050136) and cardiometabolic risk factors in the obese older population.
METHODS
Study Design and Population
This study was a cross-sectional analysis of baseline data from 165 participants in 2 lifestyle modification trials of sedentary, frail and obese (BMI ≥30 kg/m2), older (≥ 65 yrs. of age) patients. These trials were conducted at Washington University and at Raymond G. Murphy VA Medical Center, Albuquerque, NM. The study was approved by Washington University and the University of New Mexico Institutional Review Boards. Participants were recruited via radio and newspaper advertisements. Informed written consents were obtained from each participant. Participants that were included in the study were ≥65 years of age, with a BMI of ≥30 kg/m2, with a sedentary lifestyle (regular exercise <1 h/week) and stable body weight (±2 kg) for the past year, and on stable medications (≥6 months) before enrollment. The use of medications (e.g. antihypertensives, antidyslipidemics) were determined by medical history and review of medical records. Additionally, all participants were required to have mild-to-moderate frailty determined by meeting at least two operational criteria: modified physical performance test (PPT) score of 18–32, peak oxygen consumption (VO2peak) of 11–18 ml ml/kg, or difficulty in performing two instrumental activities of daily living (ADL) or one basic ADL [16]. Exclusion criteria included severe cardiopulmonary disease, musculoskeletal or neuromuscular impairments that precluded exercise training, known diagnosis of dementia, history of malignant neoplasm, and current smoking. Candidates were also excluded if they had a history of diabetes or fasting glucose of ≥ 126 mg/dL. The same inclusion and exclusion criteria were used in all clinical trials. Because this is a cross-sectional study of baseline data, they would not be influenced by potential responses to lifestyle modification.
Oral Glucose Tolerance Test (OGTT) and Fasting Blood Analyses
A standard 75-g OGTT was performed after an overnight fast. Venous blood samples measurements for glucose and insulin were obtained in fasted state and at time points 30, 60, 90, and 120 minutes after glucose ingestion using glucose oxidase method (YSI Inc., Yellow Springs, OH, USA) and radioimmunoassay. Areas under the curve (AUC) were calculated using the trapezoid method . An insulin sensitivity index [17] was calculated by using the formula: ISI = 10 000/square root of ((fasting glucose × fasting insulin) × (mean glucose × mean insulin during the OGTT)). This index correlated (r = 0.73) with the rate of whole-body glucose disposal during a euglycemic insulin clamp study [17]. Homeostasis model assessment of insulin resistance (HOMA-IR) was also calculated as fasting glucose (mg/dL) multiplied by fasting insulin (μU/mL) divided by 22.5 [18]. Lipoprotein levels were measured using automated enzymatic/colorimetric assays (Roche/Hitachi System, Indianapolis, IN, USA). High-sensitive C-reactive protein was measured by immunoturbidimetric assay (Hitachi 917, Indianapolis, IN, USA). Interleukin-6 (HS600B; R&D Systems, Minneapolis, MN, USA) and adiponectin (EZHADP-61K; Linco Research, Inc., St Louis, MO, USA) were measured using enzyme-linked immunosorbent assay. Leptin (Leptin HL 81K; Linco Research Inc., St Louis, MO) was measured using radioimmunoassay. Coefficient of variations for these measurements was less than 10%.
Waist Circumference, Blood Pressure, and Body Weight and Composition
Waist circumference was measured horizontally at the midpoint between the highest point of the iliac crest and the lowest portion of the 12th rib in the standing position [19]. Blood Pressure (BP) was measured with a sphygmomanometer cuff of the appropriate size after participants remained in the supine position for 15 minutes. Body weight was measured in duplicate in the morning following an overnight fast. Body fat mass, fat-free mass, and trunk fat were measured by using dual X-ray absorptiometry (Hologic, Waltham, MA, USA) as described previously [16].
Metabolic Syndrome
Subjects who met ≥3 of the following criteria [20] were defined as having the Metabolic syndrome (MetS): waist circumference ≥102 cm in men and ≥88cm in women, fasting glucose ≥100 mg/dL, triglyceride ≥150 mg /dL or drug treatment for elevated triglycerides, HDL-cholesterol ≥40 mg/dL in men and ≥50 mg /dL in women or drug treatment for low HDL-cholesterol, and systolic BP ≥130 or diastolic BP ≥85mmHg or on antihypertensive treatment with a history of hypertension. MetS z-score [21, 22], which accounts for continuous differences in each component that cannot be reflected using the MetS criteria, was also calculated. For example, the formula for men was MetS z-score = (40 – HDL-cholesterol/SDHDL-C) + (TG – 150)/SDTG + (Glucose-100)/SDglucose + (Waist circumference – 102)/SDWC + (systolic BP-130)/SDSBP + (diastolic BP-85/SDDBP), where SDHDL-C, SDTG, SDglucose, SDWC, SDSBP, and SDDBP were gender-specific SDs for HDL-C, triglycerides, glucose, waist circumference, systolic BP, and diastolic BP, respectively, from all participants.
Genotyping
Genomic DNA was extracted from peripheral leukocytes using the Puregene DNA Isolation Kit (Gentra Systems, Minneapolis, MN, USA) and used as a template for genotyping procedures. For this project, we selected the SNPs that have been reported to result in differences in body weight and body composition namely: rs9939609 and rs8050136 (both at intron 1) [2-6]. Genotyping was performed by allelic discrimination using specific TaqMan SNPs genotyping assays following the manufacturer’s instructions. Briefly, ~15 μl PCR reactions were carried out containing 12.5 μl TaqMan Universal PCR Master Mix (Applied Biosystems, New Jersey, USA), 10–15.0 ng of DNA template, 1.25 μl TaqMan® Assay primers, and FAM/VIC labeled probes by Applied Biosystems as Assays-by-Design™ (Applied Biosystems, Foster City, CA 94404). The assay IDs of selected assays were: C_30090620_10 (rs993609), and C_2031259_10 (rs8050136). All assays were performed in 96-well plates including negative template controls. After PCR, end point discrimination of alleles was performed on the ABI prism 7500 using the Sequence Detection Software (Applied Biosystems, Foster City CA 94404). All genotype call rates for each assay were >95% quality value.
Statistical Analysis
Results are expressed as mean ± SD in the text and tables and mean ± SE in the figures. A P value of ≤ 0.05 was considered significant. Normality for outcome variables was verified by Shapiro–Wilks test. Group differences were compared using independent t tests or analysis of covariance (ANCOVA) for continuous variables (adjusting for age, gender, and race) as appropriate; while categorical variables were compared using chi-square analysis. All analyses were done using the IBM SPSS/PC statistical program (version 22, Amonk, NY). Subanalyses by gender did not change the results (i.e. no significant group × gender interaction effects); therefore, data for women and men were combined for each group. r2 was used to analyze for linkage between the 2 SNPs [23, 24].
RESULTS
Our clinical trial population originally consisted of 168 frail, obese (BMI 37.0 ± 5.4 kg/m2), older (70.0 ± 4.5 yrs.) adults. However, due to DNA degradation of three samples leading to unsuccessfully genotyping, our sample population was reduced to 165 obese older adults. The genotype frequencies were: CC = 66, CA = 80, and AA = 19; and were in Hardy Weinberg equilibrium. Our analysis for the rs9939609 revealed no significant influence on the parameters we were evaluating in our study population at baseline before intervention; thus, our results and discussion will be focused primarily on rs8050136 polymorphism. Linkage analysis between the 2 SNPs revealed an r2of 0.06 suggesting that these 2 SNPs are not in strong linkage disequilibrium in this specific population of frail, obese older adults.
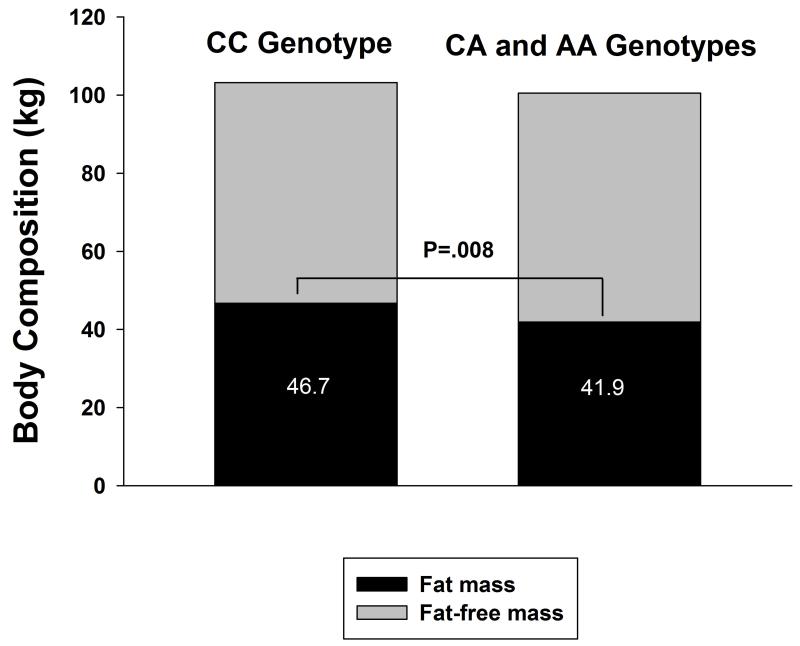
There were no significant differences in demographics, physical frailty measures, history of cardiovascular disease or smoking, and medication use (Table 1). However, there were significant differences in body weight and body composition (Table 2). Carriers of the A allele (CA/AA genotypes) had significantly lower body weight, BMI, fat mass, fat-free mass, and trunk fat compared to those without the A allele (CC genotype), after adjusting for age, gender, and race. In particular, the A allele was associated with a ~5 kg lower fat mass (Figure 1). In addition, carriers of the A allele also had significantly lower triglyceride levels and higher HDL-cholesterol with a trend for lower waist circumference, resulting in a significantly lower MetS z-score (Table 2) and lower prevalence of the MetS (Table 3). Further adjustment for use of medication did not change the results (see Table, Supplemental Digital Content 1).
Table 1.
Characteristics of the Study Population for the rs8050136 of the FTO gene*
| CC (n = 66) |
CA/AA (n = 99) |
P value† | |
|---|---|---|---|
| Age (years) | 69.0 ± 3.7 | 70.0 ± 4.9 | 0.17 |
| Female sex (%) | 45 (68.2) | 61 (62.2) | 0.87 |
| College graduate (%) | 39 (59.1) | 63 (63.6) | 0.63 |
| Married (%) | 32 (48.5) | 54 (54.5) | 0.44 |
| Race/ethnicity (%) | 0.40 | ||
| Non-Hispanic white | 48 (72.7) | 76 (76.8) | |
| Hispanic white | 12 (18.2) | 10 (10.1) | |
| African-American | 5 (7.6) | 9 (9.1) | |
| Other/multiple | 1 (1.5) | 4 (4.0) | |
| Frailty measures | |||
| PPT score | 28.3 ± 2.7 | 28.0 ± 3.3 | 0.78 |
| VO2peak (ml/kg/min) | 16.6 ± 3.6 | 17.5 ± 3.6 | 0.18 |
| FSQ score | 30.5 ± 3.3 | 30.2 ± 3.1 | 0.54 |
| Knee extension strength (Nm) | 69.9 ± 24.9 | 70.9 ± 25.0 | 0.95 |
| Knee flexion strength (Nm) | 43.4 ± 15.3 | 44.5 ± 17.8 | 0.85 |
| History of cardiovascular disease, n (%) | 21 (31.8) | 33 (33.3) | 0.84 |
| Previous cigarette use, n (%) | 17 (25.8) | 22 (22.2) | 0.60 |
| Medication use | |||
| Antihypertensive, n (%) | 34 (53.1) | 50 (51.0) | 0.80 |
| Antidyslipidemic, n (%) | 16 (24.2) | 25 (25.3) | 0.52 |
| Antacid/proton-pump inhibitor (%) | 11 (16.7) | 18 (18.2) | 0.80 |
| Antiallergic (%) | 13 (19.7) | 14 (14.1) | 0.35 |
| Analgesic/nonsteroidal (%) | 18 (25.8) | 36 (36.4) | 0.22 |
| Psychotropic (%) | 7 (10.6) | 16 (16.2) | 0.31 |
| Multivitamin/supplement (%) | 17 (25.8) | 33 (33.3) | 0.30 |
Values are given as mean ± SD or number (percentage) of participants unless otherwise specified.
P-values as calculated by independent t tests for continuous variables and Chi square test for categorical variables.
Abbreviations: PPT = Modified Physical Performance Test range from 0 to 36, with higher scores indicating better physical function. Peak oxygen consumption (VO2peak) was assessed during graded treadmill walking. Scores on the Functional Status Questionnaire (FSQ) range from 0 to 36, with higher scores indicating better functional status. Nm = Newton meter.
Table 2.
Body Composition, Metabolic and Oral Glucose Tolerance Variables, and Adipocytokines for the rs8050136 of the FTO gene*
| CC (n = 66) |
CA/AA (n = 99) |
P value† | |
|---|---|---|---|
| Body weight and composition | |||
| Weight (kg) | 105.3 ± 18.9 | 98.6 ± 15.4 | 0.003 |
| Body mass index (kg/m2) | 38.5 ± 6.1 | 35.9 ± 4.6 | 0.004 |
| Fat mass (kg) | 46.7 ± 11.7 | 41.9 ± 9.1 | 0.008 |
| Fat-free mass (kg) | 58.6 ± 13.1 | 56.5 ± 12.3 | 0.02 |
| Appendicular skeletal mass (kg/m2) | 8.9 ± 1.6 | 8.7 ± 1.5 | 0.20 |
| Trunk fat (g) | 22.9 ± 55.3 | 21.2 ± 44.0 | 0.04 |
| Metabolic syndrome variables | |||
| Waist circumference (cm) | 118.1 ± 15.7 | 114.1 ± 12.3 | 0.06 |
| Systolic BP (mm Hg) | 134.7 ± 18.7 | 137.9 ± 17.6 | 0.33 |
| Diastolic BP (mm Hg) | 72.0 ± 9.7 | 74.9 ± 10.6 | 0.13 |
| Triglycerides (mg/dL) | 157.9 ± 59.1 | 136.2 ± 64.7 | 0.03 |
| HDL-cholesterol (mg/dL) | 47.3 ± 12.7 | 51.1 ± 15.1 | 0.05 |
| Fasting glucose (mg/dL) | 99.0 ± 13.6 | 97.3 ± 12.9 | 0.32 |
| MetS z-score | 1.3 ± 2.8 | 0.4 ± 2.6 | 0.01 |
| Oral glucose tolerance variables | |||
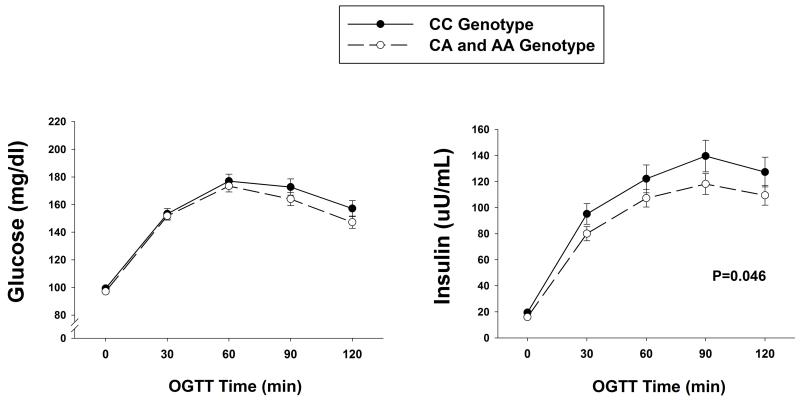
| Glucose AUC (mg·min/dL) | 18859 ± 4038 | 18018 ± 4349 | 0.19 |
| Insulin AUC (μU·min/mL) | 13032 ± 7905 | 10678 ± 6115 | 0.046 |
| HOMA-IR | 5.1 ± 4.3 | 3.9 ± 2.6 | 0.04 |
| Insulin sensitivity Index‡ | 2.5 ± 1.7 | 3.2 ± 2.4 | 0.04 |
| Adipocytokines and vitamin D | |||
| Adiponectin (ng/mL ) | 10524 ± 4863 | 12637 ± 8474 | 0.06 |
| Leptin (ug/L) | 44.4 ± 25.3 | 36.5 ± 22.3 | 0.04 |
| hs-CRP (mg/L ) | 4.0 ± 4.1 | 4.0 ± 4.1 | 0.86 |
| Interleukin-6 (pg/mL) | 2.6 ± 2.6 | 2.7 ± 2.4 | 0.86 |
| 25-hydoxyvitamin D (ng/mL) | 21.8 ± 9.5 | 24.8 ± 9.4 | 0.06 |
Values are given as mean ± SD
P-values as calculated by analyses of covariance (controlling for age, gender, and race)
Insulin sensitivity from Matsuda [17]
Abbreviations: BP = Blood Pressure; AUC, Area Under the Curve; HDL, High Density Lipoprotein; MetS, Metabolic Syndrome; HOMA-IR, Homeostasis Model Assessment of Insulin Resistance; hs-CRP, High sensitivity C-reactive protein.
Figure 1.
Body composition assessed by dual energy x-ray absorptiometry in CC genotypes and carriers of the A allele of rs8050136. CC = cytosine/cytosine, CA = cytosine/adenine, AA = adenine/adenine. Values are mean.
Table 3.
Prevalence of the Metabolic Syndrome for the rs8050136 of the FTO gene
| Participants with the Metabolic Syndrome, no (%) |
CC (n = 66) |
CA/AA (n = 99) |
P value* |
|---|---|---|---|
| 3 criteria | 26 (39.3) | 27 (27.3) | |
| 4 criteria | 19 (28.7) | 25 (25.2) | |
| 5 criteria | 5 (7.6) | 8(8.1) | |
| Total | 50 (76.6) | 60 (61.2) | 0.02 |
Between-group difference in the proportions of participants with the Metabolic Syndrome, as calculated with the use of Chi-square test.
The OGTT revealed that the insulin AUC was significantly lower in the carriers of the A allele compared to those without the A allele (Table 2, Figure 2). Despite the significantly lower insulin levels, the glucose levels during the OGTT assessed by the glucose AUC were not different between groups, providing evidence for improved insulin sensitivity. This improved insulin sensitivity was reflected in a significantly lower HOMA-IR and higher insulin sensitivity index in the CA/AA group compared to the CC group (Table 2).
Figure 2.
Plasma glucose and insulin concentrations during the oral glucose tolerance test (OGTT) in CC genotypes and carries of the A allele of rs8050136. CC = cytosine/cytosine, CA = cytosine/adenine, AA = adenine/adenine. P value indicates the significance of the difference in insulin area under the curve between groups. Values are mean ± SE.
Moreover, leptin levels were also significantly lower in the CA/AA group than in the CC group, and there was also a trend for correspondingly higher adiponectin and 25-OH vitamin D levels (Table 2). Other adipocytokines such as HS-CRP and IL-6 were not significantly different between the two groups.
Adjusting the analyses for body fat revealed that the group differences in insulin sensitivity index became borderline significant (p=0.052) while the group differences in Met-Z remained significant (P=0.03).
DISCUSSION
Our results suggest that a common polymorphism in the FTO gene (rs8050136) which was associated with high BMI and obesity in carriers of the A allele was also associated with significant differences in parameters of the metabolic syndrome among obese older adults. However in contrast to prior studies which were conducted in mostly younger subjects [15], our results indicate that carriers of the risk A allele in older adults have lower BMI, body fat, trunk fat and better metabolic profile compared to those without the A allele. In addition, older adults tend to have higher vitamin D levels relative to subjects without the A allele. Taken together our findings suggest that, unlike results from association studies in younger individuals, the risk A allele is associated with a favorable metabolic profile in obese older adults.
Two SNPs in the FTO gene (rs9939609 and rs8050136) have been reported to be associated with differences in BMI, obesity and T2D. Both of these SNPs are located in Intron 1 and were found to be in linkage disequilibrium in some population. Patients carrying the risk A allele (A/T) for rs9939609 and the risk A allele (A/C) for rs8050136 are found to have a higher risk for obesity and T2D [4-6]. Observations of differences in food intake or energy consumption according to genotype mostly among children and adolescents carrying the A allele have been reported [10, 25]. Furthermore, these polymorphisms are also linked to the development of truncal obesity, hypertension, and an increased risk of the metabolic syndrome [11, 25, 26]. Other authors likewise reported a higher risk of developing overt T2D in women carrying the A allele who had a history of gestational diabetes [27]. Collectively, the above findings suggest the importance of the FTO gene on influencing factors related to carbohydrate metabolism and this seems to be consistent among different racial/ethnic groups [5-8]. These studies were, however, predominantly conducted in younger individuals across a spectrum of body weight while little information is available in the elderly population. In the few studies that included older individuals, the authors concluded that these polymorphisms may have a greater effect on body weight in younger compared to older adults implying that these polymorphisms may not be relevant in the elderly [13, 14, 28]. In a study among persons 70 year of age, Jacobson et al [13] failed to demonstrate that a single SNP or a combination of SNPs in the FTO gene had any association with body weight, daily energy intake, or body composition. Although these patients were mostly overweight, they were not obese; thus, it remains uncertain whether these polymorphisms would have any influence on life-threatening cardiometabolic risk factors in the elderly who are already obese.
Our results indicate that in fact in the elderly who are also obese, carriers of the risk A allele have lower body weight, BMI, fat mass, trunk fat, and fat-free mass. The difference in fat mass was especially striking (~5 kg less in carriers of A allele) despite that all our older adults were already obese (BMI ≥30 kg/m2). Although this difference in body fat had no influence on degree of frailty (i.e. similar low scores in the PPT and VO2peak), it had an important influence on the cardiometabolic risks of these obese older adults. In particular, older adults with the A allele had lower triglyceride levels, higher HDL-cholesterol levels, and tended to have lower waist circumference than those without the A Allele, thus, had lower Met S z-scores and importantly, lower prevalence of the metabolic syndrome. Accordingly, since insulin resistance has been implicated as the principal mediator of the metabolic syndrome [29], carriers of the risk A allele also had greater insulin sensitivity (e.g. lower HOMA-IR, higher ISI from the OGTT), perhaps mediated by their lower leptin and/or higher adiponectin [30] and vitamin D [31] levels associated with lesser obesity. Leptin binds to specific receptors in the hypothalamus to alter the expression of several neuropeptides that regulate neuroendocrine function and energy intake and expenditure [32]. Moreover, leptin plays a central role in adipocytokine metabolism and has been proposed to be a mediator of insulin resistance and a component of the metabolic syndrome, which itself is associated with an increased risk of death [33]. Accordingly, a recent study showed that older men with high leptin levels had higher risks of CV mortality than do those with lower leptin levels [34]. As a group, our results suggest that the “risk A allele” in this subset may confer protection from unwanted cardiometabolic risk factors which this allele is associated with in younger subjects.
Our findings suggest that, in contrast to what was reported in younger individuals, the A allele, is associated with a more favorable cardiometabolic risk profile in older individuals. It is possible that despite the genetic susceptibility among carriers of the A allele for obesity and metabolic derangement during childhood and younger adult age, by the time these patient reach old age, they may have acquired some safeguards to prevent further metabolic deterioration while those with the CC genotype are predisposed to a more accelerated metabolic decline with aging and increasing weight. Another possible explanation for the paradoxical findings is that the present cohort of older obese adults may represent selective survival into very old age, such that younger obese individuals in whom the risk A allele confers worse cardiovascular disease would not have survived to this age group [35]. Although at this juncture we do not have any physiologic explanation for our findings, with the recent recognition of of epigenetics as influencing health outcomes [36], one of the future directions for this project is to explore the effect of gene methylation in the metabolic profile of our subjects. It is likely that changes in the methylation with aging and increase in body weight may result in altered protein function resulting in variable cardiometabolic profiles. Other possible biological mechanisms for the obesity paradox found in elderly persons in other studies include that obese persons have an earlier diagnosis of their disease (lead time bias), better nutritional reserve and higher lean mass in obese persons, and that since most obesity-related sequelae take years to develop, those who become obese in old age may die of non-obesity-related diseases before the adverse effects of obesity become apparent (time discrepancy of competitive risk factors) [37]. Older adults have higher risk of experiencing competing comorbidities and mortality risks than younger subjects which may lead to reduced longevity, regardless of obesity [38].
An important clinical implication for our findings is whether response to lifestyle intervention intended to modify cardiometabolic risk factors in obese older adults will be dependent on the polymorphisms in the FTO gene. In this regard, the Look AHEAD trial recently reported that FTO predicted weight regain in the support and education group although not in the intensive lifestyle intervention [39]. However, the subjects in the study were selected for T2D, and were mostly middle-aged (mean age ~59 yrs.) rather than older adults. Moreover, changes on the metabolic risk factors as it relates to the FTO gene were not included in the report. As suggested in the present and other studies [13, 14], the effects of the FTO polymorphism in the older population may be less or even opposite to that in the younger population.
To our knowledge, this is the first study exploring the effects of genetic variations in the FTO gene on the cardiometabolic risk factors in obese older adults. However, our study has important limitations. Our study is cross-sectional and does not establish a cause and effect relationship between FTO rs8050136 and cardiometabolic risk factors. Morever, our study is limited by small sample size; thus, our findings need validation in a larger population of obese older adults. Finally, our subjects are predominantly Caucasians and our results need to be confirmed in other racial groups.
In summary, we found that in a subset of obese older subjects, carriers of the A allele for the FTO rs8050136 have better cardiometabolic risk profile compared to subjects without the A allele (CC genotype). This is in contrast to what has been reported for younger individuals across a varying spectrum of body weight where the A allele was associated with greater risk of obesity, metabolic syndrome, and T2D. Considering the above findings, it would be clinically relevant, if long term, these differences in cardiometabolic profiles among the genotypes result in differences in hard outcomes such as cardiovascular events and mortality and whether this SNP modifies response to lifestyle intervention in the specific population of obese older adults.
Supplementary Material
Supplemental Digital Content 1. Table that illustrates the differences in body composition, metabolic and glucose tolerance variables, and adipocytokines for the rs8050136 of the FTO gene with further adjustment for medication use
Acknowledgments
We thank the study participants for their cooperation and the staff of the Clinical and Translational Sciences for their skilled assistance in the performance of this study.
Funding: Supported by grants RO1-AG025501, AG031176, UL 1-RR024992, UL1TR000041, P30-DK020579 and resources at the New Mexico VA Health Care System and Michael E DeBakey VA Medical Center
Footnotes
COMPETING INTERESTS
The authors declare that they have no competing interests
AUTHORS’ CONTRIBUTIONS
RA, NN, and DTV: conceived of and designed the study; DTV: obtained funding; NW, LEA, VK, GC and NN: carried out the molecular genetic studies as well as the body composition and metabolic phenotyping of all participants. RA, NW, LEA, VK, NN, RA, GC, CQ, and DTV: analyzed and interpreted the data, drafted the manuscript, and critically revised the manuscript for intellectual content. RA and DTV supervised the study. RA, DTV, and CQ performed the statistical analyses.
REFERENCE LIST
- 1.Waters DL, Ward AL, Villareal DT. Weight loss in obese adults 65years and older: A review of the controversy. Exp Gerontol. 2013;48:1054–1061. doi: 10.1016/j.exger.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond ) 2008;32:1431–1437. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- 3.Bouchonville MF, Villareal DT. Sarcopenic obesity: how do we treat it? Curr Opin Endocrinol Diabetes Obes. 2013;20:412–419. doi: 10.1097/01.med.0000433071.11466.7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, Perry JR, Elliott KS, Lango H, Rayner NW, Shields B, Harries LW, Barrett JC, Ellard S, Groves CJ, Knight B, Patch AM, Ness AR, Ebrahim S, Lawlor DA, Ring SM, Ben-Shlomo Y, Jarvelin MR, Sovio U, Bennett AJ, Melzer D, Ferrucci L, Loos RJ, Barroso I, Wareham NJ, Karpe F, Owen KR, Cardon LR, Walker M, Hitman GA, Palmer CN, Doney AS, Morris AD, Smith GD, Hattersley AT, McCarthy MI. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dina C, Meyre D, Gallina S, Durand E, Korner A, Jacobson P, Carlsson LM, Kiess W, Vatin V, Lecoeur C, Delplanque J, Vaillant E, Pattou F, Ruiz J, Weill J, Levy-Marchal C, Horber F, Potoczna N, Hercberg S, Le SC, Bougneres P, Kovacs P, Marre M, Balkau B, Cauchi S, Chevre JC, Froguel P. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet. 2007;39:724–726. doi: 10.1038/ng2048. [DOI] [PubMed] [Google Scholar]
- 6.Scuteri A, Sanna S, Chen WM, Uda M, Albai G, Strait J, Najjar S, Nagaraja R, Orru M, Usala G, Dei M, Lai S, Maschio A, Busonero F, Mulas A, Ehret GB, Fink AA, Weder AB, Cooper RS, Galan P, Chakravarti A, Schlessinger D, Cao A, Lakatta E, Abecasis GR. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet. 2007;3:e115. doi: 10.1371/journal.pgen.0030115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chauhan G, Tabassum R, Mahajan A, Dwivedi OP, Mahendran Y, Kaur I, Nigam S, Dubey H, Varma B, Madhu SV, Mathur SK, Ghosh S, Tandon N, Bharadwaj D. Common variants of FTO and the risk of obesity and type 2 diabetes in Indians. J Hum Genet. 2011;56:720–726. doi: 10.1038/jhg.2011.87. [DOI] [PubMed] [Google Scholar]
- 8.Hotta K, Kitamoto T, Kitamoto A, Mizusawa S, Matsuo T, Nakata Y, Kamohara S, Miyatake N, Kotani K, Komatsu R, Itoh N, Mineo I, Wada J, Yoneda M, Nakajima A, Funahashi T, Miyazaki S, Tokunaga K, Masuzaki H, Ueno T, Hamaguchi K, Tanaka K, Yamada K, Hanafusa T, Oikawa S, Yoshimatsu H, Sakata T, Matsuzawa Y, Nakao K, Sekine A. Association of variations in the FTO, SCG3 and MTMR9 genes with metabolic syndrome in a Japanese population. J Hum Genet. 2011;56:647–651. doi: 10.1038/jhg.2011.74. [DOI] [PubMed] [Google Scholar]
- 9.Hinney A, Nguyen TT, Scherag A, Friedel S, Bronner G, Muller TD, Grallert H, Illig T, Wichmann HE, Rief W, Schafer H, Hebebrand J. Genome wide association (GWA) study for early onset extreme obesity supports the role of fat mass and obesity associated gene (FTO) variants. PLoS ONE. 2007;2:e1361. doi: 10.1371/journal.pone.0001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cecil JE, Tavendale R, Watt P, Hetherington MM, Palmer CN. An obesity-associated FTO gene variant and increased energy intake in children. N Engl J Med. 2008;359:2558–2566. doi: 10.1056/NEJMoa0803839. [DOI] [PubMed] [Google Scholar]
- 11.Haupt A, Thamer C, Machann J, Kirchhoff K, Stefan N, Tschritter O, Machicao F, Schick F, Haring HU, Fritsche A. Impact of variation in the FTO gene on whole body fat distribution, ectopic fat, and weight loss. Obesity (Silver Spring ) 2008;16:1969–1972. doi: 10.1038/oby.2008.283. [DOI] [PubMed] [Google Scholar]
- 12.Cheung CY, Tso AW, Cheung BM, Xu A, Ong KL, Law LS, Wat NM, Janus ED, Sham PC, Lam KS. Genetic variants associated with persistent central obesity and the metabolic syndrome in a 12-year longitudinal study. Eur J Endocrinol. 2011;164:381–388. doi: 10.1530/EJE-10-0902. [DOI] [PubMed] [Google Scholar]
- 13.Jacobsson JA, Almen MS, Benedict C, Hedberg LA, Michaelsson K, Brooks S, Kullberg J, Axelsson T, Johansson L, Ahlstrom H, Fredriksson R, Lind L, Schioth HB. Detailed analysis of variants in FTO in association with body composition in a cohort of 70-year-olds suggests a weakened effect among elderly. PLoS ONE. 2011;6:e20158. doi: 10.1371/journal.pone.0020158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graff M, Gordon-Larsen P, Lim U, Fowke JH, Love SA, Fesinmeyer M, Wilkens LR, Vertilus S, Ritchie MD, Prentice RL, Pankow J, Monroe K, Manson JE, Le ML, Kuller LH, Kolonel LN, Hong CP, Henderson BE, Haessler J, Gross MD, Goodloe R, Franceschini N, Carlson CS, Buyske S, Buzkova P, Hindorff LA, Matise TC, Crawford DC, Haiman CA, Peters U, North KE. The influence of obesity-related single nucleotide polymorphisms on BMI across the life course: the PAGE study. Diabetes. 2013;62:1763–1767. doi: 10.2337/db12-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng S, Zhu Y, Xu F, Ren X, Li X, Lai M. FTO gene polymorphisms and obesity risk: a meta-analysis. BMC Med. 2011;9:71. doi: 10.1186/1741-7015-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Villareal DT, Banks M, Siener C, Sinacore DR, Klein S. Physical Frailty and Body Composition in Obese Elderly Men and Women. Obes Res. 2004;12:913–920. doi: 10.1038/oby.2004.111. [DOI] [PubMed] [Google Scholar]
- 17.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 19.WHO STEPwise approach to sureillance (STEPS). Generva, World Health Organization (WHO), 2008. 2013. [Google Scholar]
- 20.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC., Jr. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 21.Johnson JL, Slentz CA, Houmard JA, Samsa GP, Duscha BD, Aiken LB, McCartney JS, Tanner CJ, Kraus WE. Exercise training amount and intensity effects on metabolic syndrome (from Studies of a Targeted Risk Reduction Intervention through Defined Exercise) Am J Cardiol. 2007;100:1759–1766. doi: 10.1016/j.amjcard.2007.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wijndaele K, Beunen G, Duvigneaud N, Matton L, Duquet W, Thomis M, Lefevre J, Philippaerts RM. A continuous metabolic syndrome risk score: utility for epidemiological analyses. Diabetes Care. 2006;29:2329. doi: 10.2337/dc06-1341. [DOI] [PubMed] [Google Scholar]
- 23.Devlin B, Risch N. A comparison of linkage disequilibrium measures for fine-scale mapping. Genomics. 1995;29:311–322. doi: 10.1006/geno.1995.9003. [DOI] [PubMed] [Google Scholar]
- 24.Lewontin RC. On measures of gametic disequilibrium. Genetics. 1988;120:849–852. doi: 10.1093/genetics/120.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haupt A, Thamer C, Staiger H, Tschritter O, Kirchhoff K, Machicao F, Haring HU, Stefan N, Fritsche A. Variation in the FTO gene influences food intake but not energy expenditure. Exp Clin Endocrinol Diabetes. 2009;117:194–197. doi: 10.1055/s-0028-1087176. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, Dong S, Xu H, Qian J, Yang J. Genetic variants in FTO associated with metabolic syndrome: a meta- and gene-based analysis. Mol Biol Rep. 2012;39:5691–5698. doi: 10.1007/s11033-011-1377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ekelund M, Shaat N, Almgren P, Anderberg E, Landin-Olsson M, Lyssenko V, Groop L, Berntorp K. Genetic prediction of postpartum diabetes in women with gestational diabetes mellitus. Diabetes Res Clin Pract. 2012;97:394–398. doi: 10.1016/j.diabres.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 28.Qi L, Kang K, Zhang C, van Dam RM, Kraft P, Hunter D, Lee CH, Hu FB. Fat mass-and obesity-associated (FTO) gene variant is associated with obesity: longitudinal analyses in two cohort studies and functional test. Diabetes. 2008;57:3145–3151. doi: 10.2337/db08-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirk EP, Klein S. Pathogenesis and pathophysiology of the cardiometabolic syndrome. J Clin Hypertens (Greenwich ) 2009;11:761–765. doi: 10.1111/j.1559-4572.2009.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yadav A, Kataria MA, Saini V, Yadav A. Role of leptin and adiponectin in insulin resistance. Clin Chim Acta. 2013;417:80–84. doi: 10.1016/j.cca.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 31.Mezza T, Muscogiuri G, Sorice GP, Prioletta A, Salomone E, Pontecorvi A, Giaccari A. Vitamin D deficiency: a new risk factor for type 2 diabetes? Ann Nutr Metab. 2012;61:337–348. doi: 10.1159/000342771. [DOI] [PubMed] [Google Scholar]
- 32.Mantzoros CS. The role of leptin in human obesity and disease: a review of current evidence. Ann Intern Med. 1999;130:671–680. doi: 10.7326/0003-4819-130-8-199904200-00014. [DOI] [PubMed] [Google Scholar]
- 33.Dong M, Ren J. What fans the fire: insights into mechanisms of leptin in metabolic syndrome-associated heart diseases. Curr Pharm Des. 2014;20:652–658. doi: 10.2174/138161282004140213160930. [DOI] [PubMed] [Google Scholar]
- 34.Batsis JA, Sahakyan KR, Singh P, Bartels SJ, Somers VK, Lopez-Jimenez F. Leptin, adiposity, and mortality: results from the National Health and Nutrition Examination Survey III, 1988 to 1994. Mayo Clin Proc. 2015;90:481–491. doi: 10.1016/j.mayocp.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 35.Oreopoulos A, Kalantar-Zadeh K, Sharma AM, Fonarow GC. The obesity paradox in the elderly: potential mechanisms and clinical implications. Clin Geriatr Med. 2009;25:643–59. viii. doi: 10.1016/j.cger.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 36.Handel AE, Ebers GC, Ramagopalan SV. Epigenetics: molecular mechanisms and implications for disease. Trends Mol Med. 2010;16:7–16. doi: 10.1016/j.molmed.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Dorner TE, Rieder A. Obesity paradox in elderly patients with cardiovascular diseases. Int J Cardiol. 2012;155:56–65. doi: 10.1016/j.ijcard.2011.01.076. [DOI] [PubMed] [Google Scholar]
- 38.Batsis JA, Singh S, Lopez-Jimenez F. Anthropometric measurements and survival in older Americans: Results from the third National Health and Nutrition Examination Survey. J Nutr Health Aging. 2014;18:123–130. doi: 10.1007/s12603-013-0366-3. [DOI] [PubMed] [Google Scholar]
- 39.McCaffery JM, Papandonatos GD, Huggins GS, Peter I, Kahn SE, Knowler WC, Hudnall GE, Lipkin EW, Kitabchi AE, Wagenknecht LE, Wing RR. FTO predicts weight regain in the Look AHEAD clinical trial. Int J Obes (Lond ) 2013;37:1545–1552. doi: 10.1038/ijo.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. Table that illustrates the differences in body composition, metabolic and glucose tolerance variables, and adipocytokines for the rs8050136 of the FTO gene with further adjustment for medication use