Abstract
RATIONALE
The ability to adjust response strategies when faced with changes in the environment is critical for normal adaptive behavior. Such behavioral flexibility is compromised by experimental disruption of cortical GABAergic signaling, as well as in conditions such as schizophrenia and normal aging that are characterized by cortical hyperexcitability. The current studies were designed to determine whether stimulation of GABAergic signaling using the GABA(B) receptor agonist baclofen can facilitate behavioral flexibility.
METHODS
Male Fischer 344 rats were trained in a set-shifting task in which they learned to discriminate between two response levers to obtain a food reward. Correct levers were signaled in accordance with two distinct response rules (Rule 1: correct lever signaled by a cue light; Rule 2: correct lever signaled by its left/right position). The order of rule presentation varied, but they were always presented sequentially, with the trials and errors to reach criterion performance on the second (set shift) rule providing the measure of behavioral flexibility. Experiments determined the effects of the GABA(B) receptor agonist baclofen (i.p., 0, 1.0, 2.5 and 4.0 mg/kg) administered acutely before the shift to the second rule.
RESULTS
Baclofen enhanced set-shifting performance. Control experiments demonstrated that this enhancement was not simply due to improved discrimination learning, nor was it due to impaired recall of the initial discrimination rule.
CONCLUSIONS
The results demonstrate that baclofen can facilitate behavioral flexibility, suggesting that GABA(B) receptor agonists may have utility for treating behavioral dysfunction in neuropsychiatric disorders.
INTRODUCTION
The ability to flexibly modify one’s actions in response to changes in the environment is a critical aspect of normal adaptive behavior that is enabled by the prefrontal cortex (PFC). Deficits in behavioral flexibility are prevalent in psychiatric disorders such as schizophrenia and addiction, and are also associated with normal aging (Beas et al. 2013; Buckner 2004; Cunha et al. 2013; D'Cruz et al. 2013; Everett et al. 2001). Impairments in behavioral flexibility can result in maladaptive perseveration on response strategies that no longer produce the desired outcome, and can interfere with the ability to complete the normal activities of daily living. Despite the fact that interventions for improving behavioral flexibility could offer significant clinical benefit, no such pharmacological treatments currently exist.
Behavioral flexibility can be assessed in the laboratory using set-shifting tasks. Although the task details can vary, all involve shifting between response rules. Specifically, after acquisition of an initial response rule, that rule ceases to be reinforced and another response rule is introduced, the contingencies of which predict the correct response. Set-shifting reflects the ability to inhibit responding to the initial rule and adapt responding according to the second rule. Damage to primate dorsolateratal PFC or the rodent homologue, medial PFC (mPFC), does not impede learning of the individual response rules, but significantly impairs the ability to shift between rules (Birrell and Brown 2000; Bissonette and Powell 2012; Darrah et al. 2008; Demakis 2003; Dias et al. 1996; Floresco et al. 2008; Owen et al. 1991; Ragozzino 2007; Uylings et al. 2003). Beyond frank damage to PFC, behavioral flexibility is sensitive to perturbations in the balance of excitatory and inhibitory signaling within this brain region. Indeed, behavioral flexibility is impaired following a number of manipulations that disrupt GABAergic signaling, including neonatal ventral hippocampal lesions, intra-mPFC blockade of GABA(A) receptors, and genetically-induced GABAergic interneuron dysfunction (Enomoto et al. 2011) (Brady 2009; Cabungcal et al. 2014; Gruber et al. 2010; Lipska et al. 2003; Placek et al. 2013) (Bissonette et al. 2014; Cho et al. 2015; Sparta et al. 2014). Together, these findings suggest that pharmacologically enhancing inhibition may facilitate set-shifting.
Both ionotropic GABA(A) receptors and metabotropic GABA(B) receptors mediate inhibitory signaling in the PFC; however, GABA(B) receptors are of particular interest as a therapeutic target for improving PFC-supported cognition. Presynaptically, GABA(B) receptors are localized to both GABAergic and glutamatergic terminals, where they regulate neurotransmitter release. Postsynaptically, these receptors are localized to pyramidal neuron dendrites where they mediate slow inhibition and contribute to inhibitory tone (Bettler et al. 2004; McQuail et al. 2015; Wang et al. 2010). In transgenic mice with interneuron deficits, the selective GABA(B) receptor agonist baclofen normalizes pyramidal neuron hyperexcitability and restores gamma synchrony (Billingslea et al. 2014; Bortolato et al. 2007; Gandal et al. 2012; Henderson et al. 2012; Qin et al. 2015; Silverman et al. 2015). Moreover, drugs targeting GABA(B) receptors (including baclofen) enhance several aspects of cognitive function in preclinical animal models, including some that depend on the PFC (Banuelos et al. 2014; Beas et al. in press; Lasarge et al. 2009; Qin et al. 2015; Zhang et al. 2015). Finally, baclofen has a strong safety profile, as it is used clinically as a treatment for spasticity, and has been explored as a treatment for addiction (Colombo et al. 2004; Franklin et al. 2009; Garbutt et al. 2010; Liu and Wang 2015; Margetis et al. 2014; Morley et al. 2014).
The goal of this study was to determine whether pharmacological stimulation of GABA(B) receptors with baclofen can enhance behavioral flexibility in a set-shifting task (Beas et al. 2013; Floresco et al. 2008). Rats initially learned to discriminate between response levers on the basis of one of two discrimination rules. After acquiring one of the rules, the rats were “shifted” to the other rule following administration of baclofen or vehicle. We hypothesized that baclofen would facilitate behavioral flexibility as evidenced by enhanced acquisition of the second rule.
METHODS
Subjects
Male Fischer 344 rats (N=145 total, 5 months of age upon arrival) were obtained from Charles River and single-housed in the AAALAC-accredited vivarium facility in the McKnight Brain Institute Building at the University of Florida, in accordance with University of Florida IACUC and NIH guidelines. The facility was maintained at 25°C with a 12h light/dark cycle (lights on at 0800) with free access to food and water except as noted below. Prior to the start of experiments, rats were handled at least 3 times to minimize stress during testing. In addition, on the two days prior to drug injections, the rats were subjected to the handling procedures used during i.p. injections (although no actual injections were given).
Apparatus
All testing was conducted in 8 identical behavioral test chambers (30.5 × 25.4 × 30.5 cm, Coulbourn Instruments) composed of stainless steel front and back walls and transparent Plexiglas side walls. The floor was made of steel rods (0.4 cm in diameter) spaced 1.1 cm apart. A food pellet delivery trough was placed 2 cm above the floor in the center of the front wall. The food trough was equipped with a 1.12 W lamp for illumination and a photobeam for recording head entries. On each side of the trough, a retractable lever was located 11 cm above the floor, and a cue lamp (1.12 W) was placed 3.8 cm above each lever. Each chamber was located inside a sound-attenuating cubicle. An additional 1.12 W house light was mounted near the top of the rear wall of the cubicle. Food rewards consisted of individual 45 mg grain-based food pellets (PJAI, Test Diet) delivered into the food trough following a correct response. An infrared activity monitor was positioned above each test chamber to monitor locomotor activity. Test chambers were controlled by a computer equipped with Graphic State 3.01 software (Coulbourn Instruments).
Experimental Procedures
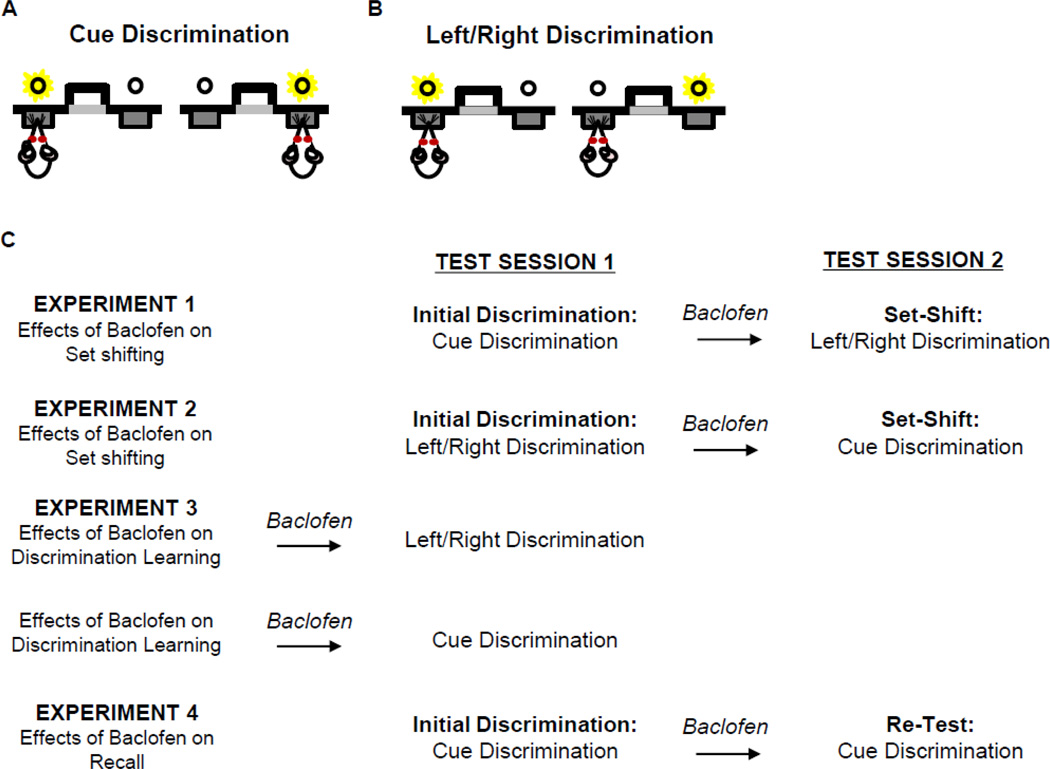
Experiment 1: Effects of systemic baclofen administration on set-shifting from visual cue to left/right discrimination
Behavioral Shaping
The design of the set-shifting task was based on that used by Floresco et al. (2008) and Beas et al. (2013). Prior to the start of testing, rats (n = 45) were reduced to 85% of their free feeding weights over the course of five days and maintained at this weight for the duration of the experiment. Rats progressed through four stages of shaping prior to the start of the set-shifting task, with new stages beginning the day immediately after completion of the previous stage. On the day prior to Shaping Stage 1, each rat was given five 45 mg food pellets in its home cage to reduce neophobia to the food reward. Shaping Stage 1 consisted of a 64-min session of magazine training, involving 38 deliveries of a single food pellet with an inter-trial interval (ITI) of 100 ± 40s. Shaping Stage 2 consisted of lever press training, in which a single lever (left or right, counterbalanced across groups) was extended and a press resulted in delivery of a single food pellet. After reaching a criterion of 50 lever presses in 30 min, rats were trained on the opposite lever using the same procedures.
Shaping Stage 3 consisted of 90 trials designed to train rats to press the levers upon their insertion into the test chamber. Each 20 s trial began with illumination of the house light and insertion of a single lever (left or right, randomly selected within each pair of trials) into the test chamber where it remained for a maximum of 10 s. A response on the lever in this time window resulted in lever retraction, delivery of a food pellet, and continued illumination of the house light for an additional 4 s. If a rat failed to respond on the lever within 10 s, the lever was retracted and the house light turned off, and the trial was scored as an omission. Rats received a minimum of four daily sessions in this stage, and were trained until reaching criterion performance of fewer than 10 omissions out of the 90 trials.
Shaping Stage 4 was designed to determine each rat’s side bias (inherent preference for one lever over the other). Each trial consisted of multiple phases. In the first phase, the house light was illuminated and both levers inserted into the test chamber. A response on either lever resulted in retraction of both levers and delivery of a single food pellet. In the second phase of a trial, both levers were again inserted, but only a response on the lever opposite to that chosen in the first phase was rewarded. A response on the same lever chosen in the first phase (i.e., “incorrect”) resulted in the levers being retracted and the house-light being extinguished. After a “correct” response in this second phase of a trial, a new trial was initiated, whereas after an “incorrect” response, the second phase was repeated until rats made a “correct” response. The session ended after a total of 45 completed trials. The side associated with the greatest number of total responses was considered a rat’s “biased” side.
Visual Cue Discrimination
Following Shaping Stage 4, rats were trained to press the lever associated with a visual cue (light). In this discrimination (Figure 1A), illumination of a cue light over one of the two response levers signaled the correct response, irrespective of the left/right position of the cue. Each 20 s trial began with illumination of one of the cue lights (left or right, randomly selected in each pair of trials). After 3 s, the house light was illuminated and both levers were inserted into the chamber (the cue light remained illuminated while the levers were extended). A press on the lever corresponding to the cue light (a correct response) resulted in the house light remaining on for 4 s, during which time the levers were retracted, the cue light was extinguished, and a single food pellet was delivered. A response on the opposite lever (an incorrect response) or failure to respond within 10 s (omission) resulted in retraction of both levers and all lights being extinguished. Rats were considered to have acquired the task upon reaching criterion performance of 8 consecutive correct trials (and at least 30 total trials, excluding omissions), with the maximum number of trials per session set at 120. If rats failed to acquire the task in a single session, they received additional sessions on subsequent days.
Figure 1. Schematic of the set-shifting task and experimental designs.
The set-shifting task employed two types of discrimination: visual cue discrimination and left/right discrimination. (A) During the visual cue discrimination, rats were required to respond on the lever illuminated by a cue light, irrespective of its left/right location. (B) During the left/right discrimination, rats were required to respond on the lever in a particular location (e.g., as in the illustration, always press the left lever), irrespective of whether that lever was illuminated by the cue light. (C) Outline of each of the four experiments.
Left/Right Discrimination (Set-shift)
After reaching criterion performance on the visual discrimination, rats were tested the next day on the left/right discrimination (i.e., the set shift; Figure 1B). In this condition, rats had to ignore the visual cue and instead choose the left or right lever (whichever was not their biased side as determined in Shaping Stage 4). Hence, accurate performance required rats to “shift” their responding away from the visual cue and toward the left/right position of the lever. Beyond the change in reward contingencies, trials were identical to those in the visual cue discrimination in all other respects (i.e., on each trial, both levers were presented, with the cue light illuminated over one lever, randomly selected in each pair of trials). Rats were considered to have acquired the task upon reaching criterion performance of 8 consecutive correct trials, excluding omissions. The maximum number of trials per session was set at 120 and rats that failed to acquire the task in a single session received additional sessions on subsequent days.
Drug Administration
Experiment 1 (Figure 1C) evaluated the effects of baclofen or vehicle on set-shifting. Rats were assigned to one of the four drug conditions on the basis of their initial (visual cue) discrimination performance, such that the groups had approximately equivalent performance. Rats received an intraperitoneal (i.p., 1.0 ml/kg) injection of either the selective GABA(B) receptor agonist baclofen (1.0 (n = 11), 2.5 (n = 9), or 4.0 (n = 6) mg/kg; Tocris, Ballwin, MO) or 0.9% saline vehicle (n = 19) 20 minutes prior to set-shifting test sessions.
Data Analyses
Data files were exported from Graphic State software and compiled using a custom macro written for Microsoft Excel (Dr. Jonathan Lifshitz, University of Kentucky). Statistical analyses were conducted using SPSS 22.0. The total numbers of trials and errors required to achieve criterion on the initial discrimination and on the set shift (excluding trial omissions) were used as indices of performance. As the task design involved presentation of the same stimuli during both the initial discrimination and the set shift, the types of errors were also examined. Specifically, comparisons between drug conditions were performed separately for errors that involved responses corresponding to previously-reinforced choices (the cue light was incongruent with the correct lever location and the rat chose based on the previous visual discrimination rule) and for errors that were never reinforced (the cue light and spatial location were congruent and the rat’s choice was not correct according to either discrimination rule (Floresco et al. 2008; Ragozzino et al. 2002)). In addition to these measures, numbers of omitted trials, response latencies (latencies to press one of the two levers after they were extended into the chamber), and locomotor activity during inter-trial intervals were recorded. Comparisons between groups were conducted using one-way ANOVA and LSD post-hoc tests when warranted. For all analyses, p values less than 0.05 were considered significant.
Experiment 2: Effects of baclofen administration on set-shifting from left/right to visual cue discrimination
Experiment 2 was designed to determine whether baclofen enhanced set-shifting performance when rats were required to shift from a left/right to a visual cue discrimination (Figure 1C). A naïve cohort of rats (n = 36) was initially shaped as described in Experiment 1. Following shaping, rats were trained on a modified version of the left/right discrimination task, which was identical to that described in Experiment 1 except that a minimum of 30 trials (in addition to performing 8 consecutive correct trials) was required to achieve criterion performance. As in Experiment 1, this first session ended when rats reached criterion performance. These rats also received an additional session of 120 trials of left/right discrimination performance on the day after reaching criterion. This session was conducted to ensure that all rats developed an attentional “set” prior to the set shift, as pilot studies suggested that initial learning of the left/right discrimination was too rapid for rats to develop a robust bias for this rule. Rats were assigned to one of four drug conditions on the basis of their initial (left/right) discrimination performance, such that the groups had approximately equivalent performance. On the following day, rats received an injection of either 0.9% saline vehicle (n = 10) or one of three doses of baclofen (1.0 (n = 10), 2.5 (n = 10), or 4.0 (n = 6) mg/kg), followed by testing in the visual cue discrimination.
Experiment 3. Effects of baclofen administration on discrimination learning
This experiment was designed to test whether baclofen enhances left/right or visual cue discrimination learning in the absence of a rule shift (Figure 1C). Two naïve cohorts of rats (n = 23 and n = 18) underwent shaping procedures as described in Experiment 1. Following completion of shaping, rats were assigned to either the left/right or visual cue discrimination task and randomly assigned to drug conditions. Rats were given an i.p. injection of either 0.9% saline vehicle or baclofen (1.0 or 2.5 mg/kg) prior to testing on acquisition of the left/right or visual cue discrimination task (i.e., in the absence of learning a prior discrimination rule). Group sizes were n = 8, 7, and 8 for groups in the left/right discrimination and n = 6, 6, and 6 for groups in the visual cue discrimination, respectively.
Experiment 4. Effects of baclofen on retrieval of a previously learned discrimination rule
This experiment was designed to test the possibility that baclofen impairs recall of a previously learned response rule (Figure 1C). A naïve cohort of rats (n = 23) was shaped and trained on the visual cue discrimination task as in Experiment 1. After reaching criterion performance on the visual cue discrimination, rats were assigned to drug conditions such that the groups had approximately equivalent performance. The following day, rats received an i.p. injection of either 0.9% saline vehicle (n = 8) or baclofen (1.0 (n = 7) or 2.5 (n = 8) mg/kg), and re-tested in the same visual cue discrimination task.
RESULTS
Experiment 1: Effects of systemic baclofen administration on set-shifting from visual cue to left/right discrimination
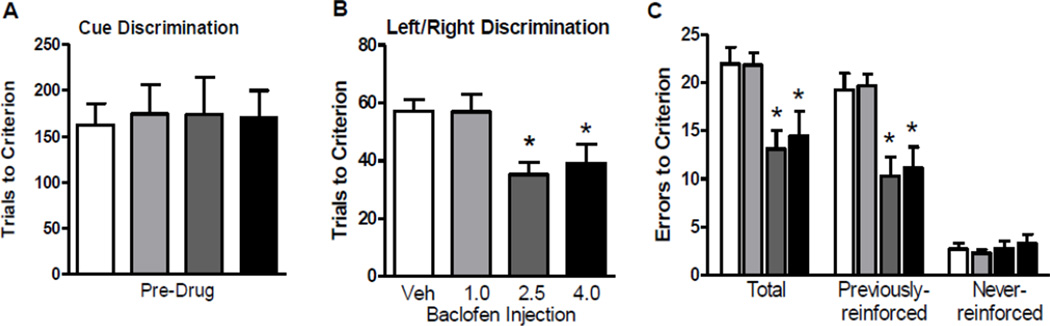
Comparisons of performance on the initial visual cue discrimination confirmed that there were no differences between groups in the number of trials needed to reach criterion (Figure 2A). On the set shift (left/right discrimination), comparisons of performance following vehicle or baclofen administration revealed significant drug effects on both trials (F(3, 44) = 4.74, p < .05; Figure 2B) and errors (F(3, 44) = 5.73, p < .05; Figure 2C) to criterion. Post hoc comparisons showed that on both measures, the 2.5 and 4.0 mg/kg doses of baclofen significantly enhanced performance compared to vehicle (ps < 0.05). Because the task design involved explicit presentation of the same set of stimuli during both the initial discrimination and the set shift, the nature of the errors committed during acquisition of the set shift was further investigated. As shown in Figure 2C, the analysis of error type revealed a main effect of drug on previously- reinforced errors (F(3, 44) = 6.32, p < .05), with post hoc comparisons showing that both the 2.5 and 4.0 mg/kg dose groups performed significantly better than the vehicle group. In contrast, there were no differences between drug groups in the number of never-reinforced errors. Considered together, these data suggest that systemic baclofen administration enhances behavioral flexibility.
Figure 2. Experiment 1: Baclofen facilitated set-shifting from visual cue to left/right discrimination learning.
(A) Trials to criterion on the visual cue (initial) discrimination. (B) Trials to criterion on the left/right (set shift) discrimination following vehicle or baclofen (1, 2.5, or 4.0 mg/kg) administration. (C) Errors to criterion on the left/right (set shift) discrimination shown for all error types total and broken out by previously- and never-reinforced error types. Data are expressed as mean + SEM. * p < 0.05 compared to vehicle.
Experiment 2: Systemic baclofen administration enhances set-shifting from left/right to visual cue discrimination
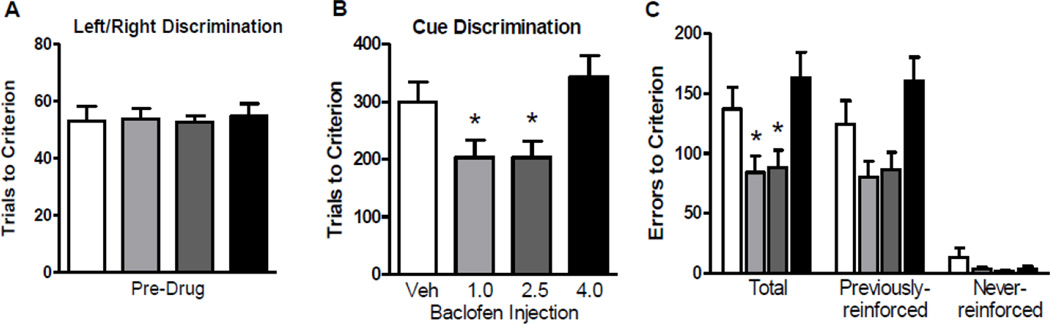
To determine whether the results of Experiment 1 were independent of the type of discrimination learning involved, a new cohort of rats (n=36) was shifted in the opposite direction from the cohort in Experiment 1. Specifically, rats were initially trained on the left/right discrimination task, and upon reaching criterion performance, received vehicle or baclofen followed by a shift to the visual cue discrimination task. Comparison of performance on the initial, left/right discrimination revealed no group differences in the number of trials required to reach criterion (Figure 3A). As in Experiment 1, comparison of performance on the set shift (visual cue discrimination) following vehicle or baclofen administration revealed significant drug effects on both trials (F(3, 32) = 4.22, p < 0.05; Figure 3B) and errors (F(3, 32) = 4.91, p < 0.05; Figure 3C) to criterion. Post hoc comparisons showed that on both measures, the 1.0 and 2.5 mg/kg baclofen groups performed significantly better than the vehicle group (ps < 0.05). An error type analysis conducted as in Experiment 1 revealed a main effect of drug on previously-reinforced errors (F(3, 32) = 4.02, p < 0.05), but no effect on never-reinforced errors.
Figure 3. Experiment 2: Baclofen facilitated set-shifting from left/right to visual cue discrimination learning.
(A) Trials to criterion on the left/right (initial) discrimination. (B) Trials to criterion on the visual cue (set shift) discrimination following vehicle or baclofen (1.0, 2.5, or 4.0 mg/kg) administration. (C) Errors to criterion on the visual cue (set shift) discrimination shown for all error types total and broken out by previously- and never-reinforced error types. Data are expressed as mean + SEM. * p < 0.05 compared to vehicle.
Experiment 3. Effects of baclofen administration on discrimination learning
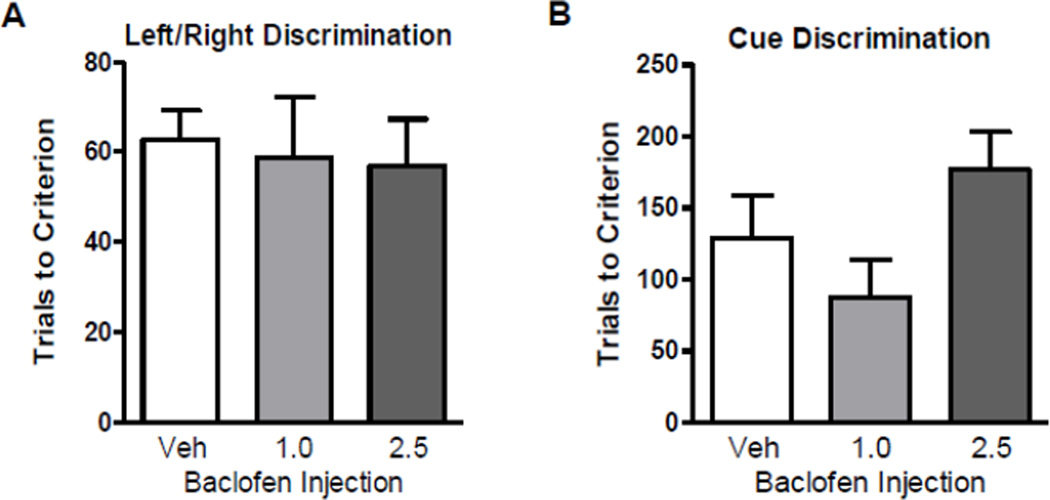
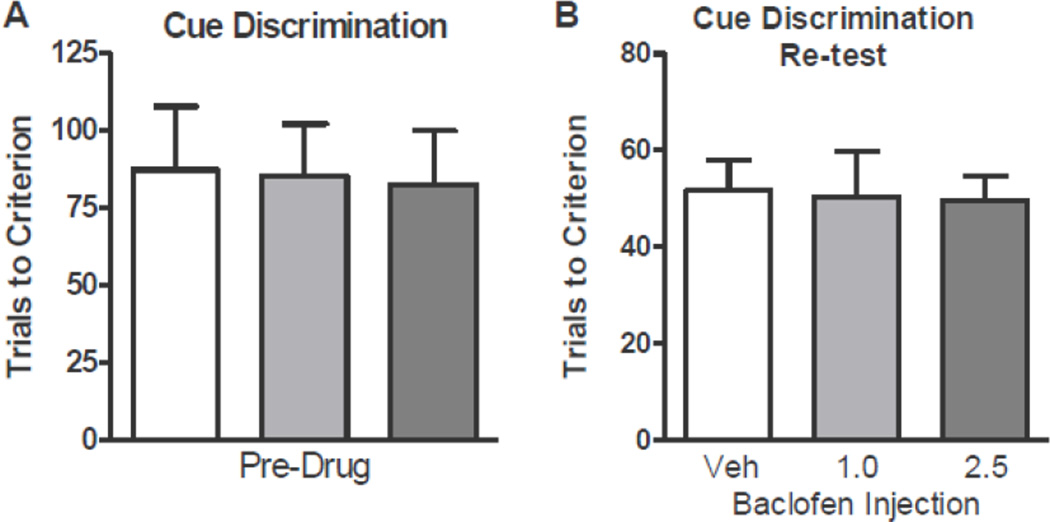
Experiments 1 and 2 showed that baclofen enhanced acquisition of both a left/right and a visual cue discrimination rule in the context of a set shift (i.e., following learning of a previous rule). These data suggest that baclofen enhances behavioral flexibility; however, an alternative explanation is that baclofen more generally enhances discrimination learning. To assess this possibility, the effects of baclofen on acquisition of the left/right and visual discrimination rules were tested in the absence of prior rule learning. Comparisons of performance revealed no effects of drug condition on the number of trials to reach criterion on either the left/right (Figure 4A) or visual cue (Figure 4B) discrimination, suggesting that the effects of baclofen on set-shifting observed in Experiments 1 and 2 were not the result of non-specific enhancement of discrimination learning.
Figure 4. Experiment 3: Baclofen had no effect on acquisition of left/right or visual cue discriminations.
(A) Trials to criterion on acquisition of the left/right discrimination following vehicle or baclofen (1.0 or 2.5 mg/kg) administration. (B) Trials to criterion on acquisition of the visual cue discrimination following vehicle or baclofen (1.0 or 2.5 mg/kg) administration Data are expressed as mean + SEM.
Experiment 4. Effects of baclofen on retrieval of a previously learned discrimination rule
As baclofen can impair memory in certain contexts (Arolfo et al. 1998; Castellano et al. 1989; Stackman and Walsh 1994), it is possible that the enhanced set-shifting performance observed in Experiments 1 and 2 resulted from effects of baclofen on recall of the initial discrimination rule. Specifically, impaired recall of the initial rule would be expected to minimize interference from the previously learned contingencies, thereby facilitating learning of the second rule. To test this possibility, rats were trained on the visual cue discrimination task. Upon reaching criterion, rats were assigned to drug groups as in Experiment 1 and then re-tested on the visual discrimination task on the following day. Comparisons of performance revealed no differences between drug groups in the number of trials required to initially acquire the visual discrimination task (Figure 5A). Following systemic administration of vehicle or baclofen, comparisons of performance revealed no differences between drug groups in trials to reach criterion during the re-test of the visual discrimination task (Figure 5B). These results show that baclofen does not impair recall of a previously-learned response rule.
Figure 5. Experiment 4: Baclofen had no effect on recall of visual cue discrimination learning.
(A) Trials to criterion on acquisition of the visual cue discrimination. (B) Trials required to re-establish criterion performance on re-test of the visual cue discrimination following vehicle or baclofen (1.0 or 2.5 mg/kg) administration. Data are expressed as mean + SEM.
Effects of Baclofen on Trial Omissions, Locomotor Activity and Response Latency
In addition to choice accuracy described above, other measures of task performance were assessed with the specific intent of determining whether non-specific effects of baclofen could account for the drug’s enhancement of set-shifting. As reported below and in Table 1, baclofen did influence trial omissions, response latency and locomotor activity in some experiments; however these effects were inconsistent across experiments and could not account for baclofen’s effects on set-shifting. In Experiment 1 (visual cue to left/right shift), baclofen produced an increase in the number of omitted trials (F(3, 44) = 4.53, p < 0.05) and mean latency to lever press (F(3, 44) = 5.26, p < 0.05), but no significant effects of baclofen were observed in Experiment 2 (left/right to visual cue shift) on any of these measures. In Experiment 3, baclofen produced a significant increase in response latency (F(2, 20) = 11.92, p < 0.05) and a decrease in locomotor activity (F(2, 20) = 9.40, p < 0.05) on the left/right discrimination but had no significant effects on the number of trial omissions. In contrast, baclofen produced a significant increase in trial omissions (F(2, 17) = 10.02, p < 0.05) on the visual cue discrimination in Experiment 3 but no changes in response latency or locomotor activity. Baclofen had no effect on any of the measures in Experiment 4.
Table 1.
Effects of baclofen on number of omitted trials, locomotor activity, and latency to respond at the lever
| Experiment | Omitted trials | Locomotion (locomotor units/ITI) | Latency |
|---|---|---|---|
| Experiment 1. | |||
| Vehicle | 3.05 (1.78) | 6.49 (1.72) | 1.71 (0.38) |
| 1.0 mg/kg Baclofen | 3.36 (2.77) | 4.22 (0.51) | 1.11 (0.74) |
| 2.5 mg/kg Baclofen | 20.55 (7.39)* | 2.24 (0.79) | 3.47 (0.62)* |
| 4.0 mg/kg Baclofen | 31.17 (17.79)* | 0.95 (0.14) | 3.93 (1.29)* |
| Experiment 2. | |||
| Vehicle | 9.90 (3.50) | 4.18 (0.95) | 1.72 (0.20) |
| 1.0 mg/kg Baclofen | 3.40 (1.60) | 5.32 (0.61) | 1.18 (0.17) |
| 2.5 mg/kg Baclofen | 11.00 (7.99) | 3.58 (0.70) | 1.56 (0.32) |
| 4.0 mg/kg Baclofen | 11.67 (4.63) | 3.41 (0.43) | 1.64 (0.16) |
| Experiment 3 (left/right discrimination). | |||
| Vehicle | 6.12 (2.03) | 7.00 (1.18) | 2.08 (0.41) |
| 1.0 mg/kg Baclofen | 1.18 (0.52) | 6.48 (1.49) | 1.76 (0.16) |
| 2.5 mg/kg Baclofen | 26.62 (14.77) | 1.03 (0.42)* | 4.42 (0.55)* |
| Experiment 3 (visual cue discrimination). | |||
| Vehicle | 1.33 (0.61) | 3.22 (0.53) | 1.69 (0.25) |
| 1.0 mg/kg Baclofen | 0.33 (0.21) | 3.91 (0.67) | 1.70 (0.10) |
| 2.5 mg/kg Baclofen | 4.17 (0.87)* | 2.03 (0.39) | 1.90 (0.25) |
| Experiment 4. | |||
| Vehicle | 8.50 (8.50) | 8.25 (1.63) | 1.24 (0.22) |
| 1.0 mg/kg Baclofen | 0.28 (0.28) | 7.05 (1.53) | 1.20 (0.21) |
| 2.5 mg/kg Baclofen | 0.12 (0.12) | 5.25 (1.09) | 1.23 (0.17) |
Asterisks indicate a significant difference from vehicle as indicated by post hoc comparisons. Values represent means (SEMs)
DISCUSSION
Baclofen is used clinically for treatment of muscle spasticity associated with multiple sclerosis and cerebral palsy (Baker et al. 2014; Overgard et al. 2015; Rekand and Gronning 2011). More recently, it has been investigated for treatment of addiction and autistic disorders. (Kahn et al. 2009; Muzyk et al. 2012)(Berry-Kravis et al. 2012; Erickson et al. 2014). It is notable that these latter conditions are characterized by behavioral inflexibility, including impairments in laboratory set-shifting tasks (Casten et al. 2011; Maes et al. 2011; Van der Molen et al. 2012). Based on this prior clinical work, the goal of the current studies was to test the utility of baclofen to specifically enhance behavioral flexibility. Indeed, impaired flexibility accompanies many neuropsychiatric diseases and can contribute to maladaptive perseverative behaviors and an inability to readily accomplish the activities of daily living (D'Cruz et al. 2013; Enomoto et al. 2011; Floresco et al. 2009; Gass et al. 2014; George et al. 2015; Gruber et al. 2010; Placek et al. 2013). The current experiments demonstrate that acute systemic baclofen administration facilitates behavioral flexibility in rats, and suggest that this drug may be of utility for clinical conditions in which behavioral flexibility is impaired.
Experiments 1 and 2 used a set-shifting task to demonstrate that systemic baclofen administration enhances behavioral flexibility. This task required the effective inhibition of an initial discrimination rule and an adaptation to response contingencies associated with a second (set shift) rule. Rats given baclofen required fewer trials and errors to reach criterion performance on the second rule compared to rats given vehicle. These data are consistent with the interpretation that baclofen enhanced the ability to shift effectively from one response strategy to another. Notably, however, an alternative explanation for this pattern of performance is that baclofen directly enhanced learning of the second rule (left/right lever discrimination in Experiment 1 and visual cue discrimination in Experiment 2), rather than facilitating behavioral flexibility per se. Indeed, in rodents, baclofen is reported to enhance performance on delayed response and radial arm maze tasks, and to reverse methamphetamine-induced deficits in object recognition (Arias et al. 2009; Escher and Mittleman 2004; Levin et al. 2004). Experiment 3 addressed this possibility by evaluating the effects of baclofen on acquisition of the left/right and visual cue discrimination in rats that had not already learned a competing response rule (i.e., when task acquisition did not require a rule shift). Under these conditions, baclofen had no effect on acquisition of either rule, demonstrating that this drug does not broadly enhance either type of discrimination learning. The distinct effects of baclofen in Experiments 1 and 2 vs. Experiment 3 are consistent with findings from prior behavioral pharmacology and lesion/inactivation studies demonstrating that different neural mechanisms mediate learning of an initial discrimination rule compared to learning to shift from one rule to another. Systemic administration of antagonists at muscarinic cholinergic and 5-HT7 receptors (Chen et al. 2004; Nikiforuk 2012), as well as acute or chronic stress (Bondi et al. 2008; Butts et al. 2013), alter set-shifting performance without affecting initial discrimination learning. Similarly, lesions or inactivation of mPFC impair set-shifting but not initial discrimination learning (Birrell and Brown 2000; Bissonette and Powell 2012; Bissonette et al. 2013; Floresco et al. 2008; Ragozzino 2007). These latter studies suggest that the mPFC is a potential site of action for the enhancing effects of baclofen on set-shifting; indeed, we recently showed that intra-mPFC baclofen administration enhances set-shifting performance in aged Fischer 344 rats in a manner similar to that in Experiment 1 (Beas et al. 2016). Future experiments in which baclofen is administered directly into the young rat mPFC prior to set-shifting would be useful for confirming the site of action for the behavioral enhancement reported here.
The fact that baclofen facilitated set-shifting irrespective of the order of the presentation of discrimination rules (Experiments 1 and 2) provides support for the conclusion that its enhancing effects are not unique to a particular set of discrimination contingencies but instead reflect improved behavioral flexibility (i.e., an enhanced ability to shift from one rule to another). It is notable, however, that while baclofen enhanced set-shifting in both conditions, the most effective doses differed somewhat depending on the direction of the shift. Both 2.5 and 4.0 mg/kg baclofen improved performance of rats shifted from the visual cue to left/right discrimination, whereas 1.0 and 2.5 mg/kg baclofen improved performance of rats shifted from the left/right to visual cue discrimination. Importantly, pharmacological manipulations that enhance cognitive performance almost always, if not always, do so in an inverted U-shaped dose response curve, such that doses that are too low have no effect and doses that are too high may also have no effect or even impair performance (Arnsten et al. 2015; Wood et al. 2014). The peaks of these curves (i.e., the most effective doses) are influenced by a variety of factors including the specific task demands, stress levels, and animal strain. In the current study, the differences in the effective doses of baclofen may relate to the relative difficulty of the two discriminations employed. A previous study showed that increasing the difficulty of the second (shift) discrimination rendered performance more sensitive to the effects of mPFC inactivation (Floresco et al. 2008). In a similar manner, the greater difficulty of the visual cue discrimination compared to the left/right discrimination in the present study (compare vehicle group performance in Figures 3B and 2B) may have rendered performance more sensitive to the effects of a lower dose of baclofen.
Accurate performance on the set-shifting task requires not only acquisition of a new response rule but also effective inhibition of a previously-learned response rule. Given that baclofen can impair memory in a variety of contexts (Arolfo et al. 1998; Castellano et al. 1989; Stackman and Walsh 1994), one explanation for its enhancing effects on set-shifting is that it may interfere with recall of the initial discrimination rule. Impaired recall of the initial rule would be expected to facilitate set-shifting as there would be less interference from this prior learning during acquisition of the second rule. Experiment 4 addressed this possibility by evaluating the effects of baclofen on retention of an initial (visual cue) discrimination rule at the same time point at which the second rule was introduced in the set-shifting task in Experiment 1. Baclofen had no effect on performance in this context, suggesting that the enhancing effects of baclofen on set-shifting cannot be attributed to impaired recall of the initial discrimination rule.
Baclofen can induce sedation and reduced locomotor activity in rodents (Beveridge et al. 2013; Li et al. 2013). Consistent with such findings, baclofen reduced locomotor activity and increased response latencies and trial omissions in some of the experiments (Table 1). For several reasons, however, it is unlikely that these effects account for baclofen’s actions on set-shifting. First, because the task employed a discrete-trials procedure, an increase in trial omissions or response latencies would not be expected to influence choice accuracy (which reflected whether trials were correct or incorrect rather than the number of correct trials, as omitted trials were excluded when calculating trials and errors to criterion). Second, the effects of baclofen on locomotion, response latencies, and trial omissions were inconsistent across experiments, suggesting that its effects on task performance were relatively weak. Most importantly, however, baclofen had no effect on locomotion, response latencies, or trial omissions in Experiment 2, in which it robustly enhanced set-shifting. This finding suggests that the effects of baclofen on these measures were unrelated to its enhancing effects on set-shifting.
It is notable that some previous studies have reported that Fischer 344 rats are more anxious in comparison to other rat strains (Faraday 2002; van der Staay et al. 2009). As baclofen has been used clinically to reduce anxiety (Knapp et al. 2007; Li et al. 2013; Morley et al. 2014) and stress/anxiety can modulate behavioral flexibility (Butts et al. 2013; Hurtubise & Howland 2016), one interesting, albeit speculative, hypothesis is that the enhancement in behavioral flexibility produced by baclofen in the current study is via its anxiolytic properties. While this represents a fertile avenue of future investigation, it is notable that baseline (vehicle) Fischer 344 set-shifting performance in the current study was actually slightly better than in a previous study from our lab using identical task parameters in Long-Evans rats (Shimp et al. 2015). As the Long-Evans strain is reported to be less anxious than others (Turner and Burne 2014), it is unlikely that excessive anxiety in the Fischer 344 strain was the sole mediating factor for the enhancing effects of baclofen on behavioral flexibility observed here.
Acknowledgments
We thank Ms. Kailey Simpson, Ms. Shannon Wall, Ms. Miranda Schwabe, and Ms. Lauren Vetere for assistance with behavioral testing. This work was supported by NIH grant R01AG029421 (JLB), the McKnight Brain Research Foundation (JLB), the NSF Graduate Research Fellowship Program DGE-0802270 (BSB) and a Diversity Supplement to NIH grant R01AG029421 (BSB).
Contributor Information
B. Sofia Beas, Email: sofiabeas@ufl.edu.
Barry Setlow, Email: setlow@ufl.edu.
Jennifer L. Bizon, Email: bizonj@ufl.edu.
REFERENCES
- Arias C, Mlewski EC, Molina JC, Spear NE. Naloxone and baclofen attenuate ethanol's locomotor-activating effects in preweanling Sprague-Dawley rats. Behav Neurosci. 2009;123:172–180. doi: 10.1037/a0014049. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Wang M, Paspalas CD. Dopamine's Actions in Primate Prefrontal Cortex: Challenges for Treating Cognitive Disorders. Pharmacol Rev. 2015;67:681–696. doi: 10.1124/pr.115.010512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arolfo MP, Zanudio MA, Ramirez OA. Baclofen infused in rat hippocampal formation impairs spatial learning. Hippocampus. 1998;8:109–113. doi: 10.1002/(SICI)1098-1063(1998)8:2<109::AID-HIPO2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Baker KW, Tann B, Mutlu A, Gaebler-Spira D. Improvements in children with cerebral palsy following intrathecal baclofen: use of the Rehabilitation Institute of Chicago Care and Comfort Caregiver Questionnaire (RIC CareQ) J Child Neurol. 2014;29:312–317. doi: 10.1177/0883073812475156. [DOI] [PubMed] [Google Scholar]
- Banuelos C, Beas BS, McQuail JA, Gilbert RJ, Frazier CJ, Setlow B, Bizon JL. Prefrontal cortical GABAergic dysfunction contributes to age-related working memory impairment. J Neurosci. 2014;34:3457–3466. doi: 10.1523/JNEUROSCI.5192-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beas BS, McQuail JA, Banuelos C, Setlow B, Bizon JL. Prefrontal cortical GABAergic signaling and impaired behavioral flexibility in aged F344 rats. Neuroscience. 2016 doi: 10.1016/j.neuroscience.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beas BS, Setlow B, Bizon JL. Distinct manifestations of executive dysfunction in aged rats. Neurobiol Aging. 2013;34:2164–2174. doi: 10.1016/j.neurobiolaging.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry-Kravis EM, Hessl D, Rathmell B, Zarevics P, Cherubini M, Walton-Bowen K, Mu Y, Nguyen DV, Gonzalez-Heydrich J, Wang PP, Carpenter RL, Bear MF, Hagerman RJ. Effects of STX209 (arbaclofen) on neurobehavioral function in children and adults with fragile X syndrome: a randomized, controlled, phase 2 trial. Sci Transl Med. 2012;4:152ra127. doi: 10.1126/scitranslmed.3004214. [DOI] [PubMed] [Google Scholar]
- Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev. 2004;84:835–867. doi: 10.1152/physrev.00036.2003. [DOI] [PubMed] [Google Scholar]
- Beveridge TJ, Smith HR, Porrino LJ. Differential development of tolerance to the functional and behavioral effects of repeated baclofen treatment in rats. Pharmacol Biochem Behav. 2013;106:27–32. doi: 10.1016/j.pbb.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billingslea EN, Tatard-Leitman VM, Anguiano J, Jutzeler CR, Suh J, Saunders JA, Morita S, Featherstone RE, Ortinski PI, Gandal MJ, Lin R, Liang Y, Gur RE, Carlson GC, Hahn CG, Siegel SJ. Parvalbumin cell ablation of NMDA-R1 causes increased resting network excitability with associated social and self-care deficits. Neuropsychopharmacology. 2014;39:1603–1613. doi: 10.1038/npp.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, Bae MH, Suresh T, Jaffe DE, Powell EM. Prefrontal cognitive deficits in mice with altered cerebral cortical GABAergic interneurons. Behav Brain Res. 2014;259:143–151. doi: 10.1016/j.bbr.2013.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, Powell EM. Reversal learning and attentional set-shifting in mice. Neuropharmacology. 2012;62:1168–1174. doi: 10.1016/j.neuropharm.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, Powell EM, Roesch MR. Neural structures underlying set-shifting: roles of medial prefrontal cortex and anterior cingulate cortex. Behav Brain Res. 2013;250:91–101. doi: 10.1016/j.bbr.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology. 2008;33:320–331. doi: 10.1038/sj.npp.1301410. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Frau R, Orru M, Piras AP, Fa M, Tuveri A, Puligheddu M, Gessa GL, Castelli MP, Mereu G, Marrosu F. Activation of GABA(B) receptors reverses spontaneous gating deficits in juvenile DBA/2J mice. Psychopharmacology (Berl) 2007;194:361–369. doi: 10.1007/s00213-007-0845-5. [DOI] [PubMed] [Google Scholar]
- Brady AM. Neonatal ventral hippocampal lesions disrupt set-shifting ability in adult rats. Behav Brain Res. 2009;205:294–298. doi: 10.1016/j.bbr.2009.07.025. [DOI] [PubMed] [Google Scholar]
- Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Butts KA, Floresco SB, Phillips AG. Acute stress impairs set-shifting but not reversal learning. Behav Brain Res. 2013;252:222–229. doi: 10.1016/j.bbr.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Cabungcal JH, Counotte DS, Lewis EM, Tejeda HA, Piantadosi P, Pollock C, Calhoon GG, Sullivan EM, Presgraves E, Kil J, Hong LE, Cuenod M, Do KQ, O'Donnell P. Juvenile antioxidant treatment prevents adult deficits in a developmental model of schizophrenia. Neuron. 2014;83:1073–1084. doi: 10.1016/j.neuron.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano C, Brioni JD, Nagahara AH, McGaugh JL. Post-training systemic and intra-amygdala administration of the GABA-B agonist baclofen impairs retention. Behav Neural Biol. 1989;52:170–179. doi: 10.1016/s0163-1047(89)90285-9. [DOI] [PubMed] [Google Scholar]
- Casten KS, Gray AC, Burwell RD. Discrimination learning and attentional set formation in a mouse model of Fragile X. Behav Neurosci. 2011;125:473–479. doi: 10.1037/a0023561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KC, Baxter MG, Rodefer JS. Central blockade of muscarinic cholinergic receptors disrupts affective and attentional set-shifting. Eur J Neurosci. 2004;20:1081–1088. doi: 10.1111/j.1460-9568.2004.03548.x. [DOI] [PubMed] [Google Scholar]
- Cho KK, Hoch R, Lee AT, Patel T, Rubenstein JL, Sohal VS. Gamma rhythms link prefrontal interneuron dysfunction with cognitive inflexibility in Dlx5/6(+/−) mice. Neuron. 2015;85:1332–1343. doi: 10.1016/j.neuron.2015.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo G, Addolorato G, Agabio R, Carai MA, Pibiri F, Serra S, Vacca G, Gessa GL. Role of GABA(B) receptor in alcohol dependence: reducing effect of baclofen on alcohol intake and alcohol motivational properties in rats and amelioration of alcohol withdrawal syndrome and alcohol craving in human alcoholics. Neurotox Res. 2004;6:403–414. doi: 10.1007/BF03033315. [DOI] [PubMed] [Google Scholar]
- Cunha PJ, Goncalves PD, Ometto M, Dos Santos B, Nicastri S, Busatto GF, de Andrade AG. Executive cognitive dysfunction and ADHD in cocaine dependence: searching for a common cognitive endophenotype for addictive disorders. Front Psychiatry. 2013;4:126. doi: 10.3389/fpsyt.2013.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Cruz AM, Ragozzino ME, Mosconi MW, Shrestha S, Cook EH, Sweeney JA. Reduced behavioral flexibility in autism spectrum disorders. Neuropsychology. 2013;27:152–160. doi: 10.1037/a0031721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrah JM, Stefani MR, Moghaddam B. Interaction of N-methyl-D-aspartate and group 5 metabotropic glutamate receptors on behavioral flexibility using a novel operant set-shift paradigm. Behav Pharmacol. 2008;19:225–234. doi: 10.1097/FBP.0b013e3282feb0ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demakis GJ. A meta-analytic review of the sensitivity of the Wisconsin Card Sorting Test to frontal and lateralized frontal brain damage. Neuropsychology. 2003;17:255–264. doi: 10.1037/0894-4105.17.2.255. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- Enomoto T, Tse MT, Floresco SB. Reducing prefrontal gamma-aminobutyric acid activity induces cognitive, behavioral, and dopaminergic abnormalities that resemble schizophrenia. Biol Psychiatry. 2011;69:432–441. doi: 10.1016/j.biopsych.2010.09.038. [DOI] [PubMed] [Google Scholar]
- Erickson CA, Veenstra-Vanderweele JM, Melmed RD, McCracken JT, Ginsberg LD, Sikich L, Scahill L, Cherubini M, Zarevics P, Walton-Bowen K, Carpenter RL, Bear MF, Wang PP, King BH. STX209 (arbaclofen) for autism spectrum disorders: an 8-week open-label study. J Autism Dev Disord. 2014;44:958–964. doi: 10.1007/s10803-013-1963-z. [DOI] [PubMed] [Google Scholar]
- Escher T, Mittleman G. Effects of ethanol and GABAB drugs on working memory in C57BL/6J and DBA/2J mice. Psychopharmacology (Berl) 2004;176:166–174. doi: 10.1007/s00213-004-1875-x. [DOI] [PubMed] [Google Scholar]
- Everett J, Lavoie K, Gagnon JF, Gosselin N. Performance of patients with schizophrenia on the Wisconsin Card Sorting Test (WCST) J Psychiatry Neurosci. 2001;26:123–130. [PMC free article] [PubMed] [Google Scholar]
- Faraday MM. Rat sex and strain differences in responses to stress. Physiol Behav. 2002;75:507–522. doi: 10.1016/s0031-9384(02)00645-5. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Block AE, Tse MT. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav Brain Res. 2008;190:85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Zhang Y, Enomoto T. Neural circuits subserving behavioral flexibility and their relevance to schizophrenia. Behav Brain Res. 2009;204:396–409. doi: 10.1016/j.bbr.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Harper D, Kampman K, Kildea-McCrea S, Jens W, Lynch KG, O'Brien CP, Childress AR. The GABA B agonist baclofen reduces cigarette consumption in a preliminary double-blind placebo-controlled smoking reduction study. Drug Alcohol Depend. 2009;103:30–36. doi: 10.1016/j.drugalcdep.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandal MJ, Sisti J, Klook K, Ortinski PI, Leitman V, Liang Y, Thieu T, Anderson R, Pierce RC, Jonak G, Gur RE, Carlson G, Siegel SJ. GABAB-mediated rescue of altered excitatory-inhibitory balance, gamma synchrony and behavioral deficits following constitutive NMDAR-hypofunction. Transl Psychiatry. 2012;2:e142. doi: 10.1038/tp.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbutt JC, Kampov-Polevoy AB, Gallop R, Kalka-Juhl L, Flannery BA. Efficacy and safety of baclofen for alcohol dependence: a randomized, double-blind, placebo-controlled trial. Alcohol Clin Exp Res. 2010;34:1849–1857. doi: 10.1111/j.1530-0277.2010.01273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Glen WB, Jr, McGonigal JT, Trantham-Davidson H, Lopez MF, Randall PK, Yaxley R, Floresco SB, Chandler LJ. Adolescent alcohol exposure reduces behavioral flexibility, promotes disinhibition, and increases resistance to extinction of ethanol self-administration in adulthood. Neuropsychopharmacology. 2014;39:2570–2583. doi: 10.1038/npp.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SA, Rodriguez-Santiago M, Riley J, Abelson JL, Floresco SB, Liberzon I. Alterations in cognitive flexibility in a rat model of post-traumatic stress disorder. Behav Brain Res. 2015;286:256–264. doi: 10.1016/j.bbr.2015.02.051. [DOI] [PubMed] [Google Scholar]
- Gruber AJ, Calhoon GG, Shusterman I, Schoenbaum G, Roesch MR, O'Donnell P. More is less: a disinhibited prefrontal cortex impairs cognitive flexibility. J Neurosci. 2010;30:17102–17110. doi: 10.1523/JNEUROSCI.4623-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson C, Wijetunge L, Kinoshita MN, Shumway M, Hammond RS, Postma FR, Brynczka C, Rush R, Thomas A, Paylor R, Warren ST, Vanderklish PW, Kind PC, Carpenter RL, Bear MF, Healy AM. Reversal of disease-related pathologies in the fragile X mouse model by selective activation of GABAB receptors with arbaclofen. Sci Transl Med. 2012;4:152ra128. doi: 10.1126/scitranslmed.3004218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtubise JL, Howland JG. Effects of stress on behavioral flexibility in rodents. Neurosci. 2016 doi: 10.1016/j.neuroscience.2016.04.007. pii: S0306-4522(16)30062-8. [DOI] [PubMed] [Google Scholar]
- Kahn R, Biswas K, Childress AR, Shoptaw S, Fudala PJ, Gorgon L, Montoya I, Collins J, McSherry F, Li SH, Chiang N, Alathari H, Watson D, Liberto J, Beresford T, Stock C, Wallace C, Gruber V, Elkashef A. Multi-center trial of baclofen for abstinence initiation in severe cocaine-dependent individuals. Drug Alcohol Depend. 2009;103:59–64. doi: 10.1016/j.drugalcdep.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Breese GR. Baclofen blocks expression and sensitization of anxiety-like behavior in an animal model of repeated stress and ethanol withdrawal. Alcohol Clin Exp Res. 2007;31:582–595. doi: 10.1111/j.1530-0277.2007.00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasarge CL, Banuelos C, Mayse JD, Bizon JL. Blockade of GABA(B) receptors completely reverses age-related learning impairment. Neuroscience. 2009;164:941–947. doi: 10.1016/j.neuroscience.2009.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Weber E, Icenogle L. Baclofen interactions with nicotine in rats: effects on memory. Pharmacol Biochem Behav. 2004;79:343–348. doi: 10.1016/j.pbb.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Li X, Risbrough VB, Cates-Gatto C, Kaczanowska K, Finn MG, Roberts AJ, Markou A. Comparison of the effects of the GABAB receptor positive modulator BHF177 and the GABAB receptor agonist baclofen on anxiety-like behavior, learning, and memory in mice. Neuropharmacology. 2013;70:156–167. doi: 10.1016/j.neuropharm.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipska BK, Lerman DN, Khaing ZZ, Weinberger DR. The neonatal ventral hippocampal lesion model of schizophrenia: effects on dopamine and GABA mRNA markers in the rat midbrain. Eur J Neurosci. 2003;18:3097–3104. doi: 10.1111/j.1460-9568.2003.03047.x. [DOI] [PubMed] [Google Scholar]
- Liu J, Wang LN. Baclofen for alcohol withdrawal. Cochrane Database Syst Rev. 2015;4:CD008502. doi: 10.1002/14651858.CD008502.pub4. [DOI] [PubMed] [Google Scholar]
- Maes JH, Eling PA, Wezenberg E, Vissers CT, Kan CC. Attentional set shifting in autism spectrum disorder: differentiating between the role of perseveration, learned irrelevance, and novelty processing. J Clin Exp Neuropsychol. 2011;33:210–217. doi: 10.1080/13803395.2010.501327. [DOI] [PubMed] [Google Scholar]
- Margetis K, Papageorgiou G, Gatzonis S, Politis K, Siatouni A, Sakas D. Intrathecal baclofen improves psychiatric symptoms in spasticity patients. J Clin Psychopharmacol. 2014;34:374–379. doi: 10.1097/JCP.0000000000000105. [DOI] [PubMed] [Google Scholar]
- McQuail JA, Frazier CJ, Bizon JL. Molecular aspects of age-related cognitive decline: the role of GABA signaling. Trends Mol Med. 2015;21:450–460. doi: 10.1016/j.molmed.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley KC, Baillie A, Leung S, Addolorato G, Leggio L, Haber PS. Baclofen for the Treatment of Alcohol Dependence and Possible Role of Comorbid Anxiety. Alcohol Alcohol. 2014 doi: 10.1093/alcalc/agu062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzyk AJ, Rivelli SK, Gagliardi JP. Defining the role of baclofen for the treatment of alcohol dependence: a systematic review of the evidence. CNS Drugs. 2012;26:69–78. doi: 10.2165/11597320-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Nikiforuk A. Selective blockade of 5-HT7 receptors facilitates attentional set-shifting in stressed and control rats. Behav Brain Res. 2012;226:118–123. doi: 10.1016/j.bbr.2011.09.006. [DOI] [PubMed] [Google Scholar]
- Overgard TM, Kjaersgaard-Hansen L, Soe M, Illum NO. Positive experience with intrathecal baclofen treatment in children with severe cerebral palsy. Dan Med J. 2015;62:A4999. [PubMed] [Google Scholar]
- Owen AM, Roberts AC, Polkey CE, Sahakian BJ, Robbins TW. Extra-dimensional versus intra-dimensional set shifting performance following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia. 1991;29:993–1006. doi: 10.1016/0028-3932(91)90063-e. [DOI] [PubMed] [Google Scholar]
- Placek K, Dippel WC, Jones S, Brady AM. Impairments in set-shifting but not reversal learning in the neonatal ventral hippocampal lesion model of schizophrenia: further evidence for medial prefrontal deficits. Behav Brain Res. 2013;256:405–413. doi: 10.1016/j.bbr.2013.08.034. [DOI] [PubMed] [Google Scholar]
- Qin M, Huang T, Kader M, Krych L, Xia Z, Burlin T, Zeidler Z, Zhao T, Smith CB. R-Baclofen Reverses a Social Behavior Deficit and Elevated Protein Synthesis in a Mouse Model of Fragile X Syndrome. Int J Neuropsychopharmacol. 2015;18 doi: 10.1093/ijnp/pyv034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Ann N Y Acad Sci. 2007;1121:355–375. doi: 10.1196/annals.1401.013. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Ragozzino KE, Mizumori SJ, Kesner RP. Role of the dorsomedial striatum in behavioral flexibility for response and visual cue discrimination learning. Behav Neurosci. 2002;116:105–115. doi: 10.1037//0735-7044.116.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekand T, Gronning M. Treatment of spasticity related to multiple sclerosis with intrathecal baclofen: a long-term follow-up. J Rehabil Med. 2011;43:511–514. doi: 10.2340/16501977-0811. [DOI] [PubMed] [Google Scholar]
- Shimp KG, Mitchell MR, Beas BS, Bizon JL, Setlow B. Affective and cognitive mechanisms of risky decision making. Neurobiol Learn Mem. 2015;117:60–70. doi: 10.1016/j.nlm.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Pride MC, Hayes JE, Puhger KR, Butler-Struben HM, Baker S, Crawley JN. GABAB Receptor Agonist R-Baclofen Reverses Social Deficits and Reduces Repetitive Behavior in Two Mouse Models of Autism. Neuropsychopharmacology. 2015;40:2228–2239. doi: 10.1038/npp.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparta DR, Hovelso N, Mason AO, Kantak PA, Ung RL, Decot HK, Stuber GD. Activation of prefrontal cortical parvalbumin interneurons facilitates extinction of reward-seeking behavior. J Neurosci. 2014;34:3699–3705. doi: 10.1523/JNEUROSCI.0235-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackman RW, Walsh TJ. Baclofen produces dose-related working memory impairments after intraseptal injection. Behav Neural Biol. 1994;61:181–185. doi: 10.1016/s0163-1047(05)80073-1. [DOI] [PubMed] [Google Scholar]
- Turner KM, Burne TH. Comprehensive behavioural analysis of Long Evans and Sprague-Dawley rats reveals differential effects of housing conditions on tests relevant to neuropsychiatric disorders. PLoS One. 2014;9:e93411. doi: 10.1371/journal.pone.0093411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Van der Molen MJ, Van der Molen MW, Ridderinkhof KR, Hamel BC, Curfs LM, Ramakers GJ. Attentional set-shifting in fragile X syndrome. Brain Cogn. 2012;78:206–217. doi: 10.1016/j.bandc.2011.12.008. [DOI] [PubMed] [Google Scholar]
- van der Staay FJ, Schuurman T, van Reenen CG, Korte SM. Emotional reactivity and cognitive performance in aversively motivated tasks: a comparison between four rat strains. Behav Brain Funct. 2009;5:50. doi: 10.1186/1744-9081-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Neubauer FB, Luscher HR, Thurley K. GABAB receptor-dependent modulation of network activity in the rat prefrontal cortex in vitro. Eur J Neurosci. 2010;31:1582–1594. doi: 10.1111/j.1460-9568.2010.07191.x. [DOI] [PubMed] [Google Scholar]
- Wood S, Sage JR, Shuman T, Anagnostaras SG. Psychostimulants and cognition: a continuum of behavioral and cognitive activation. Pharmacol Rev. 2014;66:193–221. doi: 10.1124/pr.112.007054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Xu C, Tu H, Wang Y, Sun Q, Hu P, Hu Y, Rondard P, Liu J. GABAB receptor upregulates fragile X mental retardation protein expression in neurons. Sci Rep. 2015;5:10468. doi: 10.1038/srep10468. [DOI] [PMC free article] [PubMed] [Google Scholar]