Abstract
Objectives
To compare 12 week outcomes of single-therapy tolterodine (Detrol LA ®) extended release to intravaginal estrogen (Estrace ®) for overactive bladder (OAB) symptoms and characterize 24 and 52 week outcomes in women undergoing combined therapy.
Methods
A single-site randomized, open-label trial in women with urinary frequency, urgency, nocturia, and/or urgency urinary incontinence symptoms was performed. Fifty-eight participants were randomized to oral tolterodine extended release daily or intravaginal estradiol cream nightly for six weeks then twice per week. The primary outcome was change in OAB-questionnaire (OAB-q) symptom bother score at 12 weeks. Secondary outcomes included the health-related quality of life (HRQL) of the OAB-q and a 3-day bladder diary. At 12 weeks, subjects were offered addition of the alternative therapy with follow-up at 24 and 52 weeks.
Results
There was no difference in symptom bother score improvement between the tolterodine and intravaginal estradiol groups baseline to 12 weeks (20.6±21.7, −15.8±23.3, respectively, p=0.45). There was a significant within group decrease in symptom bother score from baseline to 12 weeks (tolterodine, p < 0.0001, and intravaginal estradiol, p = 0.002). Secondary outcome improvement within groups was noted in the HRQL total, UI episodes and median voiding frequency (all p≤0.03) in the tolterodine group and in the HRQL total score (p=0.03) in the intravaginal estradiol group; with no differences between groups. Combined therapy outcomes at 24 and 52 weeks compared to single therapy at 12 weeks revealed significant improvement in symptom bother score in the intravaginal estradiol +tolterodine group at 24 and 52 weeks (20.0±23.9, p=0.008; −16.7±23.3, p=0.02, respectively)
Conclusions
Significant within group improvement in OAB-q symptom bother was noted in both the intravaginal estradiol and tolterodine groups for OAB symptoms, with no difference between groups. Greater improvement from 12 week single therapy to 24 and 52 weeks of combined therapy was noted in the group originally assigned to intravaginal estradiol. The role of combined medical therapy for OAB symptoms needs further investigation.
Keywords: overactive bladder, intravaginal estradiol cream, combined therapy, symptom severity, quality of life
Introduction
The mainstays of treatment of women with overactive bladder (OAB) syndrome are pharmacotherapy and behavioral therapy. 1–3 Pharmacotherapy with antimuscarinic medications has demonstrated an overall 60% improvement for OAB symptoms. 4 In addition to anticholinergic therapy, the use of intravaginal estrogen to treat atrophy is sporadically used as part of an overall pharmacologic treatment plan.5
Several studies have evaluated the role of estrogen in the treatment of urinary incontinence (UI), as well as the treatment of OAB symptoms.5 The lower urinary tract shares a common embryologic origin with the lower genital tract, and has been found to contain abundant estrogen receptors. Thus, an optimized estrogen milieu would be expected to have a beneficial effect on urinary tissue, symptoms and function. 6
Although in general, oral estrogen has not been noted to have a beneficial effect on UI symptoms,7 intravaginal estrogen therapy has shown a more consistent benefit for lower urinary tract symptoms. Cardozo et. al. performed a double blind placebo controlled study of 110 women using intravaginal estradiol tablets for the treatment of lower urinary tract symptoms. A significant decrease in pre-treatment sensory urinary urgency was demonstrated in the treatment group. 8 A recent systematic review of the literature concluded that compared with placebo, intravaginal estrogens improved urinary urgency, urinary frequency, stress and urgency urinary incontinence. 5
Limited data exist comparing treatment with anticholinergic therapy to intravaginal estrogen use for OAB symptoms. Our primary aim for this trial was to compare oral tolterodine extended release formulation (Detrol LA®, Pfizer, New York, NY) to low dose intravaginal estradiol cream (0.1 mg estradiol per gram, Estrace® vaginal cream, Warner Chilcott, Dublin) using the reliable, valid and responsive Overactive Bladder Questionnaire (OAB-q).9 Secondary aims include evaluating health related quality of life questionnaire (HRQL) of the OAB-q, patient satisfaction questionnaire (PSQ), global impression of improvement (PGI-I), voiding frequency, and urinary incontinence episodes. In addition, we aimed to characterize long-term, 24 and 52 week, outcomes in women undergoing combined treatment.
Materials and Methods
After Institutional Review Board approval, women were recruited from the gynecology and urogynecology clinics and enrolled from April, 2007 through July, 2009. Participants provided written informed consent forms and the study was registered on clinicaltrials.gov (NCT00465894).
Women with OAB symptoms were eligible for the study. Symptoms were assessed by questions related to patient bother on the OAB-q: “During the past 4 weeks, how bothered were you by 1. An uncomfortable urge to urinate? 2. A sudden urge to urinate with little or no warning? 3. Accidental loss of small amounts of urine? 4. Nighttime urination? 5. Waking up at night because you had to urinate? 6. Urine loss associated with a strong desire to urinate?”9 Responses were given on a 6-point scale ranging from “not at all” to “a very great deal.” Women answering “quite a bit” or greater severity on the OAB-q bother questions were considered to have OAB symptoms and were included in this study. Other inclusion criteria included: menopausal for greater than 12 months defined by having a prior bilateral oophorectomy or being one year from last period, or for women with hysterectomy and preserved ovaries must be ≥ 55 or have a documented follicle stimulating hormone level greater than 40 mIU/mL. Women also had to be ambulatory, community dwelling, and able to participate in follow up for the trial. Exclusion criteria included: post void residual > 150 cc, glaucoma, hormone replacement therapy in past 6 months, current anticholinergic therapy, breast cancer, impaired mental status, undiagnosed vaginal bleeding in past 12 months, endometrial thickness on pelvic ultrasound > 5 mm, history of thromboembolic event, gynecologic cancer, untreated urinary tract infection, stage 3 pelvic organ prolapse or greater, recent diuretic medication change (one month from change), neurological condition affecting bladder function (multiple sclerosis, Parkinson’s, spinal cord injury, spina bifida), congestive heart failure, prior pelvic irradiation, and interstitial cystitis.
At baseline, all participants underwent a physical examination and completed questionnaires including the OAB-q as well as a 3-day bladder diary. The first part of the OAB-q consists of eight questions assessing symptom bother with a possible score from 0 to 100 with a higher score indicating greater symptom bother. The second part consists of 25 questions that assesses HRQL addressing coping, concern, sleep, and social interaction where the scoring scale is from 0 to 100 with a higher score indicating a better quality of life.9 Participants also completed the Patient Global Impression of Improvement Questionnaire (PGI-I) and the Patient Satisfaction Questionnaire (PSQ) after treatment.10,11 The PGI-I consists of a single question, “Circle the one response that best describes how your urinary tract condition is now, compared to how it was before you had treatment.” There are seven possible responses ranging from “very much better” to “very much worse.” The PSQ consists of a single question, “How satisfied are you with your progress since your treatment? ” in which the three possible responses are “completely satisfied,” “somewhat satisfied,” and “not satisfied.” The bladder diary consisted of each woman completing a 3 day log of number of voids and number of urinary urgency incontinence episodes.
Women enrolled in the study and meeting inclusion criteria were randomized in a 1:1 ratio to treatment with either tolterodine extended release 4 mg oral dose daily (Detrol LA®, Pfizer, New York, NY) or low-dose estradiol topical cream 0.5 gram applied vaginally each night for six weeks and then twice per week for the duration of the study (0.1 mg estradiol per gram, Estrace® vaginal cream, Warner Chilcott, Dublin). At 12 weeks patients were offered addition of the alternative treatment regimen and were followed prospectively. Randomization was performed using a permuted block randomization schedule with sealed envelopes. Compliance (Are you taking your medication as prescribed?) and side effects were assessed by a telephone call at 4 weeks. Participants came with a bladder diary and were seen and reexamined at the primary outcome time-point of 12 weeks, and subsequently at 24, and 52 weeks where only outcome questionnaires were completed. Clinical staff administering protocol questionnaires were blinded to treatment assignment.
The two-sample t-test for analysis of the primary study outcome, change in symptom bother score between groups from baseline to 12 weeks. Changes in all outcome measures were calculated as the difference between 12 week and baseline scores (week 12 – baseline). Thus a negative difference in severity scores indicates an improvement in patient-assessed bother. A sample size of 25 per group provided 80% power to detect a difference of 20 points or more at a 0.05 level of significance. For this determination, the standard deviation of the change in severity scores was estimated to be 25 points based on prior study by Coyne, et al.9 We hypothesized a 20 point difference to be clinically meaningful because, on average, 20 was associated with a reduction of 3 or more voids per day12 (however, subsequent to trial initiation, the minimally important difference was noted to be 10 points).13 Analysis of the primary outcome was intention to treat. Bother scores at baseline and 12 weeks were compared using two-sample t-tests. At 12 weeks, subjects were offered addition of the alternative therapy with follow-up at 24 and 52 weeks utilizing the same outcome measures.
All secondary outcomes were compared between baseline and 12, 24 and 52 weeks using independent sample t-tests. We evaluated significance of changes in all scores from baseline to 12 week, 24, and 52 week follow up within each group using paired t-tests and Wilcoxon signed-rank tests. Comparisons of patient characteristics in the two study groups were conducted using the independent samples t-test for continuous measures and either the chi-square tests of association or Fisher’s exact test for categorical measures. All hypothesis tests were evaluated at a 0.05 level of significance. Raw p-values, unadjusted for multiple comparisons, are presented. SAS version 9.2 (SAS Institute Inc, Cary, NC, USA) was used for all statistical analyses.
Results
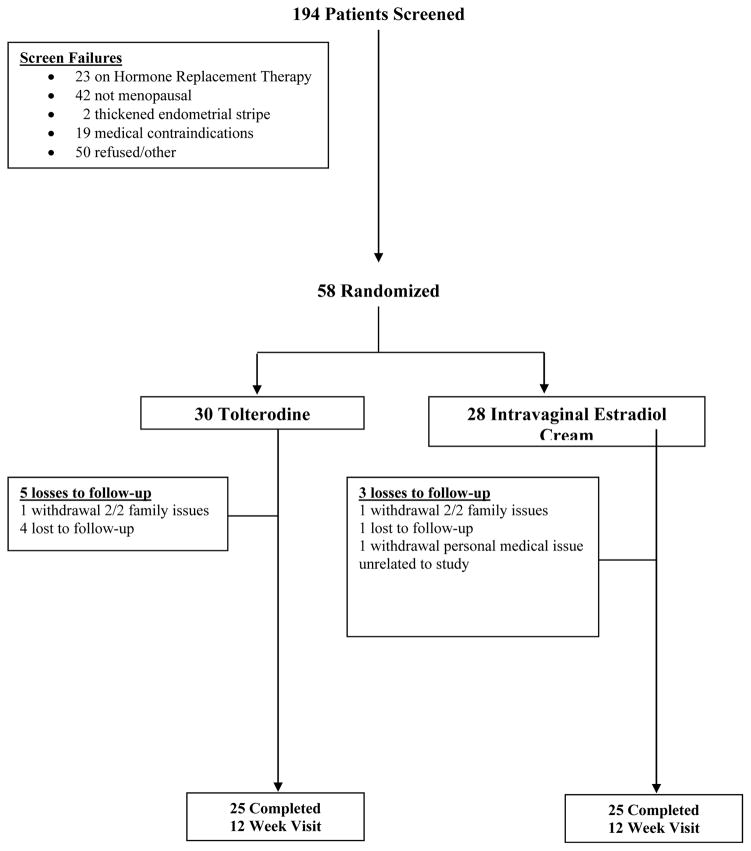
A total of 194 women were screened between April, 2007 and July, 2009 and of these 58 were eligible and randomized. Thirty women were randomized to tolterodine and 28 to intravaginal estradiol cream. Twenty-five patients in the tolterodine group and 25 patients in the intravaginal estradiol group completed the 12-week study. (Figure 1)
Figure 1.
CONSORT Diagram 26
Baseline demographics were similar for both groups including age, co-morbidities, and current treatment with medications for bladder symptoms (Table 1). A significant difference in race was noted between groups with 13.3% African American in the tolterodine versus 39.3% in the intravaginal estradiol group, p=0.02. There were no differences in baseline OAB-q symptom bother and HRQOL scores, urgency incontinence episodes of number of voids between groups at baseline (Table 2). Initial compliance was assessed at 4 weeks with a phone call from research staff and 92.9% and 80% compliance was confirmed for the intravaginal estradiol cream and the tolterodine groups, respectively.
Table 1.
Baseline Characteristics
| Characteristic | Tolterodine (n = 30) | Intravaginal Estrogen (n = 28) | p-value |
|---|---|---|---|
|
| |||
| Race/Ethnicity: n (%) | |||
| African American | 4 (13%) | 11 (39%) | 0.02 |
| Caucasian | 26 (87%) | 17 (61%) | |
|
| |||
| Marital Status: n (%) | |||
| Single/Never Married | 1 (3%) | 2 (7%) | 0.68 |
| Married | 12 (40%) | 9 (32%) | |
| Divorced | 10 (33%) | 6 (21%) | |
| Widowed | 5 (17%) | 8 (29%) | |
| Separated | 2 (7%) | 3 (11%) | |
|
| |||
| Alcohol use: n (%) | 11 (37%) | 6 (21%) | 0.20 |
|
| |||
| Cigarette use: n (%) | 2 (7%) | 5 (18%) | 0.25 |
|
| |||
| Family History: n (%) | |||
| Breast Cancer | 13 (43%) | 7 (25%) | 0.14 |
| Colon Cancer | 5 (17%) | 5 (18%) | >0.99 |
| Uterine Cancer | 9 (30%) | 6 (21%) | 0.46 |
|
| |||
| Medical conditions: n (%) | |||
| Arthritis | 14 (47%) | 14 (50%) | 0.80 |
| Asthma | 2 (7%) | 4 (14%) | 0.42 |
| Diabetes | 4 (13%) | 5 (18%) | 0.73 |
| Glaucoma | 1 (3%) | 0 (0%) | >0.99 |
| High Blood Pressure | 14 (47%) | 15 (54%) | 0.60 |
| Stress Incontinence | 18 (60%) | 16 (57%) | 0.83 |
| Thyroid Disease | 6 (20%) | 4 (14%) | 0.73 |
|
| |||
| Uterus: n (%) | 9 (30%) | 8 (29%) | 0.90 |
|
| |||
| Hysterectomy: n (%) | 18 (60%) | 16 (57%) | 0.83 |
|
| |||
| Bladder Symptoms: n (%) | |||
| Dysuria | 4 (13%) | 2 (7%) | 0.67 |
| Nocturia | 22 (73%) | 22 (79%) | 0.64 |
| Frequency | 24 (80%) | 23 (82%) | 0.84 |
| Urge Incontinence | 23 (77%) | 24 (86%) | 0.38 |
| Urgency | 28 (93%) | 26 (93%) | >0.99 |
|
| |||
| Taking Medications for Bladder Symptoms: n (%) | 10 (33%) | 6 (21%) | 0.31 |
|
| |||
| Other Treatments for Bladder Symptoms: n (%) | 3 (10%) | 4 (14%) | 0.70 |
|
| |||
| Years of Age, mean ± sd | 63.6 ± 9.1 | 60.2 ± 10.0 | 0.17 |
|
| |||
| Gravity, mean ± sd | 3.0 ± 1.6 | 2.8 ± 2.2 | 0.78 |
|
| |||
| Years of Education, mean ± sd | 13.4 ± 3.3** | 14.0 ± 2.6 | 0.41 |
Years of education was unknown for one patient
Table 2.
Analysis of OAB Symptom Scores, Bladder Diary Findings and Change from Baseline to 12 Weeks
| Baseline | Tolterodine (n = 30) | Intravaginal Estradiol (n = 28) | p-value | ||
|---|---|---|---|---|---|
|
| |||||
| OAB-q Symptom Bother Score, mean ± sd | 65.4 ± 15.3 | 61.6 ± 20.0 | 0.42 | ||
|
| |||||
| 12 Week Follow-up | (n = 25) | (n = 25) | |||
|
| |||||
| OAB-q Symptom Bother, mean ± sd | 46.7 ± 23.4 | 45.4 ± 21.0 | 0.84 | ||
|
| |||||
| Baseline | Tolterodine (n = 28) | Intravaginal Estradiol (n = 25) | p-value | ||
|
| |||||
| HRQL Total, mean ± sd | 53.1 ± 22.2 | 60.9 ± 22.5* | 0.20 | ||
| Coping | 48.9 ± 28.0 | 60.4 ± 27.0* | 0.12 | ||
| Concern | 46.8 ± 25.3 | 54.0 ± 25.2* | 0.29 | ||
| Sleep | 44.9 ± 24.3 | 50.5 ± 27.0* | 0.41 | ||
| Social | 77.1 ± 22.7 | 82.1 ± 22.8 | 0.40 | ||
|
| |||||
| Number of voids/3-days | 30.4 ± 11.6** | 26.4 ± 10.5** | 0.19 | ||
|
| |||||
| Number of accidents, mean ± sd/per 3-days | 10.8 ± 11.4** | 11.9 ± 10.5** | 0.73 | ||
|
| |||||
| 12 Week Follow-up | (n = 25) | (n = 25) | |||
|
| |||||
| HRQL Total, mean ± sd | 66.0 ± 26.6 | 69.8 ± 18.2 | 0.56 | ||
| Coping | 63.9 ± 30.1 | 67.2 ± 24.6 | 0.67 | ||
| Concern | 62.7 ± 30.4 | 63.5 ± 21.7 | 0.92 | ||
| Sleep | 58.4 ± 25.7 | 64.8 ± 24.0 | 0.37 | ||
| Social | 81.3 ± 26.5 | 87.2 ± 17.8 | 0.33 | ||
|
| |||||
| Number of voids/3-days, mean ± sd | 26.3 ± 9.7*** | 24.6 ± 10.2**** | 0.55 | ||
|
| |||||
| Number of accidents/3-days, mean ± sd | 7.2 ± 10.6*** | 7.7 ± 8.1**** | 0.86 | ||
|
| |||||
| Score Changes at 12 weeks | |||||
|
| |||||
| Baseline | Change Tolterodine (n = 25) | p-value | Change Intravaginal estradiol (n = 25) | p-value | Between group p-value |
|
| |||||
| OAB-q Symptom Bother Score, mean ± sd | −20.6 ± 21.7 | < 0.0001 | −15.8 ± 23.3 | 0.002 | 0.45 |
|
| |||||
| HRQL Total, mean ± sd | 12.8 ± 22.0 | 0.01 | 8.8 ± 18.8∘ | 0.03 | 0.50 |
| Coping | 14.6 ± 24.3 | 0.01 | 7.8 ± 25.5∘ | 0.15 | 0.35 |
| Concern | 17.3 ± 24.2 | 0.002 | 8.4 ± 21.0∘ | 0.06 | 0.18 |
| Sleep | 12.3 ± 25.3 | 0.02 | 14.2 ± 23.2 | 0.01 | 0.79 |
| Social | 4.2 ± 21.5 | 0.33 | 5.6 ± 14.4∘ | 0.06 | 0.81 |
|
| |||||
| Urinary Incontinence Episodes (total/3-days), mean ± sd | −4.2 ± 8.3∘∘ | 0.03 | −1.6 ± 7.2 | 0.26 | 0.27 |
|
| |||||
| Voiding Frequency (3 day diary), mean ± sd | −5.0 ± 14.9∘∘ | 0.1424 | −3.9 ± 10.1 | 0.07 | 0.86 |
| Median (Q1, Q3) | −4 (−10, −1) | 0.0185† | −2 (−6, 4) | 0.19† | |
24 patients with complete data
21 patients with complete data. All reported p-values are paired t-test p-values except where noted.
Reported p-value from the nonparametric signed rank test of zero median. The paired t-test did not indicate a statistically significant difference (p=0.14)
one patient had missing responses precluding subscale and total HRQL determinations,
26 patients in each group completed voiding diary at baseline,
21 patients completed voiding diary in tolterodine group at 12 weeks with complete bladder diary,
25 patients in estradiol group with complete bladder diary at 12 weeks
At 12 weeks, there was no difference in symptom bother score (46.7±23.4 vs 45.4±21.0, p=0.84) or magnitude of change (−20.6±21.7 vs −15.8±23.3, p=0.45) between the tolterodine and intravaginal estradiol groups, respectively (Table 2). There was a significant within group decrease in symptom bother score from baseline to 12 weeks (tolterodine, p < 0.0001, intravaginal estradiol cream, p = 0.002) (Table 2 and Figure 2). There were no differences between groups on the OAB-q HRQL scale or bladder diary responses (Table 2). The tolterodine group showed significant improvement in total quality of life including the subgroups of coping, concern, and sleep. The intravaginal estradiol group showed statistically significant improvement in total quality of life and in the subgroup for sleep (Table 2). In addition, within group analyses showed a statistically significant decrease in the median number of voids and urinary incontinence episodes in the tolterodine group but not in the intravaginal estradiol group (Table 2). No differences in impression of improvement and satisfaction were noted between groups. There were no significant differences between the groups regarding estimated percent of improvement and comfortability with continuation of their medication treatment (Table 3).
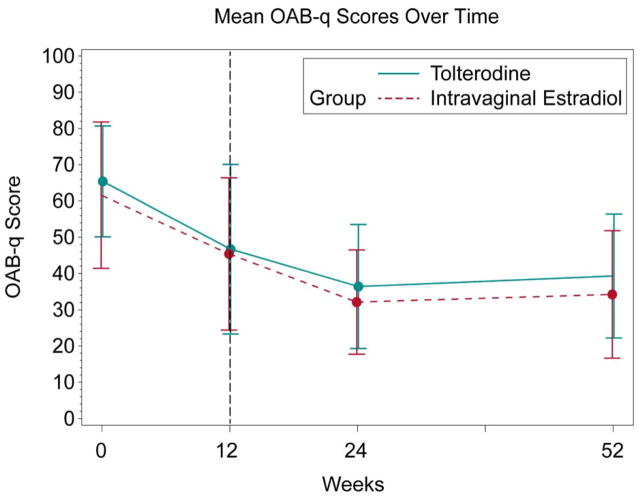
Figure 2.
Long-Term OAB-q Symptom Bother
Table 3.
Patient Impression of Improvement and Satisfaction at 12 weeks
| Impression of Improvement | Tolterodine (n = 25) | Intravaginal Estradiol (n = 24) | p-value |
|---|---|---|---|
|
| |||
| Change in Urinary Tract Condition | |||
| Very Much Better | 3 (12%) | 0 (0%) | 0.44* |
| Much Better | 8 (32%) | 7 (29%) | |
| A Little Better | 8 (32%) | 12 (50%) | |
| No Change | 5 (20%) | 5 (21%) | |
| A Little Worse | 1 (4%) | 0 (0%) | |
|
| |||
| Current Urinary Tract Condition | |||
| Normal | 2 (8%) | 0 (0%) | 0.94* |
| Mild | 9 (36%) | 11 (46%) | |
| Moderate | 10 (40%) | 11 (46%) | |
| Severe | 4 (16%) | 2 (8%) | |
|
| |||
| Patient Satisfaction | (n = 24) | (n = 24) | p-value |
|
| |||
| Satisfaction | |||
| Completely Satisfied | 5 (21%) | 3 (13%) | 0.39* |
| Somewhat Satisfied | 13 (54%) | 13 (54%) | |
| Not Satisfied | 6 (25%) | 8 (33%) | |
|
| |||
| Improvement | |||
| Much Better | 4 (17%) | 1 (4%) | 0.86* |
| Better | 11 (46%) | 13 (54%) | |
| About the Same | 6 (25%) | 9 (38% | |
| Worse | 2 (8%) | 1 (4%) | |
| Much Worse | 1 (4%) | 0 (0%) | |
|
| |||
| Patient Perception of Improvement | (n = 23) | (n = 24) | p-value |
|
| |||
| Estimated % improvement | 48.8 ± 31.7 | 42.1 ± 33.9 | 0.49 |
|
| |||
| Continued Therapy | (n = 24) | (n = 23) | p-value |
|
| |||
| Comfortable to Continue: n (%) yes) | 15 (63%) | 18 (78%) | 0.24 |
|
| |||
| Change of Therapy | (n = 22) | (n = 22) | p-value |
|
| |||
| Wish another form of treatment: n (%) yes | 14 (64%) | 13 (41%) | 0.76 |
test of trend
At 24 weeks, 28 out of 58 patients on combined therapy completed follow-up. There were significant within group change of OAB-q bother score from baseline to 24 weeks in both the tolterodine+intravaginal estradiol (p<0.0001) and intravaginal estradiol+tolterodine groups (p=0.003) (Table 4). Similar findings were noted for within group changes at 52 weeks (Table 4). However, no difference was noted in change between groups at 24 and 52 weeks (Tables 4).
Table 4.
Long Term Outcomes: Baseline to 24 and 52 Weeks
| Outcomes from Baseline to 24 Weeks | |||||
|---|---|---|---|---|---|
| Outcome | Tolterodine (+intravaginal estradiol) (n=14) | p (within group) | Intravaginal estradiol (+tolterodine) (n=13) | p (within group) | p (change between groups) |
| OAB-q Symptom Bother Score, mean±SD | −25.4 ± 11.6 | <0.0001 | −28.7 ± 20.5 | 0.0003 | 0.61 |
| HRQL total, mean±SD | 19.3 ± 16.8 | 0.0009 | 20.9 ± 15.8 | 0.0005 | 0.80 |
| Coping | 22.1 ± 16.9 | 0.0003 | 23.3 ± 19.3 | 0.001 | 0.87 |
| Concern | 24.3 ± 22.0 | 0.0012 | 20.2 ± 18.5 | 0.002 | 0.61 |
| Sleep | 16.0 ± 21.5 | 0.02 | 27.1 ± 19.9 | 0.0004 | 0.18 |
| Social | 11.1 ± 20.3 | 0.06 | 12.0 ± 16.6 | 0.02 | 0.91 |
| Outcomes from Baseline to 52 Weeks | |||||
| (n=11) | (n=14) | ||||
| OAB-q Symptom Bother Score, mean±SD | −23.4 ± 15.6 | 0.0006 | −31.3 ± 24.3 | 0.0003 | 0.36 |
| HRQL total, mean±SD, | 26.8 ± 20.5 | 0.002 | 19.7 ± 25.1 | 0.01 | 0.45 |
| Coping | 28.4 ± 19.1 | 0.0006 | 21.4 ± 27.2 | 0.01 | 0.48 |
| Concern | 31.4 ± 26.0 | 0.0025 | 19.8 ± 29.1 | 0.02 | 0.31 |
| Sleep | 22.5 ± 25.1 | 0.01 | 25.7 ± 25.9 | 0.003 | 0.76 |
| Social | 22.2 ± 19.4 | 0.004 | 10.6 ± 25.1 | 0.14 | 0.22 |
Combined long-term therapy outcomes at 24 and 52 weeks were compared to single therapy at 12 weeks (Figure 2). There was no significant within group change in the OAB-q bother score from 12 to 24 weeks in the tolterodine +intravaginal estradiol group (−7.3±14.7, p=0.10); however, a significant improvement was noted in the intravaginal estradiol+tolterodine group (−20.0±23.9, p=0.008), with no difference noted between groups (p=0.11). There were no significant changes noted in HRQL total or subscale scores in the tolterodine+intravaginal estradiol group, except in the concern subscale (p=0.04); in the intravaginal estradiol+tolterodine group, there were significant changes noted in the total and all subscales (p≤0.008), except social (p=0.16). There were no significant between-group differences (all p≥0.11). Similar findings were noted in the OAB-q bother score changes from 12 to 52 weeks with the tolterodine +estradiol cream group (−0.5±22.1, p=0.94) and the intravaginal estradiol+tolterodine group (−16.7±23.3, p=0.02), with no difference between groups, (p=0.10). There were no significant differences in the HRQL total or subscales within the tolterodine+intravaginal estradiol group (all p≥0.15); in the intravaginal estradiol+tolterodine group, there was a significant change in HRQL total (13.3±23.0, p=0.04) and in the coping (18.2±28.6, p=0.03) and concern (17.7±24.3, p=0.01) subscales. There were no significant changes between groups (all p≥0.10).
At 24 and 52 weeks (N=26), PSQ outcomes revealed that 88% and 84% of subjects, respectively, reported that they were “Completely” or “Somewhat Satisfied,” and 77% and 85% of subjects, respectively, reported that they were “Much Better” or “Better” on the PGI-I.
Adverse events were recorded at 12 weeks, 24 weeks, and 52 weeks for all patients; however, at 52 weeks the only adverse event reported was constipation, and the patient reporting had originally been in the tolterodine arm and added intravaginal estradiol at 12 weeks, and stopped tolterodine at 24 weeks. No serious study related adverse events were reported. Xerostomia, constipation, headache, gastroesophageal reflux, and mydriasis were the most commonly reported side effects at 12 weeks. (See Table 5) 56% of patients in the tolterodine arm reported xerostomia and 32% reported constipation. Interestingly, 20% of the patients in the intravaginal estradiol arm reported xerostomia as well. At 24 weeks, on combined therapy, the most common side effects reports were xerostomia and constipation.
Table 5.
Adverse Events at 12 Weeks
| 12 Weeks | ||
|---|---|---|
| Adverse Event | Tolterodine (n = 25) | Intravaginal Estradiol (n = 25) |
| Xerostomia | 14 | 5 |
| Constipation | 8 | 1 |
| Headache | 3 | 4 |
| Gastroesophageal Reflux | 4 | 1 |
| Visual Changes | 4 | 0 |
| Dry Eyes | 1 | 0 |
Discussion
This trial demonstrated that tolterodine and intravaginal estradiol both significantly improve symptom bother and HRQL in menopausal OAB patients and that there was no difference in the degree of improvement between the groups at 12 weeks. At the 12 week time-point with the addition of the alternate therapy, within group OAB symptom severity and HRQL scores remained improved significantly at 24 and 52 weeks from baseline. Interestingly, addition of tolterodine at 12 weeks to the intravaginal estradiol group resulted in further significant improvement in the OAB-q symptom bother score and the health related quality of life total and subscale scores above the single therapy effect noted at 12 weeks. These findings suggest that intravaginal estradiol therapy for OAB symptoms may for many women provide enough symptom relief, but if there is “still room for improvement” the addition of an antimuscarinic may provide additional benefit.
Additional secondary outcome measures characterized at 12 weeks included the number of voids and urgency urinary incontinence episodes as recorded by a 3-day bladder diary. There were no differences noted between groups but only the tolterodine group showed a significant decrease in number of voids and incontinence episodes at 12 weeks.
The use of intravaginal estrogen has previously been shown to decrease the incidence of urinary frequency and urgency in postmenopausal women.14–16 One meta-analysis examining the effect of estrogen for the treatment of lower urinary tract symptoms demonstrated intravaginal estrogen therapy to be associated with a statistically significant improvement in frequency, nocturia, urgency, incontinence, bladder capacity, and first sensation to void.17 The most recent meta-analysis was performed by the Cochrane group which is a revised version from 2012. Overall 34 trials were identified, including 19,676 incontinent women of whom 9599 received estrogen therapy. There were 1,464 involved in trials of intravaginal estrogen administration showing evidence that intravaginal estrogen may improve incontinence (RR 0.74, 95% CI 0.64 to 0.86). Overall there were approximately one to two fewer voids in 24 hours among women treated with intravaginal estrogen, and there was less frequency and urgency.18 The potential theories of why estrogen therapy may improve the symptoms of overactive bladder include increasing the sensory threshold of the bladder, increasing alpha-adrenergic receptor sensitivity in the urethral smooth muscle, promoting beta-3 adreno-receptor mediated relaxation of the detrusor muscle, and improving collagen quality and production in the peri-vesical and peri-urethral areas.15,19–21 Importantly, in this current study, significant OAB-q within group changes in both symptom bother and HRQL subscales met the established 10 point minimally important difference.13 This information was available subsequent to the information utilized for sample size requirements for this trial and suggests that measured changes which occurred were robust and meaningful.
Limited data exist comparing the use of intravaginal estrogen to anticholinergic medication for OAB symptoms. Nelken, et al compared an estradiol vaginal ring versus oxybutynin for the treatment of OAB. The authors reported a significant decrease in voids per day in both groups but no significant difference between the groups.22 While our study did not demonstrate a significant decrease in voids in the intravaginal estradiol group, we feel that the subjective symptom impact to be a more important primary outcome since the ultimate measure of treatment success and likelihood of long-term compliance is intrinsically linked to the patient’s perception of efficacy and symptom relief.
In our study, 63.6% of the women in the tolterodine group and 40.9% of the women in the intravaginal estradiol group reported that they requested additional treatment when asked at 12 weeks. To that end, an important issue to address was whether there would be benefit for concurrent therapy with intravaginal estrogen and an anticholinergic medication. There continues to be limited data examining this issue. Tseng et. al performed a randomized open-label trial of 80 women comparing a group treated with tolterodine alone to a group treated with tolterodine and intravaginal estrogen cream. The tolterodine plus intravaginal estrogen group had significant improvements in mean daytime frequency and volume voided after treatment but no significant difference in nocturia, urinary urgency and urgency urinary incontinence.23
An additional open-label non-randomized study compared women using tolterodine alone to women using tolterodine and intravaginal estrogen. There was no synergistic effect noted in this study.24 In our study, women on combined therapy, appeared to have an improved perception of symptom improvement as well as satisfaction. Larger more robust studies need to be conducted to assess any additive benefit of vaginal estrogen to anticholinergic or β-agonist treatment for OAB symptoms.
The significant limitation of this study is the lack of patient blinding with concomitant placebo use, however, the study was strengthened by clinician blinding, its randomized design and the use of validated outcome measures. Further, we unfortunately had significant attrition after 12 weeks as we included outcomes in the patients with complete data accrual.
Anticholinergic therapy is often discontinued due to common side effects of dry mouth, constipation, and blurry vision.25 Given that our study demonstrated no differences for improvement in symptom bother and satisfaction between the groups, intravaginal estradiol therapy might be considered an effective and well tolerated alternative to initial anticholinergic therapy for OAB symptoms in some women. It also suggests that if a synergistic benefit of vaginal estrogen therapy and anticholinergic medications is found, then this may result in improved compliance by allowing lower anticholinergic doses and thus a reduction in unwanted side effects. While inherent weaknesses in design are acknowledged, data from this study can be used to plan a larger double blind placebo-controlled trial to more robustly characterize the importance of intravaginal estrogen in optimizing the treatment of OAB symptoms.
Brief Summary.
Intravaginal estradiol therapy for OAB symptoms may for many women provide symptom relief, however, if there is still room for improvement, the addition of an anti-muscarinic may provide additional benefit
Acknowledgments
Financial Support:
Pfizer, Inc. – Investigator Initiated Research Grant
Detrol LA® medication was provided by Pfizer, Inc.
Estrace Cream® medication was provided by Warner Chilcott, Ltd.
Partially funded by the National Institutes of Diabetes and Digestive and Kidney Diseases 2K24-DK068389 – to Holly E. Richter, PhD, M.D.
Footnotes
Paper Presentation Information:
Presented as an oral poster at the 31st Annual Scientific Meeting of the American Urogynecological Society, Long Beach, CA, October 1st, 2010
Accepted as a short oral presentation at the 45th Annual Meeting of the International Continence Society, Montreal, Canada, October 9th, 2015
Accepted as an oral poster at the 36th Annual Scientific Meeting of the American Urogynecological Society (Pelvic Floor Disorders Week), Seattle, WA, October 16th, 2015
ClinicalTrials.gov Identifier: NCT00465894
References
- 1.Rovner ES, Gomes CM, Trigo-Rocha FE, et al. Evaluation and treatment of the overactive bladder. Rev Hosp Clin Fac Med Sao Paulo. 2002;57:39–48. doi: 10.1590/s0041-87812002000100007. [DOI] [PubMed] [Google Scholar]
- 2.Burgio KL, Locher JL, Goode PS, et al. Behavioral vs drug treatment for urge urinary incontinence in older women: a randomized controlled trial. Jama. 1998;280:1995–2000. doi: 10.1001/jama.280.23.1995. [DOI] [PubMed] [Google Scholar]
- 3.Gormley EA, Lightner DJ, Burgio KL, et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline. J Urol. 2012 Dec;188:2455–63. doi: 10.1016/j.juro.2012.09.079. [DOI] [PubMed] [Google Scholar]
- 4.Hay-Smith J, Herbison P, Ellis G, et al. Anticholinergic drugs versus placebo for overactive bladder syndrome in adults. Cochrane Database Syst Rev. 2002:CD003781. doi: 10.1002/14651858.CD003781. [DOI] [PubMed] [Google Scholar]
- 5.Rahn DD, Carberry C, Sanses TV, et al. Vaginal estrogen for genitourinary syndrome of menopause: a systemic review. Obstet Gyncol. 2014 Dec;124:1147–56. doi: 10.1097/AOG.0000000000000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iosif CS, Batra S, Ek A, et al. Estrogen receptors in the human female lower urinary tract. Am J Obstet Gynecol. 1981;141:817–20. doi: 10.1016/0002-9378(81)90710-9. [DOI] [PubMed] [Google Scholar]
- 7.Robinson D, Cardozo L, Milsom I, et al. Oestrogens and Overactive Bladder. Neurourol Urodyn. 2014;33:1086–91. doi: 10.1002/nau.22464. [DOI] [PubMed] [Google Scholar]
- 8.Cardozo LD, Wise BG, Benness CJ. Vaginal oestradiol for the treatment of lower urinary tract symptoms in postmenopausal women--a double-blind placebo-controlled study. J Obstet Gynaecol. 2001;21:383–5. doi: 10.1080/01443610120059941. [DOI] [PubMed] [Google Scholar]
- 9.Coyne K, Revicki D, Hunt T, et al. Psychometric validation of an overactive bladder symptom and health-related quality of life questionnaire: the OAB-q. Qual Life Res. 2002;11:563–74. doi: 10.1023/a:1016370925601. [DOI] [PubMed] [Google Scholar]
- 10.Burgio KL, Goode PS, Richter HE, et al. Global ratings of patient satisfaction and perceptions of improvement with treatment for urinary incontinence: validation of three global patient ratings. Neurourol Urogyn. 2006;25:411–7. doi: 10.1002/nau.20243. [DOI] [PubMed] [Google Scholar]
- 11.Wai C, Curto T, Zyczynski H, et al. Patient Satisfaction After Midurethral Sling Surgery for Stress Urinary Incontinence. Obstet Gynecol. 2013;121:1009–1016. doi: 10.1097/AOG.0b013e31828ca49e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coyne KS, Matza LS, Thompson CL. The responsiveness of the Overactive Bladder Questionnaire (OAB-q) Qual Life Res. 2005;14:849–55. doi: 10.1007/s11136-004-0706-1. [DOI] [PubMed] [Google Scholar]
- 13.Coyne KS, Matza LS, Thompson CL, et al. Determining the importance of change in the overactive bladder questionnaire. J Urol. 2006 Aug;176:627–32. doi: 10.1016/j.juro.2006.03.088. [DOI] [PubMed] [Google Scholar]
- 14.Cardozo L, Rekers H, Tapp A, et al. Oestriol in the treatment of postmenopausal urgency: a multicentre study. Maturitas. 1993;18:47–53. doi: 10.1016/0378-5122(93)90028-g. [DOI] [PubMed] [Google Scholar]
- 15.Zullo MA, Plotti F, Calcagno M, et al. Vaginal estrogen therapy and overactive bladder symptoms in postmenopausal patients after a tension-free vaginal tape procedure: a randomized clinical trial. Menopause. 2005;12:421–7. doi: 10.1097/01.GME.0000148645.93603.62. [DOI] [PubMed] [Google Scholar]
- 16.Lose G, Englev E. Oestradiol-releasing vaginal ring versus oestriol vaginal pessaries in the treatment of bothersome lower urinary tract symptoms. Bjog. 2000;107:1029–34. doi: 10.1111/j.1471-0528.2000.tb10408.x. [DOI] [PubMed] [Google Scholar]
- 17.Cardozo L, Drutz HP, Baygani SK, et al. Pharmacological treatment of women awaiting surgery for stress urinary incontinence. Obstetrics & Gynecology. 2004;104:511–9. doi: 10.1097/01.AOG.0000134525.86480.0f. [DOI] [PubMed] [Google Scholar]
- 18.Cody JD, Jacobs ML, Richardson K, et al. Oestrogen therapy for urinary incontinence in post-menopausal women. Cochrane Database Syst Rev. 2012;10:CD001405. doi: 10.1002/14651858.CD001405.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fantl JA, Wyman JF, Anderson RL, et al. Postmenopausal urinary incontinence: comparison between non-estrogen-supplemented and estrogen-supplemented women. Obstet Gynecol. 1988;71:823–8. [PubMed] [Google Scholar]
- 20.Kinn AC, Lindskog M. Estrogens and phenylpropanolamine in combination for stress urinary incontinence in postmenopausal women. Urology. 1988;32:273–80. doi: 10.1016/0090-4295(88)90400-1. [DOI] [PubMed] [Google Scholar]
- 21.Matsubara S, Okada H, Shirakawa, et al. Estrogen levels influence beta-3-adrenoceptor- mediated relaxation of the female rat detrusor muscle. Urology. 2002;59:621–5. doi: 10.1016/s0090-4295(01)01583-7. [DOI] [PubMed] [Google Scholar]
- 22.Nelken RS, Ozel BZ, Leegant AR, et al. Randomized trial of estradiol vaginal ring versus oral oxybutynin for the treatment of overactive bladder. Menopause. 2011;9:962–966. doi: 10.1097/gme.0b013e3182104977. [DOI] [PubMed] [Google Scholar]
- 23.Tseng LH, Wang AC, Chang YL, et al. Randomized comparison of tolterodine with vaginal estrogen cream versus tolterodine alone for the treatment of postmenopausal women with overactive bladder syndrome. Neurourol Urodyn. 2009;28:47–51. doi: 10.1002/nau.20583. [DOI] [PubMed] [Google Scholar]
- 24.Serati M, Salvatore S, Uccella S, et al. Is there a synergistic effect of topical oestrogens when administered with antimuscarinics in the treatment of symptomatic detrusor overactivity? Eur Urol. 2009;55:713–719. doi: 10.1016/j.eururo.2008.06.051. [DOI] [PubMed] [Google Scholar]
- 25.Drutz HP, Appell RA, Gleason D, et al. Clinical efficacy and safety of tolterodine compared to oxybutynin and placebo in patients with overactive bladder. Int Urogynecol J Pelvic Floor Dysfunct. 1999;10:283–289. doi: 10.1007/s001929970003. [DOI] [PubMed] [Google Scholar]
- 26.Moher D, Schulz KF, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports or parallel-group trials. Lancet. 2001;357:1191–1194. [PubMed] [Google Scholar]