Abstract
Tumors are highly heterogeneous at the patient, tissue, cellular, and molecular levels. This multi-scale heterogeneity poses significant challenges for effective therapies, which ideally must not only distinguish between tumorous and healthy tissue, but also fully address the wide variety of tumorous sub-clones. Commonly used therapies either leverage a biological phenotype of cancer cells (e.g. high rate of proliferation) or indiscriminately kill all the cells present in a targeted volume. Tumor microenvironment (TME) targeting represents a promising therapeutic direction, because a number of TME hallmarks are conserved across different tumor types, despite the underlying genetic heterogeneity. Historically, TME targeting has largely focused on the cells that support tumor growth (e.g. vascular endothelial cells). However, by viewing the intrinsic physical and chemical alterations in the TME as additional therapeutic opportunities rather than barriers, a new class of TME-inspired treatments has great promise to complement or replace existing therapeutic strategies. In this review we summarize the physical and chemical hallmarks of the TME, and discuss how these tumor characteristics either currently are, or may ultimately be targeted to improve cancer therapies.
1. Introduction
Tumors are marked by a high degree of heterogeneity both within as well as between patients. This multi-scale heterogeneity drastically diminishes the treatment efficacy of many classical cancer therapies. The most commonly used chemotherapies target a biological phenotype of cancer cells, specifically their highly proliferative nature. However such therapies leave behind resistant populations that repopulate the tumor, while also resulting in toxicity to healthy cells. Other therapies indiscriminately kill or remove all cells within a given tumor volume, such as surgical resection and radiation therapy, and these too may leave behind cells that cause tumor relapse. Recent advancements in targeted therapy typically focus on receptors that are upregulated in cancer. However, almost all of the receptors currently being targeted are also expressed in normal cell populations, leading to off-target effects and potential toxicity [1–4]. These targeted methods also drive the evolution of resistant cells, causing eventual treatment failure. Often these remaining cells are self-renewing progenitors known as cancer stem cells (CSC), which have been demonstrated to be responsible for tumor initiation and growth maintenance in cancers of the brain [5], breast [6], colon [7], and the hematopoietic system [8, 9]. Because these CSCs often share surface markers with normal tissue stem cells, and furthermore do not exhibit the high proliferative tendency of bulk tumor cells, they present several treatment challenges that are not addressed by traditional methods.
With a current therapeutic focus on biological properties of neoplastic cells, which tend to have a high degree of variance due to the highly heterogeneous nature of tumors, tumor recurrence and metastasis continue to present major challenges. However the physical and chemical hallmarks of malignancy, which often times are more consistent across different tumors than biological markers, may provide effective and reliable targets that could complement more traditional approaches. The dynamic process of tumor development results in a major restructuring of the entire tumor microenvironment (TME). As this TME develops, there is a complex and dynamic feedback between the selective stimuli acting on the tumor cells and the surrounding profiles of hypoxia and acidity, growth factors, and mechanical forces. Not only is the surrounding tumor milieu of importance in the development of cancer, it also has a profound effect on therapy efficacy. It is therefore critical to consider the underlying TME in developing more effective treatment practices that are also effective against resistant cell clones. Exploitation of the physical and chemical properties of the TME as therapeutic targets may greatly complement or enhance the efficacy of existing treatment options, however these have only recently begun to receive serious attention clinically. In this mini-review, we outline the major physical and chemical microenvironmental alterations in cancer that we believe present the most promising targets for anti-tumor therapies. We will then discuss key advances in the development of this new class of therapies that considers these alterations in tumors as therapeutic opportunities rather than hurdles to effective treatment.
2. Physical and chemical alterations in cancer
Cancer development is a dynamic process characterized by a vast array of alterations at the cellular and tissue level. This includes abnormal growth and alterations in cells, the extracellular matrix, and blood vessels, and these alterations are all highly inter-related. For example, due to the high rate of cellular proliferation, solid tumors eventually become significantly less oxygenated than normal tissues, as the rapidly growing tumor mass exhausts the local supply of oxygen (O2) [10]. The hypoxic microenvironment drives alterations in the behavior of cells, as they must adapt to enable survival in a low O2 environment. Regulation of biological pathways by hypoxia-inducible genes is controlled by hypoxia inducible factor-1 (HIF-1). Downstream signaling from HIF-1 activation (resulting from the stabilization of HIF-1α in the absence of O2), regulates cell functions such as apoptosis, cell cycle arrest, angiogenesis, glycolysis and adaptation to low pH [11]. Newly formed vascular networks within tumors are in large part driven by HIF-1 tumor signaling in response to this low O2, however the vascular growth is rapid and disordered, resulting in a network that does not deliver nutrients efficiently [12]. Angiogenesis, this process of tumor vascular growth from the surrounding vasculature, greatly alters both the physical and chemical TME. Leaky and uneven vasculature and poor lymphatic drainage contribute to a complex environment with variable interstitial pressure, hypoxic zones, and gradients of nutrients and growth factors within the tumor bulk [13]. Extracellular acidity is a primary characteristic of the TME as a result of metabolic alteration of hypoxic tumor cells, which rely on glycolysis for energy production, along with the poor perfusion associated with neoplastic growth and angiogenesis.
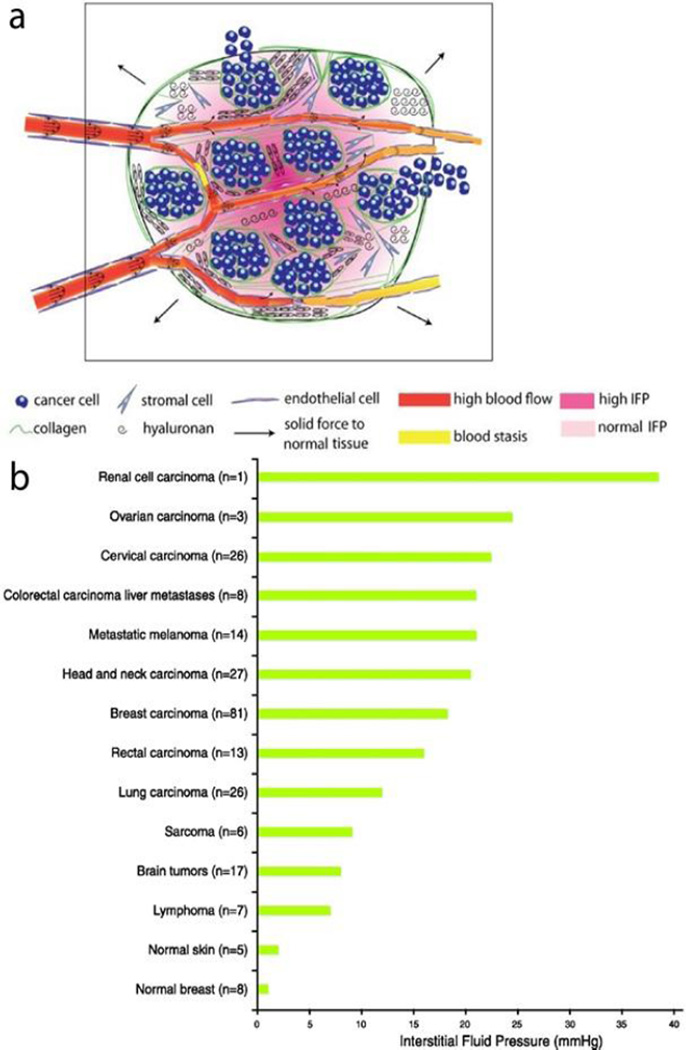
The physical tumor microenvironment is altered from normal tissue due to matrix deposition and remodeling as well as increased proliferation of both cancerous and stromal cells. The rapid division and proliferation that characterizes cancer cells leads to increased mass in a confined volume and causes increased intratumoral pressure and compression of lymphatic and blood vessels within the tumor [14]. Cancer associated fibroblasts (CAFs) form the major supporting structure for the tumorous tissue and exhibit increased proliferation, ECM production, and altered cytokine secretion compared to normal fibroblasts [15]. The deposition of highly cross-linked and oriented collagen in the ECM causes tissue stiffening in the case of liver [16], breast [17] and prostate tumors [18, 19]. Tumor tissue stiffening occurs despite higher deformability of individual cancer cells compared to normal cells, which is itself partly due to cytoskeletal reorganization [20, 21]. Tumor stiffening results in the development of both increased tissue stress and interstitial fluid pressure (IFP) as seen in Figure 1 [22]. These forces and resulting cell compressions affect many aspects of cell behavior such as gene expression, cell proliferation, invasiveness, and ECM organization [23–25]. Blood and lymphatic vessel compression resulting from the increasing solid mass contributes to the hypoxic and acidic microenvironment by obstructing the delivery of oxygen to or removal of waste products from tissue [26–28]. This demonstrates that the alterations in physical and chemical properties are actually inter-related, with bi-directional feedback.
Figure 1.
Major alterations in the mechanical tumor microenvironment. a) The rapid proliferation of cancer cells and stromal cells along with the deposition of collagen creates solid forces within the tumor. These forces cause compression of blood vessels, which leads to areas of high interstitial fluid pressure in the tumor [22]. b) A comparison of the interstitial fluid pressure in aggregated data collected from a variety of human tumors as compared to normal human tissues shows an often drastic increase in IFP in the tumor microenvironment [29]. Data was collected from human patients using the wick-in-needle technique for measurement.
While altered tissue mechanics and chemistry have received significant attention in the TME field, the electrical properties of tumor tissues also become deregulated in a number of ways. Due to the increased intracellular concentration of positively charged sodium ions and an increase in negatively charged glycocalyx on the cell membrane of tumor cells, the electrochemical properties of normal and cancer cells differ [30]. Cancer cells exhibit decreased resting transmembrane potential (TMP), in part because of these altered ion and charge compositions [31]. It has been shown that the mitotic activities of cells are related to the TMP, with cells having low TMP (referred to as depolarized) exhibiting increased proliferation [32]. This alteration is largely attributed to electron and proton transport across the cell membrane, which results in accumulation of negative charges on the cell surface [31, 33]. The increased surface area of the cancer cells as a result of membrane protrusions results in increased membrane capacitance [34]. An altered nuclear-to-cytoplasm ratio (NCR) has long been known to occur in a variety of cancer cells including cancers of the breast [35], uterus and cervix [35], brain [36, 37], prostate [38], colon, and lung, and this likely alters the intracellular electrochemical properties of cells. Finally, the ECM also undergoes alterations in electrical properties due to electrically charged semiconducting proteins and proteoglycans. These integrated effects lead to an altered electrical TME, which eventually yields higher electrical conductivity and permittivity within the tumor [39–41].
Several of these microenvironmental alterations are sufficiently universal to be used for diagnosis of cancer. Breast cancer is diagnosed by changes in tissue stiffness detected by mammography. Microenvironmental electrical alteration between healthy and tumorous tissue is used in diagnosis procedures such as electrical impedance spectroscopy (EIS) [42, 43]. Clinical impedance measurements have detected an altered electrical environment in lung cancer patients, which demonstrated cancer patients to have a reduced electrical reactance [44]. PET-CT scans commonly used in diagnosing cancer detect the increased glucose uptake that leads to the acidic microenvironment. While a number of studies have suggested the importance of these altered physical and chemical properties for detection and prognosis, few have translated these alterations into effective therapy targets. In what follows we will review the studies in which these microenvironmental alterations have been leveraged toward targeted cancer therapy, as well as propose future directions for this research.
3. Targeting the chemical tumor microenvironment
Targeting acidity
Because low pH has been implicated in enhancing malignancy in a number of tumor types, recent research has focused on targeting this chemical feature for the benefit of patients. In one approach, low pH insertion peptides (pHLIP) were used to selectively deliver drugs to the cells residing in acidic tissues [45]. These pHLIP peptides undergo membrane-associated folding when exposed to acidic conditions, which causes them to change from a membrane surface state in neutral conditions, and then to be inserted into the cell membrane in low pH environments [46]. These insertion peptides have been used to transport toxins, nucleic acids, and nanoparticles into the cytoplasm of cancerous cells resulting in reduction of tumor growth in animal models [47–51]. Other drug delivery designs have used low pH to activate the drug in order to reduce accumulation of drugs in normal tissues. One such design uses a small shielding molecule attached to the terminus of a tumor receptor specific ligand. Upon exposure to acidic tissue, the shielding molecule detaches from the ligand, and exposes the targeting ligand to bind to the targeted tumor cells [52]. Another approach has been to use pH-sensitive coatings, such as the PEG coating developed by Yang et al. for siRNA therapy. In this study the PEG coating reduced non-specific interactions under normal pH, however within an acidic microenvironment this coating was degraded [53]. Tumor acidity has also been used to activate a charge-conversional nanogel. Triggered by slightly acidic conditions, the bonds that are stable in neutral and alkali pH degrade to expose positively charged amino groups. Such a system has the benefit of having high drug-loading efficiency at physiological pH, with enhanced cellular internalization triggered by nanogel–cell interaction that occurs only under low pH [54]. These efforts, which have demonstrated improved selective targeting to the acidic microenvironment and subsequent tumor reduction, capitalize on the fact that extracellular acidity is a conserved property of the TME and a predictor of aggressiveness. Therefore, targeting tumor acidity might represent a novel approach for the delivery of therapeutic agents selectively to tumor cells most likely to resist standard therapies, as well as to tumor cells with the greatest metastatic potential.
To complement these acid-activated drugs, additional research has focused on actually buffering the tumor pH to alter tumor cellular dynamics. Because low pH is an active driver of malignancy, and tumor cells possess a competitive survival advantage under low pH, the working hypothesis has been that pH buffering may reduce or reverse malignancy. In mouse models of metastatic breast cancer, oral administration of bicarbonate increased intratumoral pH and reduced the formation of spontaneous metastases [55]. A non-bicarbonate non-volatile buffer, IEPA has also been shown to reduce metastasis in a mouse model when ingested orally [56]. These results suggest that oral administration of non-volatile buffers or bicarbonate can be effective for reducing metastasis through the mechanism of pH buffering, and suggest further research to translate and optimize this approach for human patients.
Targeting hypoxia
Due to the alterations in the TME caused by hypoxia, as well as the links between hypoxia and therapy resistance, this chemical hallmark has been considered as a prime therapeutic target. One approach has been to develop bioreductive prodrugs wherein a non-toxic drug can be converted into a toxic molecule under low O2 [57]. The general mechanism involves different chemical moieties that can be metabolized by enzymatic reduction under hypoxic conditions. This reduction produces a prodrug radical through one-electron reduction, which can participate in further reactions to become cytotoxic to cells thereby achieving hypoxia-selective killing [57–59]. Five different chemical groups: nitro groups, aromatic N-oxides, aliphatic N-oxides and transition metals have been shown to reduce to cytotoxic radicals under hypoxia, and have been used as a structural basis to devise new hypoxia-activated drugs [58]. Multiple different bioreductive drugs have demonstrated antitumor cytotoxicity against hypoxic cells [59–62].
A closely related approach to targeting hypoxia involves leveraging cellular response to low O2 levels to enhance activation within or delivery to the highly malignant hypoxic tumor niches. Since HIF-1 stimulates transcription of a large number of genes (hypoxia-responsive elements), constructs to obtain hypoxia-specific transcription of a therapeutic gene would drive expression specifically in tumors [11, 63]. One of the challenges associated with this type of therapy is efficient delivery of vectors to hypoxic cells that are located distant to blood vessels. Macrophages have been leveraged to overcome this challenge since they gather at tumor sites and also express high levels of HIF-1α [64]. Hypoxia can inhibit migration of these macrophages by inhibiting the monocyte chemotactic protein (MCP-1) once they have infiltrated the hypoxic and necrotic areas of a tumor [65]. These macrophages can be transfected ex vivo with genes that encode for anti-cancer agents (tumor antigens, anti-angiogenic agents, etc.), resulting in significant killing of tumor cells [66, 67].
Similar approaches have been developed using tissue stem cells, as well as bacterium that home to hypoxic tissues. Genetically engineered strains of certain bacteria, such as Clostridium, Bifidobacterium, Salmonella, Mycobacterium, Bacillus, and Listeria are known to localize and germinate in hypoxic regions of tumors, causing tumor cell lysis [68, 69]. Clostridium spores and Clostridium strains genetically engineered to convert nontoxic drugs into cytotoxic drugs have been injected into mouse tumors causing tumor reduction [70–72]. In another study, bacterial tumor targeting was demonstrated to occur due to the dependence of E. coli reductase activity on tumor hypoxia [57]. Bacterial strains are also beneficial for therapeutic delivery because they accumulate in areas of tumors distant from blood vessels that cannot be reached by other drugs. [57]. Bacterial therapy in combination with other more traditional treatments has the potential to be a promising approach for more effective combinatorial targeting of the hypoxic tumor niche [69, 73, 74].
4. Targeting the physical tumor microenvironment
Exploiting the EPR effect
The Enhanced Permeability and Retention (EPR) effect describes the phenomenon of tumor blood vessels facilitating transport of macromolecules into tumor tissues due to the leaky and highly permeable nature of tumor vasculature. Macromolecules larger than 40 kDa selectively leak out from tumor vessels and accumulate in tumor tissues [75]. Because this EPR effect does not occur in normal tissues, macromolecule therapies can be used to selectively target tumor tissues [76, 77], representing one example by which the physics of tumors has led the way to new therapeutic opportunities. The EPR effect has been exploited for the selective delivery of proteins, drug–polymer conjugates, micelles, liposomes, nanoparticles, DNA polyplexes and lipid particles [78–82]. By leveraging this general characteristic of the TME in designing therapies, these macromolecular drugs have demonstrated prolonged retention in the tumor and greater tumor selectivity, compared to conventional small molecule anticancer drugs, allowing for improved antitumor efficacy while limiting adverse reactions [82, 83].
The EPR effect is mediated by factors that influence vascular permeability such as angiotensin, nitric oxide (NO) and vascular endothelial growth factor (VEGF). Therefore, efforts have been made to enhance the EPR effect by tuning these factors. Angiotensin, which produces systemic hypertension through vasoconstriction, causes an increase in blood pressure in normal vessels [84]. Tumor vessels, which lack the smooth muscle support needed for vasoconstriction remain open. This allows for an increase in blood flow to tumors and a pressure buildup that forces the macromolecular drug into the tumoral space [85, 86]. Further efforts to enhance the EPR effect have also capitalized on the altered hypoxic and acidic tumor microenvironment. Nitroglycerin (NG) is an agent that liberates NO in hypoxic and acidic conditions [87]. NO, acting as a vasodilator, increases blood flow to the tumor. Because NO is selectively released in the hypoxic and acidic environment by NG, blood flow is selectively increased in the TME, leading to an increase in drug delivery to the tumor [88]. The simultaneous leveraging of multiple aspects of the altered microenvironment, such as leaky vasculature, underdeveloped blood vessel walls, hypoxia, and acidity, could result in more effective therapies, as resistance mechanisms are likely to differ between these.
Tumor ablation by pulsed electric fields
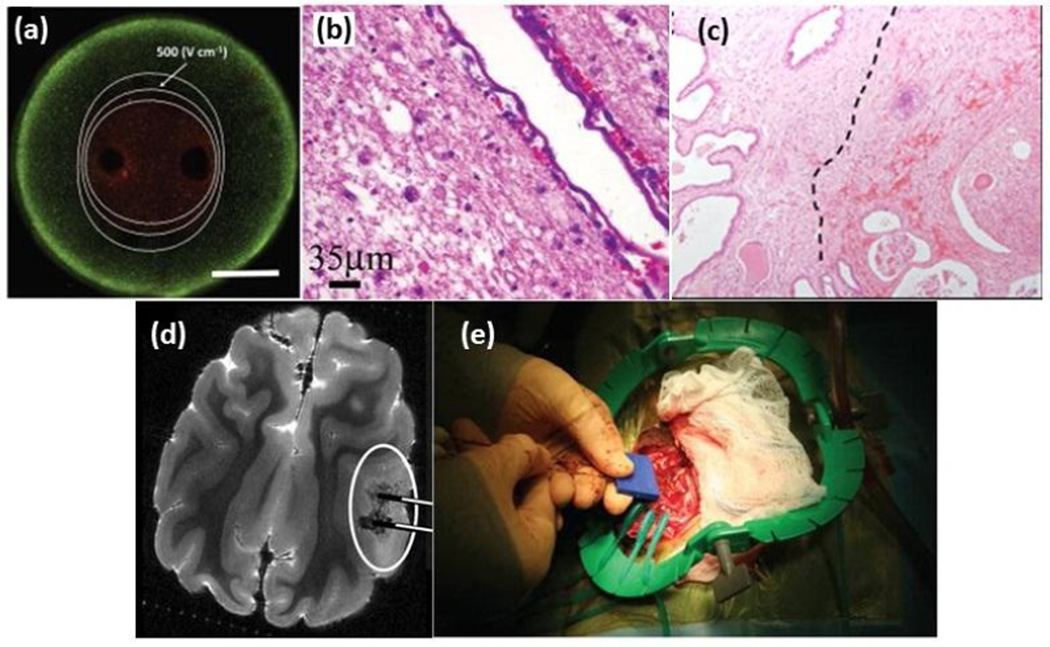
Application of pulsed electric fields (PEFs) across a tumor volume initiates a cascade of biophysical events at the cellular level that eventually yield cell death. Depending on the pulse parameters, different PEF therapies are categorized as irreversible electroporation (IRE), high frequency irreversible electroporation (H-FIRE), nanosecond-PEFs (nsPEFs), or tumor treating fields (TTFields). Electroporation is the phenomenon of inducing nanoscale pores in the cell membrane due to the application of 10–100 micro-second long PEFs of hundreds of V/cm magnitude [89]. IRE is applied at a critical electric field so the cells cannot repair the induced pores, leading to cell death. This technique has been used extensively for non-thermal ablation of tumors [90]. Figure 2 shows examples of IRE treatments in vitro and in vivo. IRE enables killing cells within the targeted area while preserving the underlying structures. However, current IRE treatments do not discriminate between tumors and healthy tissues, and rather ablate all cells within the range of the applied electric field, highlighting the significance of pretreatment planning techniques such as finite element modeling of electric field distribution. However, new studies that have been a focus of our own research groups have shown that several key physical properties of cells and their environment affect their vulnerability in response to IRE treatment. The alterations in cell membrane morphology and cell size have been used to electrically sort cells [91–93] and may be leveraged for selective treatment due to the known effects of these parameters on IRE outcome [94]. H-FIRE treatment is comprised of bursts of about 1kV/cm pulses that has been developed as an improvement to IRE without causing muscle contraction [95, 96]. Our group has demonstrated the feasibility of selectively targeting tumor cells by H-FIRE based on altered NCR [97]. Because nuclear enlargement is a highly conserved characteristic of malignant cells, H-FIRE pulses tuned to target such a phenotype can selectively kill tumorous cells while sparing healthy cells. As opposed to IRE, H-FIRE is independent of tissue conductivity due to the high frequency nature of pulses, and more predictable lesions may be attainable [98]. Recent studies have shown that electroporation changes the electrical impedance spectrum of the TME [99], which could be used for monitoring the size of the ablated tissue [100]. This would allow for precise ablation confined to the predetermined tumor area, by continued monitoring of the electro-physical properties of the TME during treatment.
Figure 2.
Irreversible electroporation from in vitro to in vivo (a) visualization of live/dead cells after IRE treatment in a 3D in vitro tumor model [101], (b) Sparing of major blood vessels after IRE treatment of canine brain [102], (c) Delineation between viable tissue (left) and reactive fibrosis and hemorrhage (right) is seen in human prostate IRE histology, adapted from [103], (d) 7.0-T MRI of IRE-treated canine brain, adapted from [104], (e) dual probe insertion during the intracranial IRE procedure in canine brain [102]
Owing to their short rise time which is faster than cell membrane charging time, nanosecond pulsed electric fields (nsPEFs) penetrate the cell membrane and damage intracellular structures, with minimal membrane electroporation [105, 106]. In vitro studies on skin cells have shown that tumor cells have a stronger response to nsPEFs than normal cells [107, 108], and suggest that an improved understanding of the electrical differences among cells may lead to more targeted therapies based on exploiting these differences.
Finally, tumor treating fields (TTFields) are a new class of electric field therapies that allow for ablation of malignant cells by targeting the highly proliferative phenotype of cancer cells. Invented in 2004 by Kirson et al. [109], this treatment is comprised of alternating electric fields with a frequency of 100–300 kHz and intensity of less than 2 V/cm. These fields interfere with mitosis by rotationally exciting the proteins involved in the formation of the cytokinetic cleavage furrow (CCF), and inducing apoptosis [110]. These fields target the cells during the cytokinesis process and therefore have specific inhibitory effects on dividing cells while leaving quiescent cells intact. The electric fields are applied locally so as to reduce the effect on quickly dividing healthy cells. This allows for a highly selective targeting of proliferative tumor cells. The optimal frequency for TTfields varies among different cell types due to variations in the cell membrane capacitance, which affects field penetration into the cell [111]. Therefore, inherent differences in the permittivity between healthy tissue and tumors could be exploited for selective application of this treatment.
Tumor targeting via thermal ablation
Thermal ablation therapies expose cells to cytotoxic heat generated from several different sources such as radiofrequency (RF), laser, microwave and high intensity focused ultrasound (HIFU). Regardless of its source, hyperthermia damages both healthy and tumor cells. However, tumors are more vulnerable to hyperthermic damage than normal tissue [112]. It has been shown that the acidic TME makes the cells more thermosensitive than the surrounding normal tissue, providing a therapeutic advantage for selective killing of tumor cells by hyperthermia [113]. The low extracellular pH in the tumor induces a low intracellular pH, which is believed to be the main cause of this observed thermosensitivity [114, 115]. Several transmembrane antiport mechanisms regulate the intracellular pH (pHi) and prohibit its equilibrium with the extracellular pH (pHe), which is necessary for its survival and proliferation. Therefore, the efficacy and selectivity of hyperthermia treatments can be improved by inhibiting the pHi regulatory mechanisms resulting in a lower pHi in the acidic TME. [116–118].
Radiofrequency ablation (RFA) is a focal ablation therapy that uses the cytotoxic heat generated from oscillating ions in the high frequency alternating electric field. Electrical and thermal properties of the TME have a significant influence on the outcome of the therapy [119, 120]. In RFA, tumors are prone to more heat generation compared to normal tissue due to their higher concentration of ions. The relatively low thermal conductivity of the tumor tissue, which is a result of dysfunctional vasculature and inefficient blood flow, hinders heat dissipation into the surrounding tissue and improves the localization of heating within the tumor [121]. Some tumors are also surrounded by low thermal conductivity tissues that act as additional thermal insulators to the tumor [122, 123]. Perfusion through the tissue decreases the extent and efficiency of RFA, because blood flow acts as a means of heat dissipation [124, 125]. Therefore, poorly perfused tumors are more easily ablated than normal tissue [126]. Experimentally it has been shown that normal tissues react to hyperthermia by enhancing blood flow; on the contrary no significant effect is observed in the blood flow in tumors [127]. These effects result in a higher temperature in tumors and even more selective cell killing.
High intensity focused ultrasound and tumor viscoelasticity
High intensity focused ultrasound (HIFU) works on the basis of mechanical wave transmission and absorption, which is highly dependent on the mechanical properties of the domain such as stiffness and damping. Depending on the frequency, magnitude, and duration, HIFU can destroy the tissue by either thermal or purely mechanical means. The former occurs by viscous absorption of the mechanical wave by the tissue and its subsequent conversion to heat [128]. Tissues with higher viscous damping are prone to more heat generation. Elastography of prostate cancer [129] and hepatocellular carcinoma [130] has shown that tumors have higher viscosity than normal tissue, which makes them more vulnerable to ultrasound-induced hyperthermia, which means enhancement of therapy targeting. On the other hand, it has been shown that tissues with lower mechanical stiffness are more vulnerable to non-thermal ultrasound-induced cavitation and destruction (histotripsy) [131, 132]. Despite the high tumor tissue stiffness, individual cancer cells present lower stiffness compared to healthy cells. Therefore, given the complex dependencies of HIFU damage mechanisms on the local tissue microenvironment, future studies are needed to explore the use of HIFU in thermal and mechanical modes to achieve preferential killing of tumor cells while preserving ECM.
5. Challenges to targeting the TME
Though the alterations in the chemical and physical TME present exciting targets for cancer therapy, there are many challenges associated with such targeting. Some of these challenges may prevent translation of individual targeting concepts; however, ongoing research is focused on overcoming these challenges for the benefit of cancer patients. As with all molecular targeted therapies, therapeutics directed to hypoxic and acidic microenvironments are only effective if they physically reach those environments. Drugs activated in acidic or hypoxic conditions may reduce side effects as compared with their non-targeted analogs, however delivery to the tumor site is still a major hurdle. Because the delivery and retention of drugs in the tumor is dependent on a large number of factors and is influenced by the complex microenvironment, in many cases targeted drug delivery only results in delivery of only a few percent of the total administered dose [133]. Similar limitations plague drugs designed to capitalize on the EPR effect for targeting. In many cases the majority of macromolecule anti-cancer drugs accumulate in the liver or spleen, with the EPR effect only resulting in very modest enhancements in tumor-selective delivery. [133] Thus it becomes an ongoing challenge to improve the overall delivery of therapeutics to the general tumor area so selective targeting designs can take effect. One new approach recently leveraged electric fields to actively enhance drug penetration into a tumor [134], and approaches such as this provide promising new avenues towards enhancement of tumor-targeted therapies broadly.
Focal ablation techniques, such as electrical, thermal and ultrasound ablation, overcome the problem of delivery that faces targeted drugs, as these ablation therapies are delivered directly at the tumor site. However, these techniques do require surgery. The size and location of the tumor should be known prior to these treatments, which is usually facilitated by ultrasound or other imaging techniques. The invasiveness, although minimal, of these procedures due to the insertion of the probe and lack of selectivity are drawbacks that should be considered. Clinical IRE and RF protocols are considered to be nonspecific ablation techniques that will ablate all tissue within a given treatment volume. In many cases the selectivity of these treatments may be improved by tuning them to capitalize on different physical and chemical features. TTFields targeting dividing cells [109] and HFIRE targeting enlarged nuclei [97] improve the selectivity of electrical ablation methods. Thermal ablation methods may gain improved selectivity by inhibiting pH regulatory mechanisms in an acidic microenvironment. HIFU treatments may have increased efficacy if combined with modulation of tissue stiffness. Further work must be done into improving both the delivery of targeted therapies and the selectivity of local therapies. In both cases, the microenvironment seems bound to play a highly important role. These therapies may ultimately be combined, e.g. as is the case in new electrochemotherapy modalities [135], for synergistic enhancements in the efficacy of each approach.
Finally, the complexity and interconnectedness of these potential physical and chemical features may result in adverse side effects that need to be considered during treatment design. Acidity, hypoxia, metabolism, and angiogenesis, for instance, are all very closely related, and impacting one of these microenvironmental features may have unintended consequences for the others. For instance, reactive oxygen species (ROS) produced as a result of aerobic glycolysis increase cell proliferation and angiogenesis. Consistent with this fact, inhibition of ROS has been shown to decrease angiogenesis and tumor growth in vivo. [136] However, inhibition of ROS with antioxidants has conflictingly been shown to increase tumor growth in mice [137]. This inconsistency in attributed to the complex role of ROS in regulating a variety of interconnected cellular processes [137]. The failure of many promising anti-angiogenesis drugs in clinical trials [138] has been attributed in part to the resulting hypoxia resulting in the outgrowth of resistant and invasive phenotypes that cause metastasis and treatment failure [139, 140]. The hypoxic microenvironment left as a result of anti-angiogenic therapy may also provide a suitable home for cancer stem cells. [141] Even if anti-angiogenic therapy is effective at initially reducing growth of the primary tumor, residual cancer stem cells or evolved invasive phenotypes may lead to repopulation of a primary, or seeding of a metastatic tumor, resulting in poor therapy outcomes. Therefore, it remains an important area of future research to clarify the potential consequences of any form of microenvironmental targeting strategy, and the development of suitable model platforms for such studies remains a key priority. It may be found that multiple interconnected features must be targeted in combination, such angiogenesis and hypoxia, for high efficacy to be achieved in the complex environment found in vivo.
6. Conclusions
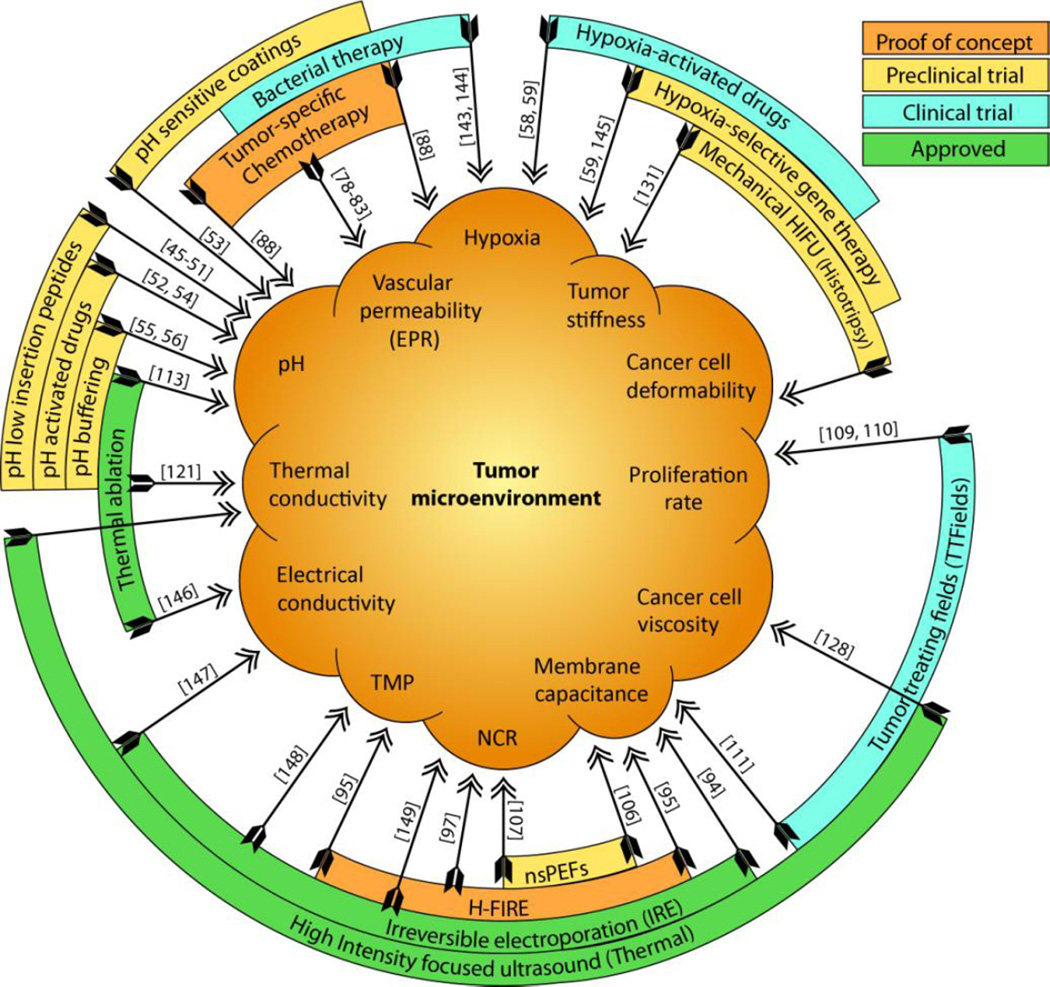
Chemical and physical characteristics of the TME act as a barrier to many traditional cancer therapies. However, there are intriguing opportunities to leverage these same hallmarks to enhance treatment efficacy with appropriately designed therapies. Figure 3 summarizes various physical and chemical alterations of the TME, and provides examples of therapies related with these alterations. The aforementioned treatments either provide proof of concept for development or translation of new methods (nsPEFs, H-FIRE), or are undergoing clinical trials (hypoxia selective drugs [59] [72], IRE [90], TTFields [142]). Despite these advancements, the area remains largely unexplored, and more basic as well as translational work is needed to advance these approaches. The TME we have discussed is often a general characteristic of tumors and does not display the same degree of variance as observed for biological markers across different tumor types. By targeting physical and chemical properties of the TME rather than the biology of neoplastic cells, the efficacy of these therapies is likely to depend on resistance processes that differ from those leading to the failure of radiation, chemotherapy, or molecular targeted therapies. These approaches will therefore complement more traditional treatments. Therapies can then be combined so that the resistant sub-populations are non-overlapping, helping to reduce tumor recurrence and increase survival times for patients. The physical and chemical hallmarks of the TME therefore can pave the way to the design of more rational and effective therapeutic regimens, and with potentially reduced side effects compared with some traditional therapies. However, further work will be required to enhance their selectivity through an improved understanding of the mechanism of individual as well as combinatorial treatments.
Figure 3.
TME-inspired therapies (outer rings) in different stages of development or translation, and their relation to the physical and chemical hallmarks targeted (inner)
Highlights.
We propose that key physical and chemical hallmarks of the tumor microenvironment (TME) should be further considered as presenting therapeutic target opportunities, as opposed to barriers for effective treatment.
We outline the chemical (low pH, low oxygen) and physical (altered tissue and cell mechanics, thermal conductivity, and electrical properties) characteristics of the TME.
We review the prior work, and suggest future studies in the targeting of each of these hallmarks, including the altered pH, oxygen, electrical, mechanical and thermal properties of the TME.
Acknowledgments
This work was supported by the R21 Award from the National Cancer Institute of the National Institutes of Health (R21CA192042), the award from Center for Innovative Technologies (MF13-037-LS), and the award from NIH (5R21 CA173092-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Malik IA, Naz N, Sheikh N, Khan S, Moriconi F, Blaschke M, Ramadori G. Comparison of changes in gene expression of transferrin receptor-1 and other iron-regulatory proteins in rat liver and brain during acute-phase response. Cell and tissue research. 2011;344:299–312. doi: 10.1007/s00441-011-1152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Fiore PP, Pierce JH, Fleming TP, Hazan R, Ullrich A, King CR, Schlessinger J, Aaronson SA. Overexpression of the human EGF receptor confers an EGF-dependent transformed phenotype to NIH 3T3 cells. Cell. 1987;51:1063–1070. doi: 10.1016/0092-8674(87)90592-7. [DOI] [PubMed] [Google Scholar]
- 3.Kraus MH, Popescu N, Amsbaugh S, King CR. Overexpression of the EGF receptor-related proto-oncogene erbB-2 in human mammary tumor cell lines by different molecular mechanisms. The EMBO journal. 1987;6:605. doi: 10.1002/j.1460-2075.1987.tb04797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nature Reviews Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 5.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 6.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proceedings of the National Academy of Sciences. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 8.Dick D. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nature Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 9.Caceres-Cortes J, Mindeni M, Patersoni B, Caligiuri MA. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:17. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 10.Höckel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. Journal of the National Cancer Institute. 2001;93:266–276. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 11.Shannon AM, Bouchier-Hayes DJ, Condron CM, Toomey D. Tumour hypoxia, chemotherapeutic resistance and hypoxia-related therapies. Cancer treatment reviews. 2003;29:297–307. doi: 10.1016/s0305-7372(03)00003-3. [DOI] [PubMed] [Google Scholar]
- 12.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 13.Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer research. 1989;49:6449–6465. [PubMed] [Google Scholar]
- 14.Stylianopoulos T, Martin JD, Chauhan VP, Jain SR, Diop-Frimpong B, Bardeesy N, Smith BL, Ferrone CR, Hornicek FJ, Boucher Y. Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. Proceedings of the National Academy of Sciences. 2012;109:15101–15108. doi: 10.1073/pnas.1213353109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karagiannis GS, Poutahidis T, Erdman SE, Kirsch R, Riddell RH, Diamandis EP. Cancer-associated fibroblasts drive the progression of metastasis through both paracrine and mechanical pressure on cancer tissue. Molecular Cancer Research. 2012;10:1403–1418. doi: 10.1158/1541-7786.MCR-12-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masuzaki R, Tateishi R, Yoshida H, Sato T, Ohki T, Goto T, Yoshida H, Sato S, Sugioka Y, Ikeda H. Assessing liver tumor stiffness by transient elastography. Hepatology international. 2007;1:394–397. doi: 10.1007/s12072-007-9012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D. Tensional homeostasis and the malignant phenotype. Cancer cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD, Rowley DR. Reactive stroma in human prostate cancer induction of myofibroblast phenotype and extracellular matrix remodeling. Clinical Cancer Research. 2002;8:2912–2923. [PubMed] [Google Scholar]
- 19.Krouskop TA, Wheeler TM, Kallel F, Garra BS, Hall T. Elastic moduli of breast and prostate tissues under compression. Ultrasonic imaging. 1998;20:260–274. doi: 10.1177/016173469802000403. [DOI] [PubMed] [Google Scholar]
- 20.Faria EC, Ma N, Gazi E, Gardner P, Brown M, Clarke NW, Snook RD. Measurement of elastic properties of prostate cancer cells using AFM. Analyst. 2008;133:1498–1500. doi: 10.1039/b803355b. [DOI] [PubMed] [Google Scholar]
- 21.Suresh S. Biomechanics and biophysics of cancer cells. Acta Materialia. 2007;55:3989–4014. doi: 10.1016/j.actbio.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain RK, Martin JD, Stylianopoulos T. The role of mechanical forces in tumor growth and therapy. Annual review of biomedical engineering. 2014;16:321. doi: 10.1146/annurev-bioeng-071813-105259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boucher Y, Kirkwood JM, Opacic D, Desantis M, Jain RK. Interstitial hypertension in superficial metastatic melanomas in humans. Cancer research. 1991;51:6691–6694. [PubMed] [Google Scholar]
- 24.Boucher Y, Baxter LT, Jain RK. Interstitial pressure gradients in tissue-isolated and subcutaneous tumors: implications for therapy. Cancer research. 1990;50:4478–4484. [PubMed] [Google Scholar]
- 25.Less JR, Posner MC, Boucher Y, Borochovitz D, Wolmark N, Jain RK. Interstitial hypertension in human breast and colorectal tumors. Cancer research. 1992;52:6371–6374. [PubMed] [Google Scholar]
- 26.Roh H, Boucher Y, Kalnicki S, Buchsbaum R, Bloomer W, Jain R. Interstitial hypertension in carcinoma of uterine cervix in patients: possible correlation with tumor oxygenation and radiation response. Cancer Research. 1991;51:6695–6698. [PubMed] [Google Scholar]
- 27.Boucher Y, Leunig M, Jain RK. Tumor angiogenesis and interstitial hypertension. Cancer Research. 1996;56:4264–4266. [PubMed] [Google Scholar]
- 28.Jain RK, Tong RT, Munn LL. Effect of vascular normalization by antiangiogenic therapy on interstitial hypertension, peritumor edema, and lymphatic metastasis: insights from a mathematical model. Cancer research. 2007;67:2729–2735. doi: 10.1158/0008-5472.CAN-06-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, Jain RK. Normalization of the Vasculature for Treatment of Cancer and Other Diseases. Physiological Reviews. 2011;91:1071–1121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cure J. Cancer an electrical phenomenon. Resonant. 1991 [Google Scholar]
- 31.Stern R, Milestone B, Gatenby R. Carcinogenesis and the plasma membrane. Medical hypotheses. 1999;52:367–372. doi: 10.1054/mehy.1997.0657. [DOI] [PubMed] [Google Scholar]
- 32.Cone CD. Unified theory on the basic mechanism of normal mitotic control and oncogenesis. Journal of Theoretical Biology. 1971;30:151–181. doi: 10.1016/0022-5193(71)90042-7. [DOI] [PubMed] [Google Scholar]
- 33.Niemtzow RC. Transmembrane potentials and characteristics of immune and tumor cells. CRC PressI Llc; 1985. [Google Scholar]
- 34.Zhao Y, Zhao XT, Chen DY, Luo YN, Jiang M, Wei C, Long R, Yue WT, Wang JB, Chen J. Tumor cell characterization and classification based on cellular specific membrane capacitance and cytoplasm conductivity. Biosensors and Bioelectronics. 2014;57:245–253. doi: 10.1016/j.bios.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 35.Brennan DJ, Rexhepaj E, O'Brien SL, McSherry E, O'Connor DP, Fagan A, Culhane AC, Higgins DG, Jirstrom K, Millikan RC. Altered cytoplasmic-to-nuclear ratio of survivin is a prognostic indicator in breast cancer. Clinical Cancer Research. 2008;14:2681–2689. doi: 10.1158/1078-0432.CCR-07-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta neuropathologica. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ko L, Koestner A, Wechsler W. Morphological characterization of nitrosourea-induced glioma cell lines and clones. Acta neuropathologica. 1980;51:23–31. doi: 10.1007/BF00688846. [DOI] [PubMed] [Google Scholar]
- 38.Maroulakou IG, Anver M, Garrett L, Green JE. Prostate and mammary adenocarcinoma in transgenic mice carrying a rat C3 (1) simian virus 40 large tumor antigen fusion gene. Proceedings of the National Academy of Sciences. 1994;91:11236–11240. doi: 10.1073/pnas.91.23.11236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blad B, Baldetorp B. Impedance spectra of tumour tissue in comparison with normal tissue; a possible clinical application for electrical impedance tomography. Physiological Measurement. 1996;17:A105. doi: 10.1088/0967-3334/17/4a/015. [DOI] [PubMed] [Google Scholar]
- 40.Foster K, Schepps J. Dielectric properties of tumor and normal tissues at radio through microwave frequencies. The Journal of microwave power. 1981;16:107–119. doi: 10.1080/16070658.1981.11689230. [DOI] [PubMed] [Google Scholar]
- 41.Laufer S, Ivorra A, Reuter VE, Rubinsky B, Solomon SB. Electrical impedance characterization of normal and cancerous human hepatic tissue. Physiological measurement. 2010;31:995. doi: 10.1088/0967-3334/31/7/009. [DOI] [PubMed] [Google Scholar]
- 42.Da Silva JE, De Sá JM, Jossinet J. Classification of breast tissue by electrical impedance spectroscopy. Medical and Biological Engineering and Computing. 2000;38:26–30. doi: 10.1007/BF02344684. [DOI] [PubMed] [Google Scholar]
- 43.Jossinet J. Variability of impedivity in normal and pathological breast tissue. Medical and Biological Engineering and Computing. 1996;34:346–350. doi: 10.1007/BF02520002. [DOI] [PubMed] [Google Scholar]
- 44.Toso S, Piccoli A, Gusella M, Menon D, Bononi A, Crepaldi G, Ferrazzi E. Altered tissue electric properties in lung cancer patients as detected by bioelectric impedance vector analysis. Nutrition. 2000;16:120–124. doi: 10.1016/s0899-9007(99)00230-0. [DOI] [PubMed] [Google Scholar]
- 45.Andreev OA, Engelman DM, Reshetnyak YK. pH-sensitive membrane peptides (pHLIPs) as a novel class of delivery agents. Molecular membrane biology. 2010;27:341–352. doi: 10.3109/09687688.2010.509285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andreev OA, Engelman DM, Reshetnyak YK. Targeting diseased tissues by pHLIP insertion at low cell surface pH. Frontiers in physiology. 2014;5 doi: 10.3389/fphys.2014.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andreev OA, Dupuy AD, Segala M, Sandugu S, Serra DA, Chichester CO, Engelman DM, Reshetnyak YK. Mechanism and uses of a membrane peptide that targets tumors and other acidic tissues in vivo. Proceedings of the National Academy of Sciences. 2007;104:7893–7898. doi: 10.1073/pnas.0702439104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yao L, Daniels J, Wijesinghe D, Andreev OA, Reshetnyak YK. pHLIP®-mediated delivery of PEGylated liposomes to cancer cells. Journal of Controlled Release. 2013;167:228–237. doi: 10.1016/j.jconrel.2013.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wijesinghe D, Arachchige MC, Lu A, Reshetnyak YK, Andreev OA. pH dependent transfer of nano-pores into membrane of cancer cells to induce apoptosis. Scientific reports. 2013;3 doi: 10.1038/srep03560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yao L, Daniels J, Moshnikova A, Kuznetsov S, Ahmed A, Engelman DM, Reshetnyak YK, Andreev OA. pHLIP peptide targets nanogold particles to tumors. Proceedings of the National Academy of Sciences. 2013;110:465–470. doi: 10.1073/pnas.1219665110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng CJ, Bahal R, Babar IA, Pincus Z, Barrera F, Liu C, Svoronos A, Braddock DT, Glazer PM, Engelman DM, Saltzman WM, Slack FJ. MicroRNA silencing for cancer therapy targeted to the tumour microenvironment. Nature. 2015;518:107–110. doi: 10.1038/nature13905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Han L, Guo Y, Ma H, He X, Kuang Y, Zhang N, Lim E, Zhou W, Jiang C. Acid Active Receptor-Specific Peptide Ligand for In Vivo Tumor-Targeted Delivery. Small. 2013;9:3647–3658. doi: 10.1002/smll.201300279. [DOI] [PubMed] [Google Scholar]
- 53.Yang X-Z, Du J-Z, Dou S, Mao C-Q, Long H-Y, Wang J. Sheddable ternary nanoparticles for tumor acidity-targeted siRNA delivery. ACS nano. 2011;6:771–781. doi: 10.1021/nn204240b. [DOI] [PubMed] [Google Scholar]
- 54.Du JZ, Sun TM, Song WJ, Wu J, Wang J. A Tumor-Acidity-Activated Charge-Conversional Nanogel as an Intelligent Vehicle for Promoted Tumoral-Cell Uptake and Drug Delivery. Angewandte Chemie. 2010;122:3703–3708. doi: 10.1002/anie.200907210. [DOI] [PubMed] [Google Scholar]
- 55.Robey IF, Baggett BK, Kirkpatrick ND, Roe DJ, Dosescu J, Sloane BF, Hashim AI, Morse DL, Raghunand N, Gatenby RA. Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer research. 2009;69:2260–2268. doi: 10.1158/0008-5472.CAN-07-5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hashim AI, Cornnell HH, Ribeiro MdLC, Abrahams D, Cunningham J, Lloyd M, Martinez GV, Gatenby RA, Gillies RJ. Reduction of metastasis using a non-volatile buffer. Clinical & experimental metastasis. 2011;28:841–849. doi: 10.1007/s10585-011-9415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nature Reviews Cancer. 2004;4:437–447. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 58.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nature Reviews Cancer. 2011;11:393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 59.Brown JM. Hypoxic cytotoxic agents: a new approach to cancer chemotherapy. Drug Resistance Updates. 2000;3:7–13. doi: 10.1054/drup.2000.0120. [DOI] [PubMed] [Google Scholar]
- 60.Iyer V, Szybalski W. Mitomycins and porfiromycin: chemical mechanism of activation and cross-linking of DNA. Science. 1964;145:55–58. doi: 10.1126/science.145.3627.55. [DOI] [PubMed] [Google Scholar]
- 61.Rockwell S, Kennedy KA, Sartorelli AC. Mitomycin-C as a prototype bioreductive alkylating agent: in vitro studies of metabolism and cytotoxicity. International Journal of Radiation Oncology* Biology* Physics. 1982;8:753–755. doi: 10.1016/0360-3016(82)90728-3. [DOI] [PubMed] [Google Scholar]
- 62.Haffty BG, Son YH, Sasaki CT, Papac R, Fischer D, Rockwell S, Sartorelli A, Fischer JJ. Mitomycin C as an adjunct to postoperative radiation therapy in squamous cell carcinoma of the head and neck: results from two randomized clinical trials. International Journal of Radiation Oncology* Biology* Physics. 1993;27:241–250. doi: 10.1016/0360-3016(93)90234-m. [DOI] [PubMed] [Google Scholar]
- 63.Brown JM. Tumor hypoxia in cancer therapy. Methods in enzymology. 2007;435:295–321. doi: 10.1016/S0076-6879(07)35015-5. [DOI] [PubMed] [Google Scholar]
- 64.Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL. The expression and distribution of the hypoxia-inducible factors HIF-1α and HIF-2α in normal human tissues, cancers, and tumor-associated macrophages. The American journal of pathology. 2000;157:411–421. doi: 10.1016/s0002-9440(10)64554-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Negus R, Turner L, Burke F, Balkwill FR. Hypoxia down-regulates MCP-1 expression: implications for macrophage distribution in tumors. Journal of leukocyte biology. 1998;63:758–765. doi: 10.1002/jlb.63.6.758. [DOI] [PubMed] [Google Scholar]
- 66.Bingle L, Brown N, Lewis C. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. The Journal of pathology. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 67.Griffiths L, Binley K, Iqball S, Kan O, Maxwell P, Ratcliffe P, Lewis C, Harris A, Kingsman S, Naylor S. The macrophage-a novel system to deliver gene therapy to pathological hypoxia. Gene therapy. 2000;7:255–262. doi: 10.1038/sj.gt.3301058. [DOI] [PubMed] [Google Scholar]
- 68.Fox M, Lemmon M, Mauchline M, Davis T, Giaccia A, Minton N, Brown J. Anaerobic bacteria as a delivery system for cancer gene therapy: in vitro activation of 5-fluorocytosine by genetically engineered clostridia. Gene therapy. 1996;3:173–178. [PubMed] [Google Scholar]
- 69.Liu S, Xu X, Zeng X, Li L, Chen Q, Li J. Tumor- targeting bacterial therapy: A potential treatment for oral cancer (Review) Oncology letters. 2014;8:2359–2366. doi: 10.3892/ol.2014.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Malmgren RA, Flanigan CC. Localization of the vegetative form of Clostridium tetani in mouse tumors following intravenous spore administration. Cancer research. 1955;15:473–478. [PubMed] [Google Scholar]
- 71.Moese J, Moese G. Oncolysis by clostridia. I. Activity of Clostridium butyricum (M-55) and other nonpathogenic clostridia against the Ehrlich carcinoma. Cancer research. 1964;24:212–216. [PubMed] [Google Scholar]
- 72.Liu S, Minton N, Giaccia A, Brown J. Anticancer efficacy of systemically delivered anaerobic bacteria as gene therapy vectors targeting tumor hypoxia/necrosis. Gene therapy. 2002;9:291–296. doi: 10.1038/sj.gt.3301659. [DOI] [PubMed] [Google Scholar]
- 73.Zhao L, Ching L, Kestell P, Baguley B. The antitumour activity of 5, 6-dimethylxanthenone-4-acetic acid (DMXAA) in TNF receptor-1 knockout mice. British journal of cancer. 2002;87:465–470. doi: 10.1038/sj.bjc.6600479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dang LH, Bettegowda C, Huso DL, Kinzler KW, Vogelstein B. Combination bacteriolytic therapy for the treatment of experimental tumors. Proceedings of the National Academy of Sciences. 2001;98:15155–15160. doi: 10.1073/pnas.251543698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer research. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 76.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Advances in enzyme regulation. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 77.Seki T, Fang J, Maeda H. Pharmaceutical Perspectives of Cancer Therapeutics. Springer; 2009. Tumor-targeted macromolecular drug delivery based on the enhanced permeability and retention effect in solid tumor; pp. 93–120. [Google Scholar]
- 78.Maeda H, Bharate G, Daruwalla J. Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. European Journal of Pharmaceutics and Biopharmaceutics. 2009;71:409–419. doi: 10.1016/j.ejpb.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 79.Maeda H, Greish K, Fang J. Polymer Therapeutics II. Springer; 2006. The EPR effect and polymeric drugs: a paradigm shift for cancer chemotherapy in the 21st century; pp. 103–121. [Google Scholar]
- 80.Iyer AK, Khaled G, Fang J, Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug discovery today. 2006;11:812–818. doi: 10.1016/j.drudis.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 81.Noguchi Y, Wu J, Duncan R, Strohalm J, Ulbrich K, Akaike T, Maeda H. Early phase tumor accumulation of macromolecules: a great difference in clearance rate between tumor and normal tissues. Japanese Journal of Cancer Research. 1998;89:307–314. doi: 10.1111/j.1349-7006.1998.tb00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vicent MJ, Ringsdorf H, Duncan R. Polymer therapeutics: clinical applications and challenges for development. Advanced Drug Delivery Reviews. 2009;61:1117–1120. doi: 10.1016/j.addr.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 83.Duncan R. The dawning era of polymer therapeutics. Nature Reviews Drug Discovery. 2003;2:347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 84.Robey IF, Baggett BK, Kirkpatrick ND, Roe DJ, Dosescu J, Sloane BF, Hashim AI, Morse DL, Raghunand N, Gatenby RA, Gillies RJ. Bicarbonate Increases Tumor pH and Inhibits Spontaneous Metastases. Cancer Research. 2009;69:2260–2268. doi: 10.1158/0008-5472.CAN-07-5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nagamitsu A, Greish K, Maeda H. Elevating blood pressure as a strategy to increase tumor-targeted delivery of macromolecular drug SMANCS: cases of advanced solid tumors. Japanese journal of clinical oncology. 2009:hyp074. doi: 10.1093/jjco/hyp074. [DOI] [PubMed] [Google Scholar]
- 86.Li C, Miyamoto Y, Kojima Y, Maeda H. Augmentation of tumour delivery of macromolecular drugs with reduced bone marrow delivery by elevating blood pressure. British journal of cancer. 1993;67:975. doi: 10.1038/bjc.1993.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kojima H, Urano Y, Kikuchi K, Higuchi T, Hirata Y, Nagano T. Fluorescent indicators for imaging nitric oxide production. Angewandte Chemie International Edition. 1999;38:3209–3212. doi: 10.1002/(sici)1521-3773(19991102)38:21<3209::aid-anie3209>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 88.Seki T, Fang J, Maeda H. Enhanced delivery of macromolecular antitumor drugs to tumors by nitroglycerin application. Cancer science. 2009;100:2426–2430. doi: 10.1111/j.1349-7006.2009.01323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rubinsky B. Irreversible electroporation. Springer Science & Business Media; 2009. [Google Scholar]
- 90.Davalos RV, Mir L, Rubinsky B. Tissue ablation with irreversible electroporation. Annals of biomedical engineering. 2005;33:223–231. doi: 10.1007/s10439-005-8981-8. [DOI] [PubMed] [Google Scholar]
- 91.Chen J, Zheng Y, Tan Q, Shojaei-Baghini E, Zhang YL, Li J, Prasad P, You L, Wu XY, Sun Y. Classification of cell types using a microfluidic device for mechanical and electrical measurement on single cells. Lab on a Chip. 2011;11:3174–3181. doi: 10.1039/c1lc20473d. [DOI] [PubMed] [Google Scholar]
- 92.Shafiee H, Sano MB, Henslee EA, Caldwell JL, Davalos RV. Selective isolation of live/dead cells using contactless dielectrophoresis (cDEP) Lab on a Chip. 2010;10:438–445. doi: 10.1039/b920590j. [DOI] [PubMed] [Google Scholar]
- 93.Salmanzadeh A, Romero L, Shafiee H, Gallo-Villanueva RC, Stremler MA, Cramer SD, Davalos RV. Isolation of prostate tumor initiating cells (TICs) through their dielectrophoretic signature. Lab on a Chip. 2012;12:182–189. doi: 10.1039/c1lc20701f. [DOI] [PubMed] [Google Scholar]
- 94.Agarwal A, Zudans I, Weber EA, Olofsson J, Orwar O, Weber SG. Effect of cell size and shape on single-cell electroporation. Analytical chemistry. 2007;79:3589–3596. doi: 10.1021/ac062049e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Arena CB, Sano MB, Rossmeisl JH, Caldwell JL, Garcia PA, Rylander MN, Davalos RV. High-frequency irreversible electroporation (H-FIRE) for non-thermal ablation without muscle contraction. Biomedical engineering online. 2011;10:102. doi: 10.1186/1475-925X-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sano MB, Arena CB, DeWitt MR, Saur D, Davalos RV. In-vitro bipolar nano-and microsecond electro-pulse bursts for irreversible electroporation therapies. Bioelectrochemistry. 2014;100:69–79. doi: 10.1016/j.bioelechem.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 97.Ivey JW, Latouche EL, Sano MB, Davalos RV, Verbridge SS. Targeted cellular ablation based on the morphology of malignant cells. Scientific Reports. 2015 doi: 10.1038/srep17157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bhonsle SP, Arena CB, Sweeney DC, Davalos RV. Mitigation of impedance changes due to electroporation therapy using bursts of high-frequency bipolar pulses. Biomedical engineering online. 2015;14:S3. doi: 10.1186/1475-925X-14-S3-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Davalos RV, Otten DM, Mir LM, Rubinsky B. Electrical impedance tomography for imaging tissue electroporation. Biomedical Engineering, IEEE Transactions on. 2004;51:761–767. doi: 10.1109/TBME.2004.824148. [DOI] [PubMed] [Google Scholar]
- 100.Bonakdar M, Latouche EL, Mahajan RL, Davalos RV. The Feasibility of a Smart Surgical Probe for Verification of IRE Treatments Using Electrical Impedance Spectroscopy. Biomedical Engineering, IEEE Transactions on. 2015:1-1. doi: 10.1109/TBME.2015.2441636. PP. [DOI] [PubMed] [Google Scholar]
- 101.Arena CB, Szot CS, Garcia PA, Rylander MN, Davalos RV. A three-dimensional in vitro tumor platform for modeling therapeutic irreversible electroporation. Biophysical journal. 2012;103:2033–2042. doi: 10.1016/j.bpj.2012.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ellis TL, Garcia PA, Rossmeisl JH, Jr, Henao-Guerrero N, Robertson J, Davalos RV. Nonthermal irreversible electroporation for intracranial surgical applications: laboratory investigation. Journal of neurosurgery. 2011;114:681–688. doi: 10.3171/2010.5.JNS091448. [DOI] [PubMed] [Google Scholar]
- 103.Neal RE, Millar JL, Kavnoudias H, Royce P, Rosenfeldt F, Pham A, Smith R, Davalos RV, Thomson KR. In vivo characterization and numerical simulation of prostate properties for non-thermal irreversible electroporation ablation. The Prostate. 2014;74:458–468. doi: 10.1002/pros.22760. [DOI] [PubMed] [Google Scholar]
- 104.Garcia PA, Rossmeisl JH, Jr, Neal RE, II, Ellis TL, Olson JD, Henao-Guerrero N, Robertson J, Davalos RV. Intracranial nonthermal irreversible electroporation: in vivo analysis. The Journal of membrane biology. 2010;236:127–136. doi: 10.1007/s00232-010-9284-z. [DOI] [PubMed] [Google Scholar]
- 105.Garon EB, Sawcer D, Vernier PT, Tang T, Sun Y, Marcu L, Gundersen MA, Koeffler HP. In vitro and in vivo evaluation and a case report of intense nanosecond pulsed electric field as a local therapy for human malignancies. International journal of cancer. 2007;121:675–682. doi: 10.1002/ijc.22723. [DOI] [PubMed] [Google Scholar]
- 106.Chen N, Schoenbach KH, Kolb JF, Swanson RJ, Garner AL, Yang J, Joshi RP, Beebe SJ. Leukemic cell intracellular responses to nanosecond electric fields. Biochemical and biophysical research communications. 2004;317:421–427. doi: 10.1016/j.bbrc.2004.03.063. [DOI] [PubMed] [Google Scholar]
- 107.Yang W, Wu Y, Yin D, Koeffler H, Sawcer D, Vernier P, Gundersen M. Differential sensitivities of malignant and normal skin cells to nanosecond pulsed electric fields. Technology in cancer research & treatment. 2011;10:281–286. doi: 10.7785/tcrt.2012.500204. [DOI] [PubMed] [Google Scholar]
- 108.Merla C, Paffi A, Apollonio F, Leveque P, d'Inzeo G, Liberti M. Microdosimetry for nanosecond pulsed electric field applications: a parametric study for a single cell. Biomedical Engineering, IEEE Transactions on. 2011;58:1294–1302. doi: 10.1109/TBME.2010.2104150. [DOI] [PubMed] [Google Scholar]
- 109.Kirson ED, Gurvich Z, Schneiderman R, Dekel E, Itzhaki A, Wasserman Y, Schatzberger R, Palti Y. Disruption of cancer cell replication by alternating electric fields. Cancer research. 2004;64:3288–3295. doi: 10.1158/0008-5472.can-04-0083. [DOI] [PubMed] [Google Scholar]
- 110.Gera N, Yang A, Holtzman T, Lee S, Wong E, Swanson K. Tumor treating fields perturb the localization of septins and cause aberrant mitotic exit. PloS one. 2015;10:e0125269. doi: 10.1371/journal.pone.0125269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Davies AM, Weinberg U, Palti Y. Tumor treating fields: a new frontier in cancer therapy. Annals of the New York Academy of Sciences. 2013;1291:86–95. doi: 10.1111/nyas.12112. [DOI] [PubMed] [Google Scholar]
- 112.Robins HI, Steeves RA, Clark AW, Martin PA, Miller K, Dennis WH. Differential sensitivity of AKR murine leukemia and normal bone marrow cells to hyperthermia. Cancer research. 1983;43:4951–4955. [PubMed] [Google Scholar]
- 113.Gerweck LE. Modification of cell lethality at elevated temperatures the pH effect. Radiation Research. 1977;70:224–235. [PubMed] [Google Scholar]
- 114.Song CW, Park H, Ross BD. Antiangiogenic Agents in Cancer Therapy. Springer; 1999. Intra-and extracellular pH in solid tumors; pp. 51–64. [Google Scholar]
- 115.Song CW, Lyons JC, Griffin RJ, Makepeace CM, Cragoe EJ. Increase in thermosensitivity of tumor cells by lowering intracellular pH. Cancer research. 1993;53:1599–1601. [PubMed] [Google Scholar]
- 116.Kitai R, Kabuto M, Kubota T, Kobayashi H, Matsumoto H, Hayashi S, Shioura H, Ohtsubo T, Katayama K, Kano E. Sensitization to hyperthermia by intracellular acidification. Journal of neurooncology. 1998;39:197–203. doi: 10.1023/a:1005996816453. [DOI] [PubMed] [Google Scholar]
- 117.Yamagata M, Tannock I. The chronic administration of drugs that inhibit the regulation of intracellular pH: in vitro and anti-tumour effects. British journal of cancer. 1996;73:1328. doi: 10.1038/bjc.1996.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lyons JC, Ross BD, Song CW. Enhancement of hyperthermia effect in vivo by amiloride and DIDS. International Journal of Radiation Oncology* Biology* Physics. 1993;25:95–103. doi: 10.1016/0360-3016(93)90150-t. [DOI] [PubMed] [Google Scholar]
- 119.Ahmed M, Liu Z, Afzal KS, Weeks D, Lobo SM, Kruskal JB, Lenkinski RE, Goldberg SN. Radiofrequency Ablation: Effect of Surrounding Tissue Composition on Coagulation Necrosis in a Canine Tumor Model 1. Radiology. 2004;230:761–767. doi: 10.1148/radiol.2303021801. [DOI] [PubMed] [Google Scholar]
- 120.Goldberg SN. Radiofrequency tumor ablation: principles and techniques. European Journal of Ultrasound. 2001;13:129–147. doi: 10.1016/s0929-8266(01)00126-4. [DOI] [PubMed] [Google Scholar]
- 121.LeVeen HH, Wapnick S, Piccone V, Falk G, Ahmed N. Tumor eradication by radiofrequency therapy: response in 21 patients. Jama. 1976;235:2198–2200. [PubMed] [Google Scholar]
- 122.Liu Z, Ahmed M, Weinstein Y, Yi M, Mahajan RL, Goldberg SN. Characterization of the RF ablation-induced'oven effect': the importance of background tissue thermal conductivity on tissue heating. International journal of hyperthermia. 2006;22:327–342. doi: 10.1080/02656730600609122. [DOI] [PubMed] [Google Scholar]
- 123.Livraghi T, Goldberg S, Meloni F, Solbiati L, Gazelle G. Hepatocellular carcinoma: comparison of efficacy between percutaneous ethanol instillation and radiofrequency. Radiology. 1999;210:655–663. doi: 10.1148/radiology.210.3.r99fe40655. [DOI] [PubMed] [Google Scholar]
- 124.Liu Z, Ahmed M, Sabir A, Humphries S, Goldberg S. Computer modeling of the effect of perfusion on heating patterns in radiofrequency tumor ablation. International journal of hyperthermia. 2007;23:49–58. doi: 10.1080/02656730601094415. [DOI] [PubMed] [Google Scholar]
- 125.Ahmed M, Liu Z, Humphries S, Nahum Goldberg S. Computer modeling of the combined effects of perfusion, electrical conductivity, and thermal conductivity on tissue heating patterns in radiofrequency tumor ablation. International Journal of Hyperthermia. 2008;24:577–588. doi: 10.1080/02656730802192661. [DOI] [PubMed] [Google Scholar]
- 126.Lagendijk JJ, Hofman P, Schipper J. Perfusion analyses in advanced breast carcinoma during hyperthermia, International journal of hyperthermia : the official journal of European Society for Hyperthermic Oncology. North American Hyperthermia Group. 1988;4:479. doi: 10.3109/02656738809027693. [DOI] [PubMed] [Google Scholar]
- 127.Song CW, Rhee JG, Levitt SH. Blood flow in normal tissues and tumors during hyperthermia. Journal of the National Cancer Institute. 1980;64:119–124. [PubMed] [Google Scholar]
- 128.ter Haar G, Coussios C. High intensity focused ultrasound: physical principles and devices. International Journal of Hyperthermia. 2007;23:89–104. doi: 10.1080/02656730601186138. [DOI] [PubMed] [Google Scholar]
- 129.Hoyt K, Castaneda B, Zhang M, Nigwekar P, di Sant’Agnese PA, Joseph JV, Strang J, Rubens DJ, Parker KJ. Tissue elasticity properties as biomarkers for prostate cancer. Cancer biomarkers: section A of Disease markers. 2008;4:213. doi: 10.3233/cbm-2008-44-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Garteiser P, Doblas S, Daire J-L, Wagner M, Leitao H, Vilgrain V, Sinkus R, Van Beers BE. MR elastography of liver tumours: value of viscoelastic properties for tumour characterisation. European radiology. 2012;22:2169–2177. doi: 10.1007/s00330-012-2474-6. [DOI] [PubMed] [Google Scholar]
- 131.Xu J, Bigelow TA. Experimental investigation of the effect of stiffness, exposure time and scan direction on the dimension of ultrasound histotripsy lesions. Ultrasound in medicine & biology. 2011;37:1865–1873. doi: 10.1016/j.ultrasmedbio.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 132.Hoogenboom M, Eikelenboom D, den Brok MH, Heerschap A, Fütterer JJ, Adema GJ. Mechanical High-Intensity Focused Ultrasound Destruction of Soft Tissue: Working Mechanisms and Physiologic Effects. Ultrasound in medicine & biology. 2015;41:1500–1517. doi: 10.1016/j.ultrasmedbio.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 133.Bae YH, Park K. Targeted drug delivery to tumors: Myths, reality and possibility. Journal of Controlled Release. 2011;153:198–205. doi: 10.1016/j.jconrel.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Byrne JD, Jajja MNR, O’Neill AT, Bickford LR, Keeler AW, Hyder N, Wagner K, Deal A, Little RE, Moffitt RA, Stack C, Nelson M, Brooks CR, Lee W, Luft JC, Napier ME, Darr D, Anders CK, Stack R, Tepper JE, Wang AZ, Zamboni WC, Yeh JJ, DeSimone JM. Local iontophoretic administration of cytotoxic therapies to solid tumors. Science Translational Medicine. 2015;7 doi: 10.1126/scitranslmed.3009951. 273ra214-273ra214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gothelf A, Mir LM, Gehl J. Electrochemotherapy: results of cancer treatment using enhanced delivery of bleomycin by electroporation. Cancer Treatment Reviews. 2003;29:371–387. doi: 10.1016/s0305-7372(03)00073-2. [DOI] [PubMed] [Google Scholar]
- 136.Xia C, Meng Q, Liu L-Z, Rojanasakul Y, Wang X-R, Jiang B-H. Reactive Oxygen Species Regulate Angiogenesis and Tumor Growth through Vascular Endothelial Growth Factor. Cancer Research. 2007;67:10823–10830. doi: 10.1158/0008-5472.CAN-07-0783. [DOI] [PubMed] [Google Scholar]
- 137.Sayin VI, Ibrahim MX, Larsson E, Nilsson JA, Lindahl P, Bergo MO. Antioxidants Accelerate Lung Cancer Progression in Mice. Science Translational Medicine. 2014;6 doi: 10.1126/scitranslmed.3007653. 221ra215-221ra215. [DOI] [PubMed] [Google Scholar]
- 138.Ebos JML, Kerbel RS. Antiangiogenic therapy: impact on invasion, disease progression, and metastasis. Nat Rev Clin Oncol. 2011;8:210–221. doi: 10.1038/nrclinonc.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rapisarda A, Melillo G. Role of the hypoxic tumor microenvironment in the resistance to antiangiogenic therapies. Drug Resistance Updates. 2009;12:74–80. doi: 10.1016/j.drup.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic Therapy Elicits Malignant Progression of Tumors to Increased Local Invasion and Distant Metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Keith B, Simon MC. Hypoxia Inducible Factors, stem cells and cancer. Cell. 2007;129:465–472. doi: 10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kirson ED, Dbalý V, Tovaryš F, Vymazal J, Soustiel JF, Itzhaki A, Mordechovich D, Steinberg-Shapira S, Gurvich Z, Schneiderman R. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proceedings of the National Academy of Sciences. 2007;104:10152–10157. doi: 10.1073/pnas.0702916104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Carey R, Holland J, Whang H, Neter E, Bryant B. Clostridial oncolysis in man. European Journal of Cancer (1965) 1967;3:37–46. [Google Scholar]
- 144.Toso JF, Gill VJ, Hwu P, Marincola FM, Restifo NP, Schwartzentruber DJ, Sherry RM, Topalian SL, Yang JC, Stock F. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. Journal of Clinical Oncology. 2002;20:142–152. doi: 10.1200/JCO.2002.20.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Binley K, Askham Z, Martin L, Spearman H, Day D, Kingsman S, Naylor S. Hypoxia-mediated tumour targeting. Gene therapy. 2003;10:540–549. doi: 10.1038/sj.gt.3301944. [DOI] [PubMed] [Google Scholar]
- 146.Goldberg SN, Ahmed M, Gazelle GS, Kruskal JB, Huertas JC, Halpern EF, Oliver BS, Lenkinski RE. Radio-Frequency Thermal Ablation with NaCl Solution Injection: Effect of Electrical Conductivity on Tissue Heating and Coagulation—Phantom and Porcine Liver Study 1. Radiology. 2001;219:157–165. doi: 10.1148/radiology.219.1.r01ap27157. [DOI] [PubMed] [Google Scholar]
- 147.Edd JF, Davalos RV. Mathematical modeling of irreversible electroporation for treatment planning. Technology in cancer research & treatment. 2007;6:275–286. doi: 10.1177/153303460700600403. [DOI] [PubMed] [Google Scholar]
- 148.DeBruin KA, Krassowska W. Modeling Electroporation in a Single Cell. I. Effects of Field Strength and Rest Potential. Biophysical Journal. 1999;77:1213–1224. doi: 10.1016/S0006-3495(99)76973-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sano MB, Arena CB, DeWitt MR, Saur D, Davalos RV. In-vitro bipolar nano- and microsecond electro-pulse bursts for irreversible electroporation therapies. Bioelectrochemistry. 2014;100:69–79. doi: 10.1016/j.bioelechem.2014.07.010. [DOI] [PubMed] [Google Scholar]