Abstract
Mouse testes contain several isoforms of cytoplasmic poly(A)-binding proteins (PABPCs), including ubiquitous PABPC1 and testis-specific PABPC2/PABPt. PABPC2 is characterized by its absence from translationally active polyribosomes and elongating spermatids. To elucidate the function of PABPC2 in spermatogenesis, we produced mutant mice lacking PABPC2. The PABPC2-null mice showed normal fertility. The processes of spermatogenesis and sperm migration in the testes and epididymides, respectively, were normal in the mutant mice. When the involvement of PABPC2 in translational regulation of haploid-specific mRNAs was examined, these mRNAs were correctly transcribed in round spermatids and translated in elongating spermatids. Moreover, immunoblot analysis revealed low abundance of PABPC2 relative to PABPC1 in spermatogenic cells. These results suggest that PABPC2 may be either functionally redundant with other PABPCs (including PABPC1) or largely dispensable for translational regulation during spermiogenesis.
Keywords: Mouse, mRNA metabolism, PABPC2, Poly(A), Spermatogenesis
Spermatogenesis is a highly specialized process of cellular differentiation for the production of spermatozoa, in which diploid spermatogonia proliferate and divide meiotically to generate haploid round spermatids that are in turn transformed into spermatozoa. The precise regulation of spermatogenesis requires a controlled program of stage-specific gene expression regulated at the transcriptional, posttranscriptional, and translational levels [1,2,3,4,5,6]. Drastic morphological changes, such as the formation of a flagellum and a highly compacted nucleus, are accomplished during spermiogenesis: the haploid phase of spermatogenesis. Transcription of the haploid genome ceases midway through spermiogenesis, concomitantly with the onset of chromatin condensation [7]. Accordingly, mRNAs encoding structural proteins required for cellular remodeling are transcribed in early haploid spermatids and stored as translationally inert messenger ribonucleoprotein particles (mRNPs). Subsequent translational activation of the repressed messages in late spermatids accompanies poly(A) shortening [8, 9]. On the other hand, poly(A) tails of some mRNAs are known to be longer in early haploid spermatids than in meiotic spermatocytes [10,11,12,13,14]. Thus, the mRNA metabolism plays a critical role in spermiogenesis.
Poly(A) tails of eukaryotic mRNAs are post-transcriptionally added to the 3′ end of mRNAs in the nucleus. After the transport of mRNA to the cytoplasm, the cytoplasmic forms of poly(A)-binding proteins (PABPCs) associate with the poly(A) tails. In mice, five PABPCs— PABPC1, PABPC2/PABPt, PABPC4/iPABPC, PABPC1L/ePABPC, and PABPC5— have been identified to date [15]. Pabpc1 is ubiquitously expressed in mammalian cells, whereas expression of intronless Pabpc2 is exclusive to spermatogenic cells [16, 17]. PABPC4 and PABPC1L are essential for erythroid differentiation and oocyte maturation, respectively [18, 19]. Moreover, X-linked PABPC5 lacks the C-terminal domain present in the other PABPC members. Among these five PABPCs, PABPC1 has been extensively studied in relation to various parameters of mRNA metabolism, including mRNA stabilization, cytoplasmic polyadenylation and deadenylation, translation initiation and termination, and microRNA-associated regulation [15, 20,21,22]. In spermatogenesis, PABPC1 is implicated in the ablation of PABPC1-interacting proteins, including DAZL, BOULE, and PABPC-interacting protein 2A (PAIP2A), which results in spermatogenic defects at different stages [5, 6].
Murine Pabpc2 is a retroposed paralogue gene that originates from Pabpc1 [16, 23]. Previously, we found that PABPC1 and PABPC2 are complexed with each other and associate nonspecifically with mRNAs during spermatogenesis [17]. Both PABPC proteins also interact with several translation-associated factors, including eukaryotic translation initiation factor 4G (eIF4G), and are capable of enhancing translation of a reporter mRNA in vitro. Despite these functional similarities, PABPC2 differs from PABPC1 in the distribution among spermatogenic cells and polyribosomes and in the expression pattern during spermatogenesis [17]. In the present study, to uncover the function of PABPC2, we produced mutant mice lacking PABPC2 and analyzed the effects of the PABPC2 knockout on mRNA metabolic pathways.
Materials and Methods
Generation of PABPC2-null mice
A targeting vector containing a 1.1-kbp expression cassette of the neomycin resistance gene neo (pMC1neopA; Stratagene, La Jolla, CA, USA), which was flanked by approximately 8.0- and 1.6-kbp genomic regions of Pabpc2 at the 5′ and 3′ ends, respectively, was constructed as described previously [14]. The herpes simplex virus thymidine kinase gene (HSV-tk) was inserted into the 1.6-kbp Pabpc2 genomic region at the 3′ end (Fig. 1A). After electroporation of the targeting vector (which had been linearized by digestion with NotI) into mouse D3 embryonic stem (ES) cells, homologous recombinants were selected using G418 and ganciclovir, as described previously [14]. Five ES cell clones carrying the targeted mutation were isolated from approximately 300 doubly-resistant colonies, and three clones were injected into blastocysts of C57BL/6 mice (Japan SLC, Shizuoka, Japan). The chimeric male mice that were born were crossed with C57BL/6 females to create heterozygous (Pabpc2+/–) mutant mice. Homozygous (Pabpc2–/–) mice were obtained by mating the heterozygous males and females.
Fig. 1.
Targeted disruption of mouse Pabpc2. (A) Physical maps of Pabpc2, targeting construct, and predicted targeted allele. The locations of the protein-coding region in Pabpc2 and of neo are boxed with black and gray colors, respectively. The open box represents HSV-tk. The restriction enzyme sites are indicated as follows: B, BamHI; E, EcoRI; H, HindIII; N, NotI; P, PstI; S, SphI; V, EcoRV; and X, XhoI. (B) Detection of the targeted allele. Genomic DNAs of original D3 ES cells (D3) and two targeted ES cell clones (#24 and #31) were double-digested with BamHI and HindIII and subjected to Southern blot analysis (upper panel) using a 32P-labeled DNA fragment (Probe S in panel A). Tail DNAs of the wild-type (+/+), heterozygous (+/–), and homozygous (–/–) mice were analyzed by PCR (lower panel) using primer sets G16/G17 and Neo/G17 (see panel A). (C) RT-PCR analysis. The protein-coding region of Pabpc2 mRNA was analyzed by RT-PCR in testicular total RNA, with Actb mRNA as a control. (D) Immunoblot analysis. Protein extracts of testicular tissues were analyzed by immunoblotting with a PABPC1- or PABPC2-specific antibody. ACTB served as a control.
PCR genotyping
Genomic DNAs of ES cell clones and mouse tails were amplified by PCR using three primers: 5′-ATGGATGACGAGACCCTGAATG-3′ (G16, see Fig. 1A), 5′-GCGCTGCGAATCGGGAGCGGCGATACCGT-3′ (Neo), and 5′-GGTCTCTGGTCAGTTTAAACAGTTGGG-3′ (G17). The PCR program consisted of 35 cycles of 94°C for 30 sec, 64°C for 1 min, and 72°C for 2 min. Approximately 1.3- and 2.1-kbp DNA fragments were produced by the primer sets G16/G17 and Neo/G17, respectively.
Southern blot analysis
Genomic DNA samples (10 μg) were digested with BamHI and HindIII, subjected to electrophoresis in agarose gels, and transferred onto Hybond-N+ membranes (GE Healthcare, Piscataway, NJ, USA). The blots were hybridized with a 32P-labeled HincII-SphI DNA fragment (Fig. 1A), as described previously [14].
Northern blot analysis
Total RNA samples (5 μg) of mouse testicular tissues were prepared using the ISOGEN Kit (Nippon Gene, Toyama, Japan). The RNA samples were denatured with glyoxal, separated on agarose gels, and transferred onto Hybond-N+ nylon membranes (GE Healthcare) [13]. The blots were probed with 32P-labeled DNA fragments.
Reverse transcription polymerase chain reaction (RT-PCR)
Total RNAs (1 μg) were digested with RQ1 RNase-free DNase (Promega, Madison, WI, USA) and reverse-transcribed in the presence of an oligo(dT)20 primer using a SuperScript III First-Strand Synthesis System (Thermo Fisher Scientific, Waltham, MA, USA). A portion of the synthesized cDNA was amplified by PCR using the following sets of primers: Pabpc2, 5′-GGAATTCGCCACCATGGCCTCGCTCTATG-3′ and 5′-CCTGCCAAAGAGGCCATTCA-3′; Actb, 5′-GGTGACAGCATTGCTTCTGTG-3′ and 5′-GGAATTCAAGTCAGTGTACAGGCCAG-3′. The PCR program consisted of 30 cycles of 98°C for 10 sec, 55°C for 15 sec, and 68°C for 1 min.
Antibodies
Affinity-purified rabbit polyclonal antibodies against the 13- and 12-residue peptides specific for mouse PABPC1 and PABPC2, respectively, were prepared as described previously [17]. A polyclonal antibody capable of recognizing both mouse PABPC1 and PABPC2 (hereafter called the anti-PABPC1/2 antibody) was also prepared using a 6 × His- and thioredoxin (TRX)-tagged fragment of the PABPC1 protein (amino acid residues 53–363) as an antigen. The 311-residue sequence is 88% identical between PABPC1 and PABPC2 [16]. Briefly, the recombinant PABPC1 was expressed in Escherichia coli and purified on a Ni-NTA His column (Merck Millipore, Billerica, MA, USA). The purified protein (400 μg) was emulsified with Freund’s complete (Becton Dickinson, Franklin Lakes, NJ, USA) or incomplete adjuvant (Wako, Osaka, Japan) and injected into female New Zealand White rabbits (Japan SLC) [17]. The antisera were fractionated with ammonium sulfate (0–40% saturation) followed by immunoaffinity chromatography on a Sepharose 4B (GE Healthcare) column conjugated with the 311-residue fragment of PABPC1 protein fused to glutathione S-transferase (GST), as described previously [24]. A mouse monoclonal antibody against murine A-kinase anchor protein 4 (AKAP4/AKAP82/PRKA4; sc-135827) and goat polyclonal antibodies against human phosphoglycerate kinase 2 (PGK2; sc-133905), human protamine 2 (PRM2; sc-23104), and mouse transition protein 2 (TNP2; sc-21106) were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Mouse monoclonal anti-β-actin (ACTB; A5441) and rabbit polyclonal anti-His tag antibodies (PM032) were obtained from Sigma-Aldrich (St. Louis, MO, USA) and Medical & Biological Laboratories (Nagoya, Japan), respectively.
Immunoblot analysis
Testicular tissues were homogenized at 4°C in 20 mM Tris-HCl, pH 7.5, containing 150 mM NaCl, 0.5% Nonidet P-40, 1 μg/ml leupeptin, 1 μg/ml pepstatin A, and 0.5 mM phenylmethanesulfonyl fluoride, using a Teflon-glass homogenizer (750 rpm, 10 strokes). After incubation at 4°C for 4 h, the homogenates were centrifuged in a microcentrifuge at 13,400 × g for 10 min at 4°C. The supernatant solution was used as protein extracts. Protein concentration was determined by means of the Coomassie Protein Assay Reagent Kit (Thermo Fisher Scientific). Protein samples (5 μg) were subjected to SDS-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes (Merck Millipore). After blocking with 2% skim milk or gelatin, the blots were probed with primary antibodies followed by incubation with horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA, USA). The immunoreactive proteins were visualized by an ECL or an ECL Prime Western Blot Detection Kit (GE Healthcare).
Histological analysis
Testicular and epididymal tissues were fixed with Bouin’s fixative and embedded in paraffin. Paraffin sections (4-μm thick) were prepared in a MICROM HM340E (Microedge Instruments, White Rock, BC, Canada), mounted on slides, deparaffinized in xylene, and hydrated in a graded ethanol series. After staining with hematoxylin and eosin (Wako), the slides were examined under a DM IRBE microscope (Leica Microsystems, Wetzlar, Germany).
Statistical analysis
The data are presented as mean ± SEM (n ≥ 3), unless stated otherwise. The Student t-test was used for statistical analysis; significance was assumed at P < 0.05.
Ethics statement
All animal experiments were approved and performed in accordance with the Guide for the Care and Use of Laboratory Animals at the University of Tsukuba (approval numbers 14-022 and 15-015).
Results
To elucidate the function of PABPC2 in spermatogenesis, we produced mice carrying a null mutation of Pabpc2 using homologous recombination in ES cells (Fig. 1A). A targeting vector was designed to delete PABPC2 by replacing the 871-nucleotide protein-coding region containing the RNA-recognition motifs 1 through 4 with the neo cassette. When genomic DNAs of selected ES cell clones were subjected to Southern blot analysis, a correctly targeted allele yielded an expected band corresponding to a 5.4-kbp BamHI-HindIII DNA fragment, in addition to a 7.2-kbp HindIII-HindIII fragment derived from the wild-type allele (Fig. 1B, upper panel). The genotypes of Pabpc2+/+, Pabpc2+/–, and Pabpc2–/– mice were also verified by PCR using tail genomic DNA as a template (Fig. 1B, lower panel). Moreover, RT-PCR analysis indicated the loss of functional Pabpc2 mRNA in Pabpc2–/– testes (Fig. 1C). Indeed, testicular extracts of Pabpc2–/– mice lacked 69-kDa PABPC2, whereas 70-kDa PABPC1 was normally present (Fig. 1D). These results prove the successful gene knockout of Pabpc2.
Intercross between the Pabpc2+/– male and female mice yielded an expected Mendelian frequency in the offspring [Pabpc2+/+: Pabpc2+/–: Pabpc2–/– = 23 (27%): 41 (49%): 20 (24%) for 84 pups from 10 litters]. Both Pabpc2–/– males and females apparently demonstrated normal behavior, body weight, and health. When the male fertility was tested by mating two of each Pabpc2+/+ and Pabpc2–/– males with wild-type females, no significant differences were observed between the Pabpc2+/+ and Pabpc2–/– males (Fig. 2A). The testicular weights of Pabpc2+/+ and Pabpc2–/– mice were also similar (Fig. 2B). Histological analysis indicated that spermatogenesis and sperm migration in the testes and epididymides, respectively, of Pabpc2–/– mice proceeded normally (Fig. 2C). Moreover, Pabpc2–/– epididymal sperm were morphologically indistinguishable from Pabpc2+/+ sperm (Fig. 2D). These results suggest that spermatogenesis and fertility are not affected by the loss of PABPC2. It should be noted that the Pabpc2–/– females also showed normal fertility and produced litters of normal size (8.7 ± 0.5 offspring for seven litters).
Fig. 2.
Characterization of the male PABPC2 knockout mice. (A) Fertility of male mice. Ten-week-old wild-type (WT) and PABPC2 knockout (KO) males were mated with 8-week-old C57BL/6 females. Two different KO males were fertile, and the pups born were counted (seven litters). (B) Testicular weight. Testicular tissues of 13-week-old WT and KO mice were weighted (n = 6). (C) Histological analysis. Testicular and epididymal slices were stained with hematoxylin and eosin and examined under a microscope. The scale bar is 50 μm. (D) Sperm morphology. Epididymal sperm of WT and KO mice were double-stained with Hoechst 33342 (blue) and MitoTracker Green FM (green) and then examined. The scale bar is 10 μm.
We next examined the effect of the PABPC2 knockout on mRNA metabolism in spermatogenic cells. Northern blot analysis indicated that the levels of five haploid-specific mRNAs, including Prm1, Prm2, and Tnp2 mRNAs, were similar among Pabpc2+/+, Pabpc2+/–, and Pabpc2–/– mice (Fig. 3A). Notably, the abundance of poly(A)-shortened forms of Prm1, Prm2, and Tnp2 mRNAs of the sizes 0.4, 0.6, and 0.6 kb, respectively, in the Pabpc2–/– testes was comparable with that in the Pabpc2+/+ and Pabpc2+/– testes. Consistent with the previous findings that poly(A) shortening accompanies translational activation of these three mRNAs [8, 9], the PRM2 and TNP2 levels were unaffected by the loss of PABPC2 (Fig. 3B). The levels of PGK2 and AKAP4 were also similar among the Pabpc2+/+, Pabpc2+/–, and Pabpc2–/– testes.
Fig. 3.
Expression and translation of haploid-specific mRNAs in Pabpc2–/– mice. (A) Northern blot analysis. Testicular total RNAs of the wild-type (+/+), heterozygous (+/–), and homozygous (–/–) mice were analyzed by Northern blot hybridization with the cDNA probes indicated. Actb served as a control. (B and C) Immunoblot analysis. Testicular protein extracts of 11-week-old mice (B) or 26-day-old (26 d) and 11-week-old mice (C) were analyzed by immunoblotting with the antibodies indicated. ACTB was used as a control. (D) Normal translational control of haploid-specific mRNAs in Pabpc2–/– mice. Testicular total RNAs of 26-day-old and 11-week-old mice were analyzed by Northern blot hybridization. Actb served as a control.
To test whether the loss of PABPC2 elicits precocious translation of Prm2, Tnp2, Pgk2, and Akap4 mRNAs in round spermatids, we conducted an immunoblot analysis (Fig. 3C). We examined the testicular protein extracts of mice 26 days and 11 weeks after birth because the 26-day-old testes contain no elongating spermatids [6]. Four proteins —PRM2, TNP2, PGK2, and AKAP4— present only in elongating spermatids were absent in the 26-day-old testes of Pabpc2+/– and Pabpc2–/– mice (Fig. 3C). In addition, the 11-week-old Pabpc2+/– and Pabpc2–/– testes contained equal amounts of these four proteins. As expected, the 0.4-kb Prm1, 0.6-kb Prm2, and 0.6-kb Tnp2 mRNAs were absent in the 26-day-old Pabpc2+/– and Pabpc2–/– testes but present in the 11-week-old testes (Fig. 3D). Thus, PABPC2 may have no effect on the regulated translation of haploid-specific mRNAs during spermiogenesis.
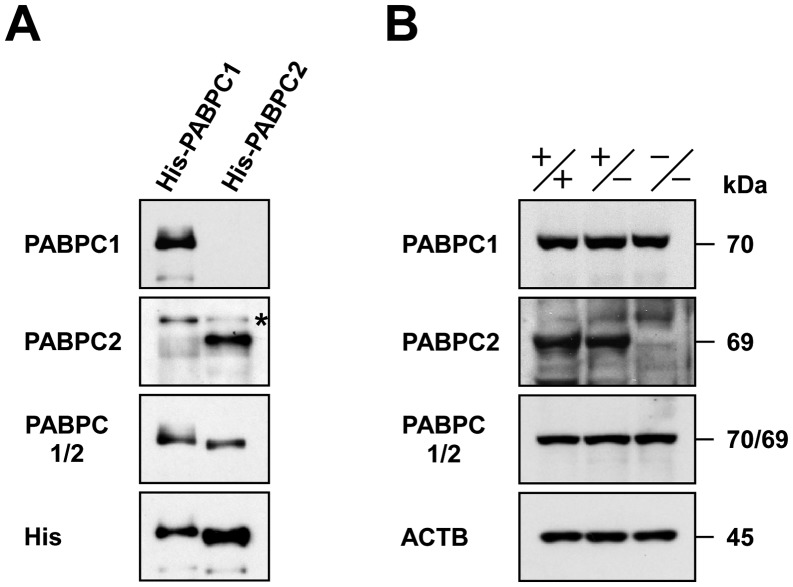
As described above, murine spermatogenic cells contain at least two isoforms of PABPCs, 70-kDa PABPC1 and 69-kDa PABPC2, that share a relatively high degree of sequence identity (approximately 80%) [16]. Although PABPC1 is distinguished from PABPC2 by the expression pattern during spermatogenesis [17], the levels of expression of these two proteins in spermatogenic cells are still unknown. Accordingly, we prepared the affinity-purified anti-PABPC1/2 antibody capable of recognizing both PABPC1 and PABPC2, using a 311-residue fragment of the PABPC1 protein as an antigen. Specificity of the anti-PABPC1/2 antibody was validated by immunoblot analysis of His-tagged recombinant PABPC1 and PABPC2 proteins (Fig. 4A). Anti-PABPC1 and anti-PABPC2 antibodies specifically recognized the recombinant PABPC1 and PABPC2, respectively, as described previously [17]. Predictably, the anti-PABPC1/2 antibody recognized these two recombinant proteins equivalently. When testicular protein extracts were analyzed by immunoblotting, the anti-PABPC1/2 antibody yielded an immunoreactive band corresponding to both PABPC1 and PABPC2 or only PABPC1 in Pabpc2+/+,Pabpc2+/–, and Pabpc2–/– mice (Fig. 4B). Densitometric analysis of the immunoblots (n = 2) indicated that the average ratio of signal intensities was 100: 113: 114 for the Pabpc2+/+, Pabpc2+/–, and Pabpc2–/– testes, respectively. Thus, only a small amount of PABPC2 may be present in spermatogenic cells as compared to PABPC1.
Fig. 4.
Abundance of PABPC2 in spermatogenic cells. Immunoblot analysis of His-tagged recombinant PABPC1 and PABPC2 proteins (A) or testicular protein extracts from the wild-type (+/+), heterozygous (+/–), and homozygous (–/–) mice (B) was carried out using the antibodies indicated. An asterisk indicates that a nonspecific protein reacted with the anti-PABPC2 antibody. ACTB served as a control.
Discussion
This study describes functional compensation for the loss of mouse PABPC2 in spermatogenic cells. We [17] previously found that PABPC2 is completely or largely absent both in elongating spermatids and in actively translating polyribosomes. These findings raise the possibility that PABPC2 protects haploid-specific mRNAs, including Prm2 and Tnp2 mRNAs, from precocious translation in round spermatids. It is also likely that the absence of PABPC2 in elongating spermatids is implicated in deadenylation of the haploid-specific mRNAs as a prerequisite for translation. Nonetheless, our present data indicate that the haploid-specific mRNAs are normally transcribed in round spermatids and translated in elongating spermatids. Thus, PABPC2 may be either functionally redundant with other PABPCs (including PABPC1) or largely dispensable for translational regulation during spermiogenesis. The low abundance of PABPC2 relative to PABPC1 in spermatogenic cells appears to support this notion.
Proteins of the PABPC family perform important functions in vertebrate cells [18, 19, 25]. Despite the presence of PABPC1, depletion of mouse PABPC4/iPABPC results in a change of the steady-state levels of some erythroid mRNAs, leading to inhibition of terminal erythroid maturation [19]. Female mice lacking germ line-specific PABPC1L/ePABPC are infertile; the oocytes fail to mature because protein synthesis is impaired by the abrogated cytoplasmic polyadenylation of maternal mRNAs [18, 26]. The oogenesis-specific defects in the PABPC1L/ePABPC-null mice may be explained by the absence of PABPC1 until four-cell embryos [27], although male germ cells contain both PABPC1 and PABPC1L/ePABPC [28]. As described above, PABPC1 and PABPC2 are both present in pachytene spermatocytes and round spermatids [17]. Thus, even if PABPC2 is involved in the translational mechanism, the loss of PABPC2 in Pabpc2–/– spermatogenic cells may be compensated by PABPC1, as in PABPC1L/ePABPC-null spermatogenic cells.
A puzzling question is why functionally redundant Pabpc2 is expressed in spermatogenic cells. Intronless genes that are specifically expressed in testes, including Pabpc2, have arisen from intron-containing progenitor genes by retroposition [23, 29, 30]. It is noteworthy that the retroposition from the X-linked genes has been suggested to be necessary as a compensation mechanism for depletion of somatic isoforms caused by meiotic X chromosome inactivation during spermatogenesis [31]. Indeed, inactivation of genes encoding polyadenylation factor CSTF2T/τCstF-64 or centriole protein CETN1 (centrin 1) results in the spermatogenic arrest [32, 33]. In contrast, Pabpc2 is believed to be retroposed from autosomal Pabpc1 [23]. One possible explanation for the exclusive expression of Pabpc2 in spermatogenic cells may be transcriptional promiscuity induced by a high concentration of RNA polymerase II holoenzyme in meiotic and early haploid cells; this state of affairs may enable transcription from the gene promoters inactive in other cells [23, 34, 35]. A similar scenario may be applicable to intronless Pabpc3/tPabp exclusively expressed in primate testes [36].
Acknowledgments
We dedicate this paper to the late Dr Masanori Kimura. We also thank Mr Yoshihiro Nakamoto and Biotechnology Research and Development, Osaka, Japan, for technical assistance and injection of ES cell clones, respectively. This work was partly supported by the Japan Society for the Promotion of Science (grant # 22580384 to S.K.) and by the Ministry of Education, Culture, Sports, Science and Technology (grant # 23013005 to SK).
References
- 1.Steger K. Transcriptional and translational regulation of gene expression in haploid spermatids. Anat Embryol (Berl) 1999; 199: 471–487. [DOI] [PubMed] [Google Scholar]
- 2.Kleene KC. A possible meiotic function of the peculiar patterns of gene expression in mammalian spermatogenic cells. Mech Dev 2001; 106: 3–23. [DOI] [PubMed] [Google Scholar]
- 3.Steger K. Haploid spermatids exhibit translationally repressed mRNAs. Anat Embryol (Berl) 2001; 203: 323–334. [DOI] [PubMed] [Google Scholar]
- 4.Kimmins S, Kotaja N, Davidson I, Sassone-Corsi P. Testis-specific transcription mechanisms promoting male germ-cell differentiation. Reproduction 2004; 128: 5–12. [DOI] [PubMed] [Google Scholar]
- 5.Idler RK, Yan W. Control of messenger RNA fate by RNA-binding proteins: an emphasis on mammalian spermatogenesis. J Androl 2012; 33: 309–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleene KC. Connecting cis-elements and trans-factors with mechanisms of developmental regulation of mRNA translation in meiotic and haploid mammalian spermatogenic cells. Reproduction 2013; 146: R1–R19. [DOI] [PubMed] [Google Scholar]
- 7.Kierszenbaum AL, Tres LL. Structural and transcriptional features of the mouse spermatid genome. J Cell Biol 1975; 65: 258–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kleene KC, Distel RJ, Hecht NB. Translational regulation and deadenylation of a protamine mRNA during spermiogenesis in the mouse. Dev Biol 1984; 105: 71–79. [DOI] [PubMed] [Google Scholar]
- 9.Kleene KC. Poly(A) shortening accompanies the activation of translation of five mRNAs during spermiogenesis in the mouse. Development 1989; 106: 367–373. [DOI] [PubMed] [Google Scholar]
- 10.Kilpatrick DL, Borland K, Jin DF. Differential expression of opioid peptide genes by testicular germ cells and somatic cells. Proc Natl Acad Sci USA 1987; 84: 5695–5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujimoto H, Erickson RP, Toné S. Changes in polyadenylation of lactate dehydrogenase-X mRNA during spermatogenesis in mice. Mol Reprod Dev 1988; 1: 27–34. [DOI] [PubMed] [Google Scholar]
- 12.Alcivar AA, Hake LE, Mali P, Kaipia A, Parvinen M, Hecht NB. Developmental and differential expression of the ornithine decarboxylase gene in rodent testis. Biol Reprod 1989; 41: 1133–1142. [DOI] [PubMed] [Google Scholar]
- 13.Kashiwabara S, Zhuang T, Yamagata K, Noguchi J, Fukamizu A, Baba T. Identification of a novel isoform of poly(A) polymerase, TPAP, specifically present in the cytoplasm of spermatogenic cells. Dev Biol 2000; 228: 106–115. [DOI] [PubMed] [Google Scholar]
- 14.Kashiwabara S, Noguchi J, Zhuang T, Ohmura K, Honda A, Sugiura S, Miyamoto K, Takahashi S, Inoue K, Ogura A, Baba T. Regulation of spermatogenesis by testis-specific, cytoplasmic poly(A) polymerase TPAP. Science 2002; 298: 1999–2002. [DOI] [PubMed] [Google Scholar]
- 15.Smith RWP, Blee TKP, Gray NK. Poly(A)-binding proteins are required for diverse biological processes in metazoans. Biochem Soc Trans 2014; 42: 1229–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kleene KC, Wang M-Y, Cutler M, Hall C, Shih D. Developmental expression of poly(A) binding protein mRNAs during spermatogenesis in the mouse. Mol Reprod Dev 1994; 39: 355–364. [DOI] [PubMed] [Google Scholar]
- 17.Kimura M, Ishida K, Kashiwabara S, Baba T. Characterization of two cytoplasmic poly(A)-binding proteins, PABPC1 and PABPC2, in mouse spermatogenic cells. Biol Reprod 2009; 80: 545–554. [DOI] [PubMed] [Google Scholar]
- 18.Guzeloglu-Kayisli O, Lalioti MD, Aydiner F, Sasson I, Ilbay O, Sakkas D, Lowther KM, Mehlmann LM, Seli E. Embryonic poly(A)-binding protein (EPAB) is required for oocyte maturation and female fertility in mice. Biochem J 2012; 446: 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kini HK, Kong J, Liebhaber SA. Cytoplasmic poly(A) binding protein C4 serves a critical role in erythroid differentiation. Mol Cell Biol 2014; 34: 1300–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brook M, Gray NK. The role of mammalian poly(A)-binding proteins in co-ordinating mRNA turnover. Biochem Soc Trans 2012; 40: 856–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goss DJ, Kleiman FE. Poly(A) binding proteins: are they all created equal? Wiley Interdiscip Rev RNA 2013; 4: 167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eliseeva IA, Lyabin DN, Ovchinnikov LP. Poly(A)-binding proteins: structure, domain organization, and activity regulation. Biochemistry (Mosc) 2013; 78: 1377–1391. [DOI] [PubMed] [Google Scholar]
- 23.Kleene KC, Mulligan E, Steiger D, Donohue K, Mastrangelo M-A. The mouse gene encoding the testis-specific isoform of poly(A) binding protein (Pabp2) is an expressed retroposon: intimations that gene expression in spermatogenic cells facilitates the creation of new genes. J Mol Evol 1998; 47: 275–281. [DOI] [PubMed] [Google Scholar]
- 24.Kanemori Y, Ryu J-H, Sudo M, Niida-Araida Y, Kodaira K, Takenaka M, Kohno N, Sugiura S, Kashiwabara S, Baba T. Two functional forms of ACRBP/sp32 are produced by pre-mRNA alternative splicing in the mouse. Biol Reprod 2013; 88: 105. [DOI] [PubMed] [Google Scholar]
- 25.Gorgoni B, Richardson WA, Burgess HM, Anderson RC, Wilkie GS, Gautier P, Martins JPS, Brook M, Sheets MD, Gray NK. Poly(A)-binding proteins are functionally distinct and have essential roles during vertebrate development. Proc Natl Acad Sci USA 2011; 108: 7844–7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozturk S, Guzeloglu-Kayisli O, Lowther KM, Lalioti MD, Sakkas D, Seli E. Epab is dispensable for mouse spermatogenesis and male fertility. Mol Reprod Dev 2014; 81: 390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seli E, Lalioti MD, Flaherty SM, Sakkas D, Terzi N, Steitz JA. An embryonic poly(A)-binding protein (ePAB) is expressed in mouse oocytes and early preimplantation embryos. Proc Natl Acad Sci USA 2005; 102: 367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ozturk S, Guzeloglu-Kayisli O, Demir N, Sozen B, Ilbay O, Lalioti MD, Seli E. Epab and Pabpc1 are differentially expressed during male germ cell development. Reprod Sci 2012; 19: 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marques AC, Dupanloup I, Vinckenbosch N, Reymond A, Kaessmann H. Emergence of young human genes after a burst of retroposition in primates. PLoS Biol 2005; 3: e357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanaka H, Baba T. Gene expression in spermiogenesis. Cell Mol Life Sci 2005; 62: 344–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turner JMA. Meiotic sex chromosome inactivation. Development 2007; 134: 1823–1831. [DOI] [PubMed] [Google Scholar]
- 32.Dass B, Tardif S, Park JY, Tian B, Weitlauf HM, Hess RA, Carnes K, Griswold MD, Small CL, MacDonald CC. Loss of polyadenylation protein τCstF-64 causes spermatogenic defects and male infertility. Proc Natl Acad Sci USA 2007; 104: 20374–20379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avasthi P, Scheel JF, Ying G, Frederick JM, Baehr W, Wolfrum U. Germline deletion of Cetn1 causes infertility in male mice. J Cell Sci 2013; 126: 3204–3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidt EE, Schibler U. High accumulation of components of the RNA polymerase II transcription machinery in rodent spermatids. Development 1995; 121: 2373–2383. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt EE. Transcriptional promiscuity in testes. Curr Biol 1996; 6: 768–769. [DOI] [PubMed] [Google Scholar]
- 36.Féral C, Guellaën G, Pawlak A. Human testis expresses a specific poly(A)-binding protein. Nucleic Acids Res 2001; 29: 1872–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]