Abstract
The importance of angiogenesis in Pancreatic Ductal Adenocarcinoma (PDAC) and its therapeutic potential have been explored in both pre-clinical and clinical studies. Human PDACs overexpress a number of angiogenic factors and their cognate high-affinity receptors, and anti-angiogenic agents reduce tumor volume, metastasis, and microvessel density (MVD), and improve survival in subcutaneous and orthotopic pre-clinical models. Nonetheless, clinical trials using anti-angiogenic therapy have been overwhelmingly unsuccessful. This review will focus on these pre-clinical and clinical studies, the potential reasons for failure in the clinical setting, and ways these shortcomings could be addressed in future investigations of angiogenic mechanisms in PDAC.
Keywords: Pancreatic cancer, Angiogenesis, Vascular endothelial growth factor (VEGF)
1. Introduction
Pancreatic Ductal Adenocarcinoma (PDAC), which comprises >85% of pancreatic cancers, is the 4th leading cause of cancer death in the United States with a 1- and 5-year relative survival of 28% and 7%, respectively [1–3]. These statistics are largely due to advanced stage at clinical presentation, the high frequency of major driver mutations, marked resistance to chemotherapy and radiation, and extensive desmoplasia that impedes drug delivery [4–8]. Because advances in screening, prevention, and treatment are limited compared to other cancers, PDAC is now projected to surpass breast, prostate, and colorectal cancers to become the second leading cause of cancer death by 2030 [9].
At presentation, only 15–20% of patients are eligible for surgical resection, the only chance for cure [1–3]. Even then, outcomes are poor, with a 5 year survival between 20–25% post-resection, since most of these patients develop disease recurrence [10]. Therefore, chemotherapy is recommended as adjuvant treatment for those undergoing surgical resection and is the mainstay of treatment for patients with locally advanced or metastatic disease [2]. The current standard of care for patients with metastatic disease includes gemcitabine plus nab-paclitaxel or fluorouracil plus leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX) [2, 11].
2. Angiogenesis
Blood vessel growth throughout adult life is primarily achieved via angiogenesis [12–18]. However, the adult vasculature is mostly quiescent as only 0.01% of the endothelium undergoes cell division at any time [12, 13, 15, 17, 18]. Examples of physiologic angiogenesis in the adult include wound healing, tissues undergoing growth, exercise induced angiogenesis in heart and skeletal muscle, the hair cycle, skeletal growth, and female reproductive processes. Pathologic examples include intraocular neovascular disorders, infantile haemangiomas, immunogenic rheumatoid arthritis, psoriasis, and tumorigenesis [12, 13, 16–20].
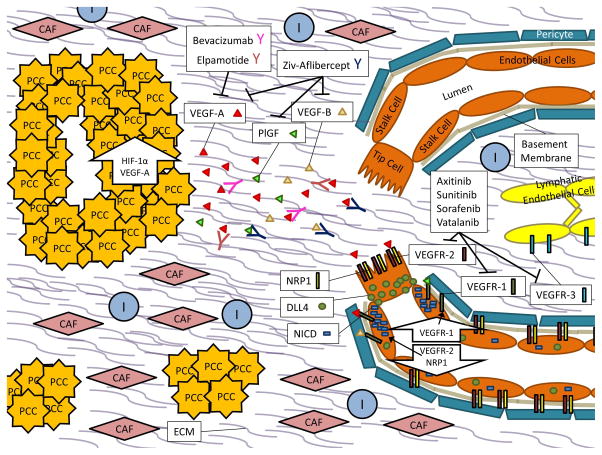
Through the use of models like the mouse retina, which becomes vascularized postnatally, we now understand many of the key players and processes involved in physiologic angiogenesis [21]. In general, activation of endothelial cells by pro-angiogenic molecules leads to the detachment of pericytes from the endothelium and remodeling of the basement membrane and cell-to-cell junctions (Figure 1) [22]. The best known pro-angiogenic molecule is vascular endothelial growth factor A (gene: VEGFA) (VEGF-A). VEGF-A binds to vascular endothelial growth factor receptor 2 (gene: KDR) (VEGFR-2) on endothelial cells, and its signaling is enhanced by the neuropilin-1 (NRP1) co-receptor, which facilitates complex internalization (Figure 1) [22]. Downstream signaling results in increased expression of the Notch ligand delta-like protein 4 (DLL4), which binds to Notch receptors on neighboring endothelial cells (Figure 1) [22]. This releases the notch intracellular domain (NICD) in these cells, which down-regulates VEGFR-2 and NRP1, and up-regulates vascular endothelial growth factor receptor 1 (gene: FLT1) (VEGFR-1), a decoy receptor for VEGF-A (Figure 1) [22].
Figure 1. PDAC Angiogenesis.
In PDAC, Pancreatic Cancer Cells (PCCs) proliferate within a desmoplastic stroma that consists of both cellular components such as Cancer Associated Fibroblasts (CAFs), Immune Cells (Is), and Endothelial Cells (ECs) as well as Extracellular Matrix (ECM) components like soluble growth factors, cytokines, collagens, fibronectin, laminin, glycoproteins, and proteoglycans.
Up-regulation of hypoxia inducible factor 1, alpha subunit (gene: HIF1A) (HIF-1α) and the pro-angiogenic molecule VEGF-A within PCCs results in secretion of VEGF-A molecules into the tumor microenvironment. When VEGF-A signals through VEGFR-2 and its NRP1 co-receptor on endothelial cells, downstream signaling results in increased expression of DLL4. DLL4 will bind to Notch receptors on neighboring cells, subsequently releasing NICD, which then down-regulates VEGFR-2 and NRP1 expression and up-regulates expression of the VEGFR-1 decoy receptor. This favors migration of a tip cell towards the VEGF-A gradient while the neighboring stalk cells become de-sensitized to the signal. In the quiescent vasculature, DLL4 and Notch signaling are balanced.
Small molecule inhibitors of angiogenesis, such as Axitinib, Sunitinib, Sorafenib, and Vatalanib primarily act on the vascular endothelial growth factor receptor complexes (VEGFR-1, VEGFR-2, and Vascular endothelial growth factor receptor 3 (gene: FLT4) (VEGFR-3)) while recombinent protein inhibitors of angiogenesis like Bevacizumab, Elpamotide, and Ziv-Aflibercept act on vascular endothelial growth factor ligands like VEGF-A, vascular endothelial growth factor B (gene: VEGFB) (VEGF-B), and/or placenta growth factor (gene: PGF) (PlGF).
The goal of this process is to isolate one cell that will migrate toward the pro-angiogenic gradient (called the tip cell), while de-sensitizing neighboring cells to the same signal. It is believed that DLL4 and Notch signaling are balanced in the quiescent vasculature, and that tip cells will offset the balance in response to pro-angiogenic signals [14]. The cells adjacent to the tip cell are called stalk cells, and they proliferate behind the tip cell to elongate the sprout and form a lumen (Figure 1) [22]. Once two tip cells on different sprouts meet, they will anastomose to form a perfused branch (Figure 1) [22]. Basement membrane then forms, and pericytes are recruited to cover the vessel (Figure 1) [22]. The process is dynamic in that endothelial cells will compete for the tip position with different cells displaying the phenotype over time.
3. Tumor Angiogenesis
Whereas physiologic angiogenesis is tightly controlled and comes to a resolution, pathologic angiogenesis is abnormal and does not resolve [13, 16, 17, 20, 21]. Because cells need nutrients and oxygen from nearby capillaries to function and survive, early tumor growth is often restricted to a volume of only a few cubic millimeters until they are able to switch to an angiogenic phenotype [13, 16, 17, 19, 20, 23, 24]. Activation of angiogenesis occurs when pro-angiogenic molecules predominate over anti-angiogenic molecules, whereas, inactivation occurs when the anti-angiogenic molecules dominate [12, 13, 25]. In tumorigenesis, the observed activation from a quiescent state is often described as an “angiogenic switch” [12, 13, 25].
The vessels formed during tumor angiogenesis are tortuous or disorganized, immature, and convoluted with excessive vessel branching that lacks pericyte coverage rendering them fragile and leaky with bleeding and exudation of plasma proteins [15–18, 21, 22, 24, 26]. The distribution of new vessels in the tumor is also heterogeneous with some areas demonstrating intense neovascularization [15, 19, 20, 22, 26]. The vessels are often functionally defective with low blood flow and reduced oxygen delivery due to high interstitial pressure [15, 18, 22, 26]. The resulting hypoxic environment exacerbates the pathologic condition by further up-regulating pro-angiogenic molecules [15, 22, 26]. While one might assume that neovascularization would improve delivery of chemotherapeutic agents to the tumor, the poor perfusion and compression of the vascular supply actually impedes drug delivery [15, 16, 18, 20, 22]. Therefore, in addition to inhibiting angiogenesis and causing vessel regression, anti-angiogenic agents can enhance the effects of simultaneously administered chemotherapeutic drugs by normalizing the remaining vasculature [15, 16, 18, 20–22, 26].
4. PDAC is Hypovascular
Though the previously discussed concepts are generalities common to many cancers, we now specifically consider concepts relevant to PDAC. Using the KrasLSL−G12D/+; Trp53LSL−R172H/+; Pdx-1-Cre (KPC) PDAC mouse model, which has oncogenic kirsten rat sarcoma viral oncogene homolog (Kras) and mutated transformation related protein 53 (Trp53) in the pancreas due to Cre-mediated recombination, Olive et al. showed that KPC tumors are poorly vascularized, poorly perfused, and have impaired drug delivery when compared to KPC transplant models or normal mouse pancreas [27]. Likewise, using both KrasLSL−G12D/+; Pdx-1-Cre (KC) mice, which have oncogenic Kras in the pancreas due to Cre-mediated recombination, and KPC mice, Provenzano et al. reported that in addition to having reduced vascularity, KC and KPC tumors have a paucity of large diameter (>10 um) vessels when compared to normal mouse pancreas [28]. This is likely due to vascular collapse caused by the presence of very high interstitial fluid pressures in these tumors, in the range of 75–130 mm Hg, compared to 8–13 mm Hg in normal mouse pancreas [28]. This observation also offers an explanation for the poor perfusion and drug delivery observed by Olive et al. [27]. Human PDAC samples were also shown to be poorly vascularized compared to normal human pancreas or adjacent normal human pancreas, and to have fewer large diameter vessels compared to adjacent normal human pancreas [27, 28].
Because PDAC is inherently hypovascular, it might be assumed that this cancer either does not demonstrate significant angiogenesis or that it is not likely to benefit from anti-angiogenic agents. However, both concepts have been disproven in other cancers [29]. All tumor types need sufficient levels of nutrients and oxygen and are growth limited unless they are able to induce angiogenesis. This is also true of hypoxic tumors, which likely have increased requirements to drain away toxic by-products released by cancer cells. Instead of measuring angiogenesis, microvessel density (MVD) rather reflects the metabolic burden of the supported tumor cells [29]. In fact, because the oxygen consumption rate is often lower in tumors compared to the corresponding normal tissue, it is not uncommon for tumors to have lower MVDs as we see in PDAC [29]. This is also the case for renal cell carcinoma, a cancer known clinically to respond to anti-angiogenic therapy [29]. Both poorly and highly vascularized cancers have been shown to respond to anti-angiogenic therapy [29].
5. Correlation of VEGF-A Expression or Microvessel Density with Health Outcomes in PDAC
VEGF-A, a potent inducer of angiogenesis, was first discovered as a secreted protein that can enhance vascular permeability [12]. Many different isoforms exist, and their different binding affinities for heparan sulfate proteogylycans (HSPGs) function to create a gradient for guiding vessels during vascular development [16]. In recent years, more insight into the alternative splicing and translation of the gene has revealed that anti-angiogenic forms and a translational read through can also be produced [30, 31].
Using immunohistochemistry (IHC), several groups found that between 60–65% of human PDAC samples have a substantial amount of VEGF-A immunoreactivity [32–34]. In terms of gene expression, Ikeda et al. found that 27/40 (67.5%) human PDAC samples overexpress VEGFA compared to a colon cancer cell line while Itakura et al. found a 5.2 fold increase in VEGFA expression in human PDAC samples (n=7) compared to normal human pancreas samples (n=4) [32, 34]. More recently, by RNA-Seq, The Cancer Genome Atlas (TCGA) dataset shows that only 8 out of 178 (4%) human PDAC samples overexpress VEGFA, suggesting that this molecule may not be as important in PDAC as was first surmised [8, 35, 36].
MVD has not been shown to be an accurate measure of angiogenesis in other cancers [29]; nonetheless, three [32–34] of four [37] studies of human PDAC samples have shown an association between VEGFA mRNA or VEGF-A protein (IHC) expression and the amount of vascularity seen in the tumor. Patients with high levels of VEGFA mRNA or VEGF-A protein (IHC) also had increased liver metastasis [33], larger tumors [34], enhanced local spread [34], and decreased survival in two [32, 33] out of four [34, 37] studies. Lastly, one [32] out of two [37] studies reported that increased vascularity was associated with decreased patient survival.
6. Pre-Clinical Studies Targeting VEGF Signaling in PDAC
Many studies have examined the potential role of targeting VEGF signaling using subcutaneous or orthotopic nude mouse models of human PDAC. Injection of human PDAC cells expressing an anti-sense VEGFA into the flanks of nude mice led to an 80% reduction in tumor size compared to controls [38]. When diphtheria toxin, which inhibits protein synthesis in target cells, was fused with VEGF-A to target it to the vasculature in orthotopic nude mouse models of human PDAC, it led to reduced tumor volume, tumor spread, and MVD, and improvement in survival in 1 of 2 models [39]. Injection of adenovirus vectors encoding the soluble form of the decoy receptor VEGFR-1 into subcutaneous tumor xenografts of human PDAC in SCID mice also resulted in reduced tumor growth and MVD [40]. Additionally, injection of adenovirus vectors encoding soluble VEGFR-1 or soluble VEGFR-1 plus a soluble fibroblast growth factor receptor 1 (gene: FGFR1) (FGFR-1) into subcutaneous tumor xenografts of human PDAC in nude mice resulted in reduced tumor growth [41].
The tyrosine kinase inhibitor PTK 787/ZK222584 (vatalanib) targets VEGF receptors, the platelet-derived growth factor receptors (PDGFRs), the mast/stem cell growth factor receptor Kit (gene: KIT) (SCFR), and macrophage colony-stimulating factor 1 receptor (CSF1R). Use of this compound in an orthotopic nude mouse model of human PDAC led to reduced tumor volume and MVD, and increased survival [42]. Moreover, use of VEGF-Trap (ziv-aflibercept), which is a recombinant fusion protein of the extracellular portions of VEGFR-1 and VEGFR-2 and the Fc fragment of human immunoglobulin IgG1, resulted in reduced tumor growth and MVD in subcutaneous tumor xenografts of human PDAC and reduced tumor growth and metastasis in an orthotopic nude mouse model of human PDAC [43]. These promising results provide support for the testing of anti-VEGF agents in human PDAC clinical trials.
7. Clinical Studies in PDAC
To date, many phase II and phase III human PDAC clinical trials using different anti-angiogenic agents have been completed. Several of these involved bevacizumab, an anti-VEGF-A monoclonal antibody, that has already been FDA approved for the treatment of several other cancer types, including persistent, recurrent, or metastatic cervical cancer, metastatic colorectal cancer, or non-small cell lung cancer in combination with chemotherapy; metastatic renal cell carcinoma in combination with interferon alpha; or in glioblastoma as a second-line therapy.
An initial Phase II trial of bevacizumab plus gemcitabine in untreated advanced PDAC patients showed a 21% objective response rate (ORR), a 6-month survival rate of 77%, and a median survival of 8.8 months (Table 1) [44]. Because these were favorable numbers compared to the pivotal trial for gemcitabine approval [45], which observed an ORR of 5%, a 6-month survival rate of 46%, and a median survival of 5.7 months, several other Phase II and Phase III studies were launched.
Table 1.
Phase II Clinical Trials using Anti-Angiogenic Agents in PDAC
| Ref | Phase | Group | Drug | Experimental Arm | Active Comparator Arm | Hazard Ratio |
|---|---|---|---|---|---|---|
| [44] Kindler et al. 2005 | II | advanced | Bevacizumab (anti-VEGF-A monoclonal antibody) |
|
NA | NA |
| [46] Ko et al. 2008 | II | metastatic | Bevacizumab (anti-VEGF-A monoclonal antibody) |
|
NA | NA |
| [47] Javle et al. 2009 | II | advanced | Bevacizumab (anti-VEGF-A monoclonal antibody) |
|
NA | NA |
| [48] Crane et al. 2009 | II | locally advanced (unresectable) | Bevacizumab (anti-VEGF-A monoclonal antibody) |
|
NA | NA |
| [49] Fogelman et al. 2011 | II | advanced | Bevacizumab (anti-VEGF-A monoclonal antibody) |
|
NA | NA |
| [50] Small et al. 2011 | II | localized | Bevacizumab (anti-VEGF-A monoclonal antibody) |
|
NA | NA |
| [52] Astsaturov et al. 2011 | II | metastatic | Bevacizumab (anti-VEGF-A monoclonal antibody) |
|
|
NA |
| [51] Van Buren II et al. 2013 | II | localized (potentially resectable) | Bevacizumab (anti-VEGF-A monoclonal antibody) |
|
NA | NA |
| [67] Spano et al. 2008 | II | advanced | Axitinib (SMI of VEGFRs) |
|
|
|
| [69] O’Reilly et al. 2010 | II | metastatic (second-line therapy) | Sunitinib (SMI of VEGFRs, PDGFRs, SCFR) |
|
NA | NA |
| [70] Reni et al. 2013 | II | metastatic (maintenance therapy) | Sunitinib (SMI of VEGFRs, PDGFRs, SCFR) |
|
|
|
| [71] El-Khoueiry et al. 2012 | II | metastatic | Sorafenib (SMI of BRAF, VEGFR-2, PDGFRB) |
|
|
NA |
| [72] Kindler et al. 2012 | II | advanced | Sorafenib (SMI of BRAF, VEGFR-2, PDGFRB) |
|
NA | NA |
| [74] Cascinu et al. 2014 | II | advanced | Sorafenib (SMI of BRAF, VEGFR-2, PDGFRB) |
|
|
|
| [76] Dragovich et al. 2014 | II | advanced (second-line therapy) | Vatalanib (SMI of VEGFRs, PDGFRs, SCFR, CSF1R) |
|
NA | NA |
Ref, Reference; SMI, small molecule inhibitor; ORR, objective response rate; OS, overall survival; PFS, progression free survival; TTP, time to progression; HR, hazard ratio; m, month(s); d, days(s); (95% confidence interval); *, statistically significant; VEGF-A, vascular endothelial growth factor A (gene: VEGFA); VEGFR, vascular endothelial growth factor receptor; PDGFR, platelet-derived growth factor receptor; SCFR, mast/stem cell growth factor receptor Kit (gene: KIT); BRAF, serine/threonine-protein kinase B-raf; VEGFR-2, vascular endothelial growth factor receptor 2 (gene: KDR); PDGFRB, platelet-derived growth factor receptor beta; CSF1R, macrophage colony-stimulating factor 1 receptor
Several Phase II trials added bevacizumab to any existing regimen that had previously shown any sort of modest activity in PDAC. These regimens included: cisplatin and gemcitabine [46]; capecitabine and gemcitabine [47]; capecitabine, radiation, and gemcitabine [48]; oxaliplatin and gemcitabine [49]; gemcitabine and radiation [50, 51], and docetaxel [52] (Table 1). However, results from the Phase III trial directly comparing bevacizumab plus gemcitabine to placebo plus gemcitabine in advanced PDAC patients showed that the addition of bevacizumab does not result in an improvement in overall survival (OS) or progression free survival (PFS) or differences in the ORR (Table 3) [53].
Table 3.
Phase III Clinical Trials using Anti-Angiogenic Agents in PDAC
| Ref | Phase | Group | Drug | Experimental Arm | Active Comparator Arm | Hazard Ratio |
|---|---|---|---|---|---|---|
| [66] Van Cutsem et al 2009 | III | metastatic | Bevacizumab (anti-VEGF-A monoclonal antibody) |
|
|
|
| [53] Kindler et al. 2010 | III | advanced | Bevacizumab (anti-VEGF-A monoclonal antibody) |
|
|
|
| [68] Kindler et al. 2011 | III | advanced | Axitinib (SMI of VEGFRs) |
|
|
|
| [75] Gonçalves et al. 2012 | III | advanced | Sorafenib (SMI of BRAF, VEGFR-2, PDGFRB) |
|
|
|
| [77] Rougier et al. 2013 | III | advanced | Ziv-Aflibercept (recombinant fusion protein that traps VEGF-A, VEGF-B, PlGF) |
|
|
|
| [79] Yamaue et al. 2015 | III | advanced or metastatic | Elpamotide (epitope peptide of VEGFR-2) |
|
|
|
Ref, Reference; SMI, small molecule inhibitor; ORR, objective response rate; OS, overall survival; PFS, progression free survival; HR, hazard ratio; m, month(s), (95% confidence interval); *, statistically significant; VEGF-A, vascular endothelial growth factor A (gene: VEGFA); VEGFR, vascular endothelial growth factor receptor; BRAF, serine/threonine-protein kinase B-raf; VEGFR-2, vascular endothelial growth factor receptor 2 (gene: KDR); PDGFRB, platelet-derived growth factor receptor beta; VEGF-B, vascular endothelial growth factor B (gene: VEGFB); PlGF, placenta growth factor (gene: PGF)
The difference between the Phase II and Phase III results was suggested to be due to the Phase II trial recruiting a more fit population [53]. Because such disparities are common in trials of PDAC, it was also suggested that the use of a single-arm Phase II trial is not ideal [53]. The majority of Phase II trials with other regimens were single-arm trials, and thus, most of them also concluded that the addition of bevacizumab produced questionable benefit.
In addition to VEGF-A, epidermal growth factor receptor (EGFR) and its ligands are commonly overexpressed in human PDAC, and high expression levels are also associated with worse outcomes [54–57]. The addition of cetuximab, a monocloncal antibody targeting EGFR, to gemcitabine has not led to improvements in ORRs, PFS, or OS [58], but the addition of erlotinib, a small molecule inhibitor of EGFR, to gemcitabine has been shown to provide a statistically significant improvement in survival [59]. However, the clinical relevance of this result is often questioned since the median gain in survival is only 10 days [59].
There is also evidence for EGFR’s role in angiogenesis and simultaneous inhibition of EGFR and VEGFR-2 has been shown to be synergistic [54, 56, 60–62]. Therefore, several regimens combining cetuximab or erlotinib with bevacizumab have been tried with limited success (Table 2) [63–65]. A Phase III trial comparing bevacizumab plus erlotinib plus gemcitabine to placebo plus erlotinib plus gemcitabine in metastatic PDAC patients did not show benefit in OS, but it did show a statistically significant one month improvement in the median PFS (Table 3) [66]. Therefore, there is some rationale for using this drug combination in metastatic PDAC patients.
Table 2.
Phase II Clinical Trials using an Anti-Angiogenic Agent + EGFR Inhibitor in PDAC
| Ref | Phase | Group | Drug | Experimental Arm | Active Comparator Arm | Hazard Ratio |
|---|---|---|---|---|---|---|
| [63] Ko et al. 2010 | II | metastatic | Bevacizumab (anti-VEGF-A monoclonal antibody) |
|
NA | NA |
| [64] Ko et al. 2012 | II | advanced | Bevacizumab (anti-VEGF-A monoclonal antibody) |
|
|
NA |
| [65] Watkins et al. 2014 | II | advanced | Bevacizumab (anti-VEGF-A monoclonal antibody) |
|
NA | NA |
| [73] Cardin et al. 2014 | II | advanced | Sorafenib (SMI of BRAF, VEGFR-2, PDGFRB) |
|
NA | NA |
Ref, Reference; SMI, small molecule inhibitor; ORR, objective response rate; OS, overall survival; PFS, progression free survival; TTP, time to progression; HR, hazard ratio; m, month(s); d, days(s); (95% confidence interval); EGFR, epidermal growth factor receptor; VEGF-A, vascular endothelial growth factor A (gene: VEGFA); BRAF, serine/threonine-protein kinase B-raf; VEGFR-2, vascular endothelial growth factor receptor 2 (gene: KDR); PDGFRB, platelet-derived growth factor receptor beta
Additional anti-angiogenic agents that have been tried in human PDAC include axitinib, sunitinib, sorafenib, vatalanib, ziv-aflibercept, and elpamotide. The Phase II or III trial comparing axitinib, a VEGFR tyrosine kinase inhibitor, plus gemcitabine to gemcitabine alone did not provide a significant improvement in overall or PFS (Table 1, Table 3) [67, 68].
Sunitinib is a small molecule tyrosine kinase inhibitor of VEGFRs, PDGFRs, and SCFR. Though a Phase III study has not been done, this molecule has been tested in the metastatic setting as either a second-line therapy [69] or as a maintenance therapy in patients who did not progress after first-line chemotherapy [70]. Interestingly, in these patient groups, the drug did not do well as a second-line therapy (Table 1), but produced a statistically significant improvement in PFS compared to observation alone in the maintenance setting (hazard ratio (HR) 0.51 [95% confidence interval (CI): 0.29–0.89], p-value < 0.01) [70]. Because the duration of first-line chemotherapy is often debated due to its cumulative toxicity and unproven efficacy, sunitinib may offer an advantage in the maintenance setting.
Similarly, sorafenib is a small molecule tyrosine kinase inhibitor of serine/threonine-protein kinase B-raf (BRAF), VEGFRs, and platelet-derived growth factor receptor beta (PDGFRB) that has been tested in many different settings without benefit (Table 1, Table 2) [71–74]. These observations were confirmed in a Phase III trial that observed no improvement in overall or PFS upon the addition of sorafenib to gemcitabine in the treatment of advanced PDAC patients (Table 3) [75].
Vatalanib is also a multi-kinase inhibitor targeting VEGFRs, PDGFRs, SCFR, and CSF1R. In a Phase II trial, it was used as a second-line therapy in advanced PDAC patients and produced a favorable 6 month survival rate of 29% compared to historic controls (Table 1) [76]. However, it was only a single-arm trial, and with the failure of several other similar receptor tyrosine kinsase inhibitors, it remains to be seen whether this drug will pan out.
Ziv-aflibercept, a recombinant fusion protein consisting of the extracellular portions of VEGFR-1 and VEGFR-2 and the Fc fragment of human immunoglobulin IgG1, is another drug that targets the VEGF pathway by trapping VEGF-A, VEGF-B, and PlGF. This drug yielded negative results in a Phase III trial compared to gemcitabine alone (Table 3) [77].
Elpamotide, a VEGFR-2 peptide, is a vaccine immunotherapy that can induce a cellular immune response against VEGFR-2 expressing endothelial cells [78, 79]. In a Phase II/III trial (Table 3) of locally advanced or metastatic pancreatic cancer patients, there were no improvements in overall or PFS compared to gemcitabine alone, but a subgroup with severe injection site reactions tended to do better, suggesting that this may be a sign of immune response to the vaccine [79].
Thus, targeting the VEGF pathway alone is not an efficacious route in PDAC. Even targeting multiple players in the neoplastic process, like EGFR or other receptor tyrosine kinases, produced marginal benefit, with only two trials showing an improvement in PFS, but not OS [66, 70].
8. Reasons for Failure
The overwhelming failure of anti-angiogenic agents in the clinic leads us to speculate on the reasons for the failure. Over the last 20 years, efforts in targeting angiogenesis in cancer have focused almost entirely on the pro-angiogenic molecule VEGF-A, and there are now several FDA approved drugs for various cancers [15, 18, 21, 22, 26, 80]. In reality, despite very convincing pre-clinical data, some cancers are resistant to such therapy or develop resistance over time [15, 18, 21, 22, 25, 26, 80]. This suggests that other angiogenic pathways that we have yet to address are involved. Indeed, other pro-angiogenic molecules include fibroblast growth factors (FGFs), platelet-derived growth factors (PDGFs), angiopoietins (ANGPTs), transforming growth factor beta (gene: TGFB1) (TGF-β), and cytokines like interleukin-8 (gene: CXCL8) (IL-8) [61, 81]. Thus, to block angiogenesis effectively, we need to target multiple molecules simultaneously.
Because many pro-angiogenic growth factors such as VEGF-A, FGF2, PDGFs, TGF-β, and heregulin (gene: NRG1) (HRG) bind to HSPGs to facilitate their signaling, another targetable common denominator would be these proteoglycans [61, 81]. The validity of this strategy has been shown with KrasLSL−G12D/+; Cdkn2aLoxP/LoxP; Pdx-1-Cre (KIC) mice that were null for glypican-1 (Gpc1), one of the HSPGs. KIC mice have oncogenic Kras and deleted cyclin-dependent kinase inhibitor 2A (Cdkn2a), which encodes for the p16INK4a cell cycle inhibitor and the p19Arf tumor suppressor, in the pancreas due to Cre-mediated recombination. KIC mice null for Gpc1 showed attenuated tumor growth, progression, and invasiveness, and decreased expression of pro-angiogenic genes compared to KIC mice that were wild type for Gpc1 [82].
Another major contributor to the lack of efficacy is the fact that drug delivery in PDAC is impaired due to high interstitial pressures and collapsed vessels [28]. It is possible that efficacy could be improved if anti-angiogenic therapy was administered simultaneously with a stromal depleting agent known to increase perfusion. Out of three recent pre-clinical studies that depleted various components of the stroma, two resulted in improved perfusion [27, 83, 84], while only one did not cause other untoward effects [28, 85]. This was the study that utilized recombinant hyaluronidase (PEGPH20) to deplete the stroma, an agent now fast-tracked by the Food and Drug Administration (FDA) to be used as an investigative therapy in combination with gemcitabine and nab-paclitaxel for the treatment of patients with metastatic pancreatic cancer [28, 85]. Initial Phase II results combining PEGPH20 with nab-paclitaxel/gemcitabine have shown a statistically significant doubling of the ORR, with a trend towards improved PFS and OS in patients with high levels of hyaluronan [86]. Another strategy to promote better drug delivery would be to normalize the vasculature via stromal remodeling instead of depletion [87], or via vascular promotion, a mechanism which involves administering agents that enhance angiogenesis, flow, and the leakiness of vessels [88].
Additionally, it has been shown that the tumor microenvironment of transplantable models is not the same as that seen in a genetically mouse model (GEMM) [27]. In the transplantable models, there is a lack of stroma and the pancreatic cancer cells are close to the vessels [27]. For that reason, many cytotoxic agents that were shown to be ineffective in human trials initially showed efficacy when tested in xenograft models [27, 89]. Later, it was found that such agents were just as ineffective when used in GEMMs [27, 89]. It is perhaps the same story with the anti-angiogenic agents, as they were primarily only tested in subcutaneous or orthotopic nude mouse models of human PDAC. Future studies should also utilize the increasing number of available GEMMs for PDAC [90, 91].
As is often observed in many clinical trials, patient responses are variable, with only a subset of patients benefiting from the therapy, while overall, no positive effect may be seen. It would be useful if we could identify those patients who might benefit the most via the use of predictive biomarkers. Though some trials have attempted to look for correlations between certain known pro-angiogenic molecules circulating in the plasma and treatment response, none have been successful to date [44, 46, 52, 76]. With an increasing number of studies utilizing high throughput technologies like RNA sequencing (RNA-Seq) to profile human tumors, it is possible that a gene expression signature could be used. In fact, we have already identified such a signature by using TCGA RNA-Seq data [92].
Because most approved indications for bevacizumab involve concomitant administration with some form of cytotoxic chemotherapy, at least one clinical study suggested that even if bevacizumab was effective at normalizing the vasculature sufficiently to improve drug delivery, the fact still remains that we lack any effective chemotherapeutic or targeted agent for the treatment of PDAC [53].
In summary, future studies of angiogenesis in PDAC should consider potential resistance mechanisms to targeted therapies, use appropriate pre-clinical models that can recapitulate the microenvironment seen in human PDAC, and use biomarkers or gene signatures to select patients for clinical trials.
Supplementary Material
Highlights.
Pancreatic Ductal Adenocarcinoma (PDAC) is hypovascular.
Vascular endothelial growth factor A (gene: VEGFA) (VEGF-A) is overexpressed in PDAC.
Increased VEGF-A or microvessel density is associated with poor patient outcome.
Blocking VEGF-A in mouse models reduces tumor volumes and improves survival.
Clinical trials targeting angiogenesis elicit marginal survival benefit.
Acknowledgments
This work was supported by the National Cancer Institute (NCI) of the National Institutes of Health (NIH) under award number F30CA200301 to K.E.C. and by a US Public Health Service Grant from the NCI under award number CA-75059 to M.K.
Abbreviations
- ANGPT
angiopoietin
- BRAF
serine/threonine-protein kinase B-raf
- CAF
Cancer Associated Fibroblast
- Cdkn2a
cyclin-dependent kinase inhibitor 2A
- CI
confidence interval
- CSF1R
macrophage colony-stimulating factor 1 receptor
- DLL4
delta-like protein 4
- EC
Endothelial Cell
- ECM
Extracellular Matrix
- EGFR
epidermal growth factor receptor
- FDA
Food and Drug Administration
- FGF
fibroblast growth factor
- FGF2
fibroblast growth factor 2
- FGFR-1
fibroblast growth factor receptor 1 (gene: FGFR1)
- FOLFIRINOX
fluorouracil plus leucovorin, irinotecan, and oxaliplatin
- GEMM
genetically engineered mouse model
- Gpc1
glypican-1
- HIF-1α
hypoxia inducible factor 1, alpha subunit (gene: HIF1A)
- HR
hazard ratio
- HRG
heregulin (gene: NRG1)
- HSPG
heparan sulfate proteogylycan
- I
Immune Cell
- IHC
immunohistochemistry
- IL-8
interleukin-8 (gene: CXCL8)
- KC
KrasLSL−G12D/+; Pdx-1-Cre
- KIC
KrasLSL−G12D/+; Cdkn2aLoxP/LoxP; Pdx-1-Cre
- KPC
KrasLSL−G12D/+; Trp53LSL−R172H/+; Pdx-1-Cre
- Kras
kirsten rat sarcoma viral oncogene homolog
- MVD
microvessel density
- NCI
National Cancer Institute
- NICD
notch intracellular domain
- NIH
National Institutes of Health
- NRP1
neuropilin-1
- ORR
objective response rate
- OS
overall survival
- PCC
Pancreatic Cancer Cell
- PDAC
Pancreatic Ductal Adenocarcinoma
- PDGF
platelet-derived growth factor
- PDGFR
platelet-derived growth factor receptor
- PDGFRB
platelet-derived growth factor receptor beta
- PFS
progression free survival
- PlGF
placenta growth factor (gene: PGF)
- RNA-Seq
RNA sequencing
- SCFR
mast/stem cell growth factor receptor Kit (gene: KIT)
- TCGA
The Cancer Genome Atlas
- TGF-β
transforming growth factor beta (gene: TGFB1)
- Trp53
transformation related protein 53
- VEGF
vascular endothelial growth factor
- VEGF-A
vascular endothelial growth factor A (gene: VEGFA)
- VEGF-B
vascular endothelial growth factor B (gene: VEGFB)
- VEGFA
vascular endothelial growth factor A (protein: VEGF-A)
- VEGFR
vascular endothelial growth factor receptor
- VEGFR-1
vascular endothelial growth factor receptor 1 (gene: FLT1)
- VEGFR-2
vascular endothelial growth factor receptor 2 (gene: KDR)
- VEGFR-3
vascular endothelial growth factor receptor 3 (gene: FLT4)
Footnotes
Conflict of Interest
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kelly E. Craven, Email: kelgalla@iupui.edu.
Jesse Gore, Email: ajgore@iu.edu.
Murray Korc, Email: mkorc@iu.edu.
References
- 1.Adsay NV, Thirabanjasak D, Altinel D. Pancreatic Cancer, chap. Spectrum of Human Pancreatic Neoplasia, M.D. Anderson Solid Tumor Oncology Series. Springer; 2008. p. 5. [Google Scholar]
- 2.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371(11):1039–49. doi: 10.1056/NEJMra1404198. ryan, David P Hong, Theodore S Bardeesy, Nabeel eng Review 2014/09/11 06:00 N Engl J Med. 2014 Sep 11;371(11):1039–49. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society. Cancer Facts & Figures. Atlanta: American Cancer Society; 2015. [Google Scholar]
- 4.Rishi A, Goggins M, Wood LD, Hruban RH. Pathological and molecular evaluation of pancreatic neoplasms. Seminars in Oncology. 2015;42(1):28–39. doi: 10.1053/j.seminoncol.2014.12.004. rishi, Arvind Goggins, Michael Wood, Laura D Hruban, Ralph H P50-CA-62924/CA/NCI NIH HHS/Semin Oncol. 2015 Feb;42(1):28–39. Epub 2014 Dec 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Apte MV, Xu Z, Pothula S, Goldstein D, Pirola RC, Wilson JS. Pancreatic cancer: The microenvironment needs attention too! Pancreatology. 2015;15(4 Suppl):S32–8. doi: 10.1016/j.pan.2015.02.013. apte, M V Xu, Z Pothula, S Goldstein, D Pirola, R C Wilson, J S Switzerland IAP Pancreatology. 2015 Jul;15(4 Suppl):S32–8. Epub 2015 Mar 21. [DOI] [PubMed] [Google Scholar]
- 6.Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL, Miller DK, Wilson PJ, Patch AM, Wu J, Chang DK, Cowley MJ, Gardiner BB, Song S, Harliwong I, Idrisoglu S, Nourse C, Nourbakhsh E, Manning S, Wani S, Gongora M, Pajic M, Scarlett CJ, Gill AJ, Pinho AV, Rooman I, Anderson M, Holmes O, Leonard C, Taylor D, Wood S, Xu Q, Nones K, Fink JL, Christ A, Bruxner T, Cloonan N, Kolle G, Newell F, Pinese M, Mead RS, Humphris JL, Kaplan W, Jones MD, Colvin EK, Nagrial AM, Humphrey ES, Chou A, Chin VT, Chantrill LA, Mawson A, Samra JS, Kench JG, Lovell JA, Daly RJ, Merrett ND, Toon C, Epari K, Nguyen NQ, Barbour A, Zeps N, Kakkar N, Zhao F, Wu YQ, Wang M, Muzny DM, Fisher WE, Brunicardi FC, Hodges SE, Reid JG, Drummond J, Chang K, Han Y, Lewis LR, Dinh H, Buhay CJ, Beck T, Timms L, Sam M, Begley K, Brown A, Pai D, Panchal A, Buchner N, De Borja R, Denroche RE, Yung CK, Serra S, Onetto N, Mukhopadhyay D, Tsao MS, Shaw PA, Petersen GM, Gallinger S, Hruban RH, Maitra A, Iacobuzio-Donahue CA, Schulick RD, Wolfgang CL, et al. Australian Pancreatic Cancer Genome I. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491(7424):399–405. doi: 10.1038/nature11547. biankin, Andrew V Waddell, Nicola Kassahn, Karin S Gingras, Marie-Claude Muthuswamy, Lakshmi B Johns, Amber L Miller, David K Wilson, Peter J Patch, Ann-Marie Wu, Jianmin Chang, David K Cowley, Mark J Gardiner, Brooke B Song, Sarah Harliwong, Ivon Idrisoglu, Senel Nourse, Craig Nourbakhsh, Ehsan Manning, Suzanne Wani, Shivangi Gongora, Milena Pajic, Marina Scarlett, Christopher J Gill, Anthony J Pinho, Andreia V Rooman, Ilse Anderson, Matthew Holmes, Oliver Leonard, Conrad Taylor, Darrin Wood, Scott Xu, Qinying Nones, Katia Fink, J Lynn Christ, Angelika Bruxner, Tim Cloonan, Nicole Kolle, Gabriel Newell, Felicity Pinese, Mark Mead, R Scott Humphris, Jeremy L Kaplan, Warren Jones, Marc D Colvin, Emily K Nagrial, Adnan M Humphrey, Emily S Chou, Angela Chin, Venessa T Chantrill, Lorraine A Mawson, Amanda Samra, Jaswinder S Kench, James G Lovell, Jessica A Daly, Roger J Merrett, Neil D Toon, Christopher Epari, Krishna Nguyen, Nam Q Barbour, Andrew Zeps, Nikolajs Kakkar, Nipun Zhao, Fengmei Wu, Yuan Qing Wang, Min Muzny, Donna M Fisher, William E Brunicardi, F Charles Hodges, Sally E Reid, Jeffrey G Drummond, Jennifer Chang, Kyle Han, Yi Lewis, Lora R Dinh, Huyen Buhay, Christian J Beck, Timothy Timms, Lee Sam, Michelle Begley, Kimberly Brown, Andrew Pai, Deepa Panchal, Ami Buchner, Nicholas De Borja, Richard Denroche, Robert E Yung, Christina K Serra, Stefano Onetto, Nicole Mukhopadhyay, Debabrata Tsao, Ming-Sound Shaw, Patricia A Petersen, Gloria M Gallinger, Steven Hruban, Ralph H Maitra, Anirban Iacobuzio-Donahue, Christine A Schulick, Richard D Wolfgang, Christopher L Morgan, Richard A Lawlor, Rita T Capelli, Paola Corbo, Vincenzo Scardoni, Maria Tortora, Giampaolo Tempero, Margaret A Mann, Karen M Jenkins, Nancy A Perez-Mancera, Pedro A Adams, David J Largaespada, David A Wessels, Lodewyk F A Rust, Alistair G Stein, Lincoln D Tuveson, David A Copeland, Neal G Musgrove, Elizabeth A Scarpa, Aldo Eshleman, James R Hudson, Thomas J Sutherland, Robert L Wheeler, David A Pearson, John V McPherson, John D Gibbs, Richard A Grimmond, Sean M 13031/Cancer Research UK/United Kingdom 2P50CA101955/CA/NCI NIH HHS/P01CA134292/CA/NCI NIH HHS/P50 CA101955/CA/NCI NIH HHS/P50 CA102701/CA/NCI NIH HHS/P50CA062924/CA/NCI NIH HHS/R01 CA097075/CA/NCI NIH HHS/R01 CA97075/CA/NCI NIH HHS/U54 HG003273/HG/NHGRI NIH HHS/Cancer Research UK/United Kingdom Wellcome Trust/United Kingdom England Nature. 2012 Nov 15;491(7424):399–405. Epub 2012 Oct 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P, Johns AL, Miller D, Nones K, Quek K, Quinn MC, Robertson AJ, Fadlullah MZ, Bruxner TJ, Christ AN, Harliwong I, Idrisoglu S, Manning S, Nourse C, Nourbakhsh E, Wani S, Wilson PJ, Markham E, Cloonan N, Anderson MJ, Fink JL, Holmes O, Kazakoff SH, Leonard C, Newell F, Poudel B, Song S, Taylor D, Waddell N, Wood S, Xu Q, Wu J, Pinese M, Cowley MJ, Lee HC, Jones MD, Nagrial AM, Humphris J, Chantrill LA, Chin V, Steinmann AM, Mawson A, Humphrey ES, Colvin EK, Chou A, Scarlett CJ, Pinho AV, Giry-Laterriere M, Rooman I, Samra JS, Kench JG, Pettitt JA, Merrett ND, Toon C, Epari K, Nguyen NQ, Barbour A, Zeps N, Jamieson NB, Graham JS, Niclou SP, Bjerkvig R, Grutzmann R, Aust D, Hruban RH, Maitra A, Iacobuzio-Donahue CA, Wolfgang CL, Morgan RA, Lawlor RT, Corbo V, Bassi C, Falconi M, Zamboni G, Tortora G, Tempero MA, Gill AJ, Eshleman JR, Pilarsky C, Scarpa A, Musgrove EA, Pearson JV, Biankin AV, Grimmond SM. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518(7540):495–501. doi: 10.1038/nature14169. waddell, Nicola Pajic, Marina Patch, Ann-Marie Chang, David K Kassahn, Karin S Bailey, Peter Johns, Amber L Miller, David Nones, Katia Quek, Kelly Quinn, Michael C J Robertson, Alan J Fadlullah, Muhammad Z H Bruxner, Tim J C Christ, Angelika N Harliwong, Ivon Idrisoglu, Senel Manning, Suzanne Nourse, Craig Nourbakhsh, Ehsan Wani, Shivangi Wilson, Peter J Markham, Emma Cloonan, Nicole Anderson, Matthew J Fink, J Lynn Holmes, Oliver Kazakoff, Stephen H Leonard, Conrad Newell, Felicity Poudel, Barsha Song, Sarah Taylor, Darrin Waddell, Nick Wood, Scott Xu, Qinying Wu, Jianmin Pinese, Mark Cowley, Mark J Lee, Hong C Jones, Marc D Nagrial, Adnan M Humphris, Jeremy Chantrill, Lorraine A Chin, Venessa Steinmann, Angela M Mawson, Amanda Humphrey, Emily S Colvin, Emily K Chou, Angela Scarlett, Christopher J Pinho, Andreia V Giry-Laterriere, Marc Rooman, Ilse Samra, Jaswinder S Kench, James G Pettitt, Jessica A Merrett, Neil D Toon, Christopher Epari, Krishna Nguyen, Nam Q Barbour, Andrew Zeps, Nikolajs Jamieson, Nigel B Graham, Janet S Niclou, Simone P Bjerkvig, Rolf Grutzmann, Robert Aust, Daniela Hruban, Ralph H Maitra, Anirban Iacobuzio-Donahue, Christine A Wolfgang, Christopher L Morgan, Richard A Lawlor, Rita T Corbo, Vincenzo Bassi, Claudio Falconi, Massimo Zamboni, Giuseppe Tortora, Giampaolo Tempero, Margaret A Gill, Anthony J Eshleman, James R Pilarsky, Christian Scarpa, Aldo Musgrove, Elizabeth A Pearson, John V Biankin, Andrew V Grimmond, Sean M C29717/A17263/Cancer Research UK/United Kingdom C596/A18076/Cancer Research UK/United Kingdom P50 CA062924/CA/NCI NIH HHS/P50 CA62924/CA/NCI NIH HHS/England Nature. 2015 Feb 26;518(7540):495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Cancer Genome Atlas Network. The Cancer Genome Atlas. 2015. [Google Scholar]
- 9.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Research. 2014;74(11):2913–21. doi: 10.1158/0008-5472.CAN-14-0155. rahib, Lola Smith, Benjamin D Aizenberg, Rhonda Rosenzweig, Allison B Fleshman, Julie M Matrisian, Lynn M Cancer Res. 2014 Jun 1;74(11):2913–21. [DOI] [PubMed] [Google Scholar]
- 10.American Cancer Society. Cancer Facts & Figures. Atlanta: American Cancer Society; 2014. [Google Scholar]
- 11.Garrido-Laguna I, Hidalgo M. Pancreatic cancer: from state-of-the-art treatments to promising novel therapies. Nat Rev Clin Oncol. 2015;12(6):319–34. doi: 10.1038/nrclinonc.2015.53. garrido-Laguna, Ignacio Hidalgo, Manuel P30CA042014-23/CA/NCI NIH HHS/England Nat Rev Clin Oncol. 2015 Jun;12(6):319–34. Epub 2015 Mar 31. [DOI] [PubMed] [Google Scholar]
- 12.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86(3):353–64. doi: 10.1016/s0092-8674(00)80108-7. hanahan, D Folkman, J eng Research Support, U.S. Gov’t, P.H.S. Review 1996/08/09 Cell. 1996 Aug 9;86(3):353–64. [DOI] [PubMed] [Google Scholar]
- 13.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3(6):401–10. doi: 10.1038/nrc1093. bergers, Gabriele Benjamin, Laura E England Nat Rev Cancer. 2003 Jun;3(6):401–10. [DOI] [PubMed] [Google Scholar]
- 14.Eilken HM, Adams RH. Dynamics of endothelial cell behavior in sprouting angiogenesis. Current opinion in cell biology. 2010;22(5):617–25. doi: 10.1016/j.ceb.2010.08.010. eilken, Hanna M Adams, Ralf H England Curr Opin Cell Biol. 2010 Oct;22(5):617–25. [DOI] [PubMed] [Google Scholar]
- 15.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146(6):873–87. doi: 10.1016/j.cell.2011.08.039. potente, Michael Gerhardt, Holger Carmeliet, Peter Cancer Research UK/United Kingdom Cell. 2011 Sep 16;146(6):873–87. [DOI] [PubMed] [Google Scholar]
- 16.Chung AS, Ferrara N. Developmental and pathological angiogenesis. Annu Rev Cell Dev Biol. 2011;27:563–84. doi: 10.1146/annurev-cellbio-092910-154002. chung, Alicia S Ferrara, Napoleone Annu Rev Cell Dev Biol. 2011;27:563–84. Epub 2011 Jul 13. [DOI] [PubMed] [Google Scholar]
- 17.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. hanahan, Douglas Weinberg, Robert A eng Research Support, N.I.H., Extramural Review 2011/03/08 06:00 Cell. 2011 Mar 4;144(5):646–74. [DOI] [PubMed] [Google Scholar]
- 18.Jeltsch M, Leppanen VM, Saharinen P, Alitalo K. Receptor tyrosine kinase-mediated angiogenesis. Cold Spring Harb Perspect Biol. 5(9) doi: 10.1101/cshperspect.a009183. jeltsch, Michael Leppanen, Veli-Matti Saharinen, Pipsa Alitalo, Kari Cold Spring Harb Perspect Biol. 2013 Sep 1;5(9). pii: a009183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1(1):27–31. doi: 10.1038/nm0195-27. folkman, J eng Review 1995/01/01 Nat Med. 1995 Jan;1(1):27–31. [DOI] [PubMed] [Google Scholar]
- 20.Folkman J. Seminars in Medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. The New England journal of medicine. 1995;333(26):1757–63. doi: 10.1056/NEJM199512283332608. folkman, J N Engl J Med. 1995 Dec 28;333(26):1757–63. [DOI] [PubMed] [Google Scholar]
- 21.Welti J, Loges S, Dimmeler S, Carmeliet P. Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer. J Clin Invest. 2013;123(8):3190–200. doi: 10.1172/JCI70212. welti, Jonathan Loges, Sonja Dimmeler, Stefanie Carmeliet, Peter J Clin Invest. 2013 Aug 1;123(8):3190–200. Epub 2013 Aug 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10(6):417–27. doi: 10.1038/nrd3455. carmeliet, Peter Jain, Rakesh K P01-CA80124/CA/NCI NIH HHS/R01-CA115767/CA/NCI NIH HHS/R01-CA126642/CA/NCI NIH HHS/R01-CA85140/CA/NCI NIH HHS/England Nat Rev Drug Discov. 2011 Jun;10(6):417–27. [DOI] [PubMed] [Google Scholar]
- 23.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. hanahan, D Weinberg, R A eng Research Support, U.S. Gov’t, Non-P.H.S. Research Support, U.S. Gov’t, P.H.S. Review 2000/01/27 Cell. 2000 Jan 7;100(1):57–70. [DOI] [PubMed] [Google Scholar]
- 24.Yang Y, Sun M, Wang L, Jiao B. HIFs, angiogenesis, and cancer. J Cell Biochem. 2013;114(5):967–74. doi: 10.1002/jcb.24438. yang, Yongzhi Sun, Mingjuan Wang, Lianghua Jiao, Binghua J Cell Biochem. 2013 May;114(5):967–74. [DOI] [PubMed] [Google Scholar]
- 25.Baeriswyl V, Christofori G. The angiogenic switch in carcinogenesis. Semin Cancer Biol. 2009;19(5):329–37. doi: 10.1016/j.semcancer.2009.05.003. baeriswyl, Vanessa Christofori, Gerhard England Semin Cancer Biol. 2009 Oct;19(5):329–37. Epub 2009 May 29. [DOI] [PubMed] [Google Scholar]
- 26.Saharinen P, Eklund L, Pulkki K, Bono P, Alitalo K. VEGF and angiopoietin signaling in tumor angiogenesis and metastasis. Trends Mol Med. 2011;17(7):347–62. doi: 10.1016/j.molmed.2011.01.015. saharinen, Pipsa Eklund, Lauri Pulkki, Kristina Bono, Petri Alitalo, Kari England Trends Mol Med. 2011 Jul;17(7):347–62. Epub 2011 Apr 12. [DOI] [PubMed] [Google Scholar]
- 27.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, Denicola G, Feig C, Combs C, Winter SP, Ireland-Zecchini H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Ruckert F, Grutzmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M, Hruban RH, Whitebread N, McGovern K, Adams J, Iacobuzio-Donahue C, Griffiths J, Tuveson DA. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324(5933):1457–61. doi: 10.1126/science.1171362. olive, Kenneth P Jacobetz, Michael A Davidson, Christian J Gopinathan, Aarthi McIntyre, Dominick Honess, Davina Madhu, Basetti Goldgraben, Mae A Caldwell, Meredith E Allard, David Frese, Kristopher K Denicola, Gina Feig, Christine Combs, Chelsea Winter, Stephen P Ireland-Zecchini, Heather Reichelt, Stefanie Howat, William J Chang, Alex Dhara, Mousumi Wang, Lifu Ruckert, Felix Grutzmann, Robert Pilarsky, Christian Izeradjene, Kamel Hingorani, Sunil R Huang, Pearl Davies, Susan E Plunkett, William Egorin, Merrill Hruban, Ralph H Whitebread, Nigel McGovern, Karen Adams, Julian Iacobuzio-Donahue, Christine Griffiths, John Tuveson, David A CA084291/CA/NCI NIH HHS/CA101973/CA/NCI NIH HHS/CA105490/CA/NCI NIH HHS/CA111292/CA/NCI NIH HHS/CA114028/CA/NCI NIH HHS/CA15704/CA/NCI NIH HHS/F32 CA123939/CA/NCI NIH HHS/F32 CA123939-03X1/CA/NCI NIH HHS/F32CA123887-01/CA/NCI NIH HHS/F32CA123939-02/CA/NCI NIH HHS/K08 CA106610/CA/NCI NIH HHS/New York, N.Y. Science. 2009 Jun 12;324(5933):1457–61. Epub 2009 May 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21(3):418–29. doi: 10.1016/j.ccr.2012.01.007. provenzano, Paolo P Cuevas, Carlos Chang, Amy E Goel, Vikas K Von Hoff, Daniel D Hingorani, Sunil R CA109552/CA/NCI NIH HHS/CA114028/CA/NCI NIH HHS/CA152249/CA/NCI NIH HHS/CA161112/CA/NCI NIH HHS/K08 CA114028/CA/NCI NIH HHS/K08 CA114028-03/CA/NCI NIH HHS/P01 CA109552-05/CA/NCI NIH HHS/P30 DK056465/DK/NIDDK NIH HHS/R01 CA129357/CA/NCI NIH HHS/R01 CA161112/CA/NCI NIH HHS/R01 CA161112-01/CA/NCI NIH HHS/R21 CA152249/CA/NCI NIH HHS/R21 CA152249-02/CA/NCI NIH HHS/Cancer Cell. 2012 Mar 20;21(3):418–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hlatky L, Hahnfeldt P, Folkman J. Clinical application of antiangiogenic therapy: microvessel density, what it does and doesn’t tell us. J Natl Cancer Inst. 2002;94(12):883–93. doi: 10.1093/jnci/94.12.883. hlatky, Lynn Hahnfeldt, Philip Folkman, Judah eng Review 2002/06/20 10:00 J Natl Cancer Inst. 2002 Jun 19;94(12):883–93. [DOI] [PubMed] [Google Scholar]
- 30.Harper SJ, Bates DO. VEGF-A splicing: the key to anti-angiogenic therapeutics? Nat Rev Cancer. 2008;8(11):880–7. doi: 10.1038/nrc2505. harper, Steven J Bates, David O eng BS/06/005/20340/British Heart Foundation/United Kingdom Review England 2008/10/17 09:00 Nat Rev Cancer. 2008 Nov;8(11):880–7. Epub 2008 Oct 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eswarappa SM, Potdar AA, Koch WJ, Fan Y, Vasu K, Lindner D, Willard B, Graham LM, DiCorleto PE, Fox PL. Programmed translational readthrough generates antiangiogenic VEGF-Ax. Cell. 2014;157(7):1605–18. doi: 10.1016/j.cell.2014.04.033. eswarappa, Sandeepa M Potdar, Alka A Koch, William J Fan, Yi Vasu, Kommireddy Lindner, Daniel Willard, Belinda Graham, Linda M DiCorleto, Paul E Fox, Paul L eng P01 HL029582/HL/NHLBI NIH HHS/P01 HL076491/HL/NHLBI NIH HHS/P30 CA043703-23/CA/NCI NIH HHS/R01 DK083359/DK/NIDDK NIH HHS/R01 GM086430/GM/NIGMS NIH HHS/R21 HL094841/HL/NHLBI NIH HHS/S10 RR031537/RR/NCRR NIH HHS/Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t 2014/06/21 06:00 Cell. 2014 Jun 19;157(7):1605–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ikeda N, Adachi M, Taki T, Huang C, Hashida H, Takabayashi A, Sho M, Nakajima Y, Kanehiro H, Hisanaga M, Nakano H, Miyake M. Prognostic significance of angiogenesis in human pancreatic cancer. British journal of cancer. 1999;79(9–10):1553–63. doi: 10.1038/sj.bjc.6690248. ikeda, N Adachi, M Taki, T Huang, C Hashida, H Takabayashi, A Sho, M Nakajima, Y Kanehiro, H Hisanaga, M Nakano, H Miyake, M SCOTLAND Br J Cancer. 1999 Mar;79(9–10):1553–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seo Y, Baba H, Fukuda T, Takashima M, Sugimachi K. High expression of vascular endothelial growth factor is associated with liver metastasis and a poor prognosis for patients with ductal pancreatic adenocarcinoma. Cancer. 2000;88(10):2239–45. doi: 10.1002/(sici)1097-0142(20000515)88:10<2239::aid-cncr6>3.0.co;2-v. seo, Y Baba, H Fukuda, T Takashima, M Sugimachi, K Cancer. 2000 May 15;88(10):2239–45. [DOI] [PubMed] [Google Scholar]
- 34.Itakura J, Ishiwata T, Friess H, Fujii H, Matsumoto Y, Buchler MW, Korc M. Enhanced expression of vascular endothelial growth factor in human pancreatic cancer correlates with local disease progression. Clin Cancer Res. 1997;3(8):1309–16. itakura, J Ishiwata, T Friess, H Fujii, H Matsumoto, Y Buchler, M W Korc, M CA-40162/CA/NCI NIH HHS/Clin Cancer Res. 1997 Aug;3(8):1309–16. [PubMed] [Google Scholar]
- 35.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–4. doi: 10.1158/2159-8290.CD-12-0095. cerami, Ethan Gao, Jianjiong Dogrusoz, Ugur Gross, Benjamin E Sumer, Selcuk Onur Aksoy, Bulent Arman Jacobsen, Anders Byrne, Caitlin J Heuer, Michael L Larsson, Erik Antipin, Yevgeniy Reva, Boris Goldberg, Arthur P Sander, Chris Schultz, Nikolaus eng GM103504/GM/NIGMS NIH HHS/P41 GM103504/GM/NIGMS NIH HHS/P41 RR031228/RR/NCRR NIH HHS/R01 CA132744/CA/NCI NIH HHS/R21 CA135870/CA/NCI NIH HHS/R21CA135870/CA/NCI NIH HHS/U24 CA143840/CA/NCI NIH HHS/U24CA143840/CA/NCI NIH HHS/Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t 2012/05/17 06:00 Cancer Discov. 2012 May;2(5):401–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1. doi: 10.1126/scisignal.2004088. gao, Jianjiong Aksoy, Bulent Arman Dogrusoz, Ugur Dresdner, Gideon Gross, Benjamin Sumer, S Onur Sun, Yichao Jacobsen, Anders Sinha, Rileen Larsson, Erik Cerami, Ethan Sander, Chris Schultz, Nikolaus eng GM103504/GM/NIGMS NIH HHS/P41 GM103504/GM/NIGMS NIH HHS/P41 RR031228/RR/NCRR NIH HHS/R01 CA132744/CA/NCI NIH HHS/R01 MH097062/MH/NIMH NIH HHS/R21 CA135870/CA/NCI NIH HHS/R21CA135870/CA/NCI NIH HHS/U01 CA168409/CA/NCI NIH HHS/U24 CA143840/CA/NCI NIH HHS/U24CA143840/CA/NCI NIH HHS/Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t 2013/04/04 06:00 Sci Signal. 2013 Apr 2;6(269):pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ellis LM, Takahashi Y, Fenoglio CJ, Cleary KR, Bucana CD, Evans DB. Vessel counts and vascular endothelial growth factor expression in pancreatic adenocarcinoma. Eur J Cancer. 1998;34(3):337–40. doi: 10.1016/s0959-8049(97)10068-5. ellis, L M Takahashi, Y Fenoglio, C J Cleary, K R Bucana, C D Evans, D B ENGLAND Oxford, England : 1990 Eur J Cancer. 1998 Feb;34(3):337–40. [DOI] [PubMed] [Google Scholar]
- 38.Luo J, Guo P, Matsuda K, Truong N, Lee A, Chun C, Cheng SY, Korc M. Pancreatic cancer cell-derived vascular endothelial growth factor is biologically active in vitro and enhances tumorigenicity in vivo. International journal of cancer. Journal international du cancer. 2001;92(3):361–9. doi: 10.1002/ijc.1202. luo, J Guo, P Matsuda, K Truong, N Lee, A Chun, C Cheng, S Y Korc, M CA-40162/CA/NCI NIH HHS/Int J Cancer. 2001 May 1;92(3):361–9. [DOI] [PubMed] [Google Scholar]
- 39.Hotz HG, Gill PS, Masood R, Hotz B, Buhr HJ, Foitzik T, Hines OJ, Reber HA. Specific targeting of tumor vasculature by diphtheria toxin-vascular endothelial growth factor fusion protein reduces angiogenesis and growth of pancreatic cancer. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2002;6(2):159–66. doi: 10.1016/s1091-255x(01)00040-3. discussion 166, hotz, Hubert G Gill, Parkash S Masood, Rizwan Hotz, Birgit Buhr, Heinz J Foitzik, Thomas Hines, O Joe Reber, Howard A J Gastrointest Surg. 2002 Mar–Apr;6(2):159–66; discussion 166. [DOI] [PubMed] [Google Scholar]
- 40.Hoshida T, Sunamura M, Duda DG, Egawa S, Miyazaki S, Shineha R, Hamada H, Ohtani H, Satomi S, Matsuno S. Gene therapy for pancreatic cancer using an adenovirus vector encoding soluble fit-1 vascular endothelial growth factor receptor. Pancreas. 2002;25(2):111–21. doi: 10.1097/00006676-200208000-00001. hoshida, Tohru Sunamura, Makoto Duda, Dan G Egawa, Shinichi Miyazaki, Shukichi Shineha, Ryuzaburo Hamada, Hirofumi Ohtani, Haruo Satomi, Susumu Matsuno, Seiki Pancreas. 2002 Aug;25(2):111–21. [DOI] [PubMed] [Google Scholar]
- 41.Ogawa T, Takayama K, Takakura N, Kitano S, Ueno H. Anti-tumor angiogenesis therapy using soluble receptors: enhanced inhibition of tumor growth when soluble fibroblast growth factor receptor-1 is used with soluble vascular endothelial growth factor receptor. Cancer gene therapy. 2002;9(8):633–40. doi: 10.1038/sj.cgt.7700478. ogawa, Tadashi Takayama, Koichi Takakura, Nobuyuki Kitano, Seigo Ueno, Hikaru England Cancer Gene Ther. 2002 Aug;9(8):633–40. [DOI] [PubMed] [Google Scholar]
- 42.Solorzano CC, Baker CH, Bruns CJ, Killion JJ, Ellis LM, Wood J, Fidler IJ. Inhibition of growth and metastasis of human pancreatic cancer growing in nude mice by PTK 787/ZK222584, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinases. Cancer Biother Radiopharm. 2001;16(5):359–70. doi: 10.1089/108497801753354267. solorzano, C C Baker, C H Bruns, C J Killion, J J Ellis, L M Wood, J Fidler, I J CA16672/CA/NCI NIH HHS/R35-CA42107/CA/NCI NIH HHS/Cancer Biother Radiopharm. 2001 Oct;16(5):359–70. [DOI] [PubMed] [Google Scholar]
- 43.Fukasawa M, Korc M. Vascular endothelial growth factor-trap suppresses tumorigenicity of multiple pancreatic cancer cell lines. Clin Cancer Res. 2004;10(10):3327–32. doi: 10.1158/1078-0432.CCR-03-0820. fukasawa, Mitsuharu Korc, Murray CA-102687/CA/NCI NIH HHS/Clin Cancer Res. 2004 May 15;10(10):3327–32. [DOI] [PubMed] [Google Scholar]
- 44.Kindler HL, Friberg G, Singh DA, Locker G, Nattam S, Kozloff M, Taber DA, Karrison T, Dachman A, Stadler WM, Vokes EE. Phase II trial of bevacizumab plus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2005;23(31):8033–40. doi: 10.1200/JCO.2005.01.9661. kindler, Hedy L Friberg, Gregory Singh, Deepti A Locker, Gershon Nattam, Sreenivasa Kozloff, Mark Taber, David A Karrison, Theodore Dachman, Abraham Stadler, Walter M Vokes, Everett E N01-CM-17101/CM/NCI NIH HHS/J Clin Oncol. 2005 Nov 1;23(31):8033–40. [DOI] [PubMed] [Google Scholar]
- 45.Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403–13. doi: 10.1200/JCO.1997.15.6.2403. burris, H A 3rd Moore, M J Andersen, J Green, M R Rothenberg, M L Modiano, M R Cripps, M C Portenoy, R K Storniolo, A M Tarassoff, P Nelson, R Dorr, F A Stephens, C D Von Hoff, D D J Clin Oncol. 1997 Jun;15(6):2403–13. [DOI] [PubMed] [Google Scholar]
- 46.Ko AH, Dito E, Schillinger B, Venook AP, Xu Z, Bergsland EK, Wong D, Scott J, Hwang J, Tempero MA. A phase II study evaluating bevacizumab in combination with fixed-dose rate gemcitabine and low-dose cisplatin for metastatic pancreatic cancer: is an anti-VEGF strategy still applicable? Investigational new drugs. 2008;26(5):463–71. doi: 10.1007/s10637-008-9127-2. ko, Andrew H Dito, Elizabeth Schillinger, Brian Venook, Alan P Xu, Zhidong Bergsland, Emily K Wong, Derrick Scott, Janet Hwang, Jimmy Tempero, Margaret A Invest New Drugs. 2008 Oct;26(5):463–71. Epub 2008 Apr 1. [DOI] [PubMed] [Google Scholar]
- 47.Javle M, Yu J, Garrett C, Pande A, Kuvshinoff B, Litwin A, Phelan J, 3rd, Gibbs J, Iyer R. Bevacizumab combined with gemcitabine and capecitabine for advanced pancreatic cancer: a phase II study. British journal of cancer. 2009;100(12):1842–5. doi: 10.1038/sj.bjc.6605099. javle, M Yu, J Garrett, C Pande, A Kuvshinoff, B Litwin, A Phelan, J 3rd Gibbs, J Iyer, R England Br J Cancer. 2009 Jun 16;100(12):1842–5. Epub 2009 Jun 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crane CH, Winter K, Regine WF, Safran H, Rich TA, Curran W, Wolff RA, Willett CG. Phase II study of bevacizumab with concurrent capecitabine and radiation followed by maintenance gemcitabine and bevacizumab for locally advanced pancreatic cancer: Radiation Therapy Oncology Group RTOG 0411. J Clin Oncol. 2009;27(25):4096–102. doi: 10.1200/JCO.2009.21.8529. crane, Christopher H Winter, Kathryn Regine, William F Safran, Howard Rich, Tyvin A Curran, Walter Wolff, Robert A Willett, Christopher G U10 CA21661/CA/NCI NIH HHS/U10 CA32115/CA/NCI NIH HHS/U10 CA37422/CA/NCI NIH HHS/J Clin Oncol. 2009 Sep 1;27(25):4096–102. Epub 2009 Jul 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fogelman D, Jafari M, Varadhachary GR, Xiong H, Bullock S, Ozer H, Lin E, Morris J, Cunningham P, Bennett B, Abbruzzese JL, Wolff RA. Bevacizumab plus gemcitabine and oxaliplatin as first-line therapy for metastatic or locally advanced pancreatic cancer: a phase II trial. Cancer Chemother Pharmacol. 2011;68(6):1431–8. doi: 10.1007/s00280-011-1601-4. fogelman, David Jafari, Mehrdad Varadhachary, Gauri R Xiong, Henry Bullock, Susie Ozer, Harold Lin, E Morris, Jeffrey Cunningham, Patti Bennett, Bronwyn Abbruzzese, James L Wolff, Robert A Germany Cancer Chemother Pharmacol. 2011 Dec;68(6):1431–8. Epub 2011 Apr 9. [DOI] [PubMed] [Google Scholar]
- 50.Small W, Jr, Mulcahy MF, Rademaker A, Bentrem DJ, Benson AB, Weitner BB, Talamonti MS. Phase II trial of full-dose gemcitabine and bevacizumab in combination with attenuated three-dimensional conformal radiotherapy in patients with localized pancreatic cancer. International journal of radiation oncology, biology, physics. 2011;80(2):476–82. doi: 10.1016/j.ijrobp.2010.02.030. small, William Jr Mulcahy, Mary F Rademaker, Alfred Bentrem, David J Benson, Al B Weitner, Bing Bing Talamonti, Mark S Int J Radiat Oncol Biol Phys. 2011 Jun 1;80(2):476–82. [DOI] [PubMed] [Google Scholar]
- 51.Van Buren G, 2nd, Ramanathan RK, Krasinskas AM, Smith RP, Abood GJ, Bahary N, Lembersky BC, Shuai Y, Potter DM, Bartlett DL, Zureikat AH, Zeh HJ, James Moser A. Phase II study of induction fixed-dose rate gemcitabine and bevacizumab followed by 30 Gy radiotherapy as preoperative treatment for potentially resectable pancreatic adenocarcinoma. Ann Surg Oncol. 2013;20(12):3787–93. doi: 10.1245/s10434-013-3161-9. van Buren, George 2nd Ramanathan, Ramesh K Krasinskas, Alyssa M Smith, Ryan P Abood, Gerard J Bahary, Nathan Lembersky, Barry C Shuai, Yongli Potter, Douglas M Bartlett, David L Zureikat, Amer H Zeh, Herbert J James Moser, A Ann Surg Oncol. 2013 Nov;20(12):3787–93. Epub 2013 Aug 1. [DOI] [PubMed] [Google Scholar]
- 52.Astsaturov IA, Meropol NJ, Alpaugh RK, Burtness BA, Cheng JD, McLaughlin S, Rogatko A, Xu Z, Watson JC, Weiner LM, Cohen SJ. Phase II and coagulation cascade biomarker study of bevacizumab with or without docetaxel in patients with previously treated metastatic pancreatic adenocarcinoma. Am J Clin Oncol. 2011;34(1):70–5. doi: 10.1097/COC.0b013e3181d2734a. astsaturov, Igor A Meropol, Neal J Alpaugh, R Katherine Burtness, Barbara A Cheng, Jonathan D McLaughlin, Sue Rogatko, Andre Xu, Zhiheng Watson, James C Weiner, Louis M Cohen, Steven J P30 CA006927/CA/NCI NIH HHS/P30 CA006927-44/CA/NCI NIH HHS/Am J Clin Oncol. 2011 Feb;34(1):70–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, Hurwitz H, Innocenti F, Mulcahy MF, O’Reilly E, Wozniak TF, Picus J, Bhargava P, Mayer RJ, Schilsky RL, Goldberg RM. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303) J Clin Oncol. 2010;28(22):3617–22. doi: 10.1200/JCO.2010.28.1386. kindler, Hedy Lee Niedzwiecki, Donna Hollis, Donna Sutherland, Susan Schrag, Deborah Hurwitz, Herbert Innocenti, Federico Mulcahy, Mary Frances O’Reilly, Eileen Wozniak, Timothy F Picus, Joel Bhargava, Pankaj Mayer, Robert J Schilsky, Richard L Goldberg, Richard M CA 32291/CA/NCI NIH HHS/CA17145/CA/NCI NIH HHS/CA21115/CA/NCI NIH HHS/CA31946/CA/NCI NIH HHS/CA33601/CA/NCI NIH HHS/CA41287/CA/NCI NIH HHS/CA45418/CA/NCI NIH HHS/CA47559/CA/NCI NIH HHS/CA47577/CA/NCI NIH HHS/CA77440/CA/NCI NIH HHS/CA77651/CA/NCI NIH HHS/J Clin Oncol. 2010 Aug 1;28(22):3617–22. Epub 2010 Jul 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xiong HQ, Abbruzzese JL. Epidermal growth factor receptor-targeted therapy for pancreatic cancer. Semin Oncol. 2002;29(5 Suppl 14):31–7. doi: 10.1053/sonc.2002.35645. xiong, Henry Q Abbruzzese, James L Semin Oncol. 2002 Oct;29(5 Suppl 14):31–7. [DOI] [PubMed] [Google Scholar]
- 55.Tobita K, Kijima H, Dowaki S, Kashiwagi H, Ohtani Y, Oida Y, Yamazaki H, Nakamura M, Ueyama Y, Tanaka M, Inokuchi S, Makuuchi H. Epidermal growth factor receptor expression in human pancreatic cancer: Significance for liver metastasis. International journal of molecular medicine. 2003;11(3):305–9. tobita, Kosuke Kijima, Hiroshi Dowaki, Shoichi Kashiwagi, Hiroyuki Ohtani, Yasuo Oida, Yasuhisa Yamazaki, Hitoshi Nakamura, Masato Ueyama, Yoshito Tanaka, Makiko Inokuchi, Sadaki Makuuchi, Hiroyasu Greece Int J Mol Med. 2003 Mar;11(3):305–9. [PubMed] [Google Scholar]
- 56.Papageorgio C, Perry MC. Epidermal growth factor receptor-targeted therapy for pancreatic cancer. Cancer Invest. 2007;25(7):647–57. doi: 10.1080/07357900701522653. papageorgio, Chris Perry, Michael C Cancer Invest. 2007 Oct;25(7):647–57. [DOI] [PubMed] [Google Scholar]
- 57.Longo R, Cacciamani F, Naso G, Gasparini G. Pancreatic cancer: from molecular signature to target therapy. Crit Rev Oncol Hematol. 2008;68(3):197–211. doi: 10.1016/j.critrevonc.2008.03.003. longo, R Cacciamani, F Naso, G Gasparini, G Ireland Crit Rev Oncol Hematol. 2008 Dec;68(3):197–211. Epub 2008 Apr 23. [DOI] [PubMed] [Google Scholar]
- 58.Philip PA, Benedetti J, Corless CL, Wong R, O’Reilly EM, Flynn PJ, Rowland KM, Atkins JN, Mirtsching BC, Rivkin SE, Khorana AA, Goldman B, Fenoglio-Preiser CM, Abbruzzese JL, Blanke CD. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol. 2010;28(22):3605–10. doi: 10.1200/JCO.2009.25.7550. philip, Philip A Benedetti, Jacqueline Corless, Christopher L Wong, Ralph O’Reilly, Eileen M Flynn, Patrick J Rowland, Kendrith M Atkins, James N Mirtsching, Barry C Rivkin, Saul E Khorana, Alok A Goldman, Bryan Fenoglio-Preiser, Cecilia M Abbruzzese, James L Blanke, Charles D J Clin Oncol. 2010 Aug 1;28(22):3605–10. Epub 2010 Jul 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W Australian Pancreatic Cancer Genome I, National Cancer Institute of Canada Clinical Trials G. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25(15):1960–6. doi: 10.1200/JCO.2006.07.9525. moore, Malcolm J Goldstein, David Hamm, John Figer, Arie Hecht, Joel R Gallinger, Steven Au, Heather J Murawa, Pawel Walde, David Wolff, Robert A Campos, Daniel Lim, Robert Ding, Keyue Clark, Gary Voskoglou-Nomikos, Theodora Ptasynski, Mieke Parulekar, Wendy J Clin Oncol. 2007 May 20;25(15):1960–6. Epub 2007 Apr 23. [DOI] [PubMed] [Google Scholar]
- 60.Bruns CJ, Harbison MT, Davis DW, Portera CA, Tsan R, McConkey DJ, Evans DB, Abbruzzese JL, Hicklin DJ, Radinsky R. Epidermal growth factor receptor blockade with C225 plus gemcitabine results in regression of human pancreatic carcinoma growing orthotopically in nude mice by antiangiogenic mechanisms. Clin Cancer Res. 2000;6(5):1936–48. bruns, C J Harbison, M T Davis, D W Portera, C A Tsan, R McConkey, D J Evans, D B Abbruzzese, J L Hicklin, D J Radinsky, R CA 69676/CA/NCI NIH HHS/CA16672/CA/NCI NIH HHS/CA67952/CA/NCI NIH HHS/Clin Cancer Res. 2000 May;6(5):1936–48. [PubMed] [Google Scholar]
- 61.Korc M. Pathways for aberrant angiogenesis in pancreatic cancer. Molecular cancer. 2003;2:8. doi: 10.1186/1476-4598-2-8. korc, M England Mol Cancer. 2003 Jan 7;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tonra JR, Deevi DS, Corcoran E, Li H, Wang S, Carrick FE, Hicklin DJ. Synergistic antitumor effects of combined epidermal growth factor receptor and vascular endothelial growth factor receptor-2 targeted therapy. Clin Cancer Res. 2006;12(7 Pt 1):2197–207. doi: 10.1158/1078-0432.CCR-05-1682. tonra, James R Deevi, Dhanvanthri S Corcoran, Erik Li, Huiling Wang, Su Carrick, Francine E Hicklin, Daniel J Clin Cancer Res. 2006 Apr 1;12(7 Pt 1):2197–207. [DOI] [PubMed] [Google Scholar]
- 63.Ko AH, Venook AP, Bergsland EK, Kelley RK, Korn WM, Dito E, Schillinger B, Scott J, Hwang J, Tempero MA. A phase II study of bevacizumab plus erlotinib for gemcitabine-refractory metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2010;66(6):1051–7. doi: 10.1007/s00280-010-1257-5. ko, Andrew H Venook, Alan P Bergsland, Emily K Kelley, R Kate Korn, W Michael Dito, Elizabeth Schillinger, Brian Scott, Janet Hwang, Jimmy Tempero, Margaret A Germany Cancer Chemother Pharmacol. 2010 Nov;66(6):1051–7. Epub 2010 Feb 4. [DOI] [PubMed] [Google Scholar]
- 64.Ko AH, Youssoufian H, Gurtler J, Dicke K, Kayaleh O, Lenz HJ, Keaton M, Katz T, Ballal S, Rowinsky EK. A phase II randomized study of cetuximab and bevacizumab alone or in combination with gemcitabine as first-line therapy for metastatic pancreatic adenocarcinoma. Investigational new drugs. 2012;30(4):1597–606. doi: 10.1007/s10637-011-9691-8. ko, Andrew H Youssoufian, Hagop Gurtler, Jayne Dicke, Karel Kayaleh, Omar Lenz, Heinz-Josef Keaton, Mark Katz, Terry Ballal, Shaila Rowinsky, Eric K Invest New Drugs. 2012 Aug;30(4):1597–606. Epub 2011 Jun 1. [DOI] [PubMed] [Google Scholar]
- 65.Watkins DJ, Starling N, Cunningham D, Thomas J, Webb J, Brown G, Barbachano Y, Oates J, Chau I. The combination of a chemotherapy doublet (gemcitabine and capecitabine) with a biological doublet (bevacizumab and erlotinib) in patients with advanced pancreatic adenocarcinoma. The results of a phase I/II study. Eur J Cancer. 2014;50(8):1422–9. doi: 10.1016/j.ejca.2014.02.003. watkins, D J Starling, N Cunningham, D Thomas, J Webb, J Brown, G Barbachano, Y Oates, J Chau, I Cancer Research UK/United Kingdom England Oxford, England : 1990 Eur J Cancer. 2014 May;50(8):1422–9. Epub 2014 Mar 6. [DOI] [PubMed] [Google Scholar]
- 66.Van Cutsem E, Vervenne WL, Bennouna J, Humblet Y, Gill S, Van Laethem JL, Verslype C, Scheithauer W, Shang A, Cosaert J, Moore MJ. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol. 2009;27(13):2231–7. doi: 10.1200/JCO.2008.20.0238. van Cutsem, Eric Vervenne, Walter L Bennouna, Jaafar Humblet, Yves Gill, Sharlene Van Laethem, Jean-Luc Verslype, Chris Scheithauer, Werner Shang, Aijing Cosaert, Jan Moore, Malcolm J J Clin Oncol. 2009 May 1;27(13):2231–7. Epub 2009 Mar 23. [DOI] [PubMed] [Google Scholar]
- 67.Spano JP, Chodkiewicz C, Maurel J, Wong R, Wasan H, Barone C, Letourneau R, Bajetta E, Pithavala Y, Bycott P, Trask P, Liau K, Ricart AD, Kim S, Rixe O. Efficacy of gemcitabine plus axitinib compared with gemcitabine alone in patients with advanced pancreatic cancer: an open-label randomised phase II study. Lancet. 2008;371(9630):2101–8. doi: 10.1016/S0140-6736(08)60661-3. spano, Jean-Philippe Chodkiewicz, Catherine Maurel, Joan Wong, Ralph Wasan, Harpreet Barone, Carlo Letourneau, Richard Bajetta, Emilio Pithavala, Yazdi Bycott, Paul Trask, Peter Liau, Katherine Ricart, Alejandro D Kim, Sinil Rixe, Olivier England Lancet. 2008 Jun 21;371(9630):2101–8. Epub 2008 May 29. [DOI] [PubMed] [Google Scholar]
- 68.Kindler HL, Ioka T, Richel DJ, Bennouna J, Letourneau R, Okusaka T, Funakoshi A, Furuse J, Park YS, Ohkawa S, Springett GM, Wasan HS, Trask PC, Bycott P, Ricart AD, Kim S, Van Cutsem E. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. The lancet oncology. 2011;12(3):256–62. doi: 10.1016/S1470-2045(11)70004-3. kindler, Hedy L Ioka, Tatsuya Richel, Dirk J Bennouna, Jaafar Letourneau, Richard Okusaka, Takuji Funakoshi, Akihiro Furuse, Junji Park, Young Suk Ohkawa, Shinichi Springett, Gregory M Wasan, Harpreet S Trask, Peter C Bycott, Paul Ricart, Alejandro D Kim, Sinil Van Cutsem, Eric England Lancet Oncol. 2011 Mar;12(3):256–62. [DOI] [PubMed] [Google Scholar]
- 69.O’Reilly EM, Niedzwiecki D, Hall M, Hollis D, Bekaii-Saab T, Pluard T, Douglas K, Abou-Alfa GK, Kindler HL, Schilsky RL, Goldberg RM Cancer, B. Leukemia Group. A Cancer and Leukemia Group B phase II study of sunitinib malate in patients with previously treated metastatic pancreatic adenocarcinoma (CALGB 80603) Oncologist. 2010;15(12):1310–9. doi: 10.1634/theoncologist.2010-0152. o’Reilly, Eileen M Niedzwiecki, Donna Hall, Margaret Hollis, Donna Bekaii-Saab, Tanios Pluard, Timothy Douglas, Kathe Abou-Alfa, Ghassan K Kindler, Hedy L Schilsky, Richard L Goldberg, Richard M CA04326/CA/NCI NIH HHS/CA08025/CA/NCI NIH HHS/CA11789/CA/NCI NIH HHS/CA31946/CA/NCI NIH HHS/CA32291/CA/NCI NIH HHS/CA33601/CA/NCI NIH HHS/CA35113/CA/NCI NIH HHS/CA35279/CA/NCI NIH HHS/CA41287/CA/NCI NIH HHS/CA45389/CA/NCI NIH HHS/CA45418/CA/NCI NIH HHS/CA45808/CA/NCI NIH HHS/CA47577/CA/NCI NIH HHS/CA47642/CA/NCI NIH HHS/CA77298/CA/NCI NIH HHS/CA77406/CA/NCI NIH HHS/CA77440/CA/NCI NIH HHS/CA77597/CA/NCI NIH HHS/CA77651/CA/NCI NIH HHS/CA77658/CA/NCI NIH HHS/CA86726/CA/NCI NIH HHS/Oncologist. 2010;15(12):1310–9. Epub 2010 Dec 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reni M, Cereda S, Milella M, Novarino A, Passardi A, Mambrini A, Di Lucca G, Aprile G, Belli C, Danova M, Bergamo F, Franceschi E, Fugazza C, Ceraulo D, Villa E. Maintenance sunitinib or observation in metastatic pancreatic adenocarcinoma: a phase II randomised trial. Eur J Cancer. 2013;49(17):3609–15. doi: 10.1016/j.ejca.2013.06.041. reni, Michele Cereda, Stefano Milella, Michele Novarino, Anna Passardi, Alessandro Mambrini, Andrea Di Lucca, Giuseppe Aprile, Giuseppe Belli, Carmen Danova, Marco Bergamo, Francesca Franceschi, Enrico Fugazza, Clara Ceraulo, Domenica Villa, Eugenio England Oxford, England : 1990 Eur J Cancer. 2013 Nov;49(17):3609–15. Epub 2013 Jul 27. [DOI] [PubMed] [Google Scholar]
- 71.El-Khoueiry AB, Ramanathan RK, Yang DY, Zhang W, Shibata S, Wright JJ, Gandara D, Lenz HJ. A randomized phase II of gemcitabine and sorafenib versus sorafenib alone in patients with metastatic pancreatic cancer. Investigational new drugs. 2012;30(3):1175–83. doi: 10.1007/s10637-011-9658-9. el-Khoueiry, A B Ramanathan, R K Yang, D Y Zhang, W Shibata, S Wright, J J Gandara, D Lenz, H J N01 CM-62209/CM/NCI NIH HHS/N01 CM062209/CM/NCI NIH HHS/P30CA033572/CA/NCI NIH HHS/P30CA14089/CA/NCI NIH HHS/Invest New Drugs. 2012 Jun;30(3):1175–83. Epub 2011 Mar 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kindler HL, Wroblewski K, Wallace JA, Hall MJ, Locker G, Nattam S, Agamah E, Stadler WM, Vokes EE. Gemcitabine plus sorafenib in patients with advanced pancreatic cancer: a phase II trial of the University of Chicago Phase II Consortium. Investigational new drugs. 2012;30(1):382–6. doi: 10.1007/s10637-010-9526-z. kindler, Hedy Lee Wroblewski, Kristen Wallace, James A Hall, Michael J Locker, Gershon Nattam, Sreenivasa Agamah, Edem Stadler, Walter M Vokes, Everett E N01-CM-62201/CM/NCI NIH HHS/Invest New Drugs. 2012 Feb;30(1):382–6. Epub 2010 Aug 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cardin DB, Goff L, Li CI, Shyr Y, Winkler C, DeVore R, Schlabach L, Holloway M, McClanahan P, Meyer K, Grigorieva J, Berlin J, Chan E. Phase II trial of sorafenib and erlotinib in advanced pancreatic cancer. Cancer Med. 2014;3(3):572–9. doi: 10.1002/cam4.208. cardin, Dana B Goff, Laura Li, Chung-I Shyr, Yu Winkler, Charles DeVore, Russell Schlabach, Larry Holloway, Melanie McClanahan, Pam Meyer, Krista Grigorieva, Julia Berlin, Jordan Chan, Emily P30CA68485/CA/NCI NIH HHS/Cancer Med. 2014 Jun;3(3):572–9. Epub 2014 Feb 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cascinu S, Berardi R, Sobrero A, Bidoli P, Labianca R, Siena S, Ferrari D, Barni S, Aitini E, Zagonel V, Caprioni F, Villa F, Mosconi S, Faloppi L, Tonini G, Boni C, Conte P, Di Costanzo F, Cinquini MC. Italian Group for the Study of Digestive Tract, Sorafenib does not improve effcacy of chemotherapy in advanced pancreatic cancer: A GISCAD randomized phase II study. Digestive and liver disease : offcial journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2014;46(2):182–6. doi: 10.1016/j.dld.2013.09.020. cascinu, Stefano Berardi, Rossana Sobrero, Alberto Bidoli, Paolo Labianca, Roberto Siena, Salvatore Ferrari, Daris Barni, Sandro Aitini, Enrico Zagonel, Vittorina Caprioni, Francesco Villa, Federica Mosconi, Stefania Faloppi, Luca Tonini, Giuseppe Boni, Corrado Conte, Pierfranco Di Costanzo, Francesco Cinquini, Michela (GISCAD) Netherlands Dig Liver Dis. 2014 Feb;46(2):182–6. Epub 2013 Nov 2. [DOI] [PubMed] [Google Scholar]
- 75.Goncalves A, Gilabert M, Francois E, Dahan L, Perrier H, Lamy R, Re D, Largillier R, Gasmi M, Tchiknavorian X, Esterni B, Genre D, Moureau-Zabotto L, Giovannini M, Seitz JF, Delpero JR, Turrini O, Viens P, Raoul JL. BAYPAN study: a double-blind phase III randomized trial comparing gemcitabine plus sorafenib and gemcitabine plus placebo in patients with advanced pancreatic cancer. Annals of oncology : official journal of the European Society for Medical Oncology/ESMO. 2012;23(11):2799–805. doi: 10.1093/annonc/mds135. goncalves, A Gilabert, M Francois, E Dahan, L Perrier, H Lamy, R Re, D Largillier, R Gasmi, M Tchiknavorian, X Esterni, B Genre, D Moureau-Zabotto, L Giovannini, M Seitz, J-F Delpero, J-R Turrini, O Viens, P Raoul, J-L England Ann Oncol. 2012 Nov;23(11):2799–805. Epub 2012 Jul 5. [DOI] [PubMed] [Google Scholar]
- 76.Dragovich T, Laheru D, Dayyani F, Bolejack V, Smith L, Seng J, Burris H, Rosen P, Hidalgo M, Ritch P, Baker AF, Raghunand N, Crowley J, Von Hoff DD. Phase II trial of vatalanib in patients with advanced or metastatic pancreatic adenocarcinoma after first-line gemcitabine therapy (PCRT O4-001) Cancer Chemother Pharmacol. 2014;74(2):379–87. doi: 10.1007/s00280-014-2499-4. dragovich, T Laheru, D Dayyani, F Bolejack, V Smith, L Seng, J Burris, H Rosen, P Hidalgo, M Ritch, P Baker, A F Raghunand, N Crowley, J Von Hoff, D D CA017094/CA/NCI NIH HHS/P30 CA023074/CA/NCI NIH HHS/P50 CA95060/CA/NCI NIH HHS/Germany Cancer Chemother Pharmacol. 2014 Aug;74(2):379–87. Epub 2014 Jun 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rougier P, Riess H, Manges R, Karasek P, Humblet Y, Barone C, Santoro A, Assadourian S, Hatteville L, Philip PA. Randomised, placebo-controlled, double-blind, parallel-group phase III study evaluating aflibercept in patients receiving first-line treatment with gemcitabine for metastatic pancreatic cancer. Eur J Cancer. 2013;49(12):2633–42. doi: 10.1016/j.ejca.2013.04.002. rougier, Philippe Riess, Hanno Manges, Robert Karasek, Petr Humblet, Yves Barone, Carlo Santoro, Armando Assadourian, Sylvie Hatteville, Laurence Philip, Philip A England Oxford, England : 1990 Eur J Cancer. 2013 Aug;49(12):2633–42. Epub 2013 Apr 30. [DOI] [PubMed] [Google Scholar]
- 78.Wada S, Tsunoda T, Baba T, Primus FJ, Kuwano H, Shibuya M, Tahara H. Rationale for antiangiogenic cancer therapy with vaccination using epitope peptides derived from human vascular endothelial growth factor receptor 2. Cancer Research. 2005;65(11):4939–46. doi: 10.1158/0008-5472.CAN-04-3759. wada, Satoshi Tsunoda, Takuya Baba, Toshiyuki Primus, F James Kuwano, Hiroyuki Shibuya, Masabumi Tahara, Hideaki Cancer Res. 2005 Jun 1;65(11):4939–46. [DOI] [PubMed] [Google Scholar]
- 79.Yamaue H, Tsunoda T, Tani M, Miyazawa M, Yamao K, Mizuno N, Okusaka T, Ueno H, Boku N, Fukutomi A, Ishii H, Ohkawa S, Furukawa M, Maguchi H, Ikeda M, Togashi Y, Nishio K, Ohashi Y. Randomized phase II/III clinical trial of elpamotide for patients with advanced pancreatic cancer: PEGASUS-PC Study. Cancer Sci. 2015;106(7):883–90. doi: 10.1111/cas.12674. yamaue, Hiroki Tsunoda, Takuya Tani, Masaji Miyazawa, Motoki Yamao, Kenji Mizuno, Nobumasa Okusaka, Takuji Ueno, Hideki Boku, Narikazu Fukutomi, Akira Ishii, Hiroshi Ohkawa, Shinichi Furukawa, Masayuki Maguchi, Hiroyuki Ikeda, Masafumi Togashi, Yosuke Nishio, Kazuto Ohashi, Yasuo England Cancer Sci. 2015 Jul;106(7):883–90. Epub 2015 May 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mittal K, Ebos J, Rini B. Angiogenesis and the tumor microenvironment: vascular endothelial growth factor and beyond. Semin Oncol. 2014;41(2):235–51. doi: 10.1053/j.seminoncol.2014.02.007. mittal, Kriti Ebos, John Rini, Brian Semin Oncol. 2014 Apr;41(2):235–51. Epub 2014 Feb 28. [DOI] [PubMed] [Google Scholar]
- 81.Whipple C, Korc M. Targeting angiogenesis in pancreatic cancer: rationale and pitfalls. Langenbecks Arch Surg. 2008;393(6):901–10. doi: 10.1007/s00423-008-0280-z. whipple, Chery Korc, Murray CA-101306/CA/NCI NIH HHS/CA-102687/CA/NCI NIH HHS/Germany Langenbecks Arch Surg. 2008 Nov;393(6):901–10. Epub 2008 Jan 22. [DOI] [PubMed] [Google Scholar]
- 82.Whipple CA, Young AL, Korc M. A KrasG12D-driven genetic mouse model of pancreatic cancer requires glypican-1 for efficient proliferation and angiogenesis. Oncogene. 2012;31(20):2535–44. doi: 10.1038/onc.2011.430. whipple, C A Young, A L Korc, M eng CA-R37-075059/CA/NCI NIH HHS/F32 CA-144579/CA/NCI NIH HHS/R37 CA075059/CA/NCI NIH HHS/R37 CA075059-14/CA/NCI NIH HHS/Research Support, N.I.H., Extramural England 2011/10/15 06:00 Oncogene. 2012 May 17;31(20):2535–44. Epub 2011 Sep 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, Dekleva EN, Saunders T, Becerra CP, Tattersall IW, Westphalen CB, Kitajewski J, Fernandez-Barrena MG, Fernandez-Zapico ME, Iacobuzio-Donahue C, Olive KP, Stanger BZ. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25(6):735–47. doi: 10.1016/j.ccr.2014.04.021. rhim, Andrew D Oberstein, Paul E Thomas, Dafydd H Mirek, Emily T Palermo, Carmine F Sastra, Stephen A Dekleva, Erin N Saunders, Tyler Becerra, Claudia P Tattersall, Ian W Westphalen, C Benedikt Kitajewski, Jan Fernandez-Barrena, Maite G Fernandez-Zapico, Martin E Iacobuzio-Donahue, Christine Olive, Kenneth P Stanger, Ben Z CA046952/CA/NCI NIH HHS/CA136526/CA/NCI NIH HHS/CA157980/CA/NCI NIH HHS/CA169123/CA/NCI NIH HHS/CA177857/CA/NCI NIH HHS/DK034933/DK/NIDDK NIH HHS/DK083111/DK/NIDDK NIH HHS/DK083355/DK/NIDDK NIH HHS/DK088945/DK/NIDDK NIH HHS/DP2 DK083111/DK/NIDDK NIH HHS/K08 DK088945/DK/NIDDK NIH HHS/P30 CA015083/CA/NCI NIH HHS/P30 CA016520/CA/NCI NIH HHS/P30 DK034933/DK/NIDDK NIH HHS/P30 DK050306/DK/NIDDK NIH HHS/P30CA013696/CA/NCI NIH HHS/P30DK050306/DK/NIDDK NIH HHS/R01 CA136526/CA/NCI NIH HHS/R01 CA136673/CA/NCI NIH HHS/R01 CA157980/CA/NCI NIH HHS/R01 CA169123/CA/NCI NIH HHS/R01 CA177857/CA/NCI NIH HHS/R01 DK083355/DK/NIDDK NIH HHS/R01 HL112626/HL/NHLBI NIH HHS/S10 RR025482/RR/NCRR NIH HHS/S10RR025686/RR/NCRR NIH HHS/T32CA009503/CA/NCI NIH HHS/Cancer Cell. 2014 Jun 16;25(6):735–47. Epub 2014 May 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C, Novitskiy SV, De Jesus-Acosta A, Sharma P, Heidari P, Mahmood U, Chin L, Moses HL, Weaver VM, Maitra A, Allison JP, LeBleu VS, Kalluri R. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25(6):719–34. doi: 10.1016/j.ccr.2014.04.005. ozdemir, Berna C Pentcheva-Hoang, Tsvetelina Carstens, Julienne L Zheng, Xiaofeng Wu, Chia-Chin Simpson, Tyler R Laklai, Hanane Sugimoto, Hikaru Kahlert, Christoph Novitskiy, Sergey V De Jesus-Acosta, Ana Sharma, Padmanee Heidari, Pedram Mahmood, Umar Chin, Lynda Moses, Harold L Weaver, Valerie M Maitra, Anirban Allison, James P LeBleu, Valerie S Kalluri, Raghu CA155370/CA/NCI NIH HHS/CA163191/CA/NCI NIH HHS/DK55001/DK/NIDDK NIH HHS/DK81976/DK/NIDDK NIH HHS/P30 CA016672/CA/NCI NIH HHS/P50 CA127003/CA/NCI NIH HHS/R01 CA125550/CA/NCI NIH HHS/R01 CA155370/CA/NCI NIH HHS/R01 DK055001/DK/NIDDK NIH HHS/R01 DK081576/DK/NIDDK NIH HHS/U01 CA151925/CA/NCI NIH HHS/U54 CA163191/CA/NCI NIH HHS/UO1 CA151925/CA/NCI NIH HHS/Cancer Cell. 2014 Jun 16;25(6):719–34. Epub 2014 May 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jacobetz MA, Chan DS, Neesse A, Bapiro TE, Cook N, Frese KK, Feig C, Nakagawa T, Caldwell ME, Zecchini HI, Lolkema MP, Jiang P, Kultti A, Thompson CB, Maneval DC, Jodrell DI, Frost GI, Shepard HM, Skepper JN, Tuveson DA. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2013;62(1):112–20. doi: 10.1136/gutjnl-2012-302529. jacobetz, Michael A Chan, Derek S Neesse, Albrecht Bapiro, Tashinga E Cook, Natalie Frese, Kristopher K Feig, Christine Nakagawa, Tomoaki Caldwell, Meredith E Zecchini, Heather I Lolkema, Martijn P Jiang, Ping Kultti, Anne Thompson, Curtis B Maneval, Daniel C Jodrell, Duncan I Frost, Gregory I Shepard, H M Skepper, Jeremy N Tuveson, David A Cancer Research UK/United Kingdom England Gut. 2013 Jan;62(1):112–20. Epub 2012 Mar 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hingorani SR, Harris WP, Hendifar AE, Bullock AJ, Wu XW, Huang Y, Jiang P. High response rate and PFS with PEGPH20 added to nab-paclitaxel/gemcitabine in stage IV previously untreated pancreatic cancer patients with high-HA tumors: Interim results of a randomized phase II study. ASCO Meeting Abstracts. 2015;33(15 suppl):4006. [Google Scholar]
- 87.Nagathihalli NS, Castellanos JA, Shi C, Beesetty Y, Reyzer ML, Caprioli R, Chen X, Walsh AJ, Skala MC, Moses HL, Merchant NB. STAT3 Mediated Remodeling of the Tumor Microenvironment Results in Enhanced Tumor Drug Delivery in a Mouse Model of Pancreatic Cancer. Gastroenterology. doi: 10.1053/j.gastro.2015.07.058. Nagathihalli, Nagaraj S Castellanos, Jason A Shi, Chanjuan Beesetty, Yugandhar Reyzer, Michelle L Caprioli, Richard Chen, Xi Walsh, Alex J Skala, Melissa C Moses, Harold L Merchant, Nipun B R01 CA161976/CA/NCI NIH HHS/Gastroenterology. 2015 Aug 6. pii: S0016-5085(15)01090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wong PP, Demircioglu F, Ghazaly E, Alrawashdeh W, Stratford MR, Scudamore CL, Cereser B, Crnogorac-Jurcevic T, McDonald S, Elia G, Hagemann T, Kocher HM, Hodivala-Dilke KM. Dual-action combination therapy enhances angiogenesis while reducing tumor growth and spread. Cancer Cell. 2015;27(1):123–37. doi: 10.1016/j.ccell.2014.10.015. wong, Ping-Pui Demircioglu, Fevzi Ghazaly, Essam Alrawashdeh, Wasfi Stratford, Michael R L Scudamore, Cheryl L Cereser, Biancastella Crnogorac-Jurcevic, Tatjana McDonald, Stuart Elia, George Hagemann, Thorsten Kocher, Hemant M Hodivala-Dilke, Kairbaan M C18270/A12888/Cancer Research UK/United Kingdom C18270/A14355/Cancer Research UK/United Kingdom C82181/A12007/Cancer Research UK/United Kingdom G0901609/Medical Research Council/United Kingdom Cancer Cell. 2015 Jan 12;27(1):123–37. [DOI] [PubMed] [Google Scholar]
- 89.Singh M, Lima A, Molina R, Hamilton P, Clermont AC, Devasthali V, Thompson JD, Cheng JH, Bou Reslan H, Ho CC, Cao TC, Lee CV, Nannini MA, Fuh G, Carano RA, Koeppen H, Yu RX, Forrest WF, Plowman GD, Johnson L. Assessing therapeutic responses in Kras mutant cancers using genetically engineered mouse models. Nature Biotechnology. 2010;28(6):585–93. doi: 10.1038/nbt.1640. singh, Mallika Lima, Anthony Molina, Rafael Hamilton, Patricia Clermont, Anne C Devasthali, Vidusha Thompson, Jennifer D Cheng, Jason H Bou Reslan, Hani Ho, Calvin C K Cao, Timothy C Lee, Chingwei V Nannini, Michelle A Fuh, Germaine Carano, Richard A D Koeppen, Hartmut Yu, Ron X Forrest, William F Plowman, Gregory D Johnson, Leisa Nat Biotechnol. 2010 Jun;28(6):585–93. Epub 2010 May 23. [DOI] [PubMed] [Google Scholar]
- 90.Qiu W, Su GH. Challenges and advances in mouse modeling for human pancreatic tumorigenesis and metastasis. Cancer and Metastasis Reviews. 2013;32(1–2):83–107. doi: 10.1007/s10555-012-9408-2. qiu, Wanglong Su, Gloria H R01 CA109525/CA/NCI NIH HHS/Netherlands Cancer Metastasis Rev. 2013 Jun;32(1–2):83–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guerra C, Barbacid M. Genetically engineered mouse models of pancreatic adenocarcinoma. Mol Oncol. 2013;7(2):232–47. doi: 10.1016/j.molonc.2013.02.002. guerra, Carmen Barbacid, Mariano Netherlands Mol Oncol. 2013 Apr;7(2):232–47. Epub 2013 Feb 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gore J, Craven KE, Wilson JL, Cote GA, Cheng M, Nguyen HV, Cramer HM, Sherman S, Korc M. TCGA data and patient-derived orthotopic xenografts highlight pancreatic cancer-associated angiogenesis. Oncotarget. 2015;6(10):7504–21. doi: 10.18632/oncotarget.3233. gore, Jesse Craven, Kelly E Wilson, Julie L Cote, Gregory A Cheng, Monica Nguyen, Hai V Cramer, Harvey M Sherman, Stuart Korc, Murray CA-075059/CA/NCI NIH HHS/P30 CA082709/CA/NCI NIH HHS/Oncotarget. 2015 Apr 10;6(10):7504–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.