Abstract
Aortic aneurysm (AA) is one of the life-threatening aortic diseases, leading to aortic rupture of any cause including atherosclerotic and non-atherosclerotic diseases. AA is diagnosed in a variable proportion of patients with dilated aorta by imaging modality. The etiopathogenesis of AA remains unclear in many aortic diseases. Furthermore, although it may be difficult to explain all phenotypes of patients even if genetic mutation could be identified in some proteins such as smooth muscle cell α-actin (ACTA2), myosin heavy chain 11 (MYH11) or SMAD3, individualized consideration of these factors in each patient is essential on the basis of clinicopathological characteristics.
Keywords: fibrillin-1, TGFβ, ACTA2, MYH11, SMAD3
Introduction
Aortic aneurysm is potentially lethal conditions that require prompt evaluation and treatment. It is caused by the lesion on the basis of atherosclerotic and non-atherosclerotic mechanism. Atherosclerotic mechanisms consist of typically true aneurysms with atheroma and subadventitial pseudoaneurysm due to progression of the penetrating atherosclerotic ulcer (PAU). Non-atherosclerotic mechanisms include inflammation and degeneration. Histopathological features of these conditions are meant to assist physicians who bring their own judgment in diagnosis by using imaging modality for individual patient. Therefore this article focuses on the clinicopathological features including etiology of aortic aneurysm.
Atherosclerosis
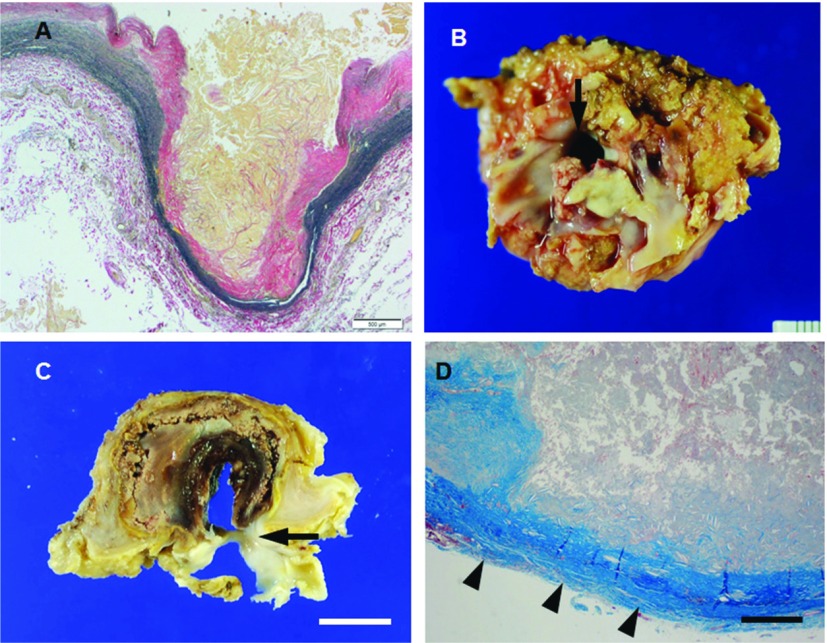
Abdominal aortic aneurysms (AAA) most commonly involve the infrarenal segment and typically true aneurysms that consist of three layers of aortic wall with atheroma. The prevalence of AAA increases with age and is most common in men.1) When atheromatous lesion extends into the media with loss of elastic fibers, the aortic wall is thinned and results in aneurysmal formation (Fig. 1A). Risk factors for developing AAA include positive family history, peripheral vascular disease, and smoking. Among the most important are atherosclerosis, dissection and aneurysm formation (dissecting aneurysm). Virchow et al. considered the major cause of dissecting aneurysm to be atheromatous ulceration with dissection of blood between intima and media. The thrombotic hematoma began at primary intimal rupture, leading to an atheromatous ulcer in four of his cases.2) In 1986, Stanson et al. described an ulceration of atherosclerotic plaque that allows blood from the aortic lumen to contact the media of the aortic wall as PAU of the thoracic aorta.3) PAU also may resolve spontaneously, but can progress to an enlarging intramural hematoma, dissection and dissecting aneurysm or subadventitial pseudoaneurysm (Figs. 1B–1D).
Fig. 1.
(A, B) Microphotograph of the abdominal aortic wall section stained with elastic van Gieson from patient (69 y.o., male) with atherosclerotic aneurysm. Atheromatous lesion extends into the media with loss of elastic fibers, the abdominal aortic wall is thinned and results in aneurysm. (B–D) Macrophotographs (B, C) and microphotograph (D, Masson’s trichrome) of the thotacic aorta from patient (79 y.o., female) with PAU. Ulceration of atherosclerotic plaque that contact to the adventitia (arrowheads in D) with intimal disruption (arrows in B and C) of the thoracic aorta, leading to subadventitial pseudoaneurysm. Scale bars: Panel A: 500 µm; Panel C: 1 cm; Panel D: 200 µm. PAU: penetrating atherosclerotic ulcer
Inflammation
Infected aortic aneurysm
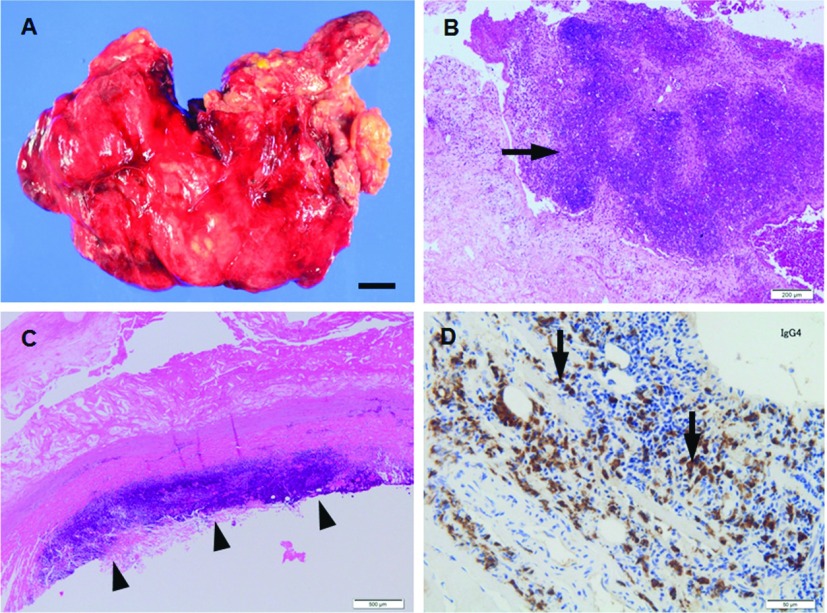
Osler, 1885, reported a 30-year-old patient with endocarditis who died of one of four aneurysms of the thoracic aorta as the term mycotic aneurysm.4) He described findings as bacterial endocarditis with subsequent bacterial embolization leading to an infected arterial wall and aneurysmal with fungus vegetations. Infected aortic aneurysm is sometimes termed “mycotic” aortic aneurysm. However, only a minor fraction of this disease is caused by fungus. The prevalence is considered to comprise 0.7%–2.6% of all cases of aortic aneurysm.5) About 70% of the infected aneurysms were found to be located in the thoracic and abdominal aorta at or above the renal arteries. In general, infected aortic aneurysm appears usually as a saccular form aneurysm with irregular configuration (Fig. 2A). Inflammation with bacterial colony and neutrophils infiltrate are characteristic features in the aorta with medial destruction due to infection via the vasa vasorum (Fig. 2B). Early and timely surgery with perioperative antimicrobial treatment is thought to be mandatory.
Fig. 2.
(A, B) Macrophotograph (A) and microphotograph (B, H.&E.) of the abdominal aortic wall from patient (69 y.o., male) with infected aortic aneurysm. Aortic aneurysm shows saccular form aneurysm with irregular configulation (A) and neutrophils infiltrate with medial destruction (arrow in B). (C, D) Microphotographs (C, H.&E.; D, immunohistochemistry for IgG4) of the abdominal aortic wall sections from patient (69 y.o., male) with IAAA. Fibrosing adventitia shows lymphoplasmacytic infiltrate that appears as a bands and clusters (C). Large population of plasma cells shows positive reactivity for IgG4 (D). Scale bars: Panel A: 1 cm; Panel B: 200 µm; Panel C: 500 µm; Panel D: 50 µm. IAAA: Inflammatory abdominal aortic aneurysm
Inflammatory abdominal aortic aneurysm (IAAA)
In 1972, Walker et al. first used the term IAAA to describe some aneurysms that are characterized by diffuse thickness of the aneurysmal wall and extensive fibrous adhesion.3) In comparison to atherosclerotic aneurysm, IAAA has different characteristics such as younger age of the patients, symptoms such as backache, low-grade fever, or weight loss. Histological characteristic features are extensive adventitial thickening and fibrosis with medial and adventitial lymphoplasmacytic infiltrate that appears as a bands and clusters (Fig. 2C). Adventitia shows entrapment of nerves and adjacent inflammatory infiltrate in dense periaortic fibrosis, which may be adherent to the surrounding retroperitoneal structures.6) Most aneurysms are found to be located in the infrarenal abdominal aorta and in male patients.7) Although the cause of IAAA is not known, histopathologic findings suggest that lipids or products of lipid oxidation are present in the aortic adventitia. It is reported that the adventitia shows the presence of immunoglobulin (Ig) G4-positive plasma cells (Fig. 2D) in some IAAA.8)
Aortic vasculitis
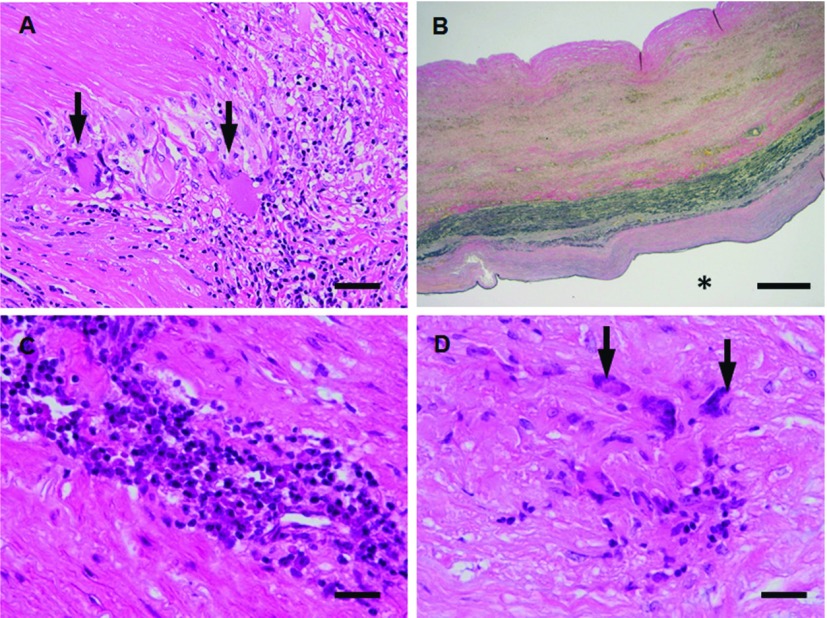
Specific disorders that may present with aortitis include Takayasu arteritis (TA) and giant cell (temporal) arteritis (GCA). TA is a primary inflammatory disease of elastic arteries such as aorta, its branches and pulmonary artery trunk. This disease was reported in 1908 by an ophthalmologist, Mikito Takayasu. In 1948, Shimizu and Sano called it the “pulseless disease’’. The etiology remains obscure so far. This disease is reported to be prevalent in India, Thailand, Japan, China, Korea and Israel. The sex ratio was 1:8 (M:F). Nasu and LupiHerrera classified into four main types, respectively as Table 1.8,9) The characteristic features are irregular luminal narrowing caused by the intimal proliferation and fibrotic contraction of the media and adventitia. Aneurysmal formation is the less commonly known complication. However, the incidence of dilated lesions is increasing in ascending aorta of prolonged cases. Granulomatous inflammation with coagulative necrosis and elasticophagia is an important finding in addition to intimal proliferation and obliteration without fibrinoid necrosis of the vasa vasorum (Fig. 3A). Inflammatory lesions originate in the outer area of the media and a part of the adventitia through the vasa vasorum, and gradually progress to fibrosis (Fig. 3B). When fibrosis is delayed, the aortic wall is thinned and often results in aneurysmal formation. Differential disorders are GCA, isolated thoracic aortitis (ITA) as well as mesoaortitis syphilitica, tuberculous aortitis, Behcet disease, Buerger disease and rheumatic aortitis. In contrast with TA, GCA exclusively affects adults more than 50 years of age. It typically involves the extracranial branches of the carotid arteries (temporal arteries), but can also involve the thoracic aorta commonly (10%–15% of GCA). Inflammation is most pronounced in the inner one half of the aorta in GCA. The precise etiology of ITA is largely unknown, although recently increasing published case reports. Some cases of ITA may be associated with IgG4-related systemic disease. The cellular infiltration was associated with granulomatous lesions (Figs. 3C and 3D), mucoid accumulation and elastic fiber fragmentation in addition to medial and adventitial fibrosis without obliteration of the vase vasorum.10)
Table 1.
Classification of Takayasu arteritis
| Nasu |
| Type I Cranial branches |
| II Ascending aorta to aortic arch |
| III Abdominal aorta and its main branches |
| IV Abroad portion of the arteries |
| LupiHerrera |
| Type I Aortic arch and its main branches |
| II Thoracoabdominal aorta sparing the aortic arch |
| III Combination of type I and type II |
| IV Pulmonary artery involvement associated with type I, II, or III |
Fig. 3.
(A, B) Microphotographs (A, H.&E.; B, elastica van Gieson) of the ascending aortic wall sections from patient (63 y.o., female) with TA. The media shows Langhans type giant cells infiltrate around coagulative necrosis (arrows in A). (C, D) Microphotographs (C, H.&E.; D, H.&E.) of the ascending aortic wall sections from patient (88 y.o., female) with ITA. The media shows lymphoplasmacytic infiltrate (C) associated with multinucleated giant cells infiltrate around necrotic tissue (arrows in D). Scale bars: Panel A: 50 µm; Panel B: 500 µm; Panel C: 25 µm; Panel D: 25 µm. * represents vascular lumen. TA: Takayasu arteritis; ITA: isolated thoracic aortitis
Degeneration
Marfan syndrome (MFS)
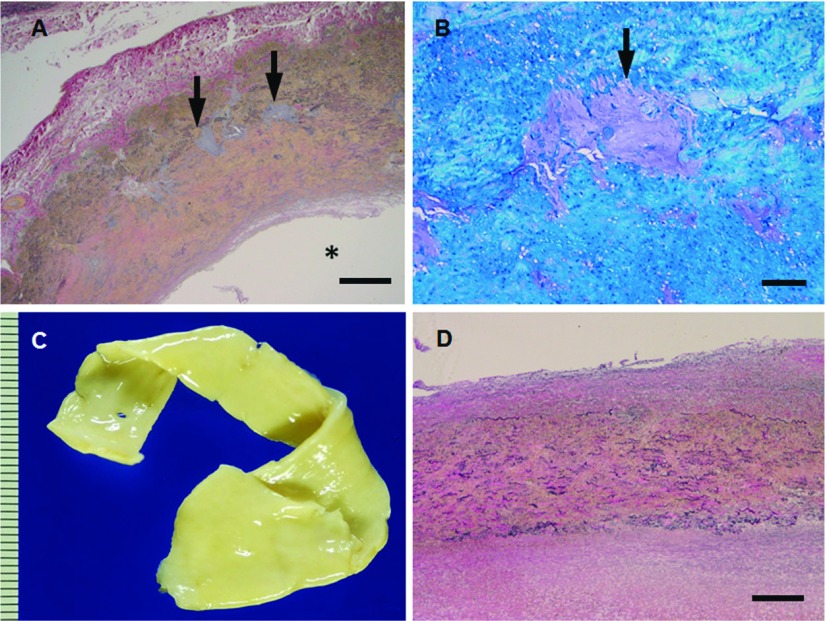
Marfan syndrome is recognized autosomal dominant connective tissue disorder resulting from mutations in the fibrillin-1 (FBN1). The syndrome includes some features such as particularly tall, lens dislocation, pectus deformity, scoliosis, long fingers, dural ectasia and aortic root aneurysm.11) FBN1 is the extracellular matrix protein. The manifestation of MFS is caused by weakness of the tissues due to loss of structural integrity resulted from heterozygous mutations in the FBN1 gene. Histological characteristic features are cystic medial degeneration with elastic fiber fragmentation of the aorta (Figs. 4A and 4B). FBN1 contributes to the increased TGFβ signaling. Indeed, aortic dilatation could be improved in patients and mice with FBN1-deficiency by TGFβ antagonist in vivo.12,13)
Fig. 4.
(A, B) Microphotographs (A, elastica van Gieson; B, toluidine blue) of the thoracic aortic wall sections from patient (30 y.o., male) with MFS. The media shows cystic medial degeneration with proteoglycan deposits and elastic fiber fragmentation of the aorta (arrows in A, B). (C, D) Macrophotograph (C) and microphotograph (D, elastica van Gieson) of thoracic aortic wall sections from patient (23 y.o., male) with LDS. Aortic wall is nonatherosclerotic and its media shows diffuse medial degeneration with elastic fiber fragmentation. Scale bars: Panel A: 500 µm; Panel B: 100 µm; Panel D: 500 µm. * represents vascular lumen. MFS: Marfan syndrome; LDS: Loeys-Dietz syndrome
Loeys-Dietz syndrome (LDS)
Loeys-Dietz syndrome is an autosomal dominant connective tissue disorder resulting from mutations in the transforming growth factor β receptors 1 and 2 (TGFBR1 and TGFBR2).14) The syndrome is characterized hypertelorism (widely spaced eyes), bifid uvula and/or cleft palate, clubfoot deformity, translucent skin with easy bruising and dystrophic scars, cervical spine malformation or instability and arterial tortuosity with aneurysm and dissection throughout the arterial tree. The aortic wall macroscopically shows non-atherosclerotic lesion with smooth surface in the lumen (Fig. 4C). Histological characteristic features are more diffuse medial degeneration with elastic fiber fragmentation and less cystic medial necrosis of the aorta compared with MFS and significantly more collagen deposition than control samples (Fig. 4D). Tissues derived from LDS patients show increased expression of nuclear enrichment of phosphorylated Smad2, indicating of increased TGFβ signaling.15)
Mutations in smooth muscle cell α-actin (ACTA2)
All vertebrates encode six tissue-specific actin isoforms including skeletal muscle (ACTA1), cardiac muscle (ACTC), vascular smooth muscle (ACTA2), visceral smooth muscle (ACTG2) and nonmuscle (ACTB and ACTG1). ACTA2 is the single most abundant protein in smooth muscle cells (SMCs).16) ACTA2 deficiency leads to impaired vascular SMC contractility and hypotension in mice. In 2007, Guo et al. identified a large family with autosomal dominant inheritance of thoracic aortic aneurysm and dissections (TAAD). It has been proposed that ACTA2 mutations in humans disrupt α-actin polymerization and lead to decreased contractility of aortic SMCs and this dysfunction leads to thoracic aortic aneurysm and dissection. Six affected members in two families had patent ductus arteriosus (PDA) and three individuals in other two families had bicuspid aortic valve. Histological characteristic features are medial degeneration with increased proteoglycan accumulation, elastic fiber fragmentation and loss, decreased numbers of SMCs, disarrangement of SMCs of aorta (Fig. 5A). The vasa vasorum shows increased size and fibromuscular dysplasia (FMD) due to SMC hyperplasia (Fig. 5B).17) These findings are reminiscent of myocyte disarray in hypertrophic cardiomyopathy (HCM).18)
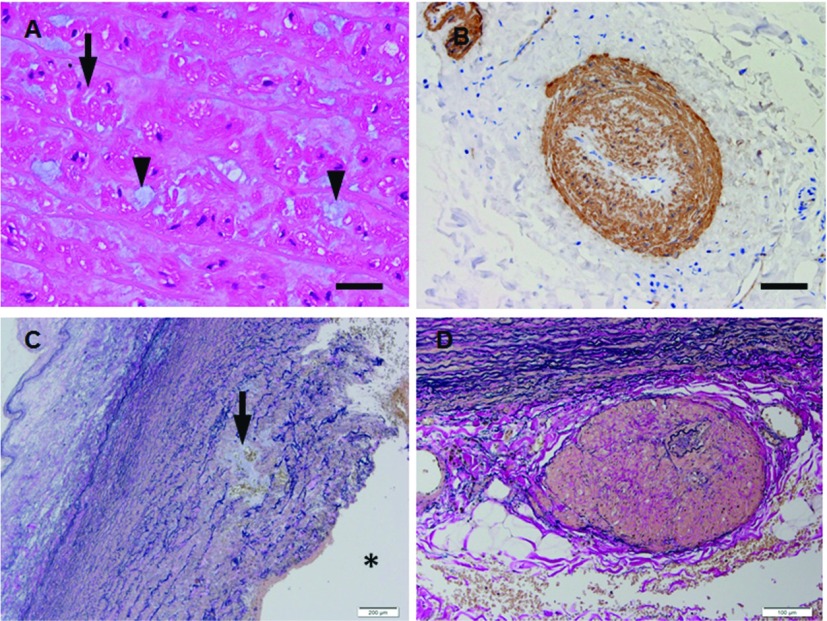
Fig. 5.
(A, B) Microphotographs (A, H.&E.; B, immunohistochemistry for a-smooth muscle actin) of the thoracic aortic wall sections from patient (50 y.o., male) with ACTA2 mutation. The aortic wall shows disarrangement of vacuolated (arrow in A) of SMCs with abundant extracellular matrix (arrowheads in A) in the media and vasa vasorum with smooth muscle cell hyperplasia (FMD) in adventitia (B). (C, D) Microphotographs (C, elastica van Gieson; D, elastica van Gieson) of the ascending aortic wall sections from patients (51 y.o., male; 56 y.o., male) with MYH11 mutation. The aortic wall shows elastic fiber fragmentation with cystic medial degeneration in the media (arrow in C) and vasa vasorum with medial thickening (FMD) in the adventtiia (D). Scale bars: Panel A: 25 µm; Panel B: 50 µm; Panel C: 200 µm; Panel D: 100 µm. * represents vascular lumen. SMC: smooth muscle cell; FMD: fibromuscular dysplasia
Mutations in myosin heavy chain 11 (MYH11)
MYH is a major and specific contractile protein composed of N-terminal globular head and C-terminal coiled-coil rod regions of SMC. In 2005, Zhu et al. have described two kindreds with MYH11 mutation affecting the rod region, presenting TAAD and PDA. The altered interaction between wild-type and mutant SM-MHC provides evidence that the mutant act via a dominant-negative mechanism. Furthermore, MYH11 heterozygous mutation leads to an early and severe decrease in the elasticity of the aortic wall. Histological characteristic features are elastic fiber fragmentation and loss, decreased numbers of SMCs, cystic medial degeneration and FMD with increased size of vasa vasorum of the aorta (Figs. 5C and 5D).19) It has also identified excessive hyperplasia of SMCs and strong positive reactivity for smooth muscle α-actin compared to control.20) MYH11 and ACTA2 mutations revealed a role for enhanced TGFβ signaling in familiar TAAD.21) Although SM-MHC-deficient mice present thin-walled bladder and abnormal intestinal movement, such anomalies are not observed in human so far.
Mutations in SMAD3
SMAD3 mutations were identified for 2% of family with autosomal dominant inheritance of TAAD with intracranial and abdominal aortic aneurysms.22) This gene encodes a protein of TGF-β pathway that is essential for TGF-β signal transmission initiated by binding to its receptors (TGFBR1 and TGFBR2). In almost all patients with aneurysms osteoarthritis syndrome, early-onset joint abnormalities including osteoarthritis, intervertebral disc degeneration, osteochondritis dissecans and menisceal anomalies are present. The craniofacial abnormalities in these individuals were hypertelorism, abnormal palate and/or uvula and dental malocclusion, dolichostenomelia, arachnodactyly, scoliosis, hypermobility and acetabular protrusion. Arterial tortuosity of the cerebral, thoracic and/or abdominal arterial tree was present in a majority of the cases. Histological characteristic features are disarray, fragmentation and loss of elastic fibers with increased collagen deposition in the media of the aorta. These heterozygous mutations lead to increased expression of the several protein of the TGF-β pathway including phosphorylated SMAD2, SMAD3, TGF-β1 in the thoracic aorta.23) SMAD3 knockout mice show a reduction of TGF-β signaling in the intervertebral discs.24)
Conclusion
Aortic aneurysm include a wide spectrum of diseases as above that may have lethal conditions, most notably aortic rupture if not diagnosed precisely and promptly. Familiarity with acute aortic syndrome, it is important to detect the aortic aneurysm by using imaging modality such as computed tomography (CT) and magnetic resonance imaging (MRI) in the standpoint of prevention for aortic rupture. The appropriate imaging on the basis of histopathological findings can lead to prompt evaluation and diagnosis, resulting in accurate treatment of patient.
Disclosure Statement
Author has no conflict of interest.
References
- 1).Bengtsson H, Bergqvist D, Sternby NH. Increasing prevalence of abdominal aortic aneurysms. A necropsy study. Eur J Surg 1992; 158: 19-23. [PubMed] [Google Scholar]
- 2).Shennan T. Dissecting aneurysms. Special report series n 193 Medical Research Council; (Great Britain: ). P86, 1933-1934. [Google Scholar]
- 3).Stanson AW, Kazmier FJ, Hollier LH, et al. Penetrating atherosclerotic ulcers of the thoracic aorta: natural history and clinicopathologic correlations. Ann Vasc Surg 1986; 1: 15-23. [DOI] [PubMed] [Google Scholar]
- 4).Osler W. The Gulstonian Lectures, on Malignant Endocarditis. Br Med J 1885; 1: 467-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Hsu RB, Chen RJ, Wang SS, et al. Infected aortic aneurysms: clinical outcome and risk factor analysis. J Vasc Surg 2004; 40: 30-5. [DOI] [PubMed] [Google Scholar]
- 6).Pennell RC, Hollier LH, Lie JT, et al. Inflammatory abdominal aortic aneurysms: a thirty-year review. J Vasc Surg 1985; 2: 859-69. [PubMed] [Google Scholar]
- 7).Walker DI, Bloor K, Williams G, et al. Inflammatory aneurysms of the abdominal aorta. Br J Surg 1972; 59: 609-14. [DOI] [PubMed] [Google Scholar]
- 8).Kasashima S, Zen Y, Kawashima A, et al. A new clinicopathological entity of IgG4-related inflammatory abdominal aortic aneurysm. J Vasc Surg 2009; 49: 1264-71; discussion 1271. [DOI] [PubMed] [Google Scholar]
- 9).Nasu T. Takayasu’s truncoarteritis. Pulseless disease or aortitis syndrome. Acta Pathol Jpn 1982; 32: 117-131. [PubMed] [Google Scholar]
- 10).Lupi-Herrera E, Sánchez-Torres G, Marcushamer J, et al. Takayasu’s arteritis. Clinical study of 107 cases. Am Heart J 1977; 93: 94-103. [DOI] [PubMed] [Google Scholar]
- 11).Laco J, Steiner I, Holubec T, et al. Isolated thoracic aortitis: clinicopathological and immunohistochemical study of 11 cases. Cardiovasc Pathol 2011; 20: 353-360. [DOI] [PubMed] [Google Scholar]
- 12).Dietz HC, Pyeritz RE. Mutations in the human gene for fibrillin-1 (FBN1) in the Marfan syndrome and related disorders. Hum Mole Genet 1995; 4: 1799-1809. [DOI] [PubMed] [Google Scholar]
- 13).Habashi JP, Judge DP, Holm TM, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 2006; 312: 117-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Brooke BS, Habashi JP, Judge DP, et al. Angiotensin II blockade and aortic-root dilation in Marfan’s syndrome. N Engl J Med 2008; 358: 2787-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Loeys BL, Chen J, Neptune ER, et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet 2005; 37: 275-81. [DOI] [PubMed] [Google Scholar]
- 16).Maleszewski JJ, Miller DV, Lu J, et al. Histopathologic findings in ascending aortas from individuals with Loeys-Dietz syndrome (LDS). Am J Surg Pathol 2009; 33: 194-201. [DOI] [PubMed] [Google Scholar]
- 17).Fatigati V, Murphy RA. Actin and tropomyosin variants in smooth muscles. Dependence on tissue type. J Biol Chem 1984; 259: 14383-8. [PubMed] [Google Scholar]
- 18).Guo DC, Pannu H, Tran-Fadulu V, et al. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet 2007; 39: 1488-93. [DOI] [PubMed] [Google Scholar]
- 19).Ahmad F, Seidman JG, Seidman CE. The genetic basis for cardiac remodeling. Annu Rev Genomics Hum Genet 2005; 6: 185-216. [DOI] [PubMed] [Google Scholar]
- 20).Zhu L, Vranckx R, Khau Van Kien P, et al. Mutations in myosin heavy chain 11 cause a syndrome associating thoracic aortic aneurysm/aortic dissection and patent ductus arteriosus. Nat Genet 2006; 38: 343-9. [DOI] [PubMed] [Google Scholar]
- 21).Renard M, Callewaert B, Baetens M, et al. Novel MYH11 and ACTA2 mutations reveal a role for enhanced TGFβ signaling in FTAAD. Int J Cardiol 2013; 165: 314-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Regalado ES, Guo DC, Villamizar C, et al. Exome sequencing identifies SMAD3 mutations as a cause of familial thoracic aortic aneurysm and dissection with intracranial and other arterial aneurysms. Circ Res 2011; 109: 680-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).van de Laar IM, Oldenburg RA, Pals G, et al. Mutations in SMAD3 cause a syndromic form of aortic aneurysms and dissections with early-onset osteoarthritis. Nat Genet 2011; 43: 121-6. [DOI] [PubMed] [Google Scholar]
- 24).Li CG, Liang QQ, Zhou Q, et al. A continuous observation of the degenerative process in the intervertebral disc of Smad3 gene knock-out mice. Spine 2009; 34: 1363-9. [DOI] [PubMed] [Google Scholar]